Chapter 1 Principles of cell biology
Anatomy and physiology are scientific terms used to describe the study of the structure of the body (anatomy) and how the body actually ‘works’ (physiology). In this section, we will study the anatomy and physiology of the dog and cat. In Section 2, the anatomy and physiology of some of the most commonly kept exotic species and of the horse are covered. We start by looking at the basic unit of the body – the cell – and then work our way through the tissues, organs and systems until the picture is complete.
Animal classification
When studying any aspect of biology it is important to have a basic understanding of the classification system used to group animals. How the species that one may meet in a veterinary practice fit into this classification system should also be understood. Classification is the way in which we ‘sort’ species into orderly groups, depending on how closely they are related in terms of their evolution, structure and behaviour. The science of classification is known as taxonomy.
If organisms have certain basic features in common they are grouped together into a kingdom. For example, if an organism is composed of more than one cell, i.e. it is multicellular, and obtains its food by ingestion, it is placed in the animal kingdom. Other kingdoms include plants and fungi. The animal kingdom is then further subdivided, based upon similarities of organisms, into a hierarchical system (Table 1.1). This narrows the classification down until we eventually reach a particular genus and species. Most living organisms are identified by a genus and species – a method known as the binomial system and invented by the Swedish scientist Carl Linnaeus.
Table 1.1 Classification of the domestic dog and cat
| Taxonomic group | Dog | Cat |
|---|---|---|
| Kingdom | Animal | Animal |
| Phylum | Chordata (Vertebrate) | Chordata (Vertebrate) |
| Class | Mammalia (Mammal) | Mammalia (Mammal) |
| Order | Carnivora | Carnivora |
| Family | Canidae | Felidae |
| Genus | Canis | Felis |
| Species | familiaris | catus |
| Common name | Domestic dog | Domestic cat |
All the species within the animal kingdom are divided into those with backbones – the vertebrates – and those that do not have backbones – the invertebrates, e.g. insects, worms, etc. The vertebrates are divided into eight classes. The classes that are of the most veterinary importance are:
These classes are then further divided into orders, and so on, until a species is identified, as in Table 1.1.
Most of this section of the book concerns the mammals, because the majority of animals seen in veterinary practice will be from this class. The distinctive features of mammals are the production of milk by the mammary glands and the possession of hair as a body covering. Examples of mammalian orders include:
Generally speaking, all mammals have a similar basic structural plan in terms of anatomy and physiology, but each species has been modified to suit its specific lifestyle. In other words, mammals have become specialised for activities such as running, digging, gnawing, jumping and eating specific foods.
Anatomical definitions
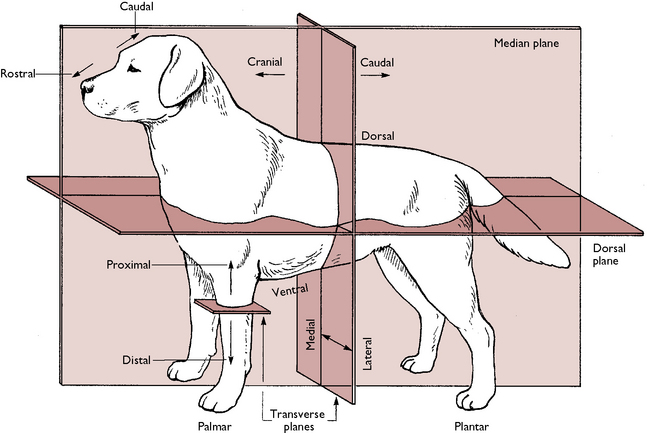
When studying anatomy and physiology it is important to understand the terms that are used to describe where structures lie in relation to one another. These are illustrated in Figure 1.1 and named as follows:
The basic plan of the body
The body is made up of a number of systems and each of them has a specific job, enabling the body to function effectively. These systems can be placed in one of three groups depending on their function:
Structural systems
Coordinating systems
Visceral systems
Each system of the body is made up of a collection of specific types of tissue arranged as organs. Each tissue is composed of a specialised type of cell (the smallest unit of the body).
The mammalian cell
Cells are the minute units of a tissue that can only be seen under a microscope. Cells can be considered to be the basic structural and functional unit of an organism. In fact they are like ‘little bodies’ themselves because they carry out a number of basic functions such as taking in nutrients and excreting waste, respiring or ‘breathing’, and reproducing. These and other functions are carried out by various structures that make up the cell – mainly by the organelles, or ‘little organs’, that float within the cytoplasm of the cell.
Cell structure and function
The components of a cell are shown in Figures 1.2 and 1.3 and are as follows:
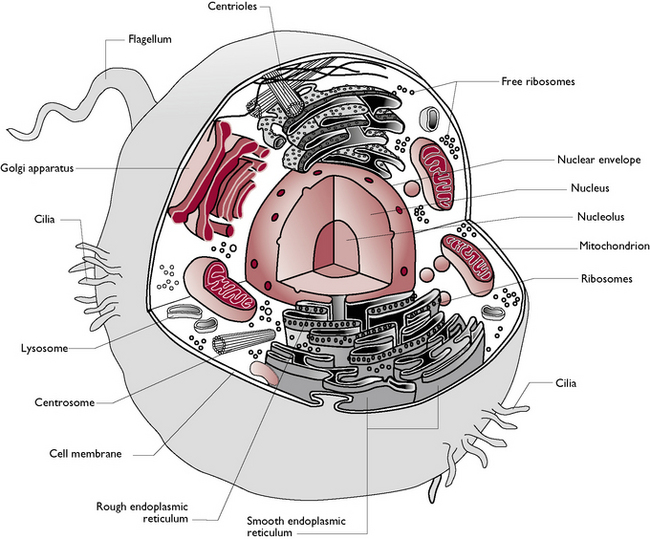
Fig. 1.2 Components of the mammalian cell.
(With permission from Colville T, Bassett JM 2001 Clinical anatomy and physiology for veterinary technicians. Mosby, St Louis, MO, p 11.)
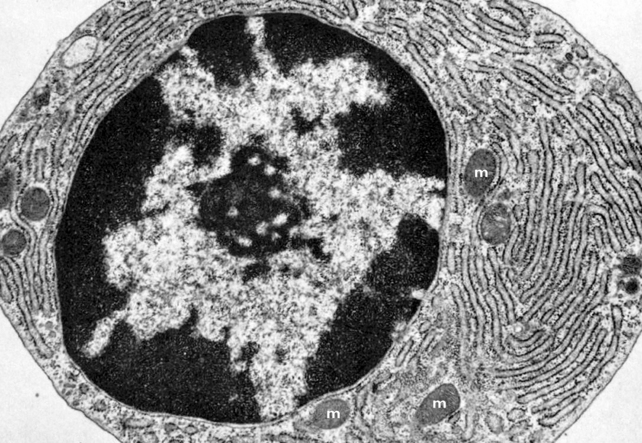
Fig 1.3 Transmission electron micrograph of a plasma cell showing extensive rough endoplasmic reticulum and scattered mitochondria (m).
(With permission from Samuelson DA 2007 Textbook of veterinary histology. Saunders-Elsevier, St Louis, MO, p 86.)
Cell membrane
The cell membrane covers the surface of the cell and may also be called the plasma membrane. It is responsible for separating the cell from its environment and controls the passage of substances in and out of the cell. Carbohydrates are found on the surface of the cell membrane and it is believed that these help cell recognition, e.g. they enable a cell to recognise whether or not it is in contact with another cell of the same type. The cell membrane of a mammalian cell is composed of a phospholipid bilayer (Fig. 1.4). This is a double layer of phospholipid molecules and has protein molecules embedded within it.
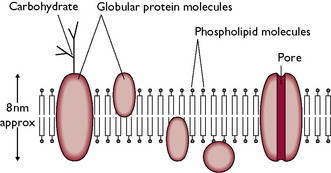
Fig. 1.4 Structure of the cell membrane showing the phospholipid bilayer. This structure is also known as the ‘fluid mosaic model’.
The nature of its structure means that the cell membrane is selectively permeable, allowing some substances to pass through it while others may either be excluded or must travel across the membrane by means of specialised transport systems. These include:
Cytoplasm
This is the fluid that fills the interior of the cell, providing it with support. The nucleus and organelles are found within the cytoplasm, along with solutes such as glucose, proteins and ions.
Nucleus
The nucleus is the information centre of the cell. It is surrounded by a nuclear membrane and contains the chromosomes. Chromosomes are the bearers of the hereditary material, DNA, which carries the information for protein synthesis. DNA is the ‘set of instructions’ that tells the cell how to function, and these instructions are then passed on to the cell’s descendants. The nucleus also contains several nucleoli, where the ribosomes (see below) are manufactured.
Organelles
 Rough endoplasmic reticulum (Fig 1.3) is so called because it has numerous ribosomes attached to its surface and thus appears ‘rough’ when viewed under a microscope. The function of rough ER is to transport the proteins that have been synthesised by ribosomes. Some of these proteins are not required by the cell in which they are made but are ‘exported’ outside the cell, e.g. digestive enzymes and hormones.
Rough endoplasmic reticulum (Fig 1.3) is so called because it has numerous ribosomes attached to its surface and thus appears ‘rough’ when viewed under a microscope. The function of rough ER is to transport the proteins that have been synthesised by ribosomes. Some of these proteins are not required by the cell in which they are made but are ‘exported’ outside the cell, e.g. digestive enzymes and hormones.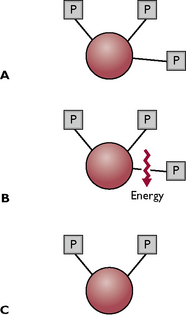
Fig. 1.5 The conversion of ATP to ADP to release energy. A The ATP molecule has three phosphate groups (P) attached by chemical bonds; energy is stored within the bonds. B One of the phosphate groups is ‘snapped off’, releasing energy. C The remaining molecule (ADP, with two phosphate groups) goes back into the metabolic cycle and has a phosphate group reattached, becoming ATP again.
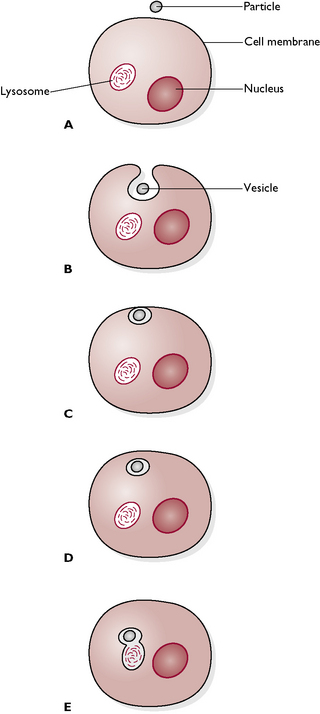
Fig. 1.6 Phagocytosis. A A small particle (e.g. bacterium) is present outside the cell. B The cell membrane invaginates and starts to enclose the particle. C The cell membrane completely surrounds the particle and seals it off in a vesicle. D The vesicle detaches from the membrane and enters the cell. E A lysosome, containing digestive enzymes, fuses with the phagocytic vesicle containing the particle and the particle is destroyed.
Materials can either be taken into the cell or exported out of it. These processes are called endocytosis and exocytosis respectively. There are two types of endocytosis: phagocytosis or ‘cell eating’ and pinocytosis or ‘cell drinking’. During both these processes the cell surface folds to make a small pocket that is lined by the cell membrane (Fig. 1.6). The pocket seals off, forming a vesicle that contains the material being brought into the cell. This separates from the cell surface, moves into the cell’s interior and fuses with a lysosome, containing lysozymes, which digest the vesicle contents. The process of phagocytosis is also used to remove foreign particles such as invading bacteria (see blood cells).
Cell division
The cells of the body are classified into two types:
Mitosis
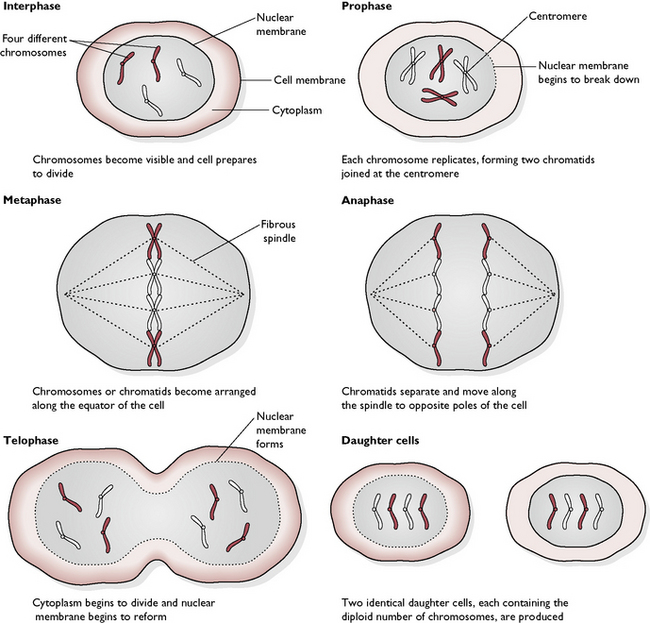
The tissues of the body grow, particularly when the animal is young, and are able to repair themselves when damaged. This is achieved by the process of mitosis in which the somatic cells of the body make identical copies of themselves. The cells replicate by dividing into two – a process called binary fission. However, before they can do this they must first make a copy of all the hereditary or genetic information that the new cell will need in order to function normally. This information is carried in the DNA (deoxyribonucleic acid) of the chromosomes within the nucleus of the parent cell. The normal number of chromosomes is described as the diploid number and before cell division takes place the chromosomes are duplicated (Fig. 1.7).
Mitosis can be divided into four active stages, followed by a ‘resting’ stage (called interphase), during which the new daughter cells grow and prepare for the next division. Interphase is not actually a resting stage because it is during this stage that the DNA replicates in preparation for the next mitosis. The centrioles have also replicated by the start of the new mitotic division. The four active stages of mitosis are:
Meiosis
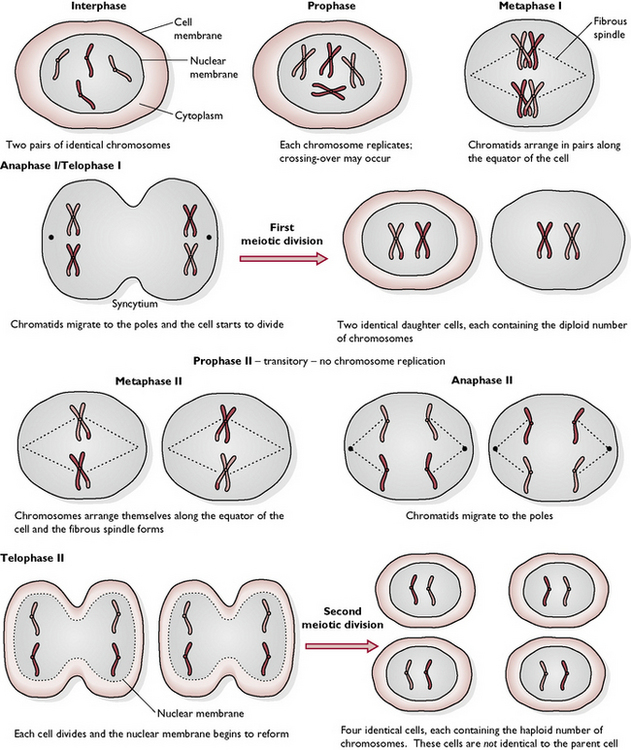
This is the process by which the germ cells divide within the ovary of the female and the testis of the male. Meiosis results in the production of ova or sperm containing half the normal number of chromosomes (the haploid number). Meiosis must occur before fertilisation, when a sperm penetrates the ovum and the two nuclei fuse. If those two nuclei had the diploid number of chromosomes then the nucleus of the resulting gamete would have twice the normal number and abnormalities would develop.
The resting cell is in interphase before meiosis begins. The eight stages are as follows (see also Fig. 1.8):
The chemistry of the body
The cells, and therefore the tissues and organs, which are all made of cells, are composed of chemicals. It is important to be able to understand these chemicals and the reactions in which they take part within the body. Chemical compounds can be divided into two groups:
Both groups are found in the body, but let us first look at the most biologically important inorganic compound of the body – water (H2O).
Water content of the body
An individual mammalian cell contains approximately 80% water. In fact, 60–70% of the whole body’s weight is water, which is divided into two main body compartments: intracellular and extracellular water.
Intracellular fluid (ICF) is that which is found inside the cells of the body and can be subdivided into the fluid within the blood cells and the fluid in all other cells. ICF takes up 40% of total body weight.
Extracellular fluid (ECF) is that which lies outside the cells, i.e. the surrounding environment of the cells. ECF takes up 20% of total body weight and includes the fluid in which the blood cells are suspended (the plasma), the fluid within the lymphatic system and the cerebrospinal fluid (the transcellular fluid), and the fluid that surrounds all the other cells of the body (the interstitial or tissue fluid).
Plasma takes up about 5% of body weight. It forms the medium in which the blood cells are transported within the blood-vascular system. It is rich in proteins, termed plasma proteins. Transcellular fluid is formed by active secretory mechanisms and its volume varies. It is considered to take up about 1% of body weight and it includes fluids such as cerebrospinal fluid, digestive juices and lymph. Interstitial fluid takes up 15% of body weight and lies outside the blood vascular system, surrounding the cells. It is formed from the blood by a process of ultrafiltration – small molecules and ions are separated from larger molecules and cells. The pressure in the blood vascular system forces the fluid through the walls of the capillaries. This acts like a sieve, holding back the large plasma protein molecules and the cellular components of the blood and allowing everything else to go through. Thus, interstitial fluid is similar to plasma but without the blood cells and protein molecules. Interstitial fluid is the medium in which the cells are bathed and from it the cells extract all that they need, such as oxygen and nutrients. They get rid of all their unwanted waste products into it.
Water or fluid provides the medium in which all the body’s biochemical reactions take place and is thus essential to maintain the body’s internal environment in a state of balance – this is a process known as homeostasis. Body water and the chemical substances within it constantly move around the body. The biological processes that are responsible for this movement are diffusion and osmosis.
Diffusion
Diffusion (Fig. 1.9A) is the movement of molecules of a liquid or a gas down a concentration gradient, i.e. from a region where they are at a high concentration to a region where they are at a lower concentration. Diffusion will occur until an equilibrium is reached, i.e. until the concentration equalises out. Diffusion takes place where there is no barrier to the free movement of molecules orions and is very important in their movement in and out of cells. However, it can only occur if the particle size is small enough to pass through the cell membrane. If the molecules are too large, then another process takes place in order to achieve equilibrium – this is known as osmosis.
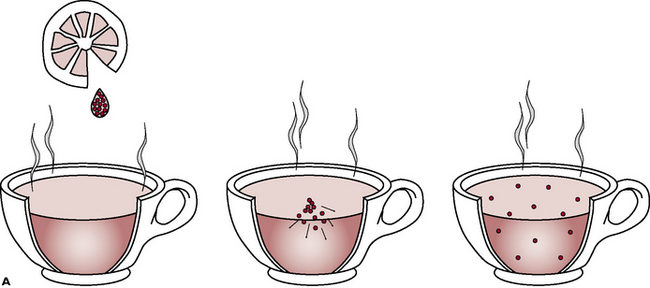
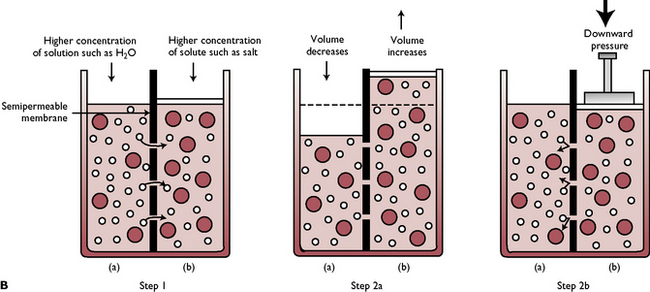
Fig. 1.9A Diffusion. Molecules in solution are active and constantly collide into one another. With time, they become evenly distributed throughout the liquid, having moved down concentration gradients from areas of high concentration to those of low, until equilibrium is reached. Diffusion occurs when there is no barrier to free movement and it occurs more rapidly in hot liquids than in cold ones as molecules are more active at higher temperatures.
(With permission from Colville T, Bassett JM 2001 Clinical anatomy and physiology for veterinary technicians. Mosby, St Louis, MO, p 24.)
Osmosis
Osmosis (Fig. 1.9B) is the movement of water through a semi-permeable membrane from a fluid of low concentration to one of a higher concentration, which continues until the two concentrations are equal. The water can be considered to be diffusing along a concentration gradient. A semi-permeable membrane allows some substances through but not others. Osmosis is responsible for water movement from the interstitial fluid into the cells.

Fig. 1.9B Osmosis. Step 1: Smaller molecules of solution in side (a) can pass through the semi-permeable membrane into side (b), but the larger molecules of solute cannot. Step 2a: As solution moves from side (a) to side (b), the volume of side (b) increases until the concentration of solute is the same on both sides. Step 2b: Osmosis can be reversed by filtration, when hydraulic pressure is placed on side (b). This forces solution back through the semi-permeable membrane to side (a).
(With permission from Colville T, Bassett JM 2001 Clinical anatomy and physiology for veterinary technicians. Mosby, St Louis, MO, p 25.)
A solution consists of the molecules of one substance (the solute) dissolved in another substance (the solvent). In the body, the solvent is water so osmosis is a significant factor in the maintenance of the fluid volume within the body fluid compartments. A solution can be described as having an osmotic pressure. This is the pressure needed to prevent osmosis from occurring and is dependent on the number of particles, both dissolved and undissolved, in the solution; e.g. if the osmotic pressure of the plasma is high, water will flow into the blood to equalise the concentration; if the osmotic pressure of the plasma is low, water will flow out of the blood into the tissue spaces.
The osmotic pressure or tonicity of a rehydrating fluid is described relative to the osmotic pressure of blood plasma as follows:
This is important in selecting fluids for rehydration therapy – most fluids used are isotonic. The replacement fluid must be as close as possible in tonicity and electrolyte content to what has been lost from the body.
Fluid balance
Water is constantly moving within the body, e.g. from the interstitial fluid into the cells, from the plasma to the tissue fluid, etc., but it is also continually lost from the body and must be replaced to ensure that the total fluid balance in the body is maintained. Water is lost through the respiratory system (expired air contains water vapour), and in the urine and faeces. Dogs and cats do not sweat appreciably but do lose heat and water through panting. Water is also lost in the tears, which are produced constantly to moisten the eye, and in vaginal secretions. Water is taken into the body through drinking fluids and from the water content of food.
Fluid losses may be increased in sick or injured animals, e.g. vomiting, diarrhoea, vaginal discharge (as seen with an open pyometra) or blood loss. This can lead to dehydration, which may have serious consequences such as reduction of the circulating blood volume, known as hypovolaemic shock. In a normal adult animal, about 60% of the total bodyweight is water. This percentage will be slightly lower if the animal is old or very obese (fatty tissue contains little water) and slightly higher in young or thin animals.
Typical daily water loss is: 20 mL per kg bodyweight in the urine; 10–20 mL per kg bodyweight in the faeces; and 20 mL per kg bodyweight through the loss of water vapour in expired air and panting and in body secretions – a total of 50–60 mL of water per kg of bodyweight daily. Thus an adult healthy animal should take in 50–60 mL of water per kg bodyweight per day to balance the normal fluid loss, e.g. an animal weighing 20 kg will need 1000–1200 mL of water each day.
Inorganic compounds
There are a number of other inorganic compounds that are also essential to the functions of the body: minerals, acids and bases. It is important to be familiar with some basic chemical definitions when considering these substances. Everything is composed of atoms, and an element is a substance that is composed of only one kind of atom, e.g. the element oxygen consists only of oxygen atoms. Molecules consist of two or more atoms linked by a chemical bond. A substance whose molecules contain more than one type of atom is called a compound.
When dissolved in water, the molecules of many substances break apart into charged particles, called ions. This charge may either be negative or positive: ions with one or more positive charges are called cations and ions with one or more negative charges are called anions.
An electrolyte is a chemical substance that, when dissolved in water, splits into ions and is thus capable of conducting an electric current. Sodium chloride (NaCl) is an example of an electrolyte in the body, its ions being sodium (Na+) and chloride (Cl−) in solution.
Minerals
The principal cations in the body are sodium (Na+), potassium (K+), calcium (Ca2+) and magnesium (Mg2+). The principal anions include chloride (Cl−) and bicarbonate (HCO3 −). These ions are essential to the functions of the body and it is vital that they are present in sufficient and balanced quantities. Sodium and chloride are mainly found in the extracellular fluid, while potassium is mainly found in the intracellular fluid (i.e. inside the cells). The concentration of these ions is important in the regulation of fluid balance between the intracellular and extracellular fluid. This balance is maintained by special ‘pumps’ in the cell membrane. An imbalance will lead to significant problems; e.g. sodium affects the osmotic pressure of the blood and so influences blood volume and pressure; a high concentration of potassium in the extracellular fluid can disrupt heart function.
Calcium, phosphorus and magnesium are important minerals that are found in storage in bone tissue. Calcium is essential for many processes in the body, such as muscle contraction, nerve conduction and blood clotting. Iron and copper are also essential to normal body function, iron being an essential component of the haemoglobin in red blood cells.
Acids and bases
An acid is a compound that can release hydrogen ions when dissolved in solution. Compounds that can accept or take in hydrogen ions are called bases or alkalis. The acidity of a solution is expressed as its pH, which is the measure of the hydrogen ion concentration. The pH scale is from 0–14, with a pH of 7 being neutral. A solution with a pH less than 7 is acidic (the lower the number the higher the acidity, i.e. the greater the concentration of hydrogen ions). A solution with a pH above 7 is basic or alkaline (the higher the number the more alkaline the solution).
The pH of body fluids is 7.35 and it is important that the body maintains this level. Within the respiratory system and kidney there are homeostatic processes to maintain the correct acid/base balance.
When the normal pH of the body is disrupted the animal may show an acidosis, i.e. a decreased blood pH, or an alkalosis, i.e. an increased blood pH. A respiratory acidosis may develop if the animal holds its breath, allowing carbon dioxide levels to rise and oxygen levels to fall; a respiratory alkalosis occurs during rapid panting, which lowers carbon dioxide levels. A metabolic acidosis may occur as a complication of diabetes mellitus and a metabolic alkalosis as a result of excessive vomiting and diarrhoea.
Organic compounds
These are compounds that are based on the element carbon. The other main elements found in organic compounds are oxygen and hydrogen, and in some instances nitrogen. The principal organic compounds found in the body are carbohydrates, proteins and fats.
Carbohydrates
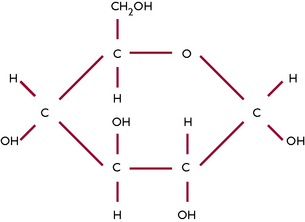
Carbohydrates contain carbon, hydrogen and oxygen and are also known as sugars. Sugars are an important source of energy and the most common simple sugar in the body is glucose (Fig. 1.10). Simple sugars can join together to form more complex carbohydrates; when many sugars join together they form a polysaccharide, e.g. glycogen – which is the form in which glucose is stored in the body. Carbohydrates are obtained from food and are then broken down during digestion into simple sugars so that they can be absorbed through the mucous membrane of the digestive system into the blood and utilised by the body.
Lipids
Lipids include the fats, which are compounds of fatty acids and glycerol (Fig. 1.11) and are also made up of carbon, hydrogen and oxygen. Fatty acids are the main form in which fats are transported in the blood after the breakdown of lipids obtained from food. Although carbohydrates provide the most direct source of energy for the body, fats can also yield a large amount of energy. They are an important means of energy storage for the body, to be used when required. Other functions of lipids include insulation of the body itself and of nerves, and in the formation of cell membranes and synthesis of steroids.
Proteins
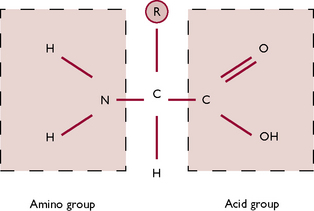
Proteins are built up from subunits called amino acids (Fig. 1.12). Proteins differ from carbohydrates and lipids in that they always contain nitrogen in addition to carbon, hydrogen and oxygen. They may also contain other elements such as sulphur, phosphorus and iodine. When two amino acids are joined together by a peptide link they form a dipeptide. The addition of more amino acids (a process called polymerisation) leads to the formation of a polypeptide. A protein consists of one or more polypeptide chains, which are then coiled and folded to give the specific structure of a particular protein (Fig. 1.13).
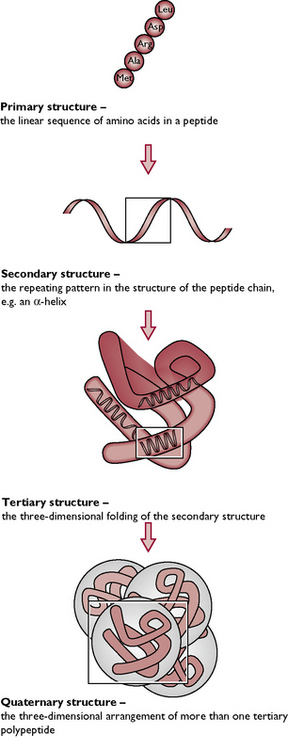
Fig. 1.13 The structure of a protein. It is not only the sequence of amino acids, but also the arrangement of the polypeptide chains, which determines the characteristics of a protein.
Proteins generally fall into one of two groups:
By means of digestive enzymes the body breaks down the proteins acquired from the diet into their constituent amino acids, which can then be absorbed through the mucous membrane of the digestive system into the blood.
Chemical reactions in the body
Most of the chemical reactions that take place in the body require the presence of a functional protein compound called an enzyme. Enzymes are organic catalysts that speed up and control chemical reactions in the body. Enzymes are involved in the breakdown of food in the digestive system but are also involved in the many metabolic processes that are carried out within cells.
A chemical reaction that requires an input of energy is called an anabolic reaction. A chemical reaction that releases energy is a catabolic reaction. The sum of the energy use, i.e. the gain and loss, is the total metabolism.
All animals require energy and this is provided by raw materials obtained from food. This is then converted by the body into a form that it can use – ATP. Energy cannot be created or destroyed, it is just moved around or else changes its form; for example, electrical energy can be converted to heat energy or it can be stored as potential energy that is released when the compound in which the energy is stored is broken down. In the body of an animal, energy comes from the oxidation of glucose, i.e. a reaction involving oxygen and glucose.