Chapter 7 Normal labour and delivery
MYOMETRIAL ACTIVITY: PREGNANCY
During pregnancy the uterus is usually in a quiescent state. In the third trimester women experience low amplitude, poorly synchronised Braxton Hicks or practice contractions. Substances which contribute to the inhibition of myometrial activity include progesterone from placental syncytiotrophoblast and chorion; myometrial parathyroid hormone-related peptide; and nitric oxide and relaxin from the myometrium, decidua, chorion and amnion in addition to prostacyclins. The process of myometrial contraction requires activation of calmodulin, a calcium-binding protein with calcium ions which then in turn activates the enzyme myosin light chain kinase to produce adenosine triphosphate to power actin and myosin filaments to slide over each other to produce shortening. Inhibitors of myometrial activity act by increasing intracellular levels of cyclic nucleotides to prevent release of calcium ions from intracellular stores or by reducing myosin light chain kinase activity.
Towards term a number of processes occur which predispose to activation or preparation of the myometrium for onset of labour. Formation of gap junctions by increases in contraction-associated proteins such as connexin-43 enhance cell to cell coupling. Receptors for oxytocin and stimulatory prostaglandins are also increased. These changes are associated with myometrial stretch when the uterus enlarges and with the higher levels of oestrogens derived partly from placental conversion of fetal dehydroepiandrosterone (DHEAS) where they also exert a local oestrogen effect. In addition to increased DHEAS production, activation of the fetal hypothalamic–pituitary–adrenal axis in late pregnancy results in more fetal cortisol biosynthesis. Fetal cortisol competes to reduce the local progesterone effect and stimulate synthesis of corticotrophin-releasing hormone from placenta and fetal membranes for production of prostaglandins by the latter structures.
MYOMETRIAL ACTIVITY: LABOUR
Initiation of labour remains unclear, but prostaglandins have been implicated in myometrial contraction. Isoforms of phospholipase A2 or C, activated by varying requirements for calcium ions, liberate arachidonic acids from membrane phospholipids. Prostaglandin synthase in amnion and chorion convert arachidonic acids to primary prostaglandins. For prostaglandin E2 (PGE2) there are four main receptor subtypes labelled from EP-1 to EP-4. These receptors are distributed in the myometrium in varying concentrations. Stimulation of EP-1 and EP-3 results in contractions whereas stimulation of the other two receptors leads to relaxation. These together with corticotrophin-releasing hormone-related cyclic AMP in the lower uterine segment contribute to fundal dominance.
Onset of labour is associated with a substantial increased myometrial sensitivity to oxytocin stimulation and a three- to five-fold increase in oxytocin production by chorio-decidual tissue. Oxytocin raises concentrations of free calcium in the myocytes to promote myometrial contraction.
Fetal membranes covering the internal cervical os exhibit a decreased production of the 15-hydroxyprostaglandin dehydrogenase enzyme which metabolises prostaglandins. More local prostaglandin production is available for cervical effacement and dilatation. This effect is supplemented by release of collagenase through increased cytokine activity.
Onset of labour is most likely between midnight and 0500 hours when maternal secretion of oxytocin peaks and the myometrium is most sensitive to oxytocin and prostaglandin. These changes in steroid and protein concentrations return to non-pregnant levels 48–72 hours post partum.
Uterine work
Myometrial contraction exerts a pull in circular and longitudinal directions (Figure 7.1)
The term fundal dominance is used to describe travel of myometrial contractions from the fundus of the uterus towards the cervix. The starting point or pacemaker for myometrial activity is situated near the point of insertion of the fallopian tubes into the uterus. The spread of myoelectrical activity through the uterus requires 1 minute. Approximately another minute is needed for adequate relaxation. Blood vessels must traverse the myometrium to reach the placenta. Contractions occurring more than once every 2 minutes contribute to poor myometrial relaxation and reduce blood and hence oxygen supply to the fetus. When two contractions are noted every 10 minutes for an hour consider possible onset of labour. In established labour the rate of contractions ranges between 3 and 5 per 10 minutes.
In early labour the uterus is not working at maximum capacity. This may be because the number of myometrial cells contracting is limited or contraction is poorly synchronised or of short duration. As labour progresses contractions becomes more efficient and uterine work increases. Capacity for work cannot, however, increase indefinitely. A stable phase for ability to work results once maximal work output is achieved for the individual mother. When the stable phase is achieved (Figure 7.2a) additional stimulation by oxytocics is of little value and can be harmful. Uterine work has been expressed as:
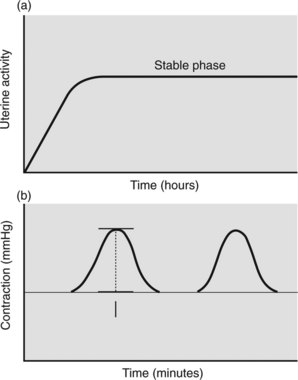
Figure 7.2 (a) Graph of uterine activity with stable phase (plateau) for individual woman. (b) Montevideo unit = intensity (amplitude of recorded contraction) × contractions per 10 minutes. The Alexandra unit, a refinement, takes into consideration duration of the contraction.
Intrauterine pressure
The viscoelastic myometrium always exerts pressure on the amniotic fluid. The resting pressure or tone is usually 6–12 mmHg. Amniotic fluid is not compressible.
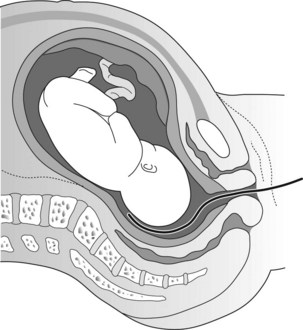
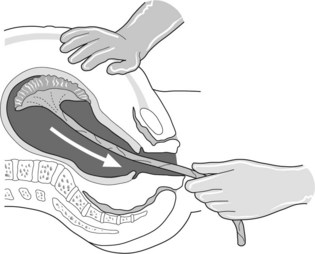
Figure 7.3 Intrauterine pressure measured by a catheter-tipped pressure transducer placed above presenting part is less affected by movement.
Intensity of uterine contractions increases throughout pregnancy. Towards term pressures up to 30 mmHg may be recorded. During labour intrauterine pressure increases to levels of 60–80 mmHg. Contractions of this intensity are still detectable for 48 hours post partum but the frequency diminishes after 12 hours.
General comments
Effect of uterine activity on the cervix
Before labour
In the weeks before labour that portion of the uterus between the internal os and the reflection of the uterovesical fold of the peritoneum is attenuated by stretch and Braxton Hicks contractions to form the lower segment. This allows the presenting part of the fetus, particularly in primiparous women, to settle or engage into the pelvis.
In labour
Labour is traditionally divided into two stages:
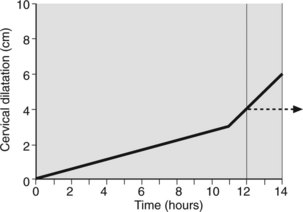
Effacement precedes cervical dilatation. Initially uterine activity is expanded to achieve effacement. This period, the latent phase of labour, takes on average 9 ± 6 hours in the primiparas and 5 ± 4 hours in multiparas. The cervix is usually up to 2 cm dilated when labour begins. At the end of the latent phase cervical dilatation is usually between 3 cm and 4 cm. Once effaced further uterine activity produces rapid cervical dilatation of at least 1 cm per hour in both parous and nulliparous women. This, the active phase of labour takes on average 6 hours to reach full cervical dilatation (Figure 7.4). Multiparous women approach labour with more cervical effacement hence a shorter latent phase and thus a shorter labour.
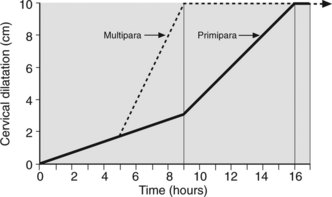
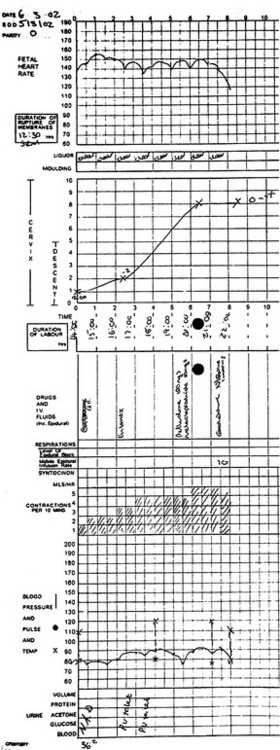
Figure 7.4 Partogram. Primipara and multipara showing latent phase and steep active phase to full cervical dilatation.
It is common practice to plot the rate of cervical dilatation to indicate the progress of labour. This is usually charted on a single sheet of paper designed for recording labour events over a 24-hour period. Provision is also made for registering fetal heart rate (every 30 minutes), uterine contractions (every 30 minutes), rate of head descent and medication. Cervical dilatation detected by vaginal examination is recorded every 2–4 hours. This graphic representation of parturition (partogram) summarises and depicts, visually, the events in labour (Figure 7.5). Cervical dilatation lagging 2–3 hours behind that expected from the normal graph (Figure 7.6), should suggest to medical attendants the need for reassessment.
Effect of uterine activity on the fetus
Flexion and entry
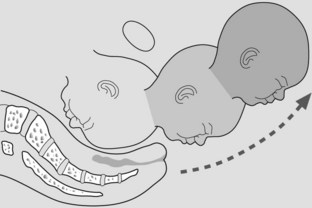
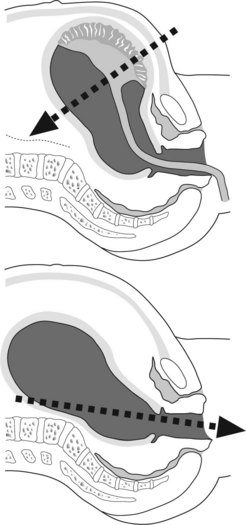
Uterine contractions cause further flexion and entry of the fetal head into the pelvis, usually in the occipito-transverse (Figure 7.7).
Descent and internal rotation
Descent occurs to the level of the ischial spines when levator ani muscles assist internal rotation to align the sagittal sutures for delivery through the widest anteroposterior diameter of the outlet.
Extension and delivery of the head
Distension of the lower part of the vagina evokes reflexes which stimulate the urge to push. Pushing is achieved by the Valsalva response and contraction of diaphragmatic and abdominal muscles. The head stretches the vagina and vulva as it delivers. Extension occurs once the head passes beneath the symphysis pubis. Fetal membranes usually rupture before this stage (shown in sequence in Figure 7.8).
Restitution, external rotation and delivery of the shoulders
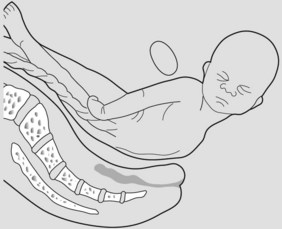
The head rotates to occipitolateral or restitutes to align naturally perpendicular to the shoulders. The shoulders, having entered the pelvis in the oblique diameter, rotate so the bisacromial diameter of the shoulder delivers in the anteroposterior diameter of the pelvic outlet. Further rotation of the head laterally accompanies rotation of the shoulders (Figure 7.9).
Delivery of the body
The shoulders deliver assisted by lateral flexion of the body. Once this is achieved the rest of the fetus delivers readily as the uterus contracts down (Figure 7.10).
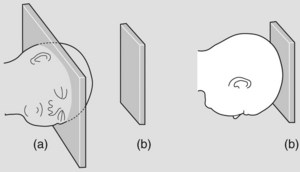
Figure 7.7 The widest part of the inlet (a) (transverse diameter) and outlet (b) (anteroposterior diameter) is represented geometrically. The fetal head enters in the occipito-transverse then rotates to address anteroposterior diameter of outlet.
Management of the delivery
Position
The squatting posture is well suited to delivery. A woman adopting the lithotomy position propped up with pillows and her legs drawn back essentially achieves this posture but has the added advantage of allowing attendant access to assist delivery.
Aseptic conditions
Gloves and gown are mandatory for the attendant conducting the delivery. Women should be draped and the vulval area cleansed. Deliver onto sterile towels.
Medical attendant
The medical attendant is usually stationed on the woman’s right. Place the left hand on the fetal head as it ‘crowns’ (passage of the biparietal eminences through the vulva) to maintain flexion and prevent expulsive delivery.
The right hand is used to guard the perineum and cover the anus with a sanitary pad. If necessary assist extension of the fetal head by lifting the chin. Consider if an episiotomy is needed.
Delivery of the head
Instruct the woman to pant once the head is crowned. This prevents forceful pushing, and repeated gentle increase in intra-abdominal pressure created by panting nudges the head out. Aspirate the mouth and then the nose of the baby at the first opportunity. Give 0.5–1 ml of Syntometrine (a combination of the synthetic oxytocic Syntocinon 5 units/ml and ergometrine maleate, 0.5 mg/ml) intramuscularly to the mother. The oxytocic effect is evident in 2.5 minutes (Syntocinon) to 7 minutes (ergometrine). It assists uterine contraction and placental separation and thereby controls blood loss. For mothers at risk of haemorrhage, give ergometrine intravenously (oxytocic effect in 1 minute).
Delivery of the shoulders
Once external rotation is completed, direct the head gently downwards to assist delivery of the shoulders. When the anterior shoulder appears beneath the symphysis insert a finger into the anterior axilla and lift the body upwards watching the perineum at the same time to avoid extension of the episiotomy or tearing of the perineum.
Umbilical cord
Cut the cord within 1 minute of birth. This interval allows additional transfusion of more than a third of the fetal blood volume. Do not wait for cessation of pulsation as the placenta may be separated and fetal exsanguination can result. Free any loose cord around the neck over the head or shoulders and proceed with the delivery. If the cord is tight around the neck or fetal resuscitation is anticipated clamp, cut the cord at once and deliver the baby.
Management of the third stage
The third stage is the internal between delivery of the fetus and delivery of the placenta. Placental separation is shown by:
Delivery of the placenta
The placenta usually separates within 3 minutes and is delivered within 5 minutes after birth.
Effect of uterine activity on the mother
Management guidelines
Box 7.3 Administration of entonox
Fox H. Pathology of the Placenta. London: WB Saunders, 1997.
Grammatopoulos DK, Hillhouse EW. Role of corticotrophin-releasing hormone in onset of labour. Lancet. 1999;354:1546-1549.
Howell CJ, Kidd C, Roberts W, et al. A randomised controlled trial of epidural compared with non-epidural analgesia in labour. British Journal of Obstetrics and Gynaecology. 2001;108:27-33.
Lye SJ, Ou CW, Teoh TG. The molecular basis of labour and tocolysis. Feto-Maternal Medical Review. 1998;10:121-136.
Patel FA, Clifton VL, Chawalisz K, et al. Steroidal regulation of prostaglandin dehydrogenase activity and expression in human term placenta and chorio decidua in relation to labour. Journal of Clinical Endocrinology and Metabolism. 1999;84:291-299.
Petraglia F, Florio P, Nappi C, et al. Peptide signalling in human placenta and membranes: autocrine, paracrine, and endocrine mechanisms. Endocrine Reviews. 1996;17:156-186.
Sangha RK, Walton JC, Ensor CM, et al. Immunohistochemical localisation, mRNA abundance, and activity of 15-hydroxyprostaglandin dehydrogenase in placenta and fetal membranes during term and preterm labour. Journal of Clinical Endocrinology and Metabolism. 1994;78:982-989.
Sparey C, Robson SC, Bailey J, et al. The differential expression of myometrial connexin-43, cyclooxygenase-1 and -2 and Gsa proteins in the upper and lower segments of the human uterus during pregnancy and labour. Journal of Clinical Endocrinology and Metabolism. 1999;84:1705-1710.
Steer PJ, Carter MC, Gordon AJ, et al. The use of catheter-tip pressure transducers for the measurement of intrauterine pressure in labour. British Journal of Obstetrics and Gynaecology. 1978;85:561.
UK Amniotomy Group. A multi-centre randomised trial comparing routine versus delayed amniotomy in spontaneous first labour at term. British Journal of Obstetrics and Gynaecology. 1994;101:307-309.
Wu YW. Systematic review of chorio-amnionitis and cerebral palsy. Mental retardation and Developmental Disabilities Research Reviews. 2002;8:25-29.