52 Hepaciviruses and hepeviruses
Hepatitis C and E viruses; non-A, non-B hepatitis
Key points
• Hepatitis C virus (HCV) is principally transmitted by parenteral routes. The development of serological and PCR-based methods for the diagnosis of HCV infection is of major value in blood donor screening and investigation of clinical hepatitis.
• HCV infection is persistent in a large proportion of those exposed. Significant liver disease develops slowly (20–30 years) and can lead to cirrhosis, end-stage liver failure and hepatocellular carcinoma.
• Chronic HCV infection can be successfully treated in a proportion of individuals by combination therapy with pegylated interferon-α and ribavirin.
• Hepatitis E virus produces an acute infection prevalent in South-East Asia, north and central Africa, India and Central America, transmitted by faecal contamination, often affecting water supplies and caused by a single-stranded, non-enveloped RNA virus.
• Most cases are recognized in those aged 15–40 years. Large outbreaks occur; a severe form with fulminant hepatitis may be seen in the third trimester of pregnancy.
Hepatitis C virus
Hepatitis C virus (HCV), discovered in 1989, was the long sought-after and highly elusive causative agent of post-transfusion non-A, non-B hepatitis. This hugely important discovery allowed rapid development of serological screening assays for HCV infection and their adoption for blood donor screening. Consequently transmission of HCV by blood transfusion has been virtually eliminated, as has the occurrence of transfusion-associated hepatitis.
Infection with HCV is widespread throughout the world, and is particularly associated with risk groups for parenteral (blood-borne) exposure. Amongst these, drug users sharing needles are numerically the most significant in Western countries. HCV infection is frequently persistent, and leads to the development of significant liver disease, such as cirrhosis and hepatocellular carcinoma (HCC), but only after a long asymptomatic carrier phase. There are currently intensive efforts to develop effective antiviral treatment for chronic infection, and protective vaccines.
Properties
Structure
HCV is a small, enveloped virus with a single stranded RNA genome of positive (coding) polarity (Fig. 52.1). HCV has been visualized in the plasma of HCV-infected individuals as small (50 nm), round particles. The surface of the virus particle contains a number of small projections thought to be formed from complexes of the virally encoded envelope glycoproteins, E1 and E2. The RNA genome is approximately 9400 bases in length, of which over 98% contains protein-coding sequence. In common with many other small RNA viruses, the gene sequences of HCV are translated in a single block to produce a large (>3000 amino acid) polyprotein. During and after translation, proteases cleave this precursor into a total of 9 mature proteins, which are involved in virus replication (NS2–NS5B), or form structural components of the virus particle (core, E1 and E2).
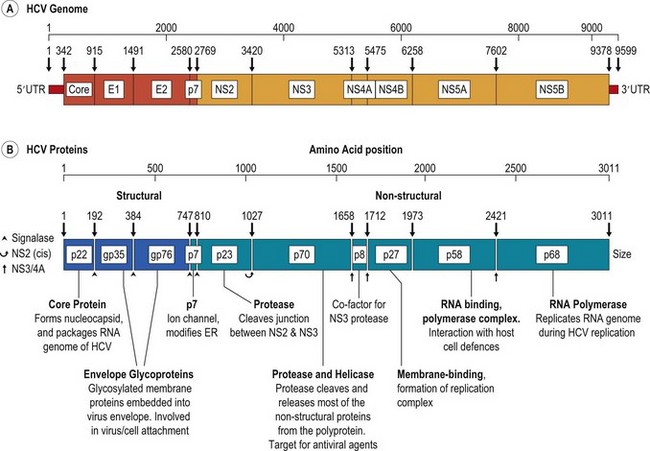
Fig. 52.1 Organisation of HCV genome, showing (A) the structural (core, E1 and E2) and non-structural genes (NS2–NS5B), and (B) the proteins produced from them by proteolytic cleavage of the translated polyprotein. Properties and functions of the HCV proteins, where known are summarised below. ER, endoplasmic reticulum; UTR, untranslated terminal region.
Replication
Information on the replication of HCV and its assembly into infectious virus particles remains limited because it still remains problematic to culture the virus in vitro. However, investigation of individual HCV-encoded proteins and comparison with related viruses have allowed the functions of most to be ascertained (Fig. 52.1). For example, NS5B is the RNA polymerase required by HCV for replication of its genetic material through a negative-stranded intermediate. NS3 contains protease and helicase activities; the former is required for the majority of cleavage reactions in the processing of the polyprotein after translation. The three proteins at the left hand end of the genome are structural proteins. Multiple copies of the core protein assemble to form a nucleocapsid that packages the viral RNA, while E1 and E2 are synthesized on internal membranes within the infected cell, become heavily glycosylated and form the HCV envelope as the nucleocapsid buds out of the cell.
The extreme ends of the HCV genome are non-coding, and play a number of roles in the transcription and translation of the virus genome. Details of the factors that initiate and regulate HCV transcription remain unclear at present, although the non-coding ends are likely to be involved in protein complexes with NS5B, NS3 and other HCV and cellular proteins to mediate binding and initiation of RNA copying. The 5′ untranslated region (5′UTR) plays an important role in directing the translation of the HCV polyprotein from an internal (methionine) codon. RNA secondary structure formation allows direct binding of the host cell ribosome to internal sequences in the 5′UTR (known as the internal ribosomal entry site, or IRES), bypassing the conventional attachment to the (capped) end of the RNA molecule, as is found in the translation of cellular mRNAs.
Hepatocytes are the principal sites of HCV replication in vivo, although there may be extrahepatic sites of replication including haemopoietic cells such as B lymphocytes, and stem cells in the bone marrow. The entry mechanism of HCV into hepatocytes (and other cell types) is complex and incompletely understood. A variety of cellular proteins are involved in the initial attachment of HCV to the cell (e.g. the LDL receptor, heparin sulphate, DC-SIGN, L-SIGN). Interaction with other host factors such as CD81, the scavenger receptor B-1 (SRB-1), claudin 1 and occludin leads to viral internalisation through endocytosis of the bound virion in a clathrin-coated endosome, and fusion of viral and endosomal membranes. In vivo, this likely occurs on the basolateral membrane surface of hepatocytes to allow concentration of the virion. On entering the cytoplasm, the RNA genome is released from the viral capsid and translated. The newly synthesized RNA replicating and unwinding enzymes, such as NS5B, NS5A and NS3, subsequently initiate transcription of the genomic RNA into a full-length negative-polarity copy, which in turn acts as the template for the production of multiple positive-stranded RNA copies. These can be used for further rounds of transcription, or used for the production of further viral proteins. Factors that regulate negative- and positive-strand synthesis and the use of the latter transcripts for translation remain undetermined, and are likely to be complex.
Replication of HCV takes place in membranous webs associated with the NS4B protein and cellular endoplasmic reticulum (ER). The core protein, however, remains within the cytoplasm after cleavage from E1, E1 and E2 are embedded in the ER membrane, and their extracellular domains are glycosylated. Details of the subsequent stages of capsid assembly and maturation, the insertion of HCV RNA, and the budding of HCV through the ER into extracytoplasmic space and release from the cell await further studies.
Classification
HCV shows the greatest similarity in structure, size and genome organization with the flaviviruses. Together, these viruses are classified as members of the Flaviviridae (Table 52.1), currently divided into four named genera. The Hepacivirus genus contains HCV, a recently discovered canine homologue of HCV (canine hepacivirus; CHV) and GB virus-B, originally isolated from a captive tamarind, in which it causes acute hepatitis and liver disease similar to that of HCV in humans. Both GBV-B and CHV are currently under evaluation as possible experimental models for HCV vaccines and antiviral treatment.
Table 52.1 Members of the Flavivirus family
| Genus | Examples |
|---|---|
| Hepacivirus | |
| Pegivirus | |
| Pestivirus | |
| Flavivirus (examples of the 68 members) |
Human pegivirus (HPgV, originally described as GB virus C or hepatitis G virus) is classified as a member of the recently described Pegivirus genus. This virus is widely distributed in humans and was originally thought to be a further agent involved in post-transfusion and chronic hepatitis of unexplained aetiology. This association has subsequently been disproved, and infection appears to be entirely asymptomatic, despite being persistent in a significant proportion of those it infects. Its genome shows a number of similarities to that of HCV, including a 5’UTR that has similar ribosomal binding and internal initiation (although a different RNA secondary structure), while the coding region contains homologues to HCV structural and non-structural proteins. However, it lacks a protein corresponding to the core protein of HCV that forms the nucleocapsid. Viruses similar to HPgV are widely distributed in a range of non-human primate species (simian pegiviruses; formerly described as GBV-A) and in bats (bat pegivirus; originally described as GBV-D). The pestivirus genus contains viruses which are structurally similar to HCV and infect a number of domestic and wild species of ruminants such as pigs, sheep and cows.
Genetic variation
Nucleotide sequences of HCV frequently show substantial differences from each other. This has led to the current genotypic classification of HCV, in which variants from a variety of geographical locations can be classified into 6 main genotypes and a very rare genotype 7, and a number of subtypes (Fig. 52.2). Genotypes show approximately 30% sequence divergence from each other, differences that greatly modify their antigenic properties, and their biology (see Treatment).
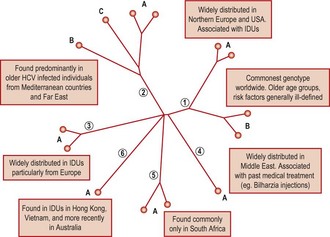
Fig. 52.2 Comparison of complete genome sequences of the common genotypes of HCV plotted as a phylogenetic tree, showing the 6 main genetic groups (genotypes 1–6) and a number of the commoner subtypes (A, B, C). The distribution of the different genotypes in the principal risk groups for HCV infection is indicated.
Some HCV genotypes (types 1a, 2a, 2b) show a broad worldwide distribution, while others such as type 5a and 6a are only found in specific geographical regions (South Africa and South East Asia respectively). HCV infection in blood donors and patients with chronic hepatitis from countries in Western Europe and the USA frequently involves genotypes 1a, 1b, 2a, 2b and 3a. The relative frequencies of each may vary geographically, such as the trend for more frequent infection with type 1b in southern and Eastern Europe, and the association of genotype 1a and 3a infection with infection through drug use. HCV genotypes can be identified by analysis of sequences from the 5′UTR or from coding regions. Methods for rapid genotyping of HCV have been developed, and play a role in the pre-treatment assessment of patients receiving antiviral treatment (See Treatment).
Virus stability
HCV is inactivated by exposure to chloroform, ether and other organic solvents and by detergents. The effectiveness of a number of virus-inactivating procedures have been demonstrated by studies of the infectivity of products manufactured from plasma such as the factor VIII and IX concentrates used to treat haemophiliacs. For example, dry-heat treatment at 80°C or wet-heat treatment at 60°C, organic solvents (n-heptane) and detergents have been shown to efficiently remove HCV infectivity.
Laboratory diagnosis
Chronic infection with HCV is associated with the presence of both plasma viraemia and antibody to HCV. Methods to detect both antibody and HCV directly are used for diagnosis of HCV infection.
The technical simplicity of antibody testing has favoured its use for general screening and diagnostic testing. Antibody tests for HCV are based upon cloned HCV RNA sequences of HCV genotype 1a originally derived from an experimentally infected chimpanzee. Recombinant proteins expressed from these clones have formed and remain the basis for almost all assays for antibody to HCV since then. Current assays incorporate antigens from the core, NS3, NS4 and NS5 regions to produce assays of high sensitivity and specificity for antibody to HCV of all HCV genotypes.
Testing for antibody to HCV is normally carried out as a two stage procedure. An ELISA format is used for initial testing of serum or plasma from patients or blood donors. Repeatedly reactive samples are then re-tested by a second ELISA in a different format or by a supplementary assay such as the recombinant immunoblot assay (RIBA) that contains a number of separate HCV antigens. Currently used serological tests for anti-HCV are now highly sensitive and specific in most patient groups, although individuals who are immunosuppressed, such as those co-infected with HIV, on renal dialysis, or transplant patients, and those with congenital immunodeficiencies can produce false-negative serological test results. In these cases, direct detection methods for viral RNA or antigen are required.
Direct detection methods
While current screening and supplementary serological tests will detect the vast majority of infections, there remains a considerable window period in HCV infection between exposure and development of antibody detectable by the best current ELISAs (Fig. 52.3). Also, the presence of antibodies to HCV does not allow distinction between past, cleared infection and current infection. For these reasons, further direct methods for the detection of HCV antigens or RNA sequences are required for the effective diagnosis of HCV infection in acute hepatitis, for diagnosis of HCV infection in immunosuppressed individuals who do not mount a detectable antibody response, and to identify current, as opposed to past, infection. Genome detection methods have also been widely adopted in addition to serological tests for the routine screening of blood donors for HCV in most Western countries.
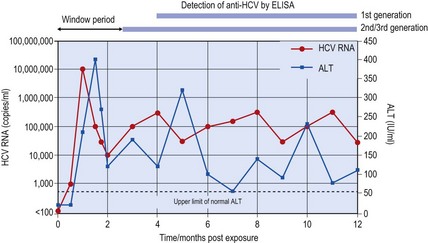
Fig. 52.3 Virological and biochemical markers of acute HCV infection. HCV RNA (red line) and abnormal ALT levels (blue line) appear approximately 4–6 weeks after exposure to HCV in a typical individual. The subsequent development of chronic hepatitis is indicated by persistent viraemia and by fluctuating abnormal ALT levels. Antibody to HCV first appears after the onset of acute hepatitis, in this example leading to ‘window periods’ of around 100–150 days for the first generation serological assay (containing only NS3 and NS4 proteins) and approximately 60–80 days for second and third generation assays (containing additional NS3, NS5 and core proteins).
Direct detection methods are based upon either the detection of HCV RNA sequences by the polymerase chain reaction (PCR) or other nucleic acid amplification methods, or of viral antigens by ELISA. Reverse transcriptase PCR is the most commonly used method for direct detection of HCV. Diagnostic PCR methods are commercially available and are capable of high sensitivity and specificity for the detection of HCV RNA sequences in plasma (or liver biopsy) specimens. Alternative, non-PCR-based methods, such as transcript-mediated amplification, have recently been developed and provide comparable sensitivity. An alternative to nucleic acid detection is the use of ELISA-based methods for the detection of HCV core protein in plasma, and more recently combined antibody/antigen detection methods.
Direct virus detection is the only reliable method of monitoring the effect of treatment with antiviral drugs in patients with chronic infection since normalization of liver biochemistry is not always associated with virus clearance, and chronic HCV infection with associated disease may arise in patients with normal liver functions tests. Quantitation of the virus genome titre is now increasingly possible with commercial tests based on real-time PCR, other amplification methods or hybridization, and is used as a predictor for response to antiviral therapy (see Treatment).
Epidemiology
In Western Europe, Australia and North America, most HCV-infected patients have a history of parenteral exposure to the virus, and the majority are, or have been, injecting drug users (IDUs). The infection rate of IDUs has been estimated at 20% per year, so long-term drug users are almost invariably HCV-infected. Drug use was uncommon before the 1960s and so infected drug users tend to be younger than patients infected by blood transfusion or other routes. Most drug users have asymptomatic infection with no history of jaundice but have chronic hepatitis; few have overt clinical signs or symptoms of liver disease or liver failure.
Other blood-borne routes of HCV transmission include blood transfusion before 1991 (when universal donor screening was initiated), and receipt of pooled plasma products such as factor VIII, and immunoglobulin manufactured before 1986 (when virus-inactivation procedures for plasma-derived blood products became widely used). Other at-risk groups include transplant recipients, haemodialysis patients, and healthcare workers from needle-stick injuries. Tattooing and acupuncture may also be responsible for some percutaneous exposure, and in countries of high prevalence, the use of unsterilized needles for cultural rituals, medical treatment or vaccination programmes may result in HCV infection.
The lowest frequencies of HCV infection are found in Scandinavian and other Northern European countries such as the United Kingdom (0.3–0.4%), with slightly higher prevalences in North America (1%) and Australia. Prevalence is intermediate in Eastern and Southern European countries, higher in Japan, and most prevalent in the Middle East; frequencies of HCV infection of up 30% have been recorded in areas of Egypt. In this latter case, bilharzia treatment using reusable and unsterile needles in the 1960s has been identified as the main source of infection.
There is little evidence for non-parenteral transmission of HCV. For example, there is little convincing evidence for transmission by sexual contact where compounding factors have been removed. Mother-to-child transmission of HCV occurs at frequencies between 3–10% in the majority of studies. Transmission occurs generally at birth, presumably through contact with blood (some evidence suggests that elective Caesarian section may prevent transmission).
Clinical features
Hepatitis C infection causes an indolent and slowly progressive liver disease that is asymptomatic until the development of cirrhosis and decompensated liver disease, or liver cancer (Fig. 52.4).
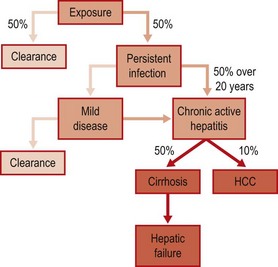
Fig. 52.4 Natural course of HCV infection, showing approximate frequencies and time course of persistence, and of progression to clinically significant disease.
Acute hepatitis
Exposure to HCV usually results in an asymptomatic infection without jaundice, with only 25–50% patients clearing the infection spontaneously – the remainder become chronically infected. Most studies have reported an interval of around 8 weeks to the development of abnormal liver function tests (such as alanine aminotransferase (ALT) levels) (Fig. 52.3). Clinically, hepatitis caused by HCV is indistinguishable from that caused by other hepatitis viruses; jaundice may develop, but more usually symptoms are non-specific such as fatigue, anorexia and nausea. Viraemia can be detected in the early stages of acute hepatitis, appearing at the same time or slightly earlier than abnormal ALT levels, while seroconversion for antibody may be delayed for several weeks or months after the onset of hepatitis. Histologic features of acute HCV are similar to those associated with acute HAV and HBV infection; liver biopsy is rarely indicated to make a diagnosis of acute HCV infection.
Chronic hepatitis
The frequency of chronic infection following exposure to HCV is between 50 and 75% (Fig. 52.4). Chronic infection with HCV is generally associated with persistent and progressive hepatitis, with fluctuating or continuously abnormal ALT levels. Although viraemia is invariably detected in patients with chronic hepatitis, there is no correlation between level of viraemia and the severity of liver disease, ALT levels or other biochemical abnormalities associated with hepatitis.
HCV infection causes a range of characteristic histological changes in the liver, although few allow a specific diagnosis of HCV infection to be made. These include lymphoid follicles within the portal tracts, a dense periportal inflammatory process, bile duct damage, and lobular hepatitis, with lymphocyte infiltration within sinusoids surrounding the hepatocytes. Chronic infection with genotype 3 HCV is particularly associated with the development of fatty change in the liver (steatosis). There are a variety of ‘scoring systems’ or histological activity indices (HAI) available (e.g. Knodell, Ishak, Metavir), designed to assess the degree of liver damage as seen on biopsy.
The percentage of chronically infected individuals who progress to cirrhosis and liver failure is not known. When chronic hepatitis does progress to clinically significant liver damage, then progression is almost invariably very slow, although faster progression may be associated with risk factors such as alcohol ingestion, older age, and immunodeficiency. Particularly aggressive HCV-associated liver disease has been observed in immunosuppressed organ transplant recipients, and in patients with inherited immunodeficiency states. Cirrhosis is rarely observed within 10 years of infection, but around 20% of infected patients have cirrhosis after 20 years follow-up. Cirrhosis may be complicated by liver failure (decompensated cirrhosis), manifested as jaundice, portal hypertension and variceal bleeding; these manifestations of liver failure are shared with other forms of cirrhosis. Hepatocellular carcinoma (HCC) frequently complicates chronic hepatitis C, although HCC is rare within 15 years of initial infection. In many Western countries such as Spain and Italy, and, in Japan, HCV infection is found in 60–90% cases of HCC.
Extrahepatic manifestations
In a minority of infected patients, HCV may be responsible for extrahepatic clinical manifestations and disease. These include certain types of vasculitis and glomerulonephritis caused by immune complex deposition. Associations between HCV infection and Sjogren’s syndrome, essential mixed cryoglobulinaemia and membranoproliferative glomerulonephritis type 1 have been suggested.
Treatment and control
Interferon-α (IFN-α) covalently linked to polyethylene glycol (PEG-IFN), combined with oral Ribavirin (RBV) is the current standard treatment for chronic HCV infection. Pegylation prolongs the half-life of the interferon, so dosing is restricted to once weekly injection. Response to therapy is monitored by quantitative PCR for detection of viral RNA. Three patterns are observed – non-response, response but with relapse after cessation of therapy, and sustained virological response (SVR, see Fig. 52.5). Duration of therapy, and likelihood of a successful outcome, are governed by viral genotype (see below). Overall, around 50% of patients achieve an SVR, i.e. viral RNA is not detectable 6 months after the end of therapy, which equates to having been cured. In an attempt to improve response rates, and to avoid the unnecessary treatment of potential non-responders, clinical and virological features associated with SVR have been defined. The most important factors follow.
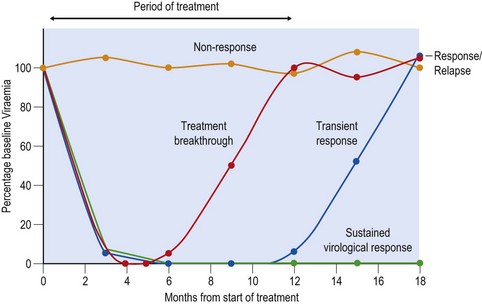
Fig. 52.5 Outcomes of combination therapy with pegylated interferon and ribavirin in chronic hepatitis C. Non-responders (NR) are those where HCV RNA remains detectable at high levels during and after cessation of treatment. Among those initially achieving virus clearance, a proportion will relapse during therapy (treatment breakthrough) and many more will relapse at conclusion of therapy. These patients are collectively referred to as responder/relapsers (RR). The desired outcome is a sustained virological response (SVR), where individuals remain non-viraemic for 6 months (and longer) from the cessation of treatment.
HCV genotype
Sensitivity to current standard-of-care therapy varies significantly between genotypes. Six months therapy in patients infected with genotypes 2a, 2b and 3a results in SVR rates of the order of 75–80%, whereas even prolonged (12 months) therapy in patients infected with genotypes 1 or 4 still only results in around 45% achieving SVR. Limited data indicates an intermediate response rate for genotypes 5 and 6. The mechanism by which different genotypes differ in their susceptibility to treatment remains unknown, particularly as the mechanisms of action of PEG-IFN and RBV are unknown in this context. It is unlikely that the greater response rates achieved with types 2 and 3 are simply secondary to differences in disease severity. Pre-treatment assessment of genotype is essential in order to specify the dose and duration of therapy likely to give the best chance of obtaining a sustained clearance of viraemia.
IL-28B genotype
Recent genome-wide association studies have identified a series of linked single nucleotide polymorphisms close to IL-28B (also known as interferon Lambda-3), a recently characterised antiviral cytokine distantly related to type I IFNs and IL-10, that are associated with treatment response. The frequencies of the ‘good’ and ‘bad’ responder alleles differ markedly in populations of different ethnic origins, which largely accounts for the observation that response rates in Caucasians are invariably higher than those in, say, black African-Americans. These polymorphisms are also associated with differences in spontaneous clearance of HCV infection after exposure. They likely represent, or are closely genetically linked to, key factor(s) governing infection outcomes of HCV.
Immunosuppression
Subjects co-infected with HIV-1 (even when controlled with anti-retroviral therapy) and immunosuppressed individuals (genetic immunodeficiencies, iatrogenic, those with systemic disease such as leukaemia) all show reduced response rates to therapy, emphasising the necessary role of the adaptive immune system in virus clearance.
Disease progression
Patients with advanced HCV-related disease such as cirrhosis or decompensated liver disease respond poorly to therapy; the latter are best referred for transplant (see below).
Baseline viral load and early treatment response
High viral loads are associated with a reduced likelihood of achieving a sustained response to treatment. Monitoring of viral loads early in treatment is also predictive of outcome; rapid reduction, particularly if associated with clearance of detectable viraemia by 4 weeks of therapy, a so-called rapid virological response (RVR), is the best single predictor of eventual SVR. Patients with a low pretreatment viral load who achieve an RVR may be given shortened duration of therapy (e.g. only 6 months for genotype 1, or 16 weeks for genotype 2 or 3) without altering their chances of eventual success.
A new generation of drugs with specific antiviral actions against HCV are under active development. The first two of these agents, telaprevir and boceprevir, were licensed for clinical use in 2011 as an adjunct to PEG-IFN-α/RBV therapy of genotype 1 infections. The three main classes of anti-HCV drugs currently leading in development are inhibitors of the viral NS3/4A protease (e.g. Telaprevir, Boceprevir), inhibitors of the NS5B RNA polymerase (e.g. Mericitabine), and inhibitors of the multi-functional NS5A protein (e.g. Alisporivir, BMS-790052). Analogous to the antiretrovirals used for HIV therapy, polymerase inhibitors are either nucleoside analogues that cause chain termination of RNA transcripts, or non-nucleoside protein binding inhibitors of RNA polymerase. Also analogous to HIV therapy is the potential for rapid development of antiviral resistance during therapy through mutations in the genes encoding the target viral proteins.
To date, almost all trials have evaluated the additive effect of antiviral drugs to the efficacy of PEG-IFN-α/RBV therapy. For example, addition of Telaprevir increases the clearance rate in genotype 1-infected trial subjects from around 50% to nearly 80%. Addition of protease inhibitors additionally substantially increases the effectiveness of re-treatment in those who had previously failed with conventional therapy. In the longer term, it may perhaps be possible to treat with multiple antiviral drug combinations (without interferon) to suppress virus replication sufficiently before drug resistance develops. HCV genotypes respond differently to certain classes of antivirals; for example, genotypes 1 and 4 are more effectively inhibited by current protease inhibitors than genotypes 2 and 3. This differential susceptibility is opposite to the genotype-associated differences in responsiveness to PEG-IFN/RV therapy described above.
Liver transplantation
Liver transplantation is indicated for patients with decompensated HCV cirrhosis, and for some patients with hepatocellular carcinoma complicating HCV infection. Liver transplantation does not cure HCV infection, and re-infection of the graft, with subsequent rapidly progressive liver disease, is frequently encountered.
Prevention
Screening of blood donors has proved to be effective at preventing transmission of HCV infection through blood transfusion. A combination of blood donor screening and virus inactivation has virtually eliminated HCV transmission by blood products such as clotting factor concentrates and immunoglobulins.
The main continuing risks for HCV transmission are injecting drug use and the use of unsterile needles for medical and dental procedures, tattooing and other percutaneous exposures. Much of this could be prevented by education, greater availability of disposable needles, and for drug users by needle exchange programmes. Many of the public health measures adopted to prevent transmission of HIV by parenteral routes will assist efforts at controlling HCV.
Immunization
The development of a vaccine for HCV faces a series of formidable obstacles, amongst which viral heterogeneity, and the difficulty in evaluating candidate vaccines in suitable animal models, are the most acute. Recombinant envelope proteins (E1 and E2) have been shown to induce a short-lived specific anti-E1 and E2 response in immunized chimpanzees, and transient protection from challenge with the same virus strain. There is, however, little prospect of an effective vaccine for human use in the coming decade.
Infection with HCV is a growing medical problem worldwide. A combination of public health preventative measures, improved diagnosis, screening, antiviral treatment and immunization will undoubtedly all be required to combat its spread in the future.
Hepatitis E virus
Description
Hepatitis E virus was characterized in 1991. The virion is non-enveloped, with a genome of single-stranded RNA of positive sense. It is classified as a Hepevirus. Virion structure and genome organization resemble those of the caliciviruses; there is also some similarity with rubella and other togaviruses. There is a single serotype with strong cross-reactions among the four known human species (HEV-1, -2, -3 and -4) and also with other unidentified viruses of mammals as well as an avian virus.
Clinical features
The disease has an incubation period of 15–60 days (average 40 days). The clinical features are those of acute hepatitis with anorexia, fever, abdominal pain, nausea and vomiting. The urine darkens and the stools lighten. Liver function tests show hepatocellular damage, and jaundice develops.
Most clinical cases occur in those aged 15–40 years. Infections in younger patients are mostly mild and anicteric.
The virus is acquired by the oral route; virus excretion can be detected about 4 weeks after infection and persists for about 2 weeks. Evidence of liver damage can be found after about 3 months. The overall mortality rate is 1–3%, but can reach 15–25% in the third trimester of pregnancy.
Chronic infection has been described recently in the context of heavily immunosuppressed patients e.g. liver transplant recipients or HIV-infected individuals.
Laboratory diagnosis
Antibodies to the virus can be measured and show the usual profile of IgM and IgG responses. Reverse transcriptase–PCR can be used to detect viral RNA, although it is not widely available.
Epidemiology and transmission
The virus is acquired orally and outbreaks, often involving many thousands of cases, are linked to faecal contamination of water supplies or food. Unlike hepatitis A, there is little evidence of person-to-person transmission. Several large water-borne outbreaks have been recognized in Africa, India, China and Mexico. Infection is endemic in undeveloped areas of the world, and HEV is the cause of more than 50% of cases of sporadic hepatitis. In the developed world the prevalence of antibody to the virus is less than 2%, and most cases are seen in travellers returning from endemic areas, although there is good evidence that there are endemic HEV strains within the UK and other Western European countries, as several cases of acute infection have been described in individuals with no travel history.
Antibody studies suggest that several animal species, including primates, pigs, cows, sheep, goats and rodents, may be infected with HEV or related viruses. Strains from swine are related to human strains 3 and 4. The virus may be maintained in endemic areas by person-to-person transmission or in a non-human reservoir.
Treatment and control
There is no specific therapy, although ribavirin has been shown to be effective in cases of chronic infection. Prevention requires the provision of safe clean water and raising standards of food hygiene. Travellers should take the same precautions as recommended for protection from any faecal–oral infection.
Passive immunization is not available. Studies to develop a vaccine are under way.
Alter MJ. Epidemiology of hepatitis C virus infection. World Journal of Gastroenterology. 2007;13:2436–2441.
Dalton HR, Bendall R, Ijaz S, Banks M. Hepatitis E: an emerging infection in developed countries. Lancet Infectious Diseases. 2008;8:698–709.
Hofmann WP, Zeuzem S. A new standard of care for the treatment of chronic HCV infection. Nature Reviews Gastroenterology & Hepatology. 2011;8:257–264.
Houghton M, Abrignani S. Prospects for a vaccine against the hepatitis C virus. Nature. 2005;436:961–966.
Lindenbach BD, Rice CM. Unravelling hepatitis C virus replication from genome to function. Nature. 2005;436:933–938.
Poenisch M, Bartenschlager R. New insights into structure and replication of the hepatitis C virus and clinical implications. Seminars in Liver Disease. 2010;30:333–347.
Vermehren J, Sarrazin C. New HCV therapies on the horizon. Clinical Microbiology and Infection. 2011;17:122–134.