Chapter 25 Female reproductive physiology
After reading this chapter, you will be able to:
Introduction
Knowledge of the complexities underpinning reproductive physiology enables the midwife to assess the woman’s general and sexual health and apply this to the individual’s menstrual and ovarian cycles, and conception.
This chapter includes a detailed account of the sequence of events within the ovaries, before, during and after ovulation, and ovarian neuroendocrine regulation of hypothalamic–pituitary gonadotrophin responsiveness, across the infertile cycle. The chapter concludes with ovarian steroid regulation of the uterine and mammary cycles and recent markers of female reproductive health. More in-depth information on the underpinning physiology is available on the website.
From puberty to menopause, ovarian activity is characterized by cyclic development of several follicles; maturation and ovulation of a selected follicle, and subsequent formation and regression of the corpus luteum. This episodic pattern of ovulation requires cyclical release of steroid and peptide hormones that imposes a corresponding cyclicity on many organ systems; levels of sexual arousability; and differing behaviours (Chapman et al 1998, Chidambaram et al 2002, Haselton et al 2007, Salonia et al 2005, Tarin et al 2002, Webb 1986).
Evidence of increased sexual motivation during the periovulatory phase of the cycle has been found in humans and other primates, but these behaviours are far less evident and reliable than the monthly shedding of bloody endometrial tissue at the end of the luteal phase. Consequently, the term menstrual cycle is used as an indirect measure of the ovarian cycle. Both cycles are driven by the dynamic pattern of negative and positive feedback between ovarian steroids and peptide hormones, and gonadotrophins from the anterior pituitary gland and hypothalamus.
The ovarian cycle
The ovarian cycle has three distinct phases:
In humans, the first and third phases take approximately 14 days each, while the second takes about 15 minutes (Lousse & Donnez 2008) (Fig. 25.1).
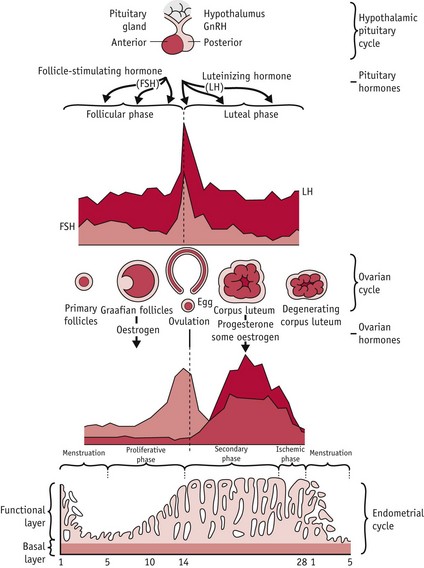
Figure 25.1 Hypothalamic–pituitary–ovarian–endometrial changes during an ovulatory cycle.
(Reproduced with permission from Blackburn 2007:36.)
Fertility depends on ovarian production of one dominant follicle per cycle, with the capacity to ovulate a fertilizable oocyte. From puberty to menopause, a few primary follicles are recruited every day into a pool of growing follicles, producing a continuous trickle of developing follicles.
Of the 15–20 follicles recruited, one selected follicle demonstrates increased cell proliferation and differentiation; expansion of thecal vascularity; rapid accumulation of antral fluid; and rising capacity for steroid and peptide hormone secretion (Geva & Jaffe 2000, Gougeon 1996, Seifer et al 2002). Following selection, this growing follicle regulates changes in hypothalamic–pituitary gonadotrophin activity during oocyte maturation and ovulation, and following the mid-cycle LH/FSH surge, when it differentiates into a functional corpus luteum or postovulatory follicle (Baker & Spears 1999, Hodgen 1982, Kunz et al 1998, Nakao et al 2007) (Fig. 25.2).
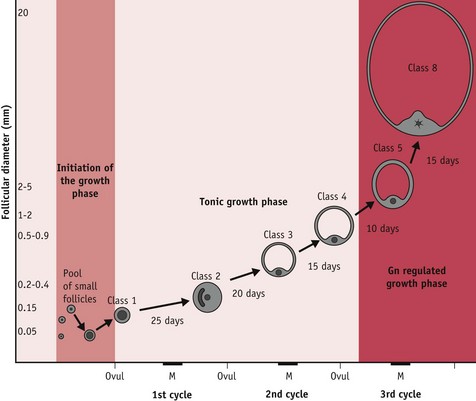
Figure 25.2 Complete follicular growth trajectory across three successive cycles. Progression through stages 1–4 relies to some extent on pituitary gonadotrophins while stages 5–8 occur during the follicular phase following the third menses from initiation of the growth phase. Exponential growth, selection and dominance are regulated by rising levels of FSH and by varying sensitivity to FSH within the selected cohort. Key: M, Menses; Ovul, ovulation; Gn, gonadotrophin.
(Reproduced with permission from Blackburn, 2007:55.)
At mid-cycle, the dominant follicle becomes visible to the naked eye as a large bulge under the surface epithelium of the ovary. The enclosed oocyte acquires meitotic and developmental competence and subsequently undergoes a profound reorganization of the nucleus and cytoplasm, in preparation for fertilization and blastocyst formation (Canipari 2000, Gilchrist et al 2008).
Ovarian regulation of gonadotrophins
The ovary contains thousands of highly differentiated cells and functions as a complex neuroendocrine–autocrine–paracrine gland, synchronizing hypothalamic, pituitary, ovarian, uterine and mammary activities, throughout the cycle (Behrens et al 1995, Hirshfield 1991, Kunz et al 1998, Nakao et al 2007, Armstrong & Webb 1997).
The ovarian cycle temporarily modulates the secretory patterns of pituitary and hypothalamic gonadotrophins, through a changing balance of hormonal secretions, in successive cohorts of antral follicles, the dominant follicle and its successor, the postovulatory follicle or corpus luteum (Baker & Spears 1999, Hodgen 1982). Across the cycle, ovarian steroid and peptide hormones act through negative and positive feedback mechanisms on the synthesis and pattern of release of hypothalamic gonadotrophin-releasing hormone (GnRH); pituitary gonadotrophins FSH and LH and their responsiveness to GnRH, in conjunction with other modulatory hormones and neurotransmitters operating on pituitary and hypothalamic gonadotrophin cells (Ball 2007, Nakao et al 2007, Smith & Jennes 2001).
Antral fluid has a key role in follicular growth, oocyte maturation, ovulation and fertilization (Tse et al 2002).
As the dominant follicle increases in size (Fig. 25.3), rising synthesis of oestrogens and progesterone lead to a parallel rise in circulating concentrations.
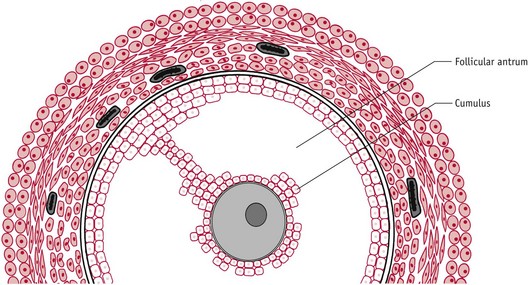
Figure 25.3 Expanded antral follicle with a fully developed follicular antrum leaving the oocyte surrounded by a distinct layer of granulosa cells.
(Reproduced with permission from Johnson 2007:84.)
Mid-cycle LH/FSH surge
The LH/FSH surge is temporally associated with attainment of peak secretion of oestrogens following a rapid rise in progesterone 12 hours earlier (Micevych & Sinchak 2008, Stouffer 2003). In the hypothalamus, oestrogens induce a rise in synthesis of neuroprogesterone and neuroprogesterone receptors, and these events initiate the pulsatile release of GnRH, which then stimulates the surge release of LH needed for ovulation (Micevych & Sinchak 2008, Smith & Jennes 2001) (Fig. 25.4).
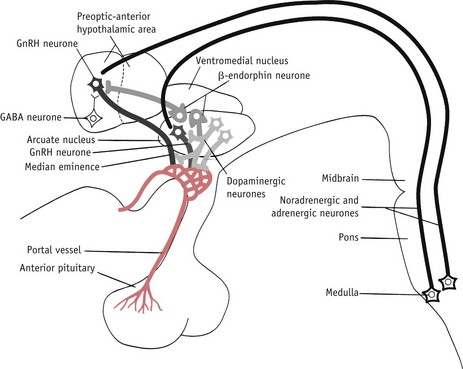
Figure 25.4 Some of the postulated neurochemical reactions that may control GnRH secretion.
(Reproduced with permission from Johnson 2007:116.)
A wide variety of peptides have also been shown to interact with steroids, ions and other paracrine factors, as well as GnRH, in regulating LH and FSH secretion, particularly around ovulation (see website).
During the ovulatory cycle, oxytocin levels vary in the hypothalamo-hypophysial complex, medulla and cerebrospinal fluid (Evans 1996, Salonia et al 2005). In the peripheral circulation, higher levels of oxytocin coincide with the mid-cycle LH surge – variations have been found in oxytocin and oxytocin receptors in granulosa and stromal cells, as well as in uterine and cervical tissue across the ovarian cycle (Behrens et al 1995, Kunz et al 1998). Evidence suggests that central oxytocin plays a key role in regulating the LH surge, by acting directly on GnRH neurons and stimulating sexual behaviour around ovulation.
Uterine and cervical oxytocin cooperate in conjunction with prostaglandin E2 (PGE2)and the preovulatory surge in oestrogens from the dominant follicle. These interactions promote fertilization, by softening cervical tissue and stimulating cervico-fundal contractions, to rapidly transport waves of spermatozoa from the posterior vaginal fornix to the isthmic portion of the fallopian tube (Behrens et al 1995, Drobnis & Overstreet 1992, Kunz et al 1998).
The preovulatory follicle
Following the LH/FSH surge, fibroblasts within the theca externa lay down connective tissue and theca interna and mural granulosa cells begin to differentiate from a predominantly oestrogen-secreting tissue into a highly vascularized corpus luteum with progesterone as its major steroid hormone and a rapid rise in progesterone receptors (Stouffer 2003).
During the critical preovulatory period, the oocyte also secretes molecules that enhance oestrogens and inhibit progesterone secretion by cumulus granulosa cells (Gilchrist et al 2008, Sutton et al 2003) which also express oxytocin receptors. Oxytocin appears to modulate progesterone secretion while stimulating progesterone receptors on granulosa cells and a variety of regulatory molecules, including growth factors, integrins, prostaglandins and intracellular messengers involved in the process of ovulation.
Oocyte maturation
Throughout the growth phase of the oocyte, progression of meiosis is arrested at the diplotene or germinal vesicle stage of prophase 1. Within 12 hours of the LH/FSH surge, the fully grown oocyte is reactivated to briefly resume meiosis, while complex maturational changes occur in the cytoplasm to support fetilization and the surrounding cumulus cells expand (Sutton-McDowall et al 2010). FSH receptors are present on the entire surface of human oocytes, suggesting that FSH has direct control of oocyte maturation (Meduri et al 2002).
Preparation for the corpus luteum
Prior to ovulation, mural granulosa and theca interna cells differentiate predominantly into progesterone-secreting theca-lutein and granulosa-lutein cells that remain in the ovary following ovulation, and rapidly expand to form the highly vascularized corpus luteum (Niswender et al 2000, Stouffer 2003) (see website).
Ovulation
The LH/FSH surge dramatically increases blood flow and vascular permeability in thecal capillary networks that descend to the basal lamina. The rapid increase in size and vascularization of the follicle is accompanied by the local release of the prostaglandin PGE2 and of vasodilatory substances like histamine and bradykinin. PGE2 initiates the breakdown of collagen fibres within the thecal compartment, and other molecules cause an inflammatory reaction from within. FSH and progesterone also initiate proteolytic enzyme activity that loosens, distends and finally erodes the follicle wall at its weakest point (Brannstrom et al 1996).
Within 12 hours of the LH/FSH surge, progesterone replaces oestrogens as the dominant steroid hormone synthesized by theca and mural granulosa cells. This shift in steroid hormone production is accompanied by a rise in prostaglandin output which activates enzymes that weaken and distend the follicle wall.
Between 36 and 42 hours following the surges, the apex of the follicle begins to bulge below the surface of the ovary, rupturing at its weakest point, allowing antral fluid to flow into the peritoneal cavity, carrying the expanded oocyte–cumulus complex into the fallopian tube (Brannstrom et al 1996).
At ovulation, the dominant follicle releases the hugely expanded oocyte–cumulus complex from the surface of the ovary (Fig. 25.5). Immediately afterwards, the cumulus cell mass regulates acceptance of the oocyte by the fimbriae of the fallopian tube and subsequently functions as a hormonal and metabolic unit in the lumen of the uterine cavity, optimizing conditions for the enclosed oocyte and zona pellucida to undergo final maturational changes in preparation for fertilization, implantation, and embryo formation (Talbot et al 2003).
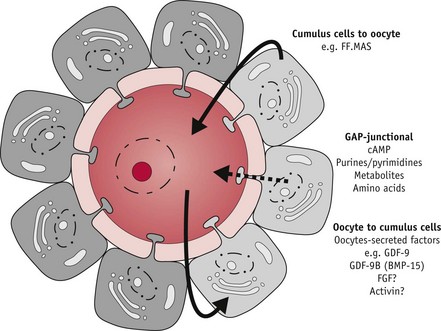
Figure 25.5 Bi-directional oocyte–cumulus cell communication: paracrine (bold arrow) and gap-junctional (dashed arrow) communication between oocyte and cumulus cells. Factors transmitted include follicular-fluid meiosis-activating sterol (FF-MAS); growth differentiation factor-9 (GDF-9), fibroblast growth factor (FGF) and activin.
(Reproduced with permission from Sutton et al 2003:37.)
The corpus luteum – reprogramming the postovulatory follicle
When the cumulus–oocyte complex leaves the ovary, residual cells of the dominant follicle undergo rapid remodelling, including cell growth, proliferation and final differentiation, accompanied by a rapid increase in blood flow and lymphatic drainage (Alexander et al 1998, Redmer & Reynolds 1996, Stocco et al 2007). These changes allow the corpus luteum to expand rapidly to become the most active and highly vascularized autocrine–paracrine–endocrine gland in the body, with a lifespan of approximately 14 days (Duncan 2000, Stocco et al 2007).
At the peak of its activity, in the mid-luteal phase of the cycle, the corpus luteum measures up to 2 cm in diameter and produces up to 25 mg progesterone per day, although the majority of its cells are non-luteal in origin (Duncan 2000, Niswender et al 2000, Redmer & Reynolds 1996).
The convoluted ovarian arterial branches are enmeshed by a responsive venous network surrounding the ovary, providing an effective means of adapting local blood flow to the dynamic state of the enclosed follicles across successive cycles (Alexander et al 1998) (see website).
Luteinization
The preovulatory LH surge triggers luteinization or terminal differentiation of theca and granulosa cells within a few hours of ovulation (Stocco et al 2007). Both cells synthesize progesterone as the primary steroid hormone. This acts locally to sustain luteal cell function and stimulate its own secretion (Stocco et al 2007). Androgens and oestradiol are also synthesized (Niswender et al 2000).
Cyclical changes in reproductive organs
Ovarian regulation of gonadotrophs before, during and after ovulation imposes a corresponding cycle on the functional layer of the endometrium and fallopian tubes, mucosal secretions of the cervix, and the structure and secretions of vaginal tissue (Gipson et al 2001). The functional zone of the endometrium undergoes successive phases of proliferation, secretion and regression, while mammary epithelial cells undergo proliferation and involution across the ovarian cycle (Herbison & Pape 2001, Russo & Russo 1987, Smith 2001, Smith & Jennes 2001).
The endometrial cycle
The endometrial cycle (Fig. 25.6) is divided into three phases, which normally take place over 28–30 days:
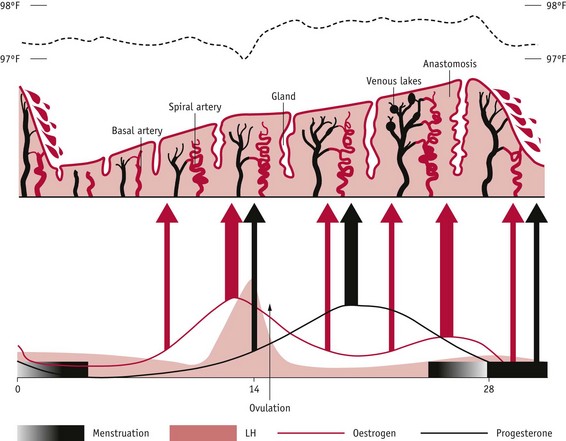
Figure 25.6 Changes in human endometrium during the menstrual cycle. Underlying ovarian steroid hormone changes are indicated below and basal temperature is indicated above. Thickness of arrows indicates strength of action.
(Reproduced with permission from Johnson 2007:151.)
Cyclical endometrial activity
Cyclical regeneration of the functional layer of the endometrium that culminates with pre-decidualization at around 8–9 days after ovulation, begins towards the end of menses. During the follicular phase, rising concentrations of oestrogens stimulate an intense proliferation of epithelial and stromal cells. This is followed by differentiation of glandular and stromal cells, and continued growth and tubal formation of endometrial vascular cells, which is largely regulated by rising levels of progesterone, following ovulation. During the luteal phase, the functional zone is characterized by changes in cell morphology and extracellular matrix composition that transform it into a secretory, paracrine/autocrine gland. Throughout the secretory phase, the endometrium clearly differentiates into three layers, with numerous gap junctions:
As illustrated in Figure 25.6, from the mid-secretory phase to the end of menstruation, thickness of the functional layer declines from 5–8 mm to 1–3 mm, as the functional layer is shed along with its connective tissue matrix and the highly specialized arterioles are reduced to around one-third of the length reached by the onset of menses (Bakos et al 1993, Dockery et al 1990, Rogers 1996, Smith 2000, Starkey 1993). During the premenstrual phase, endometrial cells release increasing levels of proteolytic enzymes that degrade the extracellular matrix, and highly potent and long-lasting vasoconstrictors called endothelins that act on the spiral arterioles (Ohbuchi et al 1995). From the second day after bleeding commences, remaining stromal cells respond to the reduced oxygen tension by synthesizing vascular endothelial growth factor (VEGF), which stimulates repair of the vascular bed and elongation of the remaining blood vessels, by the fifth day of the cycle (Maas et al 2001). These blood vessels have an essential role in tissue reconstruction during the proliferative phase of the cycle (Rogers 1996, Smith 2001).
The proliferative phase
By around day 5 of the endometrial cycle, cell proliferation commences when endometrial and myometrial oestrogen receptors are stimulated by increased secretion of oestrogens from the cohort of developing follicles. During this phase, oestrogens stimulate a rise in the number of ciliated cells in the luminal epithelium and expression of a variety of mitogenic factors, including VEGF, that stimulate a marked proliferation of luminal epithelial, glandular and vascular endothelial cells (Ferrara & Davis-Smith 1997). At the same time, oestrogens directly inhibit endometrial angiogenesis, while vascular permeability rises and endometrial blood flow increases, to peak just before ovulation (Ma et al 2001).
Stromal fibroblasts enlarge and show signs of increased protein synthesis and association with microfibrils of collagen that become denser and thicker just before ovulation. An insoluble pericellular matrix of collagen and fibronectin forms a tight meshwork primarily around glandular and basement membranes of the luminal epithelium (Dockery et al 1990, Fraser & Peek 1992, Shiokawa et al 1996, Starkey 1993). With the surge in oestrogens that follows selection of the dominant follicle, a three- to fivefold increase occurs in endometrial thickness, and oestrogen-dependent intracellular receptors for progesterone are synthesized. During the late proliferative phase, secretory glands enlarge and become thicker and more convoluted, while proliferation in epithelial and stromal cells continues until 3 days following ovulation (Irwin & Giudice 1998, Strauss & Coutifaris 1999).
The secretory phase
Within the uterus, endometrial glandular cells accumulate glycogen, regulatory proteins, sugars and lipid droplets, and their secretory activity reaches a maximum around 6 days after ovulation. In anticipation of fertilization, these molecules prepare for implantation and provide essential nutritional and regulatory secretions for the conceptus (Budak et al 2006, Burton et al 2007, Cheon et al 2001). At the same time, progesterone stimulates glandular and stromal cells to release human chorionic gonadotrophin (hCG) and increase expression of two potent angiogenic factors: angiogenin in stromal cells and VEGF in stromal cells and neutrophils associated with microvessel walls (Alexander et al 1998, Gargett et al 2001, Ma et al 2001). As illustrated in Figure 25.7, a subepithelial capillary plexus has formed into a complex network of vessels by the mid-secretory phase and newly regrown arterioles become increasingly more spiral as they lengthen more rapidly than the endometrium thickens (Gargett et al 2001, Strauss & Coutifaris 1999).
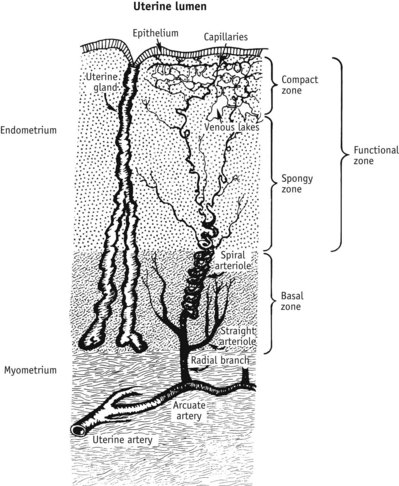
Figure 25.7 The arterial supply to the uterine endometrium. These specialized endometrial blood vessels arise within the myometrium, as the arcuate arteries. Small straight arterioles supply the basal unchanging layer of the endometrium. As they leave the basal portion and enter the spongy and compact tissue that underlies the luminal epithelium, their thick smooth muscle coat formed by circular and longitudinal layers becomes progressively thinner, and by the time these blood vessels reach the subepithelial surface of the endometrium, they consist only of endothelial cells (Abberton et al 1999).
(Reproduced with permission from Strauss & Coutifaris 1999:219.)
Pre-decidualization
During the secretory phase, stromal cells synthesize and release a growing number of new matrix proteins together with surface expression of their receptors, including laminin, fibronectin and integrins, while the earlier cross-linking collagen fibrils are degraded. This process of matrix remodelling creates a looser and more soluble structure and, from the mid-secretory phase onwards, stromal cells also express a variety of regulatory peptides involved in cell replication and haemostasis. Stromal tissue undergoes further differentiation, and individual cells become larger and oedematous, which contributes to the overall thickening of the endometrium.
From the late secretory phase, further changes occur within the endometrium which are regulated by relaxin, progesterone, prolactin and leptin (Budak et al 2006, Gubbay et al 2002, Haig 2008). The endometrial stroma immediately surrounding the arterioles undergoes decidualization as it synthesizes a number of hormones and other molecules and is infiltrated by lymphoid cells.
In the event of fertilization, these changes provide an appropriate nutritive, regulatory and immunoprotective environment for the emerging embryo until 9–10 weeks’ gestation (Alexander et al 1998, Gonzalez et al 2003, Gubbay et al 2002, Lane et al 1994, Starkey 1993).
Cyclical changes in the cervix and vagina
Significant changes occur in the cervix and vagina throughout the cycle, which facilitate the passage of spermatozoa. During the follicular phase, the muscles of the cervix relax, causing the cervical os to dilate slightly (to around 3–4 mm) at the time of ovulation. The epithelial cells begin to secrete clear watery and ‘stretchable’ mucus mid-cycle.
Under the influence of rising levels of progesterone following the mid-cycle LH/FSH surge, the cervix becomes firmer and more tightly closed and cervical secretions become scant, viscous and cellular, making it more difficult for spermatozoa to enter the uterus. In addition, relaxin and progesterone relax muscle layers in the isthmic portion of the tube, which assists movement of the conceptus towards the uterine cavity (Downing & Hollingsworth 1993, Johnson 2007).
Lined with stratified squamous epithelium, the vagina is also responsive to oestrogens and progesterone. During the follicular phase, vaginal cells proliferate and begin to accumulate glycogen, which is fermented to lactic acid by the normal bacterial flora. This provides a slightly acid environment, which acts as an anti-infective agent. During sexual excitation, the acidity of vaginal fluid is partly neutralized by the increased blood flow to the pelvic region, and this alters the pH, making it more receptive for the ejaculated sperm.
The mammary cycle
The female mammary gland undergoes a surge of cell division during puberty and a cyclical pattern of proliferation and involution until approximately 35 years. During this period, hormonally induced increases in cell proliferation and apoptosis (programmed cell death) do not return the gland to the starting point of the previous cycle but provide for a cumulative budding of new lobules (Vorherr 1974).
During each cycle, episodes of increased mitosis and apoptosis follow a contrasting pattern to that of the endometrium (Fig. 25.8). Mammary epithelium shows decreased DNA synthesis and mitotic divisions during the first half of the cycle and maximal proliferation that peaks during the luteal phase of the cycle and is followed by a shorter period of increased apoptosis (Russo & Russo 1987). A contrasting pattern of cellular activity in uterine and mammary epithelium is reflected in cyclical differences in steroid hormone receptor concentrations in the two organs of reproduction. Mammary tissue receptors for oestrogen (as in the endometrium) decline during the second half of the cycle; those for progesterone remain fairly constant throughout both phases of the cycle (Soderqvist et al 1993). During the second half of the cycle, secretory activity may also occur together with increases in breast volume, because of the significant rise in extracellular levels of VEGF that stimulates angiogenesis and increases vascular permeability (Dabrosin 2003).
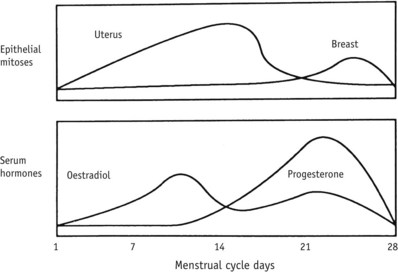
Figure 25.8 Changes in epithelial mitosis of breast and endometrium, and serum oestradiol and progesterone levels during the menstrual cycle.
(From Yen, 1999:210.)
Conclusion
It is important to understand the complex mechanisms and neurohormonal influences that synchronize the ovarian, menstrual and mammary cycles, and how these impact on the woman’s body. This ensures that the midwife can accurately calculate the expected date of delivery; help the woman to understand how the calculation is made; and enable her to see how these cycles begin the physiological preparation for pregnancy, childbirth and lactation.