Chapter 35 Physiological changes from late pregnancy until the onset of lactation
After reading this chapter, you will be able to
Introduction
Over the last 4 weeks of pregnancy, all women experience significant emotional and cognitive changes associated with ‘nesting’ and other signs of the emergence of maternal responsiveness and affiliation (Brunton & Russell 2008, Grattan 2002, Neumann 2009). At the same time, maternal pain threshold rises, the hypothalamic–pituitary axis (HPA) becomes hyporesponsive to various stressors, and women often experience periods of heightened apprehension relating to fetal and neonatal wellbeing (Douglas 2010, Douglas et al 2005, Gintzler & Liu 2001, Kask et al 2008, Neumann 2009, Russell & Brunton 2006).
During late pregnancy and lactation, anxiety behaviours in response to stressful stimuli are attenuated by central oxytocin and prolactin, while aggressive defensive behaviours and reduced fearfulness in response to perceived threats to the fetus and neonate increase from late pregnancy to advanced labour and peak during lactation (Kinsley 2008, Neumann 2009) (see website). Several neurotransmitters and neuropeptides are implicated in regulating maternal aggression, including local release of oxytocin within selected areas of the hypothalamus during and after birth (Brunton & Russell 2008, Neumann 2009, Russell & Brunton 2009).
Maternal brain adaptations are driven by a cascade of transformations beginning during late pregnancy, and continuing during labour and following birth. These are regulated by vaginocervical stimulation during contractions, and maternal–infant sensory interactions and suckling from birth until weaning (see website).
Current findings on the affective and behavioural manifestations of these altered patterns of neuronal interactions provide insights into the sensibilities and vulnerabilities of mothers, before, during and after birth (Neumann 2009). A variety of studies indicate that maternal and neonatal outcomes are positively influenced by women’s chosen place of birth; emotional security and seclusion during labour; vaginal birth; and undisturbed maternal–infant sensory contact, particularly during the first hours of extrauterine life (DeVries 2002, Erlandsson & Fagerberg 2005, Hodnett et al 2008, Johnson & Davis 2005, Klein et al 1995, Swain et al 2008).
Acquiring brain capacities for mothering
Between mid-pregnancy and the first few days after birth, the maternal hypothalamus and limbic system are exposed to a progressive increase in placental steroid and peptide hormones, and a selective increase in their multiple receptor concentrations.
This includes central and peripheral levels of oestrogens, progesterone, placental lactogen (hPL), and prolactin (see website).
Maternal HPA axis, social aggression and anxiety-related behaviours
Evidence from human and animal studies indicate that pregnancy and lactation are characterized by an enhanced parasympathetic tone, suppression of sympathetic responses to stressful stimuli, and attenuated response of the hypothalamic–pituitary–adrenal (HPA) axis to various stressors (Brunton et al 2008, Douglas 2010). This is manifested by the woman’s different responses to stressors and perceived dangers to herself and her baby. A key regulator of maternal anxiety postpartum appears to be physical separation of mother and infant (see website).
Prolactin – maternal stress and anxiety
In humans, levels of prolactin and hPL rise throughout pregnancy. During advanced labour, prolactin levels decline rapidly but rise again soon after birth (see website). Following placental separation, hPL disappears from the maternal circulation, but suckling provides the stimulus for ongoing prolactin secretion that peaks within the first 3 months of human lactation (Diaz et al 1989, Grattan 2002).
Prolactin has been found to initiate key elements in the repertoire of behaviours involved in nest-building during late pregnancy and nurturing, protecting and nourishing the infant during lactation (Lucas et al 1998): reduction of maternal fearfulness and anxiety, and reduced anxiety and stress during lactation (see website).
The maternal emotional brain – oxytocin and prolactin
In pregnancy, magnocellular oxytocin neurons in the supraoptic nucleus (SON) and paraventricular nucleus (PVN) are restrained from premature activation, to prevent pre-term labour and preserve accumulating oxytocin stores in the neurohypophysis, in preparation for labour, birth and the onset of lactation (Higuchi & Okere 2002, Russell & Brunton 2006, 2009, Russell et al 2003) (see website).
Gestational analgesia
A significant rise in maternal pain threshold occurs between late pregnancy and 24 hours following birth (Gintzler & Liu 2001, Whipple et al 1990). Maternal pain threshold rises gradually from 30 weeks’ gestation, accelerates during the last 3–4 weeks of pregnancy, rises further during labour, and then falls precipitously within 24 hours of birth (Gintzler & Liu 2001, Ohel et al 2007). Evidence indicates that placental steroids augment pelvic afferent tone. These nerves entering the spinal cord from the cervix and uterus activate multiple analgesic synergies between spinal κ/δ opioid systems, non-opioid peptides and spinal noradrenergic pathways descending from the brainstem (Liu & Gintzler 2003) (see website).
Painless nocturnal contractions occur in women from around 30 weeks’ gestation, coinciding with higher circulating levels of oxytocin and melatonin, and a lower oestrogen/progesterone ratio, which increases myometrial sensitivity to oxytocin during the early hours of darkness (Fuchs et al 1992, Murphy Goodwin 1999, Sharkey et al 2009).
Neuroendocrine and central oxytocin systems – late pregnancy to weaning
There are complex anatomical and physiological adaptations during the last few weeks of pregnancy and through labour through to the process of weaning.
During pregnancy, secretion of oxytocin and secretory response of magnocellular neurons to various physiological stimuli are progressively restrained at several levels by opioid systems which are largely stimulated by allopregnanolone – a neurosteroid metabolite of progesterone (Brunton & Russell 2008, Douglas 2010, Higuchi & Okere 2002, Russell & Brunton 2009). In late pregnancy, brainstem and forebrain neuronal projections to the SON and PVN are activated in preparation for labour, birth, the induction of maternal behaviours and lactation (de Kock et al 2003, Douglas et al 2002, Ortiz-Miranda et al 2005; Russell et al 2003) (see website).
Central oxytocin
A distinct and independently regulated oxytocin system exists within the brain which is highly activated during the peripartum period. Central oxytocin acts as a neurotransmitter within PVN neurons that project to the forebrain, limbic system and autonomic centres in the brainstem and spinal cord (Neumann 2009, Russell & Brunton 2009). Oxytocin is also released in much larger quantities from soma and dendrites of magnocellular neurons within the SON, PVN and other associated nuclei throughout labour and lactation (Russell et al 2003) (see website).
Myometrial quiescence
Throughout pregnancy, the smooth muscle of the myometrium undergoes a series of adaptations that facilitate proliferation and hypertrophy while the capacity for contractility is deactivated (Shynlova et al 2009). This state of quiescence facilitates implantation, placental formation, subsequent growth of the fetus and placenta, and the progressive accumulation of amniotic fluid (Price & Lopez Bernal 2001).
An array of factors maintain uterine quiescence until the end of pregnancy, including human chorionic gonadotrophin (hCG), progesterone, corticotrophin-releasing hormone (CRH), relaxin, nitric oxide and melatonin. hCG inhibits formation of gap junctions; oxytocin stimulates contractions and stimulates enzymes that synthesize relaxatory prostaglandins, until receptor concentrations for hCG decline with the onset of labour (Sparey et al 1999, Ticconi et al 2006, Zuo et al 1994).
Localized myometrial contractions occur spontaneously in response to uterine distension, towards the end of pregnancy, when uterine growth declines relative to the fetus (Shynlova et al 2009). This increases uterine wall stretch, facilitating a pre-labour rise in myometrial oxytocin receptors. Prostacyclin increases the expression of gap-junction and contractile proteins, and melatonin receptor expression declines relative to non-pregnant values, attenuating its suppressive effects on myometrial oxytocin receptors (Fetalvero et al 2008, Lindstrom & Bennett 2005, Terzidou et al 2005).
Placental steroids
Plasma concentrations of oestrogens and progesterone increase progressively throughout pregnancy and labour, but target tissue responsiveness is controlled by changes in expression and activation of their nuclear and non-nuclear receptor subtypes, and by pregnancy-induced expression of progesterone metabolites, which maintain uterine quiescence by binding directly to membrane-bound receptors and inhibiting signalling pathways (Condon et al 2003, Mesiano 2001, Mesiano et al 2002, Mesiano & Welsh 2007, Sheehan et al 2005). The capacity of progesterone to maintain uterine quiescence is also enhanced by functional inactivation of sympathetic nerves in the myometrium and increased receptors for peptides and neurotransmitters that promote relaxation and inhibit the contractile effects of oxytocin (Casey et al 1997, Dong et al 1999, 2003, Ferguson et al 1998, Grammatopoulos et al 1996, Owman 1981, Price & Lopes Bernal 2001) (see website).
This complex balance between the oestrogen and progesterone ratios ensures that there is less myometrial responsiveness, desensitizing it to oestrogen-induced formation of gap junctions, contraction-associated proteins and responsiveness to oxytocin. At the same time, oestrogens increase myometrial responsiveness to the progesterone receptor, subtype B (PR-B) (Mesiano 2001, Mesiano et al 2002, Mesiano & Welsh 2007) (see website). Progesterone contributes to myometrial quiescence by modulating the expression of genes that encode a number of contraction-associated proteins, including oxytocin receptors and formation of myometrial gap-junctions, until the end of pregnancy (Mesiano et al 2002, Mesiano & Welsh 2007).
For most of pregnancy, myometrial contractions remain localized, since few intracellular connections are formed until the last trimester (see website).
Placenta and fetal membranes
During pregnancy, spontaneous, oxytocin- and prostaglandin-induced myometrial contractions are inhibited by the placenta and chorioamniotic membranes surrounding the uterus. The placenta produces atrial natriuretic peptide (ANP), while the chorion and amnion produce brain natriuretic peptide (BNP). Both peptides inhibit oxytocin-induced contractions (Carvajal et al 2006, 2009; Cootauco et al 2008). These are ideally positioned to protect the fetus from oxytocin and other inflammatory mediators, with the capacity to stimulate myometrial contractility (Keelan et al 2003) (see website).
Amnion, chorion and decidua also express enzymes to synthesize and metabolize oestrogens and progesterone. Current evidence suggests that the dominance of PR-B is maintained in these tissues until the end of pregnancy, when enzymatic changes stimulate concurrent increases in the most biologically active oestrogen and the most inactive progestogen (Blanks et al 2003). The fetoplacental membranes and maternal decidual tissues therefore establish endocrine–paracrine networks regulating the length of gestation and the onset of labour (Chibbar et al 1995, Cootauco et al 2008, Henderson & Wilson 2001, Jaffe 2001, Rehman et al 2007, Smith 2007, Ticconi et al 2006).
From pregnancy to labour
Fetal preparations for labour and lactation
The progressive nocturnal rhythm in uterine activity during the last trimester gradually shifts the fetus towards the lower pole of the uterus and the presenting part descends into the pelvis. This helps the fetus to increase flexion and descent, and follow the curve of Carus. Activation of the fetal HPA axis during the third trimester produces physiological increases in cortisol, which interacts with other hormones to induce maturational changes in organs like the lungs, liver, pancreas and gut, thyroid axis, and thermogenic proteins in brown adipose tissue (Fowden et al 1995, Freemark 1999, Garbrecht et al 2006, Liggins 1994). In the brain, catecholaminergic neurons have a key role in cortical differentiation and maturation of respiratory neural networks in the brainstem (Fujii et al 2006). Cortisol and adrenaline also stimulate a gradual increase in blood pressure, in preparation for pulmonary expansion and cessation of the fetoplacental circulation soon after birth (see website).
During the last couple of days before the onset of labour, fetal breathing activity is reduced and lung fluid is produced at a gradually decreasing rate (Bland 2001). Fetal breathing may be depressed by endogenous opioids and rising concentrations of prostaglandin E2 (PGE2), while the decline in lung liquid volume is associated with increased production of cortisol and catecholamines, from late pregnancy to birth (Jain & Duddell 2006, Lagercrantz & Herlenius 2002). It is suggested that the fetal brain is protected from reduced oxygen and glucose supplies around the time of birth, by an increase in central oxytocin, beginning just before the onset of labour and peaking around 2 hours before birth (Brown & Grattan 2007). The fetal brain is exposed to elevated levels of oxytocin, which triggers a transient but significant switch of the GABA neurotransmitter, from excitatory to inhibitory. This reduces nutrient and oxygen requirements of the brain during transition to air-breathing and suckling (Khazipov et al 2008, Tyzio et al 2006). Current evidence suggests that the oxytocin is derived from both mother and fetus (Khazipov et al 2008).
The fetal adreno-placental ‘clock’
Duration of pregnancy is strongly associated with the rising profile of placental CRH in the maternal circulation (Smith 2007, Tyson et al 2009). CRH levels rise exponentially in maternal and fetal circulatory systems during the last 12 weeks of pregnancy, peak during labour, and fall precipitously following birth (Chan et al 1993, Goland et al 1986). In individual women, the exponential increase tends to mirror the duration of pregnancy: women who give birth prematurely have higher mid-pregnancy levels of CRH than those who give birth at term (McLean et al 1995, Smith 2007). The bioavailability of CRH is regulated by a circulating binding protein, which declines at the end of pregnancy, further increasing maternal and fetal tissue exposure to CRH (Grammatopoulos 2008) (Fig. 35.1).
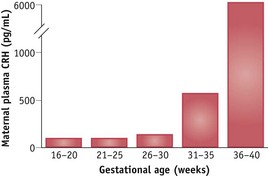
Figure 35.1 Mean plasma CRH concentrations in eight women followed sequentially during the second half of pregnancy.
(Reproduced with permission from Goland et al 1994:1289.)
During pregnancy and labour, placental CRH targets a number of maternal, placental and fetal organ systems. In the fetal compartment, CRH receptors have been identified in the pituitary gland, adrenal cortex, lungs, placenta and membranes (Grammatopoulos 2007, 2008). In the adrenals, placental CRH directly stimulates the fetal zone to produce DHEA-S and the definitive zone to produce cortisol, in a dose-dependent manner (Rehman et al 2007). The maternal pituitary–adrenal axis is also a target organ for CRH, while the myometrium is both a source and target for CRH and a related family of urocortin peptides (Goland et al 1994, Grammatopoulos 2007, Markovic et al 2007, Smith 2007) (see website).
Myometrial actions of placental CRH
During pregnancy, human myometrial cells express a large number of CRH and CRH-related urocortin peptides, and their major receptor subtypes CRH-R1 and CRH-R2 (see website).
As term approaches, oxytocin and inflammatory cytokines also stimulate expression of many variants of the CRH-R1 receptor with reduced signalling capacities (Grammatopoulos & Hillhouse 1999, Hillhouse & Grammatopoulos 2001). Recent work suggests that expression of CRH-R1 variants with reduced signalling capacity are only increased in the lower uterine segment with the onset of labour (Markovic et al 2007).
In contrast to CRH-R1, activation of CRH-R2 stimulates signalling pathways that enhance myometrial contractility. Recent experiments on gene profiles in different regions of the uterus have identified expression of fundal genes for CRH-R2 that increase significantly during labour (Grammatopoulos 2008, Stevens & Challis 1998). These findings indicate that dynamic changes in the balance of myometrial CRH-R1 receptor subtypes at term stimulate concomitant physiological changes in the fundus and lower uterine segment from late pregnancy to birth. While muscles in the upper segment generate coordinated forceful contractions, those in the lower segment have reduced stimulatory influences, which facilitates increased contractility of the fundus, elongation of the lower segment over the presenting part and progressive cervical dilation, as labour advances (Bukowski et al 2006).
CRH activity in placenta and membranes
The placenta and membranes also express two major CRH receptor subtypes: CRH-R1 and CRH-R2. In the placenta, the CRH-R1 subtype seems to increase expression of type 2 cyclo-oxygenase (COX-2), which stimulates biosynthesis of prostaglandin precursors and decreases expression of prostaglandin dehydrogenase (PGDH) – the key enzyme produced by the placenta and membranes that metabolizes active primary prostaglandins to an inactive form and inhibits production of progesterone (Amash et al 2009, Gao et al 2008, Grammatopoulos 2008) (see website).
Uterine oxytocin receptors
During pregnancy, myometrial receptors for oxytocin (OTR) increase from 27.6 fmol/mg DNA in the non-pregnant state to 171.6 fmol/mg DNA at mid-gestation, and 1391 fmol/mg DNA at term (Fuchs et al 1984). This represents a 50-fold increase within the uterus before the onset of labour (see website).
Maximum receptor concentrations have been found in early labour at term, 3583 fmol/mg DNA – significantly higher than before labour begins (Fuchs et al 1982). Concentrations of decidual receptors are relatively low in mid-pregnancy and reach maximal values following the onset of labour. Within the fetal membranes, increased OTR binding has been found between late pregnancy and labour, with highest increases in the amnion (Takemura et al 1994). Myometrial receptor concentrations are highest in the fundus and corpus, significantly lower in the lower segment, and lowest in the cervix, while decidual receptors are highest in sections surrounding the corpus and lowest around the lower segment (Blanks et al 2003).
During early labour, myometrial receptor concentrations are uniformly high in the upper segment and progressively lower in the isthmus and cervix, while those in the decidua are highest in the corpus, followed by the fundus and the isthmus (Fuchs et al 1984, Fuchs & Fuchs 1991, Hirst et al 1993) (see website).
There is an important role for gap junctions in coordinating cellular responsiveness to oxytocin. At term, higher concentrations of myometrial gap-junctions occur in the fundus compared to the lower segment, and the difference becomes increasingly pronounced during labour. This creates increasing fundal dominance during the course of labour and regulates progressive conductance of electrical activity, from fundus to cervix, to propagate multicellular synchronization of myometrial responsiveness to neuroendocrine, pulsatile and intrauterine oxytocin systems (Blanks et al 2003, Fuchs et al 1991, Kimura et al 1996, Russell et al 2003, Shmygol et al 2006).
During spontaneous labour, myometrial and decidual OTR concentrations decline significantly in advanced labour, particularly in the lower segment (Fuchs et al 1984). While findings in the lower segment are unreliable because of progressive incorporation of the cervix into the lower segment, available evidence suggests that oxytocin receptor mRNA significantly declines in the lower segment with increasing duration of labour. In spontaneous labour, the decline occurs gradually over 12–16 hours, but in oxytocin-induced and oxytocin-augmented labour, it is much steeper, especially when the infusion is constant rather than pulsatile (Phaneuf et al 2000, Robinson et al 2003, Willcourt et al 1994) (see website).
Nocturnal myometrial activation and cervical ripening
The uterus has a well-defined 24-hour rhythm of contractility and electrical and endocrine activation (Schlabritz-Loutsevitch et al 2003). In human pregnancy, increased contractile activity has been observed between 20:30 and 02:00 from 24 weeks’ gestation (Fuchs et al 1992, Germain et al 1993, Moore et al 1994, Sharkey et al 2009). Current research suggests the emergence of a nocturnal surge in rhythmic myometrial contractions is a key indication of uterine activation in preparation for the shift from pregnancy to labour. Nocturnal surges in oestradiol, melatonin and oxytocin occur from around 35–36 weeks’ gestation and these coincide with the 24-hour rhythm of spontaneous birth (Fuchs et al 1992, Schlabritz-Loutsevitch et al 2003, Tamura et al 2008). The nocturnal surge in oestriol, which originates almost exclusively from fetal adrenal DHEA-S, occurs from 35 weeks’ gestation; nocturnal plasma melatonin rises from 36 weeks’ gestation and nocturnal peaks in plasma concentrations of oxytocin occur from 37–39 weeks’ gestation (Fuchs et al 1991, 1992, Germain et al 1993, Moore et al 1994, Murphy Goodwin 1999, Schlabritz-Loutsevitch et al 2003, Tamura et al 2008). Oestradiol and melatonin increase gap junctions and oxytocin receptors, and melatonin also synergizes with oxytocin, increasing oxytocin-induced contractility in a dose-dependent manner (Sharkey et al 2009).
From approximately 36 weeks onwards, structural alterations become more apparent in cervical stroma and mucosal tissues, which alters its dimensions in relation to the lower uterine segment (House et al 2009). Within cervical connective tissue, alterations occur in the composition and concentration of the gel-like material called ground substance (proteoglycans) in which connective tissue cells and fibres are embedded. At the same time, an increase occurs in enzymes that degrade collagen. The concentration of ground substance relative to collagen is thought to reach a maximum during cervical softening prior to the onset of labour. This overall increase is characterized by the emergence of a higher proportion of molecules with a weaker affinity for collagen fibrils (see website).
Cervical and uterine muscles
During late pregnancy and the latent phase of labour, myometrial components of the cervix contract in characteristic short, high-frequency pressure increases, that are independent of the rest of the uterus, until the onset of established labour (Rudel & Pajntar 1999). These contractions may stimulate local connective tissue changes associated with cervical ripening (Olah et al 1993, Pajntar 1994). In primiparous women, softening of cervical tissue proceeds alongside effacement and is thought to occur in response to increased formation of gap junctions between adjacent cells, in the myometrium of the uterine cavity. Gap junctions are composed of symmetrical portions of plasma membrane from adjacent cells. These form intercellular channels for passage of ions and small molecules, facilitating rapid intracellular transmission of electrical impulses and chemical signals between cells. Gap junctions emerge in late pregnancy and undergo further increases in size and number during early labour. Formation and permeability of gap junctions are stimulated by oestrogens, prostaglandins and melatonin, and inhibited by progesterone, hCG and relaxin (Ambrus & Rao 1994, Burghardt et al 1993, Chow & Lye 1994, Sharkey et al 2009). Before labour begins, myometrial expression of gap junctions is much higher in the fundus than in the lower segment and this difference accelerates during the course of labour (Sparey et al 1999).
By facilitating the propagation of action potentials from cell to cell, gap junctions synchronize myometrial activity. Tension is transmitted from the myometrium by the outer layer of muscle that extends along the periphery of the supravaginal portion of the cervix (Pajntar 1994). This facilitates stretching of the lower uterine segment, which elongates as pressure is exerted by the fetus during descent into the pelvis. These combined forces seem to produce a differential rate of tissue uptake in the cervix and the adjacent lower segment of the uterus. Maximum uptake occurs at the lower peripheral end of the cervix, producing a gradual upward movement of soft cervical tissue that eventually merges with the lower segment (Gee & Olah 1993, Havelock et al 2005) (Fig. 35.2).
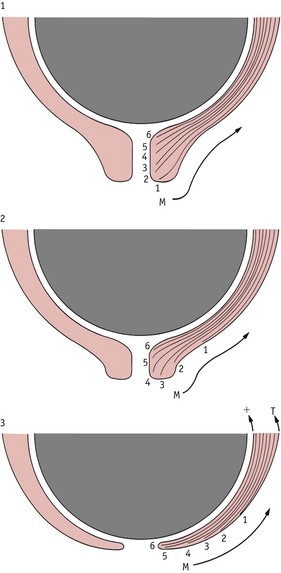
Figure 35.2 Diagram representing a hypothesis concerning differential movement of tissue planes at the time of cervical effacement and dilatation. M, direction of movement of collagen bundles; T, +, differential tension across the myometrium.
(Reproduced with permission from Gee 1981, Fig. 14.5.)
In a recent study on women following induction and augmentation of labour, a number of significant features were associated with the presence or absence of cervical contractions in response to myometrial activity. Cervical contractions predominantly occurred in women with lower measures of cervical effacement and dilatation and a longer latent phase, compared to those in whom cervical contractions were absent (Rudel & Pajmtar 1999). These indicate the importance of coordinated changes, from the fundus to the cervix, during the transition from pregnancy to labour (Havelock et al 2005, Olah et al 1993, Pajntar 1994, Rudel & Pajntar 1999).
Uterocervical changes and inflammation
Local pro-inflammatory changes accompany the remodelling and stretching of uterine muscle and cervical connective tissue during the latter part of pregnancy. The progressive release of inflammatory mediators like nuclear factor kappa B (NF-κB), cytokines and interleukins seems to gradually overwhelm the selective suppression of inflammatory and immune responses established from the beginning of pregnancy by progesterone, prolactin and cortisol (Gubbay et al 2002, Johnson et al 2008, Lindstrom & Bennett 2005, Pepe & Albrecht 1995, Rosen et al 1998, Shynlova et al 2009, Vaisanen-Tommiska et al 2003). Remodelling of the cervical connective tissue; stretching of the lower uterine segment and of the fetal membranes overlying the cervix, produce local alterations in the relative activity of mediators of inflammatory and anti-inflammatory reactions (Allport et al 2001, Bennett et al 2001, Moore et al 2006, Vaisanen-Tommiska et al 2003).
These include increased concentrations of a key cytokine, interleukin (IL)-8, in the cervix and lower uterine segment with cervical ripening; higher concentrations of enzymes that synthesize prostacyclin, PGE2 and PGF2α in the lower segment compared to the fundus, before and during labour; raised cervical production of cytokines and nitric oxide (NO) at term and during labour; increased expression of NF-κB and decreased expression of glucocorticoid receptors in cervical tissue from late pregnancy to birth; and downregulation of placental cortisol receptors (Allport et al 2001, Bennett et al 2001, Johnson et al 2008, Keelan et al 2003, Lindstrom & Bennett 2005, Norman et al 1998, Sparey et al 1999). The COX-2 enzymes that stimulate prostaglandin synthesis are activated in cervical tissue by NO and NF-κB. The higher concentrations of these enzymes in the lower compared with the upper segment before labour may also increase collagenolytic activity of cervical tissue, thus contributing to cervical ripening. With the onset of labour, these enzymes increase further in the lower but not the upper segment, which suggests that prostaglandins may actively promote relaxation of the lower uterine segment throughout the course of labour (Myatt &Lye 2004, Sparey et al 1999, Zuo et al 1994).
Remodelling gestational tissues
Fetal membranes consist of amnion and chorion layers connnected by an extracellular matrix (ECM) of collagen fibres that provides the main tensile strength of the membranes. Current findings suggest that membranes undergo a regulated process of tissue remodelling similar to the cervix, from late pregnancy to birth (Moore et al 2006). In the cervix and membranes, remodelling and maturation processes involve changes in collagen fibres.
The amnion lies in direct contact with amniotic fluid, which contains elevated concentrations of pro- and anti-inflammatory cytokines from early pregnancy (Keelan et al 2003). During the third trimester, surfactant proteins and phospholipids also enter amniotic fluid in increasing quantities and these have macrophage-activating properties that stimulate NF-κB activity, which regulates expression of MMP enzymes. Cytokines stimulate the prostaglandin H synthase 2 enzyme, which stimulates synthesis of prostaglandins, and concentrations of PGF2α increase significantly in the amnion from around 38 weeks’ gestation (Keelan et al 2003, Lee et al 2008, Smith 2007). NO has been found to stimulate release of PGE2 in amnion-like cells and fetal membranes, and recent evidence suggests that oxytocin is involved in stimulating the release of NO and pro-inflammatory cytokines from fetal membranes during labour (Ticconi et al 2004).
The amnion is separated from the myometrium by the chorion and decidua. Research findings suggest that the chorion, decidua and placenta produce anti-inflammatory cytokines and the placenta and chorion also produce the enzyme prostaglandin dehydrogenase (PGDH) which is a potent inactivator of prostaglandins (Amash et al 2009, Keelan et al 2003). Late in pregnancy, chorionic PGDH activity declines while the expression of the inducible isoform of the prostaglandin-generating enzyme COX-2 increases significantly in the adjacent amnion (Ticconi et al 2006). Many anti-inflammatory cytokines are produced in the decidua, which decrease local prostaglandin production, and their levels remain elevated following the onset of labour, while those in the placenta seem to decline during the course of labour (Keelan et al 2003) (see website).
From late pregnancy to birth
The transition from nocturnal myometrial contractions to the onset of labour extends from around 30 weeks’ gestation, particularly in primigravid women. During this period, cervical tissues become less resistant, interrelated anatomical changes occur in the cervix and lower uterine segment, and the myometrium is activated nocturnally by episodes of rhythmic contractions (House et al 2009). Progesterone dominance declines within uterine tissues and related forces that promote myometrial quiescence and inhibit multicellular interactions are progressively modulated, in a region-specific manner, within the myometrium and surrounding intrauterine tissues, from late pregnancy to birth (Bukowski et al 2006, Henderson & Wilson 2001, Mesiano & Welsh 2007, Sparey et al 1999).
From around 32 weeks, the onset of increased nocturnal release of oxytocin coincides with the decrease in the plasma oestrogen/progesterone ratio and the rise in oxytocin receptor density in the uterus. Under these conditions, a small rise in the pulsatile release of oxytocin seems to stimulate episodes of uterine contractions during the hours of darkness (Fuchs et al 1991, Germain et al 1993, Moore et al 1994). Expression of oestrogen-, melatonin- and prostacyclin-induced gap-junction proteins in the myometrium also increases from around 37 weeks, particularly in the fundus, and these provide low-resistance pathways between smooth muscle cells that increase the coordination of contractile activity throughout the uterus (Chow & Lye 1994, Fetalvero et al 2008, Sharkey et al 2009) (see website).
These dynamic changes progressively generate functionally distinct sections of the uterus from late pregnancy to birth. Towards the end of pregnancy, the recurring episodes of nocturnal contractions become stronger and more frequent, until the functionally differentiated myometrium expresses its intrinsic capacity to propagate progressively stronger contractions from the fundus to the cervix during labour and birth (Bukowski et al 2006, Sparey et al 1999, Ticconi et al 2006).
Maternal–fetal readiness for labour
In women, the transition from nocturnal rhythms in uterine contractions to latent labour is highly variable and is influenced by a host of additional factors, including maternal cognitive activity, fetal position and emotional readiness for labour (Wuitchik et al 1989). Throughout the last 4 weeks of pregnancy, physiological adaptations seem to be enhanced when women take time out in the evenings for relaxation, to enhance the duration of sleep (Lee & Gay 2004). Using favoured ways of relaxing in late pregnancy, particularly during the early hours of darkness, facilitates the nocturnal rise in oxytocin and melatonin, which regulate the physiological increase in myometrial activity (Fuchs et al 1992, Sharkey et al 2009). This phase of preparatory changes in uterine smooth muscle and cervical tissue accelerates at term, and the onset of spontaneous labour indicates the combined readiness of maternal and fetal organ systems for labour and birth (Majzoub & Karalis 1999).
Reduced cognitive stimulation, relaxation and sleep are key elements of preparation because of the positive association between low cognitive activation and expressions of maternal love; sleep duration in late pregnancy and shorter duration of labour; and the positive association between chronic anxiety, heightened levels of fear, pain perception and labour complications (Bartels & Zeki 2004, Haddad et al 1985, Lee & Gay 2004, Saisto et al 2001). Research evidence also indicates that low cognitive anxiety enables women to experience less discomfort during latent labour, suggesting that the absence of fear modulates maternal pain perception (Wuitchik et al 1989). Once established labour begins, mothers need to be with their trusted companion, who maintains a quiet, warm, low-lit environment and communicates with minimal cognitive stimulation (Hodnett et al 2008). These conditions are conducive to the release of central oxytocin, which induces a timeless hypnotic state that deepens as labour progresses. Maintaining a calm, secure, low-lit environment also prevents a stress-induced rise in catecholamines, which have been shown to inhibit oxytocin and attenuate uterine contractions (Levinson & Shnider 1979, Peled 1993).
Neuroendocrine oxytocin
The release of oxytocin from the neurohypophysis during labour occurs in response to neuronal feedback to the brainstem from the uterus, cervix and vagina. During late pregnancy and labour, innervation is low in the body of the uterus and significantly higher in the cervix, vagina and adjacent parts of the pelvic cavity. Stretching and distension of these areas activates sensory afferent nerve pathways that transmit signals via the spinal cord and brainstem to oxytocin neurons in the hypothalamus. These respond with discrete bursts of accelerated discharge that transport the stored hormone along axons of hypothalamic neurons that each give rise to numerous varicosities or large vesicles in the neurohypophysis. From here, oxytocin is released intermittently into the general circulation (Rossoni et al 2008) (Fig. 35.3).
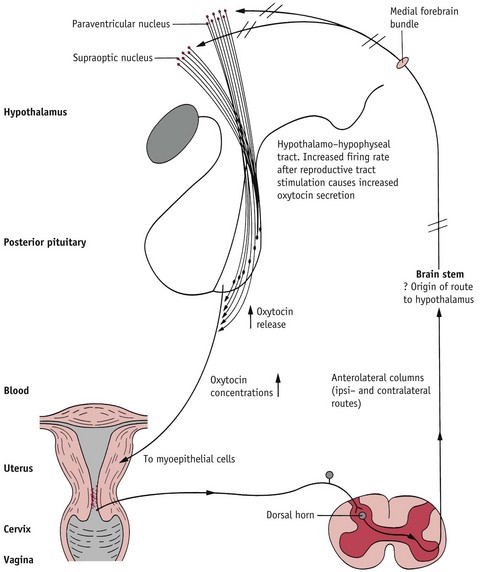
Figure 35.3 The neuroendocrine reflex underlying oxytocin synthesis and secretion. Stretching of the cervix and lower segment (black line) activates the reflex leading to oxytocin release (red line)
(Reproduced with permission from Johnson 2007: 248.)
Throughout labour and birth, increasing vaginocervical stimulation produced by downward pressure of the fetus transmits nerve impulses via the vagal and pelvic nerves, through spinal and brainstem pathways, to the hypothalamus (Russell et al 2003). These recurring episodes of sensory stimulation trigger characteristic bursts of magnocellular oxytocin neurons, resulting in minute-to-minute variation in plasma oxytocin levels during spontaneous labour (Fuchs et al 1991). As illustrated in Figure 35.4, pulse frequency increases significantly during labour but pulse amplitude remains low until the active phase, when it rises sharply, particularly during the final moments around birth. After birth, this pattern of oxytocin release accelerates in response to sensory contact and suckling, and peaks during the middle of lactation (Fuchs et al 1991, Johnston & Amico 1986, Rossoni et al 2008) (see website).
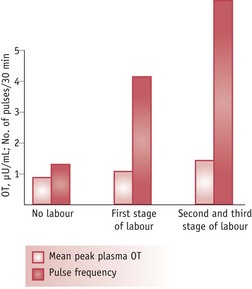
Figure 35.4 Mean oxytocin pulse frequency and amplitude determined in women at term, not in labour (n = 11), during the first stage of labour (n = 13) and combined second and third stages (n = 8). n = number in each group.
(Reproduced with permission from Fuchs et al 1991:1520.)
Oxytocin receptors are regulated by oxytocinase, an enzyme that rapidly degrades oxytocin. Placental oxytocinase is released into the maternal circulation during pregnancy and reaches highest levels just before the onset of labour (Ito et al 2001, Nomura et al 2005). This enzyme prevents receptor desensitization during prolonged oxytocin release, as happens during labour and lactation. Oxytocinase may also suppress the pain of uterine contractions before and after birth, by rapidly inactivating oxytocin following its release (see website). Because of the increase in oxytocinase before the onset of labour, receptor concentrations remain elevated for approximately 20 hours of labour, before they begin to decline (Ito et al 2001).
Towards the expulsive phase
Just before the cervix has been fully incorporated into the lower segment, the frequency of contractions may slow down, particularly in primigravid women. When maternal and fetal events are not completely synchronized, this presents as a slowing down of the labour process and may require experimenting with different maternal positions to allow the fetus to fully descend into the pelvic outlet. A slow pace of descent allows the simultaneous surge in fetal adrenaline to complete the removal of lung liquid which enhances respiratory adaptations immediately following birth (Bland 2001).
In biological terms, the expulsive phase is reached when the cervix has become incorporated into the lower uterine segment, which becomes progressively thinner, as expulsive contractions set up positive afferent nerve pathways to the hypothalamus. This reflexive mechanism stimulates increased central release of oxytocin, and, with increasing flexion and anterior rotation of the fetal head, pressure from the vertex against the gutter-shaped pelvic floor muscles stimulates their stretch receptors that activate both neuroendocrine and neurotransmitter oxytocin systems (Sansone et al 2002). During the expulsive phase, vaginocervical stretch activates magnocellular and parvocellular neurons. The former release oxytocin in characteristic pulses into the systemic circulation, while the latter release oxytocin into the spinal cord, which stimulates sympathetic neurons that project to radial muscles of the iris, producing dilation of the pupils (Komisaruk & Sansone 2003) (Fig. 35.5).

Figure 35.5 Schematic view of the pathway mediating the reflexive release of oxytocin in response to vaginocervical stimulation and the pathway mediating the pupil dilation response to vaginocervical stimulation.
(Reproduced with permission from Komisaruk & Sansone 2003:247.)
Unresolved anxieties or perceived threats or dangers may stimulate catecholamines, particularly adrenaline, which inhibits the pulsatile release of oxytocin, leading to a decline or cessation of uterine contractions, excessive blood loss following birth, and delay in the onset of suckling and lactation (Chen et al 1998, Levison & Shnider 1979, Odent 1992, Peled 1993). Current evidence suggests that a private, warm, low-lit environment will enhance oxytocin secretion and minimize the rise in maternal cortisol and catecholamines activated by heightened fear and anxiety that may occur just before expulsive contractions begin (Chen et al 1998, Levinson & Shnider 1979, Wuitchik et al 1989).
The expulsive phase is accompanied by a physiological increase in maternal catecholamine levels, particularly noradrenaline. This rise increases cardiac output and pulmonary circulation, providing the physical energy that usually coincides with the final moments of labour (Odent 1987). Many women find that they focus on rhythmic breathing during the expulsive phase and this ‘hypnotic-like’ state enables them to follow their bodies’ expulsive efforts. This encourages the woman to move the rest of the fetal body down the birth canal, without undue stress on herself or her baby.
Spontaneous maternal breathing
To maintain uterine blood flow and adequate oxygenation to the fetus during the expulsive phase, maternal hyperventilation and breath-holding need to be avoided. Over prolonged periods, breath-holding (Valsalva manoeuvre) increases intrathoracic pressure, which reduces venous return to the heart. Consequently, cardiac output falls and blood pressure drops, leading to a reduction in uteroplacental perfusion (Blackburn 2007). In a recent randomized study comparing Valsalva and spontaneous pushing, the expulsive phase of labour was shorter and umbilical arterial pH, PO2 and Apgar scores were higher in the spontaneous pushing group (Yildirim & Beji 2008).
Fetal responses to labour and birth
Labour and birth induce increased secretion of adrenomedullin, catecholamines, cortisol and thyroid-stimulating hormone (TSH). Adrenomedullin is a potent vasodilator of the pulmonary circulation which facilitates a rapid increase in pulmonary blood flow at birth (Boldt et al 1998). Free cortisol levels double in association with labour, rising further in the 1–2 hours following birth. As well as stimulating maturational changes in key organ systems, cortisol induces increased deiodination of thyroxine (T4) to produce triiodothyronine (T3). This works in conjunction with the dramatic surge in thyroid-stimulating hormone (TSH) at birth, stimulating striking increases in T3 during the first 24 hours of neonatal life, which is particularly important for regulating thermogenesis (Nathanielsz et al 2003, Pearson Murphy & Branchaud 1994).
In the fetus, catecholamine levels rise throughout labour, reaching 20-times adult resting values immediately following birth. The intermittent squeezing of the fetal head during human labour triggers a rapid surge in catecholamine release (see website).
Cardiovascular responses
Contractions induce transient reductions in uteroplacental perfusion that alter the pattern of fetoplacental circulation. Ultrasonic studies suggest that at the beginning of a uterine contraction, maternal venous outflow is halted and the content of the uterine veins is expressed into the maternal circulation. Simultaneously, arterial inflow that coincides with the onset of contractions is retained within the intervillous space. During contractions, this blood forms an increased pool that creates marked distension and vascular engorgement in the intervillous space. Transient reduction in uteroplacental perfusion during contractions may be partly compensated by the increased volume of maternal blood made available for gaseous exchange. To ensure the perfusion of the placenta, maternal blood pressure and cardiac output also rise in response to contractions. During the phase of uterine relaxation following each contraction, an increased blood flow has been observed, which may also compensate for decreased oxygen delivery during the preceding contraction (Bleker et al 1975, Robson et al 1987).
The circulation of a healthy fetus in spontaneous labour is not thought to be compromised by contractions. The umbilical circulation does not seem to be altered by changes in intrauterine pressure or by short-term changes in fetal–placental or maternal–placental blood flow that accompany contractions. Fetal cardiac output rises in response to increased intrauterine pressure during contractions, allowing fetal blood pressure to maintain a relatively constant pressure difference between the inside and outside of its vascular system. Concurrently, raised levels of fetal adrenaline specifically act to facilitate increases in heart rate and blood pressure, both of which serve to increase the rate of fetoplacental blood flow between contractions (see website).
The effect of fetal head pressure and the resulting catecholamines activates the parasympathetic system and inhibits cardiac pacemakers, resulting in decreased cardiac output, slowing of the heart rate and reduced blood pressure. Slowing of the heart rate during contractions reduces the oxygen requirements of cardiac muscle. Parasympathetic influences on heart rate can be counteracted by adrenaline but not by noradrenaline. In the fetus at term, sufficient levels of adrenaline may be released to produce variable increases or decreases in heart rate, in response to uterine contractions (see website).
Birth and placental separation
As the fetus leaves the uterine cavity, the surface area of the contracting uterus declines rapidly to produce a uterine diameter of around 10 cm. This reduction encompasses the site of placental attachment to the decidual lining, leading to compression of placental tissue and uteroplacental blood vessels, including approximately 100 spiral arteries that have supplied the placenta at a rate of 500–800 mL/min throughout the course of labour (Letsky 1998). Tonic myometrial compression of these blood vessels following birth is greatly facilitated by placental-induced adaptations in the constituents of decidual and myometrial segments of the spiral arteries (Kawamata et al 2007). During the first and second trimesters, structural transformations of the vessel walls replace elastic lamina and smooth muscle layers with a matrix containing fibrin (Matijevic et al 1996).
When the mother is free to reach down and take the baby into her arms at birth, she brings herself into an upright position. This prevents compression of uterine blood flow returning to the heart via the inferior vena cava and allows gravity to assist the process of placental ejection, while the sight, sounds and sensory contact between mother and infant stimulate a significant increase in central and peripheral release of oxytocin, as opioid restraint on maternal oxytocin neurons is removed immediately after birth. Basal levels of oxytocin rise significantly and sensory stimulation and suckling increase pulse frequency and amplitude of oxytocin release, compared to labour and birth (Matthiesen et al 2001).
The enhanced release of oxytocin into the peripheral circulation following birth stimulates tonic contraction and retraction of the myometrium, which squeezes the spongy placental tissue and forces blood in the collapsing intervillous spaces back into the veins of the decidua (Kawamata et al 2007, Shynlova et al 2009). The uninterrupted flow of blood through the intact umbilical cord further reduces placental size, by transferring approximately 120 mL of blood into the neonatal circulation (Dunn 1985). As illustrated in Figure 35.6, the intact cord continues to provide oxygenated blood to the infant as placental separation is progressing. This allows volume adjustments to supply new capillary beds, which are opened by the dramatic fall in pulmonary vascular resistance following the rise in PO2 as lung capillaries dilate with the onset of ventilation immediately following birth (Boldt et al 1998, Dunn 1985).
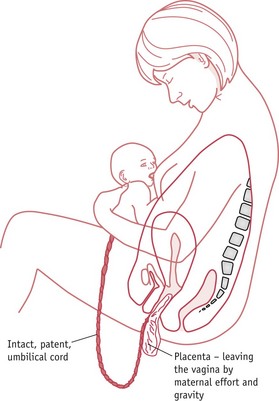
Figure 35.6 Maternal–infant sensory contact, placental separation and the transition from placental to mammary nutrition.
(Reproduced with permission from Inch 1989.)
With sustained myometrial contraction, the congested decidual veins are severed and sealed by the shearing forces of the criss-cross network of muscle fibres surrounding them. As illustrated in Figure 35.7, the placenta is simultaneously torn from the uterine wall, at the line of the decidua basalis, and falls into the uterine cavity, peeling off the membranes as it descends towards the cervix, and falls into the vagina (Benirschke 1992). Placental separation leaves behind a surface wound of 300 cm2 containing approximately 100 severed arteries in which maternal blood coagulates rapidly because of a major increase in the concentration of several coagulation factors and decreased fibrinolytic activity which characterizes pregnancy, labour and the first couple of hours following birth (Letsky 1998). Pregnancy is also accompanied by marked increases in clotting factors VII, VIII, X and XII and by a significant rise in vascular and placental plasminogen activator inhibitors (PAIs) leading to a marked increase in plasma fibrinogen by the third trimester (Dalaker 1986, Letsky 1998).
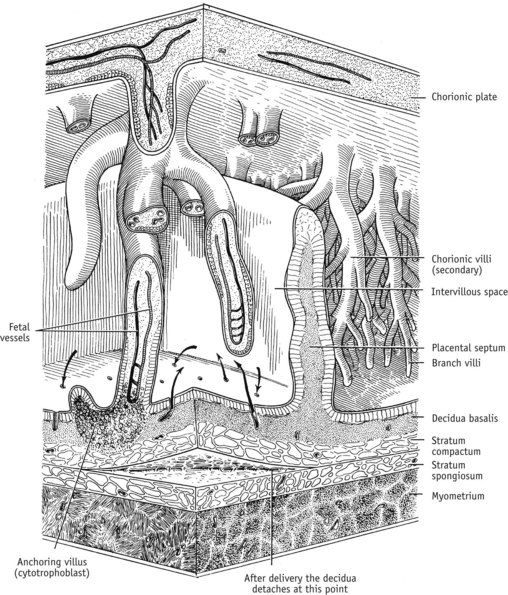
Figure 35.7 Diagrammatic representation of placental, decidual and myometrial tissue near term, illustrating the line of placental separation. Arrows indicate blood flow from uteroplacental arteries to the intervillous space and back to the uteroplacental veins
(Reproduced with permission from Blackburn 2007; Fig. 3-11; 93.)
Before and after birth, these systemic haemostatic changes are accompanied by a local activation of clotting factors V and VIII and increased levels of fibrinogen, which results in a pronounced shortening of whole blood clotting time that is more pronounced in uterine than in peripheral blood. Because of their combined effects, torn blood vessels are sealed and the site of placental separation is rapidly covered by a fibrin mesh that represents 5–15% of total circulating fibrinogen (Letsky 1998). Prostaglandin metabolites are simultaneously released into the general circulation from the torn surface tissues of the decidua basalis at the site of separation where they stimulate sustained uterine contractions (Noort et al 1989).
When women do not experience prolonged stress-induced catecholamine release or severe vaginal or perineal laceration during labour and birth, immediate blood loss from the vagina constitutes a small proportion of the increase in plasma and red cell volume that occurs during pregnancy. Under these conditions, most of the pregnancy-induced increase in blood volume is lost over a longer time period, through diuresis during the first 48 hours and lochia discharge from the placental site over the first 2 months postpartum (Hytten 1995). While visual estimates of blood loss within the first hour following birth are highly inaccurate, the significance of estimated blood loss at birth needs to be judged in relation to the expansion in blood volume during pregnancy (Bloomfield & Gordon 1990, Gyte 1992).
From fetus to neonate
Until birth, the fetus is essentially a parenterally nourished organism receiving a fairly constant supply of simple nutrients from the maternal circulation across the fetoplacental barrier. During this period of enhanced anabolic metabolism, the maternal circulation supplies the placenta with glucose, amino acids and relatively smaller amounts of essential and non-essential fatty acids for selective uptake and transfer to the fetus (Hay 1995, Herrera 2000). Soon after birth, placental transfer of nutrients and gaseous exchange between maternal and fetal circulatory systems ends along with placental hormonal interactions with the fetal adrenal gland (Ben-Davis et al 2007) (see website).
The expression of innate mother–infant interactions, particularly during the early weeks after birth, is critical for the infant’s long-term general health and emotional wellbeing (Moriceau & Sullivan 2006, Neumann 2009, Stern 1997). Close body contact reduces energy loss, regulates homeostatic mechanisms, promotes a physiological increase in glucocorticoid and mineralocorticoid brain receptors, and prevents a rise in the infant’s stress axis, which is highly sensitive to periods of separation, particularly during the first 3 days after birth (Bystrova et al 2003, Christensson et al 1995, Hofer 1994, Sarrieau et al 1988).
Initiation of maternal behaviour and attachment
Following spontaneous vaginal birth, mother and baby are in an ideal state to develop the relationship needed to initiate bonding and lactation. This includes an oxytocin-induced synaptic reorganization of the hippocampus, which improves maternal spatial memory, enabling the mother to develop a ‘laser-like’ focus for everything relating to the needs of her infant (Kinsley 2008, Monks et al 2003, Pedersen & Boccia 2002, Tomizawa et al 2003) (see website).
Women’s stated preference for intimate ‘social affection’ over participating in large social groups is mediated by suckling-induced activation of the mesocorticolimbic dopamine system, which seems to be regulated by oxytocin (see website). The pleasurable effects of intimate contact between mother and infant set up the desire for further maternal stimulation and infant suckling, which reinforces and strengthens the maternal–infant bond, partly by increasing central oxytocin receptors and release of oxytocin into the central nervous system (see website).
Conclusion
The physiology of late pregnancy and labour is characterized by a complex interplay between maternal–fetoplacental systems that is finely regulated by neurohormonal systems. It is essential that midwives are knowledgeable about recent findings regarding the onset and processes of labour, and the onset of lactation, to provide care that complements the unfolding processes involved.