Chapter 43 Diseases of the Reproductive System
FEMALE REPRODUCTIVE DISORDERS
NONPATHOGENIC INFERTILITY
THE BREEDING SEASON
Mares
The mare is a seasonally polyestrous animal, breeding during seasons of long day length. Annual breeding and nonbreeding seasons are divided by fall and spring transitional periods, which are characterized by erratic reproductive behavior and irregular estrous cycles.
Clinical Signs
During the breeding season, mares ovulate every 21 days (range 19 to 22 days).1 Estrus (5 to 7 days, but variable) is characterized by the presence of an ovarian follicle, serum progesterone level of less than 1 ng/mL, and sexual receptivity. During estrus the cervix is palpably relaxed and the uterus is edematous. One or two follicular waves occur per cycle, and preovulatory follicles are 45 to 60 mm in diameter, often with a cone-shaped appearance on ultrasonography.2 Ovulation occurs 24 to 48 hours before the end of estrus and may be accompanied by ovarian sensitivity.1 The ruptured follicle is replaced by a corpus luteum (CL). Diestrus (luteal phase) is predictable in length because regression of the CL, caused by release of endometrial prostaglandin F2α (PGF2α), occurs 14 to 15 days after ovulation.3 During diestrus and early pregnancy the cervix is tight and the uterus is firm and tubular. Diestrous ovulations occur and may be fertile.4 First postpartum estrus (“foal heat”) begins in the week after foaling, and ovulation occurs in most mares 7 to 15 days postpartum.
Ruminants
COWS
Cows are polyestrous, but seasonal differences in fertility may be caused by climate. The estrous cycle averages 21 days (range 17 to 25 days), and the duration of estrus averages 12 to 16 hours (range 6 to 24 hours). Cows are unique among domestic animals in that they ovulate spontaneously after the end of estrus, 24 to 30 hours after the beginning of estrus.5
In the absence of a bull, estrus can be detected in cows by their homosexual (bisexual) activity. Cows that stand to be mounted by another cow are in estrus (standing heat). Secondary signs that may be helpful in detecting estrus include restlessness and increased activity, vulvar hyperemia and edema, and a clear mucous discharge. Errors in heat detection are a common cause of infertility on large dairy farms.6 The optimum time for insemination of cows is between 16 and 24 hours after the onset of estrus. Insemination of cows on the basis of standing to be mounted results in a higher pregnancy rate than if it is based on secondary signs of estrus.7 Well-managed dairy cows with uncomplicated periparturient experiences may ovulate approximately 20 to 25 days after calving, whereas beef cows with nursing calves usually do not ovulate until 40 or more days after calving. The presence of the calf appears to be responsible for the difference in return to cyclicity.8
SHEEP
Coarse-wooled breeds of ewes are seasonally polyestrous during the autumn and winter (short photoperiod) in temperate climates. Ovulation can be induced in seasonally anestrous ewes by artificial simulation of day length and temperatures characteristic of autumn (reduced photoperiod and reduced ambient temperature), but the long latency period required for response to manipulation of light and temperature makes the procedure impractical. Ewes of fine-wooled breeds may be polyestrous throughout the year if adequately nourished. The estrous cycle of ewes averages 17 days (range 14 to 19 days), and the duration of estrus averages 36 hours. Ovulation occurs spontaneously 24 hours after the onset of estrus. Ewes display few, if any, signs of estrus unless a male is present. The primary signs of estrus include seeking the ram and standing for mating. Secondary signs include restlessness and rapid tail switching; vulvar edema and discharge of clear cervical mucus may be observed occasionally. Lambing ordinarily occurs during the anestrous season; therefore ewes do not return to estrus until the next breeding season.5
GOATS
Does in temperate climates are seasonally polyestrous from late summer until early spring (short photoperiod). Onset of the breeding season in yearling does can be advanced by exposing them to 19 hours of artificial light per day for 70 days beginning in mid to late winter. Termination of artificial light results in a relative decrease in day length and stimulation of estrus and ovulation.5 Alternatively, the breeding season may be hastened by exposing does to 14 to 18 hours of light per day for 3 months, followed by a reduction to 6 hours of light per day.7 The estrous cycle averages 21 days, and estrus lasts 18 to 36 hours. Ovulation occurs spontaneously 24 hours after the onset of estrus. An intact male or male pheromone is usually necessary for estrous detection. The primary signs of estrus are seeking the buck and standing for service. Secondary signs of estrus in does include rapid tail switching, restlessness, increased frequency of urination and vocalization, transient decrease in appetite and milk production, and edema and hyperemia of the vulva. As in sheep, parturition takes place during the anestrous season, and return to cyclicity in does is delayed until the next breeding season.5
CAMELIDS
South American camelids bred in North America are nonseasonal. In keeping with management decisions, they are often bred in a seasonal manner to avoid having newborn crias during the hottest or coldest months of the year. Some consider the South American camelids to be polyestrous, whereas others argue that they do not have a true estrous cycle.9 These discrepancies arise from the fact that camelids are induced ovulators. Cyclic ovarian activity (e.g., transition from estrus to diestrus) is caused by coital activity. Unbred female camelids essentially exhibit estrous behavior continually, with perhaps short, occasional, unpredictive intervals of 1 to 2 days of decreased receptivity. Follicular waves in alpacas and llamas last around 17 days. Small follicles (≤3 mm) are always present on the surface of the ovaries. After recruitment, follicles grow to approximately 5 mm in size. Dominance is established when one of the follicles reaches the size of 6 mm, and the female will show signs of receptivity to the male as long as a dominant follicle is present. The sizes of preovulatory follicles are 9 to 13 mm and 8 to 12 mm in the llama and alpaca, respectively. Females maintain fertile, dominant follicles for an average of 8 days before undergoing regression. Usually the next follicular wave has already produced the next dominant follicle before the previous preovulatory follicle begins to regress. For this reason, the female camelid usually maintains estrous behavior continually until she is mated.9
CYSTIC FOLLICULAR DEGENERATION
Cows
Ovarian cysts are follicle-like ovarian structures that arise because of failure of ovulation.5 They are usually larger than 25 mm in diameter and persist in the absence of a CL for 10 days or more. Follicular cysts have thin walls and may be single, multiple, or multilocular structures on one or both ovaries. Partially luteinized cysts tend to be single, unilateral structures with thicker walls because of the presence of luteal tissue.
The mechanism by which ovulation fails and cysts develop is not known. Failure of ovulation may result from inadequate release of gonadotropins or ovarian dysfunction. Increased stress of conditions such as retained placenta, metritis, and hypocalcemia around the time of calving and postpartum ketosis have been associated with an increased prevalence of cystic follicular degeneration (CFD), as has a hereditary predisposition.10
Clinical Signs and Diagnosis
Approximately 70% to 80% of cows affected by CFD are anestrus, whereas 20% to 30% display frequent or intense estrus (nymphomania). Cystic ovarian disease affects 10% to 30% of dairy cows. The condition is rare in commercial beef cows because of rigid culling for reproductive failure.
The physical appearance of cows with CFD depends on the duration of the condition. No changes are apparent after a short time, but in long-standing cases relaxation of the pelvic ligaments may result in prominence of the tailhead and masculine characteristics such as a crested neck.
The diagnosis of CFD is based on an accurate history and clinical examination. A history of constant or frequent estrus, short interestrous intervals, or anestrus may suggest CFD. Examination of the ovaries by palpation per rectum reveals the presence of enlarged fluid-filled structures raised above the surface of the ovary that greatly increase total ovarian size. Ovarian cysts are larger (>25 mm) than preovulatory follicles (15 to 25 mm). Differentiation between a single large cyst and several smaller cysts on the same ovary may not be possible, nor may be recognition of the presence of partially luteinized cysts (based on peripheral progesterone concentrations) unless ultrasonography is used. Ovarian cysts appear to be dynamic structures; those that develop early in the postpartum period may regress without treatment, and a normal estrous cycle may follow, or another cystic structure may develop.
During palpation of the ovaries, several normal structures may complicate the diagnosis of CFD. Normal preovulatory follicles may approach 25 mm in diameter and have palpable characteristics similar to those of small cysts. During the follicular phase of the estrous cycle, however, the uterus responds to palpation by becoming more turgid, whereas the uterus of a cow with CFD is typically flaccid and unresponsive. In neglected cases of CFD, mucometra may develop and must be differentiated from pregnancy. During the first 5 to 7 days of the estrous cycle, the developing CL may be smooth and soft and is commonly mistaken for an ovarian cyst. More mature CLs are solid and liver-like in consistency, often feature a palpable ovulatory papilla at the apex, and are more easily differentiated from ovarian cysts. However, 10% to 20% of mature CLs may lack an ovulatory papilla, making them more easily confused with ovarian cysts. Ultrasonography is generally more accurate in identifying subtle structural differences than transrectal palpation. Salpingitis, hydrosalpinx, oophoritis, ovarian abscesses, ovarian neoplasms, and cysts of the fimbria are other causes of enlargement of the ovary and surrounding structures that must be differentiated from ovarian cysts.11
Histories that may erroneously suggest CFD include apparently short interestrous intervals because of inaccurate detection of estrus.12 Oxytocin administered to stimulate milk letdown may result in short interestrous intervals and suggest CFD. The estrous cycle may be shortened by administering 100 IU of oxytocin per day on days 2 through 613 or on days 3 through 7 or 8.14 Heifers treated with 100 IU of oxytocin per day returned to estrus in an average of 12.9 days versus 20.3 days in untreated controls.
Clinical Pathology
Plasma progesterone concentrations are low in cows with follicular cysts. Partial luteinization may occur, and progesterone concentrations may increase over time but remain lower than those of cows with normal CLs. Estrogen concentrations in the plasma of cows with CFD are variable.
Treatment and Prognosis
The goal in treating CFD is to induce luteinization of the cyst and reestablish normal estrous cycles. Several methods have been recommended.
SPONTANEOUS RECOVERY
Spontaneous recovery from CFD occurs in up to 60% of cows that develop CFD before the first ovulation after calving but in only approximately 20% of cases that develop after the first postpartum ovulation. Evaluation of therapeutic agents for CFD may be confounded by spontaneous recovery.
LUTEINIZING HORMONE
Recommended doses of human chorionic gonadotropin (hCG) range from 5000 IU either intravenously (IV) or intramuscularly (IM) to 10,000 IU IM. Of cows treated with a single dose of hCG, 65% to 80% establish a normal estrous cycle within 3 to 4 weeks; a second or third dose may be required in cows that do not respond after 3 to 4 weeks or in cases in which nymphomania persists. Anaphylaxis after repeated treatments with a larger protein hormone such as hCG can occur. Antibodies to hCG may reduce the effectiveness of sequential treatments. Therapeutic response, both endocrinologically and clinically, is essentially equivalent between hCG and gonadotropin-releasing hormone (GnRH). The practical disadvantage of hCG is its higher price.
GONADOTROPIN-RELEASING HORMONE
Currently the most common treatment for ovarian cysts, especially follicular cysts, is an injection of GnRH (100 mcg IM). Cows responding to this treatment have an average interval to estrus of one estrous cycle or 18 to 24 days. The treatment to breeding interval can be shortened by administering GnRH at the time of diagnosis, followed by a luteolytic dose of prostaglandin 10 days to 2 weeks later. With this regimen it is not critical whether the cyst is follicular or luteal or even whether it is a misdiagnosed large, smooth CL with or without a fluid-filled central cavity. Most veterinarians agree that accurate differential diagnosis by rectal palpation among follicular cysts, luteal cysts, and some CLs can be a problem.
PROSTAGLANDIN F2α
Luteal-type cysts can be treated with the luteolytic activity of PGF. The advantage is the quicker return to estrus for those cows able to respond and the lower cost of PGF. Cysts that luteinize in response to GnRH regress at a time similar to that of normal CLs. Treatment with PGF may be used to reduce the interval from treatment with GnRH to estrus from 18 to 24 days to an average of 12 to 14 days by administering PGF 9 days after GnRH. Most clinicians are only approximately 50% accurate in determining the degree of luteinization of cysts by palpation per rectum; therefore measurement of concentrations of progesterone in milk or of plasma from affected cows allows the selection of GnRH or hCG for treatment of follicular cysts and PGF for the treatment of luteinized cysts. Ultrasonography can also be used to make an accurate diagnosis.15
MANUAL RUPTURE
Thin-walled follicular cysts may be inadvertently ruptured during examination of the ovaries, and some practitioners may intentionally attempt cyst rupture. Recovery rates after manual rupture have rarely been studied in well-designed controlled experiments but are generally within the range reported for spontaneous recovery. Deliberate manual rupture of ovarian cysts is considered an obsolete form of treatment by some veterinary clinicians, but others routinely use the procedure—especially as an initial treatment for cysts found during the voluntary waiting period. Manual rupture of cysts may be followed by hemorrhage and adhesions between the ovary and surrounding structures. These complications appear to be much more common with use of digital pressure to enucleate CLs than with manual rupture of ovarian cysts.
Ewes and Does
Cystic ovaries appear to be more common in goats than in sheep. In one study 12% of goats had cystic ovaries when examined at a slaughterhouse.16 The condition is often overdiagnosed by owners observing nymphomania. Treatment typically consists of administering exogenous ovulation-hastening drugs (hCG and GnRH).17 LH surge is noted usually within 2 hours of GnRH administration. PGF may be administered 10 days later to bring the doe into heat (55 hours after PGF).
Camelids
Hemorrhagic follicles are observed in nonbred llamas and usually regress within 22 days.18 Follicles larger than 12 mm are considered by some authors as pathologic cysts. These structures may, however, be anovulatory follicles. They may have a negative influence on the emergence of other follicular waves, but this influence seems to last for only approximately 8 days.9,19
POOR NUTRITION
As with other species, poor body condition, depressed energy intake, and decreased vitamin and mineral intake suppress reproductive activity in ewes and does. Lowered energy balance results in poor or weak signs of estrus, depressed ovulation, abnormal cycle, and delayed puberty. Deficiencies in energy, protein, vitamins A and E, phosphorus, and many trace minerals (iodine, copper) are commonly seen. These deficiencies are most commonly associated with irregular estrous cycles.
PLANT TOXICITY
HEAT STRESS
Fertility in lactating cows is decreased during the hot seasons of the year. Heat stress may cause decreased estrous detection, impair follicular development, disrupt function of the reproductive tract, affect oocyte competence, and lead to early embryonic death. Embryos develop resistance to heat shock as they age. Bovine morulae to blastocyst stages are unaffected by heat shock.19a
ANESTRUS
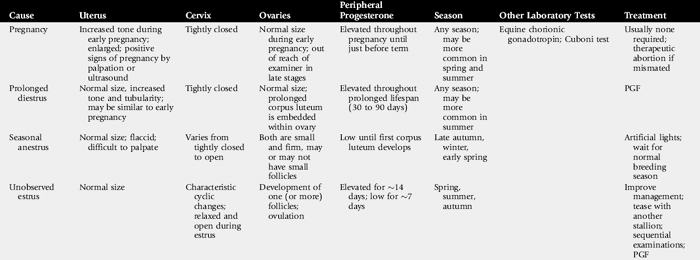
Anestrus, may be defined as ovarian inactivity. The causes of anestrus are multiple and include diseases of the reproductive and other systems. In addition, the problem is complicated by management factors that cause estrus to pass undetected, even though the animal’s estrous cycles and estrous behavior are normal. Common causes of anestrus in mares are summarized in Table 43-1.
MARES
PUBERTY
The mare likely undergoes puberty between the ages of 12 and 24 months. Because the horse is not an agricultural production animal, there has been little interest in studying the onset of puberty as in other species, where hastening puberty increases production. Most mares are not bred until they are at least 3 years old, thus making prepubertal status an unlikely differential diagnosis for infertility.
SEASONAL ANESTRUS
Clinical Signs and Diagnosis
The mare is a seasonally polyestrous animal, showing anestrus during the shorter days of the year and cycling regularly during the longer days. Length of anestrus varies from one to several months, although some mares, particularly in the tropics, may cycle year round.20 In California, Australia, and South Africa 18% to 25% of mares cycle year round.1,21-23 Anestrous mares may be indifferent to teasing and do not show regular estrous behavior. Ovaries are small and firm on palpation, and the uterus is flaccid with a thin endometrium. The cervix has mild tone and may be indistinct. On speculum examination of the vagina, the vaginal mucosa is pale and dry, and the cervix usually appears closed but is occasionally open or may be easily opened. Mares that experience seasonal anestrus will go through a transition period in late winter and early spring, characterized by the development of waves of antral follicles that regress without ovulation because the ovulatory surge of luteinizing hormone (LH) is absent.23 Transitional mares exhibit signs of estrus, including clitoral “winking,” tail flagging, and urinating in the presence of the stallion. Eventually, increasing LH concentration coincides with a large follicle, resulting in ovulation. After the first ovulation of the season, the mare will continue to ovulate on successive estrous cycles. A transition period is also observed during the fall as the mare changes from a polyestrous condition to the winter anestrus. Differential diagnoses for seasonal anestrus and transitional mares are listed in Table 43-1.
Treatment and Prognosis
As day length increases, most mares ovulate and begin regular cyclicity without treatment. Methods to advance the onset of regular ovulatory periods are discussed in the following sections.
ARTIFICIAL LIGHTING
The vernal transition can be moved but not shortened beyond its physiologic length of 6 to 8 weeks by exposure of mares to artificial light. A common artificial lighting regimen is to expose the mares to 16 hours of light and 8 hours of dark by extending the photoperiod in the evening starting in late November to initiate ovulation by February (in the Northern Hemisphere). Light should be added to the end of the day, or split between the beginning and end of the day, as opposed to adding light only at the beginning of the day.6 An alternative regimen is to expose mares to 1 hour of artificial light 9.5 to 10.5 hours after the onset of darkness.24 Use of one 200-watt incandescent bulb or two 40-watt fluorescent tubes at a height of 7 to 8 feet in a 12- by 12-ft box stall has been recommended.25 Paddock lighting has been described.5
GONADOTROPIN-RELEASING HORMONE
Treatment with GnRH or a GnRH analogue for mares in anestrus or spring transition has been shown to induce ovulation.26-30 Twice-daily injections of a GnRH agonist induced ovulation in a majority of mares within 2 to 3 weeks.30 Mares that are in deep anestrus (January and February, Northern Hemisphere) can be expected to return to anestrus after treatment.
DOPAMINE ANTAGONISTS
Domperidone and sulpiride have been reported to stimulate follicular activity and advance the first ovulation of the year in seasonally anestrous mares.31,32 However, the efficacy of dopamine antagonists in advancing follicular growth and ovulation in anestrous mares has recently been questioned, and it has been suggested that adjustments in light and climactic conditions may influence the efficacy of the treatment.33,34
STEROIDS
Exogenous progestins suppress the release of LH from the anterior pituitary and may be used for estrous regulation during the vernal transition. After treatment of mares for 10 to 14 days, withdrawal of progestin may result in LH release from the pituitary and estrus beginning in 4 to 5 days, with ovulation within 10 days after cessation of treatment. Mares should be in mid to late transition and have a follicle at least 25 mm in size to respond to treatment. Progestins will not induce estrus or ovulation in anestrous mares.35 The recommended dose of progesterone in oil is 150 to 300 mg daily by intramuscular injection. The synthetic progestin, altrenogest, is administered orally (PO) at 0.044 mg/kg daily. Progestins may be used in combination with extended photoperiod and gonadotropins. Products that are ineffective or unavailable include repositol progesterone, melengestrol acetate, chlormadinone acetate, proligestone, medroxyprogesterone acetate, hydroxyprogesterone acetate, and norgestomet implants.
Synchronization of the first ovulation (or any ovulation during the cyclic season) can be accomplished by the administration of a combination of progesterone in oil (150 mg/day IM) and estradiol-17β (10 mg/day IM) once daily for 10 days.36 Treatment is most effective if given after mares have been under lights for 45 to 60 days. PGF2α should be given on the last day of steroid treatment. Treated mares will ovulate within 8 to 10 days after the last treatment.
GONADOTROPINS
At a dose of 2500 to 3000 IU, hCG may induce ovulation within 48 hours when administered to a mare with a follicle larger than 35 mm in diameter37 and may reduce time to first ovulaton in transitional mares, particularly when used in combination with lights and/or progesterone treatment. Ovulation response here is less predictable than that induced with hCG during the breeding season.
FOLLICULAR ASPIRATION
Ultrasound-guided transvaginal follicular aspiration of follicles <35 mm has been shown to hasten the onset of cyclicity in transitional mares.38 Follicular aspiration resulted in the formation of an active CL, and subsequent treatment with PGF2α resulted in estrous behavior and ovulation of a dominant follicle.
PROLONGED LUTEAL PHASE AND PSEUDOPREGNANCY
Mares that experience embryonic loss in the presence of endometrial cups (days 35 to 150 of gestation) are said to be pseudopregnant, (pseudopregnancy, may also refer to that condition in which a conceptus was lost after maternal recognition of pregnancy and before the development of endometrial cups, resulting in prolonged luteal life). In spite of the loss of the fetus and placental tissue, endometrial cups remain in place and continue to secrete equine chorionic gonadotropin (eCG) for a similar period to that in a pregnant mare, to 100 to 150 days of gestation.39 The primary and secondary CLs occasionally regress after embryonic loss40 but usually remain during eCG secretion, maintaining high levels of peripheral progesterone.
Treatment and Prognosis
In untreated mares, cyclic activity is reestablished after the cessation of eCG secretion. Repeated daily injections of PGF products have been reported to cause luteal regression in pseudopregnant mares,41 but only CLs older than 5 days respond to the treatment, which may prevent mares returning to estrus. Pregnancies have occurred in the face of high eCG,42 but fertility of treated mares is usually low.
LACK OF BEHAVIORAL ESTRUS (SILENT ESTRUS)
Behavioral estrus may not be detected in otherwise normal mares as a result of inadequate estrous detection or a failure on the part of the mare to show obvious signs of estrus. The latter may occur in up to 15% of mares on well-managed farms.43 Inadequate estrous detection may be a result of human apathy or ignorance or a result of using a low-libido or inexperienced stallion. Teasing mares as a group may make detection of estrus more difficult, especially for nervous mares, mares with foals, and mares of low social rank. Use of anabolic steroids may suppress behavioral estrus.
Clinical Signs and Diagnosis
The mare fails to show estrus on adequate teasing with a stallion. Differential diagnoses are listed in Table 43-2.
Table 43-2 Irregularities of the Equine Estrous Cycle: DifferentialDiagnosis
| Etiology | Distinguishing Features |
|---|---|
| FAILURE TO CYCLE WITH LOW PROGESTERONE | |
| Winter anestrus | Season; inactive ovaries |
| Gonadal dysgenesis | Small, hard, inactive ovaries; karyotype; underdeveloped tubular tract; small body |
| Pituitary adenoma | Systemic signs; inactive ovaries |
| Granulosa-theca cell tumor | See prolonged or irregular behavioral estrus |
| Behavioral | Intimidated by stallion; recently foaled; low social rank |
| FAILURE TO CYCLE WITH HIGH PROGESTERONE | |
| Pregnancy | Presence of embryonic vesicle or fetus |
| Persistent CL | CL fails to regress; responds to PGF |
| Diestrous ovulation | CL immature at time of endogenous PGF; responds to exogenous PGF |
| Pseudopregnancy | Conceptus loss after maternal recognition of pregnancy; responds to exogenous PGF |
| Iatrogenic | History of exogenous progestin or nonsteroidal antiinflammatory drug administration |
| Pyometra | Uterus palpably enlarged |
| SHORT LUTEAL PHASE | |
| Uterine infection | Pyometra or endometritis causing premature endogenous PGF secretion |
| Systemic endotoxemia | Systemic signs; endotoxin-mediated release of endogenous PGF |
| Iatrogenic | History of uterine manipulation, infusion, invasive procedure, or exogenous PGF |
| PROLONGED OR IRREGULAR BEHAVIORAL ESTRUS | |
| Transitional period | Season, variable ovarian activity |
| Granulosa-theca cell tumor | Affected ovary large and multicystic, contralateral ovary small; elevated inhibin and/or testosterone; anestrus, nymphomaniac or stallion-like behavior |
| Gonadal dysgenesis | Occasionally irregular cyclicity; as above |
| Behavioral nymphomania | Otherwise normal mare |
| Normal mare | Mares in winter anestrus and pregnancy may show estrous signs |
CL, Corpus luteum; PGF, prostaglandin F2α.
Treatment and Prognosis
Management should be examined to ensure a competent teasing routine. When approached by a stallion, mares in estrus stand still with ears held forward; they may elevate the tail, rhythmically evert the clitoris (“winking”), assume a squatting posture, urinate, and lean against the teasing chute toward the stallion. Mares that are not in estrus move about and hold their ears back; they may strike, kick, squeal, swish their tails, and forcefully void small amounts of urine. Experienced personnel should handle both stallion and mare. The teaser stallion should have adequate libido without being aggressive. Transrectal palpation and ultrasonography should supplement teasing. Some mares are indifferent to teasing, and records of sequential palpation must be relied on for breeding. Prostaglandins can be used to control estrus. Progesterone concentrations of less than 1 ng/mL are consistent with estrus but may also occur in anestrous mares. Mares that fail to show behavioral estrus should be bred by artificial insemination (AI) (if allowed by the breed register) or appropriate restraint used for natural cover.
BEHAVIORAL NYMPHOMANIA
Abnormal estrous behavior and aggression may be demonstrated by otherwise normal mares at any stage of the estrous cycle. Cause is unknown, although exaggerated response to ovarian steroids has been proposed.44
Clinical Signs and Diagnosis
Exaggerated signs of estrus occur, initially during estrus and then throughout the cycle. Mares may develop behavioral anomalies and become aggressive. Differential diagnoses are listed in Table 43-2. It is important to differentiate abnormal estrous behavior from unrelated behavioral problems.
Treatment and Prognosis
Exogenous progestins have been used to limited effect. Short-term dexamethasone treatment (5 to 10 mg) may alleviate signs for 3 to 4 days.44 Bilateral ovariectomy may be successful in some cases.
RUMINANTS
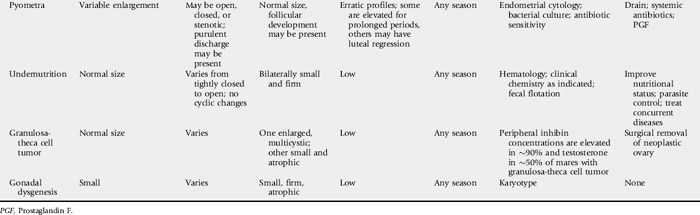
Common causes of anestrus in cows are summarized in Table 43-3.
UNOBSERVED OR SILENT ESTRUS
Cow
Failure of a cow to display, or a manager to observe, the signs of estrus contributes significantly to reproductive inefficiency. When the presenting history suggests anestrus (failure to have a normal estrous cycle), the clinician must determine if the cause is failure of the manager to detect estrus in normal cows or failure of the cow to cycle because of some abnormal process. In dairy herds approximately 90% of cows presented for examination because of a history of anestrus have evidence of normal cyclic ovarian changes, whereas only approximately 10% are affected by an abnormality that suspends the estrous cycle (i.e., only approximately 10% are in true anestrus).
Nearly 90% of well-managed dairy cows have initiated normal-length estrous cycles by 60 days after calving, but only approximately 60% are detected correctly to be in estrus by that time.45 Rates of estrous detection by twice-daily observation range from 50% to 73% depending on the skill of the observer.
Mounting activity and estrous behavior are reduced by hot and cold ambient temperatures and during the times of milking and feeding. More mounts are observed when cows are kept on dirt than on concrete. Estrous behavior varies with the time of day and may be an inverse reflection of extraneous activity interfering with cow behavior. In one study, 43% of cows showed heat between midnight and 6 AM; 22% between 6 AM and noon; 10% between noon and 6 PM; and 25% between 6 PM and midnight.46
Estrus in dairy cows averages 7 to 16 hours in length but ranges from 0.5 to 36 hours. Sixty-five percent of cows are in estrus for less than 16 hours, and 25% are in estrus for less than 8 hours.47 The number of mounts per hour ranges from 2 to 8, and the total number of mounts during estrus ranges from 11 to 56. Total number of mounts per estrus increases with the number of cows simultaneously in estrus.
Clinical Signs and Diagnosis
Dairy herds in which infertility is caused by inaccurate estrous detection are usually characterized by prolonged intervals from calving to first breeding and between services; insemination intervals of 10 to 15 days and 30 to 35 days; records of examinations that confirm cyclic ovarian changes, but in which observation of estrus is not recorded; and finding more than 15% of cows presented for pregnancy examination to be nonpregnant. Insemination during the luteal phase of the estrous cycle may occur in 10% to 20% of cows and is not likely to result in conception; insemination of pregnant cows may be followed by abortion.
The diagnosis of unobserved estrus requires sequential examination of affected cows and accurate records. Other causes of anestrus are eliminated. Conditions such as CFD, pyometra, mummified fetuses, granulosa-theca cell tumors, and segmental aplasia that cause anestrus affect individual animals. Anestrus caused by undernutrition is characterized by depressed milk production and low body condition score.
Treatment and Prognosis
PGF is widely used in clinical management of unobserved estrus. Mature CLs (approximately day 6 through day 18 of the estrous cycle) are responsive to PGF-induced luteolysis. Estrus occurs an average of 3 days (range 2 to 5 days) after administration of PGF, depending on the follicular status of the ovaries at the time of injection. The endocrine events surrounding the controlled estrus are indistinguishable from those surrounding spontaneous estrus and ovulation. Treatment with PGF shortens the intervals from treatment to first breeding and from treatment to conception but has no effect on fertility. The benefits of PGF treatment are limited by inaccurate palpation of the temporary ovarian structures, injection during the wrong phase of the cycle, and failure of the manager to observe estrus in treated cows (timed AI can be used to overcome this problem).
Measurement of progesterone concentrations in milk samples taken on the day of breeding is useful in herds with a history of reduced fertility to confirm that cows being inseminated are not in the luteal phase of the estrous cycle. If more than an occasional cow presented for insemination has an elevated concentration of progesterone, the methods of estrous detection should be reviewed. Enzyme immunoassay kits for measuring concentrations of progesterone in milk and plasma of cows and other female animals have been described and are commercially available.
Various heat detection aids have been developed. Several use devices mounted on the tailhead to record that a cow has stood to be ridden. Pressure-sensitive devices that are glued to the tailhead and change color after sustained pressure by the weight of a mounting cow are commonly used. Similarly, pressure-sensitive devices glued to the tailhead can send a record of riding events directly to a computer. Chalk, cattle crayon marker, or paint applied to the tailhead are inexpensive aids that are rubbed off when the animal is mounted when she is in heat. These methods require daily maintenance and twice daily evaluation to function effectively. Detection aids that measure changes in activity (pedometers), mucous conductivity, or body temperature can be used successfully. Accuracy is enhanced when measurements are related to previous estrous activity and progesterone concentrations.48
Prevention and Control
Because unobserved estrus is primarily a problem of management, efforts to reduce time lost from delayed breeding are directed at improving efficiency of heat detection. Accurate records are required to identify cows that have not been observed in estrus by 40 days after calving. Cows not observed in estrus by 40 days after calving should be examined, and abnormalities of the reproductive organs that cause anestrus treated as indicated. The time of estrus can be predicted by palpation of the temporary ovarian structures, or estrus can be controlled with PGF. The most significant benefit of a planned herd health program is stimulation of improvements in management that decrease the interval from calving to conception as a result of improved estrous detection.
Anestrus after insemination is frequently interpreted as a clinical sign of pregnancy. However, unobserved estrus in cows that have failed to conceive or have experienced early embryonic death (postservice anestrus) contributes significantly to increased calving intervals. Clinical management of postservice anestrus depends on diligent observation of cows 18 to 24 days after breeding and identification of nonpregnant cows as early as possible after the infertile service so they may be reinseminated with minimum delay. Nonpregnant cows may be accurately identified by ovarian palpation or ultrasonography for absence of a mature CL, by low milk or plasma progesterone at the time of the first expected postservice estrus (approximately 21 days after breeding), or by palpation of the uterus per rectum before the second expected postservice estrus (30 to 42 days after breeding).
Ewe
The breeding season of most breeds of sheep maintained at temperate latitudes is restricted to late summer, autumn, and early winter, although some breeds cycle all year long. There is almost no homosexual interaction among ewes; therefore a male must be present to stimulate display of estrus.5
Introduction of a ram (either intact or vasectomized) into a flock of ewes advances the breeding season. Most ewes ovulate by 3 to 6 days after introduction of rams. The induced ovulation is seldom accompanied by estrus, but the subsequent estrus approximately 17 days later is ovulatory and fertile. The “ram effect” is lost when rams are allowed to associate with ewes throughout the year.
Return to estrus after mating may be detected in a flock of ewes by fitting the ram with a brisket device that marks serviced ewes. Return of an excessive number of ewes to service after breeding alerts the owner to the possibility of infertility.
AI of ewes is rare in the United States but more popular in other countries. Detection of estrus for AI depends on use of teaser rams mingled with the ewes or led through the flock several times daily.
Doe
The breeding season of does is similar to that of ewes (i.e., it surrounds the autumnal equinox). During periods of short daylight, the normal estrous cycle of does is 20 to 21 days. Homosexual interaction among estrous does rarely occurs; so signs of estrus must be elicited by teasing. Signs of estrus may also be evoked by exposure to male pheromones by way of a “buck jar” prepared by rubbing a cloth over the scent glands caudomedial to the horns of a mature buck during the breeding season and storing the cloth in a tightly closed container. If estrus is not observed in does exposed to a mature buck or to a buck jar during the physiologic breeding season, pregnancy or pseudopregnancy might be considered as possible causes of anestrus. Severely parasitized or inadequately nourished does do not have normal estrous cycles. Deficiencies of phosphorus, iodine, and manganese have been suggested as causes of anestrus in does.
Introduction of bucks into a flock of does early in the breeding season results in initiation of estrous cycles and some degree of synchrony of estrus approximately 10 days after introduction of the bucks.5 In contrast to ewes, however, the first ovulation after exposure to males is accompanied by estrus and fertile mating.
Camelids
Most female South American camelids, although showing signs of ovarian cyclicity as early as 5 months of age, have decreased fertility until approximately 15 months of age. A female camelid should be at least 60% of her expected adult weight before she is bred. Male camelids have a preputial attachment of the penis that is not separated until 2 to 3 years of age. Some males may actually detach as early as 15 months of age. Before this time they will show mounting behavior but will not be capable of intromission.9
INFERTILITY CAUSED BY ABNORMALITIES OF THE FEMALE GENITAL ORGANS
ABNORMALITIES CAUSED BY PROBLEMS WITH SEXUAL DIFFERENTIATION
Sexual differentiation occurs in three stages, each stage dependant on the previous one:
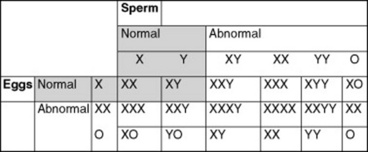
Chromosomal sex is determined at fertilization. All normal mammalian oocytes contribute an X chromosome in addition to one of each representative autosomal maternal chromosome. Sperm cells contribute either an X or a Y sex chromosome in addition to one of each representative autosomal paternal chromosome. Abnormalities in chromosomal sex occur because of nondisjunctional errors during either mitosis or meiosis. Fig. 43-1 shows some examples of chromosomal sex anomalies.
Monosomy X (XO)
Monosomy X is also known as Turner’s syndrome., Owing to the lack of a Y chromosome and the consequent Sry, gene, but the presence of the Dax1, gene on the present X chromosome, the phenotype is female. Monosomy X is the most commonly reported chromosomal abnormality in mares.49 Animals with this syndrome often have a history of poor performance and lack of or sporadic reproductive cyclicity. Ovaries are typically inactive, small, smooth, and firm. The uterus and cervix are usually hypoplastic. Externally the mare’s genitalia may appear normal or underdeveloped.
XXY Syndrome
The genotype for XXY syndrome is part of Klinefelter’s syndrome. Because of the presence of a Y chromosome and the consequent Sry, gene, affected individuals are phenotypically male; but probably owing to the presence of two copies of the Dax1, gene on the two X chromosomes, they generally have hypoplastic genitalia and reproductive organs. It is thought that some factor (Dax1, or some other) on the X chromosome must escape the inactivation process, which happens very early in development (around day 7 or 8 in the horse). Testicular development and spermatogenesis are inhibited, resulting in small, flaccid testes and azoospermia. The testes may be retained or descended, but they are often small and soft. The penis may be normal or smaller than usual. Affected males often show normal libido and sexual behavior. Low testosterone concentrations may be noted. Infertility always accompanies this syndrome. Reports have been made in numerous species, including the horse.50,51
XXX
More is not better. A report of an infertile mare with the 65,XXX genotype confirms this.52 The mare had bilaterally small, inactive ovaries, and a hypoplastic uterus and cervix.
Mosaics
Mosaics are individuals that have at least two cell lines with different karyotypes arising from the same zygote (Fig. 43-2).
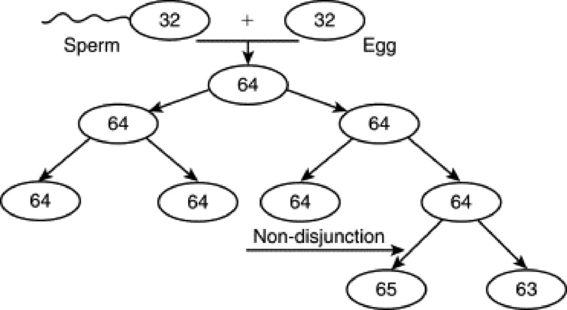
Fig. 43-2 Process of how a mosaic may occur. The haploid sperm and egg form a diploid zygote. Through the process of mitosis, diploid cells are replicated to always produce the same number of chromosomes. If, however, a chromosome pair does not separate during anaphase, called a nondisjunction event, cell lines that do not reflect a correct representation of the genome will be created and potentially propagated. If this happens with the sex chromosomes in the germ cell line, fertility of the individual or its offspring may be affected.
Phenotypes vary in accordance with the degree of mosaicism. Varying degrees of hermaphroditism and pseudohermaphroditism have been reported in many domestic species. Mosaics often have mixed gonadal dysgenesis, with an ovary and a testis, or ovotestes, owing to sex chromosome mosaic cell lines.53,54 These are true hermaphrodites.
Chimeras
Chimeras are individuals having cell lines from two different embryonic sources. This can occur experimentally or from the natural fusion of blastocysts in utero. The possibility has been reported from a suspected double ovulation and fertilization followed by blastomere fusion in the horse (64,XX/64,XY and 63,XO/64,XY genotypes reported). Freemartinism is a common occurrence in ruminants resulting in chimeric twins.
Freemartins
Freemartinism is a phenomenon in ruminants in which an infertile female is twin to a male. The dizygotic occurrence happens when the blastodermic vesicles of the two zygotes fuse early in development (day 18 to 20 in cattle) and share embryonic tissue. The placentas fuse (day 30 to 50 in bovines), and they share blood throughout gestation. This occurs before gonadal differentiation at day 40 to 50. Both individuals are XX/XY chimeras. The Sry, gene of the male twin causes the freemartin gonads to develop at least partially toward the male testis. The degree of differentiation varies with each freemartin, and many freemartin gonads remain undifferentiated. The shared circulation allows testosterone and antimüllerian hormone (AMH; discussed later) from the male twin, and possibly from the chimeric freemartin, to affect the freemartin genitalia, and so she lacks a cervix, uterus, uterine tubules, and cranial vagina. The vulva is fairly normal. The yearling freemartin fails to exhibit estrus, the udder and teats remain small, and the freemartin externally resembles a steer (only with a vulva). Diagnosis can be made by establishing a blind end to the vagina (no cranial vagina, no cervix). Of heifers born co-twin to a male, 92% will be freemartins.55
The male twin may develop into a fertile adult, but these individuals show a higher incidence of infertility than bulls with a 60,XY genotype. Most male twins to freemartins become steers.
Freemartinism is less common in sheep than in cattle, but it does occur. It has also been reported in goats and pigs. With increased fecundity in modern animals, we observe the phenomenon more often than was reported in the past. This is because ovine freemartinism is rare with twins or triplets but much more common with quadruplets or quintuplets. A notable difference between ovine and bovine freemartinism is the marked masculinity of the ovine freemartin. Gonads within the inguinal canal resemble normal prepubertal testes, and those within the abdomen resemble cryptorchid testes from rams of normal XY gonadal sex. Many ovine freemartins also have epididymides, vasa deferentia, vesicular glands, and even cremaster muscles.
GONADAL SEX
Chromosomal sex determines gonadal sex. The Y chromosome has very few genes, and all of the ones studied play a role in sex differentiation. The gene located at the sex-determining region on the Y chromosome (Sry,) has a DNA binding domain high mobility group (HMG) box. It produces a protein called the HY antigen, and its action appears to be regulated by the transcription of other genes. Their actions initiate differentiation of bipotential embryonic gonadal tissue into testicular tissue. Other genes act downstream of Sry, to support gonadal differentiation, including Sox9, Gata4, and Wt1, and Sf1, which act synergistically to promote testicular differentiation. Sox9, is a powerful promoter of testicular tissue differentiation. It is hypothesized that Sry, upregulates Sox9, but there is currently no direct evidence to support this. In the absence of the Sry, gene, the dual copies of the Dax1, gene on the X chromosomes suppress the formation of testicular tissue by antagonizing the Sry, gene and the synergy between Sf1, and Wt1., Another gene, Wnt4, has been shown to support female development, and the absence of this gene in female mice results in masculinization.56 This evidence refutes the notion that female development is strictly a default process caused by the absence of Sry., Further research may elucidate other factors that actively promote the formation of ovarian tissue. Factors affecting Sry, or any of the other genes downstream of Sry, or any factors governing female development will affect phenotypic sex.
Abnormalities of Gonadal Sex: Sex Reversals
Sex reversals occur when the chromosomal sex and gonadal sex do not agree with each other. XX sex-reversed males and XY sex-reversed females are reported in many domestic species. These individuals are either XX males (testicular tissue), XY females (ovarian tissue), or true hermaphrodites (both ovarian and testicular tissue on separate gonads or the same gonad [ovotestes]). Sex reversals are relatively common in the horse, and all three of the types discussed in the following sections are reported. Sex reversal is considered to occur in horses both sporadically and with familial inheritance.
XY Sex-Reversed Females (Gonadal Dysgenesis)
Reported XY sex-reversed females have been reported to arise because of an absent or mutated (and nonfunctional) Sry, gene. It is believed that the Sry chromosome is missing because of an abnormal meiotic exchange with the X chromosome. It is thought that in the sire two crossing-over events occurred between X and Y chromosomes during spermatogenesis. These animals are infertile and have a normal female appearance to their external genitalia. The ovaries and uterus tend to be hypoplastic. The gonads may, in fact, be completely undifferentiated (“streak gonads”). These animals may be true hermaphrodites (ovotestes) or XY females (only ovarian tissue).
Sry,-positive XX Sex-Reversed Males
Many XX sex-reversed males conversely arise from a translocation of the Sry, gene onto the X chromosome. These animals may be true hermaphrodites or XX males (only testicular tissue).
Sry,-negative XX Sex-Reversed Males
There are reports in horses of Sry,-negative XX sex-reversed males.57,58 The exact mechanism of masculinization is still uncertain. Possibilities include Y-specific sequences other than Sry, (which would require an XY individual with an inactivated or absent Sry, gene), XX/XY chimerism within the testicular tissue, and a mutation in an autosomal or X-linked gene farther down the cascade of genes responsible for sex determination. XX sex-reversed males may have ambiguous sexual characteristics and may be true hermaphrodites or XX males (only testicular tissue).
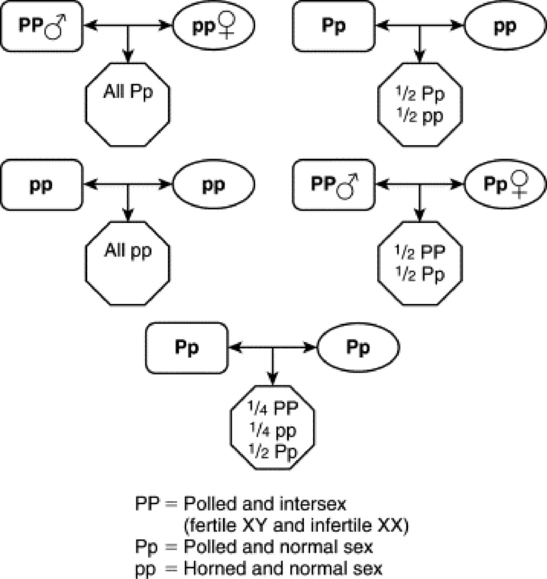
Goats (especially Alpine, Saanen, and Toggenburg) present another classic, common example of Sry,-negative XX sex-reversed males. The “polled” (hornless) gene is either very closely linked to an intersex locus, or the polled gene itself is pleomorphic and controls both the hornless and intersex traits. This close linkage or pleomorphism is called the polled/intersex syndrome, (PIS). A partial reason for the sex reversal is the deletion that affects a noncoding RNA (Pisrt1) and a transcription factor (FoxL2). The mechanism of testis induction has not been discovered yet. Elucidating this mechanism may be a big step in describing autosomal sex-determining factors in other species, including humans. The intersex gene is currently thought to work by mimicking the Sry, gene and codes for the HY antigen.
The polled gene shows a dominant autosomal inheritance pattern, whereas the intersex trait shows a recessive autosomal inheritance pattern. A single dose of the P polled gene is enough to cause the polled trait, but a double dose is required to cause intersexuality in XX individuals. Therefore PP animals are hornless and infertile (if XX, fertile if XY); Pp individuals are hornless (although sometimes have horny bosses) and fertile; and pp individuals are horned and fertile. Intersex individuals are always sterile and have a shortened vagina, large clitoris, bucklike head and neck, buck odor, and buck behavior. The gonads are testes or ovotestes and may be scrotal, inguinal, or abdominal. Even very masculinized intersexes with scrotal testes are azoospermic. A PP polled goat that has an XY genotype is usually initially fertile but often develops sperm granulomas later in life. The only way to avoid the intersex condition is to always breed a polled individual to a horned individual (Fig. 43-3).
PHENOTYPIC SEX
Gonadal sex determines phenotypic sex. Initially each embryo has both müllerian (paramesonephric) ducts and wolffian (mesonephric) ducts. Within testicular tissue, Sox9 triggers Sertoli cells to secrete müllerian inhibiting substance (MIS; also known as antimüllerian hormone, [AMH]), which initiates the irreversible regression of the paramesonephric ducts. The action of MIS or AMH is further regulated by other genes and their proteins (Sf-1, Gata factors, Wt-1, Dax-1, and FSH). Wt-1 and Sf-1 synergistically enhance AMH transcriptional activity. Gata-4 enhances AMH promoter activity by directly binding to DNA and by synergistically interacting with Sf-1.
Leydig cells secrete testosterone that is converted by 5α-reductase to dihydrotestosterone. These two steroids promote the differentiation of male genitalia. Testosterone influences the differentiation of the wolffian ducts into the internal male genitalia (vasa deferentia and epididymides), whereas dihydrotestosterone stimulates the formation of the seminal vesicles and male urethra from the urogenital sinus, and the penis from the genital tubercle. In the absence of these testicular hormones, the wolffian ducts regress and the müllerian ducts become the female internal genitalia.
In the presence of two X chromosomes, the double dose of Dax-1 has an inhibitory effect on the synergistic relationships between Sf-1 and Wt-1 and Gata-4, preventing their support of AMH production.59 The primitive sex cords (gonadal cords) degenerate in the medulla and remain in the cortex (opposite in the horse). Subsequently there is no communication between the gonad and the mesonephros. In the absence of AMH, the müllerian ducts persist as the oviducts and fuse to form the uterus and cranial vagina. In the absence of testosterone, the wolffian ducts regress. Vestigial traces of these are located in the mesentery of the ovary, the epoophoron, the paroophoron, and Gartner’s ducts. The tissues that form the round ligament of the uterus are analogous to the male gubernaculum.
Abnormalities of Phenotypic Sex
Abnormalities of phenotypic sex occur when the chromosomal and gonadal sex agree (XX with ovaries or XY with testes) but the external and/or internal genitalia do not correlate or are ambiguous. Affected animals are the male and female pseudohermaphrodites. The condition can occur because of insensitivity of androgen receptors or because of any abnormality along the pathway that may affect the intervening hormones, such as the conversion of 5α-reductase to dihydrotestosterone.
TESTICULAR FEMINIZATION
Testicular feminization is reported in domestic species, including the horse.60 Patients have external genitalia that are either female or ambiguous in appearance. The vagina may be blind-ending, or the uterus hypoplastic. The gonads are testicles, although they are usually abdominal or inguinal. Male behavior may be reported in horses. The problem lies in the gene for the androgen receptor, located on the X chromosome received from the dam. This X-linked recessive inheritance has been demonstrated in both humans and horses. Affected individuals are male pseudohermaphrodites; the condition has been diagnosed in multiple horse breeds. Serum testosterone is often elevated as a baseline because of loss of negative feedback, or at least in response to an hCG test.
ABNORMALITIES OF THE OVARIES
MARES
ABNORMALLY SMALL OVARIES
The most common causes of bilaterally small ovaries in the mare are (1) seasonal anestrus, (2) immaturity, (3) advanced age, (4) use of anabolic steroids, (5) gonadal dysgenesis, (6) hypothalamopituitary dysfunction, and (7) severe malnutrition. Clinical signs and treatment of malnutrition, hypothalamopituitary dysfunction, and seasonal anestrus are discussed elsewhere in this chapter. The average age at which fillies reach puberty is 18 months, with a wide range of 10 to 24 months. Many older mares (>20 years of age) are still reproductively active, but some mares reach ovarian senescence when they grow older.61,62 In addition, infertility in aged mares may be a result of defects inherent in ovulated oocytes that result in embryos of lowered viability.63 Anabolic steroids are derivatives from androgens that have been altered to provide high anabolic activity with minimal androgenic side effects. A suppression of gonadotropin secretion has been documented when mares have been treated with these drugs.64 Aberrations of meiosis involving the X and Y chromosomes may lead to genotype abnormalities accompanied by gonadal dysgenesis. Abnormal genotype is most commonly 63X0; but 63X/64XX, 63X/64XY, 65XXX, and 64XY sex reversed have been reported.65
Clinical Signs and Diagnosis
IMMATURITY
Fillies younger than 2 years of age with inactive ovaries and a flaccid and relaxed reproductive tract may be too young to cycle and should be reexamined at a later time. The condition should be differentiated from gonadal dysgenesis. Karyotyping may be indicated if puberty is delayed beyond 24 months.
ADVANCED AGE
Older mares (≥20 years) frequently begin cycling later in the season than younger mares.61 Older mares that cycle often have a longer follicular phase, and subsequently, a longer interovulatory interval.61,66 Some aged mares develop large, anovulatory follicles. In one study, significantly more mares aged 16 to 20 years developed anovulatory follicles than mares aged 6 to 10 years.67 Alternatively, older mares may have fewer follicles on their ovaries and elevated serum gonadotropins. It is postulated that mares with these reproductive characteristics frequently become reproductively senescent.68 Aged, senescent mares typically have small, inactive ovaries (follicles ≤5 mm) and a flaccid uterus and cervix. Although senescent mares may still show behavioral signs of estrus, similar to anestrous or ovariectomized mares, ovarian function is completely absent.
Oocytes collected from aged donor mares, cultured, and transferred to young recipient mares were less likely to result in pregnancy than oocytes from young donor mares.63 Furthermore, oocytes obtained from old mares and examined using transmission electron microscopy had more morphologic abnormalities than oocytes from young mares.69 Results from these studies suggest that oocyte quality declines as mares age, and this factor will contribute to poor fertility in older mares.
EXOGENOUS HORMONE TREATMENT
Anabolic steroid administration may affect both estrous behavior and ovarian function. The treatment of mares with low doses of anabolic steroids can cause aggressive or stallion-like behavior, whereas high doses can inhibit ovarian activity and result in failure of follicular development and ovulation.64 Prolonged treatment of prepubertal mares with anabolic steroids results in hypertrophy of the clitoris.70
Progestins are commonly given to cycling mares for the suppression of estrus or synchronization of ovulation. Mares may continue to ovulate during progestin administration, especially if treatment is started late in the luteal phase. A high incidence of persistent CL formation has been observed in mares that ovulate during progestin treatment.71 Administration of the potent GnRH agonist deslorelin acetate (Ovuplant, Ft. Dodge) to induce ovulation has been associated with delayed follicular development and a prolonged interovulatory interval.72,73 Deslorelin acetate is very effective in inducing ovulation, but treatment appears to cause a temporary downregulation of follicle stimulating hormone (FSH) secretion. The low FSH concentrations have been associated with a prolonged period of decreased follicular growth. Administration of PGF2α7 to 8 days after ovulation appears to increase the risk of delayed follicular development. It has been suggested that PGF2αadministration “resets” the timing of the estrous cycle during a period when limited follicular activity is present.
GONADAL DYSGENESIS
Chromosomal abnormalities occur in all breeds of horses. Mares are usually small and phenotypically female. The ovaries are small, firm, smooth, and inactive. The tubular tract is thin and flaccid. Endometrial hypoplasia is a common finding. Diagnosis is confirmed by physical findings and karyotype.
EQUINE CUSHING’S DISEASE
Mares with hypertrophy, hyperplasia, or adenoma formation in the pars intermedia of the pituitary (equine Cushing’s disease [ECD]) have been reported to have abnormal estrous cycles, infertility, or both.74,75 The mechanisms by which ECD causes reproductive abnormalities have not been determined. Potential cause(s) may be destruction of the gonadotrophs of the anterior pituitary owing to compression by the enlarged pars intermedia76 or suppression of gonadotropin secretion owing to elevated levels of glucocorticoids or androgens produced by the adrenal cortex.76 In support of the glucocorticoid hypothesis, administration of dexamethasone to intact mares results in reduced estrous behavior, LH concentrations, follicular growth, and incidence of ovulation.77 In addition, administration of dexamethasone to ovariectomized mares results in suppression of pituitary LH and FSH secretion,78 and treatment of pony mares with dexamethasone during the winter eliminates estrous behavior.79 A majority of horses diagnosed with ECD are older, with the average age being approximately 20 years. Consequently the decrease in reproductive efficiency in mares with ECD may be partly a result of advanced age.
Clinical signs of ECD include hirsutism and abnormal hair-coat shedding patterns, polyuria, polydipsia, and hyperhidrosis.80 Diagnostic tests for ECD include measurements of serum glucose, insulin, adrenocorticotropic hormone (ACTH) cortisol levels, dexamethasone suppression, ACTH stimulation, and thyrotropin-releasing hormone response tests.81 The measurement of single samples for basal cortisol or ACTH concentrations is of limited value in the diagnosis of ECD.
Treatment and Prognosis
IMMATURITY AND ADVANCED AGE
No treatment is available for age-related ovarian inactivity. Because aged mares often experience a delayed seasonal onset of ovarian activity, they benefit from an artificial light regimen starting 60 to 90 days before the breeding season. GnRH has been used to stimulate follicular growth in mares with poor follicular development attributable to anestrus or transition. Several studies (reviewed by Ginther23) have examined the effects of GnRH administered at different doses and intervals to stimulate follicular development in mares. In general, the number of mares responding to GnRH therapy increased as the size of follicle or time of year at the onset of therapy increased.
EXOGENOUS HORMONE TREATMENT
Ovarian inactivity in mares treated with anabolic steroids is reversible, and pituitary and ovarian function eventually return to normal in most mares after withdrawal of the treatment. Mares intended for breeding should not be treated with anabolic steroids.
Removal of the deslorelin implant after ovulation has been detected will decrease the incidence of prolonged interovulatory intervals.82
EQUINE CUSHING’S DISEASE
The medical management of ECD includes the administration of pergolide mesylate, a dopamine receptor agonist, at a dose of 0.5 to 2 mg every 24 hours in an adult horse. The serotonin antagonist cyproheptadine has also been used, but it may not be as efficacious as pergolide. The dose of cyproheptadine is 0.25 mg/kg every 24 hours, given once in the morning.
ABNORMALLY ENLARGED OVARIES
The most common causes of enlarged ovaries in the mare are (1) tumors, (2) anovulatory follicles, (3) ovarian hematomas, and (4) pregnancy.
Ovarian Tumors
The majority of equine ovarian tumors can be categorized as sex cord-stromal tumors (granulosa-theca cell tumors), epithelial tumors (cystadenomas), or germ cell tumors (dysgerminomas and teratomas).
GRANULOSA-THECA CELL TUMORS
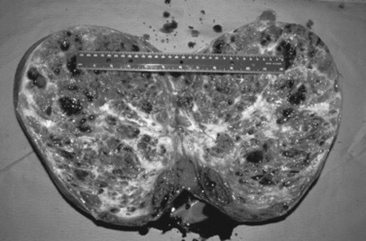
The granulosa-theca cell (GCT) tumor is the most common ovarian tumor in the mare (Fig. 43-4). It is usually slow growing, unilateral, and benign. It occurs in mares of all ages and is occasionally found in pregnant mares. The tumor arises from the steroidogenic cells of the follicle, resulting in abnormal secretion of inhibin and testosterone.
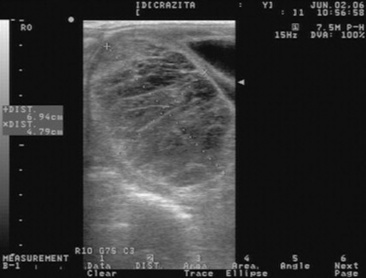
Clinical Signs and Diagnosis
Transrectal sonographic examination of the affected ovary often reveals a multicystic or honeycombed structure, but the tumor may also occur as a solid mass or as a single large cyst. The contralateral ovary is usually small and inactive, although mares with a GCT on one ovary and a functional contralateral ovary have been reported.83 Granulosa cell tumors are hormonally active, and clinical diagnostic assays for the detection of a GCT include the measurement of inhibin, testosterone, and progesterone.84-87α-Inhibin is elevated in approximately 90% of the mares with a GCT.85,87 It has been hypothesized that inhibin produced by the GCT is responsible for inactivity of the contralateral ovary through the suppression of pituitary FSH release. However, recent reports on a poor correlation between dimeric inhibin and the presence of GCT raise questions about the mechanism by which the contralateral ovary is suppressed in affected mares.87,88 Serum testosterone in a single blood sample can be expected to be elevated (100 to 200 pg/mL) in approximately 50% to 60% of affected mares. Daily fluctuations in testosterone concentrations have been reported, and repeated samples may have to be obtained on different days in order to detect elevated testosterone.87 Progesterone concentrations in mares with a GCT are almost always below 1 ng/mL because normal follicular development, ovulation, and CL formation do not occur. As a result of hormone secretion from the tumor, mares may show anestrus, constant estrus, irregular estrus, or stallion-like behavior. They are infertile in the presence of the tumor. Final diagnosis is histologic.
Differential diagnoses for abnormal cyclicity are listed in Table 43-2 and for ovarian enlargement in Table 43-4.
Table 43-4 Unilaterally Large Ovary: Differential Diagnosis
| Etiology | Distinguishing Features |
|---|---|
| Granulosa-theca cell tumor | Sonographically multilocular, high inhibin and/or testosterone; small contralateral ovary |
| Other ovarian tumor | See text |
| Ovarian hematoma | Ovulation fossa still palpable, cycles normally |
| Ovarian abscess | Large, hard ovary; sonographically echogenic |
| Ovarian follicle | Normal cycling mare, ovulates |
| Anovulatory hemorrhagic follicle | Free-floating echogenic spots |
Treatment and Prognosis
The affected ovary should be surgically removed. Surgical approaches for tumor removal include colpotomy, flank and ventral midline laparotomy, and laparoscopy. The prognoses for both life and reproductive use are good. Return to cyclicity varies, but most mares cycle in the year after ovariectomy.
CYSTADENOMAS
The most common tumor of the surface epithelium of the equine ovary is the cystadenoma. They are rare, benign, hormonally inactive tumors from the surface epithelium of the ovulation fossa. The tumor is unilateral, and the contralateral ovary is normal.
Clinical Signs and Diagnosis
Mares with cystadenomas cycle normally from the opposite ovary and may even become pregnant. Rectal palpation and ultrasonography reveal the presence of one enlarged multicystic ovary, which may appear similar to a granulosa-theca cell tumor, and one normal ovary. Differential diagnoses for ovarian enlargement appear in Table 43-4. Final diagnosis is histologic.
GERM CELL TUMORS
Dysgerminomas and teratomas are rare ovarian tumors of germ cell origin. Both tumors are unilateral and hormonally inactive. Teratomas are considered to be benign, whereas dysgerminomas are potentially malignant.
Clinical Signs and Diagnosis
Both tumors make the affected ovary unilaterally enlarged and multicystic. Dysgerminomas are malignant and often metastasize to the peritoneal and thoracic cavities. Teratomas may arise from all three germinal layers, and the neoplastic ovary may contain bone, cartilage, teeth, hair, muscle, and nerves. Teratomas do not cause clinical signs, interrupt the estrous cycle, or alter the behavior of the mare. Dysgerminomas and teratomas are often detected in association with a routine reproductive examination. Differential diagnoses for ovarian enlargement are listed in Table 43-4. Final diagnosis for germ cell tumors is histologic.
Anovulatory Follicles
Clinical Signs and Diagnosis
Ovulation failure is a normal physiologic event for the mare during the spring and fall transition periods, but it may also occur occasionally during the physiologic breeding season. Persistent anovulatory follicles (PAFs) may be quite large (5 to 15 cm in diameter), persist for up to 2 months, and result in abnormal estrous behavior and prolonged interovulatory intervals.89 The cause of ovulation failure has been suggested to be endocrine in nature. Absence of sufficient pituitary gonadotropin stimulation to induce ovulation, or insufficient estrogen production from the follicle, has been proposed as a possible mechanism. PAFs were reported in a recent study to occur in approximately 8.2% of estrous cycles.67 The formation of an anovulatory follicle was preceded by development of normal endometrial folds or edema in 78.3% of these cases. Initial growth patterns of follicles destined to become anovulatory were usually normal, and the first indication of a problem was the detection of echogenic particles within the follicular fluid. The incidence of PAFs was also found to increase with age.
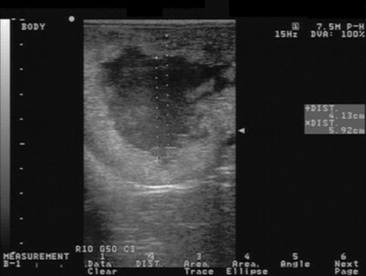
PAFs may contain blood and have been termed hemorrhagic anovulatory follicles., The hemorrhage can be detected ultrasonically as scattered free-floating echogenic spots within the follicular fluid. The follicular fluid may form a gelatinous, hemorrhagic mass within the follicular lumen. Ultrasonographically these structures may contain echogenic fibrous bands traversing the follicular lumen (Fig. 43-5). A thickening of the follicular wall may be observed in anovulatory follicles. This thickening is often associated with luteinization of the follicular wall, and 85.7% of PAFs were found to be luteal structures based on elevations in plasma progesterone concentrations.67
Treatment and Prognosis
The administration of prostaglandins may result in the destruction of the luteal cells in mares with luteinized PAFs. A majority of nonluteinized PAFs spontaneously regress in 1 to 4 weeks. Treatment with hCG (2500 IU IV) or a GnRH agonist (Ovuplant 2.1 mg subcutaneously [SC]) to induce ovulation or luteinization of the anovulatory follicle is generally not effective. Pregnancy does not usually occur if a persistent follicle eventually spontaneously ovulates or is induced to ovulate. This is likely a result of degeneration of the oocyte over time. Pregnancy obviously will not occur if the follicle becomes hemorrhagic or luteinized without ovulation.
Ovarian Hematoma
Hemorrhage into the follicular cavity is a normal occurrence at ovulation. Occasionally hemorrhage is severe, resulting in the formation of an ovarian hematoma that may be 10 cm in diameter or larger.
Clinical Signs and Diagnosis
Affected mares continue to cycle normally. Transrectal palpation and ultrasonography reveal an enlarged ovary that is initially irregularly hypoechoic and then echogenic with organization of the hematoma. The ovulation fossa usually remains distinguishable on the affected ovary, and the contralateral ovary remains active.
Treatment and Prognosis
Ovarian hematomas regress spontaneously over a period of weeks or months. The functional lifespan of the luteal tissue in a hematoma is normal, and ovarian activity is unaffected.90 Because the affected ovary is not permanently damaged and the contralateral ovary is unaffected, the prognosis for fertility is undiminished.
Pregnancy
Multiple secondary CLs form in pregnant mares at 40 to 180 days of gestation, resulting in bilaterally enlarged ovaries that may be mistaken for ovarian pathology.
Clinical Signs and Diagnosis
Ovarian enlargement is commonly bilateral. Pregnant mares may show stallion-like behavior associated with increased testosterone production from the fetus during midgestation. Pregnancy should always be considered in a mare with stallion-like behavior, elevated serum testosterone concentrations, and enlarged ovaries. Pregnancy is diagnosed by rectal palpation and ultrasonographic examination.
PERSISTENT CORPUS LUTEUM
The CL usually regresses 14 to 15 days after ovulation.3 Although luteal cells appear to be sensitive to PGF2α almost immediately after ovulation, complete luteolysis and return to estrus in response to endogenous or exogenous PGF2α will not occur until 5 days after ovulation. Because the functional CL is lysed by PGF2α from the endometrium, the CL will continue to function in the following situations.
Clinical Signs and Diagnosis
This syndrome may be suspected clinically in mares that are not expressing normal estrous behavior during the physiologic breeding season, and it must be differentiated from mares with silent heat. Diagnosis of a persistent CL is made by transrectal sonographic examination of the ovaries. The CL appears as a well-defined hyperechoic structure on the ovary. Mares with a persistent CL will have good cervical and uterine tone on palpation, and the cervix will appear tight and dry on vaginal speculum examination because of the influence of progesterone. The diagnosis may be confirmed by analysis of plasma progesterone concentrations or a clinical response to prostaglandin administration. Progesterone concentrations >1 ng/mL1 are indicative of the presence of active luteal tissue. Differential diagnoses are listed in Table 43-2.
Treatment and Prognosis
After pregnancy has been ruled out with sonographic examination, luteolysis can be achieved with administration of 10 mg of the PGF product dinoprost tromethamine or similar PGF analog. The mare must be at least 5 days postovulation to respond reliably to treatment. If the mare is treated with PGF in the presence of a mature diestrous follicle (≥35 mm), she may ovulate the follicle without signs of estrus in response to declining progesterone concentrations that allow LH to be secreted from the anterior pituitary. An ultrasonographic or rectal examination of ovarian follicular activity should therefore always be performed before PGF is administered to induce estrus.
SHORTENED LUTEAL PHASE (PREMATURE LUTEOLYSIS)
Premature (<15 days) luteolysis is associated with an early onset of estrus and a decrease in the interovulatory interval. The most common cause of premature luteolysis in the mare is endometritis. Inflammation of the endometrium results in an acute activation of inflammatory mediators. One of these mediators is PGF2α, which in addition to its inflammatory effect also may cause luteolysis and return to estrus. Consequently a mare that exhibits a shortened diestrus should be examined for endometritis. A culture, biopsy, and cytologic examination of the uterus may be indicated.
LUTEAL INSUFFICIENCY
Primary luteal insufficiency implies a deficiency in progesterone production. Luteal insufficiency has been suggested to be a cause of subfertility in mares.94 Maintenance of pregnancy in some habitually aborting mares after administration of exogenous progestogens offers circumstantial evidence that progesterone insufficiency may be responsible for some cases of pregnancy loss.
Data are limited and not supported by scientific evidence from controlled studies.
Luteal insufficiency secondary to PGF2α release in mares with endotoxemia has been reported95 and should be considered in pregnant mares with gram-negative infection and/or endotoxemia-associated colic. Reports on the effect of exogenous administration of PGF2α during the periovulatory period suggest that PGF2α can delay the formation of a functional CL. Suboptimal concentrations of progesterone were found in mares after treatment with PGF2α during the first 2 days after ovulation.96-100 Although the CL eventually became functional and progesterone concentrations had returned to normal levels at day 14, pregnancy rates were significantly lower in treated mares.97,98
Clinical Signs and Diagnosis
The minimum concentration of progesterone required to maintain pregnancy in the mare has been suggested to be 2 ng/mL.95 Repeated samples are necessary to diagnose luteal insufficiency because progesterone is released episodically.
Treatment and Prognosis
The most common treatment for luteal insufficiency is supplementation with the synthetic progestogen altrenogest (Regumate) at a dose of 0.044 mg/kg PO once daily. Options for duration of altrenogest supplementation include treatment until day 80 to 120 of pregnancy or greater and measurement of endogenous progesterone level of >2 ng/mL (progesterone and altrenogest do not cross-react on radio immunoassay [RIA]), or treatment until the end of gestation. It is important to emphasize the need to monitor fetal well-being when mares are kept on progestin supplementation to maintain pregnancy. A case of fetal mummification of a 5-month-old fetus in a pregnant mare at term has been reported.101 The mare had been maintained on altrenogest throughout gestation.
RUMINANTS
PROLONGED LUTEAL FUNCTION
Cow
Spontaneous prolongation of luteal function in the presence of a normal, nongravid uterus does not occur in cows. But several conditions affecting the uterus do suspend the luteolytic mechanism, resulting in prolonged luteal function, persistently elevated progesterone concentrations, and anestrus. Common causes of prolonged luteal function in cows include pregnancy, pyometra, mummified fetus, and segmental aplasia, including uterus unicornis.
Clinical Signs and Diagnosis
Pregnancy resulting from an unobserved or unrecorded breeding must always be considered as a possible cause of anestrus. Examination of the uterus by transrectal palpation or ultrasonography for one of the positive signs of pregnancy (fetal membrane slip, amnionic vesicle, placentomes, or fetus) must precede administration of PGF.
Pyometra is characterized by accumulation of variable amounts of mucopurulent exudate within the uterine lumen, failure of luteolysis, and subsequent anestrus. An enlarged uterus as a result of fluid accumulation and a thickened uterine wall in the absence of any positive signs of pregnancy can be used to differentiate uterine enlargement caused by pregnancy from that caused by pyometra. Fluid associated with pyometra is more viscous than fetal fluid and can be manipulated from horn to horn. Because the cervix is nearly always closed, there is generally no vaginal discharge.
Fetal mummification is occasionally encountered in dairy and beef cows and is characterized by fetal death, failure of expulsion, absorption of fetal fluids, and persistence of the CL. Cows with fetal mummification are usually presented when they do not deliver a calf at the expected time. The condition can be differentiated from pregnancy by palpation per rectum of a dried, leather-like fetus within the involuted uterus. Fetal membranes cannot be slipped, and fetal fluids and placentomes are absent.
Clinical Pathology
No remarkable changes in hematology and clinical chemistry are associated with pregnancy, mummified fetus, or pyometra in cows. In all three conditions, peripheral concentrations of progesterone remain elevated above 1 ng/mL of plasma until spontaneous luteolysis occurs or the condition is treated.
Treatment and Prognosis
Unwanted pregnancy is seldom encountered in dairy cows, but when cows or heifers are mated by accident or to an undesired sire, abortion may be reliably induced with PGF products (25 mg of dinoprost tromethamine,* or 500 mcg of cloprostenol;† two injections 8 to 12 hours apart) after 7 days and before 150 days of gestation or with a combination of PGF and dexamethasone (20 mg) beyond 150 days of gestation. The PGF products are also the treatment of choice for pyometra in cows. Mummified fetuses are usually expelled 3 to 5 days after treatment with PGF. The mummy may pass through the cervix, but the vagina is likely to be dry and may not dilate sufficiently, so the mummified fetus may be retained. If the mummified fetus is not expelled by 5 days after treatment, a vaginal examination should be performed, and the mummy delivered by gentle traction if necessary. The prognosis for fertility after delivery of a mummified fetus is good.
OVARIAN HYPOPLASIA
Ovarian hypoplasia occurs sporadically as an autosomal recessive trait in the cow. The condition has incomplete penetrance; therefore it may be partial or complete and unilateral or bilateral. The affected gonad varies in size from a cordlike thickening in the cranial edge of the mesovarium to a bean-sized structure. The tubular genital organs remain infantile in animals with complete bilateral hypoplasia or may develop to near-normal size in heifers with unilateral or partial hypoplasia. Individuals affected with complete bilateral ovarian hypoplasia are sterile, whereas those affected by partial or unilateral hypoplasia may be subfertile. With partial hypoplasia the uterine pole of the ovary is typically affected. On direct observation by laparotomy or laparoscopy or on slaughter, the uterine pole is flat and triangular and shows converging striations. The affected part is devoid of follicles. Animals with partial ovarian hypoplasia can be expected to have a reduced superovulatory response to gonadotropin treatment. Heifers with abnormal karyotypes are also affected by ovarian hypoplasia. The condition should be differentiated from nonfunctional ovaries and anestrus associated with malnutrition or debilitating diseases. Treatment of ovarian hypoplasia is not successful.102
FREEMARTINISM
Cow
A freemartin is a phenotypic female, born co-twin to a male, that is sterile because of arrested development of the reproductive tract. The term “freemartin” is said to have originated in England, where it referred to a heifer that was not pregnant after the summer breeding season and therefore “free” for fattening and slaughter at Martinmas, a fall festival in honor of St. Martin.11 Freemartinism arises as a result of anastomoses between the placental circulations of twin fetuses of opposite sexes. Sexual differentiation of the male embryo occurs earlier than that of the female; therefore the male twin may sterilize the female by transfer of HY antigen, which inhibits development of the female gonad. The ovaries of a freemartin are underdeveloped and contain seminiferous tubules. Abnormalities of the tubular genital organs vary in severity, and the structures range from cordlike bands to near-normal uterine horns. In most freemartins there is no cervix; therefore no communication exists between the uterus and vagina. The latter is a short blind pouch. Frequently, seminal vesicular glands of varying size are present.
As many as 92% of phenotypic females born co-twins to males are freemartins55; the history suggests a diagnosis in most cases. Singleton freemartins are possible if male and female twins are conceived and the male is lost after 30 days of gestation. Palpation per rectum of breeding age freemartins reveals aplasia or hypoplasia of the tubular genital organs and hypoplastic ovaries. If animals too small for palpation per rectum are presented, examination of the vagina with a small glass speculum (or test tube) reveals that the vagina of a freemartin is short (6 to 7 cm in freemartins versus nearly double that length in normal heifers). A definitive diagnosis may be made by karyotyping the suspected individual; varying percentages of male cells are found in freemartins.102 More details on freemartinism are discussed elsewhere in this chapter.
Ewe
Although freemartinism is possible in sheep, the condition is rare, despite the high incidence of multiple births. The condition can be confirmed by determination of blood cell chimerism.
Doe
Twinning is also common in goats, but vascular anastomosis is either uncommon or occurs after the critical period for sexual differentiation. Caprine freemartins comprise approximately 6% of intersexes.5
INTERSEX
Doe
Intersexes are common among Saanen, Toggenburg, and Alpine goats. The fetal testes appear to be unable to fully masculinize the duct system or external genitalia. Parts of both the mesonephric and paramesonephric ducts persist; therefore the phenotype of affected individuals may approach that of either sex. Intersexes are most frequent among polled goats, and the condition is thought to be caused by a recessive gene linked to that for polledness; however, intersexuality may rarely be seen in horned goats. Diagnosis is based on a history of abnormal sexual behavior, and identification of abnormal genital development is by physical examination. There is no satisfactory treatment, but the prevalence of the condition may be reduced by preventing mating between two polled animals.5 More detailed information can be found elsewhere in this chapter.
OVARIAN TUMORS
Granulosa Cell Tumor
COW
Although rare, various ovarian neoplasms have been described in cows; granulosa cell tumors appear to be most common.11
Clinical Signs and Diagnosis
Granulosa-theca cell tumors are characterized by unilateral ovarian enlargement, with the affected ovary being greater than 10 cm in diameter. The surface may be smooth or coarsely lobulated. Function of the contralateral ovary may be suppressed. The behavior of affected cows ranges from anestrus to nymphomania to bull-like behavior. Udder development and lactation may occur in affected heifers. Very few bovine granulosa cell tumors are malignant, and they rarely metastasize. Among the other ovarian neoplasms that have been reported in cattle are dysgerminomas, interstitial cell tumors,11 and teratomas. Causes of ovarian enlargement that must be differentiated from ovarian neoplasia include ovarian cysts, oophoritis, ovarian abscesses, and parovarian cysts.
EWE
Granulosa-theca cell tumors have been reported, but tumors of the genital organs of ewes appear to be rare.11 Animals with ovarian tumors may exhibit all types of behavioral and physiologic abnormalities (nymphomania, inappropriate lactation). Diagnosis is based on ultrasonographic evaluation of ovaries per rectum or transabdominally. Treatment is ovariectomy.
OVARIAN HEMORRHAGE
Cow
Ovulation tags develop after ovulation, resulting from blood loss associated with rupture of the follicle.11 Fine adhesions may develop between the ovarian surface and surrounding structures. Most ovulation tags resolve spontaneously and have no effect on fertility. Severe ovarian hemorrhage may follow attempts to manually enucleate the CL. Adhesions between the ovary and its bursa interfere with their normal function. Enucleation of CLs for treatment of anestrus and pyometra has been superseded by treatment with PGF products.
INFERTILITY CAUSED BY ABNORMALITIES OF THE FEMALE TUBULAR GENITALIA
SALPINGITIS
Inflammation of the oviducts is characterized by macroscopic enlargement. Lesions are frequently bilateral and consist of infiltration by lymphocytes, plasma cells, and neutrophils, and desquamation of epithelial cells.11
Most cases of salpingitis follow infections of the uterus. Necrotizing and granulomatous salpingitis may follow infection by Arcanobacterium pyogenes, Mycobacterium tuberculosis, and Brucella abortus., Mild inflammation of the uterine tubes that does not usually result in permanent damage accompanies uterine infection caused by Campylobacter fetus, subsp. venerealis, and Tritrichomonas foetus., Salpingitis may be a sequela to manipulations of the ovaries and uterine tubes by palpation per rectum, transvaginal ovum pickup, aggressive irrigation of an infected uterus, and inappropriate treatment with estrogenic hormones. Migrating larvae of Strongylus edentatus, have been proposed as a possible cause of nonobstructive infundibulitis in mares, but their role is speculative.11
Pyosalpinx is characterized by segmental accumulation of pus within the lumen of the oviduct after mechanical blockage of either end. Pyosalpinx frequently follows severe cases of uterine infection and may be complicated by perimetritis and localized peritonitis.
Hydrosalpinx is characterized by accumulation of thin mucus within the lumen of the oviduct. Hydrosalpinx and adhesions to perisalpingial tissues are common sequelae to chronic salpingitis.
Clinical Signs and Diagnosis
The usual history associated with diseases of the uterine tubes is one of infertility. Additional history may include uterine infection or traumatic therapy such as uterine irrigation, enucleation of CLs, or administration of exogenous estrogen during CL function. Salpingitis is an uncommon clinical finding in the mare. However in one study, up to 88% of mares were found to have macroscopic lesions in the oviduct, including adhesions, fibrous bands, and parovarian cysts, which may or may not have affected fertility.103 Accumulations of cells and debris may form intraluminal masses; however, their role in infertility has not been adequately tested.104 The pathology of the oviduct has been reviewed.105 Similarly, moderate lesions of uterine tube disease may escape diagnosis by physical examination in cows, but the results of abattoir studies suggest that lesions of the oviducts are not uncommon.11 In cows, lesions involving adhesions among the ovary, ovarian bursa, oviduct, and surrounding tissues may be identified per rectum by inserting two or three fingers into the ovarian bursa and rolling the oviduct between the fingers and thumb. Easy identification of the oviduct by palpation per rectum is sometimes considered indicative of abnormalities. Diagnosis of diseases of the oviducts in ewes and does is impossible by physical examination. Although a history of infertility after one of the predisposing causes might suggest oviductal lesions, diagnosis is made by exploratory laparotomy, peritoneoscopy, or necropsy.
Lesions of the oviductal or perisalpingial tissues must be differentiated from other causes of abnormal enlargements such as ovarian neoplasia, parovarian cysts, cystic ovarian disease, and ovarian hematomas. Neoplasia of the oviducts in domestic animals is extremely rare.
Clinical Pathology
Several tests that determine oviductal patency of mares106 and cows107 have been described, but neither the starch test nor the phenolsulfonphthalein dye test is very reliable or consistently diagnostic. For suspected unilateral blockage,108 each uterine horn may be catheterized individually with a Foley catheter placed at the base of the horn on different days.
Embryo Recovery
Embryo recovery after either a single ovulation or superovulation is objective evidence that one or both uterine tubes are patent and functional. Improved reproductive performance of cows may follow uterine lavage; therefore embryo recovery as a diagnostic test may have therapeutic benefits as well.109
Treatment and Prognosis
Treatment of diseases of the oviducts is not likely to be successful. Appropriate treatment for concurrent uterine infections should be instituted. A period of sexual rest may be beneficial and is indicated in valuable animals. The prognosis for reproduction in cases of bilateral obstruction of the oviducts is poor. In vitro fertilization of ova harvested from affected females is a therapeutic option. Affected females can also serve as embryo recipients.
Prevention and Control
Traumatic manipulation of the ovaries, irrigation of the endometrial cavity with large volumes of fluid (over 100 mL in heifers or 150 mL in cows) or irritating chemicals, and administration of estrogenic hormones to luteal phase females should be avoided.102 Because abnormalities of the oviducts are frequently associated with uterine infections, reduction of the prevalence of uterine infections results in fewer tubal infections as well.
UTERINE ABNORMALITIES
Retained Fetal Membranes
MARES
Retention of the fetal membranes beyond a period of 3 hours is an abnormal occurrence in the mare. The mare has an epitheliochorial placenta characterized by diffuse microvilli that interdigitate with endometrial crypts. After delivery, blood flow through the placental vessels is reduced, and placental microvilli shrink and disengage from endometrial crypts. The condition is more common after abortion, dystocia, cesarean section, and fetotomy. The pathophysiology of the disease is poorly understood but may involve disturbances of normal prepartum endocrine events or myometrial contractility. Partial placental retention may be localized to well-defined areas of continued placental attachment. The most common site of partial retention is the previously nongravid horn.
Equine placentas should be spread on a flat surface after expulsion and examined to ensure that the complete membrane is present. Areas of placental necrosis are common near the tips of the uterine horns, and the fragile area may be incarcerated by the rapidly contracting uterus.
Clinical Signs and Diagnosis
Retained fetal membranes (RFMs) are usually visible at the vulva. However, small tags of placental tissue may remain attached to the uterus without being apparent and may be a nidus for infection, resulting in severe metritis, endotoxemia, and laminitis hours to days postpartum.
Treatment and Prognosis
The severity of sequelae makes early intervention essential. Treatment should begin if fetal membranes are not passed within 3 hours of foaling. Most instances respond to vigorous early pharmacologic treatment. Occasional cases require several days of persistent treatment.
MANUAL REMOVAL
Manual removal is contraindicated because trauma induces placental tearing, leaving microvilli in endometrial crypts.
OXYTOCIN
Oxytocin induces myometrial contractions, which may aid placental expulsion. Oxytocin may be administered by intravenous injection (5 to 20 IU every 15 to 30 minutes) or intramuscular injection (20 to 40 IU every 30 to 60 minutes), or it may be infused slowly (30 to 80 IU in 500 mL of warm saline over 30 to 60 minutes). Care should be taken to avoid overdosage, which may result in signs of abdominal pain and which will cause tetanic rather than orchestrated uterine contraction.
ALLANTOCHORIONIC INFUSION
If the chorioallantois is intact, the chorioallantoic cavity may be filled to distention with 3 to 4 gallons of warm saline or water through the cervical star.111 The opening in the placenta is held closed until the mare exerts abdominal pressure. Oxytocin may be used in conjunction with this treatment.
UTERINE LAVAGE
If the chorioallantois is not intact, uterine lavage with an isotonic saline solution will clear debris, encourage myometrial contractions, and may help to free the retained fragment of the fetal membrane. The isotonic saline solution may be infused into the uterus through an equine nasogastric tube (dedicated to reproductive use) manually held in place. Approximately 5 L may be placed in the uterus of a full-grown, postpartum mare at one time, but care should be taken to observe the mare for signs of discomfort and to gauge the amount of fluid felt within the uterus. During fluid infusion the operator may carefully explore the uterus manually, searching for retained fetal membrane remnants. Once the uterus is relatively full of fluid, the fluid is siphoned out. Care must be taken to protect the fragile endometrium from damage incurred by the strong suction force of the siphon. The operator’s hand should guard the end of the tube from direct contact with the endometrium. This process should be repeated until the effluent is clear. Uterine lavage should be performed at least once daily until 12 to 24 hours after the RFM remnants are retrieved.
ADJUNCT THERAPY
Concurrent therapy directed at controlling or minimizing common sequelae to retained placenta is often indicated.
Some cases of RFMs are refractory to treatment, and membranes may remain firmly attached to the endometrium for several days. Aggressive attempts at manual removal should be eschewed, because severe endometrial damage may follow. Persistent treatment with antibiotics, antiinflammatory drugs, and oxytocin is indicated until the placenta is expelled and bacterial infection of the uterus is controlled.
The prognosis for RFMs is generally good but is reduced if treatment is delayed or if retention is accompanied by infection with virulent pathogens. Sequelae to RFMs include metritis, endometrial fibrosis, invagination of a uterine horn, uterine prolapse, and laminitis.
RUMINANTS
Cow
The cotyledonary placenta of cows is usually expelled within 3 to 8 hours after calving and is considered retained if not expelled by 12 hours. RFMs are more commonly seen in dairy than in beef breeds. In dairy cattle the reported prevalence ranges from 8% to 12% after spontaneous delivery of single calves. RFMs are more likely after deliveries of male calves or twins and deliveries complicated by dystocia. Parturition after a shorter- or longer-than-normal gestation length is accompanied by an increase in the incidence of RFMs.112
The cause of RFMs in cattle is failure of fetal cotyledons to separate from crypts of maternal caruncles; the process of separation normally begins during the last months of pregnancy. Villi shrink after blood flow is interrupted by rupture of the umbilical vessels. Strong myometrial contractions continue during the third stage of labor, and changes in the size and shape of maternal caruncles contribute to separation of the placenta from the endometrium. A number of factors have been associated with separation failure, but the precise reasons for separation failure are unknown.5 Deficiencies of selenium, vitamin E, and vitamin A are associated with an increased prevalence of RFMs.5
Clinical Signs and Diagnosis
The majority of affected cows show no serious clinical signs other than a transient decrease in appetite and milk production. However, 20% to 25% of cows affected by RFMs develop moderate to severe metritis. The most objectionable clinical signs are the malodorous discharge and objectionable tissue hanging from the genital tract. RFMs are usually expelled by 4 to 10 days after calving when the caruncular tissue has become necrotic and is sloughed. Some affected cows show signs of endotoxemia, including depression, fever, ruminal stasis, and inappetence, as a result of RFMs.
Treatment and Prognosis
A variety of treatments have been suggested for RFMs in cows, including aggressive attempts at manual removal, myometrial stimulants, intrauterine and systemic antibiotics (alone or in combination with other approaches), and no therapy whatsoever. Because the processes that culminate in RFMs begin during late gestation, it is not unreasonable that treatment initiated at calving has little effect on the loosening process. Most treatments for RFMs are directed toward controlling the intrauterine bacterial population.
MANUAL REMOVAL
Manual removal of the placenta is indicated only when gentle traction is sufficient to withdraw the membranes in a short time. Attempts at manual removal are contraindicated if the patient shows clinical signs of septicemia. Trauma caused by manual removal inhibits phagocytosis by uterine neutrophils and predisposes to severe sequelae, including septic metritis and peritonitis.
MYOMETRIAL STIMULANTS
Administration of a single dose of oxytocin does not reduce the prevalence of RFMs in cows that calve spontaneously or in cows that require assistance at delivery.113,114 Cows with RFMs have an elevated plasma concentration of estrogen during the period of retention; therefore administration of additional estrogen for treatment of RFMs may be of questionable value.115 Intravenous calcium solutions are indicated in cases of RFMs secondary to hypocalcemia.
PROSTAGLANDIN
In one trial, treatment with fenprostalene resulted in a shorter period of retention in treated cows, reduced the number of treatments subsequently required for metritis, and slightly reduced the intervals to first service and conception.116 However, other researchers found that fenprostalene produced no changes in myometrial activity between days 1 and 4 after calving and concluded that uterotonic agents are unlikely to hasten placental expulsion because uterine effort is already increased in animals that have RFMs.117 An imbalance between synthesis of PGF2 and PGI2 between 30 and 60 minutes after parturition has been demonstrated in cows affected by RFMs.118 Prostaglandin at the time of calving does not reduce the incidence of RFMs or improve reproductive performance.119
ANTIBIOTICS
Intrauterine tetracycline may reduce fertility,120 or the reproductive performance of treated cows may be as good as that of untreated herdmates.7 Intrauterine treatment with 4 to 6 g of oxytetracycline per day until the placenta is expelled may reduce the prevalence of metritis associated with RFMs, but pyometra may develop in treated cows.121 Bacterial putrefaction and the disagreeable odor of RFMs may be reduced by intrauterine antibiotics, but the placenta is released only after necrosis of the caruncles. Systemic and intrauterine antibiotics are indicated in cases of RFMs in which the cow has a fever, is off feed, or has a drop in milk production.
Cows that retain their membranes for more than 12 hours after calving are more likely to develop metritis than are cows that promptly expel the membranes. However, reproductive performance of cows that rapidly return to normal after RFMs is similar to that of their unaffected herdmates, indicating that in the absence of a secondary reproductive abnormality, RFMs have a minimum effect on future fertility.
COLLAGENASE
An alternative approach to the treatment of RFMs is the injection of collagenase into the umbilical arteries of the retained membranes.122 This treatment is aimed directly at the lack of cotyledonary proteolysis. Intrauterine infusion of collagenase is not effective. Bacterial collagenase from Clostridium histolyticum, is used and is commercially available (Type XI, Sigma Chemical, St. Louis, Mo.). However, collagenase is not currently approved for use in food-producing animals in the United States.
Ewe and Doe
Fetal membranes are considered retained in ewes and does if not expelled within 12 hours after delivery of the last fetus. The prevalence in does is approximately 6% after spontaneous delivery but may be higher when delivery is complicated by dystocia or abortion. Selenium deficiency has been suggested as a cause.
The clinical signs of RFMs in ewes and does are usually obvious.5 Does may ingest their placentas, complicating identification of cases of partial retention. RFMs may accompany retention of a fetus within the uterus, and does and ewes should be carefully examined.
Other tissues that may be exposed from the vulva in association with parturition are a prolapsed uterus, a prolapsed or everted urinary bladder, prolapse of some portion of the digestive tract through a uterine rupture, prolapsed rectum, prolapsed vagina, or a twin fetus.
Treatment and Prognosis
Manual separation of cotyledons from caruncles is impossible in ewes and does; therefore manipulative attempts to remove the placenta are limited to gentle traction on exposed membranes at daily intervals. Treatments with intrauterine and systemic antibiotics, oxytocin (10 to 20 IU) at 12-hour intervals until the placenta is expelled, and antiinflammatory drugs have been suggested. Prophylaxis against tetanus is indicated.
Camelids
The placenta is usually passed within 1 to 2 hours of parturition. Camelid placentas resemble equine placentas (diffuse, microcotyledonary, epitheliochorial), with the exception that the left horn is almost always the pregnant horn. RFMs in camelids are most commonly seen as sequelae to dystocia or other disorders of parturition.9 Treatment is similar to that described for the mare.
UTERINE INFECTIONS
MARE
Endometritis
A failure of the uterine defense mechanisms to effectively eliminate an antigen (bacteria or spermatozoa) and inflammatory products from the uterus results in persistent endometritis, which is a major cause of reduced fertility in broodmares.123 In the normal mare the uterus is well protected from external contamination by physical barriers consisting of the vulva, the vestibule, the vagina, and the cervix, and any compromise of these barriers may predispose the mare to a chronic uterine infection.124 Breeding is another source of uterine contamination. Intrauterine deposition of semen causes an inflammatory reaction resulting from bacterial contamination of the ejaculate or from spermatozoa.125 Approximately 15% of a normal population of thoroughbred broodmares developed persistent endometritis after breeding.126 Natural resistance to experimentally induced bacterial contamination has been demonstrated in young mares, whereas a population of multiparous and barren mares developed persistent endometritis after bacterial contamination of the uterus.127,128 Based on these studies, mares have been classified as either susceptible or resistant to persistent uterine infection.127 Endometritis has severe effects on the fertility of affected mares. A persistent inflammation may interfere directly with the survival of an embryo or may cause premature luteolysis and embryonic loss because of increased PGF concentrations.129
Several classes of immunoglobulins have been isolated from the equine uterus. Although antibody-mediated uterine defense may be important for effective elimination of bacterial contaminants from the uterus in susceptible mares, concentrations of immunoglobulins in uterine secretion are similar or even elevated compared with those of resistant mares.130-134 Polymorphonuclear neutrophils (PMNs) are the first inflammatory cells to enter an inflamed site.135 Chemoattractive properties of uterine fluid have been described in vitro in horses, and the uterus responds quickly to an antigen with release of PMN-chemotactic mediators, which results in a rapid migration of PMNs into the uterine lumen.136 Complement products and leukotriene B4 (LTB4), PGE, and PGF may all serve as chemoattractants for PMNs in the uterus.136-140 Studies on the role of local uterine factors in PMN function suggested that an impaired phagocytosis by uterine PMNs in susceptible mares is the result of insufficient opsonization in uterine secretion rather than a primary dysfunction of the PMNs.141
Mechanical aspects of the uterine defense system are currently believed to be a major contributor in uterine clearance of bacteria and inflammatory products.142-144 Through use of intrauterine inoculations of a combination of radioactive-labeled microspheres and bacteria, impaired uterine clearance was demonstrated in susceptible but not in resistant mares.142 Studies using scintigraphic measurements of intrauterine clearance of radioactive colloids further defined a delayed physical clearance in susceptible mares.143 Through use of electromyography (EMG) to register myometrial activity, it was observed that the impaired uterine clearance in susceptible mares was caused by reduced myometrial activity in response to the inflammation (Fig. 43-6).144 The dependent position of the mare’s uterus may also interfere with effective clearance.
Fig. 43-6 Myoelectrical activity before and after uterine inoculation of Streptococcus zooepidemicus, in mares susceptible ( ) and resistant (
) and resistant ( ) to persistent endometritis. Time 0 indicate time of inoculation. Susceptible mares had impaired myoelectrical activity after inoculation.
) to persistent endometritis. Time 0 indicate time of inoculation. Susceptible mares had impaired myoelectrical activity after inoculation.
Modified from Troedsson MH, Liu IK, Ing M, et al: Multiple site electromyography recordings of uterine activity following an intrauterine bacterial challenge in mares susceptible and resistant to chronic uterine infection, J Reprod Fertil, 99:307, 1993.
Based on pathogenesis, persistent endometritis can be divided into (1) sexually transmitted diseases (STDs), (2) persistent uterine infection, (3) persistent breeding-induced endometritis, and (4) chronic degenerative endometritis (endometrosis).
SEXUALLY TRANSMITTED DISEASES
Few true STDs are known in the horse. Contagious equine metritis (CEM) is an example of a true STD.145,146 The disease is caused by Taylorella equigenitalis, a highly contagious and pathogenic microorganism. Although the present status of a mare’s uterine defense mechanism is important for the manifestation of the disease, this bacterium is highly resistant and capable of overcoming the mare’s normal disease barriers.
PERSISTENT UTERINE INFECTION
Bacteria most commonly isolated from the uterus of the mare are beta-hemolytic streptococci (Streptococcus zooepidemicus, and Streptococcus equisimilis,), Escherichia coli, Pseudomonas aeruginosa, and Klebsiella pneumoniae., Other aerobic bacteria isolated from reproductive tracts of mares include alpha-hemolytic streptococci, Corynebacterium, species, Staphylococcus, species, Enterobacter, species, Actinobacter, species, Proteus, species. Citrobacter, species, Candida, species, and Aspergillus, species are the organisms most commonly associated with yeast or fungal endometritis. The role of viruses, mycoplasmas, ureaplasma, and anaerobic bacteria in endometritis is poorly understood. P. aeruginosa, K. pneumoniae, and possibly S. zooepidemicus, and E. coli, can be sexually transmitted in horses, but the consequences of exposure to these microorganisms are determined by the particular strain involved and active participation of all facets of the mare’s uterine defense mechanisms. In contrast to a true STD, persistent infectious endometritis is often the result of contamination of the uterus by the mare’s fecal and genital flora in combination with compromised uterine defense.147,148
PERSISTENT BREEDING-INDUCED ENDOMETRITIS
Bluegrass
Intrauterine deposition of semen causes an inflammatory reaction resulting from spermatozoa.149,150 The mechanism of the induced inflammation is similar to endometritis caused by bacteria, involving activation of the complement cascade.150 The role of spermatozoa in breeding-induced endometritis implies that a transient uterine inflammation is a physiologic reaction to semen, and it appears to be a normal process by which excess sperm and bacterial contamination are eliminated from the mare’s reproductive tract.148,151 Transport of spermatozoa from the uterus to the oviduct is completed within 4 hours after breeding, and only a small portion of the ejaculated or inseminated semen reaches the oviduct.152,153 The rapid transport of spermatozoa to the oviduct coincides with increased uterine activity.151 Increased myometrial contraction in response to breeding is also responsible for rapid sperm elimination from the uterus through the cervix.154 However, not all excess spermatozoa are removed from the uterus through this mechanism. The remaining spermatozoa have to be eliminated by means of other uterine clearance mechanisms, such as PMN-phagocytosis of spermatozoa.148 However, the condition may develop into a persistent inflammation in mares with impaired uterine clearance.142,143 If sperm elimination and all physical and chemical reactions that are involved in the induced inflammation persist beyond the time when the embryo enters the uterus at 5 days after ovulation, embryonic loss will occur because of an incompatible inflammatory uterine environment. The incidence of persistent breeding-induced endometritis has been reported to be approximately 15% of a normal population of thoroughbred broodmares, and the incidence may be higher for mares bred by artifical insemination with thawed frozen semen.126
In contrast to spermatozoa, seminal plasma has a suppressive effect on complement activation, PMN-chemotaxis, and phagocytosis.155 A function of seminal plasma may be to act as an inflammatory inhibitor or modulator in the uterus, which may be of importance with regard to the transient nature of breeding-induced endometritis. The duration of breeding-induced uterine inflammation was shown in a study to be shorter when seminal plasma was included in an insemination dose, compared with when all seminal plasma was removed and replaced by a commercial semen extender.156 Although the peak numbers of PMNs were the same for both groups, significantly fewer PMNs were recovered from the uterus at 24 hours compared with 6 and 12 hours after insemination when seminal plasma was included. In contrast, there was no significant difference in the number of uterine PMNs at 6, 12, and 24 hours of insemination in the absence of seminal plasma. Another function of seminal plasma in breeding-induced endometritis may be to protect spermatozoa from being phagocytosed and destroyed in an inflammatory environment. PMNs are present in the uterine lumen by 0.5 hours after breeding, but sperm transport is not completed until 3 to 4 hours later.152,157 In addition, when mares are inseminated twice within a 24-hour period, semen from the second insemination is introduced into an inflammatory environment. This environment is detrimental to sperm motion characteristics, and motile sperm cells appear to bind to PMNs, forming large clusters of PMN and spermatozoa. Addition of seminal plasma has been shown to reduce the binding between spermatozoa and inflammatory cells in vitro.158 Recent data suggest that equine seminal plasma selectively protects viable but not dead spermatozoa from PMN-binding and phagocytosis.159,160 Selective protection of viable spermatozoa from PMN-binding and phagocytosis increases their survival in a hostile uterine environment and ensures that a sufficient number of spermatozoa reach the oviduct for fertilization, while effective sperm elimination of nonviable spermatozoa can be maintained.
CHRONIC DEGENERATIVE ENDOMETRITIS (ENDOMETRIOSIS)
Degenerative changes of the endometrium such as periglandular fibrosis and glandular dilation are often seen in older multiparous mares. The condition is associated with susceptibility to persistent endometritis161 and may result from repeated uterine inflammation. However, the condition has also been observed in older mares without any known history of endometritis, suggesting that degenerative fibrosis of the endometrium can be a process of aging rather than inflammation.162 Based on the possibility of a noninfectious cause of the disease, it was suggested that the condition should be called endometrosis, rather than degenerative endometritis.,162 It is not clear why mares with fibrotic degenerative changes to the endometrium have an impaired physical uterine clearance mechanism. Sclerotic changes in the uterine vascular bed impair blood flow to both the endometrium and the myometrium.163
Diagnostic Approach
History compatible with endometritis includes infertility after breeding to a fertile stallion. Mares with severe endometritis may have shortened interestrous intervals and may show vaginal discharge. Physical and speculum examination may show anatomic defects of the vulva or cervix. Excessively easy passage of a vaginal speculum may indicate loss of integrity of the vestibulovaginal sphincter. Discharge from the cervix and vaginal inflammation may be apparent. Transrectal palpation and ultrasonography may reveal accumulations of luminal fluid (Fig. 43-7). Diagnostically, it may be difficult to identify susceptibility to breeding-induced endometritis before breeding. Some mares have free fluid present in the uterine lumen before breeding, but most mares are not diagnosed until after they have been bred. If susceptibility to persistent breeding-induced endometritis is suspected, the mare should be monitored closely by ultrasonography per rectum at 6 to 12 hours after breeding, if possible, and at a minimum within 24 hours after breeding. If free fluid is present in the uterine lumen, the mare should be considered to have persistent mating-induced endometritis. Clearance of charcoal particles from the uterus within 48 hours of inoculation and the use of scintigraphy to measure uterine clearance have been suggested to be useful in identification of mares that are susceptible to persistent breeding-induced endometritis.143,164 However, these methods may not be practical under field conditions.
MICROBIOLOGY
Quantitative aerobic bacterial culture of the uterine lumen is necessary to identify potential pathogens and for antibiotic sensitivity testing. Samples should be taken during estrus, and the swab plated immediately on a solid medium or transported in a nonnutritive medium to the laboratory. Inadvertent contamination of cultures with bacteria from the lower reproductive tract is common, so the culture instrument should be guarded until it is within the uterus.165 A false-positive bacterial sample result may be obtained as the result of contamination (even when double-guarded swabs are used), and culture results should always be interpreted together with results from endometrial cytology. Culture alone is not diagnostic. False-negative swab sample results are frequently obtained even under optimal circumstances, and laboratory results should always be interpreted in light of clinical findings. Use of culture and histologic interpretation of an endometrial biopsy appears to be the most accurate method to diagnose persistent infectious endometritis.166 Cultures may also be performed on endometrial biopsy samples. Culture samples for T. equigenitalis, should be taken from the endometrium, cervix, clitoral fossa, and sinuses. Samples should be placed in Amies medium with charcoal or Steward’s medium and be kept refrigerated until delivered to the laboratory.
ENDOMETRIAL CYTOLOGY
PMNs migrate into the uterine lumen in response to inflammation, so endometritis is rapidly and accurately diagnosed by examination of exfoliated endometrial cells. A sample may be taken with a guarded swab, a cytology brush, or by infusion and aspiration of a small amount of fluid. Air-dried smears are stained with new methylene blue or modified Wright-Giemsa stain. Epithelial cells may be shed singly or in rafts. Arbitrary definitions of endometritis have been established on the basis of relative numbers of PMNs. Using these criteria, more than one PMN per 10 epithelial cells is consistent with endometritis. Endometrial cytology from normal mares may contain PMNs and spermatozoa for several days after breeding. It has been suggested that eosinophils are associated with fungal endometritis and pneumovagina. Urine crystals indicate urovagina.
ENDOMETRIAL BIOPSY
Endometrial biopsy is an accurate diagnostic and prognostic tool for endometritis. The biopsy sample should be taken during the breeding season, should be of adequate size, and should be fixed in Bouin’s solution for 24 hours and then transferred to 10% formalin. Chronic endometritis is characterized by infiltration of the endometrium with mononuclear cells and deposition of layers of fibrosis around endometrial glands. Fibrosis of the endometrium is a degenerative change and is permanent. The severity of endometrial changes is inversely related to reproductive performance. A system to classify histologic changes has been described and is widely used5 (Table 43-5). Special stains such as periodic acid—Schiff and Gomori’s methenamine silver may be used to identify the presence of fungi in endometrial biopsies.
Table 43-5 Endometrial Biopsy Grade and Fertility Prognosis (Kenney and Doig)
| Biopsy Category | Degree of Change | Predicted Foaling Rate (%) |
|---|---|---|
| I | None | 80–90 |
| IIA | Mild | 50–80 |
| IB | Moderate | 10–50 |
| III | Severe | <10 |
TRANSRECTAL ULTRASONOGRAPHY
The presence of free intraluminal fluid before breeding strongly suggests susceptibility to persistent endometritis.167 Ultrasonographic examination of the uterus is helpful to assess both the quantity and quality of accumulated fluid in the uterine lumen. Normal mares may retain fluid up to 6 to 12 hours after mating. If fluid is present at 12 hours or more after breeding, the mare should be considered to have a persistent mating-induced endometritis.168 Increased echogenicity of the fluid is associated with the presence of inflammatory cells and debris.
Treatment and Prognosis
SEXUALLY TRANSMITTED DISEASES
Mares with CEM should be treated with intrauterine infusions of antibiotics based on sensitivity tests, in combination with local treatments of the clitoral fossa and sinuses. Best results can be expected when treatment is initiated when the mare is in estrus and is combined with uterine lavage if inflammatory debris or intraluminal fluid is present. Cleansing of the vulva and the clitoris daily for 5 days with a 4% chlorhexidine or nitrofurazone ointment has been recommended.169 Sinusectomy can also be performed.170 Import regulations in countries free from CEM serve to prevent outbreaks of the disease. The spread of CEM on farms in endemic countries is best prevented by implementation of strict hygiene, screening of breeding stallions before the breeding season, and the use of AI, if allowed by the breed registry.
PERSISTENT UTERINE INFECTION
Treatment of mares with persistent uterine infections needs to be directed toward the underlying breakdown of the uterine defense and against the microbial agent. The first therapeutic concern should be to remove predisposing causes, such as a breakdown of external genital barriers. Persistent uterine infection frequently follows degenerative or traumatic anatomic changes and loss of integrity of the barriers of ascending infection. Therefore Caslick’s surgery, repair of cervical damage and perineal lacerations, and correction of urovagina should precede specific endometrial treatment. All potential sources of contamination including intrauterine passage of diagnostic and treatment implements should be minimized. In some mares, recovery follows with sexual rest and no further treatment. Mares that are susceptible to persistent uterine infections should be bred using minimal contamination techniques to avoid bacterial contamination of the uterus.171 Antibiotics may be administered by either local or systemic routes. Intraluminal fluid and inflammatory debris should be removed by uterine lavage before local treatment. Drugs and doses are summarized in Table 43-6. Treatment should be based on sensitivity. Mares should be treated during estrus when natural defense is maximal, and strict aseptic technique should be used. The volume of fluid used for antibiotic therapy is dependent on the size of the uterus. A total volume of 30 to 60 mL is usually sufficient. Treatment should continue daily for 4 to 6 days during the duration of estrus. Bacterial resistance may follow inadequate dosage, and follow-up cultures should be performed. Repeated contamination may indicate an unsuccessfully resolved predisposing cause. Removal of the primary microorganism may result in overgrowth of a second bacteria or fungus (superinfection). Critical studies of the efficacy of systemic antibiotics are limited, although effective levels are produced in the endometrium after systemic administration.172 Parenteral administration may be easier, and the opportunity to introduce uterine contamination or cause uterine irritation with treatment is eliminated. Treatment of fungal infections is generally more challenging than treatment of bacterial infections. Culture and sensitivity will determine the choice of antifungal drugs (Table 43-6). Fungal endometritis may require daily intrauterine infusions for 7 to 10 days to effectively resolve the infection. In order to avoid intrauterine infusions in the presence of high circulating concentrations of progesterone, treatment can be initiated 1 or 2 days after an injection of PGF2α in diestrual mares and continues until 1 or 2 days after ovulation. A single dose of a benzyoylphenyl urea (lufenuron) has recently been suggested to effectively treat mares with fungal endometritis.173 Initial data from four mares were encouraging and need to be confirmed by controlled studies using larger groups of mares.
Table 43-6 Antibacterial Drugs Used for Intrauterine Administration for Treatment of Uterine Infections in Mares
| Drug | Dose (Intrauterine Administration) | Comments |
|---|---|---|
| Amikacin sulfate | 2 g | Gram-negative spectrum; buffer with equal volume of 7.5% bicarbonate |
| Ampicillin | 3 g | Gram-negative spectrum; may irritate the endometrium |
| Carbenicillin | 2–6 g | Gram-negative spectrum; may irritate the endometrium; buffer with equal volume of 7.5% bicarbonate |
| Gentamicin sulfate | 1–3 g | Gram-negative spectrum; buffer with equal volume of 7.5% bicarbonate |
| Kanamycin sulfate | 1–3 g | Escherichia coli, spermatocidal |
| Neomycin sulfate | 3–4 g | Useful against sensitive E. coli, |
| Potassium penicillin G | 5 million U | Streptococcus zooepidemicus, |
| Polymyxin B | 1 million U | Pseudomonas, |
| Ticarcillin | 6 g | Broad spectrum |
| Ticarcillin/clavulanic acid | 6 g/200 mg | Broad spectrum |
| Ceftiofur | 1 g | Broad spectrum (S. zooepidemicus), |
| Antimycotics | ||
| Nystatin | 500,000 U | Dissolve in 30 mL 0.9% saline solution; daily for 7 to 10 days |
| Clotrimazole | 500 mg | Suspension or cream; daily for 1 wk |
| Miconazole | 500 mg | Effective against yeast |
| Amphotericin B | 200–250 mg | Daily for 1 wk |
| Vinegar | 2% | 20 mL wine vinegar to 1 L 0.9% saline solution; used as uterine lavage |
| Lufenuron | 540 mg (single dose) | Suspend in 60 mL sterile water |
Treatment with immunostimulatory agents (Propionibacterium acnes), has been reported to improve pregnancy rates in mares with persistent endometritis, but the mechanism is not fully understood.174
PERSISTENT BREEDING-INDUCED ENDOMETRITIS
Management of mares susceptible to persistent breeding-induced endometritis should include limiting uterine exposure to semen and bacteria and assisting the uterus to physically clear contaminants and inflammatory products after breeding.136,173,175 Preexisting uterine infections should be resolved before the mare is bred. Exposure to semen should be limited to a single breeding per cycle, if possible. This can be accomplished by closely monitoring follicular development and hormonal treatment to induce ovulation of mature follicles. Physical clearance can be assisted by the use of uterotonic drugs. Oxytocin or PGF2α treatment 4 to 8 hours after breeding has been shown to aid in uterine clearance, resulting in improved pregnancy rates in susceptible mares.136,167,173,176 Care must be taken with regard to the timing of PGF2α treatment. Recent reports have demonstrated that PGF2α can cause a delay in the formation of a functional CL when administered within 2 days after ovulation.96-100 This was associated with pregnancy failure in two of the reports.97,98 Large-volume uterine lavage 6 to 24 hours after breeding will also effectively assist the uterus in clearing fluid and inflammatory products.175 Because sperm transport to the oviduct is completed within 4 hours after breeding, uterine lavage 6 to 24 hours after breeding will not have a negative effect on fertility.152 Manual dilation of the cervix in mares with poor cervical dilation may help these mares to more effectively clear the uterus of fluid.
The use of corticosteroids in mares with excessive inflammation in response to breeding has been suggested.177 The authors administered acetate 9α-prednisolone (0.1 mg/kg) twice daily during estrus, starting when a follicle >35 mm was detected and ending when ovulation was confirmed. Preliminary results are encouraging, and further research is needed to clarify the mechanism of action for this treatment alternative.
Electroacupuncture has been used clinically to increase uterine contractility in mares with delayed uterine clearance. Anecdotal reports are encouraging, and research is needed to confirm the efficacy of this treatment alternative.
It is important for the clinician to keep in mind that a transient inflammatory response to semen is normal and required for normal fertility. Postbreeding treatments of these mares will most likely not improve fertility but may cause even further contamination and interfere with pregnancy. Only 10% to 15% of all broodmares develop a pathologic persistent form of breeding-induced endometritis.126 Attention should be given to identify and manage these mares appropriately in order to optimize reproductive efficiency.
CHRONIC DEGENERATIVE ENDOMETRITIS (ENDOMETROSIS)
Several treatments have been suggested for degenerative fibrosis of the endometrium, but consistent results have not been reported. Mechanical or chemical irritation of the endometrium has been used, but concerns that trauma to the endometrium may produce more scar tissue than repair have limited the popularity of such methods. Infusion of dimethyl sulfoxide (DMSO) into the uterine lumen has been shown to improve fibrosis in mares with chronic degenerative endometritis178; however, other researchers were not able to repeat these results.179
Prognosis for fertility after endometritis varies with the severity of inflammation and fibrosis and the inciting cause. Prognosis should take into account the age of the mare and the level of reproductive management, in addition to the cause and likely response to treatment.
Metritis
Metritis is classically defined as inflammation of all layers of the uterine wall. Metritis occurs in the first 2 weeks after foaling and commonly follows abortion, dystocia, and RFMs. In mares, metritis is often accompanied by endotoxemia and laminitis. Transluminal adhesions between endometrial folds may follow severe metritis.
Clinical Signs and Diagnosis
Metritis is characterized by uterine accumulation of postpartum secretions, bacteria, and the products of inflammation, with discharge from the cervix and possibly the vulva. Discharge is usually fluid and red-brown and may be fetid. Systemic signs of depression accompanied by neutropenia and leukopenia are apparent with development of endotoxemia. Differential diagnoses include normal lochia and causes of profound depression in the postpartum period as a result of uterine tears and abdominal catastrophes.
Treatment and Prognosis
Treatment is directed toward removing contamination and microorganisms from the uterus while providing systemic treatment for endotoxemia. Broad-spectrum systemic antibiotics, antiinflammatory drugs, and fluid therapy are indicated. Uterine contamination may be removed by gentle intraluminal infusion of warm water or saline and siphoning off of uterine contents. Vigorous lavage should be avoided, particularly during acute systemic disease. Prognosis depends on severity of clinical signs. If metritis is diagnosed quickly and treatment is instituted, prognosis for fertility and systemic health is good. Prognosis is guarded once endotoxemia and laminitis develop.
Pyometra
Pyometra in mares is an accumulation of purulent exudate in the lumen of the uterus. Impedance to mechanical uterine outflow, such as cervical fibrosis and adhesions of cranial parts of the tract ventrally into the abdomen, may contribute to the development of pyometra. If endometrial irritation causes release of endogenous endometrial PGF, diestrus will be shortened. In some mares, endometrial destruction is so severe that PGF release is inadequate and luteal life is prolonged.92 A variety of bacteria may be involved, including E. coli, Pseudomonas, species, and Streptococcus, species. Cultures may be negative.
Clinical Signs and Diagnosis
A purulent vaginal or cervical discharge may be seen. The mare may demonstrate a short diestrus, a normal interestrous interval, or a prolonged diestrus. Occasional mares with pyometra have mild leukopenia and normocytic-normochromic anemia, secondary to mild suppression of erythropoiesis.92 Transrectal palpation and ultrasonography reveal a fluid-filled uterus. The uterine wall may be thin and flaccid or thick.
Treatment and Prognosis
Treatment should involve correction of predisposing causes, fluid evacuation, and local antibiotic treatment. Evacuation of large amounts of fluid from the uterus may result in redistribution of fluid and circulatory shock. The mare should be monitored for signs of circulatory shock, and intravenous fluid may be administered during evacuation of large amounts of fluid from the uterus. The prognosis for life is excellent; however, the prognosis for return to normal fertility is guarded to poor because the conditions that predispose to development of pyometra in mares (cervical stenosis and adhesions) are difficult to treat and because severe endometrial destruction may develop. Endometrial biopsy should precede vigorous treatment. Hysterectomy should be considered if treatment is unsuccessful and if discharge is unacceptable or if adhesions impair athletic ability.
RUMINANTS
Parturition
Deliveries complicated by dystocia or RFMs may be followed by severe bacterial infections of the uterus. The most sanitary environment possible should be provided for calving. The use of a clean pasture may be most appropriate on some farms, whereas the use of roomy, well-bedded, indoor maternity pens that are cleaned after each delivery may be appropriate on others.
Cows with abnormalities around the time of calving such as hypocalcemia, dystocia, and RFMs are more likely to develop uterine infections than are cows that calve normally. Routine treatment of cows with antibacterial drugs and chemicals has not been shown to be beneficial and in some cases has reduced fertility. Postpartum uterine infections may be prevented, or the number of such infections reduced, by strict attention to sanitation in the calving environment and during assistance with delivery, along with proper management during the dry period.180
Bovine Uterine Infection
Bovine uteri are normally contaminated by a wide variety of microorganisms during the puerperium. Most of the organisms are transient residents of the reproductive tract and are soon eliminated from the involuting uteri of normal cows. Arcanobacter (Actinomyces) pyogenes, can persist in the uteri of cows and act with Fusobacterium necrophorum, and Bacteroides, species to cause uterine infections. Coliforms, P. aeruginosa, hemolytic streptococci, and gram-positive and gram-negative anaerobic bacteria are also frequently isolated from animals with postpartum uterine disease. A. pyogenes, and Clostridium, species occasionally colonize the postpartum uterus synergistically, causing severe gangrenous metritis. Other organisms that appear to have little effect on fertility may colonize the uterus and produce penicillinase, thus influencing the selection and route of administration of drugs used to treat uterine infections.121
Clinical Signs and Diagnosis
Lochia is normally expelled during the first 2 weeks after calving and may range from dark red or brown to white to clear. If uterine involution is delayed, discharge of lochia may continue until 30 days after calving. Discharge of lochia is not abnormal unless the fluid is fetid or the cow develops other abnormal clinical signs. Abnormalities of uterine involution cannot be diagnosed by palpation per rectum during the first several days after calving when both normal and abnormal uteri are out of reach and cannot be safely retracted. By 10 to 15 days after calving the entire uterus can be palpated if involution is normal. Fluid should not be palpable within the uterine lumen by 14 to 18 days after calving. Gross reduction in size and histologic repair of the endometrium are complete in dairy cows by 40 to 50 days after calving.
Postpartum metritis in cows is characterized by the presence of variable amounts of lochia within the uterine lumen that may be discerned by palpation per rectum. A vaginal discharge is usually present, but it may become obvious during palpation. Septic metritis is characterized by clinical signs of toxemia that may include fever, depression, partial or complete anorexia, and laminitis. Milk yield is depressed, and cows may be unwilling or unable to rise. Some cases may be complicated by tenesmus. Vaginitis and cervicitis may accompany metritis. Discharges associated with septic metritis vary from scanty white mucus to copious amounts of red to red-black, watery, malodorous fluid. In some cases inflammation may spread through the uterine wall and cause perimetritis and peritonitis.181 Septic metritis in ewes and does is characterized by fever, depression, anorexia, and tenesmus.
Endometritis in cattle is usually observed between 2 and 8 weeks after calving. Discharge can range from white pus to estrual mucus. Purulent exudate may be observed only with palpation or may be found in the cranial vagina and cervical canal on examination with a speculum. The history may indicate that the cow has failed to conceive after several services but the patient is otherwise healthy.
Culture of endometrial fluid is not usually done in individual cases of bovine uterine infection but may be indicated to determine the antibiotic susceptibility of microorganisms on a particular farm or as a part of the diagnostic plan when the incidence of postpartum metritis or endometritis increases suddenly.
Endometrial biopsies are rarely used in cows but have been recommended when complete evaluation is required of the reproductive tract of cows that do not conceive or that conceive but do not complete their pregnancies.5
Treatment and Prognosis
To be useful in treating uterine infections in cows, an antibiotic must be active against the primary uterine pathogens (A. pyogenes, and gram-negative anaerobes), in the presence of organic debris, and in the anaerobic environment of the postpartum bovine uterus.182 Organisms infecting the uteri of cows are usually susceptible to penicillin, but during the first month after calving, contaminating microorganisms may produce penicillinase. Therefore penicillin is not likely to be effective if given locally during the early postpartum period. By 30 days after calving, organisms that produce penicillinase are usually eliminated from the uterus, and intrauterine treatment with penicillin may be beneficial. The daily intrauterine dose of penicillin required to reach the minimum inhibitory concentration of common bacteria such as A. pyogenes, is 1 × 106 IU.182 Oxytetracycline is active against many of the microorganisms that infect the bovine uterus, and its activity is only slightly reduced by organic debris and absence of oxygen. Intrauterine treatment with administration of 4 to 6 g of oxytetracycline per day has been recommended. Some preparations of oxytetracycline irritate the endometrium, cervix, and vagina. Intrauterine antibiotic treatment of dairy cows results in residues in their milk.183 For example, oxytetracycline has been found in milk from 44184 to 96185 hours after intrauterine administration.
Penicillin by systemic administration is effective for treatment of some uterine infections in cows. Daily doses of penicillin required to reach the minimum inhibitory concentration of A. pyogenes, are 10,000 to 20,000 IU/kg/day. Minimum inhibitory concentration of oxytetracycline for A. pyogenes, in the uterus is usually higher than the concentration that can be achieved by systemic administration of the drug.
Treatment of septic metritis should be directed toward controlling septicemia. Large doses of broad-spectrum systemic antibiotics are indicated, along with fluids and other supportive therapy. Attempts to remove RFMs or irrigate the uterus are contraindicated during the acute phase of the disease. After the patient has recovered from acute septicemia, intrauterine therapy may be considered.
ANTISEPTIC CHEMICALS
A variety of antiseptic chemicals have been infused into the uterine lumen of cows in attempts to treat metritis and endometritis, but few controlled trial evaluations are available. These are attractive as a way to avoid antibiotic residues in the milk intrauterine infusion.186 Dilute solutions of povidone-iodine (one part povidone-iodine stock solution to 10 to 20 parts saline) have been suggested as being useful in treating fungal endometritis.44 Povidone-iodine is generally available as a 10% solution with 1% free iodine (10,000 ppm of free iodine), so that a dilution of 20:1 saline:povidone-iodine yields a flush with 500 ppm of free iodine, which should be bactericidal.
UTERINE LAVAGE
Lavage of the uterine lumen with large volumes of warm saline (40° to 45° C [104° to 118° F]) removes accumulated fluid and debris. Uterine lavage has been used as an adjunct to antibiotic, antiseptic, and plasma treatment. Catheters designed for nonsurgical embryo recovery are suitable for uterine lavage. Saline is infused into the endometrial cavity in 0.5- to 1-L increments, allowed to reflux through the catheter, and collected for inspection. A milk hose and larger fluid volumes may be used in cattle with larger postpartum uteri, but care must be taken not to enter far into the uterus because it is friable and easily perforated. Massage or partial retraction of the uterus by palpation per rectum may be necessary to increase fluid recovery. The uterine lumen is lavaged repeatedly until the fluid returning through the catheter is no longer turbid.
PROSTAGLANDINS
In cows, repeated administration of PGF results in shortened estrous cycles and may mimic the shortened luteal phase of patients with acute endometritis. PGF therapy may be sufficient in mild cases of endometritis or may be used in combination with intrauterine or systemic therapy. In cases of chronic bovine endometritis, treatment with PGF one or two times at 10- to 14-day intervals decreased the number of days open.187
The prognosis in cows for recovery from endometritis is usually good if the condition does not progress to a more severe form of uterine disease. Septic metritis after dystocia or RFMs may result in permanent impairment of reproductive function, laminitis, or death of the patient in spite of aggressive therapy.
Pyometra
In dairy cows, pyometra is likely to develop in cows that ovulate before microorganisms that infect the uterus during the postpartum period are eliminated. The CL that develops after the first postpartum ovulation at approximately 15 to 18 days after calving persists, possibly because the abnormal uterine contents suspend release of PGF from the endometrium or sequester it within the uterine lumen. The uterus is brought under the influence of progesterone, which depresses phagocytic activity of uterine neutrophils and closes the cervix, allowing the bacterial infection to persist.188 Pyometra rarely endangers the general health or life of affected cows. Postcoital pyometra may be caused by T. foetus.,189
PGF is the treatment of choice for bovine pyometra. Treatment with PGF is followed in 3 to 6 days by uterine evacuation in 85% to 90% of treated cows. Response to PGF treatment may be raised with a second injection of PGF in 6 to 12 hours. After endometrial lesions are allowed to heal for 30 days, fertility is restored in most patients.
Treatment of cows with GnRH 2 weeks after calving improves fertility in some but not all situations. In herds with a high prevalence of postpartum uterine infections, treatment with GnRH may decrease fertility by inducing ovulation and CL development; thus the uterus is brought under the influence of progesterone before contaminating bacteria are removed, leading to pyometra.
Perimetritis
Perimetritis may occur in all species as a sequela to severe uterine infections, uterine rupture, penetration of the vagina during mating, traumatic insemination or obstetric procedures, and cesarean section.102,190 Perimetritis is characterized by inflammation of the peritoneal surface of the uterus and may be accompanied by localized or diffuse peritonitis. Adhesions then develop between the uterus and other pelvic and abdominal organs.
Clinical Signs and Diagnosis
The clinical signs of perimetritis are those of peritonitis and may include fever, depression, partial or complete anorexia, stasis of the gastrointestinal tract, and evidence of abdominal pain. Abdominal pain is typified by colic in mares and by grinding the teeth (odontoprisis) in cows. In cows the condition should be differentiated from traumatic reticuloperitonitis, displacements of parts of the digestive tract, abomasal ulcers, postpartum metritis, and abdominal fat necrosis. Perimetritis in mares must be differentiated from other causes of severe abdominal pain. Antemortem diagnosis of perimetritis is difficult in sheep and goats that are presented mainly with fever, depression, anorexia, and odontoprisis.
Clinical Pathology
Cases of acute perimetritis are accompanied by leukopenia, neutropenia, and a degenerative left shift. Further evidence of peritonitis is obtained when peritoneal fluid is obtained by paracentesis and examined for its cellular and microbiologic content.
Treatment and Prognosis
The cause of perimetritis should be treated if possible. Cases of severe metritis should be treated appropriately and uterine ruptures sutured if possible. Repair of uterine ruptures inaccessible by flank incisions may be facilitated by intentional prolapse of the uterus after administration of epinephrine, provided the tear is not too close to the cervix (see next section). Treatment with broad-spectrum systemic antibiotics is indicated. Lavage to remove peritoneal exudate has been recommended but is difficult to accomplish, especially in cows in which the rumen, abomasum, and greater omentum make ventral drainage almost impossible and in which fibrinous peritonitis with loculation of infection occurs rapidly. Other supportive treatments such as intravenous fluids and antiinflammatory drugs should be administered as indicated.
The prognosis depends on the severity of lesions. Fatalities can occur in spite of prompt treatment, and surviving animals may be infertile because of mechanical interference with gamete transport caused by adhesions between the genital organs and other pelvic and abdominal tissues. In general, the prognosis for fertility in affected animals is fair at best.
Prevention and Control
Perimetritis occurs sporadically in individual animals; therefore prevention depends on avoiding the causes. Immature females, especially heifers and fillies, should not be allowed at pasture with adult males, to prevent undesired mating complicated by penetration of the vagina. Traumatic obstetric, insemination, and uterine lavage procedures must be avoided. Uterine tears that occur at parturition must be sutured immediately. Postpartum metritis must be treated promptly and appropriately before it progresses to perimetritis.
SMALL RUMINANTS
Uterine infections may follow dystocia and RFMs in sheep and goats but are not frequently a cause of infertility because lambing and kidding are followed by a period of up to 6 months of sexual rest before the next breeding season. RFMs and metritis follow abortion in ewes caused by Listeria monocytogenes, C. fetus, subsp. fetus, and Chlamydia psittaci.,
Ewes and does affected with metritis are usually treated with systemic antibiotics such as penicillin or sulfamethazine. Early and aggressive treatment is indicated.190
CAMELIDS
Metritis and Endometritis
Camelids are induced ovulators, and females are usually receptive to males unless they are pregnant. Females do not, however, always have a preovulatory follicle present. Therefore it is frequently the case that females are bred at a time when they do not have a fertile follicle present. When camelids breed, the penis is inserted through the cervix and deep into the uterine horns. Unnecessary matings, or overbreeding, is the most important factor causing damage and contamination to the uterus.191 Other major contributing factors include RFMs, rectal vaginal tears, and unsanitary obstetric manipulations.191
Chronic endometritis will often not cause evident clinical signs, whereas acute, postpartum endometritis may cause fever, depression, and signs of toxic shock.191 A thick, mucoid lochial discharge is normal in the postpartum female for up to a week postpartum. Thin, watery, fetid discharge is a sign of endometritis.191
Transrectal ultrasonography and vaginoscopy are helpful in diagnosing endometritis and metritis. Inflamed, thickened uterine walls and hyperechoic, intraluminal fluid may be present on ultrasound examination. Vaginoscopy may reveal cervical discharge. Transrectal ultrasonography may usually be performed in llamas as in horses or cattle. In alpacas, because of smaller size, an extension probe will be necessary to facilitate transrectal ultrasonographic evaluation.
Uterine culture and cytology samples from the llama may be obtained in methods similar to those in the mare and cow. A double-guarded swab prevents environmental contamination. In the alpaca the swab should be passed through the cervix via visualization using a vaginoscope. These diagnostics should be performed during the peak follicular phase to ensure ease of passage through the open cervix and more reliable test results.191 The most common bacteria isolated from the uteri of camelids with endometritis are E. coli, S. zooepidemicus, β-hemolytic streptococci, Enterococcus, coagulase-negative Staphylococcus, Proteus, species, Enterobacter aerogenes, K. pneumoniae, and A. pyogenes,.191
Uterine biopsy can be a very useful diagnostic tool for evaluation of metritis and endometritis in camelids. Endometrial biopsy samples in llamas may be obtained as in the mare. The left horn is perhaps better to target unless a particular pathology is suspected in the right horn, because camelid pregnancies almost always occur in the left horn.191 Pathologic changes, evaluation, and prognosis are all assumed to be similar to those in the mare.191
Treatment for endometritis and metritis is also similar to that described for the mare. Uterine lavage with a warm, isotonic saline solution and oxytocin injection (5 to 10 IU) are the major components of treatment. Intrauterine antibiotic infusion is done after uterine lavage. The most common antibiotics used are penicillin K (1.5 × 106 U), gentamicin sulfate (200 to 300 mg), and ceftiofur sodium (250 to 500 mg).191 Antibiotics should be diluted in sterile water or saline (saline should not be used with ceftiofur) and given once daily for 5 to 7 days. Females should be evaluated after cessation of treatment and completion of 2 weeks of sexual rest.
Prevention of endometritis often requires a “minimum contamination breeding technique.”191 This entails monitoring follicular growth via transrectal ultrasound until the follicle is of preovulatory size, breeding only once, following breeding with an injection of hCG (750 IU) or GnRH, and administering an intrauterine infusion of antibiotics 24 hours after breeding. Females should be evaluated for pregnancy 12 to 14 days after breeding.
Further preventative measures include performing prebreeding examinations on all maiden animals to avoid breeding animals that are too young or do not have follicular activity; breeding only females exhibiting strong receptive behavior (as opposed to mere submissive behavior); performing complete gynecologic examinations of all females with a history of infertility, obstetric problems, or postpartum complications; and observing strict rules of hygiene during breeding and obstetric manipulations.191
Pyometra
Pyometra caused by delayed uterine involution may be observed shortly after parturition. Chronic pyometra is often associated with vaginal or cervical adhesions that may be the sequelae to trauma after dystocia or aggressive obstetric manipulation.191
ANATOMIC DEFECTS AS A CAUSE OF UTERINE INFECTION
The most common anatomic defect associated with genital infections is pneumovagina. Other defects include urovagina and perineal lacerations. These defects should be corrected to prevent contamination with environmental and fecal organisms.
ENDOMETRIAL CYSTS AND LACUNAE
MARES
Endometrial cysts and lymphatic lacunae are common degenerative changes of the endometrium that are more prevalent in mares older than 11 years of age than in younger mares.192 Endometrial cysts can originate from endometrial glands or obstructed lymphatics. Glandular cysts are small (<10 mm) and are believed to be the result of periglandular fibrosis. Lymphatic cysts can reach several centimeters in diameter. The cause of lymphatic cysts is not fully understood, but lacunae are thought to arise after interference with normal lymph drainage from the genital tract.5
Clinical Signs and Diagnosis
Endometrial cysts can be visualized by ultrasonography or by hysteroscopy. Cysts may mimic pregnancy when mares are examined per rectum by palpation or ultrasonography.193 Sequential examination reveals that size of endometrial cysts remains static, whereas amniotic vesicles enlarge. Large, discrete, fluid-filled cysts may be identified by palpation of the uterus per rectum. Smaller cysts and lacunae may be observed in endometrial biopsy sections. Uteri affected by lymphatic lacunae are enlarged and have a thicker wall than normal.
Treatment and Prognosis
Endometrial cysts do not require treatment unless they are suspected to interfere with pregnancy. Endometrial cysts have been suggested to cause embryonic death and abortion if large or numerous. However, one study failed to find an association between the presence or number of cysts and fertility.192 Obliteration of endometrial cysts using endoscopic guided laser surgery removes the cysts permanently, but the long-term effect on fertility has not been critically evaluated. Needle aspiration, mechanical rupture of the cyst, uterine curettage, or intrauterine infusion of hypertonic saline solution have all been suggested to effectively remove endometrial cysts. However, the cysts often recur after treatment.
UTERINE PROLAPSE
Uterine prolapse occurs when the previously gravid uterine horn becomes invaginated after delivery of the fetus(es) and protrudes from the vulva.
MARES
Uterine prolapse is an uncommon sequela to normal foaling, dystocia, or RFMs in the mare. The tip of the previously gravid horn invaginates to form uterine eversion. Eversion is accompanied by pain and abdominal straining. The myometrium may contract around the ring of invaginated tissue. Transrectal palpation confirms the diagnosis, and the everted tissue may be replaced manually. Eversion may progress to complete uterine prolapse, accompanied by rapid onset of systemic signs.
Treatment and Prognosis
The prolapsed uterus should be washed with clean saline and replaced manually in the standing mare as rapidly as possible. Replacement is aided by sedating the mare and administering epidural anesthesia. The uterus should be supported on a clean sheet held at the level of the pelvis. The uterus should be replaced, beginning with the uterine body and working gradually, replacing the tip of the horns last. Correct positioning of the uterus is important to prevent the prolapse from recurring. Replacement should be followed by treatment with broad-spectrum antibiotics, antiinflammatory drugs, and intravenous isotonic fluids. Treatment with oxytocin (10 to 20 IU IM) facilitates uterine involution. Prognosis is related to development of sequelae such as uterine tears, metritis, and endometrial damage.194
RUMINANTS
In cows most cases of uterine prolapse occur within a few hours after calving. The condition is invariably associated with hypocalcemia, which results in lack of uterine tone and delayed cervical involution. In addition, dystocia frequently precedes uterine prolapse.
Elective uterine prolapse can be induced within 6 to 12 hours after calving by administration of epinephrine to relax the uterus for the repair of uterine tears. A 10-mL amount of epinephrine (1:1000) is diluted to 250 mL in sterile saline and administered slowly IV. After 100 mL of the solution has been given, the operator reaches through the cervix, grasps the uterine wall and caruncles, and everts the uterine horn toward the cervix. When sufficient uterine tissue has entered the cervical canal, the patient responds by straining, which assists in completion of the prolapse. Epidural anesthesia is administered to abolish further straining after, the prolapse is complete.
Uterine prolapse in does has been associated with dystocia, hypocalcemia, and lack of exercise.5 The predisposing factors are probably similar for ewes.
Clinical Signs and Diagnosis
Clinical signs of uterine prolapse are obvious. The membranes may remain attached. Immediately after prolapse occurs the tissues are nearly normal, but within a few hours they become enlarged and edematous. The endometrium is usually contaminated with feces and bedding material. In some cases the prolapsed tissue may be lacerated or severely traumatized and may contain loops of intestines.
Clinical signs that may accompany uterine prolapse include straining, abdominal pain, restlessness, anorexia, and increased pulse and respiratory rates.102 Parturient paresis is common in affected dairy cows. In most patients these signs are transitory, but shock may complicate some cases.
Clinical Pathology
Uterine prolapse in cows is frequently accompanied by hypocalcemia and a significant increase in the packed cell volume.195
Treatment and Prognosis
The prolapsed tissue should be protected from further damage by wrapping it in wet towels or covering it with a plastic bag. Beef cows should be restrained where they are found, to prevent trauma to the uterus or rupture of the large uterine vessels should an animal try to escape on arrival of the clinician. Treatment of hypocalcemia is usually indicated before replacement of the uterus if the cow is recumbent and semicomatose; otherwise calcium gluconate is administered after replacement. Epidural anesthesia is frequently (but not always) required.
The prolapsed tissue is washed with a mild presurgical scrub. The membranes are removed if they can be easily separated from the endometrium but are left in place if removal is difficult. Some clinicians recommend that cows stand during replacement, whereas others have found that the organ can be replaced in recumbent cows if the patient is placed on her sternum with the hind legs drawn straight out behind. The prolapse is placed between the extended limbs.196 In fresh cases, replacement is relatively easy and is begun at the cervical pole of the organ; the dorsal and ventral parts are massaged alternately back into their normal position. After the ovarian pole has been replaced, the previously prolapsed horn must be straightened, and eversion of the uterus corrected. Administration of clenbuterol is reported to relax the uterus, facilitate replacement, and reduce the need for epidural anesthesia, but its use is illegal in the United States.197 If the patient has been neglected, accumulated fluid must be reduced by lubricating the tissue with an emollient ointment then carefully but vigorously massaging the tissue from the ovarian pole toward the cervical pole. The hygroscopic action of sugar when applied liberally to prolapsed uteri is of limited value and vastly overrated.
Oxytocin is frequently administered to stimulate myometrial contractions after the uterus has been replaced. Metritis is a frequent sequela, and appropriate antibiotic treatment is indicated in most cases. Temporary closure of the vulva with heavy sutures after replacement is not necessary102 but is practiced by many clinicians.5 If replacement of the prolapsed uterus is impossible or the tissue is severely traumatized, amputation may be indicated; in this case it is important that the uterine arteries be double ligated.198
The prognosis varies but is generally favorable if there has been no serious damage to the uterus.5,196 Fatalities can occur in cases complicated by shock or by rupture of large uterine vessels. The culling rate from infertility of cows with uterine prolapse is higher than that of their herdmates, and the calving interval is prolonged in affected cows. Barring hypocalcemia, the risk of uterine prolapse at a subsequent calving is no greater than for other cows in the herd.
Prevention and Control
Because the condition is associated with hypocalcemia in cows, provision of a properly balanced ration before calving is indicated. Although uterine prolapse can occur after an apparently normal delivery, it is more commonly associated with dystocia and forced extraction; therefore prolapse should be anticipated, and the dam observed so affected patients may receive prompt treatment.
CAMELIDS
Uterine prolapse in camelids usually is a consequence of dystocia, RFMs, or excessive obstetric manipulation. Treatment is the same as described for other species and includes gentle, clean manipulation of the prolapsed uterus, replacing the organ starting at the cervix. Replacement is easier with early cases. Difficulty increases and prognosis decreases greatly with chronicity.9
UTERINE TUMORS
Neoplasia uncommonly affects the uterus of domestic animals. Tumors may arise from within uterine tissues or metastasize from other organs.11 Leiomyomas are usually benign and arise from the outer smooth muscle of the uterus without need for a preparatory event. The multicentric form of lymphosarcoma may affect the uterus of cattle. Lymphosarcoma also affects does, but a predilection for the uterus is not apparent. Carcinomas, chorionepitheliomas, fibromas, fibrosarcomas, rhabdomyosarcomas, and adenosarcomas are rarely reported.
Small tumors may escape detection, whereas larger ones may be palpable per rectum in mares and cows. Leiomyomas are not necessarily associated with reproductive failure, and tumors and fetuses can coexist. Uterine walls affected by lymphosarcoma may contain discrete neoplastic nodules or be diffusely infiltrated. Tumor masses must be differentiated from normal fetuses, mummified or macerated fetuses, placentomes, abscesses, and fat necrosis.
Solitary leiomyomas thought to interfere with fertility may be removed. Other forms of uterine neoplasia are usually not treated. The prognosis is generally poor.