Neonatal Care of the Puppy, Kitten, and Foal
When you have completed this chapter, you will be able to:
1 Describe special requirements for examination of neonates.
2 List normal physiologic and behavioral parameters of neonatal puppies.
3 Describe diagnostic sampling techniques used for neonatal puppies.
4 Define hypothermia and describe problems related to hypothermia in neonatal puppies and kittens.
5 Explain how neonatal isoerythrolysis occurs and is managed in kittens.
6 List the symptoms, causes, and treatments of fading puppy or kitten syndrome.
7 Describe considerations for the care of orphan puppies and kittens.
8 Describe the care of the high-risk perinatal mare and list supplies needed to attend a high-risk foaling.
9 Describe the events that occur in each of the stages of labor in the mare.
10 Describe the normal development and routine nursing care, medical care, and diagnostic procedures in the neonatal foal.
NEONATOLOGY OF PUPPIES AND KITTENS
Because of the unusual or nonspecific clinical signs associated with ill puppies and kittens, it is of great importance to obtain a comprehensive history not only of the patient, but also of the littermates, parents, and other relatives. The history should include: the number of ill animals, the method by which they were raised, their normal environment, behavior of each puppy or kitten within the litter, body weight curves, duration and type of clinical signs, and medications given. The queen’s or bitch’s history should include vaccination dates, estrous cycle (intervals and duration), breeding practice, medications or supplements given during pregnancy, and problems during pregnancy or birth. Has the disorder that the patient is experiencing been seen in previous litters or in any of the relatives? In certain cases, clients are advised to bring the whole litter or at least one healthy littermate including the mother so that the patient can be compared with its littermate(s). If the patient or its littermates have not been vaccinated, it may be better to have them come in the back door to prevent exposure to all the infectious diseases that may linger in the waiting room.
PHYSICAL EXAMINATION
The physical examination of the neonate and the juvenile patient can be challenging. Owners and littermates that were brought in for comparison can be quite distracting. Usually, one cannot expect cooperation from the youngest of our patients, especially those that are already aware of their surroundings. They are so distracted by the new environment that something as simple as a menace reflex is difficult to elicit. Whereas everyone knows how to perform a physical examination on an adult, the following two paragraphs focus on examination techniques for the neonate. Some specific examples are given, but keep in mind that the list is not complete. However, some of the developmental landmarks described later may be helpful in determining abnormalities.
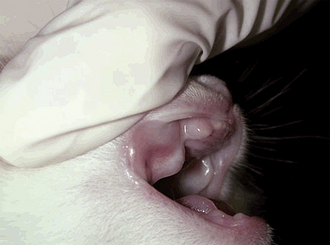
For the physical examination of a neonate, a pediatric stethoscope with a 2-cm bell is helpful. In addition, a digital thermometer allows a rapid measurement of the body temperature, without causing great discomfort. Because the neonate can have a body temperature lower than 94° F, a digital thermometer that measures as low as 85° F is practical. Neonates cannot regulate their body temperature during the first 2 weeks of life. They should be examined on a warm, clean surface rather than the cold metal table. Checking the oral mucous membranes assesses hydration in the neonate because the skin turgor is not developed as it is in adults (Figure 10-1). Moist mucous membranes are present in an adequate state of hydration. The neonate is born with hair that covers most of the body except the ventral abdominal skin. A lack of hair or a sparse hair coat may indicate either a genetic abnormality of the skin or premature birth (Figure 10-2). The neonate normally has nonhaired, dark-pink, ventral abdominal skin. Bluish or dark red discolorations are indicative of a neonate in distress (cyanosis or sepsis, respectively). Other than urine and feces, a discharge from any orifice is abnormal in the neonate. The neonate’s head, body, limbs, and tail are examined for symmetry and normal conformation. The head is specifically examined for open fontanelles, cleft palates, bulging eyes from behind closed eyelids (an infection behind closed eyelids), and formation of the nose and external ears. The presence of flattening or malformations of the chest are noted (e.g., swimmer syndrome, pectus excavatum) as are bulges in the neck area (e.g., gas in the esophagus, ectopic heart, goiter). Neonatal puppies are mildly pudgy, and neonatal kittens are generally on the lean side. Neither of them should ever be bloated, which would be a sign of distress. The abdomen and urachus are especially examined for defects of the abdominal wall and ventral urine scalding, such as cannibalism as a result of an overzealous mother, ventral closure defects, and a persistent urachus. The genitals and the anus are checked for patency by stimulating urination and defecation using a moistened cotton ball. The presence of hair coat abnormalities over the dorsum may indicate the presence of a spina bifida. The tail is examined for muscle tone, length, curliness, and kinks. Abnormalities in tone may be indicators for associated defects or problems, such as abnormal innervation of the distal pelvis.
NORMAL DEVELOPMENT
During the first week of life, newborn kittens and puppies sleep throughout most of the day (80%) and nurse vigorously for a short period of time every 2 to 4 hours. Because the brain is not completely developed at birth, neuromuscular reflexes are missing and the only motor skills present are crawling, suckling, and distress vocalization. The neonates only respond to stimuli such as odor, touch, and pain. The queen or bitch initiates urination and defecation by licking the urogenital area. At 3 days of age, kittens and puppies should be able to lift their head and by 1 week crawl in a coordinated manner. Puppies and kittens are unable to maintain their body temperature during the first few days of life, and the shiver reflex does not develop until after the first week of life. Their body temperature at birth (94.5° F to 97.3° F) is lower than in adults and rises to 94.7° F to 100.1° F during the first week of life. Heart and respiratory rates may be irregular at birth (pulse [P] = 160 to 200 beats/min [bpm], respiration [R] = 10 to 20 breaths/min), and there is no abdominal component to their breathing. During the first week, the neonates begin to adjust to the new, extrauterine environment, and their physiology adjusts (P = 200 to 220 bpm, R = 16 to 35 breaths/min). The umbilical cord dries out during the first day of life and should have fallen off by day 2 to 3. The flexor tone present at birth switches over to extensor tone after the fourth day of life (Box 10-1). Although sex determination in normal newborn puppies is unambiguous, it can be challenging in kittens. The sex of kittens can be determined at birth by evaluating the anogenital distance, which is shorter in females (7.6 ± 1 mm) than in males (12.9 ± 1.5 mm). Male kittens are born with descended testicles, which are able to move freely in and out of the scrotum until 5 to 7 months of age. In dogs, the testicles do not descend until 6 weeks of age.
During the second week of life, kittens and puppies begin to crawl, and their body temperature slowly rises toward normal adult levels. Kittens and puppies will have doubled their birth weight by 7 to 10 and 10 to 12 days, respectively. They begin to open their eyes at 7 to 12 days of age, and the external ear canals open at 14 to 16 days of age. The iris is not well pigmented, has a blue-gray color, and the cornea is slightly cloudy as a result of an increased water content. Kittens may have divergent strabismus. By the end of 3 weeks of age, puppies and kittens are able to stand and have good postural reflexes. Refer to Box 10-2 for details about the development of specific organ systems.
DIAGNOSTICS
Blood can be easily obtained from the jugular vein in neonates. However, no more than 10% of the circulating volume should be drawn over the course of a week. In other words, if a neonate weighs 250 g, no more than 2.5 ml of blood should be drawn in 1 week. If a neonate remains in the hospital for several days, it may be worthwhile posting a chart next to the patient where every nurse can record the amount of blood drawn to prevent causing the patient to become anemic. This is especially helpful in neonates that cannot maintain proper blood glucose levels and need to be subjected to multiple blood draws (one drop of blood equals about 0.1 ml). Because of the small amount of sample drawn at a time, the appropriate-size collection tube should be used to ensure that the ethylenediaminetetraacetic acid (EDTA) does not dilute the sample resulting in false laboratory data.
To obtain urine samples, the neonate can simply be stimulated to urinate by gently rubbing the genital area with a moistened cotton ball. Alternatively the bladder can be carefully expressed. It is rarely necessary to obtain sterile urine samples by cystocentesis, which should be avoided because of the fragility of neonates’ skin and organs.
Imagining techniques include radiography and ultrasonography. An ultrasound examination is best performed using a 7.5-MHz transducer, and neonates generally tolerate this imaging technique better than radiography. Radiography of neonates requires high-detail intensifying screens and single emulsion films. For optimal contrast, no whole body radiographs should be taken, and the kilovoltage should be reduced to half that of an adult because there is little body fat and poor mineralization at this age. If possible, a normal littermate should be radiographed for comparison.
COMMON CONCERNS AND DISORDERS IN THE PUPPY AND KITTEN (BOX 10-3)
When puppies and kittens are born, they have almost no subcutaneous fat and thus little insulation. Initially, body heat is produced by brown fat metabolism, which is under the control of the sympathetic nervous system (nonshivering thermogenesis). Because of their relatively large surface area when compared with older animals, heat loss is much greater in neonates. As long as neonates are close to their dams and the mammary glands, there is not much heat loss, so they can maintain thermal balance. However, as the neonate begins to take up food, its metabolic rate increases, which in turn elevates its body temperature. Shivering and vasoconstrictive mechanisms may begin around 6 to 8 days, but by about 6 weeks, puppies and kittens are good homeotherms and have a body temperature that is similar to that of adults.
Hypothermia in the neonate is a serious problem. Gut motility slows with a decreasing body temperature, ultimately causing an ileus. When hypothermic neonates are tube fed, the milk replacer is either regurgitated and aspirated resulting in pneumonia, or the ingesta may ferment leading to bloat. This causes increased pressure to the thorax, which in turn causes labored breathing. Most neonates in pain or respiratory distress swallow air, which exacerbates a bloated condition. In this way, a downward spiral forms that ultimately results in circulatory collapse and death. Hypothermia also inhibits cellular immune functions, which may lead to an increased susceptibility to infections. A neonate is considered hypothermic if its body temperature drops below 94° F at birth, below 96° F at 1 to 3 days of age, or below 99° F at 1 week of age. Clinical signs in a chilled neonate with a body temperature above 88° F include restlessness, continuous crying, red mucous membranes, and skin that is cool to the touch. However, muscle tone is still good; the respiratory rate is greater than 40 breaths/min and the heart rate greater than 200 bpm. When the body temperatures fall into the range of 78° F to 85° F, the neonate appears lethargic and uncoordinated, but responsive. Moisture is seen around the corners of the lips, the heart rate drops below 50 bpm, and the respiratory rate is between 20 to 25 breaths/min. No abdominal sounds are heard, and metabolism is impaired, resulting in hypoglycemia (see later discussion). Below 70° F, the neonate appears to be dead. If extreme measures of arousal result in a response, treatment may be attempted. Hypoxia also contributes significantly to hypothermia. Therefore the neonate should be provided with proper ventilation or oxygen administration whenever possible.
The treatment consists of slowly (= 2° F/hr) reheating the patient by providing the appropriate ambient temperature and humidity. Heating pads, heat lamps, warm water gloves, rice bags, and incubators can be used, but it is essential that the temperature be controlled and monitored carefully, especially when using heating lamps or heating pads because the neonate cannot escape if the temperature is too warm. Warm air and oxygen in a human neonatal incubator or veterinary oxygen cage is optimal for rewarming the hypothermic neonate. Warm IV fluids can also be given, but at no more than 2° F above body temperature. Do not give anything orally until the patient has audible gut sounds and is moderately rewarmed. Rapid rewarming will result in heat prostration with increased respiratory rate and effort. Eventually the patient will become cyanotic and have diarrhea and seizures. Raising the neonatal body temperature more than 4° F is usually fatal because of delayed organ failure. Thermal burns may also occur if the surrounding temperature is not properly monitored.
Dehydration
Any disease process or imbalance of fluids or electrolytes will quickly lead to dehydration in the neonate because of increased body water, increased water turnover, and immaturity of the renal system. As indicated before, checking the oral mucous membranes assesses hydration in the neonate because the skin turgor is not yet developed as it is in adults. Moist mucous membranes are present in an adequate state of hydration, but tacky to dry mucous membranes indicate 5% to 7% dehydration. At 10% dehydration, the mucous membranes are dry, and there is a noticeable decrease in skin elasticity.
Fluid requirements are high in neonates, but total volumes that can be given are low. All fluids should be warmed to 98° Fto 99° F before administration unless the neonate is substantially colder. In that case, the fluids should be warmed to 2° F more than the current body temperature. Boluses can be given at 3.3 ml per 100 g of the kitten’s weight over 5 to 10 minutes. The maintenance dose is 6 ml/kg/hr. To this, 50% of the deficit is added over 6 hours (deficit = body weight [BW] × %dehydrated). Fluids can be given intravenously (IV), intraosseously (IO), intraperitoneally (IP), or subcutaneously (SQ). It is often easiest to place a short 23- or 25-G catheter in the jugular vein for fluid administration. Another option is IO fluid delivery: the bone is still soft enough that an 18- or 19-G needle can be placed in the proximal tibia or proximal femur, and fluids are given at the same rate as IV fluids. It is important that each bone not be punctured more than once because the fluid will leak out of the other hole. Administering fluids at a constant rate is best accomplished by using either a syringe pump or a pediatric drip (60 drops/min). If IV or IO access is not available, fluids are given IP or SQ. However, absorption rates are slow with both routes, and they are not ideal for long-term fluid therapy. When giving fluids IP or SQ, the volume should be divided in 2 to 3 boluses per day. In many cases, the neonate is acidotic, but because of limited liver functions, the neonate has difficulty in metabolizing lactate into bicarbonate. In most cases, lactated Ringer’s with 20 mmol/L of maintenance potassium is sufficient.
Hypoglycemia
The risk of hypoglycemia is great because the neonate is born with little glycogen stores and has poor gluconeogenesis in the liver. As long as the neonate is healthy, it can maintain normal blood glucose concentrations for up to 24 hours without nursing. However, the failure to suckle will result in hypoglycemia after 24 to 36 hours as a result of the depletion of hepatic stores. A variety of clinical signs may occur in the hypoglycemic (serum glucose <30 mg/dl) neonatal patient, including tremors, crying, irritability, increased appetite, dullness, lethargy, coma, stupor, and seizures.
The treatment consists of giving dextrose slowly IV or IO at 0.5 to 1 g/kg as part of a 5% to 10% dextrose solution in normal saline. Care should be taken giving 5% dextrose mixed with lactated Ringer’s solution because the mixture will become hypertonic and volume replacement will have to be monitored carefully. Higher concentrations of IV dextrose should be avoided because of its irritant nature (phlebitis). Dextrose can be given at higher concentrations directly to the mucous membranes of the mouth if the neonate is not dehydrated or hypothermic (1 to 2 ml of a 5% to 15% dextrose solution). Dextrose solutions should never be given SQ because they may cause tissue damage. After the treatment, blood glucose levels should be monitored because of the risk of hyperglycemia as a result of poor regulatory mechanisms in the neonate.
Neonatal Isoerythrolysis in Kittens
Cats with blood type A have low titers of naturally occurring antibodies against blood type B red blood cells. Therefore blood type B kittens born to blood type A queens do not show any clinical signs of incompatibility reactions after the ingestion of colostrum-containing alloantibodies. However, all blood type B cats have high titers of naturally occurring antibodies against type A red blood cells. This may lead to incompatibility reactions when blood type A kittens receive colostral antibodies from a blood type B queen. Clinical signs are variable and range from jaundice and death within the first 2 days of life to no signs at all (which is rare). Sometimes the tail tip becomes necrotic and falls off at 10 to 14 days of life. Studies at the University of Pennsylvania indicate that kittens at risk for neonatal isoerythrolysis must be removed from their queens only during the first day of life.
The kittens can be tested at birth using handy blood-typing cards (DMS Laboratories, Flemington, N.J.), and if blood type B kittens are born to blood type A queens, they can be fostered to a blood type B queen or hand-raised during the first 24 hours of life. If kittens have neonatal isoerythrolysis during the first day of life, they should be removed from the queen for 24 hours and given supportive care.
Malnutrition
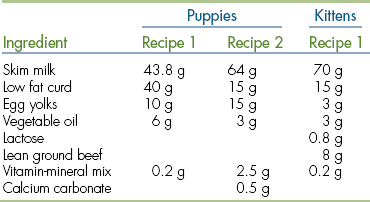
Many milk replacers can easily cover the daily caloric requirements of both puppies and kittens. However, it seems that the fluid requirements are not easily met. Regular feeding is important to maintain good hydration in the neonate. If only three feedings per day can be provided, then use a commercial milk replacer with a formulation that comes closest to the bitch’s or queen’s milk (Table 10-1) and provide the extra fluids SQ. Overfeeding or a high lactose content in the milk replacer often cause diarrhea. Nothing is better than mother’s milk; it also contains bile-salt–activated lipase, which is necessary for proper digestion. After each meal, the neonate should be encouraged to urinate and defecate by stimulating the anogenital region with a moistened cotton ball. Neonates should be weighed daily on a suitable scale until 3 weeks of age to ensure proper weight gain.
TABLE 10-1
Requirements Overview and Milk Comparison

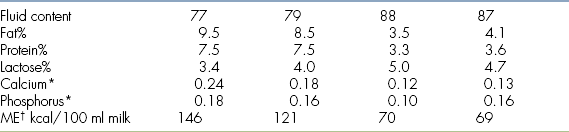
∗g/100 ml
†Metabolizable energy
‡When mixing cow’s or goat’s milk as a milk replacer for neonates be aware that to cover the fat or protein requirements, the lactose concentrations will be far too high and cause diarrhea. Commercial milk replacers are a better choice.
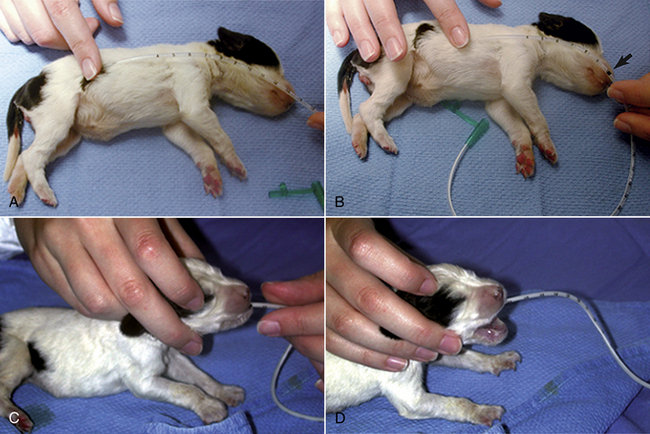
Occasionally a neonate will not nurse from a bottle or will not gain the expected weight because of illness or malformations. Tube feeding is necessary in these cases. However, a puppy or kitten should never be tube fed if its body temperature is lower than normal for its age. If the body temperature is too low, the gut shuts down and the ingested material will start to form gas, which in turn will bloat the neonate and lead to serious distress. Tube feeding is performed by first measuring the distance from the tip of the neonate’s nose to the end of the chest (Figure 10-3). Using a felt-tip pen or a piece of tape, a mark is made at 75% of this distance on the feeding tube measuring from the distal end of the feeding tube. Insert this length of a clean and dry feeding tube gently into the mouth of the neonate while holding the patient upright. No force is needed because most of the neonates will swallow the feeding tube easily. The syringe with the milk replacer is connected to the tube, and the plunger is pulled back gently. Negative pressure indicates that the feeding tube is indeed in the lower esophagus and not in the lungs. When feeding milk replacer alone to puppies or kittens, use a 5-French feeding tube. As a rule of thumb, about 5 ml of milk replacer can be given per feeding to a 160-g puppy or kitten.
The Fading Puppy or Kitten Syndrome
The fading puppy or kitten syndrome is characterized by anorexia, lethargy, emaciation, death, and birth defects in cats and dogs. Kittens or puppies may be stillborn or born small, weak, and unable to nurse, resulting in dehydration, hypothermia, hypoglycemia, and death within the first few days of life. Other neonates appear healthy during the first weeks of life; become weak, depressed. and anorectic; and die of starvation at the time of weaning.
Causes of neonatal deaths include poor management, malnutrition, inappropriate environmental conditions, congenital and genetic defects, and infections. Management and environmental problems can be easily detected by obtaining a detailed history or inspecting the facility. Problems may include poor hygiene, inappropriate temperature and humidity, overcrowding, frequent introduction of new animals, inappropriate use of medication, or exposure to chemical toxins. Nutritional deficiencies can be caused by inadequate diets for the mothers or the neonates. The treatment involves supportive care for the sickly neonate and removal of the inciting causes. If causes are not immediately apparent, necropsies of the neonates that have died are recommended.
Orphan Care
Fostering neonatal kittens and puppies is time consuming, considering that the neonate is completely helpless and requires almost 24-hour care. It is rewarding, however, once the happy, healthy puppy or kitten is ready for its new home. Materials needed are warm and clean bedding, milk bottles with a variety of different rubber nipples, feeding tubes, syringes, a gram scale, cotton balls, hand sanitizers, fur clippers, and the possibility to isolate the orphan from the other animals. Kittens or puppies need to be hand-raised because of maternal death or abandonment, a lack of milk in the mother, maternal aggression, large litter size, malformations, or trauma. When more than one neonate is taken care of, it is essential that each patient be uniquely identified so that progress can be assessed. Each orphan must be weighed daily, and records should be kept. An ambient temperature and humidity of 84° F to 90° F and 55% to 60%, respectively, should be provided during the first week of life, if possible. During the second week, the ambient temperature can be lowered to 79° F to 84° F and to 73.4° F to 79° F during the third week. Shivering reflexes will set in during the second week of life and will contribute to an increase in body temperature. The neonate must be kept warm and well hydrated at all times, following the principles outlined earlier. Proper nutrition or supplementation (Box 10-4) and clean and dry housing should be provided. After feeding, the orphan must be stimulated to urinate and defecate.
Common pitfalls are overfeeding and underfeeding. Overfeeding milk replacers often results in diarrhea and underfeeding in dehydration and a lack of weight gain. Homemade formulas are often deficient in growth factors, amino acids, and other nutrients essential for growth. Many of the commercial milk replacers are made using cow’s milk as a base and are therefore not always complete. The energy density of the formula might be too high, and the fluid requirements will not be fulfilled and vice versa; the energy density is too low, and the stomach capacity is too small for the required amount. Therefore commercial milk replacers should be carefully evaluated, and homemade milk replacers should only be made using proven recipes (Table 10-2). Other problems that may arise are housing sick animals in the same room with orphans; overcrowding; improper hygiene (not washing hands between patients); too many fosters per person; damp blankets; and a cold, drafty environment. Lastly, because of the differences in physiology, medications will not be taken up and metabolized at the same rate as in adults. Care should be taken when choosing medications and administering them. Box 10-5 outlines the main physiologic differences between neonates and adults.
NEONATOLOGY OF FOALS
Breeding a mare and delivering a foal is an exciting time for many horse owners. Some clients will breed a mare they have owned for years and want to raise the foal for riding. Other owners will breed the mare to obtain a foal intended for racing. The desired outcome is the same in both instances: deliver a healthy foal and recognize when problems arise in the neonatal period. This section will focus on two critical areas: the perinatal period, as the late-term pregnant mare is preparing to deliver a foal, and the neonatal period, when the foal is prone to certain diseases. A foal’s normal development will be discussed followed by a foal’s potential diseases and referral for intensive care at a veterinary hospital. Sick foals referred to a veterinary hospital require a team approach, and the important role of the veterinary technician will be highlighted.
THE PERINATAL PERIOD AND THE HIGH-RISK MARE
Many mares have routine pregnancies and deliver healthy foals. However, sometimes the mare will develop a problem in late-term pregnancy and require treatment. These mares are referred to as “high-risk mares” and often exhibit early warning signs, indicating a problem with the pregnancy.
The average gestation’s length in a mare is 340 days, but a normal gestation’s length can range from 320 days to 400 days. When mares have multiple pregnancies, they often deliver around the same time each pregnancy, so this is an important part of the history to obtain. When a mare is bred, she will be evaluated at regular intervals by a veterinarian early in the pregnancy. The veterinarian will perform a rectal exam and transrectal ultrasound around 15 days, 30 days, and then 90 days. These examinations will ensure that the mare is retaining the embryo, check for the presence of twins, and evaluate amniotic and allantoic fluid levels surrounding the embryo. As long as the mare is maintaining the pregnancy and findings are within normal limits, she will be monitored closely for any changes, but will often not be evaluated again until the end of her pregnancy. In late-term pregnancy (7 to 10 months), the mare will be reevaluated by a veterinarian and closely watched for any abnormal signs. During this time, the mare should be comfortable, bright, alert, and eating readily. Exercise is important to maintain the mare’s physical condition because delivery (parturition) is explosive in horses and the foal should be delivered within 30 minutes of stage 2 labor (water breaking). She should have no vaginal or udder discharge. If a vaginal discharge is present, it may indicate a problem, such as placentitis or urine pooling, and the mare needs to be evaluated. Toward the end of gestation, the mare’s udder will slowly enlarge, and before delivery, beads of milk will appear on the end of her teats (waxing of teats). Unless delivery is imminent, the mare should not have milk dripping or streaming from her udder. There are three reasons for this clinical sign: twins, placentitis, or the owner calculated the wrong delivery date, and delivery actually is imminent.
A veterinarian needs to evaluate the mare to determine the cause for dripping milk. The most common reason is that the mare has an ascending placentitis, and subsequent inflammatory changes cause premature lactation. Older mares are especially prone to this problem as a result of poor vulvar conformation as they age. Performing a transrectal ultrasound will allow the veterinarian to evaluate the placental thickness near the cervical star (the area of placenta next to the cervix most often affected by ascending placentitis) and fetal fluid amount and appearance. The veterinarian can also evaluate gross fetal movement and measure the eye orbit to estimate fetal age. A transabdominal ultrasound will also aid in evaluating the foal and placenta and can provide valuable information.A vaginal examination in a late-term pregnant mare is not recommended unless delivery is imminent because this can actually induce ascending placentitis. If the placenta appears thickened, detached, and/or the fetal fluid is more hyperechoic (more white noted on ultrasound from presence of cellular debris), the mare has placentitis and requires treatment. A typical treatment includes the use of broad-spectrum antimicrobials, such as trimethoprim sulfa, and nonsteroidal antiinflammatory medications, such as flunixin meglumine and progesterone (Regu-Mate is most commonly used in mares). This treatment is designed to kill any bacteria present, decrease the inflammation associated with placentitis, and help maintain the pregnancy until term. Many mares respond to this treatment quickly, and abnormal clinical signs, such as premature udder development and dripping milk, will resolve. If left untreated, most of these mares will deliver premature foals, with a poor chance for survival. If diagnosed and treated early enough, there may be no long-term effects on the foal, but this depends on how long the foal was affected and the extent of damage to the placenta. These mares are classified as “high risk” and need to be monitored closely for foaling. An attended foaling is important to ensure that the foal has the best chance for survival and to treat the foal quickly if any problems are noted. Other reasons for mares to be classified as “high risk” include the presence of twins and a prior history of foaling problems.
High-risk mares may be referred to a foaling facility or veterinary hospital to have an attended foaling and prompt delivery of medical care. Methods to monitor high-risk mares include a video camera placed in the stall to observe the mare’s behavior without human interaction and telemetry to monitor the mare’s and foal’s heart rates. It is important for the mare to feel as comfortable as possible. Mares can begin stage 1 labor (exhibit behavioral changes indicating impending labor), but if they feel threatened, they can stop the labor and resume later. A video camera offers the optimal way to unobtrusively observe the mare’s behavior. Telemetry to measure the foal’s and mare’s heart rates is a noninvasive way to assess fetal and maternal health. In late-term pregnancy, the foal will have a heart rate between 40 to 150 bpm. There will be periods when the foal’s heart rate is low (e.g., 40 bpm) because the foal is sleeping and other times when the foal’s heart rate is 120 bpm or higher during periods of exercise. The important point to note is that the foal should have a range of heart rates over time, not a sustained low or high heart rate, which can indicate that the foal is stressed in utero. Over the course of gestation, the resting fetal heart rate will gradually decrease and often stay around 40 to 80 bpm during periods of rest. A late-term pregnant mare should have a heart rate around 40 to 50 bpm with higher rates during periods of exercise (e.g., pasture turnout).
It is often difficult to predict exactly when a mare will foal. As parturition (foaling) approaches, the mare’s udder will slowly enlarge and fill with milk, her vulva will lengthen, and pelvic ligaments will relax. Many mares will develop “wax” (drops of dried milk) on the tip of their teats. Often mares foal at night, when the stable is more quiet, but some mares will readily deliver a foal in the afternoon surrounded by noise. Knowing when a multiparous mare has foaled in the past is helpful, and they will often foal around the same date again. Also knowing her prior behavior changes will provide clues to when she might foal. A maiden mare (primiparous: mare has not foaled in the past) can be more challenging, but checking her vulvar relaxation, udder development, and the presence of wax on her teats will aid in predicting delivery. There are commercial kits available that measure the rise in calcium in the mare’s first milk (colostrum) and accurately predict foaling in some normal mares. The rapid rise in colostral calcium represents one of the most consistent and significant changes before parturition. Calcium levels in colostrum samples can be rapidly measured in nearly every diagnostic laboratory or even just by the use of simple hard-water test kits. Colostral calcium levels above 10 to 12 mmol/dl are considered significant enough to predict parturition within the ensuing 24 hours. However, parturition in high-risk mares is often not reliably predicted by these kits. Despite the different methods available, the best choice for determining when a mare will foal is to have a well-trained staff monitor the mare’s behavior 24 hours a day (especially by video camera in the stall) and note any changes. A docile mare may become cranky; an aggressive mare may become docile. Some mares drip milk from their udders just before foaling. Many mares show obvious stage 1 behavior changes: agitation, pacing, nickering, lifting the tail head (as a result of oxytocin release), turning and biting at her sides, and kicking her abdomen. If sweating around her shoulders is observed, the mare will foal within 30 minutes. As a veterinary technician at an assisted delivery, this time is crucial. It is best to notify the veterinarian, stay near the mare, ensure that all necessary equipment is present, and wrap the mare’s tail with brown gauze to keep it clean and out of the way. Box 10-6 lists the equipment needed when attending a high-risk foaling.
Stage 2 in mares is explosive and starts when the mare’s placenta ruptures and allantoic fluid escapes (water breaking). This is the actual labor, and the foal should be delivered within 30 minutes, but many foals are delivered within 10 to 15 minutes. If the foal is not delivered within 30 minutes, the foal will experience hypoxemia (low oxygen blood levels) and may have neurologic deficits or die. Therefore it is an emergency, and if the foal is unable to be delivered vaginally, a cesarean section is needed. If the foal’s nose is protruding from the vulva, an endotracheal tube can be placed in the foal’s trachea and breaths delivered via an Ambu bag. This will maintain the foal’s oxygen needs and permit more time to prepare for a cesarean section. Stage 3 is when the mare passes her placenta. This can be painful, and many mares lay down to expel the placenta. The placenta should be collected, weighed, and evaluated to ensure that it is completely intact. If a piece is missing (often the tip of the placenta), the mare needs to be treated for a retained placenta. When the foal is delivered, the mare should show immediate interest in the foal by nickering and cleaning him. Before she stands, it is best to strip the umbilical cord of blood and gently break it near the foal’s body, leaving a 2- to 3-inch remnant.
The mare will be thirsty and needs fresh, clean water. Additional bedding should be added to the stall because it will be slippery from fetal fluids. Many mares show mild discomfort (looking at her sides, kicking her abdomen), and a dose of a nonsteroidal antiinflammatory drug (NSAID), such as flunixin meglumine, will often provide analgesia. Mares are often protective of newborn foals, so intervention should be minimized, and they should be left alone as much as possible to permit bonding (Figure 10-4). However, in the postpartum period, if the mare is not interested in the foal or appears to be in pain (e.g., rolling in the stall), intervention is necessary. Occasionally, mares will develop complications postpartum that require immediate treatment or else the foal may be rejected by the mare. Examples of complications include: mild colic, large colon volvulus, uterine artery hemorrhage, a retained placenta, and peritonitis. Depending on the problem, the foal may be able to stay with the mare or may need to be removed and either raised as an orphan (called a bucket baby) or “grafted” onto a nurse mare.
THE NEONATAL PERIOD: THE NORMAL FOAL
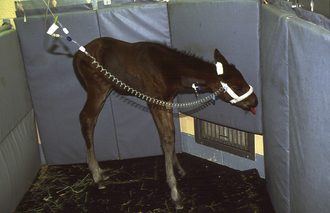
When a foal is born, many changes occur within the first 24 hours of life. In the wild, a foal must be able to stand, nurse, and run from predators within a short period of time or survival will be in jeopardy. Domesticated equine patients are similar, and a neonatal foal will develop rapidly. If not, the foal is often ill and requires treatment. Initial milestones for a foal after parturition include a suckle reflex shortly after birth (curling tongue and seeking to nurse), standing within 1 to 2 hours, and nursing successfully within 6 hours (often by 2 to 3 hours old). The first urination normally occurs around 12 hours. Foals pass meconium (black, sticky fecal material) within a few hours after birth, but some foals have difficulty and become uncomfortable, so they may require a warm-water, soapy enema. A typical enema for a 50-kg foal is 500 ml of warm water mixed with a small amount of Ivory soap. Within 24 hours of birth, the foal should be strong, alert, and capable of running. A nursing neonatal foal will urinate frequently. Urine will be dilute (specific gravity 1.001 to 1.006) because mare’s milk is mostly water. Fecal material will be soft and yellow, and foals will defecate one to two times per day.
Observing the foal’s behavior for a few minutes without human interaction is important to assess the foal’s attitude and energy level. On a physical exam, a neonatal foal should be bright, alert, and responsive. A normal temperature, pulse, and respiration (TPR) at birth will be T = 99° F to 100° F (rectal temperature), P = 60 to 80 bpm, R = 10 to 20 breaths/min. The foal’s heart rate will gradually increase to 120 bpm, and respiratory rate will gradually increase to 40 to 60 breaths/min. Mucous membranes will be pink and moist with a capillary refill time (CRT) less than 2 seconds. Eyes should be open and bright with no redness or discharge. Foals are born without a menace response, and it often takes a few weeks for a menace response to develop. The heartbeat should be strong and regular with a synchronous pulse. A quiet systolic murmur may be present (often a flow murmur), but will disappear over time. The respiratory rate and effort should be frequent and steady. Thoracic auscultation may initially sound moist, but will resolve over time. The foal’s abdomen should not be swollen and the umbilical remnant clean and dry. The foal’s legs will initially be weak, but after 24 hours, the foal should be able to stand readily and run quickly. There should be no joint swelling on palpation. A foal will quickly bond to his mother and follow at her flank. Neonatal foals will sleep about 10 to 20 minutes, then stand and nurse for 5 minutes, play, and sleep again. Normal nursing behavior includes the foal facing the mare’s tail, bumping the udder to encourage milk let-down, then nursing for about 5 minutes. The foal should readily use both teats. Palpating the mare’s udder after observing the foal nurse is important to ensure that the foal is nursing successfully from both sides of the udder. A healthy foal will nurse from both teats, and the mare’s udder will contain minimal milk. A sick foal may appear to nurse, but when the udder is palpated, it is found to be engorged and painful to the touch. Healthy foals will gain an average of 2 to 4 lb a day (1 to 2 kg) and grow rapidly.
The neurologic system of a newborn foal is quite different from that of an adult horse. When the foal is standing for the first time, a basewide stance and exaggerated steps when ambulating are considered normal. An increased response to visual, auditory, and tactile stimuli and jerky movements are also normal. When the normal standing foal is restrained, it will initially struggle and fall limp, as if sleeping, into the arms of the handler. Loosening the restraint will cause the foal to support its weight again. This specific behavior must be kept in mind by people who are restraining standing foals for procedures, such as during the placement of an IV catheter.
Routine Neonatal Therapy
Routine care for all foals includes applying 1% iodine to the foal’s umbilical remnant at birth and then three to four times daily for 2 days, monitoring the foal’s attitude, appetite, urination, and fecal production. Foals from mares not vaccinated with tetanus toxoid in the last 4 to 6 weeks of gestation should receive 1500 international units (IU) of tetanus antitoxin intramuscularly. On some farms, an enema is routinely administered after birth.
It is important to measure the foal’s antibody levels (IgG) approximately 12 hours after delivery. Foals are born without antibodies and need to drink colostrum within 12 to 24 hours after birth to gain antibodies. Colostrum contains antibodies from the mare, other proteins, growth factors, and opsonins to protect from bacterial infection. High-quality colostrum has a specific gravity of 1.080 or higher, and a sample taken shortly after parturition can be measured using a Colostrometer. Within the first 12 to 24 hours of life, the foal’s gastrointestinal (GI) tract is able to absorb antibodies from colostrum. After 24 hours, the foal can no longer absorb the mare’s antibodies. The IgG level should be greater than 800 mg/dl after 12 hours of age. The failure of passive transfer (FPT) of antibodies occurs when the foal’s IgG level is less than 800 mg/dl after 24 hours old. Reasons include poor quality colostrum from the mare, a lack of colostrum as a result of milk dripping before parturition, the foal is too weak or has limb deformities, such as carpal contracture, which prevents the foal from standing to nurse. The FPT is associated with an increased susceptibility to an infection or sepsis, and these foals must be monitored carefully (e.g., daily weight and TPR recorded) to identify problems quickly.
Several tests are available to measure IgG levels, each with advantages and disadvantages. The radial immunodiffusion test is accurate but expensive with limited availability for routine screening. The commercial IgG screening kit with an enzyme-linked immunosorbent assay (ELISA), such as SNAP ELISA, (produced by Idexx Inc., Portland, Me.) is the most commonly used test because it is quick, accurate, easily performed, and readily available. Of the many rapid test methods including zinc sulfate turbidity, latex agglutination, glutaraldehyde coagulation test, and SNAP ELISA, only the SNAP ELISA has sensitivity (and specificity) in the 800-mg/dl range.
The FPT before 24 hours is treated by giving the foal high-quality colostrum from another mare via nasogastric intubation. After 24 hours, it is treated with IV administration of plasma from an appropriate donor. Commercial plasma is available (e.g., HiGamm by Lake Immunogenics, N.Y.), and although expensive, it is the safest and most reliable product. This plasma is harvested from hyperimmunized donors and has been shown to have IgG levels that far exceed normal plasma and is tested for antiequine antibodies. If fresh plasma is given from an on-site donor, a crossmatch should be performed to lessen the risk of a significant transfusion reaction. Plasma is administered via a sterile IV catheter. It is paramount to use a blood-administration IV line with a filter to strain out any large particles from the plasma. As a general rule, 1 L of commercial plasma raises the IgG level of a 45-kg foal by 200 mg/dl.
Laboratory Evaluation
The laboratory parameters (hematology and serum chemistry values) of neonatal foals are distinctly different from those of adult horses. Coagulation values are initially different from adult horses. There are published references from the University of Florida, College of Veterinary Medicine, but because of variations in technique and equipment between different laboratories, reference ranges for values need to be established individually at each facility examining samples from veterinary patients. As foals age, many of their blood values will reach typical adult levels, so by 1 month, many parameters are similar to adult horse levels.
When evaluating a complete blood cell count (CBC), the packed cell volume of the normal foal during the first 24 hours of life (e.g., >40%) is greater than that of an adult horse. Subsequently a fall into the low normal range of an adult can be observed over the ensuing 2 weeks to 1 year. Band neutrophils are not normal in the healthy foal. Values of more than 100 to 150 band cells/dl are considered abnormal and usually indicate an acute infection, such as sepsis. Measuring plasma fibrinogen concentration can be helpful to evaluate the presence of active inflammation. Fibrinogen is an acute phase protein, produced by the liver in response to active inflammation. Fibrinogen levels vary between labs, but in general, will not exceed 420 mg/dl in foals up to 1 week of age. At birth, before the foal has nursed from the mare and has not absorbed immunoglobulins (IgG) from the mare’s colostrum, the plasma protein concentration of an equine neonate is considerably lower than that of an adult horse (e.g., between 4 to 4.5 mg/dl). After a successful passive transfer of immunoglobulins from the mare’s colostrum, the foal’s total protein will increase to approximately 5.6 mg/dl, which is below the normal range for an adult horse.
The activities of various serum enzymes are distinctly different in foals than in adult horses. Alkaline phosphatase, gamma glutamyl transferase, sorbitol dehydrogenase, alanine transaminase, and glucose levels should be consistently higher in foals than in adults. An increase in alkaline phosphatase has been attributed to increased metabolic activities in bone, intestine, and liver. Increased gamma glutamyl transferase and sorbitol dehydrogenase activities are attributed to a greater activity in the liver in foals and may be associated with the greater mass of the liver in relation to total body mass. Changes in alanine transaminase levels are of questionable clinical significance because this enzyme has not been shown to be specific for a particular organ system in the horse. The serum glucose concentration in normal foals ranges from 60 to 120 mg/dl. A decrease in serum glucose concentration below the normal reference range is a concern and is indicative of an insufficient caloric uptake of the foal or metabolic derangements in the critically ill foal. In normal foals, the serum levels of creatinine and blood urea nitrogen are often above reference values for adults for the first 36 to 72 hours of life, but will gradually decrease to adult values.
THE NEONATAL PERIOD: THE SICK FOAL
Foals are born with minimal energy reserves, such as stored fat, and can quickly deteriorate with an infection. They also need to drink good-quality colostrum from the mare within 24 hours of delivery. If there is an FPT of maternal antibodies, foals are prone to infection. There are three main sites of entry for bacteria and viruses: GI tract, respiratory tract, and umbilicus. The foal is born with sterile GI and respiratory tracts. During the first week of life, foals will eat their mother’s manure to colonize their GI tracts with proper bacteria. Since horses live in a dirty environment, foals are exposed to many pathogens. If foals are compromised for any reason, they are more likely to develop bacteremia (bacterial infection in the bloodstream) and sepsis.
Some foals develop infections as a result of their contaminated environment. Other foals are predisposed to developing infection from abnormal conditions in utero. Risk factors for compromised foals include prematurity, twin foals, a history of placentitis, the mare is sick and not producing much milk, or the foal is exhibiting abnormal behavior after delivery, such as weakness and inability to nurse, resulting in the FPT. Premature foals are born before they reach 320 days of gestational age. Signs of prematurity include low birth weight, weakness, silky hair coat, floppy ears, domed forehead, flexor tendon laxity, and angular limb deformities (e.g., incomplete ossification of cuboidal bones in the carpus and tarsus on radiographs). This incomplete ossification can result in crushing injuries of cuboidal bones from simple weight bearing, so these foals require extensive care for weeks and restrictive standing until the bones are completely ossified. The term dysmaturity usually refers to a large foal (e.g., >65 kg) with a longer than expected gestation (e.g., 400 days). The foal’s hair coat will be coarse and long, and the incisor teeth will be erupted through the mucous membranes. The foal may be weak, unable to nurse correctly, and have angular limb deformities. Both of these types of foals are often products of their mare’s abnormal placenta and have an increased susceptibility to disease and injury, requiring veterinary care.
Some foals have minor problems, such as orthopedic problems or an inguinal hernia, and require mild to moderate diagnostics and intervention. However, the classic early clinical signs of disease in critically ill foals are lethargy, depression, decreased suckle reflex, decreased nursing, and increased periods of recumbency and sleeping. Unfortunately, foals can quickly become critically ill and rapidly deteriorate, so they need to be monitored closely, especially in the first week of life. If a foal is developing any of these signs on the farm, the veterinarian needs to evaluate the foal and determine if a referral is needed for additional care. It is difficult to manage sick neonatal foals on the farm. They often require 24-hour care and extensive monitoring for optimal recovery. Sometimes, even despite the best care, critically ill foals do not survive. Since intensive care is expensive, the owner needs to decide about treatment options with the veterinarian’s guidance and long-term goals for the foal.
Admitting the Critically Ill Foal
A neonatal team including a veterinarian, a veterinary technician, and assistants are vital to the initial evaluation and treatment of the critically ill foal. Many foals may require supportive care and recover quickly, but some will not survive without intensive management. A skilled veterinary technician plays an important role in treating these cases and recognizing when complications develop. There are numerous foal neonatal intensive care units (NICUs) providing quality care to these foals, and spending time in an NICU to gain experience is invaluable.
When a foal is admitted, triage by the veterinarian and veterinary technician is important to determine the initial diagnostic evaluation and treatment. A foal able to walk into the NICU will receive less intensive care than a hypothermic, recumbent foal. Initial triage includes obtaining a foal’s history, weight, and a quick assessment of attitude, condition, TPR, and cardiovascular system (heart rate, rhythm, pulse, and limb temperature, which indicate perfusion). Ambulatory foals should be gently restrained during the examination to minimize stress and are usually stabled with their dams to permit normal nursing.
A comatose, recumbent foal should be evaluated rapidly and a treatment initiated, so many NICUs have standard protocols. Box 10-7 lists common diseases of neonatal foals. The foal is placed on a padded surface and eyes protected from trauma. At least three persons are needed to restrain the foal: one person holding the head, the second holding the front legs, and the third holding the hind legs. The foal’s attitude is assessed, and a lack of response to stimuli indicates poor brain perfusion. Mucous membranes are often dark purple with prominent vessels (injected mucous membranes). Icterus (yellow color of mucous membranes and sclerae) is commonly noted in foals with sepsis. Petechiae may be noted (small areas of hemorrhage) on oral mucous membranes or on the foal’s pinna (outer ear). The eyes may be sunken and entropion present (lower eyelids rolled inward) as a result of hypovolemia. The pupil size should be noted and eyes stained with fluorescein to check for corneal ulceration. Miotic (constricted) pupils often indicate sepsis. The foal may have tachycardia (e.g., >150 bpm) or bradycardia (e.g., <70 bpm), and the pulse may be bounding or difficult to palpate. The respiration may be fast (>60 breaths per minute) or slow (<20 breaths/min). Ribs should be carefully palpated, especially over the heart, to detect the presence of rib fractures. Auscultation of the GI tract may reveal audible borborygmi or the absence of GI sounds. The abdomen should be evaluated for distention and the umbilical remnant examined for discharge. Joints should be carefully palpated for swelling. Urine should be caught for a dipstick and specific gravity. Fecal matter should be examined, especially if diarrhea is present, for it often requires isolation of the patient. The limb temperature is important to assess. Often these foals will be hypothermic (T <99° F) and have poor perfusion to their limbs. Consequently the foal’s legs will be cold, and pulses in the limbs will be difficult to feel. The median artery (on the medial upper forelimb near the elbow) and the great metatarsal artery (on the lateral distal hind limb) are two arteries frequently used to assess pulses and perform blood gas samples. The foal’s blood pressure should be assessed.
Initial blood samples include drawing an arterial blood gas from the great metatarsal artery to assess oxygen and carbon dioxide levels. A venous sample can be taken at the time of catheter placement for a CBC, chemistry profile, glucose level, and blood culture. Typical blood work abnormalities of these critically ill foals include IgG less than 800 mg/dl, low white cell count (e.g., <5000 cells/μl), an increased fibrinogen concentration, profound hypoglycemia (<40 mg/dl), increased lactate, a low oxygen concentration, and increased carbon dioxide levels.
The initial treatment often entails administration of intranasal oxygen (oxygen insufflation), sterile placement of an IV catheter in the jugular vein, fluid therapy using crystalloid fluids (e.g., Normosol R), fluids containing dextrose (e.g., D5W), and broad-spectrum antimicrobials. Placement of a jugular catheter requires aseptic technique. The area over the vein is clipped, and sterile scrub and sterile gloves are used to clean the area for 5 minutes. Masks for the foal handlers are also helpful to prevent contamination of the area. The placement of an IV catheter offers an ideal time to draw the first sample for a blood culture using a sterile syringe and sterile gloves. Whereas many critically ill foals may have sepsis, blood cultures are only positive about 30% of the time. In the past, sepsis was usually due to a gram-negative organism, but in recent years, gram-positive sepsis is on the rise. Antimicrobial therapy should be based on the isolation of infecting organisms and antimicrobial sensitivity testing. Special considerations need to be made regarding the physiologic features of the neonate, such as reduced hepatic activity and renal immaturity. However, antimicrobial therapy must be instituted as soon as sepsis is suspected, without waiting for culture results, which take a few days to finalize. In general, using ceftiofur sodium or a combination of penicillin and an aminoglycoside (e.g., amikacin) provides appropriate coverage. Shock therapy fluids can be administered at 20 ml/kg/hr for short periods, so many critically ill foals (e.g., 50-kg foal) will receive 3 L of fluid within the first 2 hours of admission.
Often after a few liters of IV crystalloid fluids and dextrose administration, the foal’s perfusion will improve. The foal will appear more alert, sit up, and seek to nurse. To prevent corneal ulceration, ophthalmic ointment is placed in both eyes and entropion corrected if present. A hypothermic foal should be gradually warmed using blankets or forced-air warming units (Bair Hugger by Augustine Medical, Eden Prairie, Minn.). It is imperative to warm the foal slowly, or an increased metabolic demand will cause cardiovascular collapse. Blood pressure is monitored closely, and decreased blood pressure will be one of the first signs the foal is not responding to treatment. A foal’s recumbency is changed every few hours to prevent decubital ulcers and permit expansion of the down lung. Glucose measurements using a Glucometer are frequently performed to ensure that the foal’s glucose concentrations are within normal limits. If the foal remains hypoglycemic or hyperglycemic, the foal may have malmetabolism as a result of sepsis and requires close monitoring. Performing frequent urine dipsticks and assessing specific gravity will ensure that the foal is not losing glucose in the urine (glucosuria) and the kidneys are responding appropriately to fluid therapy.
Once the foal’s cardiovascular system has stabilized, additional diagnostics can be performed. Thoracic radiographs and/or an ultrasound examination may be needed to assess for pneumonia or the presence of rib fractures. Abdominal ultrasound is often used to better assess the GI tract for meconium retention, evaluate the bladder to ensure that it is intact, and examine the internal remnant of the umbilicus. The external umbilical remnant is outside of the abdomen. It contains remnants of the urachus (which connects to the urinary bladder); two umbilical arteries, which travel caudally and insert in the bladder wall; and one umbilical vein, which courses cranially to the liver. Sick foals often develop a patent urachus and omphalitis (inflammation of the umbilicus). Externally the umbilical remnant will be moist and enlarged, and the foal will strain or dribble small streams when urinating. The examination of the urogenital system also includes palpation of the umbilical, inguinal, and scrotal areas for hernias and distention. If the foal has mild colic, he will often flag his tail when defecating. The foal may also roll onto his back and fold his front legs over his chest to indicate abdominal pain. When the foal’s temperature is taken, fecal matter should be detected on the thermometer. If this does not occur and the foal exhibits signs of abdominal discomfort, the possibility of a nonpatent GI system caused by an anatomic abnormality (atresia ani, atresia coli) should be considered.
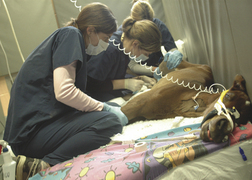
When additional venous blood samples are required, the cephalic and saphenous veins are ideal for collection, using the smallest needle and syringe possible, using a tuberculin syringe or 22-gauge, 1-inch needle and 3-ml syringe (Figure 10-5). Adequate restraint is paramount to successfully perform venipuncture in recumbent foals and usually requires three persons. Figure 10-5 depicts correct restraint of the recumbent neonatal foal to obtain a blood culture from the saphenous vein. Restraining the foal using bony areas, such as joints, offers the best approach to prevent harming soft tissue areas, such as the foal’s abdomen. A foal’s veins tend to roll, so clipping the area over the vein is helpful. After applying alcohol to the area, gently insert the needle at a 45 degree angle to obtain a blood sample. If additional arterial blood gas samples are required, the procedure is similar. Three persons are needed to restrain the foal. One person holds the foal’s head and ensures that the eyes are protected from abrasion, the second holds the front legs, and the third person holds the hind legs. An area is clipped over the great metatarsal artery and alcohol applied. Arterial samples are painful, so infusing 0.1 to 0.2 ml of lidocaine in the area using a tuberculin syringe and needle is vital to collect the sample. It is important to use a specialized blood gas syringe to collect an arterial sample. Draw back on the plunger to 0.5 ml before taking the sample to ensure that the arterial blood can flow into the syringe. Palpate the arterial pulse and insert the needle gently at a 45 degree angle. Blood should immediately flow into the syringe and appear bright red. If the blood flows slowly and appears dark, it may be a venous sample. However, if the foal is comatose with extremely cold limbs and poor pulses, the sample may indeed be an arterial sample.
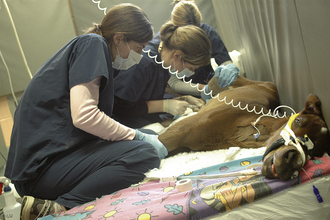
FIGURE 10-5 Correct restraint of the recumbent neonatal foal to obtain a blood culture from the saphenous vein. (Photo by Sabina Louise Pierce, University of Pennsylvania.)
Over the course of hospitalization, a foal may require additional treatment, including maintenance fluids, nutritional support, blood pressure support, treatment for orthopedic conditions, and a ventilator. Maintenance fluid rates can range from 90 to 150 ml/hr for the average 50-kg foal. Each case varies depending on the foal’s illness and clinical course. Some foals with mild to moderate illness are only hospitalized for a few days and discharged on medication. Critically ill neonatal foals are often hospitalized for a few weeks, and the treatment is often expensive. Figure 10-6 depicts a typical IV fluid setup for a critically ill foal. The best outcome for a neonatal foal is early detection of disease on the farm and rapid intervention before the foal becomes critically ill.
Monitoring and Nursing Care
Once the foal has been admitted to the veterinary hospital and initially examined, diligent monitoring is critical. Box 10-8 lists important considerations when monitoring the critically ill foal. The objective of frequent monitoring is to detect subtle changes signifying improvement or deterioration in the foal’s condition. Complications during hospitalization include resistant nosocomial (hospital-acquired) infections, additional sites of infection (septic arthritis, osteomyelitis, thrombophlebitis, pneumonia), corneal ulcers, and decubital ulcers. The severity of the patient’s illness will dictate the required frequency of monitoring. Parameters are recorded on a flow sheet for easy comparison at different time points. As a foal deteriorates, one of the earliest changes noted is decreasing blood pressure. Monitoring blood pressure frequently aids in detecting deterioration in the foal’s condition and permits treatment changes before the case spirals downward. Performing regular TPRs will also aid in assessing the foal. A recumbent foal’s temperature is often between 99° F and 101.5° F. A hypothermic foal is often unable to maintain a normal body temperature and often has a temperature less than 99° F, despite warming methods. A recumbent foal with a temperature greater than 102° F is febrile and may not be responding to the antimicrobial choice.
The hallmarks of nursing care for the foal are strict attention to asepsis and close observation of minor details. An experienced veterinary technician is invaluable and will often note changes in the foal before the veterinarian. If more than one foal is being treated by the technician, techniques, such as hand washing between patients, should be used to prevent cross contamination. Skin injections should be made only after the area has been cleaned with alcohol and dried. Intramuscular injections are limited to the semimembranosus region and should not be given in the neck, pectorals, or gluteal region. Figure 10-6 depicts a typical setup for continuous IV fluid administration in a critically ill foal. Fluid lines should be changed daily to prevent contamination. Major line changes should be performed every 3 days. If a fluid line is accidentally disconnected, it should be considered contaminated and must be replaced. All IV ports should be capped with injection caps and cleaned with alcohol swabs before a needle is inserted. Multidose vials of injectable drugs and injection caps or ports must be disinfected with an alcohol swab before needle insertion. Needles and syringes are not reused. If IV fluids are not attached continuously, catheters should be flushed with 3 ml of heparinized saline solution every 6 hours. It is important to avoid administering too much heparinized saline too frequently, or the foal may become heparinized. If IV fluids are attached continuously, 3 ml of sterile saline without heparin may be used to flush the catheter between medications. The interval for catheter changes depends on the type of catheter material used and the status of the vein. Teflon catheters should be removed and replaced within 72 hours. Silastic or polyurethane catheters are less thrombogenic and may remain in place for several weeks, but the catheter site should be monitored closely for swelling or thickening of the jugular vein. These catheters are expensive, but quickly become cost effective when compared with Teflon because of the extended time they can remain in place and the decreased likelihood of developing thrombophlebitis.
The foal should be kept clean, dry, and warm. Milk should be warmed to a tepid (not hot) temperature before it is fed to the foal. Performing physical therapy (passive range of motion) can be a helpful modality for recumbent foals, especially when one joint (often the fetlock joint) has a mild contracture. Foals should be kept in a sternal position to permit the adequate expansion of the lungs. This position can be attained by the use of pillows at the shoulder or wedge-shaped pads. Figure 10-7 demonstrates the correct positioning of a foal in sternal recumbency using a pillow at the foal’s shoulder. The recumbent foal will require frequent turning from side to side every 2 hours to encourage complete ventilation of the lungs and to prevent decubital ulcers. Foals should be encouraged to stand and ambulate, when possible. This effort may range from the handler suspending the foal for a few minutes to the foal standing on his own once assisted.
Restraint should be safe for the foal, mare, and handlers. Ambulatory foals are usually restrained with one hand under the neck and the other surrounding the rump. It is not recommended to restrain a foal by his tail because the handler may inadvertently break the foal’s tail. Bracing the foal against a wall provides more security and better control during a struggle. To lead a foal behind the mare, one person should walk the mare with a chain over her nose. The second person places a long cotton lead around the foal’s chest and places the remaining rope around the rump. Holding the rope at the foal’s withers, the person walks on the left side of the foal.
NUTRITION OF THE NEONATAL FOAL
The healthy foal will nurse readily from the mare and receive adequate nutrition. Ideally a hospitalized foal is able to nurse from the mare to receive adequate nutrition. Some foals are unable to nurse because of different problems (e.g., neonatal encephalopathy, abnormal esophageal motility leading to aspiration pneumonia, and cleft palate). Figure 10-8 demonstrates a foal with neonatal encephalopathy incorrectly seeking to nurse on a wall. An indwelling nasogastric tube can be placed and taped to the foal’s halter to permit feeding every 2 hours. It is an acquired skill to place these slender tubes because they can be difficult to palpate in the esophagus. These tubes can remain in place for weeks (or until the foal rubs it out and requires a new one). It is imperative to check a hospitalized foal’s tongue daily. When foals are unable or reluctant to swallow and treated with antimicrobials, they are prone to develop oral candidiases. This yeast infection will coat the tongue with a white plaque and requires daily treatment (debulking the plaque with a dry 4 × 4 gauze, applying potassium permanganate topically and/or oral fluconazole).
To ensure that adequate nutrition is offered, the foal should be weighed daily. Foals with mild disease will usually gain 1 to 2 lb/day (0.5 to 1 kg). Alternate options for feeding foals are bottle feeding, bucket feeding, or bonding the foal to a nurse mare. Bottle feeding foals is labor intensive and must be done properly, or the foal may develop aspiration pneumonia. This method is often used when a foal is not able to nurse from his mare temporarily. Orphan foals are raised by giving milk in a bucket. Although this method is fine initially for many foals, often behavioral problems will develop, so these foals need to be handled properly to prevent problems.
The accepted energy requirement of the compromised equine neonate is 130 to 150 kcal/kg/day. To meet this requirement, a foal will consume approximately 20% of its body weight per day. Often critically ill foals lack a normal suckle reflex and have an abnormal GI tract. These foals will not tolerate large amounts orally, so feeding should start at 60 ml every 2 hours via a nasogastric tube. If the foal tolerates these feedings, the volume of milk may be gradually increased to 5% to 10% of its body weight, divided into 12 feedings every 2 hours. If the foal does not have reflux when passing a nasogastric tube, an indwelling nasogastric tube can be placed to feed the foal. The tube placement must be checked before each feeding to ensure proper positioning. If the tube has moved, aspiration pneumonia may result. The recumbent foal is placed in sternal recumbency, fed, and kept sternally for 20 minutes after feeding to prevent aspiration of milk.
Many critically ill foals will not initially tolerate enteral nutrition, so feeding should be discontinued if regurgitation, abdominal distention, colic, or severe diarrhea occurs. Additional calories are administered IV (parenteral nutrition) to offer a sufficient energy intake for the critically ill foal. There are two sources for parenteral nutrition, either commercial products or recipes using glucose, amino acids, lipids, trace minerals, and vitamins prepared for each foal. Since parenteral nutrition contains lipids and glucose, it is an optimal medium for bacterial growth. The IV bag and line must remain sterile at all times and IV line changes done every 3 days to eliminate problems with contamination.
Fresh mare’s milk is the ideal source of oral nutrition for foals and is easily digestible. When available, mare’s milk should be used to feed healthy and critically ill neonatal foals. If the foal’s mare is in the hospital, she can be milked every 2 hours (with or without giving oxytocin before milking) to obtain milk for the foal. The mare’s udder and the caretaker’s hands should always be cleaned before milking to prevent mastitis. Proper restraint of the mare is important during milking. Lubricating the hand or udder with sterile lubricating jelly helps decrease chafing. Gentle massage or application of warm compresses before milking helps soften the udder and assist in milk expression. Milk can be collected by hand or by the use of an inverted 60-ml dosing syringe. Several alternatives to mare’s milk are available, but all have their drawbacks. Milk replacers are readily available, but have high salt content, can be difficult for the foal to digest, and can cause diarrhea. Preparations formulated for enteral nutrition of other species are generally not suitable for the foal. Goat’s milk is palatable, but causes some metabolic abnormalities and should not be used alone for extended periods. All utensils used for feeding foals should be thoroughly cleaned and disinfected before and after use because the GI tract is a potential portal for an infection. Once reconstituted, milk replacers should be kept refrigerated. The preparations should be discarded after 2 hours at room temperature.
SUMMARY
Working with neonatal foals can be rewarding. It is especially enjoyable to watch a pregnant mare deliver a healthy foal, see them bond, and eventually observe them running in the pasture together. When a foal becomes ill and requires intensive care, it can be challenging for the owner and the neonatal veterinary team to care for the foal. Figure 10-9 depicts the ultimate desired outcome for an NICU foal. Although some of these foals do not survive, many foals recover and lead normal lives as athletes, in great part because of the tremendous effort of many dedicated veterinary technicians. It is a privilege to work with many of these caring people who continue to move the veterinary profession forward.
Section on Puppies and Kittens
Casal ML: Feline Paediatrics. In Raw ME, Parkinson TJ, editors: The Veterinary Annual, 35:210-235, 1995.
Davidson, A.P. Approaches to reducing neonatal mortality in dogs. In: Concannon P.W., England G., Verstegen J., III., et al, eds. Recent advances in small animal reproduction. Ithaca NY: International Veterinary Information Service, 2003.
Gunn-Moore, D. Small animal neonatology: they look normal when they are born and then they die. Prague: Czech Republic; 2006. WSAVA Proceedings
Hoskins, J.D. Veterinary pediatrics: dogs and cats from birth to six months. Philadelphia: WB Saunders; 2001.
Hotston Moore, P., Sturgess, C.P. Care of neonates and young animals. In: Simpson G.M., England G.C., Harvey M.J., eds. BSAVA manual of small animals. Cheltenham, United Kingdom: British Small Animal Veterinary Assoc, 1998.
Section on Neonatology in the Foal
Axon J, Palmer JE, Wilkins PA: Short and long term athletic outcome of neonatal intensive care unit survivors. In Proceedings of the annual American Association of Equine Practitioners convention, vol 45, Lexington, Ky, 1999.
Barton, M.H., Morris, D.D., Crowe, N., et al. Hemostatic indices in healthy foals from birth to one month of age. J Vet Diagn Invest. 1995;7:380–385.
Barton, M.H., Morris, D.D., Norton, N., et al. Hemostatic and fibrinolytic indices in neonatal foals with presumed septicemia. J Vet Intern Med. 1998;12:26–35.
Bentz, A.I., Wilkins, P.A., MacGillivray, K.C., et al. Thrombocytopenia in two thoroughbred foals with sepsis and neonatal encephalopathy. J Vet Intern Med. 2002;16:494–497.
Clabough, D.L. Disease of the equine neonate. J Equine Vet Sci. 1988;8(1):5–10.
Drummond, W.H. Bridging the gap between the human and equine neonate. In: Rossdale P.D., ed. The application of intensive care therapies and parenteral nutrition in large animal medicine. Deerfield, Ill: Travenol Labs, 1986.
Koterba, A.M. IV fluid therapy and nutritional support in the sick neonate. Equine Vet Educ. 1991;3(1):33–39.
Koterba, A.M., Drummond, W.H., Kosch, P.C. Equine clinical neonatology. Philadelphia: Lea and Febiger, 1990.
Madigan, J.E. Manual of equine neonatal medicine, ed 3. Woodlawn, Calif: Live Oak Publishing; 1997.
Marsh, P.S., Palmer, J.E. Bacterial isolates from blood and their susceptibility patterns in critically ill foals: 543 cases (1991-1998). J Am Vet Med Assoc. 2001;218:1608–1610.
McClure JT, Miller J, Deluca JL: Comparison of two ELISA screening tests and a non-commercial glutaraldehyde coagulation screening test for the detection of failure of passive transfer in neonatal foals. In Proceedings of the annual American Association of Equine Practitioners convention, vol 49, Lexington, Ky, 2003.
McKenzie, H.C., III., Furr, M.O. Equine neonatal sepsis: the pathophysiology of severe inflammation and infection. Compend Contin Educ Pract Vet. 2001;23:661–670.
Paradis, M.R. Update on neonatal sepsis. Vet Clin North Am Equine Pract. 1994;10(1):109–135.
Peek, S.F., Semrad, S., McGuirk, S.M., et al. Prognostic value of clinicopathologic variables obtained at admission and effect of antiendotoxin plasma on survival in septic and critically ill foals. J Vet Intern Med. 2006;20:569–574.
Pierce SW: Foal care from birth to 30 days: a practitioner’s perspective. In Proceedings of the annual American Association of Equine Practitioners convention, vol 49, Lexington, Ky, 2003.
Vaala, W.E., House, J.K., Madigan, J.E. Initial management and physical examination of the neonate. In Smith B.P., ed.: Large animal internal medicine: diseases of horses, cattle, sheep, and goats, ed 3, St Louis: Mosby, 2002.
 TECHNICIAN NOTE
TECHNICIAN NOTE