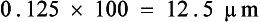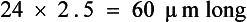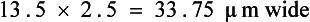
Parasitology
When you have completed this chapter, you will be able to:
1 Define the following terms: intermediate host, definitive host, reservoir host.
2 State the scientific and common names for the nematode, cestode, trematode, and protozoal parasites commonly encountered in small and large animal practice.
3 List the definitive and intermediate hosts for the nematode, cestode, trematode, and protozoal parasites commonly encountered in small and large animal practice.
4 Describe the collection and handling of fecal samples from small and large animals.
5 Describe the procedures for the gross examination and direct examination of fecal samples and state the advantages and limitations of the procedure.
6 List the methods commonly used to concentrate parasitic material in fecal samples.
7 Describe the procedure for performing standard fecal flotation and centrifugal flotation.
8 List and describe the procedures for the identification of blood parasites.
9 List the scientific and common names for ectoparasites commonly encountered in small and large animal practice.
10 List and describe the procedures for the collection of samples and the identification of ectoparasites.
ENDOPARASITES OF DOMESTICATED ANIMALS
Endoparasites live within an animal. They derive their nutrition and protection at the expense of the infected animal, which is called the host. The various internal parasites have many different life cycles. Each parasite’s life cycle is distinctive and is composed of various developmental stages, all of which may occur within the same host or separately within sequential hosts.
The host that harbors the adult (sexually mature) stage of a parasite is called the definitive host. The dog, for example, is the definitive host for Dirofilaria immitis; adult male and female heartworms are found in the right ventricle and pulmonary arteries of the dog’s heart. The host that harbors the immature (asexual) stages of a parasite is called the intermediate host. For example, the mosquito is the intermediate host for D. immitis because first, second, and third larval stages of D. immitis are found within this insect.
The life cycle of most parasites has at least one stage at which the parasite may be passed from one host to the next. Diagnostic procedures frequently detect this stage, and it is therefore called the diagnostic stage. The microfilarial stage is the diagnostic stage of D. immitis because a concentrated blood sample will frequently show the presence of D. immitis in the blood. The diagnostic stage of a parasite may leave the host in many ways; through excreta, such as feces or urine, or through the bloodstream when an arthropod, such as a mosquito, takes a blood meal. In the case of D. immitis, the female mosquito ingests microfilariae during a blood meal and transports it to the definitive host (the dog) when it later feeds on the dog.
CLASSIFICATION OF ENDOPARASITES
Nematodes often are referred to as roundworms and are one of the most important groups of parasites in veterinary parasitology. They may be found in almost any tissue of domestic animals, including the intestines, skin, lungs, kidneys, urinary bladder, nervous tissue, musculature, and blood. As a group, nematodes have diverse, complicated life cycles. They have separate sexes (male nematodes and female nematodes). Their eggs or larvae are most commonly recovered from the feces, but some species that inhabit the urinary bladder and kidney may shed their eggs in the urine. Examples of intestinal nematodes found in dogs include ascarids (Toxocara canis, Toxascaris leonina), hookworms (Ancylostoma caninum, Uncinaria stenocephala), and whipworms (Trichuris vulpis). Roundworms found in the urinary system include canine kidney worms (Dioctophyma renale). Those found in the respiratory system include the lungworms of cattle and sheep (Dictyocaulus spp. and Muellerius capillaris). Nematodes of the blood vasculature are a special group, of which the heartworm of dogs (D. immitis) is an example. Adult female heartworms give birth to small, wormlike prelarval (embryonic) stages called microfilariae. The microfilariae may be observed in a peripheral blood smear and are approximately 310 μm long. Mosquitoes acquire the microfilariae from a blood meal, and within the mosquito, the microfilariae develop into infective third-stage larvae, which are subsequently transmitted to other animals by the infected mosquitoes.
Cestodes (Tapeworms)
Cestodes (tapeworms) are long, flat, segmented worms that resemble strips of white ribbon. They are hermaphroditic, meaning that they possess both male and female sex organs and can reproduce alone. The long ribbonlike body is divided into proglottids, which are segments connected like train cars behind a scolex or “head.” The scolex allows the tapeworm to attach to the wall of the host’s intestine. Most tapeworms release proglottids from the terminal end into the lumen of the gut where they emerge from the animal during defecation and can be found in the feces. A few tapeworms release eggs rather than proglottid segments directly into the gastrointestinal tract of the host.
Proglottids can be observed with the naked eye. Because the proglottid segments have muscles that enable them to move about, it is not unusual for the owners of infected animals to see “little white worms” crawling on the pet’s feces, hair coat, or bedding. Fresh proglottids are said to resemble “cucumber seeds,” whereas dried ones resemble uncooked grains of rice.
Tapeworm proglottids often contain eggs when they are passed into the feces. Each egg contains a hexacanth embryos that contaminate the vegetation where the host has defecated. An intermediate host, such as a rabbit, ingests these hexacanth embryos as it feeds on ground dwelling vegetation and seeds. The hexacanth embryo grows within the tissues of the intermediate host to a “bladderworm” stage, which is a fluid-filled larval stage. The definitive host, such as a cat or dog, becomes infected by ingesting the intermediate host (e.g., rabbit), which contains the bladderworm larval stage. Examples of tapeworms that develop into the bladderworm stage in the intermediate host are the canine taeniid tapeworm (Taenia pisiformis) and the coenurus tapeworm (Multiceps multiceps). In some tapeworms (Echinococcus granulosus, Echinococcus multilocularis), the larval stage within the intermediate host forms a large, fluid-filled cyst called a hydatid cyst, which forms in internal organs, such as the lung, liver, kidney, spleen, and brain of the animal (or human).
When the intermediate host is an arthropod, such as a flea or a grain mite, the hexacanth embryo develops into a microscopic larval stage known as a cysticercoid. The cysticercoid stage is tiny and contains a small fluid-filled space. The definitive host becomes infected by ingesting the intermediate host, which contains the cysticercoid larval stage. The cysticercoid stage is associated with the fringed tapeworm of cattle (Thysanosoma actinoides) and the double-pore tapeworm (Dipylidium caninum) of dogs and cats.
Trematodes (Flukes)
The trematodes are leaf-shaped, flatworms with unsegmented bodies. Adults are hermaphroditic, and in domestic animals in the United States are found primarily in the intestinal tract, but some species have also been found in the liver and lungs. Flukes of veterinary importance include the liver flukes of cattle and sheep (Fasciola hepatica, Fascioloides magna, Dicrocoelium dendriticum) and the lung fluke of dogs and cats (Paragonimus kellicotti).
Adult flukes lay eggs that are passed in the feces. The end portion of many fluke eggs has a small cap or lid called an operculum, and this is easily identifiable under a microscopic examination.
Within each fluke egg is a larval stage known as a miracidium, which hatches and exits the egg through the operculum. This stage penetrates the first intermediate host, which is usually a snail. Within the snail, the miracidium develops into a sporocyst, which then produces many tiny internal structures called rediae. Each redia may produce many internal cercariae. The cercariae exit the snail and may take one of three pathways before entering the definitive host: it may be ingested by a second intermediate host and become encysted as the metacercarial stage within the second intermediate host (which is subsequently ingested by the definitive host); it may encyst on vegetation, which is subsequently ingested by the definitive host; or cercariae may directly penetrate the skin of the definitive host.
Protozoa (Unicellular Organisms)
Protozoa are unicellular, or single-celled organisms, some of which may be parasitic in domestic animals and can infect a variety of tissue sites within the definitive host. The most common sites for their detection are in blood samples and in fecal samples. If they inhabit blood, they are called blood protozoa, and if they inhabit the gastrointestinal system, they are called intestinal protozoa. The protozoan’s life cycle may be simple or complex.
Most hemoprotozoa seen in the United States are found in red blood cells (RBCs) during an examination of a stained blood smear. Ticks usually serve as intermediate hosts and transmit the hemoprotozoa from one animal to the next while feeding on multiple hosts. Babesia bigemina is a tear-shaped hemoprotozoan found within the RBCs of infected cattle. It is transmitted by the tick, Boophilus annulatus.
Trypanosomes are another group of hemoprotozoans occasionally found in the United States. Rather than being found within RBCs, trypanosomes are extracellular and “swim” within the blood. They are 3 to 10 times as long as an RBC is wide and are banana shaped. They have a lateral undulating membrane and a thin, whiplike “lash” (flagellum) that is used for swimming. These parasites are also transmitted by blood-feeding arthropods.
Acanthocephalans (Thorny-Headed Worms)
Acanthocephalans (thorny-headed worms) are uncommon parasites with complicated life cycles. Like the nematodes, they have separate sexes. On the cranial end of these helminths is a spiny proboscis, which is used to attach to the lining of the intestinal wall. Thorny-headed worms do not have a true gut; they absorb nutrients through their body wall. Acanthocephalans usually are recovered at necropsy.
A famous acanthocephalan is Macracanthorhynchus hirudinaceus, a parasite of pigs. This parasite has the dubious honor of possessing the longest scientific name among the parasites of domestic animals. Oncicola canis is an acanthocephalan found in the small intestine of dogs.
ENDOPARASITES OF DOGS AND CATS
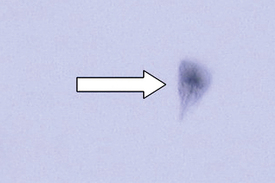
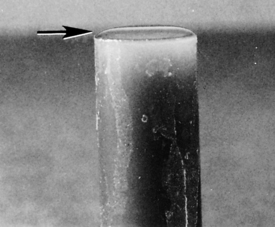
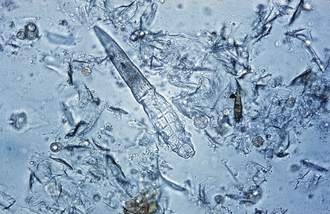
Esophageal worms: Spirocerca lupi, the esophageal worm, is a nematode that often forms nodules in the esophageal wall of dogs and cats. Occasionally, it may be found in gastric nodules in cats. Adult worms reside deep within these nodules and expel their eggs through tunnel-like openings in the nodule. Eggs are passed through the host animal’s intestine and out in the feces. The thick-shelled eggs contain a larva when they are laid; these eggs have a unique “paper clip” shape. Figure 17-1 shows the characteristic ovum of S. lupi. Eggs can usually be observed on fecal flotation and can be recovered when vomitus has been subjected to a standard fecal flotation procedure. A radiographic or endoscopic examination may also reveal characteristic granulomas within the esophagus or within the stomach.
Stomach worms: Physaloptera spp. are stomach worms of dogs and cats. Although they are occasionally found in the lumen of the stomach or small intestine, Physaloptera spp. are usually firmly attached to the mucosal surface of the stomach, where they suck blood. At this site, it is possible to view these nematodes using an endoscope. Their diet consists of blood and tissue derived from the host’s gastric mucosa. Their attachment sites continue to bleed after the parasite detaches. Vomiting; anorexia; and dark, tarry stools may be observed in affected animals.
The adult worms are creamy white, sometimes tightly coiled, and 1.3 to 4.8 cm long. They are often recovered in the pet’s vomitus and can easily be confused with the ascarids (roundworms). A quick way to differentiate these two parasites is to break open an adult specimen and (if that specimen happens to be female) examine the egg type microscopically. The eggs of Physaloptera spp. are small, smooth, thick-shelled, and contain a larva when passed in the feces. Eggs can usually be recovered on a standard fecal flotation, using solutions with a specific gravity above 1.25. If a vomiting dog is suspected of harboring Physaloptera spp., a fecal flotation can be performed on the vomitus to determine if the characteristic eggs are present.
Ollulanus tricuspis, “the feline trichostrongyle” is usually associated with vomiting in cats. This nematode is most commonly identified by examining the cat’s vomitus with a dissecting or compound microscope. Feline vomitus can also be examined using a standard fecal flotation procedure. The best flotation solution for detection is a modified Sheather’s flotation solution. Adult female O. tricuspis are 0.8 to 1.0 mm long and have three major tail cusps (i.e., toothlike projections [hence, the epithet tricuspis]). Adult males are 0.7 to 0.8 mm long and have a copulatory bursa. The female worms are larviparous (they bear live larvae). These larvae mature to the infective third-stage larvae, and they complete development to the adult stage in the cat’s stomach. Free-living stages in the external environment are not required for the completion of the life cycle. Transmission occurs through the ingestion of vomitus from infected cats.
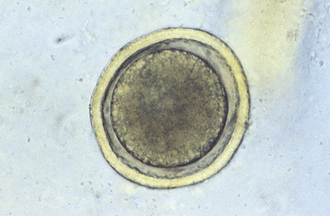
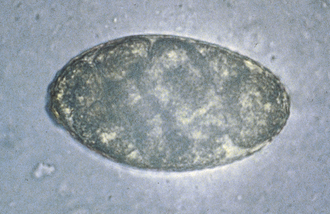
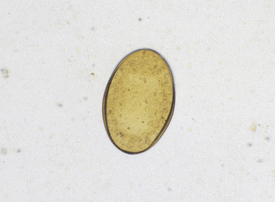
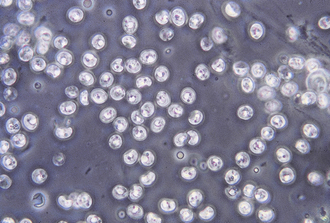
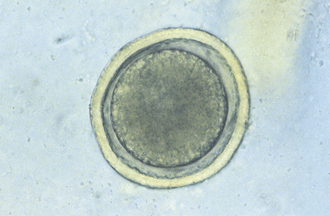
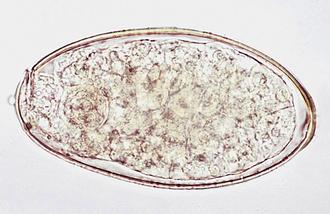
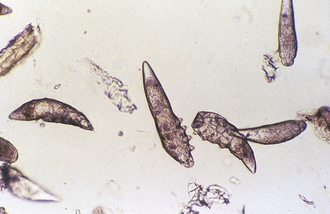
Ascarids: Toxocara canis, T. cati, and Toxascaris leonina are ascarids of dogs and cats. They are large, robust, white to cream-colored nematodes. Mature specimens measure about 3.5 to 5 cm (males) and 10 to 15 cm (females). Eggs of the Toxocara spp. are large, round to oval, and dark colored with thick, rough shells (Figures 17-2 and 17-3) T. leonina eggs are lighter in color and more egg shaped and have thick, smooth shells (Figure 17-4). All three species of ascarids are common in most geographic regions of the United States. The larval stage develops within the eggs; it will develop to the second stage larva, the infective stage for this parasite. Ascarid eggs are highly resistant to adverse conditions and in ideal environmental conditions become infective in about 2 weeks. Once ingested, Toxocara spp. hatch in the small intestine, penetrate the mucosa, migrate through the liver, pass through the heart, and go into the lungs, in which they develop within a short period. Larvae are coughed up and swallowed, and they mature to the adult stage in the small intestine within 4 to 6 weeks.
T. canis eggs hatch in the small intestine, and the larvae penetrate the intestinal mucosa to develop for about 2 to 3 months and then return to the intestinal lumen as adults. In dogs older than 3 months, most of the larvae leave the circulation and are stored in the somatic organs until the dog becomes pregnant. Between the 42 and 56 days of gestation, these larvae leave the somatic tissues, cross the placenta, enter the fetal lungs, and remain there until birth. The larvae then complete the cycle already described. Consequently a high percentage of dogs are infected via the prenatal or transplacental route. In pregnant dogs and cats, some of the activated Toxocara larvae migrate to the mammary glands. These larvae are ingested by puppies and kittens when they start to nurse. Transmammary infections are more common in cats than in dogs. The eggs of all three ascarids can be ingested by other animals (e.g., mice, chickens) and remain infective in their tissue until these animals are eaten by an appropriate canine or feline host. All three species are readily identified by several techniques, and they are amenable to treatment with several anthelmintics. Control is difficult because the eggs are resistant; control measures include thorough cleansing of kennels, runs, yards, and so forth. It is important that these eggs containing the infective larvae be removed from the premises. If these eggs are ingested by a human (e.g., a small child), the larvae will hatch from the eggs and undergo a migration through the liver and lungs or the eyes of the child. This condition is a zoonotic condition and is called visceral larva migrans.
Hookworms: Hookworms are a type of nematode found throughout the world and are common in tropical and subtropical areas of North America. A hookworm infection, which can produce severe anemia in young kittens and puppies, can be a serious problem in kennels and catteries. The prepatent period depends on the species of hookworm and the route of infection.
Ancylostoma caninum, the canine hookworm, is found in the small intestine of dogs, foxes, coyotes, wolves, raccoons, and badgers; Ancylostoma braziliense is found in dogs and cats; Uncinaria stenocephala occurs in dogs, cats, foxes, coyotes, and wolves; Ancylostoma tubaeformis is found in cats and wild Felidae. Hookworms are all short, thick parasites; adult males measure 6 to 12 mm, and females measure 6 to 20 mm. Hookworms produce strongyle-type eggs (Figure 17-5). Ancylostoma spp. are generally found in coastal areas of high rainfall, whereas U. stenocephala is found in the northern portions of North America. All hookworm species have a similar life cycle. Undeveloped eggs pass into the environment, develop and hatch, releasing first-stage larvae that undergo a free-living existence until they develop into infective third-stage larvae. Hookworms are capable of establishing themselves in the host after ingestion, but the normal mode of infection is skin penetration. After larvae penetrate, they enter the venous circulation, going ultimately to the lungs, in which they develop for a short period. They are then coughed up and swallowed, and they enter the small intestine and mature. This generally occurs within 4 to 6 weeks.
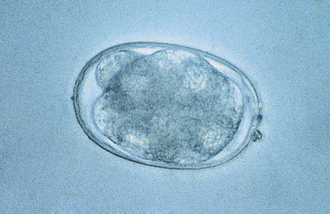
FIGURE 17-5 Characteristic hookworm ovum. The eggs of A. caninum are 56 to 75 μm by 34 to 47 μm, those of A. tubaeformis are 55 to 75 μm by 34.4 to 44.7 μm, those of A. braziliense are 75 μm by 45 μm, and those of U. stenocephala are 65 to 80 μm by 40 to 50 μm.
A. caninum has developed the additional modes of transplacental and transmammary infection. Third-stage larvae penetrate the skin and circulate in the pregnant female host, ultimately crossing the placenta. The larvae are also stored in somatic tissues until the female host becomes pregnant. In dogs, most of the somatic larvae activated at the time of pregnancy migrate to the mammary glands of the bitch and are passed on to nursing puppies. The diagnosis can be made by the identification of the eggs with a number of techniques, and these species are amenable to treatment with a number of anthelmintics. Control is difficult, especially in warm, humid geographic regions, necessitating regular thorough cleansing of yards, kennels, and other areas.
If the infective third stage hookworm larvae penetrate the skin of a human being (particularly children as they play in the sand or dirt), these larvae will migrate through the skin, producing a red, itchy, winding tunnel in the skin. This condition is a zoonotic condition and is called cutaneous larva migrans.
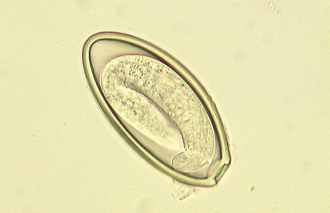
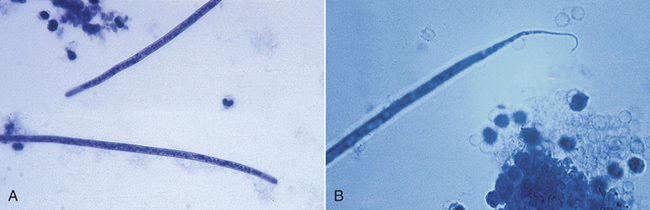
Intestinal threadworms: Strongyloides stercoralis is a nematode that is a tiny parasite that is found in the small intestinal mucosa of dogs, cats, foxes, humans, primates, and possibly other wild carnivores. Only the female nematode is parasitic; there are no parasitic males. The females reproduce by parthenogenesis (reproduction without fertilization from a male). The eggs develop in utero, and nematodes give birth to eggs that will hatch into first-stage larvae (Figure 17-6) while they are within the host. The chromosome number of the larvae determines whether they will develop into a parasitic generation that must develop to an infective third-stage larvae that must infect a new host or whether they will become a free-living male or female stage that will breed and produce eggs that will eventually develop into third-stage infective larvae that must infect a new host.
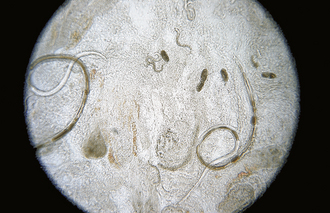
FIGURE 17-6 Parasitic adult females, eggs, and first-stage larvae of Strongyloides spp. These larvae are 280 to 310 μm long and have a rhabditiform esophagus, with a club-shaped cranial corpus, a narrow median isthmus, and a caudal bulb.
The infective larvae are capable of establishing an infection by oral ingestion, after which they penetrate the small intestine and develop there. However, the primary mode of infection is skin penetration. If the larvae penetrate the skin, they then penetrate the venous circulation, going ultimately to the lungs to develop for a short period. Larvae are then coughed up and swallowed and penetrate the mucosa of the small intestine. In immunologically compromised animals, infections may be severe. S. stercoralis is widespread in tropical and subtropical regions and in kennels and pet shops in which environmental conditions are suitable. The diagnosis is not difficult. Frequently a direct smear of fresh feces is suitable for the identification of eggs containing first-stage larvae. The treatment is not always satisfactory, and alternative anthelmintics should be considered. Control necessitates thorough cleaning of facilities and allowing the facilities to dry.
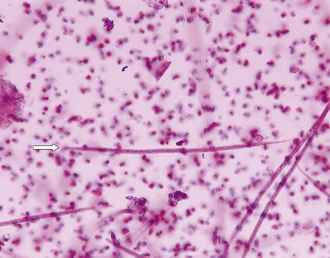
Whipworms: Trichuris vulpis occurs in the cecum of the dog, fox, and coyote. Like all whipworms, T. vulpis has a slender anterior end with its mouth at the tip and a thickened posterior extremity, resulting in its unique whiplike appearance. Male and female worms are about the same length, measuring 45 to 75 mm. The symmetrical eggs are distinctive, possessing thick, brown-yellow shells with a clear polar plug at each end (Figure 17-7). T. vulpis is widespread in temperate zones, and the incidence of infection is frequently high. The life cycle is simple and direct. The larvae develop within the eggs to the infective second stage. When the eggs are ingested, the larvae are released in the intestine, which they penetrate. Larvae develop within 8 to 10 days, return to the lumen of the intestine, migrate to the cecum, and bury their anterior ends into the mucosa of the cecum. They mature to the adult stage in an additional 60 to 80 days. The diagnosis can be effectively accomplished by a number of procedures, but eggs are quite heavy, and an interpretation of the severity of the infection based on the number of eggs present is not possible. Several treatments are available. Control is difficult because eggs are highly resistant to environmental conditions. Sanitation, as applied for ascarids, is the best approach.
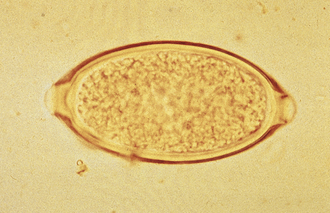
FIGURE 17-7 Characteristic ovum of Trichuris vulpis. Eggs of T. vulpis are 70 to 89 μm by 37 to 40 μm.
Whipworm infections are quite rare in cats and wild Felidae in the United States; however, Trichuris campanula has been reported to occur in the United States. Whipworm eggs can be confused with the eggs of Aonchotheca putorii, the gastric capillarid of cats. Occasionally, eggs of Capillaria spp. have been found in feces of both dogs and cats. The eggs of Capillaria spp. are similar, but not as dark in color, and on average, the eggs are somewhat smaller than those of whipworms. Frequently the eggs of trichurids or capillarids parasitize the prey of outdoor cats, such as mice, rabbits, or birds. The eggs of these nonfeline trichurids or capillarids may pass unaltered through the cat’s gastrointestinal system, remaining intact and unembryonated, thus appearing to infect the feline host. Veterinary technicians should be careful not to be fooled by these pseudoparasites when examining a cat’s feces.
Pinworms: Enterobius vermicularis is the human pinworm and does not parasitize dogs or cats. Nevertheless, the family pet is often falsely incriminated by physicians, family practitioners, or pediatricians as a source of pinworm infection in young children. The veterinary technician should remember this rule: pinworms are parasites of omnivores (mice, rats, monkeys, people) and herbivores (rabbits, horses), but never are parasites of carnivores (dogs, cats).
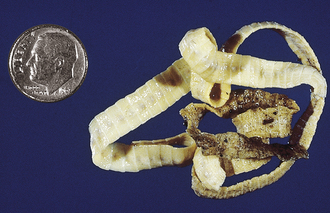
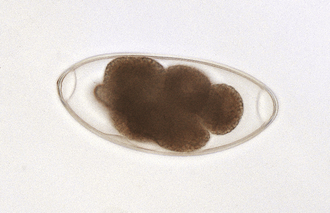
True tapeworms: Dogs, cats, the wild Canidae, and some of the wild Felidae are susceptible to infection by a number of adult tapeworms. The most commonly found tapeworm species are Dipylidium caninum, Taenia hydatigena, T. pisiformis, Taenia ovis, Taenia krabbei, Multiceps serialis, Echinococcus granulosus, and E. multilocularis. Cats, and some of the wild Felidae, are generally infected with Taenia taeniaeformis and D. caninum. The species of tapeworms found in dogs and cats depends on the pet’s geographic location and the amount of free-ranging activity the animals are allowed to pursue.
Some tapeworms use an intermediate host in which the larval stage (a microscopic cysticercoid stage) develops. D. caninum, the double-pored or cucumber seed tapeworm, uses a flea as its intermediate host; the flea contains this larval infective cysticercoid stage. T. hydatigena, T. ovis, and T. krabbei use ruminants, usually sheep, deer, elk, and moose, as intermediate hosts. In these hosts, the larval infective stage (a fluid-filled bladderworm or cysticercus) develops in the body cavity (T. hydatigena) or muscles (T. ovis and T. krabbei). The fluid-filled bladderworms or cysticerci of T. pisiformis develop in the body cavities of rabbits. M. serialis develops in subcutaneous areas or in the muscles (as a fluid-filled coenurus) of rabbits; this larval infective stage is known as Coenurus serialis. The larval infective stage of T. taeniaeformis (a strobilocercus) develops in the livers of mice, rats, and other small rodents. E. granulosus uses ruminants, such as sheep, deer and elk, and humans, as intermediate hosts. This tapeworm’s larval infective stage is a rather large, fluid-filled bladder called a unilocular hydatid cyst that is easily recognized by its large size (25 to 100 mm in diameter). This hydatid cyst is typified by the presence of numerous small pieces of larval tapeworms (brood capsules lined with thousands of protoscolices) on its inner lining surface and the presence of compartments within the body of the cyst in which daughter cysts have grown and fused together. E. multilocularis uses rodents, such as rats, mice, and voles, as intermediate hosts. This tapeworm’s infective larval stage is a fluid-filled bladder called a multilocular hydatid cyst that is easily recognized by its many compartments and invasive nature. This hydatid cyst also contains numerous small pieces of larval tapeworms (brood capsules with thousands of protoscolices) on the inner lining surface. In all instances of tapeworm life cycles, the canine or feline host becomes infected by ingesting the respective tissues containing these assorted larval infective stages.
The diagnosis of Taenia spp. and D. caninum infections in the dog or cat is normally made by finding the proglottids (tapeworm body segments), or chain of proglottids, around the host’s anal region or on its hocks. Although the eggs of these tapeworms will float in a standard fecal flotation, they are usually not released to mix with the feces. Proglottids of Taenia spp. have one genital lateral opening or pore per proglottid, whereas proglottids of D. caninum have two, one on either lateral side of the proglottid (Figure 17-8). The further identification of Taenia spp. beyond the genus designation is extremely difficult, requiring morphologic study of the intact parasite, most specifically the anterior end or scolex with its unique double row of hooklets.
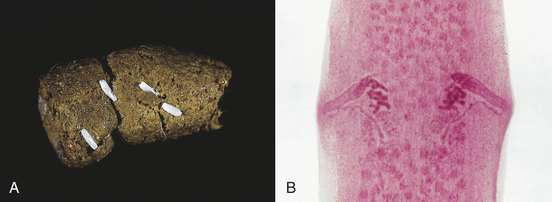
FIGURE 17-8 A, Characteristic motile, terminal, gravid proglottids of D. caninum on canine feces. In the fresh state, these proglottids resemble cucumber seeds; hence, the common name, the “cucumber seed” tapeworm. B, These proglottids of D. caninum have a lateral pore along the midpoint of each long edge, thus the second common name, double-pored tapeworm.
Gravid proglottids of D. caninum are filled with thousands of egg packets. Each egg packet may contain up to 30 hexacanth embryos. Dried proglottids of D. caninum resemble uncooked grains of rice. When water is added, the proglottids assume their natural form (Figure 17-9).
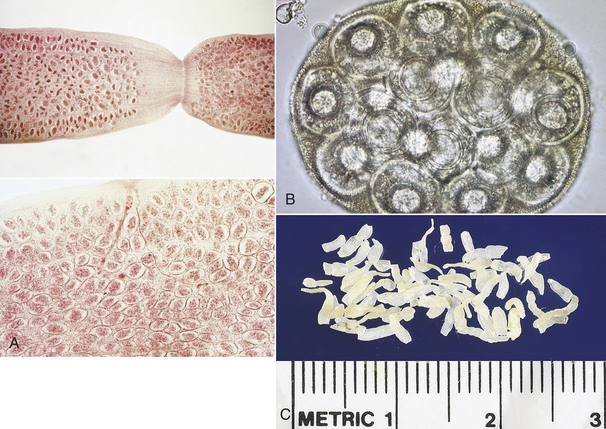
FIGURE 17-9 A, Gravid proglottids of D. caninum are filled with thousands of egg packets. B, Characteristic egg packet of D. caninum. C, Dried proglottids of D. caninum resemble uncooked grains of rice.
The eggs of Taenia spp. contain a single oncosphere with three pair of internal hooks. The oncosphere is often called a hexacanth embryo. The eggs of Taenia spp. are similar to those of Echinococcus and Multiceps spp. (Figure 17-10). These eggs are often referred to as “typical taeniid-type eggs.”
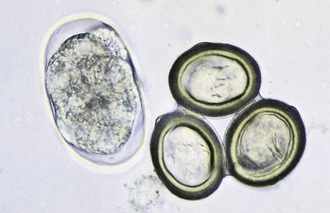
FIGURE 17-10 Characteristic ova of the taeniid tapeworms are slightly oval and 43 to 53 μm by 43 to 49 μm in diameter (T. pisiformis), 36 to 39 μm by 31 to 35 μm in diameter (T. hydatigena), and 19 to 31 μm by 24 to 26 μm (T. ovis). Eggs of Taenia spp. contain a single oncosphere with three pairs of hooks. The oncosphere is often called a hexacanth embryo. The eggs are similar to those of Echinococcus and Multiceps spp. The dissimilar ovum is that of A. caninum, the hookworm.
E. granulosus eggs frequently mix with the feces (unlike Taenia spp.), but the eggs are typical Taenia-type eggs, possessing thick, striated egg shells and containing a six-toothed (hexacanth) embryo. D. caninum eggs, if seen in feces, occur in packets or groups of 20 to 30 embryos contained within a thin-walled membrane. Several treatments are available. Control depends on the tapeworm species. The presence of D. caninum obviously necessitates the implementation of rigorous flea control programs. When Taenia, Multiceps, or Echinococcus spp. are present, dogs and cats should be restricted from consuming the flesh or viscera of the infected mammalian intermediate hosts.
Pseudotapeworms: Spirometra spp., “zipper tapeworms” or sparganum tapeworms (Figure 17-11), are often found in the small intestine of dogs and cats in Florida and along the Gulf Coast of North America. Gravid segments usually are not discharged into the pet’s feces. While the adult tapeworm is attached to the host’s jejunum, the mature proglottids will often separate along the longitudinal axis for a short distance along its length; the tapeworm appears to “unzip.” This “zipped” and “unzipped” appearance gives the tapeworm its common name, the “zipper tapeworm.” The egg of Spirometra spp. resembles that of a fluke, or digenetic trematode (Figure 17-12). This egg has a distinct operculum at one end of the pole of the shell. The eggs are oval and yellowish brown and are unembryonated when passed in the feces.
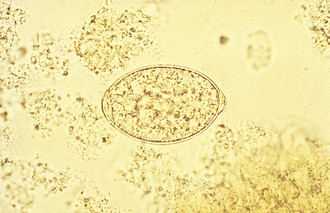
FIGURE 17-12 Characteristic ovum of S. mansonoides. The egg of Spirometra spp. resembles that of a fluke (digenetic trematode). The eggs average 60 by 36 mm and have an asymmetric appearance. These eggs tend to be rather pointed at one end.
Diphyllobothrium latum often referred to as “broad fish tapeworms,” may be 2 to 12 m long; however, it probably does not grow as large as 12 m in dogs and cats. These tapeworms continually release operculated eggs until they exhaust their uterine contents. The terminal proglottids become senile rather than gravid and detach in chains rather than individually. The egg of Diphyllobothrium latum also resembles that of a fluke (digenetic trematode). The egg is oval and light brown and possesses a distinct operculum at one end of the shell. The eggs are unembryonated when passed in the feces.
Trematodes (Flukes): Platynosomum fastosum is the “lizard poisoning fluke” of cats (Figure 17-13). The adult flukes inhabit the liver, gall bladder, bile ducts, and less commonly the small intestine. The brownish, operculated eggs are 34 to 50 μm by 20 to 35 μm.
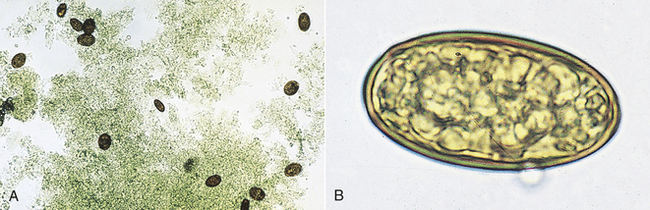
FIGURE 17-13 A, Characteristic ova of P. fastosum, the “lizard poisoning fluke” of cats. B, The brownish, operculated eggs are 34 to 50 μm by 20 to 35 μm.
Nanophyetus salmincola is the “salmon poisoning fluke” of dogs in the Pacific Northwest region of North America. The adult fluke inhabits the small intestine and serves as a vector for rickettsial agents, which produce “salmon poisoning” and “Elokomin fluke fever” in dogs. The eggs are unembryonated when laid and measure 52 to 82 μm by 32 to 56 μm (Figure 17-14). The eggs have an indistinct operculum and a small, blunt point at the end opposite the operculum.
Alaria spp. are intestinal flukes of dogs and cats and are found throughout the northern half of North America. Their ova are large, golden brown, and operculated (Figure 17-15). They are 98 to 134 μm by 62 to 68 μm.
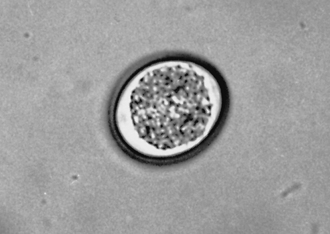
Protozoa (Unicellular Organisms): Giardia spp. are common protozoan parasites of dogs and cats in the United States. A higher incidence of infection occurs in dogs, cats, humans, and beavers than in other animals, such as deer, sheep, moose, and antelope. There are two forms of Giardia. The motile form (the trophozoite), which is approximately 12 to 17 μm long and 7 to 10 μm wide, is found in the small intestine (Figure 17-16). The cyst form (the infective stage) is approximately 9 to 13 μm long (Figure 17-17). When the cyst form is ingested, the cyst wall is digested away in the small intestine releasing the trophozoite, which immediately divides into two organisms. These organisms attach to the epithelial cells lining the small intestine and continue to multiply by binary fusion over the next 6 to 10 days until a large population exists. At that time, diarrhea develops and Giardia spp. begin to produce cysts. The diagnosis can be accomplished by means of the direct fecal-saline smear or, more effectively, with the zinc sulfate centrifugal flotation technique. A treatment is available. Giardia infection is more commonly found among young dogs and cats crowded into kennels and animal shelters. The most effective control procedure is cleanliness and disinfection with quaternary ammonium compounds.
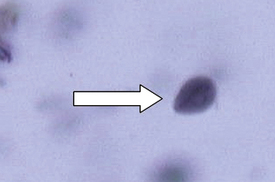
FIGURE 17-17 Cysts of Giardia spp. The mature cysts are oval and measure 8 to 10 μm by 7 to 10 μm. These have a refractile wall and four nuclei. Immature cysts, which represent recently encysted motile forms, contain only two nuclei.
Coccidia: Dogs and cats are hosts for many species of Isospora, Cryptosporidium, and Sarcocystis, and the cat is the definitive host for Toxoplasma gondii. The incidence and severity of coccidial infection depend on the host’s age and immune status, conditions in which the hosts are housed, and the diet and quality of drinking water.
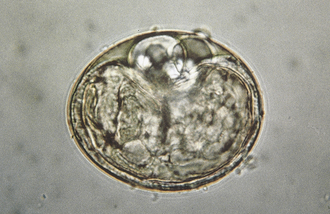
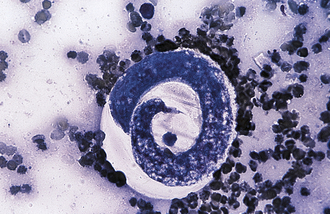
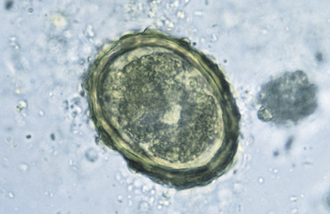
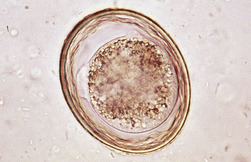
The species of Isospora have a direct life cycle. The life cycle begins with an oocyst in the feces (Figure 17-18). This oocyst must sporulate (develop into its infective form) (Figure 17-19); it does so in less than a week, given optimal conditions of warmth and moisture. Once infective, the oocyst encloses two sporocysts, each of which encloses four small, banana-shaped, infective forms called sporozoites for a total of eight infective forms in each oocyst. When ingested, the oocyst and sporocyst walls are digested in the intestine, releasing sporozoites to penetrate the intestinal epithelium and enter a cell for subsequent development. Within the intestinal cell, the sporozoites become spherical and begin to grow to a large size, the schizont, a large structure filled with the merozoites. The nucleus replicates several times, and ultimately, thousands of small, banana-shaped organisms called merozoites develop. This asexual process of reproduction is called schizogony. Once mature, the schizont ruptures, releasing its merozoites. The next step in the life cycle is species dependent, but usually the merozoites move farther down the intestine, penetrate a cell, and repeat the asexual process, but with smaller schizonts containing fewer merozoites. When released, the merozoites penetrate a cell, and some become macrogametes (ova), and some become microgametes (sperm). Once fertilization occurs, the oocyst is produced and passes in the feces to begin the life cycle again. Although the life cycle is finite (e.g., only a given number of oocysts can be produced from a single oocyst infection), the reproductive potential is great for some species.
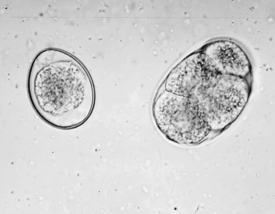
FIGURE 17-18 Unsporulated oocyst of Isospora spp. (left). Oocysts vary greatly in size. Also see Figure 17-19, A. caninum (right).
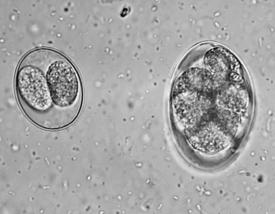
FIGURE 17-19 Sporulated oocyst of Isospora spp. The canine coccidians and their measurements are I. canis, 34 to 40 μm by 28 to 32 μm; Isospora ohioensis, 20 to 27 μm by 15 to 24 μm; and Isopora wallacei, 10 to 14 μm by 7.5 to 9.0 μm. The feline coccidians and their measurements are I. felis, 38 to 51 μm by 27 to 29 μm, and Isospora rivolta, 21 to 28 μm by 17 to 23 μm. A. caninum (right).
Species of Cryptosporidium have essentially the same type of life cycle. Cryptosporidium organisms inhabit the respiratory and intestinal epithelia of many hosts, including birds, mammals, reptiles, and fish. Dogs and cats develop an intestinal tract infection almost exclusively. Enteroepithelial (intestinal epithelial) development is limited to the luminal enterocytes; extraintestinal tissue cysts do not develop. The enteroepithelial life cycle begins with the ingestion of sporulated oocysts by a suitable host (Figure 17-20). After the ingestion of oocysts, eight sporozoites are released from each oocyst that penetrates intestinal epithelial cells. Asexual reproduction at the intestinal surface occurs with the production of merozoites that are released and penetrate other cells. Gametogony and sporogony occur, resulting in the production of thin-walled and thick-walled oocysts. Sporulated thick-walled oocysts are shed in the feces of an infected host and are immediately infective to a susceptible host. Thin-walled oocysts passed into the intestinal lumen rupture, releasing the sporozoites, which penetrate additional host cells and reinitiate the developmental cycle.
Species of Sarcocystis have essentially the same type of life cycle, except that carnivores act as hosts for the sexual stages (oocyst and sporocyst), and omnivores and herbivores act as hosts for the asexual (schizogony) stage. Infected carnivores pass a thin-walled oocyst, which will eventually rupture; the oocyst contains two small, thick-walled sporocysts in which four sporozoites have already developed and are immediately infective to the alternate host. Once ingested, the sporozoites are released and penetrate the epithelial tissue of the intestine. Generally, the sporozoites enter the circulatory system and begin the first asexual (schizogony) phase in the kidneys. The first schizont releases its small, spindle-shaped organisms, which then enter cardiac or smooth muscle, in which they develop into rather large schizonts called sarcocysts. When sarcocysts are ingested by a specific carnivore, and most species are specific for each carnivore-herbivore, the small, spindle-shaped organisms penetrate superficial epithelial cells of the intestine and immediately begin the sexual phase, terminating as a thin-walled oocyst about 11 to 14 days after the ingestion of the infected flesh.
The life cycle of Toxoplasma gondii is similar to that of Sarcocystis spp. except that most animals are suitable hosts for the development of the asexual (schizogony) stages, but only the cat is suitable as a host for the sexual stages. The typical life cycle occurs when a cat ingests the small sporulated oocyst. In the intestine, the parasite goes through two asexual stages and then into the sexual phase, producing oocysts. If, for example, a mouse should eat the oocyst, the first asexual phase occurs in this animal. When a cat eats these schizonts, the parasite goes into one asexual cycle, in the cat’s intestine, followed by the sexual cycle. If the first mouse is eaten by another mouse, Toxoplasma goes into the second asexual cycle in this mouse. When the second mouse is eaten by a cat, the parasites go directly into the sexual phase. The asexual cycle can go on indefinitely as animals eat the flesh of infected animals.
The diagnosis of an Isospora, Cryptosporidium, Sarcocystis, and Toxoplasma infection is based on recovery of the oocyst or sporocyst (for Sarcocystis) by a number of diagnostic procedures. A treatment is seldom administered for a Sarcocystis or Cryptosporidium infection, but when clinical disease occurs, a treatment is recommended for Isospora and Toxoplasma spp. infection. Control of Isospora and Cryptosporidium infections requires cleanliness, removal of the animal to clean premises, or both; however, the oocysts are extremely resistant to environmental conditions. Control of a Sarcocystis infection is generally not practiced for the carnivore host because it is considered nonpathogenic. If control is exercised, the best approach is to prevent the consumption of raw flesh from any source, including ground beef. The best control for Toxoplasma in cats is to prevent the consumption of raw flesh or carrion and to limit contact with feces of infected cats. Both Toxoplasma and Cryptosporidium spp. are zoonotic. Toxoplasma can cause birth defects in humans, and Cryptosporidium can produce a severe diarrhea, especially in immunocompromised individuals.
The Integumentary System
Rhabditis strongyloides is a free-living saprophytic nematode that normally lives in moist soil and is considered to be a facultative parasite. These nematodes are normally free living in soil mixed with moist organic debris; however, under certain circumstances, they can invade mammalian skin and develop into a parasitic existence. The females produce eggs that hatch into first-stage larvae. These larvae invade the superficial layers of damaged or scarified skin, producing mild dermatitis. Dogs become infested by lying on contaminated soil. The skin may become reddened, denuded, and covered with a crusty material on the ventrum or medial (inner) surface of the limbs.
Adult Dipetalonema (Acanthocheilonema) reconditum is a nonpathogenic nematode that resides in the subcutaneous tissues of the dog. It may also be found within the body cavity. Occasional subcutaneous abscesses and ulcerated areas have been associated with this parasite. The intermediate host for this parasite is the flea, Ctenocephalides felis. Because this parasite is found in enzootic areas where D. immitis is present, it is often necessary to differentiate the microfilariae of these two parasites from each other Adults of D. reconditum rarely are recovered from their subcutaneous sites. Microfilariae may be recovered rarely in deep skin scrapings that draw blood. When subjected to the modified Knott procedure, the microfilariae of D. reconditum average about 285 μm long, with a buttonhook tail and a blunt (broom handle–shape) cranial end.
The Circulatory System
Nematodes (Roundworms): Dirofilaria immitis, the canine heartworm, is a nematode and normally resides in the right ventricle and pulmonary arteries of its definitive host, the dog. Adult heartworms also may occur aberrantly and may be found in a variety of extravascular sites, including cystic spaces in the subcutaneous sites (Figure 17-21). When adult heartworms are found aberrantly, they are usually single, immature, isolated worms. Any female heartworms found within the cyst have not been fertilized by a male heartworm. Therefore such females are not gravid and do not produce microfilariae.
D. immitis and D. reconditum are the two filarial nematodes found commonly in dogs and the wild Canidae in the United States. D. immitis infections may also occur in cats and the wild Felidae, but not as commonly found as in dogs. The heartworm, D. immitis, is found primarily in the right ventricle and pulmonary arteries of the host, whereas D. reconditum is not found in the heart or pulmonary arteries, but instead is found in the subcutaneous tissues. Both nematodes produce a larval form called a microfilaria, which circulates in the blood (Figures 17-22 and 17-23). These filarial nematodes are found commonly in areas of the United States where the intermediate hosts (mosquitoes for Dirofilaria and fleas for Dipetalonema) occur; however, the heartworm is becoming more widespread as infected dogs and cats are brought into areas where the parasite is not normally found.
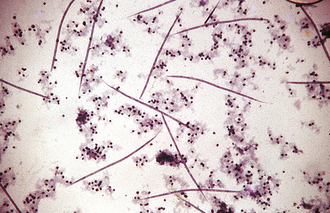
FIGURE 17-22 Microfilariae of D. immitis from a peripheral blood sample subjected to the modified Knott test. The microfilariae of D. immitis average 310 μm long. In contrast, the microfilariae of D. reconditum average 285 μm long.
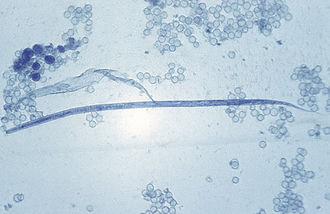
FIGURE 17-23 An individual microfilaria of D. immitis from a peripheral blood sample subjected to the modified Knott test. Note the tapered cranial end and straight tail. Microfilariae of D. reconditum have a blunt (rounded) cranial end and may exhibit a shepherd’s crook (hooked) tail.
D. immitis males measure 12 to 20 cm, and the females are 25 to 31 cm long, whereas D. reconditum males are 9 to 17 mm long, and the females are 20 to 32 mm long. Both nematodes need an intermediate host to complete the life cycle. D. immitis uses several different species of mosquito, and D. reconditum uses the common dog and cat flea. Microfilariae when ingested by the intermediate host undergo reorganization and development to the infective third-stage infective larvae. Once infective, they go into the mouthparts of the arthropod and remain there until the arthropod feeds on the susceptible host. D. immitis infective larvae enter the tissue for 85 to 120 days and develop into young adults. The larvae then go to the heart and reach sexual maturity in another 60 to 70 days, for a total of 145 to 190 days. Heartworms can also be aberrant or erratic parasites, getting “off course” en route to the heart and locating in sites other than the right ventricle and pulmonary arteries, such as the anterior chamber of the eye or in subcutaneous cysts in the skin. D. reconditum apparently goes directly into the subcutaneous tissues to develop to sexual maturity.
A microfilaria of D. immitis is 295 to 325 μm long (average = 313 μm) and 6 to 7 μm in diameter (average = 6.9 μm), whereas a microfilaria of D. reconditum is somewhat shorter and more slender, measuring 250 to 288 μm in length (average = 276 μm) and 4.5 to 5.5 μm in diameter (average = 4.6 μm).
The diagnosis of heartworm disease in the dog is usually based on the identification of microfilariae in the peripheral circulation. Various techniques have been used to detect microfilariae, including the fresh blood-saline preparation; the capillary hematocrit tube test; and modified Knott test (or the filtration concentration) test. Fresh blood-saline preparations are helpful in differentiating D. immitis and D. reconditum microfilariae. D. immitis microfilariae move in place without directional motion, whereas D. reconditum microfilariae have a directional movement across the microscopic viewing field. Concentration tests are best used for the detection of D. immitis microfilariae because they are much more accurate than fresh blood-saline preparations or capillary hematocrit tube tests. Occult heartworm infections (adult heartworms without circulating microfilariae) occur in approximately 25% of dogs and 90% of cats. Several serologic tests are available in commercial kits to test sera of dogs and cats for occult infection; these commercial kits use the ELISA as the basis for diagnosis. The treatment of a D. reconditum infection is unimportant because these parasites are nonpathogenic. The treatment for a D. immitis infection requires the use of an agent effective against adult heartworms, followed by a microfilaricide. Control of D. immitis necessitates daily or monthly heartworm preventive therapy and mosquito control in enzootic areas.
Trematodes (Flukes): Heterobilharzia americana, the canine schistosome, is a blood fluke that parasitizes the mesenteric veins of the small and large intestines and the portal veins of the dog (Figure 17-24). The blood flukes, or schistosomes, are unique flukes in that they are not hermaphroditic (most flukes are hermaphroditic). Among the blood flukes, there are separate sexes; therefore there are male schistosomes and female schistosomes. Because these flukes reside in the fine branches of the mesenteric veins, it is only natural that they should be long and slender. Females may be as long as 9 mm, and males are about 6.5 mm in length. This fluke is enzootic in the mud flats of the Mississippi delta and the coastal swampland of Louisiana. Although H. americana inhabits the blood vasculature, it manifests its presence by a bloody diarrhea. Infected dogs also exhibit emaciation and anorexia. The diagnosis is by the identification of the thin-shelled egg, about 80 by 50 μm, which contains a miracidium.
The Respiratory System
Nematodes (Roundworms): Aelurostrongylus abstrusus is the feline lungworm. The adults live in the terminal respiratory bronchioles and alveolar ducts, where they form small egg nests or nodules. The eggs of this parasite are forced into the lung tissue, where they hatch to form characteristic first-stage larvae, approximately 360 μm long. Each larva has a tail with an S-shape bend and a dorsal spine (Figure 17-25). Characteristic larvae on fecal flotation or the Baermann technique can determine their presence. Recovering the larvae on tracheal washing is also possible.
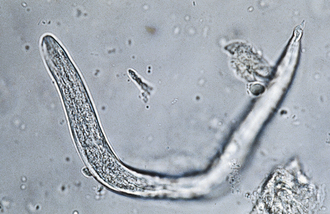
FIGURE 17-25 Characteristic first-stage larva of A. abstrusus, the feline lungworm. The diagnosis is accomplished by finding these characteristic larvae on fecal flotation or by using the Baermann technique.
Filaroides (Oslerus) osleri, Filaroides hirthi, and Filaroides milksi, the canine “lungworms,” are found in the trachea, the lung parenchyma, and the bronchioles of canids, respectively. The larva is 232 to 266 μm long and has a short, S-shape tail. Filaroides spp. are unique among the nematodes in that their first-stage larvae are immediately infective for the canine definitive host. No period of development is required outside the host. The diagnosis is by finding these characteristic larvae on fecal flotation or by using the Baermann technique. Figure 17-26 shows the unique infective larvae of F. osleri. Nodules of F. osleri are usually found at the bifurcation of the trachea, where they can be observed via an endoscopic examination.
Eucoleus aerophilus (Capillaria aerophila) is a capillarid nematode found in the trachea and bronchi of both dogs and cats. The prepatent period is approximately 40 days. In standard fecal flotations, eggs of Eucoleus spp. are often confused with those of Trichuris spp. (whipworms). Eggs of E. aerophilus are smaller than whipworm eggs (59 to 80 μm by 30 to 40 μm), more broadly barrel shape, and lighter in color. The egg also has a rough outer surface with a netted appearance.
Trematodes (Flukes): Adult Paragonimus kellicotti are flukes found in cystic spaces within the lung parenchyma of both dogs and cats. These cystic spaces connect to the terminal bronchioles. The eggs are found in sputum or feces. Adult flukes are thick, brownish-red flukes measuring up to 16 mm long by 8 mm wide. Eggs are yellowish brown, with a rather distinct operculum. The eggs are 75 to 117 μm by 42 to 67 μm; the shell at the pole opposite the operculum is somewhat thickened. See Figure 17-27 for the operculated ovum of P. kellicotti. These fluke eggs can be recovered using fecal sedimentation techniques; however, the eggs of P. kellicotti are usually recovered using standard fecal flotation solutions. The eggs of P. kellicotti can also be recovered in the sputum via tracheal washing. Cystic spaces in the lung parenchyma can also be observed via thoracic radiography.
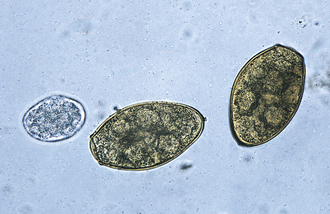
FIGURE 17-27 Characteristic ovum of P. kellicotti, the lung fluke of dogs recovered by standard fecal flotation. The eggs may be found in either sputum or feces, but often are recovered on fecal flotation. The yellowish-brown, operculated eggs measure 75 to 118 μm by 42 to 67 μm. A. caninum egg (left).
The Urogenital System
Dioctophyma renale is the “giant kidney worm” of dogs. This largest of parasitic nematodes frequently infects the right kidney of dogs and gradually ingests the renal parenchyma, leaving only the capsule of the kidney. Eggs may be recovered by centrifugation and the examination of the urine sediment. They are characteristically barrel shape, bipolar, and yellow brown. The egg’s shell has a pitted appearance. Eggs measure 71 to 84 μm by 46 to 52 μm. D. renale also may occur freely within the peritoneal cavity. When it is in this location, eggs are not passed to the external environment. The prepatent period is approximately 17 weeks.
Capillaria plica and C. (Personema) feliscati are nematodes of the urinary bladder of dogs and cats, respectively. The eggs may be found in urine or in feces contaminated with urine. Eggs are clear to yellow in color, measure 63 to 68 μm by 24 to 27 μm, and have flattened bipolar end plugs. Their outer surface is roughened. These eggs may be confused with those of the respiratory and gastric capillarids and with those of the whipworms.
The Eye and Adnexa
Thelazia californiensis is the “eyeworm” of dogs and cats. Adult parasites can be recovered from the conjunctival sac and lachrymal duct. An examination of the lachrymal secretions may reveal eggs or first-stage larvae. As mentioned previously, D. immitis may be recovered from a variety of aberrant sites, such as the anterior chamber of the eye.
The Musculoskeletal System
In the United States, canine hepatozoonosis, a protozoan, is most commonly diagnosed by a muscle biopsy rather than by the examination of peripheral blood smears for infected leukocytes. Muscle lesions consist of large cysts, pyogranulomas, and myositis. The cysts produced are round to ovoid and range from 250 to 500 μm in diameter. The center of the cyst demonstrates a basophilic nucleus surrounded by small basophilic bodies. Surrounding the nucleus and the basophilic bodies are concentric layers of fine multilaminated membranes, giving an “onion-skin” appearance. In most cases, no inflammatory response is associated with the cyst.
ENDOPARASITES OF HORSES
Horse Bots (Gasterophilus species): Gasterophilus spp. is a common parasite of horses. However, it is unusual because the adult form of the parasite (a fly) is an ectoparasite and the larval form (bots) is an endoparasite. Larval Gasterophilus spp. parasitize the stomach of horses. Because these stages are larval or immature stages of the adult flies, no demonstrable egg stage may be recovered from horse feces; however, adult flies do deposit eggs on the hairs on the legs of horses (Figure 17-28). The larval stage exits the gastrointestinal tract via the feces; therefore this stage may be recovered from the feces. The brown larvae are up to 20 mm in length and have dense spines on the cranial border of each segment. A pair of distinct mouth hooks is found on the cranial end of the first segment and a spiracular plate on the caudal end. The veterinary technician should be able to grossly identify horse bots as Gasterophilus spp. (Figure 17-29).
Stomach worms: Habronema and Draschia spp. are nematodes that are found in the stomach of horses. Habronema microstoma and Habronema muscae occur on the stomach mucosa, just beneath a thick layer of mucus; Draschia megastoma is often associated with large, thickened, fibrous nodules within the stomach mucosa. Larvae of both genera may parasitize skin lesions, causing a condition known as “summer sores.” The prepatent period for these nematodes is approximately 60 days. Larvated eggs or larvae may be recovered on standard fecal flotation. The eggs of both genera are elongated, have thin walls, and measure 40 to 50 μm by 10 to 12 μm.
Ascarids: The ascarid of horses (Parascaris equorum) has a creamy white color. Male ascarids measure about 28 cm, whereas females are about 50 cm in length. The female ascarids produce dark brown, thick-shelled oval to spherical eggs that are resistant to environmental conditions. The eggs measure 90 to 100 μm in diameter (Figure 17-30). The equine ascarid is common throughout the United States, and the incidence of infection, especially among younger horses, is frequently high. The larval stage develops within the eggs and develops to the infective second-stage larva within the egg. This development to the infective stage requires about 2 weeks. When the eggs are ingested by the horse, the larvae are released in the intestine, penetrate the intestinal mucosa, enter the circulatory system, and pass through to the liver, heart, and ultimately the lungs, in which they develop for a short period. Subsequently, larvae pass up the bronchial tree, enter the mouth, and are swallowed. They are passed into the small intestine and mature. This entire life cycle requires 10 to 12 weeks. The diagnosis is readily made by using a number of techniques, and these parasites are amenable to treatment by several anthelmintics. Control is difficult because eggs are extremely resistant to environmental conditions, and the coprophagous habits of foals tend to ensure infection.
The large and small strongyles: Strongylus vulgaris, Strongylus equinus, and Strongylus edentatus are the three species of “large strongyles,” along with 40 species of “small strongyles” of horses. The 40 or more species of small strongyles, of which there are several different genera, are bloodsucking nematodes. Strongyles vary in length from less than 12 mm (small strongyles) to 38 to 47 mm (large strongyles). However, some small strongyles, such as Triodontophorus spp., are nearly as large as S. vulgaris, the smallest of the large strongyles. All of the strongyles produce similar thin-walled eggs, each of which contains 4 to 16 brownish-colored cells when deposited (Figure 17-31). Regardless of whether these endoparasites are small strongyles or large strongyles, their eggs are virtually identical. Identification to the species level is accomplished by fecal culture and the identification of larvae. Strongyle eggs are most often observed in a standard fecal flotation. They measure approximately 70 to 90 μm by 40 to 50 μm. When these characteristic eggs are found on fecal flotation, the observation is recorded as “strongyle-type” ova, rather than as a particular species of strongyle.
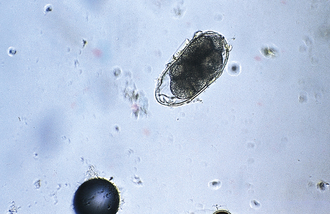
FIGURE 17-31 Strongyle-type ovum of horses. These eggs contain an 8- to 16-cell morula and measure approximately 70 to 90 μm by 40 to 50 μm.
All of the equine strongyles are common in horses throughout the United States, and the incidence of infection is generally high. The small strongyles that have been studied have a simple, direct life cycle. The eggs pass in feces, and first-stage larvae develop within the eggs. Once developed, the larvae hatch and undergo a free-living existence, developing and molting to second-stage free-living larvae. They then develop to third-stage larvae that do not feed and await ingestion. In ideal environmental conditions, development from the egg stage to the infective larval stage will occur in less than 1 week. Once small strongyles have been ingested, the larvae go to the cecum, penetrate the cecal mucosa, and develop for 1 to 2 weeks. The larvae return to the mucosal surface and mature in an additional 1 to 2 weeks. The species in the genus Strongylus all have very complex life cycles.
The development of the larval stages for large strongyles in the environment is the same as that for the small strongyles, and once ingested, large strongyles also penetrate the mucosa of the cecum and develop in a short period. S. vulgaris, the most important of the large strongyles, leaves the mucosa and migrates through the cranial mesenteric artery and its branches and develops in the lumen of the arteries over the next 6 months, becoming a young adult. It then returns to the cecum and matures; the entire prepatent period (the period after ingestion and before eggs pass in feces) requires about 180 to 200 days. S. equinus leaves the cecal mucosa and enters the peritoneal cavity. It then goes to the liver and develops into a young adult. The route taken back to the cecum is incompletely understood, but it may enter the pancreas. The entire prepatent period may be as long as 265 days.
S. edentatus leaves the mucosa and enters the subperitoneal tissue, particularly in the right dorsal flank. Eventually, it enters the venous circulation and goes to the liver. It leaves the liver and about 2 months later migrates in the mesenteries to the perirenal fat for an additional 3 months. It again migrates in the mesenteries to the large intestine, which it penetrates, and develops to maturity in the lumen of the cecum. The entire prepatent period requires 300 to 322 days. The identification of the strongyles can be accomplished by a number of techniques, and strongyles are amenable to treatment by several anthelmintics. Control is difficult because the parasites are prolific egg producers, and development of the larvae occurs rapidly. Control is best achieved by regular anthelmintic treatment and management regimen based on environmental conditions and by limiting the number of horses on the pasture.
Intestinal threadworms: Strongyloides westeri is a common parasite of horses, principally of foals 2 weeks to 6 months of age, and is widespread across the United States. These nematodes are unique; only a parthenogenetic female (one that can lay eggs without copulating with a male) is parasitic in the host. Parasitic males do not exist. The life cycle is essentially the same as that of S. stercoralis of the dog, except that the parthenogenetic female produces thin-walled eggs containing first-stage larvae when deposited. These larvated eggs measure 40 to 52 μm by 32 to 40 μm. The diagnosis can be made by a number of techniques; however, fresh feces must be used because the eggs will hatch in older feces. Control requires good hygiene together with treatment because the parasite can be transmitted by the transmammary route. The prepatent period is 5 to 7 days.
Pinworms: The pinworm of horses, Oxyuris equi, is a white to slate gray-colored nematode with a slender, sharply pointed tail. Males are small, measuring less than 12 mm, and females are 75 to 150 mm long. The eggs are slender and somewhat flattened along one side (Figure 17-32). Frequently, the eggs contain first-stage larvae when deposited. The eggs are 90 by 40 μm, with a smooth, thick shell. Pinworms are common in horses in the United States. The life cycle is simple and direct. Female parasites living in the cecum pass out though the anal sphincter and deposit masses of eggs on the perineum. Eggs are cemented into masses with a gelatinous material. Eggs drop off, either singly or in masses, landing on the ground or feed and become infective in 3 to 5 days. Once ingested, the larvae are released in the small intestine, penetrate the intestinal mucosa, and develop for several days. Larvae then return to the mucosal surface, move to the large intestine, and reach maturity about 50 days after the initial ingestion of the eggs. A diagnosis can be made effectively only by the cellophane tape technique. Pinworms are amenable to treatment with several anthelmintics. Control is difficult because of the coprophagous habits of foals. The prepatent period is approximately 4 to 5 months. The diagnosis is by finding the characteristic eggs on a microscopic examination of cellophane tape impressions or by scraping the surface of the anus.
Cestodes (Tapeworms): Anoplocephala perfoliata, Anoplocephala magna, and Paran-oplocephala mamillana are the equine tapeworms. A. perfoliata is found in the small and large intestine and cecum. A. magna is found in the small intestine and occasionally the stomach. P. mamillana also is found in the small intestine and occasionally the stomach. They are broad, thick, and white and vary in length from about 2.5 cm (A. perfoliata) to about 75 cm (A. magna). P. mamillana, the dwarf tapeworm, is only 4 to 6 mm in length. The eggs of A. perfoliata have thick walls, with one or more flattened sides, and measure 65 to 80 μm in diameter. Those of A. magna are similar, but slightly smaller, measuring 50 to 60 μm. The eggs of P. mamillana are oval and have thin walls, measuring 51 to 37 μm. Eggs of all three species have a trilayer egg shell, with the innermost lining called the pyriform apparatus. The life cycles of all three species are similar in that the eggs are ingested by a free-living mite for further development. Within the mite, a microscopic larval form, the cysticercoid, develops to the infective form in 2 to 4 months. Once ingested by the horse, the larval form is released from the mite and develops to an adult tapeworm in 6 to 10 weeks. A treatment or control is seldom practiced.
Protozoans (Unicellular Organisms): Eimeria leuckarti is a coccidian found in the small intestine of horses. This protozoan demonstrates unique, large oocysts (80 to 87 μm by 55 to 60 μm) with a thick wall, a distinct micropyle, and a dark brown color. These oocysts can be recovered on fecal flotation and are the largest coccidian oocysts. They are frequently observed on a histopathologic examination. The prepatent period ranges from 15 to 33 days.
Protozoans (unicellular organisms) of the circulatory system: Babesia equi and Babesia caballi are intracellular parasites found within the RBCs of horses. These are also referred to as the “equine piroplasms.” The diagnosis is by observing basophilic, pear-shape trophozoites in RBCs on stained blood smears. Trophozoites of B. equi may be round, amoeboid, or pyriform. Four organisms may be joined, giving the effect of a Maltese cross. Individual organisms are 2 to 3 μm long. Trophozoites of B. caballi are pyriform, round, or oval and 2 to 4 μm long. These occur characteristically in pairs at acute angles to each other.
The Respiratory System
Dictyocaulus arnfieldi, the “equine lungworm,” is found in the bronchi and bronchioles of horses, mules, and donkeys. Its eggs are ellipsoid and embryonated, measuring approximately 80 to 100 μm by 50 to 60 μm. Eggs can be recovered on fecal flotation of fresh (less than 24 hours old) feces. Larvae hatch from the eggs within a few hours after feces are passed. The prepatent period for the equine lungworm is 42 to 56 days.
The Eye and Adnexa
Thelazia lacrymalis is the “eyeworm” of horses throughout the world. Adult parasites may be recovered from the conjunctival sac and lacrimal duct. An examination of the lacrimal secretions may reveal eggs or first-stage larvae. Also in the eye of horses, the unsheathed microfilariae of Onchocerca cervicalis have been incriminated as causing periodic ophthalmia and blindness. These may be detected by an ophthalmic examination.
ENDOPARASITES OF RUMINANTS
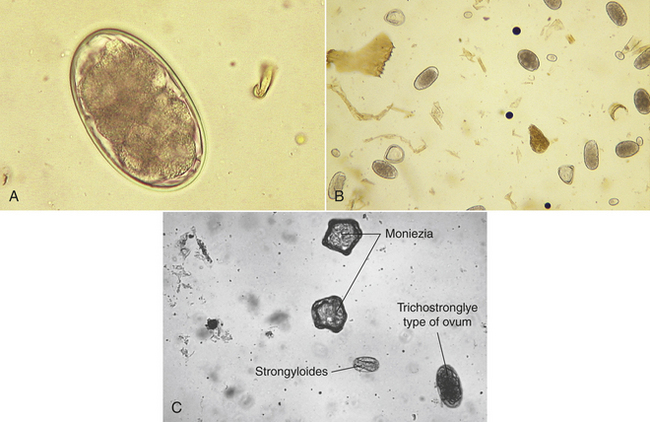
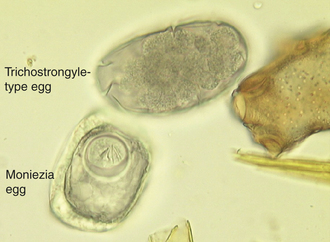
The trichostrongyles: The bovine trichostrongyles comprise several genera of nematodes within the abomasum and small and large intestine of cattle and other ruminants. Genera that produce “trichostrongyle-type eggs” are Bunostomum, Cooperia, Chabertia, Haemonchus, Oesophagostomum, Ostertagia, and Trichostrongylus spp. These seven genera (and others) produce oval, thin-shell eggs. The eggs contain four or more cells and are 70 to 120 μm long. Some of these ova may be identified to their respective genera; however, the identification is usually difficult because mixed infections of bovine trichostrongyles are quite common in ruminants (Figure 17-33).
Nematodirus spp. and Marshallagia spp. are also “bovine trichostrongyles”; however, the eggs are much larger than those of the genera mentioned previously. These eggs are the largest in the trichostrongyle family. Figure 17-34 shows the large eggs of Nematodirus spp. In a standard fecal flotation, the eggs of Nematodirus spp. are large (150 to 230 μm by 80 to 100 μm) and have tapering ends and four to eight cells. The eggs of Marshallagia spp. also are large (160 to 200 μm by 75 to 100 μm), have parallel sides and rounded ends, and contain 16 to 32 cells.
The life cycles, though somewhat variable among these species of bovine trichostrongyles, are similar. The first-stage larvae develop within the eggs and hatch to undergo a free-living existence. The larvae develop within the eggs and grow and molt to the third-stage infective form in less than 2 weeks. Once ingested by the host, the larvae generally penetrate the mucosa in the site that they normally inhabit (stomach, small intestine, large intestine) and develop in a short period, then return to the surface of the mucosa and mature.
The bovine trichostrongyles are widely distributed throughout the United States, but the incidence depends on their ability to develop in the external environment. Some, such as Haemonchus spp., need considerable warmth and moisture; whereas others, such as Ostertagia, Trichostrongylus, and Nematodirus spp., will withstand colder, drier climates.
A diagnosis can be effectively made with most techniques. Upon the identification of the characteristic eggs, the veterinary technician should record the finding as “trichostrongyle-type egg.” They should never be recorded by their individual genus names. The identification to genus and species usually can only be performed by fecal culture and larval identification.
Several treatments are available. Control is best achieved by a combination of treatment and pasture management in areas where there is an abundance of warmth and moisture to promote survival of the larval stages.
Intestinal threadworms: Strongyloides papillosus is often referred to as the “intestinal threadworm.” These nematodes are unique in that only a parthenogenetic female (a female nematode that lays eggs without copulating with a male) is parasitic in the host. Parasitic males do not exist. These females produce larvated eggs measuring 40 to 60 μm by 20 to 25 μm. Eggs usually are recovered in flotation of fresh feces. The prepatent period is 5 to 7 days.
Whipworms: Trichuris ovis is commonly called the “whipworm,” infecting the cecum and colon of ruminants. The section on nematode parasites of the gastrointestinal tract of dogs and cats contains details regarding the unique gross morphology of adult whipworms. The egg of the whipworm is described as trichinelloid or trichuroid. It has a thick, yellow-brown, symmetric shell with plugs at both ends. The eggs are unembryonated (not larvated) when laid. Eggs of ruminant whipworms measure 50 to 60 μm by 21 to 25 μm.
Cestodes (Tapeworms): Moniezia spp. are tapeworms found in the small intestine of cattle, sheep, and goats, and can reach lengths of 4 m. These tapeworms produce eggs with a characteristic cuboidal or pyramidal shape; under the compound microscope, these eggs appear to be square or triangular in silhouette. Two species are common, Moniezia benedini in cattle and Moniezia expansa in cattle, sheep, and goats. The eggs of both species can be easily differentiated using standard fecal flotation procedures. Figure 17-35 shows representative eggs of Moniezia spp. with the distinct pyriform apparatus as compared with a bovine trichostrongyle-type egg. The eggs of M. expansa appear triangular and measure 56 to 67 μm in diameter. The eggs of M. benedini appear square and are approximately 75 μm in diameter. The prepatent period for these tapeworms is approximately 40 days.
Thysanosoma actinoides is the “fringed tapeworm,” found in the bile ducts, pancreatic ducts, and small intestine of ruminants in the western regions of the United States. T. actinoides is generally 25 to 30 cm long. Its life cycle is not known. Eggs of this tapeworm measure 19 by 27 μm. The diagnosis of a T. actinioides infection can be accomplished only by the observation of the pearly white bell-shaped proglottid with a prominent fringe on its posterior margin.
Trematodes (Flukes): “Rumen flukes” are composed of two genera, Paramphistomum and Cotylophoron. These adult flukes reside in the rumen and reticulum of cattle, sheep, goats, and many other ruminants. The eggs of Paramphistomum spp. measure 114 to 176 μm by 73 to 100 μm, whereas the eggs of Cotylophoron spp. measure 125 to 135 μm by 61 to 68 μm. The prepatent period of Paramphistomum spp. is 80 to 95 days.
The common liver flukes of cattle and sheep are Fasciola hepatica and Fascioloides magna. Both trematodes are gray, flat, and leaflike in shape. F. hepatica is about 25 mm long, and F. magna is about 50 to 75 mm long. The eggs of both trematodes are similar and are large and yellow brown with an operculum or “lid” at one end (Figure 17-36). F. hepatica and F. magna are widespread throughout the United States, but only in wet, swampy, or subirrigated areas that will support substantial populations of the snail intermediate hosts. The natural hosts for F. hepatica are cattle and sheep, but the natural hosts for F. magna are members of the deer family. F. magna cannot complete its life cycle (by passing eggs into the environment) in cattle and sheep.
The life cycles of both trematodes are similar and quite complex. Eggs passing in the feces must land in water to develop. Inside each egg, a small, ciliated miracidium develops; the miracidium leaves the egg and penetrates the tissue of an aquatic snail, in which it undergoes asexual replication through larval stages called sporocysts and rediae, ultimately developing into cercariae. The cercariae leave the snail to encyst on vegetation to become metacercariae and await ingestion by the host. Once ingested, the juvenile fluke goes into the intestine, penetrates through to the body cavity, and penetrates the surface of the liver in which it wanders for several weeks. F. hepatica eventually enters the bile ducts, whereas F. magna will form a cyst wall around itself with an opening into a bile duct if it infects members of the deer family. In cattle, a calcite cyst is found, whereas in sheep, the parasite continues to wander throughout the liver. The eggs are heavy and will not float; consequently a sedimentation procedure is used for the diagnosis. An effective treatment is available. Control necessitates draining and drying wet, swampy pastures to prevent an overabundance of snails.
Dicrocoelium dendriticum is the “lancet fluke” of sheep, goats, and oxen. These tiny flukes reside within the fine branches of the bile ducts. The brown eggs have an indistinct operculum and measure 36 to 45 μm by 20 to 30 μm. Eggs may be recovered from feces using fecal sedimentation or a commercially available fluke egg recovery test.
Protozoans (Unicellular Organisms): Several species of coccidia infect cattle and sheep, and all belong to the genus Eimeria. Coccidia are common throughout the United States, and most animals are infected with at least one of the Eimeria spp. The severity of the infection depends on environmental conditions (warmth, moisture), stocking intensity, age, and previous exposure. Oocysts of the Eimeria spp. sporulate in the environment and reach the infective stage in the same manner as do Isospora spp. Eimeria spp., however, develop four sporocysts, each of which contains two sporozoites, for a total of eight infective forms per oocyst. The life cycle of Eimeria spp. is almost identical to that of Isospora spp. The diagnosis may be accomplished effectively by a number of techniques. Several treatments are available for the clinical disease. Control is difficult because oocysts are highly resistant. The proper management for coccidiosis includes the prevention of overcrowding, prevention of contamination of feed and water, and the use of dry bedding.
Ruminants serve as host to many species of coccidia belonging to the genus Eimeria. It is often difficult to identify the individual species of coccidia because their oocysts are so similar in size and shape. The two most common species of coccidia in cattle, Eimeria bovis and Eimeria zuernii, can be differentiated on a standard fecal flotation. Oocysts of E. bovis are oval, have a micropyle, and measure 20 by 28 μm, whereas those of E. zuernii are spherical, lack the micropyle, and measure 15 to 22 μm by 13 to 18 μm. When oocysts are recovered on fecal flotation, the observation is usually recorded as “coccidia.”
Cryptosporidium spp. is another coccidian parasite that parasitizes the small intestine of a variety of animals, including cattle, sheep, and goats. The sporulated oocysts in the feces are colorless and transparent and are extremely tiny, only 4.5 to 5.0 μm in diameter. The diagnosis is by standard fecal flotation and stained fecal smears. Because people may become infected with Cryptosporidium spp., feces suspected of harboring this protozoan should be handled with great care (see Figure 17-20 for features of the oocysts of Cryptosporidium spp.).
The Circulatory System
Elaeophora schneideri, the “arterial worm,” is a nematode found in the common carotid arteries of sheep in the western and southwestern United States. Microfilariae are 270 μm long and 17 μm thick, bluntly rounded cranially, and tapering caudally. They are found in the skin, usually in the capillaries of the forehead and face. Filarial dermatitis is seen on the face, poll region, and feet of sheep. The diagnosis is by the observation of characteristic lesions and the identification of microfilariae in the skin. The most satisfactory means of diagnosis is to macerate a piece of skin in warm saline and examine the material for microfilariae after about 2 hours. In sheep, microfilariae are rare and may not be found in the skin of infected animals. A postmortem examination may be necessary to confirm the diagnosis. The prepatent period is 18 weeks or longer.
Protozoans (Unicellular Parasites): Babesia bigemina is an intracellular parasite found within the RBCs of cattle. This parasite is a large piroplasm, 4 to 5 μm long by about 2 μm wide. It is characteristically pear shape and occurs in pairs, forming an acute angle within the erythrocyte. The intermediate host for this protozoan parasite is the tick, Boophilus annulatus, a tick that is reportable to the United States Department of Agriculture.
The Respiratory System
Dictyocaulus spp. are lungworms of cattle (Dictyocaulus viviparus), sheep, and goats (Dictyocaulus filaria). They are slender, white nematodes; males are 3 to 8 cm long, and females are 3 to 10 cm long. Females produce eggs containing first-stage larvae that hatch in the lungs. The first-stage larvae pass up the bronchial tree and are swallowed, passing with the feces. Lungworms occur in animals throughout the United States, but their distribution is discontinuous because the larval stages require a certain amount of warmth and moisture to survive. The life cycle is simple and direct. The first-stage larvae live on stored food granules, developing to the third-stage infective form within less than a week in optimal environmental conditions. Once ingested, the larvae enter the intestine, penetrate the intestinal mucosa, enter the lymphatic vessels, and develop for a short period in lymph nodes. They then go to the heart, enter the circulatory system, and then into the lungs to mature in a total of 25 to 30 days. Control is best achieved by proper management, ensuring that cattle and sheep do not occupy wet, swampy pastures. The prepatent period varies with the species, but is approximately 28 days.
The diagnosis is best made by use of the Baermann funnel technique. Larvae of D. filaria have brownish food granules in their intestinal cells, a blunt tail, and a cranial cuticular knob. They are 550 to 580 μm long. Larvae of D. viviparus also have brownish food granules in the intestinal cells, but have a straight tail; they lack the cranial cuticular knob. These larvae are 300 to 360 μm in length.
M. capillaris often is called the “hair lungworm.” Adults are found within the bronchioles, mostly in nodules in the lung parenchyma of sheep and goats. The eggs develop in the lungs of the definitive host, and the first-stage larvae are coughed up, swallowed, and passed out with the feces. They are 230 to 300 μm long. The larval tail has an undulating tip and a dorsal spine.
Protostrongylus spp. adults occur in the small bronchioles of sheep and goats. The eggs develop in the lungs of the definitive host, and the first-stage larvae are coughed up, swallowed, and passed out with the feces. These larvae are 250 to 320 μm long. This nematode’s larval tail has an undulating tip, but lacks the dorsal spine. The Baermann technique is used to diagnose a lungworm infection in ruminants.
The Urogenital System
Tritrichomonas foetus is a common protozoan parasite of cattle. This small, flagellated protozoan is equipped with three anterior flagella, an undulating membrane, and a trailing flagellum. Generally, T. foetus is a slender, pear-shaped organism. The bull acts as a carrier, with the parasite living on the surface of the penis or in the prepuce. When transmitted by coitus to the cow, the organism develops in the vagina and uterus, causing an abortion or fetal resorption. T. foetus multiples by binary fission; consequently, large populations can be generated in a short period. The cows, given a rest through two or three estrous cycles, will usually develop partial immunity. The diagnosis and treatment are performed on the bull. The diagnosis is difficult and complex. Control necessitates resting the cows and allowing immunity to develop, the treatment or elimination of infected bulls, and the purchase of virgin bulls for breeding.
The Eye and Adnexa
Thelazia rhodesii and Thelazia gulosa called the “eyeworms” are nematodes that parasitize the eyes of cattle, sheep, and goats. Adult parasites may be recovered from the conjunctival sac and lacrimal duct. The examination of the lacrimal secretions may reveal eggs or first-stage larvae.
The Abdominal Cavity
Setaria cervi is a nematode called the “abdominal worm” of cattle. Adults are found free within the peritoneal cavity. Their sheathed microfilariae are approximately 250 by 7 μm. The diagnosis is by the demonstration of microfilariae in blood smears.
Cysticercus tenuicollis is the metacestode or larval tapeworm of T. hydatigena and may be found attached to the greater omentum within the abdominal cavity of many ruminants. These cysticerci are usually diagnosed on a postmortem examination.
The Musculoskeletal System
Another metacestode is Cysticercus bovis, the bladderworm of Taenia saginata, the beef tapeworm of humans. It may be found within the musculature of the cattle that serves as the intermediate host. These cysticerci are colloquially referred to as “beef measles” and are usually diagnosed during a postmortem meat inspection. Humans become infected with the adult tapeworm by eating poorly cooked beef.
ENDOPARASITES OF SWINE
Ascarops strongylina and Physocephalus sexalatus are nematodes known as the “thick stomach worms” of the porcine stomach. Both of these nematodes produce thick-wall, larvated eggs that may be recovered on fecal flotation. The eggs of both species are similar. The eggs of A. strongylina are 34 to 39 μm by 20 μm and have thick shells surrounded by a thin membrane that produces an irregular outline. The eggs of P. sexalatus are 34 to 39 μm by 15 to 17 μm. The prepatent period for both species is approximately 42 days. Ascarops and Physocephalus spp. use beetles as their intermediate hosts and rarely cause problems in swine.
Hyostrongylus rubidus is referred to as the “red stomach worm” of swine. The eggs are “trichostrongyle type”; (i.e., they are oval, thin-shell eggs). They contain four or more cells and measure 71 to 78 μm by 35 to 42 μm. These eggs may be recovered on fecal flotation. As with bovine trichostrongyles, a definitive diagnosis can be made only by fecal culture and larval identification. These eggs can be confused with the eggs of Oesophagostomum. The prepatent period is approximately 20 days.
Ascaris suum, the “swine ascarid” or the “large intestinal roundworm,” is the largest nematode found within the small intestine of pigs. The eggs may be recovered on standard fecal flotation. They are oval and golden brown, with a thick albuminous shell bearing prominent projections. These eggs measure 70 to 89 μm by 37 to 40 μm (Figure 17-37).
Strongyloides ransomi, the “intestinal threadworm” of pigs, is found within the small intestine of pigs. This parasite is unique in that only a parthenogenetic female is parasitic in the host. Parasitic males do not exist. These females produce larvated eggs measuring 45 to 55 μm by 26 to 35 μm. Eggs are usually recovered in flotation of fresh feces. The prepatent period is 3 to 7 days (see Figure 17-6).
Trichinella spiralis occurs in the small intestine of many hosts, most notably the pig. Swine become infected with T. spiralis when they ingest infective larval stages (juveniles) in undercooked meat. The larvae mature into adults in the host’s small intestine in a few weeks, and the female worms give birth to larvae. (The males die after fertilizing the females, and the females die after producing larvae.) The larvae enter the bloodstream of the host and eventually end up in the pig’s musculature. See Swine Parasites of the Musculoskeletal System.
Oesophagostomum dentatum, the “nodular worm of swine,” is found in the large intestine of swine. The prepatent period is 50 days. The eggs are “trichostrongyle type”; (i.e., they are oval, thin-shell eggs). They contain 4 to 16 cells and measure 40 by 70 μm. These eggs may be recovered on a standard fecal flotation. These eggs can be confused with the eggs of Hyostrongylus. As with bovine trichostrongyles, a definitive diagnosis may be made only by fecal culture and larval identification.
Trichuris suis is commonly called the “whipworm,” infecting the cecum and colon of swine. (See the section on nematode parasites of the gastrointestinal tract of dogs and cats for details on the gross morphology of adult worms.) The egg of the whipworm is described as trichinelloid or trichuroid. It has a thick, brown, barrel-shape shell with plugs at both ends. The eggs are unembryonated (not larvated) when laid. Eggs of porcine whipworms measure 50 to 60 μm by 21 to 25 μm. The prepatent period is 42 to 49 days.
Macracanthorhynchus hirudinaceus is the “thorny-headed worm” or acanthocephalan of the small intestine of swine. It is called “thorny-headed” because of its spiny proboscis, which it embeds as an anchor into the small intestinal mucosa of the host. The eggs have a triple-layer shell, are oval, and measure 67 to 100 μm by 40 to 65 μm. The eggs may be recovered on a standard fecal flotation. The prepatent period is 60 to 90 days.
Balantidium coli is the “ciliated protozoan” found in the large intestine of swine. Although commonly observed during a microscopic examination of fresh diarrheal feces, it is generally considered to be nonpathogenic. Two morphologic stages may be found in feces: the “cyst” stage and the motile “trophozoite” stage. Both stages may vary in size. This is a large protozoan parasite. The trophozoites may be 150 by 120 μm, with a sausage- to kidney-shape macronucleus. It is covered with numerous rows of cilia and moves about the microscopic field with lively motility. The cyst is spherical to ovoid and 40 to 60 μm in diameter, with a slight greenish-yellow color. Both of these stages may be easily recognized by a microscopic examination of the intestinal contents or fresh, diarrheal feces. Figure 17-38 shows the trophozoite stage of B. coli in histopathologic section.
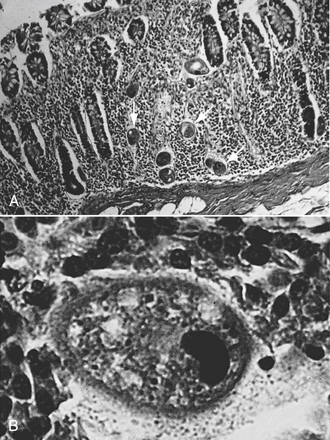
FIGURE 17-38 A, B. coli of swine in histopathologic section. This photomicrograph was taken at low magnification. Note that B. coli is quite large and easily visible (arrows). B, B. coli of swine in histopathologic section. This photomicrograph was taken at higher magnification than A.
Cryptosporidium is another coccidian parasite that parasitizes the small intestine of a wide variety of animals, including swine. The sporulated oocysts in the feces are colorless and transparent and measure only 4.5 to 5.0 μm. The diagnosis is by standard fecal flotation and stained fecal smears. Because people may become infected with Cryptosporidium spp., feces suspected of harboring this protozoan should be handled with great care (see Figure 17-20).
Isospora suis is the coccidian that parasitizes the small intestine of swine, especially young piglets. Oocysts are usually found on flotation of fresh feces. They are subspherical, lack a micropyle, and measure 18 to 21 μm (Figure 17-39). A postmortem diagnosis in piglets exhibiting clinical signs, but not shedding oocysts, can be achieved by a direct smear of the jejunum stained with Diff-Quik. The diagnosis is by the observation of the banana-shape merozoites. The prepatent period is 4 to 8 days.
Parasites of the Respiratory System: Metastrongylus apri, the “swine lungworm,” is found within the bronchi and bronchioles of pigs. The oval, thick-wall eggs measure 60 by 40 μm and contain larvae. Eggs can be recovered on fecal flotation using a flotation medium with a specific gravity above 1.25 or by using the fecal sedimentation technique. The prepatent period is approximately 24 days.
The Urinary System
Stephanurus dentatus, the “swine kidney worm,” is found in the kidney, ureters, and perirenal tissues of pigs. The eggs are “strongylus-type”; (i.e., they are oval, thin-shell eggs, containing 4 to 16 cells, and measuring 90 to 120 μm by 43 to 70 μm). Eggs may be recovered from the urine using urine sedimentation. The prepatent period is extremely long, approximately 9 to 24 months.
The Musculoskeletal System
T. spiralis is found in many species of carnivores and omnivores, but is often associated with raw or undercooked pork. Animals (including humans) become infected with T. spiralis when they ingest infective larval stages (juveniles) in meat. The larvae mature into adults in the host’s small intestine in a few weeks, and the female worms give birth to larvae. (The males die after fertilizing the females, and the females die after producing larvae.) The larvae enter the bloodstream of the host and eventually migrate to the pig’s musculature. Within the muscles, the larvae mature into infective encysted larvae. The next host becomes infected when it eats these larvae. Trichinosis is probably best known as a parasite that humans contract from eating raw or undercooked pork. It is usually diagnosed through proper meat inspection. Most recent outbreaks of trichinosis in the United States have been traced to pork products from pigs that have not been inspected and that have been slaughtered privately.
Cysticercus cellulosae, the bladderworm (larval or metacestode stage) of Taenia solium, the pork tapeworm of humans, may be found within the musculature of the porcine intermediate host. These cysticerci are colloquially referred to as “pork measles” and are usually diagnosed during a postmortem meat inspection. Humans become infected with the adult tapeworm T. solium by eating poorly cooked pork containing cysticerci. Humans may become infected with C. cellulosae in the muscles or within nervous tissue, such as the brain or the eye, by ingesting the eggs of T. solium.
DIAGNOSIS OF ENDOPARASITISM
Parasites can infect the oral cavity, esophagus, stomach, small and large intestines, and other internal organs of domesticated animals. The detection of the presence of these parasites involves the collection and microscopic examination of feces. The diagnosis is usually by finding life cycle stages of the parasite within the feces. These stages include eggs, oocysts, larvae, segments (tapeworms), and adult organisms. Veterinary technicians may perform the following procedures to detect parasitic infections.
COLLECTION OF FECAL SAMPLES
Fecal samples collected for routine examination should be as fresh as possible. Specimens that cannot be examined within a few hours of excretion should be refrigerated or mixed with equal parts of 10% formalin. The need for fresh feces stems from the fact that eggs, oocysts, and other life-cycle stages may be altered by their further development, making diagnosis extremely difficult.
Small Animal Fecal Samples
Several methods are used for collecting feces from companion animals. An owner may collect a fecal sample immediately after the animal has defecated. The feces may be stored in any type of container, such as a zippered plastic bag or a clean, small jar with a tight cap. Veterinary hospitals may dispense containers to their clients for this purpose. In either case, only a small amount of feces (1 tsp) is required for a proper examination by the technician. All specimens should be properly identified with the owner’s name, animal’s name, and species of animal.
Fecal samples also may be collected directly from the animal at the veterinary hospital, using a gloved finger or fecal loop. If a glove is used, the feces may remain in the glove, with the glove turned inside out, tied, and labeled. Samples collected with a fecal loop should be used for direct examination only because the amount collected is relatively small.
Large Animal Fecal Samples
Fecal specimens collected from livestock may be obtained either directly from an individual animal’s rectum or from a number of animals to make up a pooled sample. Samples collected directly from an individual animal using a gloved hand can remain in the glove, with the glove turned inside out, tied, and labeled.
Pooled samples are collected from a number of animals housed together and then co-mingled in a single container. These samples are used to get an impression of the degree of infection within the group. Pooled samples can be collected in any type of container as long as it is clean and can be tightly sealed. These samples should be labeled with the species, pen or group number, owner, and time of collection.
EXAMINATION OF FECAL SAMPLES
Several precautions should be taken during fecal examination:
• Fecal samples are handled with care. The feces may contain parasites, bacteria, or viruses that are zoonotic (i.e., animal diseases that may be transmitted to humans). Appropriate clothing, such as a clean laboratory coat or jacket and latex gloves, should be worn during the laboratory examination. If gloves are not worn, hands should be frequently washed with soap and water. No food or drink should be allowed in the examination area. Likewise, workers should refrain from applying makeup or adjusting contact lenses. Laboratory coats should never be worn outside of the veterinary practice; this reduces the chances of spreading any infections.
• The laboratory area is cleaned thoroughly after the fecal examinations are completed. Spilled materials create a hazardous area in which to work and could pose a serious threat to staff members’ health.
• Accurate and thorough records are maintained. Records should contain the date, owner’s name, and any parasites found in the sample. If the sample’s test results are negative, it should be recorded as such.
Gross Examination of Feces
Several characteristics of the feces also should be recorded and reported to the veterinarian. They are as follows:
• Consistency: Fresh feces should be somewhat formed, depending on the species of animal. Diarrhea or constipation could be the result of a parasitic infection.
• Color: Fecal color may be affected by the food an animal eats. Also malabsorption or a parasitic infection may alter the color of feces.
• Blood: Blood may impart a dark reddish-brown color to feces or it may appear as bright red streaks in the feces. In either case, blood may indicate a severe parasitic infection or other serious intestinal diseases. Blood in the feces is an important clinical finding and should be brought to the attention of the veterinarian. Digested blood has a dark, tarry appearance.
• Mucus: Mucus in the feces can be a result of digestive disorders or a parasitic infection. In either case, its presence should be reported to the veterinarian.
• Gross parasites: Adult parasites or tapeworm segments can be found in the feces. Adult roundworms resemble strings of spaghetti, whereas tapeworm segments look more like grains of cooked rice. Tapeworm segments may be identified by a microscopic examination. The segments of two common tapeworms infecting dogs and cats are shown in Figure 17-40.
Figure 17-41 shows common tapeworm segments, Anoplocephala spp. found in horse feces, and Moniezia spp., found in cattle feces.
FIGURE 17-41 Chains and individually mature segments of tapeworms of horses, Anoplocephala (left), and cattle, Moniezia (right).
Occasionally a client may submit a dried tapeworm segment to be identified. To identify the tapeworm species, the dried segments must be soaked in saline for 1 to 4 hours to rehydrate them. Once the segments are rehydrated, these may be identified by their unique size, shape, morphologic features, and the eggs contained within.
Segments of some tapeworm species do not contain eggs, and some segments may have expelled their eggs before the examination was conducted. In either case, a tapeworm segment may be identified as such by finding small mineral deposits (calcareous bodies) within the segment (Figure 17-42). This is done by crushing the tapewormlike segment between two glass slides and examining the material with a microscope.
Microscopic Examination of Feces
The microscopic examination of feces is the most reliable method to detect parasitic infections. A compound microscope with 4×, 10×, and 40× objectives is required for the proper examination of a fecal specimen. A mechanical stage is a necessity. An ocular micrometer is also recommended, but is not required.
Fecal specimens should be examined routinely using the 10× objective. The examination should begin at one corner of the slide and end at the opposite corner, moving over the slide in a systematic pattern (Figure 17-43).The microscope should be focused continually with the fine-tuning knob during the examination. The initial plane of focus should be that of air bubbles because most helminth eggs are found in this plane. Any material found during the initial scan, including parasite eggs, may be more closely examined using the more powerful objectives.
FIGURE 17-43 A scheme of movement of the microscopic field to examine the area under the coverslip thoroughly.
Calibration of the Microscope: The size of the various stages of many parasites is often important for correct identification. Some examples are Trichuris versus Capillaria eggs and Dipetalonema versus Dirofilaria microfilariae. Accurate measurements are obtained easily with the use of a calibrated eyepiece on the microscope. Calibration must be performed on every microscope to be used. Each objective (lens) of the microscope must be individually calibrated.
The stage micrometer is a microscope slide etched with a 2-mm line marked in 0.01-mm (10-μm) divisions (Figure 17-44). The veterinary technician should remember that 1 micron (μm) = 0.001 mm.
FIGURE 17-44 Stage micrometer (upper scale) and eyepiece scale (lower scale) used to calibrate the microscope.
The eyepiece scale is a glass disk that fits into and remains in one of the microscope eyepieces. This disk is etched with 30 hash marks spaced at equal intervals. The number of hash marks on the disk may vary with different manufacturers, but the calibration procedure is the same for all.
The stage micrometer is used to determine the distance in microns between the hash marks on the eyepiece scale for each objective lens of the microscope being calibrated. This information is recorded and labeled on the microscope for future reference.
To begin the calibration procedure, the veterinary technician should start on low power (10×) and focus on the 2-mm line of the stage micrometer. Therefore 2 mm = 2000 μm.
The eyepiece is rotated so that the hash-mark scale is horizontal and parallel to the stage micrometer scale. The zero (0) point is aligned on both scales.
The point on the stage micrometer aligned with the “10” hash mark is determined on the eyepiece scale. For example, the 0.125-mm mark might align with the “10” hash mark.
This number is multiplied by 100. In the above example,
This means that at this power (10×), the distance between each hash mark on the eyepiece scale is 12.5 μm.
These steps are repeated at each magnification (10×, 40×, 100×). The information is recorded on a label and attached to the calibrated microscope. For example:
| Objective | Distance Between Hash Marks (μm) |
| 10× | 12.5 |
| 40× | 2.5 |
| 100× | 1.0 |
To measure an object, such as a parasite egg, one end of the egg is placed on the zero mark of the ocular scale, and the number of divisions to the other end of the egg is counted. The number of divisions counted is multiplied by the calibration factor for the objective used. For example, a trichostrongylus egg is 24 divisions long and 13.5 divisions wide when measured with the 40× objective. The calibration factor for the 40× objective is 2.5. Therefore the egg is:
To measure a microfilaria, the head (the anterior end) is aligned with the zero mark of the ocular scale, and the number of divisions to the end of the tail (the posterior end) is counted. To measure round parasite eggs, the technician should measure through the middle of the egg at its greatest diameter. For more accurate measurements, the higher objective is used (40× instead of 10×).
Examination of Direct Smears: A direct smear of feces is used to rapidly estimate an animal’s parasite burden. This procedure also is used to detect some of the motile protozoa found in feces.
Advantages of direct smears include short preparation time and minimal equipment required to run the procedure. Disadvantages include the small amount of feces examined, which may not be sufficient enough to detect a low parasite burden, and the amount of extraneous fecal debris on the slide, which could be confused with parasitic material.
A fecal sample for a direct smear preparation may be obtained from an animal using a fecal loop or a rectal thermometer (after measuring the animal’s temperature). Either way, only a small amount of feces is needed.
Concentration Methods for Fecal Examination: The following methods are used to concentrate parasitic material in feces. A concentration technique makes it possible to examine a large amount of feces in a relatively short time. A low parasite burden can easily be identified. Two types of procedures are most often used in veterinary hospitals: flotation and sedimentation.
Fecal flotation methods are based on the specific gravity of parasitic material and fecal debris. Specific gravity refers to the weight of an object as compared with the weight of an equal volume of water. The specific gravity of most parasite eggs is between 1.100 and 1.200 g/ml, whereas the specific gravity of water is 1.000.
To allow for flotation of parasite eggs, oocysts, and other life-cycle stages, the flotation solution must have a higher specific gravity than that of the parasitic material. Several salt and sugar solutions work well for flotation. Most have a specific gravity of 1.200 to 1.250. In this range, heavy fecal debris sinks to the bottom of the container, and parasitic material rises to the top of the solution.
Sodium nitrate solution is the most common fecal flotation solution used in veterinary hospitals today. This solution is efficient for floating parasite eggs, oocysts, and larvae. It may be purchased with commercial diagnostic test kits or in individual aliquots. The major disadvantage of using sodium nitrate is the expense. Sodium nitrate also forms crystals and distorts eggs if allowed to sit longer than 20 minutes.
Another solution commonly used for flotation is saturated sugar solution. Sugar solution is inexpensive and does not crystallize or distort eggs. Sugar solution may be made anywhere and has a long shelf life. Although sticky to work with, spilled sugar solution may be removed with warm, soapy water.
Zinc sulfate solution is more commonly used in diagnostic laboratories. Zinc sulfate floats protozoal organisms with the least amount of distortion. It generally is used in combination with one of the previously mentioned solutions.
The least desirable solution used is saturated sodium chloride solution. This solution corrodes laboratory equipment, forms crystals, and severely distorts parasite eggs. Saturated sodium chloride solution is also a poor flotation medium because the maximum specific gravity obtainable is 1.200, allowing heavier eggs to remain submerged. A number of choices in flotation techniques exist.
Standard fecal flotation: The standard or simple fecal flotation is one of the most common flotation techniques used in veterinary hospitals. This technique uses a test tube or vial, in which feces and flotation solution are mixed (Figure 17-45). A coverslip or microscope slide is placed on top of the test tube, and the unit is allowed to sit undisturbed. Any parasite eggs in the feces float to the top and adhere to the underside of the coverslip or microscope slide. The coverslip or slide is then removed and microscopically examined for parasitic material. Although the standard flotation technique is easy to perform, it is less efficient at floating parasitic material than the centrifugal technique, described later in this chapter.
Commercial flotation kits use the same principle as the standard flotation technique. These kits contain a vial with a filter; some also include prepared flotation solution. Examples of commercial flotation kits include Fecalyzer (EVSCO Pharmaceuticals, Buena, N.J.), Ovassay Plus (Synbiotics, San Diego), and Ovatector (BGS Medical Products, Venice, Fla.) (Figure 17-46). These kits are simple to use, but expensive when compared with the simple flotation technique. Some practices reduce the expense by washing and reusing the vials and filters; this practice should be discouraged.
Centrifugal flotation: Centrifugal flotation for parasite eggs, oocysts, and other parasitic material is the most efficient method available. It requires less time to perform than the standard flotation method. The only drawback to this procedure is that it requires a centrifuge with a horizontal (nonfixed) rotor that can hold 15-ml centrifuge tubes. If such a centrifuge is available, centrifugal flotation is preferred because it is easy to perform and samples can be run individually or in batches.
If testing multiple samples, the veterinary technician may want to use a numbering system to keep the samples in order. A number is assigned to the patient, and that number is written on the corresponding centrifuge tube with a marking pen. This minimizes the chances of error.
Fecal sedimentation: Fecal sedimentation concentrates parasite eggs, oocysts, and other parasitic material by allowing them to settle to the bottom of a tube of liquid, usually water. A disadvantage of this technique is that the amount of fecal debris that mixes with the parasitic material makes a microscopic examination somewhat difficult.
This procedure is used to detect heavy eggs that would not float in flotation solution or eggs that would become distorted by the flotation solution. Trematode (fluke) eggs are often considered too heavy for flotation and often found using fecal sedimentation. Although some flotation solutions can be adjusted to a specific gravity of 1.300 to float these eggs, such solutions are not routinely used because some distortion may occur.
Quantitative Fecal Examination: Quantitative procedures are used to determine the number of eggs or oocysts present in each gram of feces. These procedures are used as a rough indication of the number of parasites present within a host. Their usefulness is limited, however, by the fact that the various parasite species produce different numbers of eggs. Egg production by the parasites may be sporadic, and the number of eggs may not correlate with the number of parasites present. The last item is the most significant disadvantage because, in most cases, clinical signs of disease are caused by immature parasites that have not yet begun producing eggs or larvae.
Several quantitative procedures can be performed in veterinary hospitals. These include the Stoll egg count technique, the modified Wisconsin double centrifugation technique, and the McMaster technique. These procedures are fairly easy to perform; each requires its own specialized equipment.
Examination of Feces for Protozoa: All of the previously described procedures may be used to detect protozoal cysts. However, some protozoans do not form cysts and pass out of the host in the trophozoite form. Trophozoites are one-cell, motile organisms that lack the rigid wall of a cyst, making flotation without distortion or death of the trophozoite impossible. Therefore the direct smear technique, using saline and a stain, is the preferred procedure for the examination of a fecal sample for protozoal organisms.
In a direct smear, a trophozoite may be recognized by its movement. B. coli, a protozoan parasite found in the feces of pigs, is bean shape and covered with tiny hairs or cilia. It moves in a slow, tumbling fashion. Giardia spp. are tear shaped and have five to eight flagella. They move with a jerky motion. Trichomonads are pear-shaped organisms with multiple anterior flagella, which produce jerky movements. Amoebae move with a flowing motion, extending a part of the body (pseudopod) and moving the rest of the body after it.
Stains also may be used to recognize certain structural characteristics of trophozoites and cysts. Lugol’s iodine and new methylene blue are common stains used with the direct smear procedure. These stains do not preserve the slide, but do facilitate the examination of the specimen, making identification easier.
If a protozoal parasite cannot be identified on direct smears, fecal smears containing protozoal trophozoites can be dried, stained with Diff-Quik, Wright, or Giemsa stain, and sent to a diagnostic laboratory. Many other procedures are used for staining and preserving protozoal trophozoites. Most of these procedures are used in diagnostic laboratories and are not explained here.
Special staining for coccidian parasites: Several coccidian parasites require special staining techniques for identification. Two procedures are discussed in this chapter.
The acid-fast staining technique is used to identify Cryptosporidium spp. in the feces. Cryptosporidium is a parasite of the gastrointestinal tract of many animals, including humans. The oocysts are 2 to 8 μm in diameter and are almost undetectable in flotation solution to the inexperienced eye. Acid-fast staining can aid detection of the oocysts in a fecal smear.
The second procedure uses Diff-Quik stain for the identification of Isospora spp. Isospora spp. are coccidia found in the gastrointestinal tract (especially the jejunum) of many animals, but they are of most concern in pigs. This parasite can cause the death of many piglets before any oocysts are found in the feces using conventional flotation methods. Therefore an intestinal mucosal scraping must be stained and examined for other diagnostic stages (schizonts, merozoites) of this parasite. This procedure involves scraping the mucosa of the jejunum and smearing the scrapings onto microscope slides. After the slides are air-dried, they are stained with Diff-Quik and examined with the oil-immersion objective.
For accurate results with either of these procedures, several samples should be examined. If such an examination is not possible, feces or intestines may be sent to a diagnostic laboratory. The collection and shipping of parasitic specimens are described later in this chapter.
Sample Collection at Necropsy
Necropsy (postmortem examination) is an important method of diagnosing many diseases, including parasitism. The types of lesions produced by immature parasites, any adult parasites found in the body cavity and tissues, and a histopathologic examination of infected tissues are used in diagnosis. Veterinary technicians are responsible for the samples collected, making sure they are contained, preserved, labeled, and shipped properly. Refer to Chapter 40 for more information about necropsy techniques.
Two methods may be used to recover parasites from the digestive tract at necropsy: the decanting method and the sieving method. With either method, the veterinary technician must separate the different parts of the digestive tract and work with the contents of each individually.
Parasites recovered from the digestive tract may be preserved in 70% alcohol or 10% neutral buffered formalin for later identification. Occasionally, bladderworms or cysticerci may be found attached to the viscera of domestic animals. A bladderworm is a fluid-filled, balloonlike structure that is actually a larval tapeworm. These should be handled with care because the fluid within the bladder can be allergenic and may also be zoonotic. To identify the parasites recovered, the veterinary technician should consult the references listed at the end of this chapter. If an in-hospital diagnosis is not possible, the samples may be preserved as previously described and sent to a diagnostic laboratory for identification.
Shipping Parasitologic Specimens
Any parasitologic specimen shipped to a diagnostic laboratory should be preserved with alcohol or formalin, unless otherwise directed by laboratory personnel. Specimens should be packaged in a leakproof container and sealed with Parafilm (American National Can, Greenwich, Conn.) or tape. If specimen containers are found leaking, the shipment will not be delivered. Feces can be sent fresh or mixed at a ratio of 1:3 with 10% formalin. Whole parasites or segments can be preserved in alcohol or formalin and placed in a leakproof container (clean small jar, medicine vial, clot tube).
All specimen containers should be labeled as to the site from which the specimen was obtained, the owner’s name, the animal’s species, name, or identification number, and the referring veterinarian (including telephone number and address). The labeled specimen container should be placed in a shockproof shipping container to prevent breakage during shipping. Styrofoam containers filled with shredded newspaper work well for shipping parasitologic specimens.
A cover letter should be included with the specimen and should contain a brief history of the animal, findings upon necropsy, and the reason for submitting the samples to the laboratory (e.g., fecal examination, special staining, and species identification). Without this background information, the diagnostic laboratory is unlikely to provide accurate results.
Miscellaneous Procedures for Detection of Endoparasites
Cellophane Tape Technique: The cellophane tape technique is used to detect the equine pinworm, O. equi. Pinworms are nematodes found in horses, rodents and rabbits, and primates including people. They live in the colon and, as adults, migrate out the rectum to lay eggs on the skin around the anus. The eggs are contained within a sticky substance and fall off as the substance hardens or as the animal scratches. For this reason, pinworm eggs usually are not seen in a routine fecal examination.
Baermann Technique: The Baermann technique is used to recover nematode larvae from feces, tissues, or soil. This technique uses warm water to stimulate larvae to move about. As the larvae do so, they sink to the bottom of the funnel for collection and identification.
This technique is performed with a Baermann apparatus (Figure 17-47). The apparatus consists of a ring stand and ring holder, a glass funnel with a piece of rubber tubing on the end, a clamp, and a wire net or cheesecloth. The sample is placed on the wire screen or cheesecloth, and warm water is added to barely cover the sample. All air bubbles are allowed to flow from the tube of the funnel by releasing the clamp. The apparatus is allowed to sit undisturbed for 12 to 24 hours.

FIGURE 17-47 Baermann apparatus is used to recover larvae of roundworms from feces, soil, or animal tissues. This apparatus is most useful in recovering larvae of lungworms.
A drop of fluid from the bottom of the funnel is removed (usually the first drop) and placed on a microscope slide. If any larvae are found swimming on the slide, the slide is carefully heated with a match to render them immobile.
Larvae recovered from fresh large animal feces are almost always those of lungworms. Larvae from S. stercoralis can be found in fresh canine feces. If the feces are not fresh, all sorts of parasitic and nonparasitic larvae and adults may be seen. For more detailed information on the descriptions of larvae, consult the reference by Bowman.
EXAMINATION OF BLOOD SAMPLES
D. immitis, the canine heartworm, is the most important parasite of the vascular system in domestic animals in the United States. For this reason, in-hospital blood examinations are commonly performed to detect heartworms.
The following procedures can be performed by veterinary technicians to identify D. immitis. As mentioned earlier, a clean environment and proper handling of samples are vital to quality control in any laboratory. Any sample handled improperly may result in inaccurate results.
Direct Blood Smear
A direct examination of the blood for microfilariae is the simplest procedure to perform. This procedure detects movement of microfilariae and other parasites among the RBCs. As with a direct examination of feces, a direct examination of blood requires only a small sample. However, unless parasites are present in large numbers, they may be missed. For this reason, the direct smear is not a good diagnostic technique for diagnosing microfilariae.
Microfilariae of primary interest are those of D. immitis, the canine heartworm, and D. reconditum, a subcutaneous parasite of dogs. Differentiation between the two is extremely important because the treatment for heartworms is expensive, somewhat stressful, and involves the use of arsenical compounds.
In a direct blood smear, microfilariae of D. immitis spp. coil and uncoil, whereas those of Dipetalonema spp. may glide smoothly across the slide. However, this is not always the case. The number of Dirofilaria spp. microfilariae in a sample is usually greater than that of Dipetalonema spp. This is not always the case. A direct examination of the blood is only used to determine the presence of microfilariae, not the type. For this, a concentration technique, which “relaxes” and stains microfilariae, is used. These procedures are discussed later in this chapter.
Microfilariae can also be found in large animals. Setaria spp. are long, white nematodes found in the serous membranes of cattle and horses. Adults are most often seen during abdominal surgery or necropsy, and microfilariae are found during a differential white blood cell count.
Thin Blood Smear: The thin blood smear is prepared and stained in exactly the same way as a blood smear for a differential white blood cell count. When doing a differential white blood cell count on an animal, the veterinary technician should note any parasitic organisms seen. Occasionally, microfilariae may be found. Because of their size, microfilariae usually are found along the feathered edge. Because differentiation of the microfilariae is not possible in a thin blood smear, other procedures must be performed for identification. Trypanosomes, protozoans, and rickettsiae also may be found among or within cells. As with the direct smear procedure, a small blood sample is used, and mild parasitic infections may be missed.
Concentration Techniques
Buffy Coat Method: The buffy coat method is a concentration technique used on a small volume of blood. When blood is placed in a microhematocrit tube and centrifuged for determining the packed-cell volume (PCV), it separates into three layers: plasma, white blood cell layer (buffy coat), and RBC layer (Figure 17-48). Microfilariae can be found on the surface of the buffy coat layer. This technique is quick and may be performed in conjunction with a PCV and total protein evaluation. However, differentiation of microfilariae is not possible.

FIGURE 17-48 Buffy coat in a hematocrit tube lies between the plasma above and packed red blood cells below. A plug of clay prevents the blood from escaping the tube during centrifugation.
The following concentration techniques may be used for differentiating D. immitis from D. reconditum.
Modified Knott Technique: The modified Knott technique is a simple procedure that allows differentiation of microfilariae. This technique concentrates, “relaxes,” and stains microfilariae while lysing RBCs to make the microfilariae more visible.
Figures 17-49 of D. reconditum and Figure 17-50 of D. immitis are helpful in identifying microfilariae. Table 17-1 shows the characteristics that may be used when differentiating these two microfilariae. The veterinary technician should always examine as many microfilariae as possible because mixed infections can occur. The most accurate method for differentiation is measuring the length and width of the body. However, with some practice, general characteristics of the microfilariae may be used for identification if a means of measuring microfilariae is not available.
TABLE 17-1
Differentiation of Microfilariae Using the Modified Knott Technique
| Dirofilaria immitis | Dipetalonema reconditum | |
| Body length | 310 μm | 290 μm |
| Midbody width | 6 μm | 6 μm |
| Head | Tapered | Blunt |
| Tail | Straight | Hooked∗ |
∗Artifact of formalin fixation.
From Hendrix CM, Sirois M: Laboratory procedures for veterinary technicians, ed 5, St Louis, 2007, Mosby.
Filter Techniques: Filter techniques are the most common method used in veterinary practices for the detection of microfilariae in the blood. An excellent diagnostic kit is available for this procedure, the Difil-Test kit (EVSCO Pharmaceuticals, Buena, N.J.). These kits come complete with filters, lysing solution, stain, and directions for use.
Most kits require 1 ml of whole blood to test for heartworms. The blood is mixed with nine parts of lysing solution and passed through a filter. The filter is rinsed, removed, and placed on a slide. A drop of stain is added, a coverslip is applied, and the filter is microscopically examined for microfilariae. Differentiation of microfilariae is possible but difficult using the filter technique. If microfilariae are present, it is best to perform other diagnostic procedures for identification purposes.
ECTOPARASITES OF DOMESTICATED ANIMALS
The ectoparasites of domesticated animals usually belong to the phylum Arthropoda. The arthropodan ectoparasites include: bugs, lice, bloodsucking flies, myiasis-inducing flies, fleas, ticks, and mites. In addition, there are a few nematodes that inhabit skin lesions and bloodsucking leeches, which belong to the phylum Annelida that may also serve as ectoparasites.
Some arthropod ectoparasites are host specific, whereas others infect any number of animal species. The diagnosis is generally based on size and the appearance of external morphologic features, with the use of taxonomic keys. Control is often difficult, sometimes necessitating the treatment of the premises and prevention by prohibiting interaction with infested animals (e.g., other companion animals with fleas, ticks, or lice within the same environment).
TRUE BUGS
Whereas some adult hemipterans (true bugs) are wingless, most adult true bugs have two pair of wings. The posterior pair of wings is membranous in appearance. The anterior pair of wings has a leathery basal portion with a membranous apical portion, which gives the appearance of a “half wing,” hence, the origin of the ordinal name: hemi-, meaning half, and -ptera, meaning wing. Two groups of true bugs are of veterinary importance: Reduviid bugs (kissing bugs) and bedbugs. Reduviid bugs (kissing bugs) are periodic parasites, making frequent visits to the host to obtain a blood meal. Kissing bugs serve as intermediate hosts for Trypanosoma cruzi, a protozoan parasite that can produce a rare disease in humans and dogs called Chagas’ disease. This disease also is called South American trypanosomiasis and rarely occurs in dogs and other animals in the United States. Kissing bugs take blood meals from an infected host and transmit the parasite as they defecate.
Bedbugs are dorsoventrally flattened, wingless true bugs that often infest homes. They are periodic parasites, making frequent visits to the host to obtain a blood meal. Although bedbugs are human parasites, they also may be found in rabbit colonies, poultry houses, and pigeon colonies. Bedbugs are not capable of naturally transmitting any human or animal pathogen.
LICE
Lice are some of the most prolific ectoparasites of domestic animals. Two orders of lice exist: the Mallophaga (chewing or biting lice) and the Anoplura (sucking lice). Lice are dorsoventrally flattened, wingless insects. They have three body divisions: the head, with its mouthparts and antennae; the thorax, with its three pairs of legs and its lack of wings; and the abdomen, the portion that bears the reproductive organs. These body divisions and their relationship to each other are important in diagnostic veterinary parasitology.
Members of the order Mallophaga, or chewing or biting lice, are smaller than sucking lice. They are usually yellow and have a large, round head. The mouthparts are mandibulate and are adapted for chewing or biting the host. Characteristically the head of every chewing louse is wider than the widest portion of the thorax. On the thorax are three pairs of legs, which may be adapted for clasping or for moving rapidly among feathers or hairs. Chewing or biting lice may parasitize birds, dogs, cats, cattle, sheep, goats, and horses (Figure 17-51).

FIGURE 17-51 Assorted chewing lice of goats and fowl. A, Damalinia caprae. B, Goniodes dissimilis. C, Goniodes gallinae.
Members of the order Anoplura (sucking lice) are larger than members of the order Mallophaga (chewing lice). These lice are red to gray; their color usually depends on the amount of blood ingested from the host. In contrast to the wide head of the mallophagans, anoplurans’ heads are narrower than the widest part of the thorax. Their mouthparts are piercing and are adapted for sucking blood. Their pincerlike claws are adapted for clinging to the host’s hairs. They are found on many species of domestic animals (Figure 17-52).
FIGURE 17-52 Assorted sucking lice. A, Solenopotes capillatus (sheep). B, Pedicinus obtusus (monkey). C, Pedicinus obtusus (monkey). D, Linognathus setosus (dog).
Anoplurans and mallophagans have the same type of life cycle. This life cycle has only three developmental stages: the egg, nymphal, and adult stages.
The egg stage is also called a “nit.” The nit is tiny, approximately 0.5 to 1.0 mm in length. It is oval, white, and usually found cemented to the hair or feather shaft of the host (Figure 17-53). Figure 17-54 shows a gravid female sucking louse and an associated nit collected from a dog. Nits hatch about 5 to 14 days after they are laid by the adult female louse.
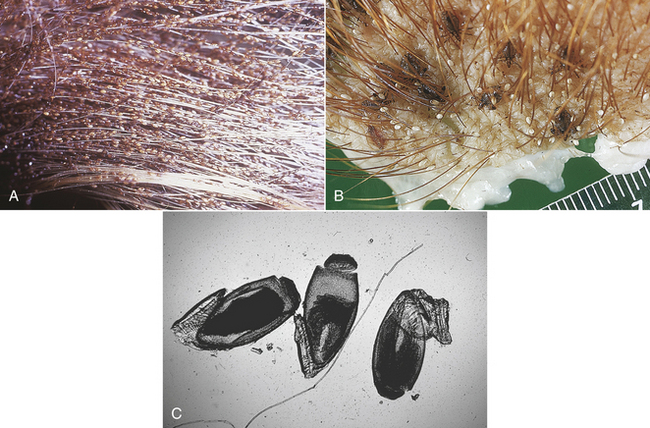
FIGURE 17-53 A, Thousands of nits can be cemented by female lice to the hair coat of domesticated animals. This calf’s tail contains thousands of nits. B, Pediculosis can be defined as infestation by either chewing or sucking lice, in this case, Haematopinus suis infestation in a pig. C, Appearance of operculated nits viewed by compound microscope.
The nymphal stage is similar in appearance to the adult. However, it is smaller and lacks functioning reproductive organs and genital openings. The three nymphal stages are each progressively larger than its predecessor. The nymphal stage lasts from 2 to 3 weeks.
The adult stage is similar in appearance to the nymphal stage, but larger. It has functional reproductive organs. Male and female lice copulate; the female lays eggs, cementing the eggs to the hair or feather; and the life cycle begins again. It takes 3 to 4 weeks to complete the cycle. Nymphal and adult stages live no longer than 7 days if removed from the host. Eggs hatch within 2 to 3 weeks during warm weather, but seldom hatch off the host.
Lice usually are transmitted by direct contact, but all life stages may be transmitted by fomites (inanimate objects, such as blankets, brushes, and other grooming equipment). Lice are easily transmitted among young, old, and malnourished animals. Veterinarians often cannot understand why certain animals in a flock or herd are heavily infested, whereas others have only a few lice. Lice are species specific (i.e., they will only parasitize their specific hosts). For example, dog lice parasitize dogs. They may temporarily reside on another species of animal, but they will not set up housekeeping on that animal.
Infestation by lice (whether mallophagan or anopluran) is referred to as pediculosis. Sucking lice can ingest blood to such a degree that they produce severe anemia; fatalities can occur, especially in young animals. The PCV can drop as much as 10% to 20%. Severely infested animals may harbor as many as one million lice. Infested animals become more susceptible to other diseases and parasites and may succumb to stresses not ordinarily pathologic to uninfested animals. When animals are poorly fed and kept in overcrowded conditions, they often become severely infested with lice and quickly become anemic and unthrifty.
A careful examination of the hair coat or feathers of infested animals easily reveals lice and the accompanying nits. Hair clippings also serve as a good source for lice. Infestation of animals with a thick hair coat may be easily overlooked. A hand-held magnifying lens or a binocular headband magnifier may aid the observation of adult or nymphal lice crawling through or clinging to hair or feathers or tiny nits cemented to individual hairs.
Any lice and/or their nits observed may be collected with thumb forceps and placed in a drop of mineral oil on a glass microscope slide. A coverslip should be placed over the specimen and the slide examined using the 4× or 10× objectives.
The identification of a louse as to genus and specific epithet is difficult and best left to a trained specialist. Veterinary technicians should be able to identify the specimen as anopluran (sucking) or mallophagan (chewing or biting) by a visual examination of the head size in relation to the width of the thorax.
FLIES
Dipterans (two-winged flies) produce two contrasting pathologic scenarios. As adults, they may feed intermittently on vertebrate blood, saliva, tears, or mucus. As larvae, they may develop in the subcutaneous tissues or within internal organs. Adult dipterans that make frequent visits to the vertebrate host to intermittently feed on blood are referred to as periodic parasites. When dipteran larvae develop in the tissue or organs of vertebrate hosts, they produce a condition known as myiasis.
As periodic parasites, blood-feeding dipterans may be classified as to which sex feeds on vertebrate blood and as to food preference. In certain dipteran groups, only the females feed on vertebrate blood; these female flies require vertebrate blood for laying their eggs. In this group are the biting gnats (e.g., Simulium, Lutzomyia, and Culicoides spp.), the mosquitoes (e.g., Anopheles, Aedes, and Culex spp.), the horseflies (e.g., Tabanus spp.), and the deerflies (e.g., Chrysops spp.).
In the second group of blood-feeding dipterans, both male and female flies require a vertebrate blood meal. These species include the stable fly Stomoxys calcitrans, the horn fly Haematobia irritans, and the sheep ked Melophagus ovinus.
This section will discuss only those dipterans that may be seen by the direct observation of either large or small animals.
Horseflies/Deerflies
Chrysops spp. and Tabanus spp. are large (up to 3.5 cm long), heavy-bodied, robust dipterans with powerful wings. Horseflies and deerflies are the largest blood-feeding dipterans. Figure 17-55 shows Tabanus spp., the largest of the blood-feeding dipterans. Horseflies are larger than deerflies. Deerflies have a dark band passing from the cranial to the caudal margins of the wings.

FIGURE 17-55 Tabanus spp., the largest blood-feeding dipteran. This tabanid is approximately 2.5 cm in length.
Horseflies and deerflies feed a number of times at multiple feeding sites before they stop feeding. When disturbed by the animal’s swatting tail or by the panniculus reflex (skin twitching), the flies leave the host, but blood continues to ooze from the open wound; these fly bites are painful. Affected horses and cattle become restless. Because they often feed on multiple hosts, these flies may acts as mechanical transmitters of anthrax, anaplasmosis, and the virus of equine infectious anemia.
Stable Fly
The stable fly, Stomoxys calcitrans, is often called the “biting housefly.” It is approximately the size of Musca domestica, the common housefly. Rather than possessing a sponging type of mouthparts, the stable fly has a bayonet-like proboscis that protrudes forward from the head (Figure 17-56). These flies are found worldwide. In the United States, they are found in the central and southeastern states, where cattle are raised. Both male and female flies are avid blood feeders, feeding on any domestic animal. They usually attack the legs and ventral abdomen and may also bite the ears. These flies also feed on the tips of the ears of dogs with pointed ears, especially the German shepherd. These dogs’ ears often demonstrate a loss of hair and the presence of dried, crusty blood on the ear tips.

FIGURE 17-56 Stomoxys calcitrans, the stable fly. Note the bayonet-like proboscis that protrudes forward from the head. The stable fly is approximately the same size as a housefly.
This fly feeds on both horses and cattle, with horses the preferred host. The fly usually lands on the host with its head pointed upward. It is a sedentary fly, not moving on the host. The flies inflict painful bites that puncture the skin and bleed freely. Stable flies stay on the host for short periods, during which they obtain the blood meals. This is an outdoor fly; however, in the late fall and during rainy weather, it may enter barns.
Stable flies are mechanical vectors of anthrax in cattle and equine infectious anemia. They are the intermediate host for Habronema muscae, a nematode found in the stomach of horses. When large numbers of stable flies attack dairy cattle, milk production can fall. Beef cattle may refuse to graze in the daytime when they are attacked by large numbers of flies; as a result, these cattle do not gain the usual amount of weight.
Haematobia
Haematobia irritans is often called the horn fly. This dark-colored fly is approximately 3 to 6 mm in length, approximately half of the size of S. calcitrans, the biting housefly. Like the stable fly, the horn fly has a bayonet-like proboscis that protrudes forward from the head. These flies are found almost exclusively on cattle throughout North America.
When the air temperature is below 70° F, horn flies cluster around the base of the horns; this is the origin of the name horn fly. In warmer climates, thousands of flies often cluster on the hosts’ shoulders, backs, and side; these areas are least disturbed by tail switching. On hot, sunny days, horn flies congregate on the ventral abdomen.
Adult horn flies spend most of their life on cattle and leave the host only to deposit eggs in fresh cow manure. Using tiny bayonet mouthparts, they feed frequently, sucking blood and other fluids, and cause considerable irritation. Female flies are more aggressive than males. Harassment by the flies and loss of blood often reduces weight gain and milk production in cattle. Horn flies probably cause greater losses in cattle in the United States than any other bloodsucking fly. Adult horn flies also cause focal midline dermatitis on the ventral abdomen of horses. These flies also serve as the intermediate host for Stephanofilaria stilesi, a filarial parasite that produces ventral, plaquelike lesions on the ventral abdomen of cattle.
Sheep Ked
Melophagus ovinus is often called the sheep ked. Members of the order Diptera usually have one pair of wings (two wings). Keds are an exception to that rule; they are wingless dipterans. Keds are hairy, leathery, and 4 to 7 mm in length. The head is short and broad, the thorax brown, and the abdomen broad and grayish brown. The legs are strong and armed with stout claws (Figure 17-57). Some observers say that keds have a “louselike” appearance, but they are not related to lice.
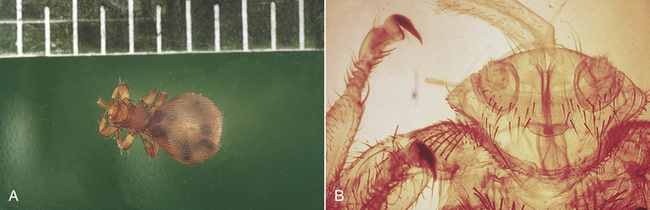
FIGURE 17-57 Melophagus ovinus, the sheep ked. Keds are hairy, leathery, and 4 to 7 mm in length. The head is short and broad. The thorax is brown, and the abdomen broad and grayish brown. The legs are strong and armed with stout claws.
Keds are permanent ectoparasites of sheep and goats. Their pupal stages are often found attached to the wool or hair of the host. Keds are avid blood feeders. Heavy infestations can reduce the condition of the host considerably and even cause anemia. Their bites cause pruritus over much of the host’s body; infested sheep often bite, scratch, and rub themselves, damaging the wool. Ked feces often stain the wool and do not wash out readily. Keds are most numerous in the cold temperatures of the fall and winter months. Their numbers decline as temperatures warm in the spring and summer months.
Louse Flies
Lynchia and Pseudolynchia spp. are often called louse flies, and like the keds, they are not related to lice. These dipterans are closely related to the wingless sheep keds, but they are found to parasitize a wide variety of birds—from songbirds to pigeons to raptors. They are found in among the feathers of these birds; however, they enjoy sucking from areas of the skin that are sparsely covered with feathers. These winged dipterans are dark brown in color, hairy, and leathery in appearance. These flies are from 4 to 6 mm in length with legs armed with stout, fierce claws. Louse flies are swift fliers and move about quickly—even attempting to get into a human’s hair.
Face Fly
The final periodic parasite among the dipteran flies is one that is not a blood feeder, but instead feeds on the mucus, tears, and saliva of large animals, particularly cattle. Face flies, Musca autumnalis (Figure 17-58), are so named because they gather around the eyes and muzzle of livestock, particularly cattle. They also may be found on the withers, neck, brisket, and sides. Face flies feed mostly on liquid media—saliva, tears, and mucus. They usually are not considered blood feeders because their mouthparts are not piercing or bayonet-like. Instead, their mouthparts are adapted for sponging up saliva, tears, and mucus. They follow blood-feeding flies, disturb them during their feeding process, and then lap up the blood and body fluids that accumulate on the host’s skin. Face flies are found on animals outdoors; they usually do not follow animals into barns.

FIGURE 17-58 A, Musca autumnalis, the face fly. Its mouthparts are adapted for sponging saliva, tears, and mucus. B, Stomoxys calcitrans head (left) and M. autumnalis (right).
Face flies produce considerable annoyance to the host. The irritation around the host’s eyes stimulates the flow of tears, which attracts even more flies. This harassment ultimately interferes with the host’s productivity. Face flies may be involved in the transmission of Moraxella bovis, a bacterium associated with infectious keratoconjunctivitis or pinkeye in cattle.
Files of the genus Hippelates feed on the mucus, tears, and saliva of large animals, particularly cattle. These tiny, fragile dipterans are nonbiting gnats with sponging mouthparts that are similar to the mouthparts of the common housefly. These tiny gnats are often found around a dog’s penis (hence, they are often referred to by the common name of, “dog penis gnats.” Because they have sponging type of mouthparts, these dipterans are not capable of biting the host, but often feed on liquid secretions from the host. Frequently, these nonbiting gnats are found around the teats of dairy cattle. They have been known to serve as mechanical vectors of a variety of bacteria.
Myiasis-Inducing Flies
The larvae or maggots of dipterans may develop in the subcutaneous tissues of many domestic animals and produce a condition known as myiasis.
Rabbit Bots and Fox Maggots: Cuterebra is known as the rabbit bot and Wohlfahrtia is known as the fox maggot. These species occasionally infest dogs, cats, rodents, rabbits, and other wildlife. Cuterebra flies usually deposit eggs around rodent burrows or runs. Eggs hatch, and the larvae penetrate the skin of the host, developing to the third stage in subcutaneous tissues without migrating in the host. The larvae then emerge from a hole in the host’s skin (Figure 17-59) and pupate in the soil. The Cuterebra larvae are known for their aberrant or erratic migrations, having been found in a variety of extracutaneous sites, the cranial vault, the eye, and the pharyngeal regions. Clinical signs vary with the site of infection or infestation. Female Wohlfahrtia deposit larvae directly on the skin of the host, which is usually a young animal. The larvae penetrate the skin and develop in the subcutaneous tissues with limited migration. When they become third-stage larvae, they drop out and pupate in the soil. The treatment consists of surgical removal of the larvae and supportive wound treatment.
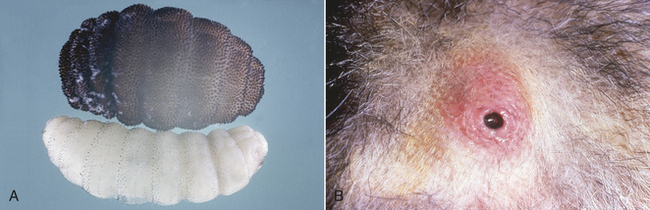
FIGURE 17-59 A, Different developmental stages of Cuterebra spp. Larval Cuterebra are either sparsely or thickly covered with tiny black spines. B, Larval Cuterebra spp. are usually found in swollen, cystlike subcutaneous sites, with a fistula (pore, or hole) communicating to the outside environment.
Horse Botflies: Gasterophilus intestinalis, Gasterophilus nasalis, and Gasterophilus hemorrhoidalis—the botflies of horses—are widespread and common wherever horses are found; but G. intestinalis is the most common species. The adult fly cements eggs to the hair of the horse (see Figure 17-28). The eggs either hatch by themselves and the larvae crawl into the horse’s mouth or the eggs are stimulated to hatch by the horse licking them. Once in the mouth, the larvae penetrate the mucosa and move down the esophagus to the stomach, where they attach. These larvae develop into the third-stage larvae (see Figure 17-29). They usually spend about 10 months as larvae. Ultimately the larvae pass out with the feces, burrow into the soil, pupate, and later emerge as adult flies, usually in late summer. The diagnosis is based on the type of egg and means of attachment of the spines on each segment (single row, G. nasalis; double row, G. intestinalis; double row, smaller spines, G. hemorrhoidalis). The treatment is generally applied in the fall with a combination of insecticides and anthelmintics or a broad-spectrum compound. Control is difficult.
Heel Flies/Cattle Grubs/Ox Warbles: The heel flies of cattle, Hypoderma lineatum and Hypoderma bovis, whose larval stages are called grubs or warbles, are widely distributed wherever cattle are found. Emergence of these flies depends on environmental conditions. For example, they are active in early January in southern Texas and in early August in Montana. When both species are present, H. bovis emerges about 1 month later than H. lineatum. After emergence, H. bovis lays single eggs attached to hair, and H. lineatum lays a row of eggs just above the hooves. The larvae hatch from the eggs, penetrate the skin, and wander in the subcutaneous tissue for 4 to 5 months. H. lineatum then goes to the esophagus for 2 months, and H. bovis goes to the epidural fat of the spinal cord for 2 months. Both then go to the subcutaneous tissue along the back for about 2 months (Figure 17-60). The larvae develop and drop out and burrow in the soil, in which they pupate for 1 month to 3 months. The diagnosis is generally based on the presence of warbles on the backs of cattle; however, the species can be identified on the basis of the morphologic appearance of the larvae. Several treatments are available, but they must be applied at a specific time of year.
Sheep Nasal Botflies: The sheep nasal botfly, Oestrus ovis, is common wherever sheep are found. Flies emerge from spring through fall and deposit first-stage larvae around the nasal opening. Larvae then enter the nasal cavity for 2 weeks to 9 months and migrate to the paranasal sinuses for a short period to complete development. The larvae leave through the nose and pupate in the soil for 15 to 60 days. The life cycle may be completed in 2 to 11 months, depending on environmental conditions. The diagnosis is based on the presence of these larvae in the nose or sinuses. There is no preferred treatment available.
Primary Screwworms: Only one fly in North America, Cochliomyia hominivorax, is a primary invader of fresh, uncontaminated skin wounds of all domestic animals. These larvae must not be confused with the larvae of the more common facultative myiasis-producing flies described previously. C. hominivorax is often referred to as the “screwworm fly” because its third larval stage resembles a wood screw and because this same larval stage burrows deep into the host tissues. Economically, it is the most important fly that may attack livestock in the southwestern and southern United States, but it may also be found in a small animal clinical situation, primarily in animals returning from foreign locales.
Adult female flies are attracted to fresh skin wounds on any warm-blooded animal, where they lay batches of 15 to 500 eggs in a shinglelike pattern at the edge of wounds. The female lays several thousand cream-color, elongated eggs during her lifetime. They hatch within 24 hours. Larvae enter the wound, where they feed for 4 to 7 days before developing into third-stage (fully-grown) larvae. They may be as long as 1.5 cm in length. When fully mature, the larvae drop from the host to the ground and pupate for about a week, after which the adult flies emerge. The adult male and female fly mate only once during their lifetime, a fact that is used to control these flies biologically, a program known as the “sterile male release technique.”
Adult flies are shiny and greenish blue, with a reddish-orange head and eyes, and are 8 to 15 mm long. Larvae often are identified by their wood-screw shape and by the deeply pigmented tracheal tubes on the posterior one third of the dorsal aspect of the caudal ends. Because of the obligatory nature of the screwworm with regard to breeding in the fresh wounds of any warmblooded animal, the veterinarian must report any screwworm infestations to both state and federal authorities. C. hominivorax has been eradicated from the United States, but occasionally surreptitiously enters the country in imported animals. The presence of this fly larva within U.S. borders must be reported to both state and federal authorities.
Secondary Myiasis (Fly Strike): This group of flies includes Musca domestica (housefly) and Calliphora, Phaenicia, Lucilia, Phormia (blowfly, bottle flies), and Sarcophaga (flesh fly) spp. The adults of these fly species are colloquially known as “filth flies” because of their propensity to fly from feces to food. The adults of these flies are “vomit drop feeders,” disgorging their stomach contents with the associated liquefaction enzymes and then lapping up the resulting liquid food. These adult flies are frequently seen in kennel situations, alighting on feces that have not been removed from dog runs.
Dipteran larvae that produce fly strike myiasis include the housefly, M. domestic; the blowflies or bottle flies Calliphora, Phaenicia, Lucilia, and Phormia spp.; and the flesh fly Sarcophaga spp. Larval stages of these flies usually are associated with skin wounds contaminated with bacteria or with a matted hair coat contaminated with feces or urine.
Under their “normal” living conditions, adult flies of these genera lay eggs in decaying animal carcasses or in feces. In “fly strike myiasis,” the adult flies are attracted to an animal’s moist wound, skin lesion, or soiled hair coat. These sites provide the adult fly with a moist medium on which it feeds. As adult female flies feed in these sites, they lay eggs. These eggs hatch, producing larvae (maggots) that move independently about the wound surface, ingesting dead cells, exudate, secretions, and debris, but not live tissue. These larvae irritate, injure, and kill successive layers of skin and produce exudates.
Maggots can tunnel through the thin epidermal layer into the subcutis. This process produces tissue cavities in the skin that measure up to several centimeters in diameter. Unless the process is halted by appropriate therapy, the infested animal may die from shock, intoxication, histolysis, or an infection. A peculiar, distinct, pungent odor permeates the infested tissues and the affected animal. Advanced lesions may contain thousands of maggots.
As adults, these flies can be pestiferous flies in a veterinary clinical setting. These flies are “vomit drop” feeders and fly from feces to food, spreading bacteria on their feet and their disgorged stomach contents. A veterinary practice with outdoor kennels is especially susceptible to the “assault” of these pestiferous adult flies.
A tentative diagnosis of maggot infestation in any domestic animal can easily be made by a veterinary technician because maggots can be observed in an existing wound or among the soiled, matted hair coat. When facultative myiasis has been diagnosed, the veterinarian must rule out the possibility of myiasis caused by the primary screwworm, C. hominivorax.
FLEAS
The most common flea of both dogs and cats is Ctenocephalides felis, the cat flea (Figure 17-61). Fleas are not host specific and will attack and may infest other animals and humans. Fleas are widely distributed, but are much more common in warm, humid environments. When environmental conditions are favorable, fleas have a great reproductive potential. Fleas thrive at low altitudes in temperature ranges of 65° F to 80° F (18.2° C to 26.6° C). Under these conditions, the flea life cycle can be completed, from hatching of an egg to the laying of the next generation of eggs, in as few as 16 days. See Figure 17-62 for a pictorial representation of the four life stages.

FIGURE 17-62 Life stages of Ctenocephalides felis, the cat flea. Left to right, Pupae, larvae, eggs, adult male (top), and female (bottom).
The female flea, C. felis, lays its eggs in the fur of dogs and cats. These flea eggs are not sticky and tend to fall out of the fur and survive in the protected places where the dog or cat sleeps or plays (Figure 17-63). The eggs will hatch into small maggotlike larvae, which are sparsely covered with fine hairs (Figure 17-64). These larvae feed on organic debris, especially the dried blood droppings (flea frass or flea dirt) defecated by adult fleas (Figure 17-65). The egg and larval stages of the flea are often recovered from the pet’s environment and brought to the practice to be identified; it behooves the veterinary technician to be able to identify these morphologic stages for the client. The flea larvae molt and form pupae that spin sticky, loose cocoons, to which sand and other environmental debris will adhere (Figure 17-66). The fleas will emerge as young and hungry adults in about 3 weeks. An important source of fleas to a dog and cat is these newly “hatched out” young fleas.
Once fleas have had a chance to establish the life cycle in a home or outdoor environment, no program that does not emphasize environmental control will be successful. Mechanical cleaning of the home and yard environment should precede any application of insecticides. In general, the same environmental control methods may be used in households with young dogs and cats as in those with adult animals. Care should always be taken that animals and people are not directly exposed to insecticides used in household extermination. All effective in-house programs should take advantage of new technologies in flea control. There are new insecticides that have truly long residuals (synthetic pyrethroids or microencapsulated products), and there are insect growth regulators (methoprene and fenoxycarb) marketed for preadult flea control.
Advances in outdoor environmental flea control have been less remarkable. At present, the use of insecticides (compounds that contain chlorpyrifos, malathion, or diazinon as their active ingredient) labeled for outdoor flea control is still the best and most economical approach. Such programs must incorporate repeated applications at 2-week intervals throughout the flea season where temperature and humidity are favorable for flea reproduction.
All topical insecticides should be used exactly according to label directions because it is not legally permissible to use or recommend the use of insecticide products beyond the label restrictions. In general, the use of organophosphate preparations on puppies younger than 16 weeks or on kittens younger than 6 months should be avoided. Any product containing lufenuron, fipronil, or imidacloprid as its active ingredient should never be administered to or used on nursing animals. Pyrethrin-based products are generally safe for frequent application; the most effective products are synergized pyrethrin sprays or foams. Very small animals and nursing animals sprayed with alcohol-based or other volatile organic solvents may be severely chilled as the solvent evaporates. Water-based sprays are preferable, and small animals and nursing animals should never be thoroughly saturated with a spray. The safest effective products are sprays and foams with microencapsulated pyrethrins. Flea collars that are safe for use on puppies or kittens are not effective in most environments. Topical treatments should be coordinated with in-home environmental flea control.
MITES
The first group of parasitic mites may be classified together as “sarcoptiform” mites. Sarcoptiform mites have several key characteristics or features in common. These mites may produce severe dermatologic problems in a variety of domestic animals. This dermatitis usually is accompanied by a severe pruritus. Sarcoptiform mites are small, barely visible to the naked eye, and approximately the size of a grain of salt. Their bodies have a round to oval shape. Sarcoptiform mites have legs with pedicels or stalks at the tip. The pedicels may be long or short. If the pedicel is long, it may be straight (unjointed) or jointed. At the tip of each pedicel, there may be a tiny sucker. Veterinarians and veterinary technicians should use the description of the pedicel (long or short, jointed or unjointed) to identify these sarcoptiform mites.
Sarcoptiform mites may be divided into two basic families: Sarcoptidae, which burrow or tunnel within the epidermis, and Psoroptidae, which reside on the surface of the skin or within the external ear canal.
Sarcoptidae
Sarcoptic mites burrow or tunnel within the epidermis of the infested definitive host. The entire four-stage life cycle is spent on the host. Male and female mites breed on the skin surface. Female mites penetrate the keratinized layers of the skin and burrow or tunnel through the epidermis. Over a 10- to 15-day period, the female deposits 40 to 50 eggs within the tunnel. After egg deposition, the female dies. Larvae emerge from the eggs in 3 to 10 days and exit the tunnel to wander on the skin surface. These larvae molt to the nymphal stage within minute pockets in the epidermis. Nymphs become sexually active adults in 12 to 17 days, and the life cycle begins again.
The disease caused by Sarcoptes scabiei is called sarcoptic acariasis (Figure 17-67). This mite causes an extremely pruritic skin condition. Certain varieties of Sarcoptes mites infest specific hosts. For example, S. scabiei variety canis infests only dogs, and S. scabiei variety suis infests only pigs. Almost every species of domestic animal has its own distinct variety of this mite.
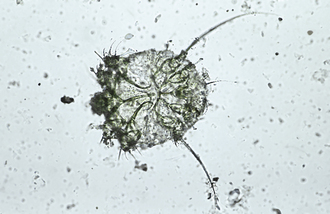
FIGURE 17-67 Adult S. scabiei mite. Note the long, unjointed pedicels (stalks), with suckers on the ends.
Scabies in dogs is caused by S. scabiei variety canis, which produces an erythematous, papular rash. Scaling, crusting, and excoriation are common. The ears, lateral elbows, and ventral abdomen are most likely to harbor mites. However, the host’s entire body may be infested, whereas some dogs may be asymptomatic carriers. Scabies is extremely contagious. The mites are spread by direct contact and can affect all dogs in a household or kennel. The dog owner can also become infested with this mite, but the infestation in humans is self-limiting. The mites burrow into human skin, producing a papulelike lesion, but they do not establish a full-blown infestation in people. Nevertheless, this mite is considered to be zoonotic. S. scabiei variety felis, which causes scabies in cats, is an extremely rare mite.
Among large animals, pigs most commonly are affected by scabies mites. Lesions caused by S. scabiei variety suis include small, red papules, alopecia, and crusts, most commonly on the trunk and ears. The mite is rare in cattle (S. scabiei variety bovis). The main infested areas are the head, neck, and shoulders. S. scabiei variety equi of horses is even more rare. The main infested area is the neck. S. scabiei variety ovis does not infest the fleece of sheep and goats; the face and muzzle are the main areas affected.
Areas with an erythematous, papular rash and crust should be scraped, especially the areas most associated with sarcoptic infestation (ears, lateral elbows, and ventral abdomen of dogs). Repeated scrapings (as many as six scrapings) may be necessary to detect mites. Adult sarcoptic mites are oval and 200 to 400 μm in diameter and have eight legs. The key morphologic feature used to identify this species is the long, unjointed pedicel with a sucker on the end of some of the legs. The anus is located on the caudal end of the body. The eggs of Sarcoptes mites are oval.
Notoedres cati infests mainly cats; but on occasion it can parasitize rabbits. This sarcoptiform mite is found chiefly on the ears, back of the neck, face, feet, and, in extreme cases, on the entire body. The life cycle is like that of S. scabiei, with the mite burrowing or tunneling in the superficial layers of the epidermis. The characteristic lesion of notoedric acariasis is a yellowing crust in the region of the ears, face, or neck.
Notoedric mites are easier to demonstrate in cats than sarcoptic mites in dogs. Likely infestation sites should be scraped. Like Sarcoptes spp., Notoedres mites have a long, unjointed pedicel with a sucker on the end of some of the legs. Adult notoedric mites are similar to sarcoptic mites, but are smaller, with a dorsal anus. The eggs of notoedric mites are oval.
Knemidokoptes pilae causes “scaly leg” in budgerigars or parakeets. This mite tunnels in the superficial layers of the epidermis of the pads and shanks of the feet. In severe cases, the beak and cere also may be affected. The mite characteristically produces a yellow to gray-white mass that resembles a honeycomb. This condition may be disfiguring to the parakeet. The parasites pierce the skin underlying the scales, causing an inflammation with exudate that hardens on the surface and displaces the scales superficially. This process causes the thickened, scaly nature of the skin. A related species, Knemidokoptes mutans, produces scaly leg in chickens and turkeys.
Infested sites should be scraped. Great care should be taken in handling infested birds because parakeets are fragile creatures. The eight-legged, globular mites are about 500 μm in diameter. Adult female mites have short legs and no suckers on the end of their legs. Adult males have longer legs and a long, unjointed pedicel with suckers on the end of some of the legs.
Psoroptidae
Members of the family Psoroptidae reside on the surface of the skin or within the external ear canal. The entire five-stage life cycle (egg, larva, protonymph, deutonymph or pubescent female, adult egg-laying female) is spent on the host. Adult male and female mites breed on the skin surface (Figure 17-68). The female produces 14 to 24 elliptic, opaque, shiny, white eggs that hatch within 1 to 3 days. The six-legged nymphs are small, oval, soft, and grayish brown. The eight-legged nymphs are slightly larger than larvae. Larval and nymphal stages may last 7 to 10 days. The life cycle is completed in 10 to 18 days. Under favorable conditions, psoroptic mites can live off of the host for 2 to 3 weeks or longer. Under optimal conditions, mite eggs may remain viable for 2 to 4 weeks.
Psoroptes cuniculi occurs most commonly in the external ear canal of rabbits, but also has been found in horses, goats, and sheep. These mites live on the surface of the skin and feed on the rabbit host by puncturing the epidermis to obtain tissue fluids. Within the external ear canal of the infested host are the characteristic dried crusts of coagulated serum. The rabbit’s ears appear to be packed with dried cornflakes. Affected animals shake their head and scratch their ears. Lesions sometimes occur on the head and legs. Severely infested animals may become debilitated. Loss of equilibrium may occur, with head tilt.
The mites within the crusty debris inside the ear can be easily isolated. The brownish-white female is large, up to 750 μm long. The mites exhibit characteristic long, jointed pedicels with suckers on the ends of some of the legs. The anus is in a terminal slit.
Psoroptes ovis, Psoroptes bovis, and Psoroptes equi are the scab mites of large animals, residing on sheep, cattle, and horses, respectively. These mites are host specific and reside within the thick-hair or long-wool areas of the animal. The mites are surface dwellers and feed by puncturing the epidermis to feed on lymphatic fluid. Serum exudes through the puncture site. After the serum coagulates and forms a crust, wool is lost. The feeding site is extremely pruritic, and the animal excoriates itself, producing further wool loss. The mites then migrate to adjacent undamaged skin. As the mites proliferate, tags of wool are pulled out, and the fleece becomes matted. Finally, patches of skin are exposed, and the skin becomes parchmentlike, thickened, and cracked. The skin may bleed easily. Infested sheep constantly rub against fences, posts, farm equipment, and anything else that might serve as a scratching post. The disease is spread by direct contact or infested premises.
P. bovis in cattle produces lesions on the withers, neck, and rump. These consist of papules, crusts, and wrinkled, thickened skin. P. equi in horses is rare and affects the base of the mane and the tail. Because of the intense pruritus and the highly contagious nature of the infestation, the occurrence of Psoroptes spp. in large animals should be reported to state and federal authorities and the United States Department of Agriculture.
These mites are host specific. Adults are up to 600 μm long. Psoroptic mites exhibit characteristic long, jointed pedicels with suckers on the ends of some of the legs.
Chorioptes equi, Chorioptes bovis, Chorioptes caprae, and Chorioptes ovis are the foot and tail mites of large animals, residing on horses, cattle, goats, and sheep, respectively. These mites are found on the skin surface on the distal (lower) part of the hind legs, but may spread to the flank and shoulder area. On cattle, they are frequently found in the tail region, especially in the area of the escutcheon. These mites do not spread rapidly or extensively. The mites puncture the skin, causing serum to exude. Thin crusts of coagulated serum form on the skin surface. The skin eventually wrinkles and thickens, although pruritus is not severe.
Infested horses stamp, bite, and kick, especially at night. Mites typically infest the pasterns, especially those of the hind legs.
The characteristic mites can be identified from skin scrapings of infested areas. Chorioptic mites have short, unjointed pedicels, with suckers on the ends of some of the legs. The female mites are about 400 μm long.
Ear mites, Otodectes cynotis, are a common cause of otitis externa in dogs, cats, and ferrets. Although they occur primarily in the external ear canal, ear mites may be found on any area of the body. A common infestation site is the tail and head region. As dogs and cats curl up to sleep, the head (and ears) are often in close proximity to the base of the tail. These mites are spread by direct contact and are highly transmissible both among and between the canine and feline species.
Ear mites are found within the external ear canal, where they feed on epidermal debris and produce intense irritation. The infection is usually bilateral. The host responds to the mite infestation by shaking its head and scratching its ears. Severe infestations may cause otitis media, with head tilt, circling, and convulsions. Auricular hematomas may develop.
Mites usually are identified by using an otoscope; through an otoscope, the mites appear as white, motile objects. The brown exudate collected by swabbing the ear may be placed in mineral oil on a glass slide and the mites observed with a low-power microscopic objective. These mites are fairly large, approximately 400 μm; they also may be easily seen with a magnifying glass or even the unaided eye. The mites exhibit characteristic short, unjointed pedicels, with suckers on the ends of some of the legs (Figure 17-69). The anus is terminal.
Demodex
Mites of the genus Demodex reside in the hair follicles and sebaceous glands of most domesticated animals and people. In many species, they are considered normal, nonpathogenic fauna of the skin. These mites are host specific and are not transmissible from one host species to another. The clinical disease, caused by an increased number of these mites, is called demodicosis.
Demodex mites resemble “eight-legged alligators.” These are elongated mites with short, stubby legs on the anterior half of the body. Adult and nymphal stages have eight legs, whereas the larvae have six. Adult Demodex mites are approximately 250 μm long (Figure 17-70). The eggs are spindle shaped or tapered at each end.
The life cycle of Demodex spp. has five stages: egg, larva, protonymph, deutonymph, and adult. The developmental periods of these various life-cycle stages are not well known.
Of all the domestic animals infested with Demodex spp., the dog is the most commonly and most seriously infested. Small numbers of these mites are considered part of the normal skin flora of all dogs. In dogs with immunodeficiencies, however, these mites proliferate and cause skin disease.
Demodicosis occurs in two forms in dogs: localized demodicosis and generalized demodicosis. The preponderant clinical sign of the localized form is patchy alopecia, especially of the muzzle, face, and forelimbs. The mites presumably are acquired during intimate contact when the dam nurses the puppy. As a result of that close contact, localized demodicosis often develops in the region of the face. Generalized demodicosis is characterized by diffuse alopecia, erythema, and secondary bacterial contamination over the entire body surface of the dog. An inherited defect in the dog’s immune system is thought to be an important factor in the development of generalized demodicosis.
Cats are infested by two species of demodectic mites: Demodex cati and an unnamed species. D. cati is an elongated mite, similar to Demodex canis. The unnamed species has a broad, blunted abdomen as compared with the elongated one of D. cati. The presence of either species on the skin of cats is rare. In localized feline demodicosis are patchy areas of alopecia, erythema, and occasionally crusting on the head (especially around the eyes), ears, and neck. In generalized feline demodicosis, the alopecia, erythema, and crusting usually involve the entire body. Demodicosis also has been associated with ceruminous otitis externa.
Demodectic mites reside in the hair follicles of other species of domestic animals, but rarely produce clinical disease. Cattle and goats are most commonly infested, but then only rarely. In cattle, Demodex bovis causes large nodules (abscesses) on the shoulders, trunk, and lateral aspects of the neck. In goats, Demodex capri occurs in small, papular or nodular lesions on the shoulders, trunk, and lateral aspect of the neck. In sheep, Demodex ovis rarely causes pustules and crusting around the coronet, nose, ear tips, and periorbital areas. In pigs, Demodex phylloides rarely produces pustules and nodules on the face, abdomen, and ventral neck. In horses, Demodex equi occurs around the face and eyes and rarely produces clinical disease.
Skin areas with altered pigmentation, obstructed hair follicles, erythema, or alopecia should always be scraped. In localized demodicosis, the areas most commonly affected are the forelegs, perioral region, and periorbital regions. In generalized demodicosis, the entire body may be affected; however, the face and feet usually are the most severely involved. In dogs, areas of apparently normal skin also should be scraped to determine if the disease is generalized. The areas should be clipped and a fold of skin gently squeezed just before scraping. Gentile scraping of affected areas should be continued until capillary blood oozing is observed because these mites live deep in the hair follicles and sebaceous glands (Figure 17-71).
Nodular lesions in large animals should be incised with a scalpel and the caseous material within smeared on a slide with mineral oil, covered with a coverslip, and examined microscopically for mites.
The mites on the slide should be counted and the live:dead ratio determined. The presence of any larval or nymphal stages or eggs should be noted. During therapy for Demodex spp., a decrease in the number of eggs and live mites is a good prognostic indicator.
Cheyletiella
Mites of the genus Cheyletiella are surface dwelling (nonburrowing), residing in the keratin layer of the skin and in the hair coat of the definitive host, which may be a dog, cat, or rabbit. These mites ingest keratin debris and tissue fluids. Cheyletiella mites are sometimes referred to as “walking dandruff” because the mites resemble large, mobile flakes of dandruff. Cheyletid mites have distinct key morphologic features. They are large (386 by 266 μm) and visible to the naked eye. With the compound microscope, the most characteristic key morphologic feature may easily be seen: the enormous hooklike accessory mouthparts (palpi). These palpi assist the mite in attaching to the host as it feeds on tissue fluids. The mite also has comblike structures at the tip of each leg. Members of the genus also are known for a characteristic body shape resembling a shield, a bell pepper, an oak acorn, or a western horse saddle when viewed from above. Eggs are 235 to 245 μm long and 115 to 135 μm wide (smaller than louse nits) and supported by cocoonlike structures bound to the host’s hair shaft by strands of fibers. Two or three eggs may be bound together on one hair shaft.
The key feature of Cheyletiella spp. infestation is often the moving white “dandruff” flakes along the dorsal midline and head of the host. A hand-held magnifying lens or Optivisor (binocular headband magnifier) often are used to view questionable dandruff flakes or hairs; these are perhaps the quickest methods of diagnosing cheyletiellosis. A fine-tooth flea comb may be used to collect mites; combing dandrufflike debris onto black paper often facilitates the visualization of these highly mobile mites. Using clear cellophane tape to entrap mites collected from the hair coat often simplifies viewing with the compound microscope.
TICKS
Ticks have dorsoventrally compressed, leathery bodies. The tick’s head, or capitulum, serves as an organ of cutting and attachment. It is made of a penetrating anchorlike sucking organ, the hypostome, and four accessory appendages, two chelicerae and two pedipalps, which act as sensors and supports when the tick fastens to the host’s body. The tick’s body may be partially or entirely covered by a hard, chitinous plate, the scutum. Mouthparts may be concealed under the tick’s body or may extend from the cranial border. Most ticks are inornate (i.e., reddish or mahogany, without markings). Some species are ornate (i.e., they have distinctive white patterns on the dark scutum background). Adult ticks have eight legs, with claws on the ends of the legs.
Ticks are important parasites because of a voracious blood-feeding activity. Ticks are important also because they can transmit many parasitic, bacterial, viral, and other diseases, such as borreliosis (Lyme disease), among animals and from animals to humans. These pathogenic organisms may be transmitted passively, or the tick may serve as an obligatory intermediate host.
The salivary secretions of some female ticks are toxic and can produce a syndrome known as “tick paralysis” in people and animals. Tick species commonly associated with tick paralysis are Dermacentor andersoni (the Rocky Mountain spotted fever tick), Dermacentor occidentalis (the Pacific Coast tick), Ixodes holocyclus (the paralysis tick of Australia), and Dermacentor variabilis (the wood tick).
Ticks of veterinary importance may be divided into two families: the argasid, or soft, ticks and the Ixodid, or hard, ticks. Argasid ticks lack a scutum, or hard, chitinous plate. The mouthparts of the adults cannot be seen when viewed from the dorsal aspect. Ixodid ticks have a hard, chitinous scutum that covers all of the male tick’s dorsum and about one third or less of the female’s dorsum. Depending on the degree of engorgement, male ticks are much smaller than female ticks.
Two species of argasid ticks are important: Otobius megnini (the spinous ear tick) and Argas persicus (the fowl tick). Thirteen economically important tick species are in the Ixodid family. These include Rhipicephalus sanguineus, Ixodes scapularis, Dermacentor spp., and Amblyomma spp. Of these species, only R. sanguineus infests buildings; the remaining ticks attack their hosts outdoors.
The specific identification of ticks is difficult and should be performed by a veterinary parasitologist or a trained arthropodologist. Ticks usually are identified by the shape and length of the capitulum, the shape and color of the body, and the shape and markings on the scutum. Male and unengorged female ticks are easier to identify than engorged females. Determining the species of larval or nymphal ticks is most difficult. Common species may be identified by size, shape, color, body markings, host, and the location on the host.
Four major stages exist in the life cycle of ticks: egg, larva, nymph, and adult. After engorgement on the host, female ticks drop off the host and seek protected places, such as within cracks and crevices or under leaves and branches, to lay eggs (Figure 17-72). The six-legged larvae, or seed ticks, hatch from the eggs and feed on a host (Figure 17-73). The larva molts to the eight-legged nymphal stage, which resembles the adult stage, but lacks the functioning reproductive organs of the adult stage. After one or two blood meals, the nymph matures and molts to the adult stage. During the larval, nymphal, and adult stages, ticks may infest one to three or even many different host species. This ability to feed on several hosts during the life cycle plays an important role in the transmission of disease pathogens among hosts. Any infestation of domestic animals by mites or ticks is referred to as acariasis.
Most ticks do not tolerate direct sunlight, dryness, or excessive rainfall. They can survive as long as 2 to 3 years without a blood meal, but females require blood before egg fertilization and deposition. Tick activity is restricted during the cold winter months.
Soft Ticks (Argasid)
Otobius megnini, the spinous ear tick, is an unusual soft tick in that only the larval and nymphal stages are parasitic. The adult stages are not parasitic, but are free living, found in the environment of the definitive host, usually in dry, protected places, in cracks and crevices, under logs, and on fence posts. The larval and nymphal stages feed on horses, cattle, sheep, goats, and dogs. These ticks usually are associated with the semiarid or arid areas of the southwestern United States. With widespread interstate movement of animals, this soft tick may occur throughout North America. As with most soft ticks, the mouthparts may not be visible when viewed from the dorsal aspect (Figure 17-74). The nymphal stage is widest in the middle, almost violin shape. It is covered with tiny, backward-projecting spines, which is the origin of the name spinous. Larvae and nymphs usually are found within the ears of the definitive host, which is the origin of the name ear tick.
FIGURE 17-74 Otobius megnini, the spinous ear tick, is an unusual soft tick in that only the larval and nymphal stages are parasitic. The mouthparts may not be visible when viewed from the dorsal aspect.
Spinous ear ticks are extremely irritating to the definitive host. They often occur in large numbers, deep within the external ear canal. These ticks imbibe large amounts of host blood; however, because they are soft ticks, they do not enlarge with feeding. Large numbers may produce ulceration deep within the ear. The ears become highly sensitive, and the animals may shake their head. The pinnae may become excoriated by the animal’s shaking and rubbing its head.
The ticks may be visualized in the ear with an otoscope. Any waxy exudate should be examined for larval and nymphal spinous ear ticks.
Hard Ticks (Ixodid)
Rhipicephalus sanguineus, the brown dog tick, is an unusual hard tick; it may invade both kennel and household environments. This tick is distributed throughout North America. It has an inornate, uniformly reddish-brown scutum, and it feeds almost exclusively on dogs. R. sanguineus also has a distinguishing morphologic feature. Its basis capitulum has prominent lateral extensions that give this structure a decidedly hexagonal appearance (Figure 17-75). The engorged female is often slate gray. In southern climates, the tick is found outdoors, but in northern climates, it becomes a serious household pest, breeding indoors.
FIGURE 17-75 Lateral expansion of the basis capitulum (base of the head) (arrows) of an adult R. sanguineus. This key morphologic feature is used to identify this parasite, which can breed in the host’s environment.
The bites of this tick can irritate dogs. Severe infestations may cause heavy blood loss. This tick is also an intermediate host for B. canis, the agent that causes piroplasmosis in dogs.
This tick may be identified by its inornate brown color and characteristic lateral projections of the basis capitulum. These ticks are unique; they may be found in indoor or kennel environments.
Dermacentor variabilis: Dermacentor variabilis, the American dog tick or wood tick, is found primarily in the eastern two thirds of the United States; however, with the increased mobility of American households, the tick may occur throughout the country. Unlike R. sanguineus, this tick only inhabits grassy, scrub brush areas, especially roadsides and pathways. This three-host tick initially feeds on small mammals and rodents; however, dogs (and people) can serve as hosts for this ubiquitous tick. This tick is a seasonal annoyance to people and domestic animals. It can serve as a vector of Rocky Mountain spotted fever, tularemia, and other diseases and may also produce tick paralysis in animals and people. This tick has a dark brown, ornate scutum with white striping. Unfed adults are approximately 6 mm long, whereas adult engorged females are about 12 mm long and blue gray (Figure 17-76).

FIGURE 17-76 Adult female Dermacentor variabilis. Unfed adults are approximately 6 mm long, whereas engorged adult females are about 12 mm long and a blue-gray color.
This tick may be identified by its morphologic features. It has a rectangular base of the capitulum and characteristic white markings on the dorsal shield.
Amblyomma americanum: Amblyomma americanum, the Lone Star tick, gets its common name from a characteristic white spot on the apex of its scutum. The spot is more conspicuous on male ticks than on female ticks. This tick is distributed throughout the southern United States, but is found also in the Midwest and on the Atlantic coast.
This three-host tick is found most often in the spring and summer months, parasitizing the head, belly, and flanks of wild and domestic animal hosts. It also feeds on people and is said to have a painful bite. It can produce anemia and has been incriminated as a vector of tularemia and Rocky Mountain spotted fever. A. americanum is easily identified by a characteristic white spot on the apex of the scutum.
Amblyomma maculatum, the Gulf Coast tick, is a three-host tick found in the ears of cattle, horses, sheep, dogs, and people. It occurs in areas of high humidity on the Atlantic and Gulf coasts. It produces severe bites and painful swellings and also is associated with tick paralysis. This tick has silvery markings on its scutum. Larval and nymphal stages occur on ground birds throughout the year. The number of adult ticks on cattle decreases during the winter and spring and increases in the summer and fall. When the ear canals of cattle and horses are infested, the pinna may droop and become deformed. A. maculatum is easily identified by the silvery markings on its scutum (Figure 17-77).
Boophilus annulatus: Boophilus annulatus is often called the Texas cattle fever tick or the North American tick. This one-host tick has historical significance in that it is the first arthropod shown to serve as an intermediate host for a protozoan parasite, B. bigemina of cattle, a discovery milestone in veterinary parasitology. This tick has been completely eradicated from the United States; however, any Boophilus spp. infestation should be reported to the proper regulatory agencies. The tick should be identified by a specialist and control methods applied. B. annulatus frequently enters the United States from Mexico.
The engorged female tick is 10 to 12 mm long and the male 3 to 4 mm. The mouthparts are short, and no festoons are on the caudal aspect of the abdomen. Because this is a one-host tick, larvae, nymphs, and adult ticks may be found on the same animal. They do not have to leave the host to complete the life cycle. Animals with heavy infestations are restless and irritated. In an attempt to rid themselves of ticks, they rub, lick, bite, and scratch themselves. Irritated areas may become raw and secondarily infected. Heavily infested cattle may become anemic. Suspect ticks from an enzootic area or from animals originating from the Texas-Mexico border should be submitted to a laboratory recommended by regulatory agencies.
RECOMMENDED READINGS∗
Bowman, D.D., Lynn, R.C., Eberhard, M.L. Georgi’s parasitology for veterinarians, ed 9. St Louis: Saunders; 2009.
Hendrix, C.M., Robinson, E. Diagnostic parasitology for veterinary technicians, ed 3. St Louis: Mosby; 2006.
Hendrix, C.M., Sirous, M. Laboratory procedures for veterinary technicians, ed 5. St Louis: Mosby; 2007.
Sloss, M.W., et al. Veterinary clinical parasitology, ed 6. Ames, Iowa: Iowa State University; 1994.
∗All figures in this chapter are originally from Hendrix CM, Robinson E:Diagnostic parasitology for veterinary technicians, ed 3, St Louis, 2006, Mosby.
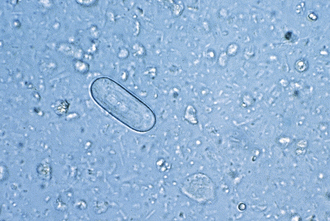
 TECHNICIAN NOTE
TECHNICIAN NOTE