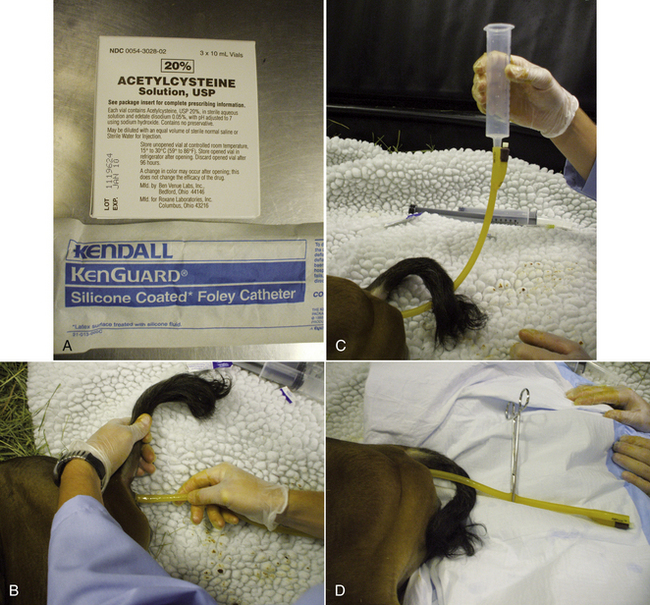
OVINE AND CAPRINE VENIPUNCTURE
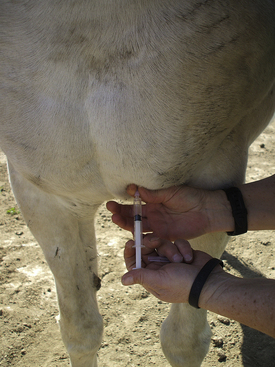
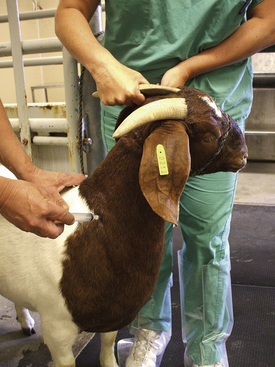
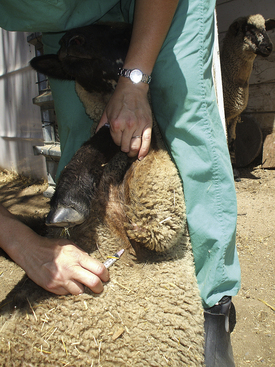
The jugular, cephalic, and femoral veins are commonly used in sheep and goats. The ear also provides an accessible site for blood sampling.
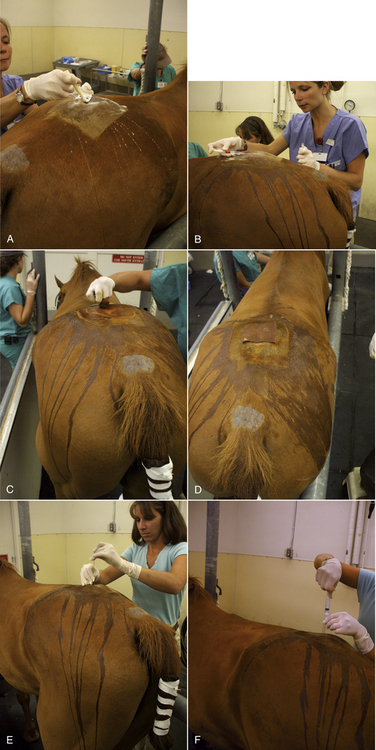
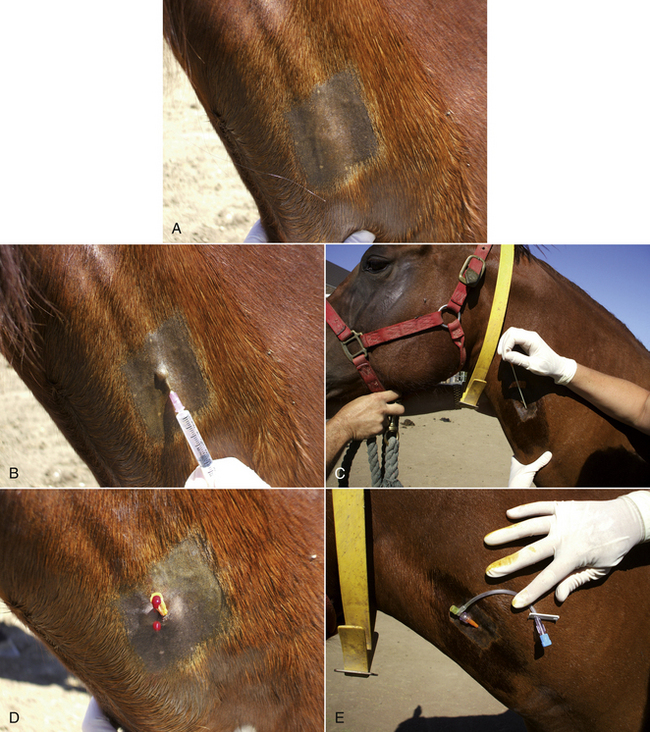
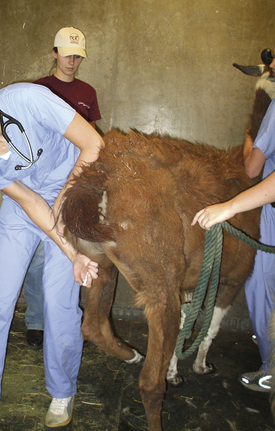
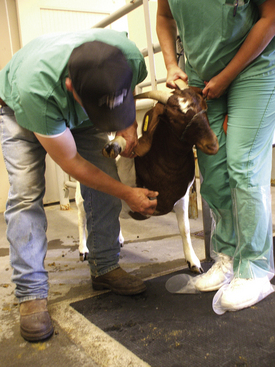
Most sheep will be restrained in a “set up” position on their rumps with their back side leaning up against the handler (Figure 20-50). Jugular, cephalic, femoral, and ear samples can be taken from this position. Jugular, cephalic, and ear samples can be obtained from some sheep while they are standing, but having sheep “set up” on their rumps will drastically reduce the amount of effort required to carry out most procedures (Figures 20-51 through 20-54). The jugular, cephalic, and ear veins can be easily accessed in goats while they are standing. The handler can restrain the animal by backing it into a corner and by straddling the goat with the handler's legs tight on either side of the neck or push the goat up against a wall. The femoral vein is accessible when the goat is in lateral recumbency.

FIGURE 20-50 Setting sheep on the rump is an effective method of restraint for venipuncture and many other procedures.

FIGURE 20-51 A technician crouches in front of a standing sheep to collect blood from the jugular vein. Note that the assistant at the rear of the sheep prevents the sheep from backing away from the technician.
PORCINE VENIPUNCTURE
Blood collection from swine is more difficult than in other large animals. They are challenging to restrain, have thick jowls, short legs, tough skin, and are fat. Sampling can be done with the animal restrained or with the animal under anesthetic. The technician should be aware that in addition to commercial hogs, many pigs that are receiving veterinary care are beloved pets, and the handling and restraint required for venipuncture and other procedures should be explained well to the owner.
The common veins used for venous blood collection in pigs include the cranial vena cava, jugular, auricular, cephalic, peripheral leg veins, and occasionally the orbital sinus or tail vein.
Cranial Vena Cava: The cranial vena cava is located in the thoracic inlet between the first pair of ribs. The right side of the animal should be used to prevent damaging the phrenic nerve, which is anatomically more protected on the right side of the animal than the left. Hitting the phrenic nerve may alter the function of the diaphragm and can result in life-threatening cardiac or respiratory problems. For piglets, a 20-gauge × 1.5-inch needle is used. An 18- to 20-gauge × 1- to 1.5-inch needle is appropriate for small pigs (up to approximately 25 kg). For pigs weighing more than 25 kg an 18- to 20-gauge × 1.5- to 3.5-inch needle is used. Large adults require a 16- to 18-gauge × 4- to 4.5-inch needle.
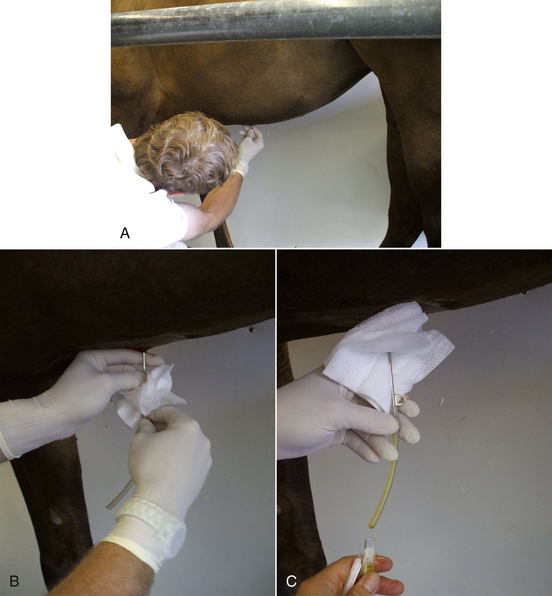
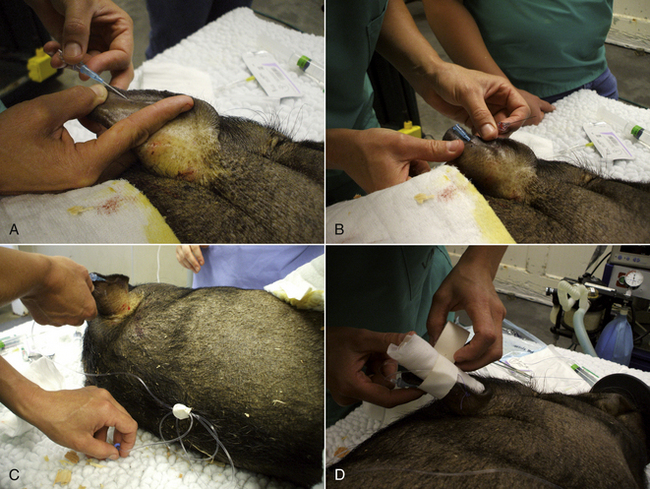
To collect blood from a small pig, the animal is placed on its back (dorsal recumbency) on a 45-degree incline with the head lower than the hips. The head is extended and front legs pulled caudally. The jugular furrow is visualized, and the needle with a syringe attached is inserted in the furrow lateral to the manubrium of the sternum. The needle is pointed toward the caudal aspect of the top of the opposite shoulder blade (Figure 20-55). As the needle is inserted, the syringe plunger should be pulled slightly to maintain negative pressure. When blood enters the syringe, the needle is held in place while the desired amount of blood is aspirated.
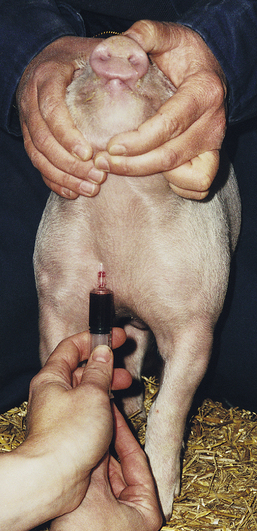
FIGURE 20-55 A blood sample from the right anterior vena cava is taken with the needle directed into the jugular fossa just lateral to the manubrium sterni.
Larger hogs are restrained with the use of a hog snare in a standing position with the head slightly elevated. The person with the syringe crouches in front of the right side of the pig facing the body of the pig or can crouch to the side of the right shoulder facing the neck. The needle is inserted into the right jugular furrow lateral to the manubrium (Figure 20-56), directed toward the shoulder. The cranial vena cava in large pigs is deep (4 inches may be required to reach the lumen).
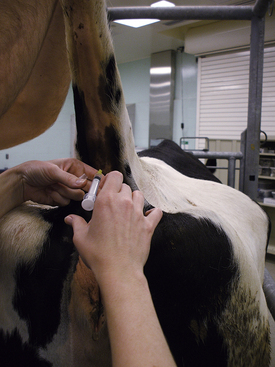
Jugular Vein: The jugular vein can be used for a blood sample collection in pigs of any age. It is located in the jugular furrow. It is not as deep as the vena cava, so fewer potential complications are associated with its use. The jugular vein has a smaller diameter than the vena cava and is more difficult to access, especially in large or heavy pigs. The needle size selected depends on the size of the animal. A jugular venipuncture in piglets can be done with a 20-gauge × 1.5-inch needle. Large pigs may require 16-gauge × 3- to 3.5-inch needles. To prevent the puncture of the phrenic nerve, the right side of the animal should be used when possible. The needle should be inserted cranially to the manubrium where the jugular furrow appears deepest. The animal is restrained as for a vena cava venipuncture. An imaginary horizontal line that passes through the shoulders and manubrium sterni is visualized. A second line is visualized that extends from the manubrium sterni to the scapula at an angle of 45 degrees with the first line. The needle is inserted perpendicular to the skin at the intersection of the second line with the deepest part of the right jugular fossa. The needle is directed caudodorsally and should not be angled toward either scapula. Because the vein is superficial, the syringe plunger should be retracted slightly as soon as the needle penetrates the skin. The needle is advanced until blood is aspirated into the syringe. Once a sufficient volume of blood has been collected, the needle is removed.
The technician should be aware that fewer complications and a lower risk for incidental injury to the animal are associated with a venipuncture of the more distal veins listed below.
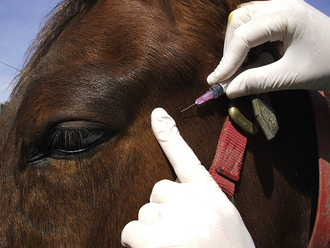
Auricular Vein: The auricular vein is located near the lateral border of the pinna of the ear. It is easily visualized on the dorsal side of the ear and can be seen even more clearly by placing digital pressure at the base of the lateral surface of the ear. The ear is held and digital pressure (or rubber-band tourniquet) applied at the base of the ear to distend the vein. A needle with a syringe attached can be inserted into the vein while gently pulling back on the plunger. To prevent the collapse of the vein with aspiration, some people prefer to insert the needle and allow the blood to drip from the needle hub directly into the uncapped collection tube. For most pigs, a 20-gauge × 1-inch needle is appropriate. For large adult pigs, an 18- to 19-gauge × 1-inch needle may be used. Vacutainer collection needles and tubes may also apply too much suction, and as in the case of excess pressure on the syringe plunger, these tend to cause the vein to collapse. When an adequate blood sample has been collected, pressure is released from the base of the ear, the needle removed, and pressure applied to the insertion site. For repeated sampling, the placement of an IV catheter should be considered.
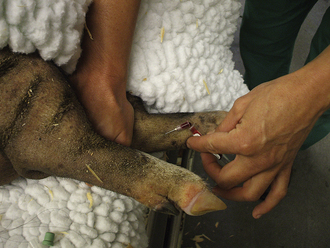
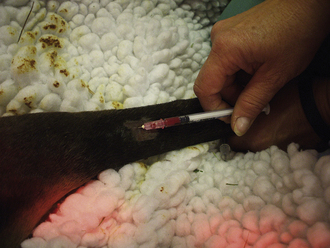
Peripheral Leg Veins: The cephalic vein on the front leg, saphenous vein on the hind leg, and branches of veins located on the lower limbs are accessible in small pigs and can be used for venous sampling. The veins can be visualized and are readily accessible in anesthetized pigs. To prevent the collapse of the vein, the needle is inserted, and blood is allowed to drip from the hub of the needle into the open collection tube (Figure 20-57). The collection of blood from the standing and restrained pig can be done by placing hand pressure or a tourniquet above the collection site to distend the vein. A 20-gauge × 1- to 1.5-inch needle is inserted at approximately a 45-degree angle to the skin in the direction of the body.
Coccygeal Vein: Though this site is not commonly used, it can be used for a venipuncture in adult pigs with intact (not docked) tails. A 20-gauge × 1-inch needle is inserted in the ventral midline of the tail perpendicular to the skin. Small blood samples can be obtained from this site.
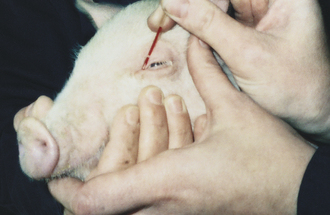
Orbital Sinus (Medial Canthus of Eye): The orbital sinus is adjacent to the medial canthus of the eye and can be used for the collection of small volumes of blood. A 20- to 22-gauge × 1 inch-needle can be used for piglets. Sampling on larger pigs may require a 16- to 18-gauge × 1.5-inch needle. The needle is inserted into the medial canthus deep into the third eyelid (nictitating membrane) and advanced at a 45-degree angle toward the opposite jaw until bone is felt. The needle is then rotated between the fingers until blood enters the hub. The syringe is attached and blood collected with gentle aspiration. When the collection is complete, the needle is removed and digital pressure applied over the medial canthus with the head elevated. A microcapillary tube with the end broken to form a rough point can be used in lieu of a needle (Figure 20-58).




 TECHNICIAN NOTE
TECHNICIAN NOTE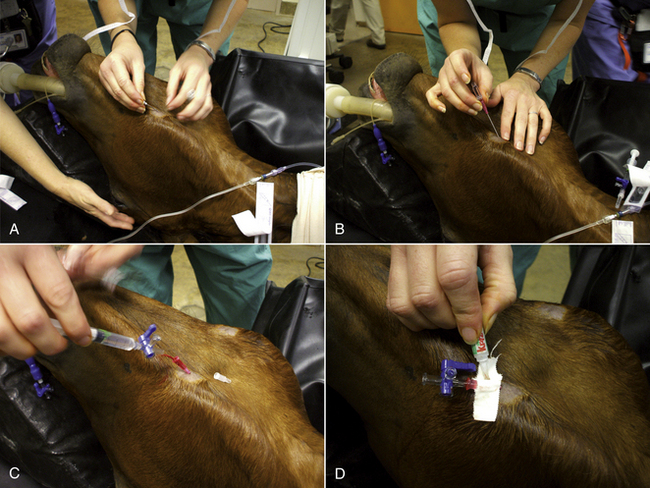





 - to ½-inch outside diameter) and estimate the necessary length as described for cattle and proceed with tube passage as described previously.
- to ½-inch outside diameter) and estimate the necessary length as described for cattle and proceed with tube passage as described previously.