Pain Management
When you have completed this chapter, you will be able to:
1 Describe methods used to recognize pain and monitor response to analgesics in small and large animals.
2 Differentiate between dysphoria and pain response and describe physiologic effects of pain on body systems.
3 Differentiate between pain and nociception and describe the phases of nociception.
4 Define hyperalgesia and allodynia and explain their role in management of chronic pain.
5 Describe the concepts of pre-emptive analgesia and multimodal analgesia.
6 List the steps in calculating constant rate infusions.
7 List the classes of medications used in management of pain and give examples of each.
8 List the routes of administration of local and systemic anesthetics, analgesics, and sedatives.
9 List the types of adjunctive medications used in pain management.
10 List and describe non-pharmacologic options of treatment of pain.
THE ROLE OF THE VETERINARY TECHNICIAN AS A PATIENT ADVOCATE
Technicians use their critical thinking, observation, and interpretation skills to make important pain management recommendations. Discussion about each case directly with the clinician might include the technician’s particular concerns about a patient or a general approach to managing different types of pain. Based on his or her interaction with patients, the technician may offer suggestions for adjustments in analgesic regimens, changes or additions to drug protocols, or the possible addition of sedatives, if needed.
Veterinary technicians often complain that their requests for patient analgesia go unheeded. The actual method of communication plays a large part in achieving a positive outcome. For example, “Can I give Charlie something for pain” is inadequate to convey the situation and often results in the response “no.” To be effective, technicians must present two sets of information; what is the patient doing that indicates painfulness and what has already been done that is considered inadequate. For example, “Dr. X … yesterday’s cruciate repair, the black lab, Charlie, is not doing as well as I would like. Despite the fact that his bladder is empty and I have offered him food and water, he seems restless and has difficulty getting comfortable. He is panting excessively, although his temperature is normal. I checked the bandage, and it does not seem too tight. The record says he received morphine last night at midnight, which allowed him to sleep for 4 hours, but has not had any since. May I give him a repeat dose to see if it makes him more comfortable?” This approach delivers the necessary information to gain the veterinarian’s confidence in the technician’s assessment skills and knowledge of the case. He or she is much more likely to agree to administer pain medication under these circumstances.
Technicians can also play a vital role in the administration of preemptive medication, which is often overlooked in a busy hospital setting. For example, “I have noticed a difference in the recovery of the animals that are given a dose of NSAID before surgery. Would you like me to give an NSAID to this patient now?” This approach also applies to the placement of transdermal analgesic patches, starting constant rate infusions (CRIs), and performing local or regional nerve blocks. Technicians should provide as much feedback as possible as to which analgesic protocols are working well and which need to be improved to increase patient comfort.
PATIENT ASSESSMENT
Historically, animal pain has been recognized and treated only in those patients that display overt behavioral signs, such as vocalization. By waiting for signs, patients are forced to prove that they are in pain before they are given analgesics. In reality, dogs and cats instinctively hide pain just as they would in the wild to avoid becoming prey. Once animals display obvious signs of pain, the pain intensity they are experiencing is likely to be severe. Old or severely debilitated patients may be too ill or weak to display changes in behavior. Patients should never be required to prove that they are in pain. A sound approach to pain management favors anticipation of the severity and duration of pain that is likely to occur with any procedure, condition, or surgery. In many cases, animals do “appear” to tolerate pain better than humans. There may be several explanations for this. In contrast to pain detection threshold (the point at which pain nerve fibers are stimulated to send signals), pain tolerance (the greatest intensity of pain that is voluntarily tolerated) varies widely between species and individuals within a species. Like humans, animals tolerate pain to a certain point before they show changes in their behavior. Awareness that patients may exhibit a wide range of pain tolerance and a broad spectrum of behaviors can improve pain recognition and treatment. Recently, research in pain management has shifted toward identifying and even predicting known painful events. For example, severe pain is expected with cervical disk herniation, extensive inflammation, medical or surgical fracture repair, limb amputation, declawing, ear canal ablation, etc. Moderate to mild pain is expected with cruciate repair, laparotomy, mass removal, castration, dental procedures, etc. This approach encourages us to treat patients who undergo painful procedures or disease processes without requiring proof. It does not, however, consider the vast variation in pain tolerance in the individual. It seems reasonable to incorporate both concepts to develop a truly effective analgesic plan (i.e., to have direction given by what are known to be painful events and be prepared to provide adequate analgesia for the expected level of pain but also to look at the individual and tailor analgesic protocols accordingly). A more complete list of anticipated levels of pain associated with surgical procedures, illness, or injuries is available in Veterinary Clinics of North America, July, 2000.
Technicians observe patients closely for extended periods of time and are usually the first to notice changes in status. Familiarity with individual patients’ personalities and usual reactions to stimuli gives insight into the meaning of behaviors. Experience establishes expectations of how particular patients may react to painful stimuli. This includes the differences in expression between dogs and cats, the young and old, and variations among certain breeds. For example, Siberian huskies and Dobermans that vocalize regularly are thought to be more “sensitive” to pain or possess a lower pain threshold than other breeds, whereas pit bulls and Labrador retrievers appear to remain stoic in the face of pain. The skilled technician factors this into his or her pain assessments.
SIGNS OF PAIN
Diagnosis of pain in veterinary medicine is seldom made on the basis of a single observation or physiologic value. Because pain is an individual, subjective experience, assessment depends on a combination of good examination skills; familiarity with species, breed, and individual behavior; knowledge of the degree of pain associated with particular surgical procedures or illnesses; and recognition of the signs of stress and pain.
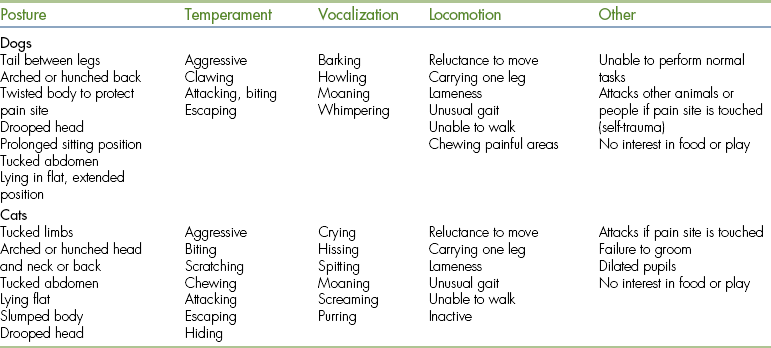
Signs of pain in animals can be categorized as physiologic or behavioral (Table 26-1). Physiologic pain signs may be obvious and include increased heart rate and blood pressure, increased respiratory rate, and vocalization. More subtle behavioral changes, such as general restlessness, decreased appetite, not sleeping, resenting handling, and not assuming a normal position, may be even more significant. Clinical signs of pain in dogs and cats most often reported include tachycardia, increased respiratory rate, restlessness, increased temperature, increased blood pressure, abnormal posturing, inappetence, aggression, frequent movement, facial expression, trembling, depression, and insomnia. Less frequently reported are: anxiety; nausea; pupillary enlargement; licking, chewing, and staring at the surgical site or wound; poor mucous membrane (MM) color; salivation; decreased CO2; and head pressing.
The clinical manifestations may be quite different between species and even among different members of the same species. For dogs, standing or sitting for long periods or sleeping in an atypical position are considered pain signs. For cats, abnormal posture, hiding, and aggression are ranked as common pain signs. Because the signs of pain are so varied and diverse, any abnormal sign(s) in a veterinary patient that cannot be attributed to another cause is (are) suspected of indicating pain.
All patients should be evaluated for pain upon admission and at regular intervals throughout the hospitalization period. The observer’s subjective opinion and physiologic signs can be described using a pain scale, such as a visual analog scale (VAS). A VAS designed for use in nonverbal human patients uses pictorial rather than numerical rating systems. The most common scale for nonverbal humans has a series of faces with varied expressions. The main difference between a human and animal VAS is that in human medicine, the patient is the reporter of his or her pain level, whereas in veterinary medicine, VAS readings are most often provided by a veterinary technician who is always a second-party reporter. Veterinary VASs typically use subjective numerical ratings where 0 correlates with no pain, and the highest number is the worst pain imaginable for that particular procedure. A new pain scale for dogs and cats has recently been developed at Colorado State University (CSU) that uses numerical, pictorial, and descriptive assessments (Figure 26-1). In addition to the VAS score, a complete patient description including physiologic signs (temperature, pulse, respiration) and behavioral signs (vocalization, posturing, eating, and sleeping habits) should be documented in the medical record.
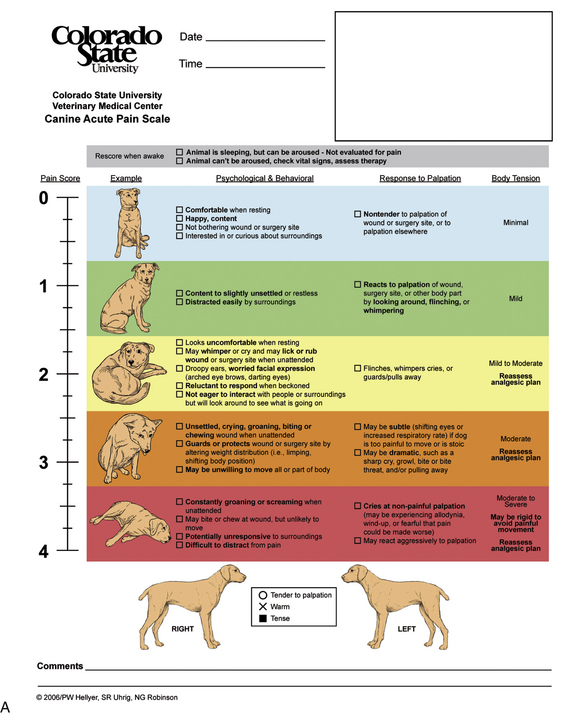
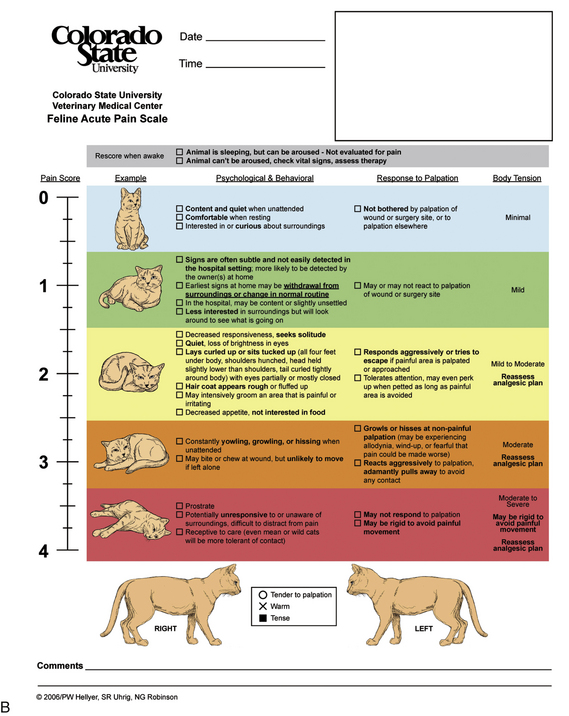
FIGURE 26-1 A and B, Colorado State University (CSU) pain scales. Acute animal pain scales developed by P. Hellyer et al at CSU for assessing pain in dogs and cats. Available at www.IVAPM.org.
Pain assessments should be made at 4- to 6-hour intervals throughout hospitalization in the general patient population. During the immediate postoperative period and throughout the critical phase, patients should be monitored as often as every 30 minutes. Ideally, assessments on an individual patient are performed by the same person over time. Repeat recorded assessments allow for evaluation of the efficacy of analgesic protocols and make response to specific drugs easier to track.
Veterinary technicians are trained to recognize animal pain. By nature, technicians are quite skillful observers of behavioral changes in their patients, noticing the subtlest expressions of potential pain. Most experienced technicians also have an innate sense of how painful most procedures, conditions, and surgeries are likely to be based on repeated prolonged exposure to animals in the recovery phase. However, it is often difficult for even the most experienced technician to distinguish between pain and other stress. For example, postoperative patients frequently display aberrant behavior for several minutes up to hours after surgery. These behaviors may include vocalization, thrashing, rolling, self-mutilation, and tachypnea. When these behaviors are thought to be related to stress other than pain, they are often referred to as dysphoria (an emotional state characterized by anxiety, depression, or unease). Dysphoria is a general term that does not specify a cause. Abnormal postoperative behaviors are sometimes referred to as “emergence delirium” attributed to residual gas anesthetics. Some animals do in fact display this response upon awakening, but anesthetic-related behaviors should resolve within several minutes. Behaviors that persist beyond a few minutes require further investigation and attention. In any case, it can be difficult to discern between pain, dysphoria, and reaction to narcotics or general anesthetics. Differentiating between pain and dysphoria following drug administration or use of anesthetics is critical to provide appropriate treatment.
Most animals in pain will be able to be temporarily soothed by a technician speaking in low tones during a petting interaction, although painful behaviors normally resume when the technician leaves the cage. These patients appear to recognize that someone is with them and usually make eye contact. A patient who stops the abnormal behaviors in the presence of a calming person is likely to be in pain rather than having a drug reaction. Animals in pain will also respond when the suspected focus of pain is gently palpated. These two findings should confirm the suspicion of pain and prompt the administration of additional analgesics. Conversely, patients who appear delirious and cannot be “calmed” are more likely to be experiencing stress or a narcotic reaction and will more likely benefit from sedation. These patients typically do not seem to recognize that a human is with them, do not make eye contact, and do not necessarily respond to light palpation of painful sites. The practice of reversing analgesics is reserved for patients who do not respond to either of the above approaches and/or whose physiologic condition is of immediate medical concern.
THE SCIENCE OF PAIN MANAGEMENT
Today in human medicine, preventing and treating pain are recognized as essential parts of overall patient management. Pain is considered to play such an important role in overall health and well-being that pain is now considered a fifth vital sign, ranking it of equal importance with temperature, pulse, respiration, and blood pressure. Not only do human health care providers view pain as a symptom of an underlying disease or condition, they view pain as an important syndrome in its own right. This is due to the vast array of negative physiologic events attributable to pain, regardless of the patient’s underlying disease or condition.
Pain triggers a series of physiologic changes that increase stress. Although the nervous system is the main target of pain transmission and provides the means for the body to react to that information, the body’s response to pain signals is not limited to the nervous system. Most, if not all, of the body’s major systems are affected by inadequately controlled pain (Box 26-1). For example, increased cortisol levels that accompany pain may interfere with wound healing and reduce the immune system’s ability to work effectively. In addition to suppressing the immune system, increased sympathetic nervous system activity associated with unrelieved pain may result in increased catabolism and metabolic rate, anorexia, ileus, and atelectasis. The cardiovascular system can also be adversely affected resulting in increased heart rate and blood pressure, irregular heart rhythms, and coagulopathies. Reducing or suppressing the stress response by managing pain can minimize adverse effects on the entire body. Given the potential consequences listed previously, it becomes obvious that, like humans, animals in pain require more intensive medical care than those in which pain is adequately managed. Recent changes to the American Animal Hospital Association’s (AAHA) standards require a pain assessment in every patient, regardless of the presenting complaint. Other requirements include making repeat regular assessments throughout hospitalization and recording of those assessments in the medical record. A full listing of the AAHA pain management standards can be found on its website at www.aahanet.org/ (Box 26-2).
PHYSIOLOGY OF PAIN
Nociception and the Pain Pathway
From a physiologic standpoint, pain is processed identically in all mammals. Nociception, derived from the Latin word nocere (to injure), includes three distinct phases: transduction, transmission, and modulation. The pain pathway begins at the point of tissue trauma, site of inflammation, injury, or surgical incision, where nociceptors (pain receptors) are stimulated. These specialized nerve endings convert mechanical, chemical, and thermal energy into electrical impulses (transduction) once their threshold is exceeded. If the noxious stimulus is large enough to exceed the nociceptor’s threshold, a nerve impulse is generated and transmitted along peripheral nerves to the spinal cord (transmission). These nerve fibers are highly specialized to carry pain information and are distinct from other nerve fibers that typically carry pleasant or neutral sensations. Once at the spinal cord, a nerve impulse is either projected upward to the thalamus and then to other parts of the brain or it may be transmitted to a nerve cell located entirely within the central nervous system (CNS) that activates sympathetic reflexes (Figure 26-2). In this way, the sensation of pain is dampened (modulation).
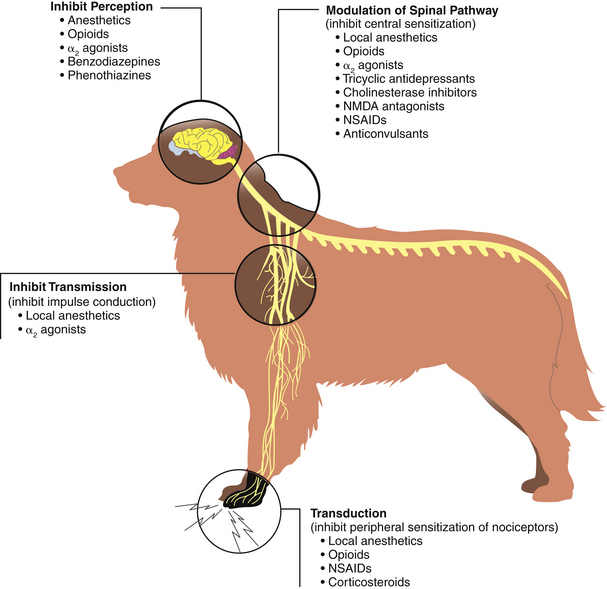
FIGURE 26-2 Nociception. Sites of analgesic action along the pain pathway. (From Tranquilli WJ, Grimm KA, Lamont LA: Pain management for the small animal practitioner, Jackson, Wyo, 2004, Teton NewMedia.)
The fourth phase of the pain pathway is perception. Perception occurs in the conscious brain and is the awareness that “I hurt.” Although the terms pain and nociception are often used interchangeably, these are not synonymous. What differentiates nociception from pain is consciousness. That means that patients under a general anesthetic (unconscious) do not perceive that they are in pain. However, nociception occurs even when an animal is in a state of unconsciousness.
As anesthesia wears off and consciousness returns, postoperative pain perception occurs. This explains why many patients display pain signs immediately upon waking. Good pain management is designed to interrupt nociception, even if the patient is under a general anesthetic and should be initiated whenever pain is anticipated. When analgesia is provided to surgical patients, recovery is typically much more comfortable.
Wind-up Phenomenon
The CNS adapts adversely to bombardment by persistent pain impulses. This can cause a profound effect on the nervous system’s architecture, altering pain processing. When spinal neurons are subjected to repeat or high-intensity nociceptive impulses, these neurons become progressively and increasingly excitable, even after the stimulus is removed. This condition is known as central sensitization or wind-up phenomenon and leads to nonresponsive or chronic intractable pain. Wind-up is the culmination of two distinct phases of change in the nervous system. First, pain-transmitting nerve fiber threshold is reset to a lower level. This resetting results in hyperalgesia where less and less stimulation is required to initiate pain. In the second phase, nerve fibers that normally carry pleasant or neutral information are recruited and become a part of the pain transmission process. This phase is termed allodynia and results in normally harmless sensations being interpreted as pain. The presence of hyperalgesia and allodynia collectively is considered wind-up phenomenon. This is apparent, for example, in the dachshund with vertebral disk disease that cries out in pain when any part of its body is touched or the cocker spaniel with a chronic ear infection that can no longer tolerate normal petting. People suffering from a migraine headache can attest to both the increase in pain sensitivity and the feeling that normally innocuous feelings (light touch, clothing, wind, etc.) have become unpleasant. Wind-up phenomenon highlights the need for effective analgesia to treat pain before it begins and at regular intervals once it occurs. Wind-up phenomenon is an important and newly understood concept in pain management. The vast majority of patients experiencing acute pain can be managed using common analgesics, such as nonsteroidal antiinflammatory drugs (NSAIDs). Patients experiencing wind-up require additional therapy just as the migraine sufferer would not likely be helped by taking two ibuprofen tablets, even though this approach would be adequate to treat a common headache. There are a variety of approaches to “unwind” the patient aimed at resetting neurologic processing so that conventional medications will work again. These will be discussed in the analgesia section of this chapter.
TREATMENT OF PAIN IN SMALL ANIMALS
ENVIRONMENTAL AND EMOTIONAL CARE
Pain has both physical and psychologic components. Fear and anxiety can exacerbate pain and vice versa. Attending to an animal’s physical and perceived emotional needs can reduce stress and consequently minimize pain levels. Environmental factors seem to affect the perception of pain in pets. The hospitalized patient in unfamiliar surroundings may be comforted by a favorite blanket or toy. Veterinary technicians must be adept at “reading” patients because the emotional needs of individual dogs and cats vary greatly. A comforting hand or a soothing voice can ease stress and make a pain assessment easier. Skilled technicians know when a visit by the owner would be therapeutic and when it would more likely create anxiety in the patient. Astute technicians can also sense which animals will recuperate better in a quiet environment and which patients are best distracted by exposure to a more active area of the hospital. The patient’s comfort can be improved by minimizing painful procedures. Many nursing procedures, such as a venipuncture and injections, cause pain. Procedures should be coordinated to minimize the total number of painful events. Increased technical skill also reduces the intensity of related pain. Policies that protect patients, such as a “two stick rule” (any individual should stick the patient no more than two times) for invasive techniques (e.g., venipuncture and catheter placement), should be instituted. Environmental care, such as providing a clean cage of appropriate size, extra padding, and careful positioning to reduce pressure on painful areas, is important. Designate the patient’s cage as a safe zone so that animals do not associate contact with an unpleasant experience. Any procedure that might be considered noxious should be performed outside of the cage, if possible. This allows the animal to feel comfortable and safe when in the cage. Nonpharmacologic actions can reduce pain by removing other stressors. However, tending to a patient’s comfort needs should not be seen as a substitute for analgesia.
PRINCIPLES OF ADMINISTERING ANALGESIA
Improved understanding of the impact of pain on the body is shaping new philosophies in managing patients’ pain. Several basic principles are used in the approach to designing analgesic protocols and are particularly important in managing pain effectively.
1. The best way to treat pain is to prevent it. This is the concept of preemptive analgesia. All research in human and veterinary medicine shows that preventing pain is unquestionably the best approach to treatment. It is an easy concept to grasp, but not so easy to remember to implement. That is because we have become used to treating animal pain on “request” (i.e., when we see overt signs of pain), even though we rationally know that once we see the signs, it is already too late. We have already missed the opportunity to most effectively manage pain in that patient. Administering preemptive analgesics whenever possible appears to be much more effective than using the same agent to treat pain once it occurs. Analgesia given before a noxious stimulus reduces postprocedure analgesia requirements, minimizes detrimental effects of pain, improves handling of patients, and potentially lowers sedation or anesthetic requirements. Reducing pain signaling helps to prevent hypersensitization at the spinal cord.
2. Drug combinations often produce better pain relief than single agents. The physiology of nociception gives rise to the concept of multimodal analgesia. Multimodal analgesia takes advantage of the synergistic effects obtained by combining two or more classes of analgesic drugs to alter more than one phase (transduction, transmission, modulation, and perception). Attacking pain from many angles is more effective than from one. Because the pain pathway has distinct phases, pain can be interrupted at various points. For example, in addition to preemptive NSAIDs (transduction), we may want to do a local block (transmission) and administrate opioids (modulation and perception). Using drugs from three different classes provides better pain control and has the added benefit of allowing use of lower doses of individual agents, thereby reducing side effects. Effective analgesia can also reduce the amount of gaseous anesthetic required for a procedure.
3. Matching analgesics (based on dosage and duration of action) to the degree of expected surgical pain rather than to the patient’s ability to express pain in a recognizable way is a more effective way to ensure pain relief.
4. Maintaining an analgesic plane once pain control is established. This may include the use of epidurals, CRIs, or continued bolus dosing. Pain management is weaned off as patients are transitioned out of the ICU. Regimented treatment (i.e., dosing at regular intervals) is helpful in maintaining an analgesic plane. Otherwise, a roller coaster effect occurs leaving the patient in varying degrees of pain between treatments. Keeping a patient out of pain is always more efficacious than continually taking the patient out of pain.
5. “Don’t quit till the pain quits.” Send pain relief home with the patient. Many professionals agree that most soft tissue procedures, such as spaying or neutering, require 3 to 4 days of postoperative analgesia, whereas orthopedic procedures probably require a 1-week supply. Of course, individual patients may vary, and owners should be advised to request additional analgesia if they perceive their pet to be in pain beyond the anticipated period.
ADMINISTRATION OF ANALGESICS AND ANALGESIC TECHNIQUES
The basic principles of current pain management have just been described to include preemptive (preventive) analgesia, multimodal analgesia (using different classes of drugs simultaneously to interrupt the pain pathway at various points), and appropriate follow-up analgesia (postoperative and take-home). Using this strategy, an analgesic plan is designed for each patient that maximizes pain control, maintains patients on an analgesic plane, and reduces unwanted side effects (Table 26-2).
TABLE 26-2
Monitoring Patients on Analgesics
| Adverse Effect | Monitoring |
| Opioids | |
| Sedation, low blood pressure, respiratory depression | Mentation, blood pressure, respiratory rate, and nature |
| Local Anesthetics | |
| None unless given by CRI. Then nausea, vomiting, neurologic signs, seizures | Observe regularly for muscle tremors and GI upset |
| NSAIDs | |
| GI disturbances, GI bleeding, renal disturbances | General observation, hydration status, stool quality, and urine production |
| α2-Agonists | |
| Bradycardia, cardiac arrhythmias, hypertension, peripheral vasoconstriction | Palpate femoral pulse rate and quality, auscultate heart, blood pressure |
Choosing the correct analgesic therapy requires an understanding of both the pharmacokinetics of a wide range of drugs and the levels or type of pain associated with various conditions. The four categories of drugs, NSAIDs, local anesthetics, opioids, and α2-agonists, are used in various combinations to inhibit the nociceptive process at more than one site. For this reason, combinations are more effective than single agents. Ultimately, pain relief, as assessed by the criteria previously described, is the only true measure of successful treatment. More recently, several classes of drugs have been added to the pain management regimen as adjunctive therapy in nonresponsive cases. This includes N-methyl-D-aspartate (NMDA)–receptor antagonists and anticonvulsants.
Anticipating pain level and duration provides a starting point for analgesic protocols. The initial approach is based on knowledge of the mechanisms of pain, drug dosages and expected duration, pain assessment, and the knowledge of the expected levels of pain for injuries, surgery, and diseases. Patients should be continually evaluated for breakthrough pain (exceeding usual protocol) or pain that persists beyond the expected period. Categorization of the expected severity (mild, moderate, severe) of pain is used to establish the initial type of analgesia and the duration of treatment. Later dosages can be adjusted according to individual patient response. For example, mild pain may be manageable with NSAIDs alone or in combination with a weak opioid, whereas moderate pain may require the addition of a stronger opioid. Severe pain might be best approached with an NSAID and a full opioid agonist or may require a CRI or additional therapies. Local and regional blocks can and should be added to the pain management plan, whenever feasible. When specific nerves cannot be identified for block, lidocaine can be administered by CRI for excellent systemic analgesia (Box 26-3).
NSAIDs
Nonsteroidal antiinflammatory drugs (NSAIDs) provide analgesia by modifying the inflammatory response. The pharmacologic actions of NSAIDs include analgesia, antipyresis (fever reduction), and control of inflammation. Because they treat the underlying problem (inflammation) and pain is diminished as a result, NSAIDs are considered a therapeutic class of analgesia used. Since NSAIDs approved for use in dogs were introduced into the market, they have been universally accepted as the treatment of choice for osteoarthritis. NSAIDs remain the most widely used analgesics in the treatment of chronic pain. However, they are also extremely effective in reducing acute pain in the perioperative period (around surgery). Recent changes in understanding animal pain and the best ways to manage it and new FDA drug approvals have led to NSAIDs becoming one of the most widely used classes of veterinary analgesics in a variety of situations.
Research shows that pretreatment with NSAIDs greatly reduces intraoperative and postoperative pain from soft tissue or orthopedic procedures. Therefore patients undergoing everything from spaying to neutering to cruciate ligament repair potentially benefit from NSAID administration, especially when given preemptively. NSAIDs have been shown to have a synergistic effect when combined with other classes of drugs, such as opioids. Often, patients with severe acute pain can be weaned to NSAIDs alone as their pain diminishes. NSAIDs have an onset of action of 45 to 60 minutes. The duration of action is typically 24 hours in the dog and varies from 8 to 96 hours in the cat. NSAIDs are best suited for mild to moderate pain, whether acute or chronic in nature. In addition to controlling postsurgical pain, NSAIDs are extremely effective in controlling inflammatory pain associated with traumatic soft tissue injury, ophthalmic conditions, otitis, gingivitis, and some cancer pain.
Current commonly used veterinary NSAIDs include: Rimadyl (carprofen), Metacam (meloxicam), Deramaxx (deracoxib), Previcox (firocoxib), and Zubrin (tepoxalin). Regardless of the NSAID, patients should be normotensive (normal blood pressure), with normal renal and liver function, without bleeding abnormalities, and without evidence or concern for gastric ulceration. Patients that receive NSAIDs should not also be receiving other NSAIDs, corticosteroids, or aspirin.
Take-Home Analgesia: The availability of NSAIDs has greatly improved outpatient pain management. These drugs are convenient to administer (once a day, chewable), relatively inexpensive, and provide long-lasting pain relief compared with other analgesics. The current anecdotal recommendation from pain management experts is that elective soft tissue procedures, such as spaying or castration, require 3 to 4 days of postoperative treatment with NSAIDs. Orthopedic procedures may require treatment for 1 week or more. Of course, each individual animal must be evaluated for presence of pain that persists beyond the expected time or that is of significant intensity as to require additional analgesics.
Local and Regional Anesthetics
Local anesthetics work by totally disrupting neural transmission of information. Blocking transmission of painful signals is one of the most effective ways of managing pain. Nerve blocks routinely performed by veterinary technicians include canine and feline dental blocks and feline declaw blocks. The use of lidocaine as a systemic blocking agent by CRI is also increasingly popular. There has been a great deal of work recently reviving the use of local or regional analgesia. Applying analgesia directly to the affected nerve endings can provide excellent pain control while reducing the need for systemic drugs.
Lidocaine, the most widely used local anesthetic, takes effect in 3 to 5 minutes and is effective for 60 to 90 minutes. The duration of lidocaine can be extended by combination with a 1:200,000 dilution of epinephrine. Epinephrine should never be used in circumferential limb blocks, such as feline declaw blocks. Marcaine (bupivacaine) takes longer to take effect (15 to 20 minutes), but its anesthetic and analgesic effects last 6 to 8 hours. Bupivacaine is not effective as a topical analgesic, but is an excellent choice for local infiltration.
Drugs, such as lidocaine and bupivacaine, are relatively safe if correctly administered. Most cases of toxicity in small animals occur as a result of accidental overdose or inadvertent IV administration. Signs of toxicity include seizures, coma, neurotoxicity, and cardiovascular collapse.
Topical: Application of topical analgesia to the surface skin or mucosa can reduce pain associated with minor procedures, such as wound suturing, venipuncture, arterial puncture, nasal cannulization, and urinary catheterization. Solutions of lidocaine, bupivacaine, tetracaine, and epinephrine can be used alone or in various combinations to provide desensitization at the application site. Gauze pads soaked with solutions can be applied directly to the site. Alternately, there are several commercially prepared topical anesthetic creams and jellies that can be applied as a thick paste. Regardless of application method, 20 to 30 minutes of direct contact time is required to ensure effective analgesia.
Local infiltration: Injection of lidocaine or bupivacaine into local tissue can reduce pain associated with various painful procedures. This technique is useful for small mass removal, digit amputation, arterial catheter placement, thoracocentesis, abdominocentesis, bone marrow sampling, etc. The entry area is infiltrated with small amounts of anesthetic before tissue penetration. An appropriate waiting time must be observed to ensure adequate desensitization of the area, as described earlier.
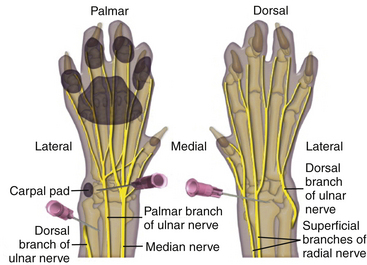
Circumferential ring block: This block is especially effective for use in feline declawing and involves subcutaneous (SQ) injections of bupivacaine or bupivacaine and lidocaine combination just above the carpal bend on the top of the paw and just above the accessory carpal pad on the underside (Figure 26-3). The dosage is 1 ml of 0.5% bupivacaine/10 lb of body weight divided among the injection sites. Sterile saline can be added to achieve sufficient coverage for smaller cats. The injections are made just above the carpal bend on the top side of the paw and just above the accessory carpal pad on the underside. The skin is tented horizontally, and the needle is fed under the skin. As the needle is withdrawn, the drug is injected slowly to leave behind a “line.” When this is done on both surfaces, the lines will connect creating a bracelet or ring block around the limb. This four-injection technique provides regional nerve block sufficient to eliminate pain for up to 8 hours after surgery.
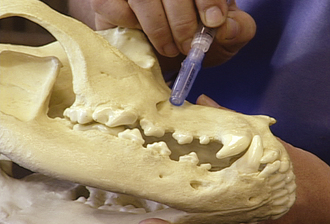
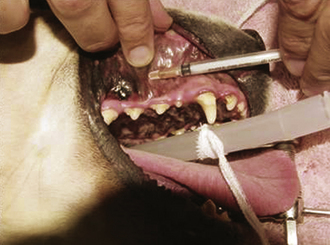
Dental nerve block: The entire muzzle can be anesthetized by blocking the infraorbital and mandibular foramen. This relatively simple technique is quite effective for dental extractions, oral mass removal, fracture repair, mandibulectomy, maxillectomy, and nasal biopsy (Figures 26-4 and 26-5). Lidocaine or bupivacaine can be used. Epinephrine can be added to reduce bleeding by coating the syringe with epinephrine before drawing up local anesthetic. Volume of administration is limited by the size of the foramen. Typically, about 0.5 ml per site is appropriate for a 50-lb dog, whereas about 0.1 ml per site is adequate in the cat.
Intraarticular (joint space): Effective analgesia in preoperative and postoperative orthopedic cases has been achieved by injection of local anesthetics directly into the joint space, such as in cruciate ligament repair. Intraarticular morphine has also been shown to effectively reduce joint pain. The effectiveness of this technique when used preoperatively is evident in the smooth plane of anesthesia maintained when the joint capsule is incised. This is in sharp contrast to the spike in heart rate and “lightness” that is observed when the capsule is entered without anesthetic, indicating these responses are likely due to pain.
Pleural space: Interpleural bupivacaine infusion following thoracotomy surgery may have some analgesic benefit. Bupivacaine (1.5 to 2 mg/kg) is injected via an indwelling chest tube into the pleural space. Analgesia is thought to occur by direct blocking of the intercostal nerves. For maximum coverage, patients are held in sternal recumbency for 5 to 10 minutes after injection and gently rolled from side to side. Drug absorption through the pleural tissue should be considered.
Epidural nerve block: Injection of opioids (morphine, fentanyl) and/or local anesthetics (lidocaine, bupivacaine) directly into the epidural space have been used to provide analgesia to the caudal half of the body while minimizing sedative effects. This is a fairly simple and safe technique. Injection is generally made at the lumbosacral space. Epidural catheters can be inserted to allow long-term analgesic administration.
Transdermal: The lidocaine transdermal patch (Lidoderm) has recently gained widespread acceptance in human medicine for management of neuropathic pain associated with back injury or surgery. Work is underway to investigate the use of transdermal lidocaine patches in veterinary medicine for specific conditions and procedures.
Intravenous: IV administration of lidocaine by CRI is an effective technique for managing a variety of pain states. At the cardiac dose of 50 to 80 μg/kg/min, lidocaine provides excellent analgesia for visceral pain (e.g., pancreatitis, parvovirus) and in procedures with extensive nerve damage, such as limb amputation. Lidocaine by CRI can be administered as a sole agent or in conjunction with other analgesics.
Opioids
Opioids are the most commonly used analgesics in hospitalized patients as a result of efficacy, rapid onset of action, and safety.
The efficacy of various opioids is determined by the specific receptors in the brain and spinal cord that they affect. The receptors are classified as mu, kappa, or sigma. Mu and kappa receptors are responsible for sedation, analgesia, and respiratory depression. Kappa receptors are responsible for analgesia and sedation. Sigma receptors are less clinically relevant and are thought to be responsible for the adverse effects of opioid administration, such as dysphoria, excitement, restlessness, and anxiety. Opioid drugs are classified as agonists (meaning that they stimulate the opioid receptors) or antagonists (meaning that they block particular opioid receptors). There are also mixed agonist-antagonist opioids that stimulate some receptors while blocking others and partial agonists with overall decreased effects at all receptor sites. In general, pure agonists are the most potent of the opioids, but also have the most severe adverse side effects. Mixed agonist-antagonist and partial agonist opioids can provide reasonably good analgesia without many of the deleterious side effects of pure agonists. Side effects may include vomiting, constipation, excitement, respiratory depression, bradycardia, and panting. The type of opioid is chosen based on the degree of analgesia required and the specific needs or limitations of the individual patient. The most commonly used pure agonists in the United States are morphine, hydromorphone, oxymorphone, and fentanyl.
Pure antagonists have the effect of reversing the narcotic properties of agonists. The availability of opioid antagonists makes opioid use extremely safe because the drug effects can be rapidly removed. Opioids are metabolized by the liver and excreted via the kidneys and should be used with caution in patients with renal or hepatic disease. Opioids are most effective when administered before the onset of pain. As a class, these drugs produce minimal side effects in animals. Virtually any animal patient experiencing pain is a candidate for opioid analgesia.
Opioids can be administered through numerous routes, ranging from IV, IM, transcutaneous, epidural, and oral routes and administration by CRI. Opioids can be administered concurrently with all other analgesics.
Morphine sulfate (pure opioid agonist): Morphine is the gold standard for pure opioid agonists. All other drugs in this class are compared with morphine in terms of efficacy, duration of action, and cost. Morphine is commonly used to provide maximal analgesia and sedation. Its relatively low cost and similar efficacy makes it preferential over other opioids in some cases. However, morphine has additional side effects, particularly systemic hypotension and vomiting, that make it less desirable in many instances. Cats are particularly sensitive to morphine, therefore lower doses are used in the cat. Typical dosage for dogs is 1.0 to 1.5 mg/kg IV q 4 to 6 hours. The dosage in cats is 0.25 to 0.1 mg/kg SQ or IM every 4 to 6 hours.
Hydromorphone (pure opioid agonist): Hydromorphone has similar properties to morphine in terms of providing analgesia, but is thought to have fewer side effects. Specifically, hydromorphone is less likely to induce vomiting or hypotension than morphine. Elevated body temperature has been noted, especially in cats. Typical dosage in dogs is 0.1 to 0.2 mg/kg SQ or IM and 0.03 to 0.1 mg/kg IV. Cat dosage is 0.05 to 0.1 mg/kg SQ or IM and 0.01 to 0.025 mg/kg IV. The duration of action is 3 to 4 hours.
Fentanyl citrate (pure opioid agonist): Fentanyl is an extremely potent synthetic opioid with rapid onset, but short duration of action when administered IV or IM. It is most efficaciously used as a transdermal patch for long-term (3 days) analgesia. Fentanyl is contained in an adhesive patch of varying concentration to deliver 25, 75, or 100 μg/hr. Once applied to shaved, cleaned skin, the drug is continually absorbed. The onset of action is from 12 to 24 hours, therefore supplemental analgesia is recommended during the initial treatment period. Use of mixed agonist-antagonist opioids will reverse the effects of the fentanyl patch and should be avoided. Fentanyl can also be delivered as a CRI and its use in this manner will be described later in this chapter.
Buprenorphine (buprenex) (partial mu agonist): Buprenorphine is a partial mu agonist that is of a longer duration than morphine as a result of its slow dissociation from receptors, providing analgesia for approximately 6 to 8 hours. Partial agonists avidly bind and partially activate mu receptors. Buprenorphine use in veterinary medicine also is increasing primarily because its duration of analgesia is significantly longer in dogs. Buprenorphine is recommended for moderate pain in the dog and moderate to severe pain in the cat. Recent work has been done to demonstrate that buprenorphine is readily absorbed across mucous membranes in the feline as a result of the unique oral pH in this species. This allows for transmucosal administration in the cat, providing analgesia for up to 8 hours from a single dose. Transmucosal buprenorphine is the primary analgesic for take-home use in cats. It is extremely easy to administer by owners and provides long-lasting analgesia (Figure 26-6).
Butorphanol tartrate (torbugesic) (mixed agonist/antagonist): Butorphanol is a kappa agonist and a mu antagonist. As a kappa agonist, it is a mild analgesic with marked sedative properties. Whereas the sedative effects of butorphanol may last for 2 or more hours, the effect of analgesia is only about 45 minutes, an important consideration when managing pain of any greater duration. As a mu antagonist, butorphanol can be used to reverse adverse events thought to be associated with mu opioid agonists, such as morphine. The dosage is 0.2 to 0.8 mg/kg SQ, IM, or IV.
Naloxone hydrochloride (pure opioid antagonist): One of the reasons that makes opioid use safe is the ability to rapidly reverse sedation and adverse side effects. Antagonists work by blocking opioid action at the receptors. Onset of reversal occurs within 1 to 2 minutes of IV administration and can last for 1 to 4 hours. Treatment may be repeated when reversing narcotics with a longer duration. Typical dosage is 2 μg/kg IV.
Tramadol: Tramadol is a synthetic drug with opioid-like analgesic effects, but fewer side effects. Tramadol has opioid-like activity at the mu receptor and appears to be quite effective in controlling moderate pain, especially when used in conjunction with an NSAID. It is available in oral form making it suitable for long-term at-home management of postoperative, cancer, orthopedic, and other chronic pain. A wide variety of dosages have been tried and reported in both the dog and the cat. Current dosing recommendation is for 4 mg/kg PO b.i.d. to t.i.d. in the dog and cat. It is also unscheduled, an additional benefit. Tramadol may be effective in management of long-term cancer patients, problem osteoarthritis cases, or postoperative prolonged pain.
α2-Agonists
Xylazine, medetomidine (Domitor), and dexmedetomidine (dexdomitor) are α2-agonists widely used in veterinary medicine. Dexdomitor is the most potent and selective α2-agonist and is playing an ever-increasing role in pain management and sedation. α2-Agonists, which are nonnarcotic and nonscheduled agents, can be useful as adjuncts in a balanced analgesic protocol. The main effect is to produce significant sedation accompanied by visceral and somatic analgesia. There are a variety of new ways to use this drug class as a premedication, rough recovery rescue and ongoing management of in-hospital pain and anxiety. α2-Agonist combinations are also extremely effective in cats for a variety of surgical and nonsurgical procedures.
α2-Agonists inhibit release of the excitatory neurotransmitter norepinephrine to produce analgesia and sedation. α2-Agonists are short-duration analgesics and can be rapidly reversed with α2-antagonists. This characteristic makes these drugs suitable for procedures requiring short-term restraint and analgesia. α2-Agonists may bind to the same receptors as opioids and act synergistically with them. The dosages of other analgesic and anesthetic agents can be significantly reduced if given concurrently with α2-agonists. α2-Agonists can have profound effects on the cardiovascular and nervous systems, but these adverse events can be minimized by using low dosages. Bradycardia and vomiting are the most common side effects with α2-agonists.
Advantages of α2-Agonists Over Other Sedatives: Other commonly used sedatives (e.g., acepromazine and diazepam) do not provide pain relief. The analgesia achieved with α2-agonists is of moderate intensity and moderate duration. Even more importantly, α2-agonists work synergistically with opioids (such as butorphanol or morphine) and improve both the intensity and the duration of pain relief. Other advantages are that the degree of sedation can be “tailored” or “titrated” by using different dosages and/or different drug combinations to provide mild to profound sedation and that α2-agonists are reversible. Antisedan (atipamezole) is most commonly used to reverse Dexdomitor. Antisedan is the safest of all of the reversal agents because it works almost exclusively by simply displacing Dexdomitor from the α2-receptors so that the nerve function can return to normal. After the administration of Antisedan, patients usually awaken in about 5 to 10 minutes and are able to stand or walk in less than 10 minutes. α2-Agonists, are used in a variety of ways to provide patient comfort including as premedicant before surgery combined with an opioid, as a stand-alone sedative or analgesic for short procedures, such as x-rays, and as a rescue drug for patients experiencing rough recovery from anesthesia. The most common use of α2-agonists in cats is in a combination called “kitty magic,” which consists of an α2-agonist, an opioid (most commonly buprenorphine), and ketamine. The addition of ketamine makes the combination a general anesthetic protocol rather than just a sedative protocol. Minor surgical procedures can be performed under kitty magic, or kitty magic can be used before a gas anesthetic for more advanced procedures. α2-Agonist can also be administered as a CRI for patients with continual anxiety or pain.
Constant Rate Infusion (CRI)
Constant rate infusion allows continuous low-dose administration of various analgesics. Optimally, CRIs are established before tissue damage (i.e., preoperatively) and run for 6 to 12 hours postoperatively. CRI analgesia is also quite effective in management of hospitalized patients with preexisting or persistent medical pain. Many agents can be delivered, but this method most commonly uses local anesthetics (lidocaine), opioids (morphine or fentanyl), and NMDA antagonists (ketamine). These drugs can be used as single agents or in combination with one another. Technicians should be adept at calculating CRIs (Box 26-4).
Morphine: The main advantage of giving morphine as a CRI is preventing the peaks and valleys typically seen with opioid bolus dosing. A lower dose of morphine can be used in a CRI, which can reduce the unwanted side effects, such as dysphoria or panting. Morphine by CRI is useful to manage any severe pain and can be safely combined with ketamine and/or lidocaine. The CRI dose for morphine in dogs: 0.2 to 0.5 mg/kg SLOW IV loading bolus followed by 0.1 to 0.3 mg/kg/hr CRI. Cats: 0.05 to 0.1 mg/kg IV loading bolus followed by 0.025 to 0.2 mg/kg/hr CRI. Fentanyl is a full opioid agonist with similar properties to morphine. The main advantage of fentanyl over morphine is a rapid onset of action and short half-life, which allows for rapid cessation of unwanted side effects. The CRI dose for fentanyl is dog: 2 to 5 μg/kg IV loading dose followed by 5 to 20 μg/kg/hr CRI intraoperatively; cats: 1 to 2 μg/kg IV loading dose followed by 5 to 20 μg/kg/hr CRI.
Lidocaine is a local anesthetic that provides excellent systemic analgesia when delivered IV. Because it is safe for use in patients with GI disturbances, lidocaine is a good choice for analgesia in patients with gastric dilation volvulus (GDV) or other similar disorders. Lidocaine seems to also provide benefits for patients undergoing procedures with excessive nerve trauma, such as complicated back surgeries or limb amputations. IV lidocaine is extremely short acting and can be discontinued without residual effect almost immediately. Lidocaine CRI should be discontinued if the patient shows signs of toxicity, including muscle tremors, seizures, nausea, or vomiting. The CRI dose for lidocaine in the dog is 1 to 2 mg/kg IV followed by 30 to 50 μg/kg/min. There are reported lidocaine CRI dosages for cats, but typically, lidocaine is not recommended for use in cats because of potential for severe cardiotoxic effects.
Ketamine is a dissociative anesthetic and an NMDA antagonist. Stimulation of NMDA receptors in the spinal cord results in firing of neurons that transmit pain signals. Prolonged bombardment of these receptors, such as occurs with intense surgical pain or long-term chronic pain, results in wind-up phenomenon. “Wind-up” will be most evident in the postoperative period once the patient has regained consciousness. However, as an NMDA receptor antagonist, ketamine given as an intraoperative CRI binds at these CNS receptors and prevents “wind up.” Because of its mechanism of action, ketamine is best used to manage neuropathic types of pain, particularly when the pain has been long standing and the patient has not responded well to other analgesics. Ketamine should always be given in combination with other analgesics and can be delivered in the same infusion. The CRI dosage for ketamine in the dog and cat is 0.5 mg/kg IV loading bolus followed by a 10 μg/kg/min CRI during surgery and 2 μg/kg/min for 24 hours following surgery.
Morphine-Lidocaine-Ketamine (MLK): The MLK infusion combines an opioid (morphine), a local anesthetic (lidocaine), and ketamine to provide optimal analgesia and treat wind-up. The recipe for MLK is:
To a 500-ml bag of lactated Ringer’s solution (LRS) add: Administer at 10 ml/kg/hr to provide
| 10 mg morphine (.66 ml) | morphine 0.2 mg/kg/hr |
| 120 mg lidocaine (6 ml 2%) | lidocaine 2.5 mg/kg/hr |
| 100 mg ketamine (1 ml) | ketamine 2 mg/kg/hr |
Adjunctive Agents
The vast majority of patients experiencing acute pain can be managed with conventional analgesics, such as NSAIDs, opioids, and local anesthetics. Patients in which pain is unmanaged or that are in preexisting pain states may require additional therapy. In addition to the classic analgesic agents, medications with other indications can be used to help manage pain. These drugs are referred to as adjunctive analgesics and come from many separate classes of pharmacologic compounds. Adjuvant analgesics are agents that can enhance analgesic drugs when co-administered, but have few or no analgesic properties when given alone. Some examples of adjunctive and adjuvant analgesics are:
• Tranquilizers (phenothiazines, benzodiazepines), which alter an animal’s response to pain and can relax muscles, are used in combination with true analgesics. These drugs also reduce anxiety and fear, which can exacerbate pain.
• NMDA receptor antagonists, such as CRI of ketamine or oral administration of amantadine, can enhance analgesia by blocking sensitization of neurons in the spinal cord and are especially useful for managing patients who have experienced wind-up phenomenon.
• Anticonvulsants. Gabapentin may play an important role in reducing neuropathic pain and central sensitization in chronic pain patients. Gabapentin is increasingly popular in both human and veterinary medicine as the first choice in patients whose pain does not respond to conventional therapies especially where neuropathic pain is suspected.
• Corticosteroids (prednisolone) have powerful antiinflammatory and immunosuppressive effects, “dampening the fires” of acute inflammation.
• Tricyclic antidepressants (amitriptyline, imipramine) are effective analgesics for chronic pain, especially neuropathic or cancer-related pain.
Nonpharmacologic Treatment Options∗
When possible, multimodal therapy that includes both pharmacologic and nonpharmacologic modalities should be used to treat pain, regardless of whether the pain is acute or chronic. Nonpharmacologic options include thermotherapy, massage, therapeutic exercises, aquatic therapy, acupuncture, electrical stimulation, therapeutic ultrasound, extracorporeal shock-wave therapy, low-level laser, etc. Many of these modalities provide direct pain relief (e.g., acupuncture), whereas others are associated with pain relief secondary to improved function and strength (e.g., many therapeutic exercises).
• Thermotherapy includes the use of both heat and cold for treatment of pain, injuries, surgical incisions, etc.
• Massage decreases pain and accelerates recovery by relieving muscle tension, increasing blood flow to the painful muscles, and mobilizing adhesions.
• Therapeutic exercises are a part of physical therapy that is designed to improve active pain-free range of motion and flexibility, improve use of limbs and reduce lameness, improve muscle mass and muscle strength, improve daily function, and help prevent further injury. Forms of therapeutic exercises include passive exercises, proprioceptive training exercises, active exercises to improve limb use, and speed and strengthening exercises.
• Aquatic therapy, such as underwater treadmill exercises and swimming, may be used for rehabilitation after orthopedic surgery, rehabilitation after neurologic injury for muscle strengthening, and for improved joint function. Water is an excellent medium for therapy because the body bears less weight in water, which reduces the load on painful joints and allows the patient to exercise more comfortably and to do exercises that were not possible for the patient on land.
• Electrical stimulation indications include pain management (especially in arthritis, spondylosis, spondyloarthrosis, recovery from orthopedic surgery, and nerve regeneration), facilitation of fracture healing, relief of muscle tension, prevention of muscle atrophy from disuse, and muscle strengthening. Treatment modalities include neuromuscular electrical stimulation (NMES), transcutaneous electrical stimulation (TENS), and electrical muscle stimulation (EMS).
• Acupuncture has been used for thousands of years to treat pain, yet the mechanism of action is still not completely understood. The efficacy of acupuncture in relieving pain, especially chronic pain, is documented in the veterinary literature, and many veterinarians support its use.
• Therapeutic ultrasound has numerous applications, but seems to be especially effective in treating diseased and dysfunctional joints and joint components and certain muscle diseases. The goals of treatment are to reduce pain, improve the elasticity of fibrous structures, increase blood flow, and improve tissue nutrition.
• Indications for extracorporeal shock-wave therapy (ESWT) include joint disease (e.g., arthritis of the hip, knee, or elbow) and tendinopathies.
• Low-level laser therapy has many possible applications, including pain relief.
TREATMENT OF PAIN IN LARGE ANIMALS
As poor as pain management can be in some small animal practices, sadly the situation in large animal medicine is generally even worse. Although we do not know exactly how many large animal patients receive analgesia in the United States, a survey of Canadian veterinarians sheds some insight on just how few horses, steers, and piglets actually receive analgesic drugs. For routine procedures, such as castrations in patients less than 6 months of age, only 0.001% of piglets, 6.9% of beef calves, and 18.7% of dairy calves received pain medication (Hewson, et al, 2007). Analgesia improved slightly in older patients where 19.9% of beef calves and 33.2% of dairy calves older than 6 months of age received some analgesic drugs. In horses, 95.8% of patients received analgesics for castration, and more than 90% of veterinarians used analgesic drugs for other equine surgeries, for cesarean sections in sows and cows, and for bovine claw amputations and omentopexies. However, even when analgesia was provided, it was often inadequate. In most cases, a sole agent was used to treat levels of pain that would have required multimodal analgesia for adequate pain control, and often that agent was a drug with a short duration of action.
IDENTIFYING AND ANTICIPATING PAIN
Why is large animal pain so grossly undertreated? Fortunately, some of the reasons are exactly the same in large animals as they are in small animals, so we can use our existing small animal knowledge to educate our large animal colleagues. However, there are also some species-specific treatment differences and differences in the economic perceptions of veterinarians and producers. Here are the main reasons that large animal pain is not addressed:
1. “Animals don’t feel pain.” As with pain in small animals, this is absolutely false. All mammals have the same pain pathway, so if a procedure or injury would be painful to you, it will be painful to other mammals—including large animals.
2. “Animals don’t show pain.” As occurs in small animals, this one is often true—but is absolutely no excuse for not treating pain. Unless an animal is in so much pain that it can no longer hide the pain, it is instinctual for them not to show pain. Remember, most farm animals are “prey,” and in their world, the weakest member of the herd might be lunch for a “predator.” It is in their best interest to be stoic. Thus each patient should be treated on the expected pain intensity and not forced to prove that it is in pain.
3. “There are a limited number of analgesic drugs available for farm animals.” This one is also often true—but absolutely no excuse for not treating pain. The analgesic drug classes that are available in small animal medicine (NSAIDs, opioids, local anesthetic agents, α2-agonists, etc.) are available in large animal medicine. However, it is true that there are some species differences in how the patients respond to a drug (e.g., horses can get quite excited after opioid administration) and some species differences in how drugs are “handled” in the body (e.g., orally administered drugs may be inactivated in the rumen). However, there are effective drugs available for each species, and they are listed in the specific species discussions later in the chapter.
4. “Owners or producers won’t pay for analgesia.” This one may be true if we let it be true. It is definitely not true for most horses, and this is reflected by the large percentage of veterinarians in the Canadian study who provided at least some analgesia for their equine patients (Hewson et al, 2007). However, most farm animals have an absolute economic value (which is generally low), and the value to the producer is in the herd rather than in each individual animal; thus individual animal medicine is not a priority, and pain relief falls in the category of individual animal medicine. But to counter that argument, many of the analgesic drugs are quite inexpensive (e.g., lidocaine) and could be used without adding significant cost. Furthermore, the stress of pain causes decreased food intake, weight loss, decreased milk production, and other side effects, which can be expensive to the producer. There is more information on this topic under the “Cattle, Sheep and Goats” section of this chapter.
As troubling as the situation seems, there are a few instances where the practice of large animal pain medicine might be slightly ahead of that practiced in small animals. For instance, equine practitioners are extremely comfortable with the use of NSAIDs for acute and chronic musculoskeletal pain and with the use of α2-agonists for the control of acute abdominal pain (or colic). Of course, this perceived comfort level with analgesics sometimes stems from issues other than concern over the welfare of the patient. As an example, NSAIDs are administered because lame horses are not highly functional and certainly will not perform well if they are in competition. Colic pain is treated because severe GI pain can make horses become extremely violent and dangerous to humans. But for whatever reason that the analgesic agents are administered, the benefit to the patient is the same. However, veterinarians and veterinary technicians should continue to stress the message that large animals do indeed feel pain and that pain should be treated in all animals (Figure 26-7).
Identifying pain in large animals can be extremely difficult because these animals evolved in a prey-predator society, and all are in the “prey” group. This means that they instinctively hide weakness (and pain is a weakness) to survive. However, because the pain pathway is the same in all mammals, the phrase “if it hurts you, it hurts them” is appropriate to determine whether or not a patient might be in pain, regardless of whether or not the patient shows pain. Unfortunately, as with small animals, the negative effects of pain (see Box 26-1) occur any time pain occurs (whether the patient shows pain or not) and cause detrimental effects to the patient, including delayed healing. Treatment of pain is not just an ethical issue, but also a medical issue. Species-specific signs of pain are listed in Box 26-5.
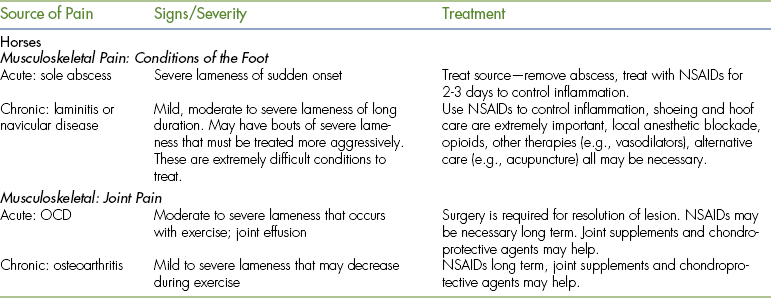
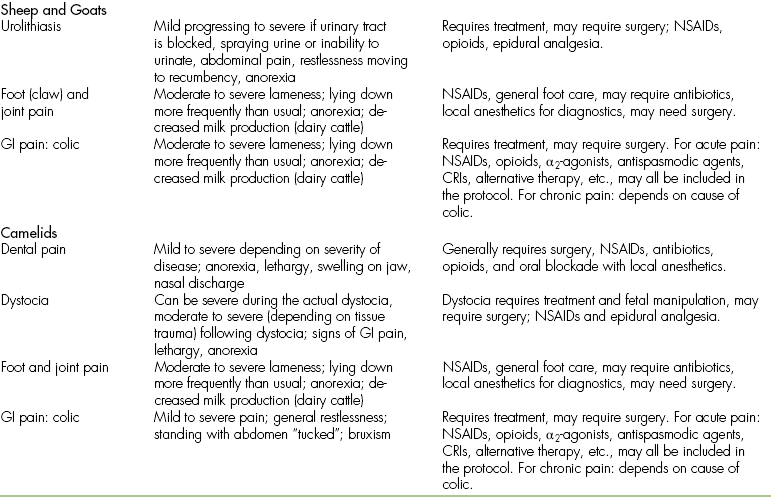
Furthermore, because these animals often do not show pain until the pain is too severe to be hidden, the sequelae of pain may well be advanced by the time that a human realizes that the patient is in pain. The degree of pain that we expect the patient to be in should be anticipated and analgesia administered based on that expectation rather than on exhibited pain. Species-specific, commonly encountered painful conditions and surgical procedures and the degree of pain expected with each condition are listed in Tables 26-3 and 26-4.
TABLE 26-4
Most Commonly Occurring Painful Surgeries in Horses, Cattle, Sheep, Goats, and Camelids
| Surgery | Pain Severity | Treatment |
| Horse | ||
| Colic surgery | Severe | Opioids (morphine?), α2-agonists, NSAIDs, CRIs |
| Joint surgery | Moderate to severe | Opioids, α2-agonists, NSAIDs, intraarticular analgesia, epidural analgesia for rear limb pain |
| Sinus surgery | Moderate to severe | Opioids, α2-agonists, NSAIDs |
| Castration | Mild to moderate | Butorphanol + α2-agonist premedication, NSAIDs, testicular block? |
| Cattle | ||
| GI or colic surgery | Severe | Opioids (morphine?), α2-agonists, NSAIDs, CRIs |
| Abomasopexy | Moderate | Local anesthetics, NSAIDs |
| Claw removal | Moderate to severe | Local anesthetics, NSAIDs, opioids + α2-agonists during surgery |
| Dehorning | Moderate to severe | Local anesthetics, NSAIDs |
| Teat surgery | Moderate to severe | Local anesthetics, NSAIDs, opioids + α2-agonists during surgery |
| Castration | Moderate | Local anesthetic block? NSAIDs? |
| C-section | Moderate to severe | Local anesthetics, epidural? NSAIDs, opioids + α2-agonists during surgery? |
| Sheep, Goats | ||
| C-section | Moderate to severe | Local anesthetics, epidural? NSAIDs, opioids + α2-agonists during surgery? |
| Perineal urethrostomy | Moderate to severe | Epidural analgesia, NSAIDs, opioids + α2-agonists during surgery? |
| Castration | Moderate | Local anesthetic blockade, NSAIDs, opioids + α2-agonists during surgery? |
| Dehorning (goats) | Moderate to severe | Local anesthetic blockade, NSAIDs, opioids + α2-agonists during surgery |
| Claw removal | Severe | See comments under cattle |
| Camelids | ||
| Dental surgery | Moderate to severe | NSAIDs, local anesthetic blockade, α2-agonists and opioids during surgery |
| C-section | Moderate to severe | Epidural analgesia, NSAIDs, opioids and α2-agonists during surgery |
TREATING PAIN IN LARGE ANIMALS
All of the analgesic drug classes used to treat small animals can be used to treat large animals, but there are fewer FDA-approved drugs and, in general, less information about the use of these drugs in large animal species (Box 26-6). However, affordable and effective analgesic drugs are available for all species, and a “lack of drugs” is not a viable excuse not to treat pain.
As with small animals, the principles of pain management include the use of:
1. Preemptive analgesia (for surgical pain)
2. Multimodal analgesia (anytime pain is moderate to severe)
3. Analgesia of a duration that covers the entire painful period
Regardless of which analgesic drugs are chosen for the patient, these principles should be addressed every time an analgesic protocol is formulated. Drug classes that can be used in large animal analgesic protocols are listed in Tables 26-5 and 26-6 and include the following.
TABLE 26-5
Analgesic Drug Dosages for Horses
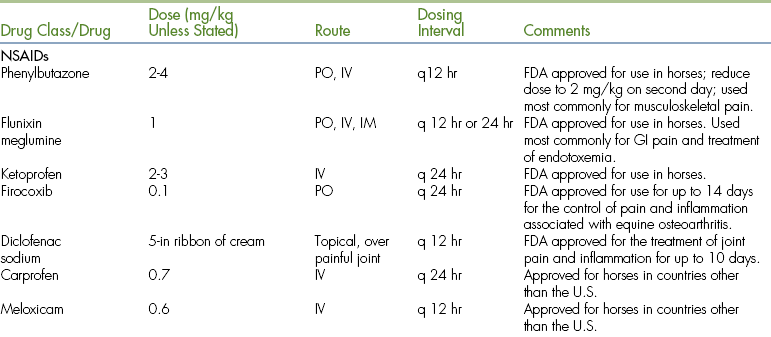
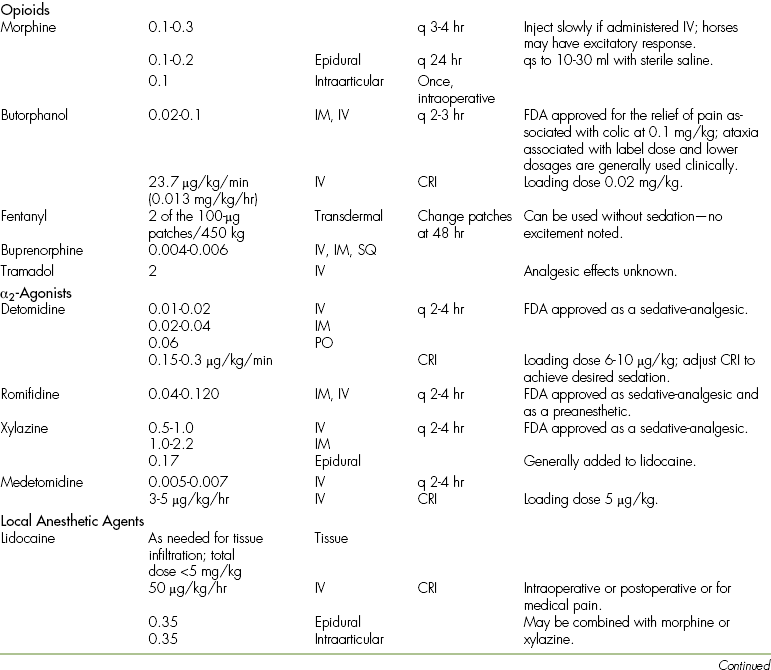

∗There are numerous products in this category, and two examples are listed here. Use of these products should be governed by proven efficacy.
TABLE 26-6
Analgesic Drug Dosages for Farm Animals (Cattle, Sheep, Goats, and Pigs_ and Camelids
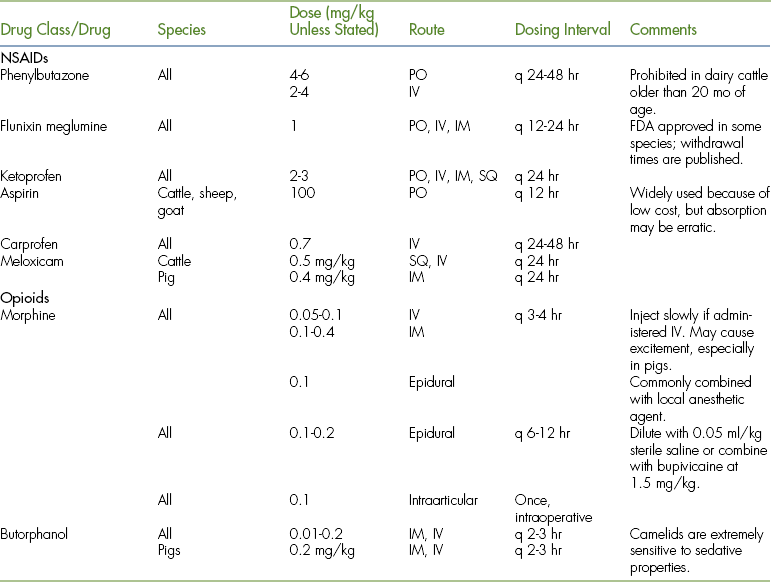
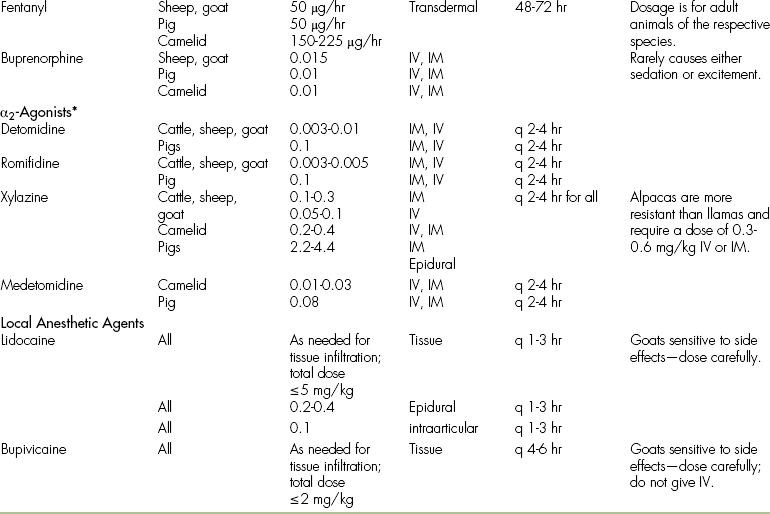
∗α2-Agonists cause profound sedation in ruminants and camelids, but pigs are fairly insensitive to α2-agonist–induced sedation.
NSAIDs
NSAIDs are an ideal choice for most painful conditions because they are antiinflammatory and analgesic. Thus anytime pain is caused by inflammation, NSAIDs are treating the source of pain and the pain itself (Figure 26-8). They are also relatively safe, easy to administer, inexpensive, and long lasting (12 to 24 hours). Because of the long duration, NSAIDs are ideal to pair with more potent but short-acting drugs, such as the opioids and the α2-agonists. Depending on which drug is chosen, NSAIDs can be used IV, IM, SQ, PO, or transdermally. The most commonly used NSAIDs in large animals are phenylbutazone (“bute”) and flunixin meglumine (“Banamin”), but other NSAIDs are available (see Tables 26-5 and 26-6). The primary side effect of NSAIDs in large animals is GI ulceration.
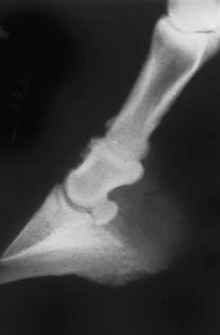
FIGURE 26-8 Radiograph of the pastern joint of a horse with “high ringbone.” This is a slowly progressive disease that will cause a mild to severe lameness (depending on the degree of pathologic condition) that will limit the usefulness of the horse. Mild pain can generally be controlled with NSAIDs and light use, but more severe pain will require multimodal therapy.
Opioids
Opioids are the most potent class of analgesic drugs and should be included in all moderate to severe cases of pain, especially acute pain. The opioid most commonly used in large animals is butorphanol, but morphine, buprenorphine, and fentanyl are also used. Opioids may cause excitement in large animal species, especially horses and pigs, so they are generally used with a sedative (e.g., xylazine, romifidine, or detomidine) in these species. Cattle, sheep, and goats generally become lightly sedated with opioids, but may have behavioral changes, such as restlessness and vocalization. Camelids are sensitive to the sedating properties of opioids. Opioids can be administered IV, IM, SQ, transdermally, epidurally, intraarticularly, and by CRI. Opioids are primarily used for acute medical or surgical pain. Other than butorphanol, opioid use in large animals has been fairly uncommon, but is increasing.
α2-Agonists
α2-Agonists, such as detomidine, xylazine, and romifidine, are used more commonly in large animals than in small animals and, although generally considered a sedative, actually provide moderate pain relief. α2-Agonists are often combined with opioids (primarily butorphanol) for improved sedation and enhanced analgesia in patients that are experiencing acute pain (e.g., patients with colic) and patients that will undergo minor procedures (e.g., wound repair). The dosage of α2-agonists is highly variable between species because the response to α2-agonists is highly variable. Ataxia secondary to profound sedation is the most common side effect of this class of drugs in large animals. The heart rate drop that often follows α2-agonist administration is rarely of concern because it is a normal physiologic response to increased blood pressure caused by α2-agonist–mediated vasoconstriction.
Local Anesthetic Agents
Local anesthetic agents are some of the most effective and least expensive (yet underused) analgesic drugs available. Local anesthetic agents are ideal for pain control in all species because they will block the transmission of painful impulses without causing systemic effects. Lidocaine, bupivacaine, and mepivacaine are all used locally in large animals, and lidocaine can be used IV as a CRI. Mepivacaine is often chosen for diagnostic nerve blocks because it has an intermediate duration of action between lidocaine and bupivacaine (Figure 26-9). For true analgesia (i.e., blocking nerves to control the pain of laminitis), longer-lasting drugs, such as bupivacaine, should be used. A lidocaine CRI is fairly commonly used to control intraoperative and postoperative pain, especially GI pain in horses.

FIGURE 26-9 Locations for blocking the nerves supplying the horns in a goat. The number 1 indicates the location to block the cornual branch of the zygomaticotemporal nerve, and the number 2 indicates the location to block the cornual branch of the infratrochlear nerve. It is important to note that both nerves must be blocked in goats, as opposed to cattle in which only the cornual branch of the zygomaticotemporal nerve supplies the horns.
Joint Supplements and Chondroprotective Agents
Joint supplements and chondroprotective agents, such as nutraceuticals, chondroitin sulfate, hyaluronic acid, and glycosaminoglycans (GAGs), are used more commonly in horses than in any other species, although they can be used in all of the species mentioned in this chapter. These products are generally not truly analgesic agents, but may help improve joint health and thereby decrease pain. Some, however, may have an antiinflammatory or other medical effect. These products are appropriate to add to a multimodal protocol (e.g., with NSAIDs) and are sometimes used alone for mild to moderate pain, especially in performing horses that cannot receive other pharmaceuticals because of drug administration rules. There are a large number of products in this category, and the choice of which one(s) to use should be based on proven efficacy of the product.
Miscellaneous Agents
Ketamine: Ketamine is an NMDA receptor antagonist that is used as a CRI to treat the pain of “wind-up” (see p. 866, general section in this chapter). Ketamine CRIs can be added to multimodal analgesic protocols in patients that are experiencing pain that is difficult to control. Ketamine CRIs have been shown to be useful in a variety of large animal species, including horses and llamas.
Alternative Therapy
Alternative therapy encompasses many modalities, including acupuncture, chiropractics, massage, low-level laser therapy, extracorporeal shock wave therapy, magnetic therapy, and many others (see “Nonpharmacologic Treatment Options” in the small animal section of this chapter). However, not all of the alternative modalities have scientific credibility, and all treatment modalities should be carefully researched before they are endorsed. These treatments may be used for acute pain, but are generally added to a multimodal protocol for chronic pain or used when pharmaceuticals are not allowed (e.g., in performance horses that will be drug tested).
Good Husbandry
Good husbandry and nursing care are always a major part of a good pain management program. This includes not only attention to comfort, as in small animals, but also attention to species-specific details, such as proper shoeing in horses with foot and limb pain and proper udder care in lactating dairy cattle.
More species-specific information on each drug class is available later in the species section and in Tables 26-5 and 26-6.
SPECIES-SPECIFIC INFORMATION
Horses generally receive better analgesic treatment than other large animals for several reasons:
1. They are more likely to be treated as “companion animals,” such as dogs and cats.
2. Horses are generally performance animals, and pain can affect their performance.
3. Horses may become violent and dangerous when in acute pain—especially with GI pain.
4. Most horses do not have an absolute economic value, such as cattle and pigs, and thus the owners are more likely to spend money on care.
More analgesic agents have been researched in the horse; thus we have more information about how to use the drugs in this species. The NSAIDs are the mainstay for both acute and chronic pain, and equine practitioners are often better at prescribing NSAIDs than small animal practitioners. Opioids are used more commonly in horses. Morphine is a potent analgesic agent that is used intraoperatively as an IV injection or used in the epidural or intraarticular space. When morphine is used parenterally in the horse, it is generally combined with an α2-agonist to reduce the chance of an excitatory response. Butorphanol is commonly combined with an α2-agonist to treat acute pain. Buprenorphine, albeit somewhat expensive in adult horses, has also been shown to provide analgesia and has a longer duration of analgesia than butorphanol. Fentanyl patches have been used in adult horses and foals for acute pain, and tramadol has been used in a few horses (with mixed success) for control of chronic pain. Other agents that are routinely used in horses include chondroprotective agents for joint pain and antispasmodic agents for GI pain. Ketamine and
lidocaine CRIs are used more and more commonly for intraoperative and postoperative pain and for medical pain (e.g., nonsurgical colic). Local and regional blockade (e.g., epidurals and intraarticular blocks) are becoming fairly standard.
CATTLE, SHEEP, AND GOATS
Food-producing herd animals, such as cattle, sheep, and goats, are the least likely large animal patients to receive analgesia. Exceptions to the lack of treatment include animals kept as individual pets, valuable beef herd sires, and high-producing dairy cows. Problems specific to food-producing animals include that these animals often have an absolute economic value with a narrow profit margin, and pharmaceutical residue is not generally accepted in the food chain. The latter issue means that some drugs are totally prohibited in this group of patients, whereas other drugs have a withdrawal time that must be observed between the administration of the drug and the time that meat and/or milk can be used.
Economics
Although it is true that many farm animals have an absolute economic value with a low profit margin, it is also true that a number of the analgesic drugs are inexpensive (e.g., local anesthetic agents), and the side effects of pain (e.g., anorexia and weight loss) can be as or more costly than the analgesia. In many other countries of the world (e.g., the United Kingdom and several Scandinavian countries), analgesia is required by law for surgical procedures done on farm animals. The most common requirement is the use of local anesthetics, which will at least provide good analgesia for the duration of the block, have been shown to decrease the side effects associated with pain, and have minimal tissue uptake so that drug residues are of decreased concern. Analgesia may be difficult to regulate in the United States because many of the routine procedures (e.g., castration in calves, pigs, and lambs) are done by producers and not by veterinarians. However, as a profession, we should strive to ensure that all procedures done by a veterinarian are accompanied by analgesia. Unfortunately, even this seemingly simple step may be difficult, as evidenced by a few of the swine practitioners that participated in the Canadian study who were concerned about the loss of professional time and income while waiting for local anesthetics to take effect—a delay that is only 2 to 5 minutes in duration.
Drug Residues in Meat and Milk
Because drug residues in the human food chain could be harmful to human beings, there are strict regulations regarding the use of many pharmaceuticals in food-producing (meat and milk) animals. For instance, the use of phenylbutazone is completely prohibited in dairy cattle 20 months of age or older. On the other hand, some forms of flunixin (but not all brands) are approved for use in cattle and pigs, with withdrawal times of 72 hours for milk and 10 days for meat in cattle and 12 days withdrawal for meat in pigs. Because regulations change, people dealing with food-producing animals should routinely update the withdrawal information of the drugs that they are using. The best source for information is the Food Animal Residue Avoidance Databank (FARAD) at www.farad.org.
Finally, many food-producing animals are ruminants, and delivery of oral drugs into the rumen can inactivate them, slow their absorption, or otherwise alter the drug or the response to the drug. Fortunately, most oral drugs that are available to treat pain can be used in ruminants, and, of course, the IV, IM, SQ, transdermal, epidural, intraarticular, and local tissue infusion routes of administration can all be used.
All of the drug classes used in horses can be used in ruminants. The most commonly used NSAIDs are phenylbutazone, flunixin, and aspirin. α2-Agonists are commonly used for acute pain, but this group of animals is extremely sensitive to the sedating effect of α2-agonists, so low dosages
should be used. Butorphanol is also commonly used for acute pain, and morphine is used occasionally. Fentanyl patches, buprenorphine, and both ketamine and lidocaine CRIs have been used to control pain in small ruminants (sheep and goats), but the use of these drugs and techniques are not as common in adult cattle simply because treatment of pain in this group is not as common. By far, the most commonly used analgesic drugs in ruminants are the local analgesic agents (primarily lidocaine and bupivacaine), and techniques include local field block (tissue block at the site of the surgery or injury), IV regional blocks, epidurals, paravertebral blocks, corneal blocks, etc. For a description of these blocks and how to perform them, see the chapter by Dr. Skarda (full reference in the Recommended Reading section of this chapter). Alternative techniques, such as acupuncture, are also appropriate for analgesia in ruminants.
CAMELIDS
Camelids (e.g., llamas and alpacas) are more likely to receive analgesic treatment than cattle, sheep, and goats (but not as likely as horses) because they are generally constrained neither by an absolute economic value nor by entry into the human food chain. These animals have a GI tract similar to that found in ruminants with a “third compartment” that functions like a rumen. Thus drug administration issues are the same in camelids as they are in ruminants, where orally administered drugs may be inactivated. All drugs used in other ruminant types of species can be used in camelids. NSAIDs are generally the first treatment for both acute and chronic pain. α2-Agonists and butorphanol are used to treat acute pain, but these drugs cause fairly profound sedation in camelids, so they are used only when sedation is appropriate. Other treatment modalities include fentanyl patches, lidocaine CRIs, ketamine CRIs, local or regional blockade, and alternative therapies, such as acupuncture.
PIGS
The lack of good pain management in swine practice rivals the lack in cattle practice, but high-producing boars and sows along with pot bellied pigs kept as pets may receive better treatment. Like ruminants, pigs generally have an absolute economic value and are affected by their likely entry into the human food chain. All of the drugs discussed with the other species can be used in pigs.
SUMMARY
Providing effective pain management is not an individual endeavor. It requires a team approach involving everyone who participates in patient care. As true patient advocates, veterinary technicians constitute a vital force in this effort. A successful technician understands the goals of pain control and the analgesic options. Combining keen observation with good technical skills, the technician appropriately assesses pain and then requests and administers analgesics to his or her patients. Attention to environmental and emotional needs and differentiating other stress from pain is part of a technician’s daily routine (Box 26-7). Veterinary technicians have played a vital role in bringing animal pain management to the forefront of veterinary practice. Through continued teaching and vigilant practice, technicians will no doubt be credited in large part with the continuing improvements in the practice of veterinary pain management. Much work is still to be done, particularly in the arena of large animal practice. Although it is true that large animals hide pain (often even better than small animals) and that there are some economic constraints and species-specific limitations in drug use or administration, it is not true that large animals do not feel pain. Pain management is often initiated at the technician’s level because the technician is often the person who spends the most time with the patient and is the most likely to recognize pain in the patient.
REFERENCES
Bockstahler, B., Levine, D., Millis, D. Essential facts of physiotherapy in dogs and cats: rehabilitation and pain management. Germany: BE VetVerlag; 2004.
Hewson, C.J., Dohoo, I.R., Lemke, K.A., et al. Canadian veterinarians’ use of analgesics in cattle, pigs, and horses in 2004 and 2005. Can Vet J. 2007;48:155–164.
Skarda, R.T. Local and regional anesthetic and analgesic techniques. In Thurmon J.C., Tranquilli W.J., Benson G.J., eds.: Lumb & Jones’ veterinary anesthesia, ed 3, Baltimore: Williams & Wilkins, 1996.
Animal Welfare Act and Regulations. USDA website with other links. Available at www.nal.usda.gov/awic/legislat/usdalegl.htm.
Bath, G.F. Management of pain in production animals. Appl Anim Behav Sci. 1998;59:147–156.
Benson G.J., Rollin B.E., eds. The well-being of farm animals: challenges and solutions. Blackwell Publishing, 2004.
Wildlife Information Network: Pain management in ruminants. Available at www.wildlifeinformation.org
Valverde, A., Gunkel, C.I. Pain management in horses and farm animals. J Vet Emerg Crit Care. 2005;15(4):295–307.
Mama, K. Pain management and anesthesia. Vet Clin North Am Eq Pract. 2002. April
∗Bockstahler B, Levine D, Millis D: Essential facts of physiotherapy in dogs and cats: rehabilitation and pain management, Germany, 2004, BE VetVerlag.
 TECHNICIAN NOTE
TECHNICIAN NOTE