Chapter 8 Musculoskeletal disorders
Benign familial joint hypermobility syndrome
Disease-modifying antirheumatic drugs (DMARDs)
Immune-mediated inflammatory diseases
Regional examination of the musculoskeletal system (REMS)
Rigid/semi-rigid, functional orthoses
Seropositive inflammatory arthritis
INTRODUCTION
This chapter is intended to provide an introduction for readers either potentially or actually involved in the care of the foot in patients with rheumatic conditions. An overview of the medical management is provided because foot pathologies do not normally present in isolation in these patients and so the systemic context is important. However, the chapter is about the foot and so focuses mainly on the foot conditions, the processes underlying them and the treatment options available. The evidence supporting many of the therapies for disorders of the foot in rheumatology is often weaker than would be desirable. This chapter represents, therefore, a digest of available literature, combined with the authors’ experience of clinical practice and research within an academic multidisciplinary rheumatology team supported by a comprehensive foot health service.
DEFINING RHEUMATOLOGY AND THE MUSCULOSKELETAL DISEASES
Rheumatology practice covers a heterogeneous range of more than 200 disorders with varied aetiologies, and which affect joints, bones, muscles and soft tissues (Linaker et al 1999, Symmons et al 2002). The rheumatic disorders have a variety of systemic features, but their common feature is their effect on the musculoskeletal system. Those working with foot problems are probably familiar with disorders arising from purely biomechanical and local factors, and these disorders are dealt with elsewhere in this book. This chapter focuses on the broader rheumatic diseases affecting the feet and their associated systemic or extended local disease processes. Many of the disorders seen in rheumatology practice are the consequence of a disordered immune response, and are therefore sometimes referred to as immune-mediated inflammatory diseases. Typical examples of immune-mediated inflammatory diseases are RA, the spondyloarthropathies, and the connective tissue diseases such as lupus and scleroderma (described later). Other systemic presentations include diseases caused by disordered metabolism, such as gout and osteoporosis; and complex, multifactorial disorders such as OA.
GENERAL EPIDEMIOLOGY OF RHEUMATIC CONDITIONS
Approximately 1 in 7 adults in the UK has ongoing musculoskeletal symptoms, with some 1 in 20 people suffering moderate to severe disability secondary to a musculoskeletal disorder (Symmons et al 2002). The prevalence of musculoskeletal disease increases steadily with age, and by the age of 75 years can be as high as one person in three (Urwin et al 1998, Symmons et al 2002). Musculoskeletal pain also presents in multiple sites, and only one-third of people report their musculoskeletal pain to be confined to a single joint (Urwin et al 1998). Unsurprisingly, the degree of impact on quality of life increases as the number of affected sites increases (Urwin et al 1998).
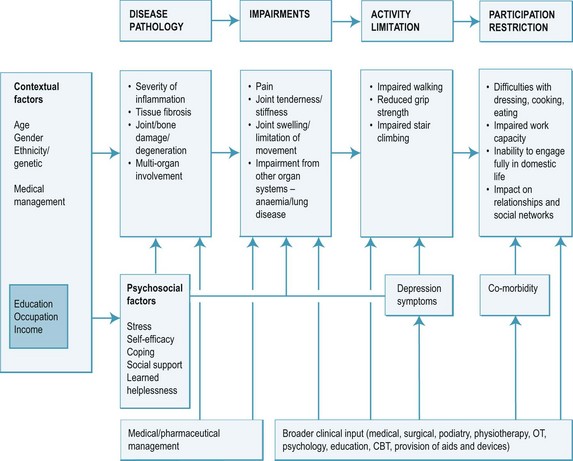
The disability pathway from the underpinning local joint inflammation through to the impact on quality of life is well described for arthritis, and is summarised in Figure 8.1. Controlling symptoms and the effects of disease on quality of life are the focus of much of the therapeutic process. It should be remembered, however, that the disability pathway is influenced not only by disease factors, and that approximately one-quarter of disability is predicted by a range of modifying factors such as age, gender, lifestyle, work demands and psychological status (Escalante & Del Rincon 1999, 2002).
BURDEN OF DISEASE
The burden of musculoskeletal disability to the health system is significant, with musculoskeletal conditions accounting for about one in five of all GP consultations, a proportion that continues to increase (Arthritis Research Campaign 2002, Symmons et al 2002). The musculoskeletal diseases are to some degree a social problem, because the prevalence is significantly higher in more deprived socio-economic groups and in workers engaged in more physical activities (Arthritis Research Campaign 2002, Urwin et al 1998).
The direct cost to the UK health service is approximately £5.5 billion each year, but indirect costs (such as the 206 million days a year lost to work, reduced incomes, social security and mortality) raise the total cost to the UK economy to £18 billion per year (Arthritis Research Campaign 2002).
THE EPIDEMIOLOGY OF FOOT PROBLEMS GENERALLY AND IN RHEUMATOLOGY
Nearly one-quarter of all people aged over 55 years report ongoing foot pain, which in the majority of cases leads to impairment to daily activities (Benvenuti et al 1995, Chen et al 2003, Gorter et al 2001, Leveille et al 1998, Thomas et al 2004). Some 20–24% of all adults have had foot pain in the past month and some 60% have had foot pain in the past 6 months, with the foot pain causing measurable disability in half of these cases (Garrow et al 2004). Musculoskeletal disorders account for more than three-quarters of all foot pain (Gorter et al 2000) or, in other words, for foot pain in up to 7% of the total population (Cunningham & Kelsey 1984).
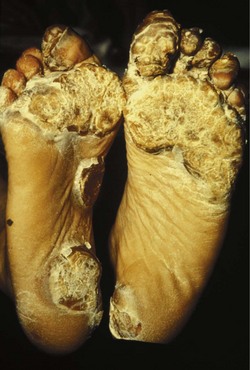
The incidence of foot problems is known to increase with age, and to be as much as five times higher in females than males (Benvenuti et al 1995, Black & Hale 1987, Dunn et al 2004, Garrow et al 2004, Leveille et al 1998, Munro & Steele 1998). Foot pathology often also coexists with other musculoskeletal morbidity such as knee or hip pain (Gorter et al 2000, Leveille et al 1998, Munro & Steele 1998, Odding et al 1995), and this should be considered in assessment and treatment planning. As well as the musculoskeletal manifestations themselves, plantar hyperkeratoses have been found in 66% of people with musculoskeletal or connective tissue disease, digital lesions in 24% and ulcerations in as many as 17% (Port et al 1980).
A BRIEF OVERVIEW OF DEVELOPMENTS IN MEDICAL RHEUMATOLOGY
In many rheumatic diseases, the classical approach of slowly escalating drug therapy has been superseded by a new paradigm of early and aggressive treatment, and combination therapies using disease-modifying antirheumatic drugs (DMARDs). This change in approach has been further enhanced in recent years by the development of a new range of pharmaceuticals, the so-called ‘biological’ immunotherapies, which provide a highly effective treatment option for patients who do not respond adequately to conventional therapies. The significant medical advances are also reflected in the management of rheumatology patients by allied health professionals, as the emphasis moves from compassionate management of physical decline to more dynamic and proactive approaches.
MEDICAL MANAGEMENT IN RHEUMATOLOGY – OVERVIEW ACROSS DISEASES
Medical management in rheumatology has undergone nothing short of a revolution in recent years. While the non-inflammatory conditions such as OA continue to be managed according to fairly traditional principles, treatment of immune-mediated inflammatory diseases has been, quite literally, turned on its head.
Between the 1950s and the mid-1990s, patients with inflammatory arthritis would be managed according to the severity of presenting symptoms, using what was described as a ‘treatment pyramid’, where drug therapy was escalated slowly and incrementally in response to treatment failure (Cannella & O’Dell 2003, Fries et al 1996, Pincus et al 1999). High doses of non-steroidal anti-inflammatory drugs (NSAIDs) would be used, with doses increasing until patients were no longer able to tolerate therapy. When disease control was inadequate using NSAIDS, other disease-modifying drugs such as the antimalarials, gold, penicillamine and sulfasalazine would be introduced sequentially to try to suppress the inflammation as far as practicable. These second-line DMARDs were often not introduced until 5–10 years of disease duration had passed and, as we now know, damage had already accrued (Wolfe et al 2001). In the 1990s, methotrexate came to prominence such that it became the recommended DMARD to be given early in the course of the disease, with rapidly escalating doses if response was suboptimal.
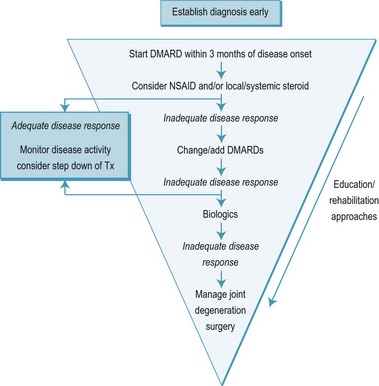
Furthermore, better understanding of the pathological processes involved, the discovery of the role of cytokines in the early 1980s, and the development of anti cytokine drugs in the past 10 years (Feldmann 2002) have led to a total paradigm shift. The new paradigm is known as the ‘inverted pyramid’ or ‘step-down bridge’ approach (Fries et al 1996, Pincus et al 1999) (Figure 8.2).
In the step-down approach early management is aggressive and combinations of DMARDs are used to suppress inflammation nearer to disease onset, so limiting irreversible joint damage (O’Dell 2004). There is compelling evidence that the new approach improves outcomes substantially in a range of immune-mediated arthropathies (Cannella & O’Dell 2003, Rao & Hootman 2004) and that the earlier that treatment is instigated the better (Bukhari et al 2003, Hazes 2003, Lard et al 2001, Puolakka et al 2004). The Arthritis and Musculoskeletal Alliance (ARMA) national standards of care for rheumatoid arthritis (RA) require that all patients with suspected inflammatory arthritis are seen by a rheumatology specialist within 12 weeks of first presentation, and preferably within 6 weeks (Arthritis and Musculoskeletal Alliance 2004a).
In inflammatory arthritis, the goal at first presentation is to establish and maintain disease remission, and this can often be achieved with standard (i.e. traditional non-biological) therapies (Bukhari et al 2003). Patients are started on NSAIDs (ibuprofen, diclofenac, indometacin or cyclo-oxygenase-2 (cox-2) inhibitors, according to risk factors), which provide immediate reduction in pain and stiffness (O’Dell 2004). NSAIDs are not used in isolation, however, as they do not prevent erosions and joint damage and so do not alter long-term prognosis (Aletaha & Smolen 2002, American College of Rheumatology Subcommittee on Rheumatoid Arthritis 2002, Choy et al 2002, Fries et al 1996). Much has been made of the potential benefits offered by the new cox-2 selective NSAIDS, although the objective evidence suggests that the effectiveness of the cox-2 inhibitors is no greater than traditional (and cheaper) NSAIDs such as ibuprofen, naproxen and diclofenac, with benefits confined to slightly better tolerance (Garner et al 2004a,b). Importantly, new data emerging have indicated a potential for increasing the risk of cardiovascular events with cox-2 drugs, and one of this new class of NSAIDs (rofecoxib) has already been withdrawn from the market (Juni et al 2004). Prescribers are therefore advised to be cautious and to avoid these drugs in anyone with a high risk, or history, of cardiovascular disease (Fitzgerald 2004). Furthermore, it is now becoming clear that even traditional NSAIDs carry an increased risk of vascular disease. These developments have led to an aversion by physicians to prescribing NSAIDs – if they are prescribed the recommendation is for the smallest possible dose for the shortest possible time.
There is good evidence that instigation of DMARD therapy within 3 months of disease onset is highly effective in controlling immune-mediated inflammatory diseases (American College of Rheumatology Subcommittee on Rheumatoid Arthritis 2002, O’Dell 2004), and so patients are normally started on a suitable DMARD immediately. The most commonly used DMARDs at present are methotrexate and hydroxychloroquine, with sulfasalazine becoming increasingly marginalised. Leflunomide is a new DMARD with good tolerability, which can be used in conjunction with methotrexate or as a fallback when patients cannot use methotrexate (American College of Rheumatology Subcommittee on Rheumatoid Arthritis 2002, Olsen & Stein 2004; Osiri et al 2004). Methotrexate is usually the DMARD of first choice because of its significant therapeutic effects when used in isolation, and because of its adjunctive effects when used in combination with other DMARDs (O’Dell 2004, Verstappen et al 2003a,b). Methotrexate is the best tolerated DMARD for long-term therapy, and many patients use methotrexate for many years (O’Dell 1997, Rau et al 1997, Verstappen et al 2003). Used in this way, methotrexate is often referred to as an ‘anchor drug’ (Pincus et al 1999).
There is some controversy on the use of oral corticosteroids as a first-line treatment because of their wide ranging systemic side-effects. Recent high-profile studies on intensive treatment regimens have used oral steroids (BeST and TICORA) but their use is not widespread in the UK. Oral steroids are useful in early disease as a ‘bridging therapy’ before the DMARDs take effect, and, given systemically, for short-term suppression of disease flares (O’Dell 2004). Low-dose systemic steroid therapy may be required on an ongoing basis in some patients but the dose is kept to a minimum (American College of Rheumatology Subcommittee on Rheumatoid Arthritis 2002).
The new, biological DMARDs inhibit cytokine activity high up in the inflammatory pathway and can be highly effective even in patients who have failed on conventional DMARD therapy (O’Dell 2004, Olsen & Stein 2004). The most common ‘biologicals’ are the agents active against tumour necrosis factor α (TNFα) (etanercept (Enbrel), infliximab (Remicade) and adalimumab (Humira)), although new ones are now appearing (Olsen & Stein 2004). The effect of anti-TNF therapy is often striking, with some patients showing a positive response within days or even hours. Biological DMARDs are expensive, however, costing £8,000 to £10,000 for a year of treatment, and so their publicly funded use is restricted to those patients with active disease who have already failed on conventional DMARDs. Because they demonstrate better efficacy than conventional DMARDS, a case is being made for their use in early disease (Emery & Seto 2003, Breedveld et al 2004), where it is contended that the significant reduction in (work-related) disability associated with biological therapy makes these therapies cost effective when viewed in the context of savings to the country from lost work days (9.4 million days, equivalent to £833 million a year for RA alone) (Arthritis Research Campaign 2002) and decreased dependence on the state benefits system.
Another family of therapies are drugs aimed at reducing the activity and number of B-cells, which are important mediators of the immune-mediated inflammatory response. Rituximab is now well established as a therapy for RA and connective tissue diseases, and good results are reported up to 30 weeks after initial injection without short-term immunocompromise (Edwards et al 2004, Tsokos 2004). Other biological therapies are emerging every year. Currently, the T-cell costimulation blocker abatacept is licensed for use in RA and the anti-interleukin-6 (anti-IL6) compound toclizumab newly available. It is likely that all these therapies will be restricted to those people who have failed on conventional (and cheaper) DMARDs but, common to all, there is an increased risk of infection and practitioners must keep this in mind when caring for patients on these drugs. We still do not know whether drugs such as those in the anti-TNF class will cause an increased tumour incidence with long-term therapy, but the initial indications are good (see box 8.1).
It is essential that those involved in the care of the feet of people with inflammatory arthritis are aware of the medication being taken by their patients, and the implications of the drug therapy. Patients with inflammatory arthritis are twice as likely as the general population to develop an infection, and there is a further increase in infection risk in patients taking DMARDs (Edwards et al 2004, Olsen & Stein 2004). Although hard evidence is conflicting there is a suggestion that patients undergoing biologic immunotherapy may be particularly at risk from infection, as they appear to be susceptible to unusually rapid progression. It is absolutely essential that the foot health clinician liaises with the rheumatologist where there is any risk of infection (e.g. from ulceration, or prior to minor surgery). Otter and Cryer (2004) have made the point well that this should not erode the practitioner’s autonomy, but it does allow the appropriate precautions to be taken – including antibiotic cover and, if necessary, temporary alteration of antirheumatic therapy (Olsen & Stein 2004).
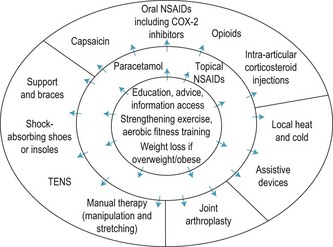
Medical management is usually combined with other approaches such as physical and occupational therapies. Exercise programmes have been found to improve outcomes in a range of rheumatological conditions, including low back pain, lupus, fibromyalgia and ankylosing spondylitis (Rao & Hootman 2004). Other physical therapies in widespread use are heat and cold therapies (baths, thermal packs etc.), therapeutic ultrasound, transcutaneous electrical nerve stimulation (TENS), mobilisation and massage.
Patients with rheumatological disorders often have to live with chronic pain. It is important to recognise the limitations of medical management, and to ensure that the standard approaches are supplemented with specific strategies directed towards living with chronic pain or disability (Rao & Hootman 2004). Cognitive–behavioural strategies are important in enabling patients to understand the condition, and moderate their beliefs and behaviours (Rao & Hootman 2004).
THE FOOT IN RHEUMATOLOGY – OVERVIEW ACROSS DISEASES
Assessing the foot
There is no validated, standardised assessment for the foot in musculoskeletal conditions. A number of validated assessments that include the foot are used in medical practice, but podiatrists tend to use more individualised assessments.
The GALS (gait, arms, legs, spine) screen is now considered a minimum standard for all musculoskeletal assessments in medical rheumatology, and this includes at least an observational assessment of the feet. Where lower-limb involvement is suspected, this may be followed up using a new validated assessment, the REMS (regional examination of the musculoskeletal system) assessment, which is being introduced to the rheumatology community (Coady et al 2004). REMS includes a fairly detailed assessment of the feet, although it must be remembered that the GALS and REMS approaches are intended to be part of a general medical work-up and are inadequate for detailed assessment of the foot.
In the absence of a standardised foot assessment, we have developed a preferred protocol for foot assessment at the Leeds centre, which is based on a history–observation–examination (or look–feel–move) model (Box 8.2).
Box 8.2 The preferred protocol for assessing the foot in rheumatology at the authors’ centre
History
This assessment protocol provides most of the information required to inform a diagnosis and management plan. However, it is sometimes helpful to supplement this assessment with objective data from more ‘high-tech’ investigations, and patients may also be referred for objective, dynamic functional assessments, such as plantar pressure evaluations, or for radiographic imaging. Gait laboratory assessments are not mainstream outside of teaching centres and are not detailed here, but radiographic imaging is central to rheumatology practice and will be accessed widely by foot practitioners.
The plain radiograph is usually still the imaging modality of first choice, although it is increasingly being supplemented by a range of alternatives. Plain films will differentiate gross radiographic features, such as joint-space narrowing, osteophyte formation or periarticular erosion, but the images are limited to two dimensions. Menz et al (2007) have produced a useful atlas of radiographic classification of joint disease in the foot which may help to standardise interpretation. Plain films are limited in the new paradigm of early and aggressive intervention, however, because they are not sensitive to many of the changes that often occur early in the disease process (Devauchelle-Pensec et al 2002, Ostergaard & Szkudlarek 2003). To image more subtle pathologies of the musculoskeletal system, other modalities are coming to prominence. Magnetic resonance imaging (MRI) allows good visualisation of soft-tissue structures such as ligaments, tendons and synovium. Methods such as T1- and T2-weighted sequences and STIR, particularly if enhanced with a contrast agent such as gadolinium-DTPA, allow for good differentiation of healthy and inflamed soft tissues, and identification of bony oedema and erosions (Bouysset et al 1995, Ostergaard & Szkudlarek 2003). Advances in imaging technology allow for sophisticated three-dimensional reconstructions that can be used to provide valuable visual and quantitative data on the severity of disease activity locally (Woodburn et al 2002a,c). MRI is expensive and time consuming, however, and cannot be considered a first resort. For high-resolution imaging of bony structures, computed tomography (CT) remains the modality of choice. However, CT requires exposure to ionising radiation and is unsuitable for screening or repeated use.
High-resolution ultrasound (HRUS) is becoming increasingly popular as a ‘bedside’ modality in rheumatology, as it is low risk, differentiates soft tissues structures well and involves no exposure to ionising radiation (Ostergaard & Szkudlarek 2003, Wakefield et al 2000). It is possible using HRUS, to reliably differentiate between inflamed and healthy soft tissues, identify erosions that are undetectable on a plain radiograph and also to investigate structures dynamically (Olivieri et al 1998, Ostergaard & Szkudlarek 2003, Szkudlarek et al 2003, Wakefield et al 2000). There are some limits to HRUS imaging where features of interest, such as those within larger joints such as the ankle, lie too deep to the surface to be imaged, or lie in the ultrasound shadow of structures that form a barrier to the imaging signal. More validation studies are needed, but HRUS imaging is an area of rapid development in musculoskeletal radiology and is proving of considerable clinical use (Riente et al 2006).
Management principles
In a dedicated rheumatology foot health service, callus reduction, footwear advice and provision, and orthosis prescription are mainstays of management. In a Bradford multidisciplinary rheumatology clinic, some 76% of patients required foot orthoses and 43% required replacement footwear (Helliwell 2003), while in a Rochdale audit the requirement (not the provision) for orthoses and footwear was estimated to be 60% and 10%, respectively. An audit of our Leeds clinic indicates that just over one-quarter of our patients are referred on for footwear intervention, with another quarter receiving orthoses. General foot care (nails, corns and callus) also accounts for one-third of our caseload, a figure lying between those reported separately by Williams & Bowden (2002) and Helliwell (2003), and illustrating the variability in caseloads between services.
Injectable steroid is useful in many cases for controlling local areas of inflammatory activity such as articular synovitis or tenosynovitis. Injection of corticosteroid has been shown to be effective in relieving plantar heel pain in the short term (Mulherin & Price 2009) and is used widely to control local sites of inflammation associated with inflammatory arthritis (Cardone & Tallia 2002, Crawford et al 1999). Local injection of corticosteroid now falls within the extended scope of practice of a number of allied health professionals and can be a valuable adjunct to traditional conservative therapies for pain in the foot. It is generally recommended that steroid injections are used sparingly because of the degenerative effects on tissues following repeated injection. Patients also need to rest the body part for 24–48 hours after injection because of the risk of local tissue damage while there are high concentrations of steroid present in the tissues and to limit dispersion of the agent (Cardone & Tallia 2002).
Traditionally, most injections of local corticosteroid have been administered ‘blind’, that is without the aid of radiographic imaging to direct the needle accurately to the desired point of deposition. Recently, the wider availability of ultrasound imaging in musculoskeletal practice has led to a move towards using ‘guided’ injections, where the agent can be delivered more accurately to the required site.
Provision of foot health services – current provision, multidisciplinary involvement, surgery
There is a clear gap between the need for foot health services generally and the provision of these services in the UK. Approximately 1 in 10 people with foot pain have nothing at all done about it, and a further 40% self-manage their painful foot problems (Gorter et al 2001). However, the fact that foot problems are substantially underreported (Gorter et al 2000, Munro & Steele 1998) makes services planning and evaluation difficult.
Only one-quarter of those needing foot health services have adequate access provided by NHS services (White & Mulley 1989) and 30–40% of people needing access to foot care services simply do not have services available to them from any source (Garrow et al 2004, Harvey et al 1997). The discrepancy is greater still in the rheumatology population, with a recent survey of 139 patients attending rheumatology outpatients at a North of England hospital finding that 89% of patients had foot problems. Sixty per cent of these patients had no access to foot care services on clinical examination (Williams & Bowden 2002). The inequity in foot health provision to patients with rheumatic disorders has been noted by rheumatologists and podiatrists alike (Helliwell 2003, Michelson et al 1994, Otter 2004).
Multidisciplinary care is important in managing rheumatology patients, and there are two sides to this. On the one hand, rheumatology patients are often complex medically, and it is essential that the practitioner managing the foot problems has a dialogue with, and good back up from, the patient’s rheumatology physician. Conversely, expertise in dealing with foot problems is often limited among rheumatologists, and a strong case can be made for better integration of foot health services into rheumatology (Korda & Balint 2004).
While multidisciplinary care is well established and of proven benefit in other disciplines such as diabetology, provision of multidisciplinary foot health services in rheumatology is highly variable. Multidisciplinary clinics will often cover the medical basics, education and coping strategies, but it is less common to find podiatry included as a core part of the team despite the level of need in this patient group nearing 80% (Prier et al 1997). We surveyed rheumatology departments in the UK and established that, while 85% of rheumatology departments include rheumatology specialist nurses in the team and 44% include physiotherapy, only 27.1% include podiatry (Redmond & Helliwell 2005). The provision of foot health services also varies widely geographically, ranging from 65% in Yorkshire and the North East; to 50% in London and the South East, to 25% and 33% in Scotland and Wales, respectively.
Only half the rheumatology departments in our survey reported having access to foot heath services for important functions such as nail care and corn/callus reduction. We have criticised the lack of coordination of foot services in rheumatology previously, noting the problems that are created with patient dissatisfaction, and impediment to the development of the service within the medical teams (Helliwell 2003). We have suggested, from local experience, that a multidisciplinary foot team should consist of at least a rheumatologist, a podiatrist and an orthotist, and it would be quite appropriate to extend team membership to orthopaedics and physiotherapy as resources permit. The Arthritis and Musculoskeletal Alliance (ARMA) and Podiatry Rheumatic Care Association (PRCA) Standards of Care (2008) provide a useful mechanism for evaluating musculoskeletal foot health services and planning strategically to best integrate medical and foot health teams. Succession planning is imperative, as small teams such as these can be highly dependent on individual people for their success, and it is all too easy for a successful service to run into problems if one of the team leaves and his or her replacement is unable to participate at the same level. To ensure a level of professional development appropriate to a specialist team it is advisable that arrangements are made for continuing professional development, and for update courses to be undertaken jointly with the rest of the rheumatology team (Otter 2004).
A good multidisciplinary rheumatology foot health service is likely to tap into considerable unmet need for foot health services (Prier et al 1997) and it is our and others’ experience that the service will expand rapidly and attract referrals for a range of conditions (Helliwell 2003, Prier et al 1997). A robust business plan should be devised prior to initiating a service in order to pre-empt these potential problems and to ensure the long-term success of such a venture. The initial business plan should also be supplemented with careful documentation of patient outcomes (Prier et al 1997) so that the merits of the service can be quantified and the cost effectiveness evaluated in the long term.
Within a rheumatology multidisciplinary team, rheumatology specialist nurses play an increasingly central role, responsible for administering, monitoring and modifying patients’ medication, education, and a valuable psychosocial support role (NICE 2009). The therapy professions, specifically physiotherapy and occupational therapy, are also important in the overall management of the symptoms associated with inflammatory arthritis, and NICE (2008, 2009) has produced explicit guidance on these roles in rheumatology. The main roles for rehabilitation therapies are to help people limit disability through skills training, exercise training, pain management, joint protection programmes, and provision of splints and orthoses (Steultjens et al 2004). The merit of providing splints as part of a regimen of occupational therapy has been demonstrated, and echoes the data for foot orthoses provided as part of podiatric management (Budiman-Mak et al 1995, Steultjens et al 2004, Woodburn et al 2002a).
Service provision
The foot health services that should be provided as part of a multidisciplinary foot health team in rheumatology fall into five categories.
Education and self-management advice
In an era of increased patient empowerment, education programmes have become more commonplace. Self-management is known to result in improved health status (Rao & Hootman 2004), but there is conflicting evidence over the merit of formal education programmes for patients with rheumatic disorders. Education is the mainstay of management in non-systemic conditions such as chronic mechanical low back pain, and provides demonstrable benefits over traditional medical management of this condition (Rao & Hootman 2004). In the inflammatory diseases the picture is less clear, however, and the measurable benefits are probably confined to short-term effects on the psychological factors and impact of disability (Riemsma et al 2004). Education in RA provides measurable improvement in knowledge but only small and non-significant changes in objective and health-related quality of life measures (Helliwell et al 1999). In OA, a small reduction in the use of healthcare services was reported after individualised education, but again no changes in patient outcomes have been found (Riemsma et al 2004, Ward 2000). Rheumatology patients can be overwhelmed with information at diagnosis, however, and it may be best to introduce education selectively, focusing on issues of particular relevance at any given time. This is equally true for foot health advice, and clear guidance on the provision of information has been provided in the ARMA/PRCA Standards of Care (2008).
General foot care, nail cutting, corn and callus reduction, provision of padding
Patients with musculoskeletal conditions have an increased need for a range of basic foot care services. Deformities of the foot associated with joint changes and soft-tissue lesions create areas of pressure that result in callus and corn formation. Arthritis in the hands may make foot care and hygiene tasks difficult, and spinal involvement can make bending to attend to basic foot care tasks impossible.
It is well accepted anecdotally and in simple cohort studies (Redmond et al 1999, Woodburn et al 2000) that debridement of symptomatic callosities and removal of cornified nuclei reduces pain in the short term. One randomised trial has provided contradictory evidence, however, suggesting that callus debridement was no better than placebo at reducing pain scores in patients with RA (Davys et al 2004). The study authors do not recommend abandoning callus debridement because the anecdotal evidence and patient demand is so overwhelming, but it is clear that more research is needed into this important area of podiatric practice. Offloading strategies are essential for managing the deformed rheumatic foot, and podiatrists can be useful in providing orthoses and/ or footwear (Korda & Balint 2004).
High-risk management of the vasculitic or ulcerative foot
This is a sometimes neglected aspect of rheumatology practice. Risk of ulceration is raised in a number of musculoskeletal diseases, such as RA, in which the point prevalence is about 3% (Firth et al 2008). Management of the high-risk foot accounts for approximately one-quarter of the Leeds foot health appointments, and in one report of the case profile of multisystem wound care service rheumatology patients made up 6% of the total caseload (Steed et al 1993). Prevention and management of the high-risk foot is an important part of the foot health service in rheumatology (Korda & Balint 2004) (Figure 8.3).
Extended scope practice and surgery
The advent of the extended scope practitioner (ESP) has created the opportunity for foot health services to be more responsive to patients’ needs. The new ESPs are able to access enhanced investigations and can intervene more proactively, making amendments to patients’ pharmacological management and using injectable steroids. More sophisticated surgical techniques and better integration of surgeons into the early management have also improved the surgical management of the foot in rheumatology.
SPECIFIC DISEASES
Seropositive inflammatory arthritis
Rheumatoid arthritis
Definition
RA is a chronic, immune-mediated inflammatory disease with polyarthritis as its main feature. Chronic inflammation leads to joint damage and functional impairment (van Gestel et al 1996). Rheumatoid factors are detectable by serological testing in a large proportion of cases.
Epidemiology
The prevalence of RA in the population is approximately 7.7 per 1000 (0.8%), a risk that is doubled for relatives of confirmed cases (Hawker 1997). Approximately two-thirds of new cases arise in females, and mostly in the fourth and fifth decades of life, although there is wide variation and some suggestion that the age of onset is increasing. In one large UK cohort reported recently, the average age at onset was 55 years (Young et al 2000).
Diagnosis
Diagnosis is made according to diagnostic criteria defined by the American College of Rheumatology (Box 8.3). Key serological markers for the systemic inflammatory response are a raised erythrocyte sedimentation rate and C-reactive protein. Testing for rheumatoid factor aids in diagnosis and estimating the prognosis (American College of Rheumatology Subcommittee on Rheumatoid Arthritis 2002). Rheumatoid factor is present in 65–80% of patients with RA, and those who are rheumatoid factor positive are predisposed to more severe disease. Radiographic changes are often not present in early disease or on plain film. Radiography may not help with diagnosis, although it does provide a useful baseline for future assessment (American College of Rheumatology Subcommittee on Rheumatoid Arthritis 2002). Enhanced visualisation of inflamed synovium is possible with modern HRUS, and this modality may be helpful in early cases.
Box 8.3 American College of Rheumatology 1987 criteria for the diagnosis of rheumatoid arthritis
Four or more of the following criteria must be present:
There is an important role for primary care practitioners in aiding with the early recognition of as yet undiagnosed inflammatory arthritis (American College of Rheumatology Subcommittee on Rheumatoid Arthritis 2002). The foot is the first site of involvement in about 1 in 8 cases of RA, and some 20% of patients report foot pain before any other manifestation of the disease (O’Brien et al 1997).
A consensus-based referral recommendation for primary care practitioners (Emery et al 2002) states that inflammatory (rheumatoid) arthritis should be considered, and patients referred rapidly for a rheumatology opinion if they demonstrate:
Pathology
The underlying histopathology is of an immune-mediated synovitis caused by a faulty autoimmune response to proteins such as immunoglobulin G. The synovial membrane of the affected joint becomes hyperplastic, and infiltrated with inflammatory cells that promote secretion of proinflammatory cytokines (including TNFα) (Feldman & Maini 2008). The synovial membrane proliferates further, forming a pannus, which increasingly intrudes into the joint space (Choy & Panayi 2001). Cells in the pannus release enzymes that degrade cartilage and the connective tissue matrix (Choy & Panayi 2001). Erosion is more extensive around the margins of joints, because the articular cartilage affords some initial protection to the subchondral bone (Gold et al 1988).
Clinical course
The disability pathway for inflammatory arthritis and the specific factors affecting the clinical course in RA are summarised in Figure 8.1. Modifiers include endogenous factors that affect the underlying pathology, biopsychosocial factors and exogenous factors.
The presence of rheumatoid factor is a key marker for subsequent disease severity and is found in about 60% of new cases (American College of Rheumatology Subcommittee on Rheumatoid Arthritis 2002, Young et al 2000). Modern antibody testing, including for anti-CCP antibodies, has further improved diagnostic specificity and prognostic prediction (van Venrooij et al 2008). Other important prognostic factors include HAQ score, delay in instigating therapy, smoking, and the presence of certain immunogenetic markers (Sanmarti et al 2003, Young et al 2000).
There appears to be some shortening of the lifespan because of the disease course itself and also because of the risks associated with therapies such as oral steroids and DMARDs (Hawker 1997). RA is associated with decreased ability to undertake both paid work, and unpaid work such as domestic duties (Backman et al 2004), and everyday activities of work and leisure take longer to perform (March & Lapsley 2001). Work disability increases with disease duration. Approximately 20% of people with RA report significant work disability within a year of diagnosis, one-third by 2 years and up to 60% within 10 years of onset (Barrett et al 2000). A third of people with RA will leave the workforce permanently within 3 years of diagnosis (Barrett et al 2000).
The clinical course of the disease improved markedly with improvements in DMARD therapy in the 1980s and 1990s, and more recently with the advent of biological immunotherapy. Patients treated with conventional DMARDs will typically do moderately well, with some 13% going into long-term remission, and just under one-half following a moderated disease course with episodes of remission and relapse (Young et al 2000). Patients following this disease course will do better than those untreated or on NSAIDs only, but joint degeneration does accrue in the long term (>10 years) and disability remains a factor in established RA (Gordon et al 2001). Joint-space narrowing occurs early in RA, and is initially symmetrical and uniform, although it becomes less regular as the joint degenerates (Kumar & Madewell 1987). Some ongoing joint degeneration will occur in all but the mildest or best-controlled cases, and joint replacement remains a mainstay of management in end-stage disease. Currently, some 1 in 10 people will require at least one joint replacement within 5 years of diagnosis (Young et al 2000), although it might be expected that this number will fall as early DMARD therapy starts to improve prognosis.
Medical management
The outline of medical management in rheumatology described in the earlier section of this chapter is derived primarily from the principles of management of RA and needs no repetition here. There are clear guidelines provided by the American College of Rheumatology Subcommittee on Rheumatoid Arthritis (2002) and in the UK by the National Institute for Clinical Excellence (NICE 2009). An abridged schema adapted from the American College of Rheumatology guidelines for the medical management of RA can be seen in Figure 8.2.
It should be noted that while this schema represents an ideal clinical pathway, complete remission is still unusual (American College of Rheumatology Subcommittee on Rheumatoid Arthritis 2002), and some patients respond particularly poorly to specific DMARDs, even to new biological agents (Feldmann 2002). Where full remission is not achieved, the management goals become symptom relief and maximisation of quality of life (American College of Rheumatology Subcommittee on Rheumatoid Arthritis 2002). Medical treatment should thus be undertaken within a multidisciplinary setting, with other disciplines providing valuable input to the chronic disease process (NICE 2009).
The foot in rheumatoid arthritis
The prevalence and impact of foot problems is strongly related to disease duration (Michelson et al 1994) and the foot is eventually affected in nearly all people with RA, usually in a symmetrical pattern. Joint pain and stiffness is the most common initial presentation, but a range of other features may also be found, including tenosynovitis, nodule formation and tarsal tunnel syndrome, reflecting the widespread soft-tissue involvement. Foot-related impact is a consequence of a combination of factors, including pain, inflammation and mechanics (Turner et al 2008), with involvement of the feet both contributing to (van der Leeden et al 2008a) and representing a marker for impaired mobility and functional capacity in RA (Wickman et al 2004). In about three-quarters of people with RA, the foot contributes to difficulty with walking, and the foot is the main or only cause of walking impairment in one-quarter (Kerry et al 1994).
Some 20–25% of all surgical operations for RA relate to manifestations of the disease in the feet (Hamalainen & Raunio 1997). Despite this high demand, the success of surgical interventions is only moderate. Fewer than 50% of patients undergoing forefoot surgery for RA report ‘very good’ or ‘excellent’ outcomes. Indeed, 13% of patients undergoing foot surgery for inflammatory arthropathy require subsequent reoperation because of poor outcomes (Hamalainen & Raunio 1997).
Hindfoot
Retrocalcaneal bursitis is common in RA, and often coexists with inflammation of the Achilles tendon and long flexor and extensor tendon sheaths (Stiskal et al 1997). This in turn leads to the development of longitudinal tendon tears and structural degeneration, which in the long term can occasionally lead to complete rupture of the tendon, especially in high-load tendons such as the tendon of tibialis posterior (Bouysset et al 1995, Kumar & Madewell 1987). Tendinopathy is best imaged using HRUS or gadolinium-enhanced MRI (see earlier), which will show a greater frequency of involvement than the clinical examination (Bouysset et al 1995). Lesions were found in the posterior tibial tendons of 53/67 patients in one recent study (Bouysset et al 2003), but complete rupture of the tendon is uncommon, even in severely deformed feet (Jernberg et al 1999, Masterton et al 1995). Spurs can be found on the plantar surface of the calcaneus in about one-third of established RA cases, with a similar prevalence of posterior spurs at the insertion of the Achilles tendon, although posterior spurs tend to be smaller and are often asymptomatic (Bouysset et al 1989). The degree of soft-tissue involvement is largely dependent on the systemic disease activity, but local areas of severe inflammation may respond to infiltration of steroid into joints or soft tissues (McGuire 2003).
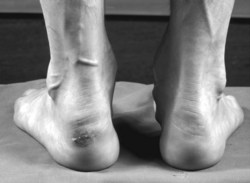
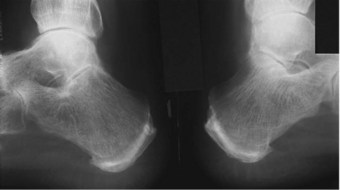
Ankle (talocrural) joint degeneration occurs relatively rarely in RA, and is confined to severe and late-stage disease (1 in 10 patients after 20 years). Ankle-joint change is almost always preceded by subtalar joint involvement (Belt et al 2001), and may therefore be a consequence of altered hindfoot alignment (Cimino & O’Malley 1999). Subtalar joint disease is suggested where there is pain on walking over uneven ground and swelling posterior to the medial malleolus or in the sinus tarsi (Bouysset et al 1995). The subtalar joint is affected in about a quarter of long-standing RA patients (Bouysset et al 1987) but rarely in early disease.
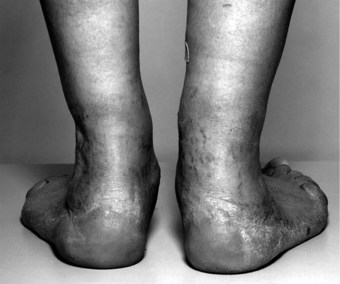
In the longer term, synovitis in the hindfoot joints leads to systematic changes in hindfoot structure and function (Woodburn et al 2002). The altered function, combined with the effect of load bearing and increased soft-tissue laxity, lead the hindfoot to function in a progressively more pronated position, with the heel becoming more inclined into valgus and the tibia more internally rotated (Bouysset et al 1987, Keenan et al 1991, Woodburn et al 2002b) (Figure 8.4).
Initially, the deformities are largely reducible, and the elastic response of the soft tissues allows for correction. Rigid functional orthoses have been shown to restore normal function at this early stage, and to slow the progression of fixed deformity (Woodburn et al 1999). If uncorrected, bony adaptation and disease-related changes in soft-tissue histology will lead to irreducible structural changes.
Varus deformities of the hindfoot occur in fewer than 1 in 30 people with RA (Kerry et al 1994, Vidigal et al 1975), and usually as a direct consequence of compensation strategies such as inversion of the forefoot to offload painful medial metatarsophalangeal joints (MTPJs).
It is important to note that health-related quality of life is better related to rearfoot pain than rearfoot deformity (Platto et al 1991), and so interventions should be provided on the basis of patient reports of impairment rather than reserved for those with the appearance of worse foot deformity.
In the valgus rheumatoid foot, synovitis and compression of tissues on the medial aspect of the hindfoot can lead to tarsal tunnel syndrome (Jernberg et al 1999). This manifests as a paraesthesia or as a burning sensation over the distribution of the tibial nerve on the plantar surface of the foot. Tarsal tunnel syndrome may respond to local injection of corticosteroid, but may require surgical decompression.
Surgical procedures most commonly performed on the rheumatoid hindfoot include tarsal tunnel decompression, and ankle, subtalar or talonavicular arthrodesis (McGuire 2003). There have been calls for surgical interventions to be considered earlier, as it is proposed that an early correction may improve local mechanics and minimise subsequent degeneration in neighbouring joints (Cimino & O’Malley 1999, Cracchiolo 1993). At present, however, most surgical interventions in the rheumatoid hindfoot are performed on feet in the late stages of disease.
Subtalar arthrodesis is often indicated following rupture of the tendon of tibialis posterior, where tendon repair is often not possible because of the degenerate nature of the tissue. In the absence of repair, the continued lack of medial stability leads to a poor prognosis unless the joint is fused surgically (Bouysset et al 1995). The salvage procedure for severe cases of hindfoot structural change is triple arthrodesis following corrective wedge osteotomies to restore better alignment (Cimino & O’Malley 1999). Ankle and hindfoot arthroplasty in RA is directed toward addressing the degenerative joint changes, and so these procedures are discussed more fully in the later section on OA.
In the presence of significant deformity, adaptive footwear might be necessary. The addition of external flanges to the heel, along with reinforcement of the heel counter and medial arch of the upper of the shoes can aid with hindfoot stability. Extra-depth shoes allow for an orthosis to be better accommodated.
Midfoot
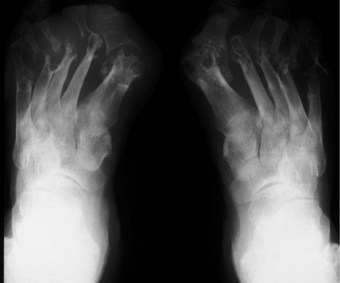
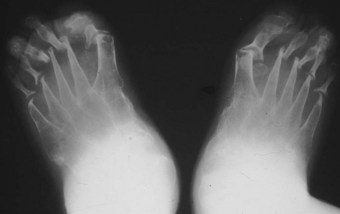
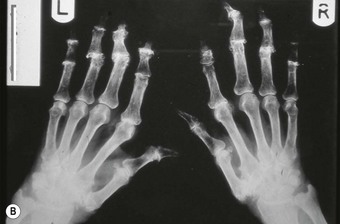
The joints of the midfoot are affected widely, with the talonavicular joint and Lisfranc’s joints affected in 15–30% of cases (Bouysset et al 1987). Diffuse joint degeneration may occur, although the characteristic erosions seen in the forefoot (Figure 8.5) are generally less common in the rheumatoid midfoot.
Forefoot
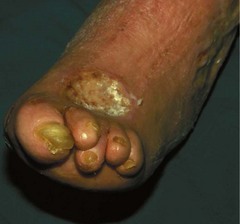
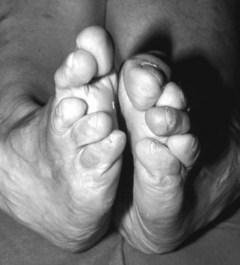
The MTPJs are the joints most commonly affected in RA (Weinfeld & Schon 1998), and MTPJ involvement often precedes that of any other joints, again with a symmetrical distribution (Pensec et al 2004, Priolo et al 1997). Early synovitis can lead to swelling of the digits (Kumar & Madewell 1987), and MTPJ synovitis, which is noted clinically as a warm boggy feeling to the joint on palpation, can lead to separation of neighbouring toes (the so-called ‘daylight sign’).
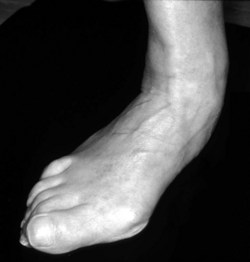
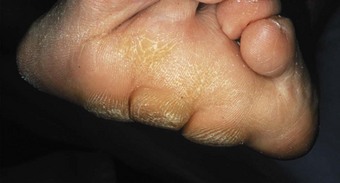
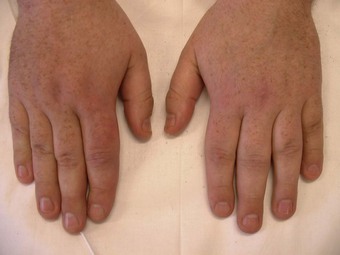
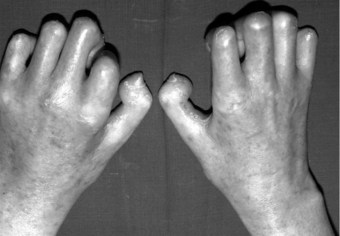
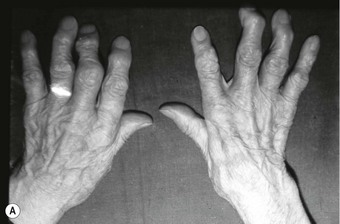
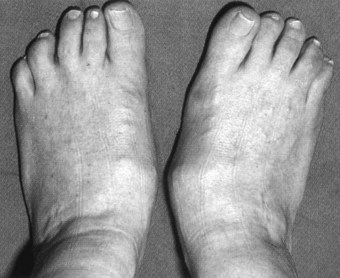
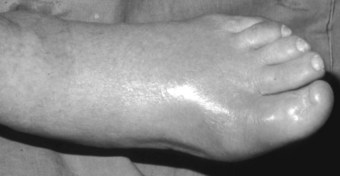
In the longer term, the acute phase will reduce (van der Leeden et al 2008b) and the rheumatoid forefoot will develop a typical marked hallux-valgus-type presentation and hammer toe deformities of the lesser toes (Figure 8.6). The hallux valgus deformity of RA is severe, with a large medial eminence, and it often results in first MTPJ subluxation (Weinfeld & Schon 1998). The second, third and fourth toes will typically exhibit lateral drift in addition to the hammer toe deformity, while the fifth toe will often be directed toward the midline, coming to lie over or under the fourth toe. It is common for the lesser digits to cease weight bearing entirely, and in combination with anterior displacement and atrophy of the plantar fat pads this leads to significantly increased pressure under the MTPJs (Turner & Helliwel 2003), which in turn is associated with greater impairment (Schmiegel et al 2008). Prominent adventitious bursae develop under the metatarsal heads, and these are frequently overlaid with callus (Figure 8.7). Bursitis at this site will be painful, and localised areas of skin ulceration are not uncommon (Figure 8.8). Plantar pressure data show the areas of increased pressure to be highly localised, and patients often describe a feeling of ‘walking on pebbles’.
The pain from the forefoot, and to a lesser extent the midfoot and hindfoot joints leads to characteristic changes in the gait of people with RA. Gait velocity decreases, as both the cadence and stride length are reduced. The double-support period extends to minimise the forces applied to the foot, and a reluctance to load the forefoot leads to delay in loading and a change in the velocity of centre-of-pressure profile (Hamilton et al 2001, Keenan et al 1991, O’Connell et al 1998, Turner & Helliwel 2003).
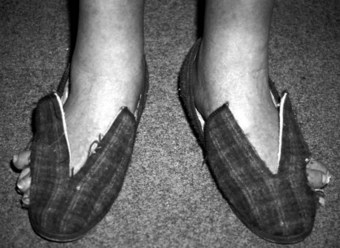
The severity of the changes that occur in the rheumatoid forefoot can often lead to a requirement for non-standard footwear (Figure 8.9). Traditionally, prescription footwear was bespoke (i.e. shoes were made to an individual cast or model) and were intended to account for specific individual features. Long-standing dissatisfaction with this approach, due to the high costs and unacceptable levels of patient (and practitioner) satisfaction (Williams et al 2007) has seen a change in approach in more recent years (Herold & Palmer 1992, Lord & Foulston 1989). One recent Dutch study indicated that about one-third of RA patients had been provided with orthopaedic footwear and, in an encouraging trend, 80% of this was in daily use (de Boer et al 2009).
Accommodative footwear can be provided off-the-shelf if the requirement is simply for extra depth or soft uppers in the toe box. The patient’s own shoes may be adapted in some cases, or bespoke shoes may still be made. Off-the-shelf shoes have been shown to provide significant improvements in pain, quality of life and gait parameters (Fransen & Edmonds 1997, Williams et al 2007), and are especially effective when combined with semi-rigid orthoses (Egan et al 2003). There is evidence for similar levels of satisfaction with bespoke and off-the-shelf shoes for people with RA (Kerry et al 1994).
Prescription footwear is usually provided in the NHS by orthotists working to a consultant prescription, but commercial alternatives provide other avenues that are increasingly available. Patient compliance is often problematic, and there is increasing evidence for benefits associated with better consideration of patient choice in the design and provision of bespoke footwear (Williams et al 2007).
Soft, flexible uppers made of soft leathers or stretchable textile can be very helpful in reducing the pressure on deformed digits (Figure 8.10), and in accommodating the increased width of the rheumatoid forefoot. The material characteristics and the presence of seams also warrant extra consideration where there is a risk of ulceration secondary to impaired arterial supply or vasculitis. The increased pressure found under the rheumatoid forefoot is aided by the addition of cushioning, and this can be provided separately or incorporated into prescription footwear (Egan et al 2003, Hodge et al 1999).
Rigid or semi-rigid, functional orthoses are known to be effective in limiting the rate of hindfoot and forefoot deformity, and in redistributing load away from the forefoot (Budiman-Mak et al 1995, Redmond et al 2004, Woodburn et al 2003), and contrary to past anecdotal opinion are well tolerated by people with RA (Woodburn et al 2002a). This approach, combined with forefoot cushioning, is effective at reducing pressure and pain in the painful forefoot (Chalmers et al 2000, Hodge et al 1999, Redmond et al 2004) and improving basic gait parameters (Kavlak et al 2003).
When joint pain is severe, the demand for painful motion in the forefoot joints during walking can be reduced by the provision of shoes with a rocker sole (Figure 8.11). A rocker sole is a straightforward adaptation little used in podiatry generally, but which can be quite effective in patients with RA.

Figure 8.11 A shoe with a rocker sole. Note: The patient had cut the heel counter of the shoe away herself to reduce pressure and discomfort.
Patients with RA affecting their back and/or hands may experience difficulties with shoe fastenings. Advice can be given when people are purchasing shoes to minimise this problem. Standard laces can be changed for elastic laces in existing shoes, removing the need for the wearer to bend and tie them. Alternative fastenings such as Velcro or elastic should be considered when choosing or prescribing orthopaedic footwear.
Many people with forefoot involvement require surgery and, because the forefoot is more affected by RA, forefoot procedures are generally performed before the hindfoot is operated on. However, if there is severe concomitant hindfoot involvement, stabilisation of the hindfoot does take precedence (Cracchiolo 1993).
The surgical approach to the forefoot joints is normally via dorsal incisions, as scarring on the weight-bearing plantar surface of the foot can lead to painful callus formation later. Plantar incisions are used when the skin quality on the dorsum of the foot might lead to problems with healing. Because of the severity of the joint degeneration in RA, excision arthroplasty is a part of most procedures, and the metatarsal heads, the base of the phalanges, or both are usually excised. Different procedures are used for the first MTPJ and lesser MTPJs. First MTPJ procedures, such as Keller’s excision arthroplasty, joint arthrodesis and replacement arthrodeses, are described elsewhere and will not be detailed here. For a severe lesser toe deformity, such as seen in RA, there are many procedures available, with those described by authors such as Clayton, Kates and Fowler forming the mainstay of rheumatoid foot surgery through the latter half of the 20th century. All lesser MTPJs are usually operated on simultaneously, because more conservative approaches involving surgery on only one of the most affected joints tend to have poorer long-term results in people with RA (Hughes et al 1991). Metatarsal head resection provides significant reduction in pain and forefoot pressure (Bitzan et al 1997, Rosenberg et al 2000), although the postoperative function cannot be considered normal and results tend to worsen in the longer term (Toolan & Hansen 1998). Modern variations, including techniques involving interposition of the plantar plate and preservation of the metatarsal head, are gradually supplanting the more traditional approaches (Cracchiolo 1993).
Subcutaneous lesions
Subcutaneous rheumatoid nodules are thought to arise in about one-quarter of patients with RA, usually those who are rheumatoid-factor positive and who have more severe disease (Gilkes 1987). The fibrotic nodules are associated with subcutaneous vasculitis and subsequent fibrosis, and arise at points of high stress, such as the extensor surfaces of elbows or knees. One study detected histological signs of rheumatoid necrosis (nodules) in the forefeet of 65% of a sample of people with RA, although most were subclinical (Berger et al 2004). Clinically evident nodules are not common in the feet, but affected sites include the posterior aspect of the heel and the dorsum of the forefoot. Rarely, nodules may break down or cause ulceration of overlying skin. If rheumatoid nodules are causing problems with shoe fitting or weight bearing, they may require surgical excision.
Skin and nails
Patients with RA of long disease duration will develop significant skin atrophy, with the skin taking on a pale, translucent quality (Gilkes 1987). The nails may be thickened and ridged, but are not usually symptomatic. The involvement of the hand, and difficulties with bending over for people with RA, mean that many patients need help with general foot health tasks such as nail cutting and maintenance of foot hygiene.
Vasculitis
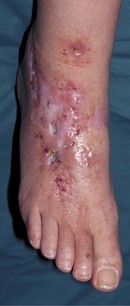
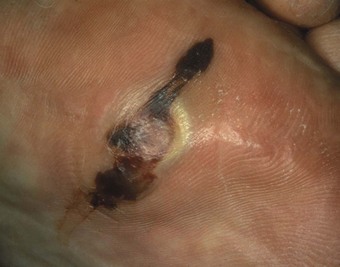
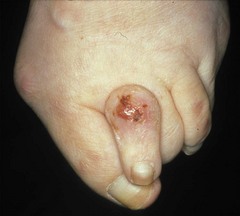
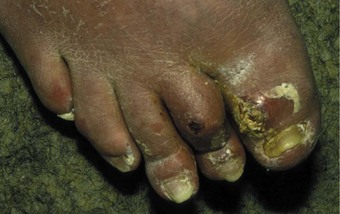
Although better disease control appears to be reducing the prevalence of RA-related vasculitis (Turesson & Matteson 2009), it is not uncommon for vasculitis and ulceration to affect the rheumatoid foot, especially in patients who are rheumatoid-factor positive or have other signs of severe disease such as rheumatoid nodules (Cawley 1987). Usually, the pedal pulses are unaffected and the vasculitis is localised, affecting only part of the foot. The most common vasculitis in RA is endarteritis obliterans, in which inflammation of the intimal layer of the small blood vessels leads to occlusion of blood flow (Cawley 1987). The clinical features of endarteritis obliterans are infarcts in the nail beds and/or toe pulps. These are initially red and uncomfortable, but often turn black and painless within a few days, although in some cases the infarct progresses to form an ulcer. A second form, necrotising vasculitis, is caused by vessel-wall destruction (Genta et al 2006). If necrotising vasculitis affects the venules, it leads to local exsanguinations under the skin (palpable purpura). Necrotising vasculitis affecting arterioles leads to larger areas of ulceration, usually on the dorsum of the foot and toes (Cawley 1987) (Figure 8.12). Vasculitis can affect the vasa nervorum, leading to peripheral sensory neuropathy in some people with RA.
The foot is at high risk of ulceration in people with RA (Genta et al 2006). The risk is elevated because of the tendency to vasculitis and the thinning of the skin, and also because of the deformity accompanying the disease, which leads to the development of areas of high pressure, both on the dorsal and on the plantar aspects of the foot (Firth et al 2008). These risk factors are also present in addition to the usual cardiovascular risk factors present in an older population. In the Leeds rheumatology foot health clinic some 15% of the patients have vascular/high-risk presentations, and they account for over a quarter of appointment slots.
The site of the ulcer will give clues as to the important underlying pathology; breakdown over obvious pressure sites, such as the dorsum of deformed interphalangeal joints, might be expected to respond well to offloading of pressure. Breakdowns on the dorsum of the foot and lower leg are more likely to be of predominantly vasculitic origin (Cawley 1987) and need multidisciplinary management, including review of antirheumatic medication and possible supplementary therapy with agents such as Iloprost (Kay & Nancarrow 1984). The vasculitis associated with RA is also reported to respond well to anti-TNF therapy (Olsen & Stein 2004).
The increased susceptibility of many rheumatology patients to infection has been discussed previously, and must be considered in the care of this group.
Juvenile idiopathic arthritis
Definition
Juvenile idiopathic arthritis is a heterogeneous group of inflammatory arthropathies indicated by the presence of an inflammatory arthropathy, with an onset at less than 16 years of age, affecting one or more joints for longer than 6 weeks.
Epidemiology
Around 12 000 children in the UK have juvenile idiopathic arthritis, and girls are almost three times more likely to be affected than boys (Arthritis Research Campaign 2002).
Classification
Juvenile idiopathic arthritis has undergone a number of different classifications in the last 20 years. Each new classification is based on emerging knowledge of the taxonomy, natural history and immunopathology of the condition. The latest classification was decided upon in Durban in 2000 and suggests seven classes for children with juvenile idiopathic arthritis: systemic arthritis, oligoarthritis, polyarthritis (two classes depending on rheumatoid factor status), psoriatic arthritis, enthesitis-related arthritis and ‘undifferentiated’ (Petty et al 1991). Better information on treatment and prognosis will be forthcoming as further studies of these groups are published.
Diagnosis
The diagnosis of juvenile idiopathic arthritis is based on the exclusion of other provisional diagnoses, such as infection and trauma. The history is usually of a chronic idiopathic synovitis, with onset in a single joint, typically the knee, ankle, hip or wrist (oligoarticular onset). Blood tests are less helpful in diagnosis than in adult-onset arthritis, but can help the clinician to decide to which subgroup the patient belongs.
Pathology
The pathophysiology is specific to the subgroup, but is essentially that of a chronic immune-mediated inflammatory synovitis. The enthesis, however, is the primary site of inflammation in enthesitis-related arthritis and psoriatic arthritis (see the section on adult seronegative arthropathy later in this chapter). The inflammatory pathways are similar in children and adults, and so the driving cytokine in many of the groups is TNFα.
Clinical course
Onset is often before 4 years of age, and the clinical course is variable and dependent on the clinical subgroup. In a good proportion of cases of oligoarticular disease, the disease may remit spontaneously with no long-term joint damage. In the majority of cases of polyarticular arthritis, the disease will continue for many years, sometimes without remission throughout the lifespan. Many cases positive for rheumatoid factor go on to a clinical course similar to RA.
Medical management
The general principle is of support rather than cure, and management is aimed at limiting symptoms, maintaining function and minimising permanent changes. NSAIDs will be the initial intervention. Intra-articular steroids are often used in severe monoarticular or oligoarticular forms. Disease-modifying drugs are used in cases with a poor prognosis, which will include those in the polyarticular group, the juvenile RA group and the systemic onset group.
The principal drugs used are methotrexate, corticosteroids and biological drugs such as anti-TNFα (Olsen & Stein 2004).
The local management of the foot and legs in these children will complement these therapies, as in the adult. It is, however, important to note the differences between children and adults with arthritis. Children are naturally stoic and will get on with their life as long as the symptoms are tolerable. Consequently, they are also more prone to loading inflamed joints and have little sense of joint protection.
The foot in juvenile idiopathic arthritis
Polyarticular type
The foot, especially the hindfoot and ankle, is involved in some two-thirds of polyarticular cases (Hendry et al 2008). Involvement of centres of ossification can lead to alterations in the shape of the growing bones and may cause deformity and brachydactyly (Moll 1987). Pes planus or pes cavus deformities may develop, as can the hallux-valgus-type picture seen in adult RA (Chen 1996).
Enthesitis-related arthritis and psoriatic arthritis
As noted previously, this presentation mirrors that of the adult seronegative arthropathies. The joints most affected in the foot are the hindfoot and interphalangeal joints, and entheseal inflammation at the insertion of the Achilles tendon and plantar fascia is common. Spinal involvement is common as the disease progresses. Inflammation in the mid- and hindfoot joints may lead to subsequent ankylosis, a not infrequent end point in this subgroup.
Particular attention should be given to the feet in juvenile idiopathic arthritis, as deformity will occur quickly. Better therapies have improved prognosis generally, but foot involvement remains common (Hendry et al 2008). Children are usually very accepting of in-shoe orthoses, but these must be reviewed annually, and more frequently during growth spurts. Joint inflammation may cause overgrowth or undergrowth of the adjacent long bones, so a careful watch for limb-length discrepancy should be maintained.
Seronegative inflammatory arthritis:
Introduction – common features
The seronegative arthritides are again a diverse group of conditions with a number of common features. There is a known association with the human leucocyte antigen (HLA) B27, and some 90% of patients with seronegative arthropathy of various types test positive for this antigen. Clinically, there is frequent involvement of the spine (axial involvement) in the seronegative arthritides, and many will first present as back pain before differentiating later. The inflammatory process in the seronegative arthropathies is also associated with enthesopathy – inflammation at the insertion of ligaments or tendons (Olivieri et al 1998). The most common sites for enthesopathy in the foot are the posterior calcaneus at the insertion of the Achilles tendon, the plantar calcaneus (the plantar fascia and the short flexor muscles), the base of the fifth metatarsal and the forefoot (McGonagle et al 2002, Olivieri et al 1998). There may be localised swelling at superficial insertions such as that of the Achilles tendon (Figure 8.13), and swellings can be especially pronounced when the enthesopathy extends to involve local bursae. In the fingers and toes the enthesitis and synovitis can present with marked dactylitis or ‘sausage digits’ (Figure 8.14). More widespread inflammation can lead to noticeable general swelling in the foot.
On radiography there may be periarticular proliferation of periosteum and bone, usually whiskery or fluffy in character, which contrasts with the bony erosions seen in RA. New bone formation may also be seen at the entheses as a consequence of the inflammatory process. It is uncommon for the foot in the seronegative arthropathies to be associated with vasculitis (Cawley 1987).
Ankylosing spondylitis (inflammatory back pain)
Definition
Ankylosing spondylitis is a progressive seronegative spondyloarthropathy that affects the axial skeleton (the spine and surrounding structures) predominantly, but which also has peripheral features that can involve the foot.
Epidemiology
The incidence of AS is approximately 1%, with men affected by AS three times as frequently as women (Braun & Sieper 2007). The ankle is affected in one-quarter of people with AS, and the forefoot in about 10% (Moll 1987). The inflammatory spondyloarthropathies cause 1.4 million working days to be lost each year, costing the UK economy some £122 million.
Diagnosis
Diagnosis is based on the modified New York criteria of the presence of one of:
However, these criteria have been criticised for having limited sensitivity (van der Heijde 2004).
Pathology
The pathology is not well understood overall, but appears to be driven by enthesitis. The sacroiliac joints are the worst affected, and initial inflammation can give way to ossification of the joints. TNF appears important in the pathological process, and overexpression of TNF precipitates an ankylosing-spondylitis-type presentation in transgenic mice. Preliminary findings from treatment studies suggest that TNF blockade appears even more beneficial in people with ankylosing spondylitis than in those with RA (Marzo-Ortega et al 2001).
Clinical course
Ankylosing spondylitis tends to manifest itself initially as low back or buttock pain because of sacroiliitis. Peripheral symptoms (including those in the foot) occur early in the disease but become less problematic over time, leaving the spondyloarthropathy as the main feature of long-standing disease. Spinal curvature increases over time as the long-standing inflammatory process leads to ankylosis and bone formation. The clinical course is generally less severe than in RA, and some cases will remit spontaneously. One notable feature of ankylosing spondylitis is the presence of fatigue as a significant factor for patients (van der Heijde 2004).
Medical management
NSAIDs are the mainstay of medical management in ankylosing spondylitis, although DMARDs, such as sulfasalazine, are also beneficial for the peripheral arthritis (DeJesus & Tsuchiya 1999, Olivieri et al 1998). Anti-TNF drugs are now established as major symptom-modifying drugs in ankylosing spondylitis. In one study of patients who had already failed to respond to conventional DMARD therapy, TNFα blockade caused complete resolution of 86% of entheseal lesions and led to substantial improvement in pain and quality of life (Marzo-Ortega et al 2001). Even supposedly end-stage ‘fixed’ spinal kyphoses can show some restoration of function once the inflammatory process has been suppressed. The Assessment in Ankylosing Spondylitis (ASAS) group have defined response criteria similar to those used for RA, which have allowed for better evaluation of therapies (Lukas et al 2009).
The foot in ankylosing spondylitis
About 20% of patients with ankylosing spondylitis will have enthesitis in the feet, although any involvement of the foot in ankylosing spondylitis is usually transient and tends not to progress to severe degenerative joint disease (Kumar & Madewell 1987, Olivieri et al 1998). Involvement of the enthesis of the plantar fascia is accompanied by soft-tissue oedema in most cases and also by bone oedema in many (McGonagle et al 2002). Retrocalcaneal bursitis occurs fairly frequently in ankylosing spondylitis (Moll 1987).
If the foot is involved more extensively, the presentation has much in common with the other seronegative arthropathies, and erosions, periosteal proliferation and, characteristically, joint ankylosis are all seen. Affected joints will be swollen and tender, and radiographic investigation reveals that initial periarticular erosion is followed by bony proliferation and bone spur formation (Kumar & Madewell 1987).
The management of ankylosing spondylitis in the foot centres on systemic disease control supplemented by local measures. Where isolated sites in the foot are symptomatic, local injection of corticosteroid may reduce inflammation, and padding or orthoses may provide mechanical relief. Achilles tendon insertional enthesitis in seronegative spondyloarthropathy can, unfortunately, be intractable, although there is promise of effective therapy with anti-TNF drugs (Olivieri et al 2007).
Psoriatic arthritis
Definition
The diagnosis of psoriatic arthritis is usually used to refer to an inflammatory arthropathy occurring in the presence of psoriasis and in the absence of rheumatoid factor. In practice, it is not always possible to be so precise about the definition, as the joint involvement may be variable, and in some cases may even pre-date the development of skin lesions. Spinal involvement is characteristic, but peripheral joint involvement may range from the widespread and severe arthritis seen in the form arthritis mutilans (Figure 8.15), to a relatively mild monoarthritis. One form of psoriatic arthritis follows a disease course almost indistinguishable from that of RA.
Epidemiology
Psoriasis of the skin or nails affects 2–3% of the population, with approximately 12% of dermatological cases developing arthritis. There is no association between joint involvement and type of psoriatic presentation in the skin. Psoriatic arthritis is the second most common inflammatory arthropathy after RA (Veale & FitzGerald 2002).
Diagnosis
There are no widely agreed diagnostic criteria for psoriatic arthritis, and so diagnosis is based on clinical features and the often difficult exclusion of other inflammatory arthritides such as RA and ankylosing spondylitis (Brockbank & Gladman 2002). Serology is not as useful as in RA, because the association between inflammatory markers and disease activity is less clear (Brockbank & Gladman 2002). Recently, new classification criteria for psoriatic arthritis have been developed by a large international group, and it appears that these criteria may also function well as diagnostic criteria (Taylor et al 2006).
Pathology
The immunogenetics of skin psoriasis and psoriatic arthritis are complex, and at present seem only marginally interrelated, although the cellular features of inflammatory cell infiltration and membrane hyperplasia show some similarities (Veale & FitzGerald 2002). The precise pathological pathway leading to psoriatic arthritis is not well understood, although it is known that the process relates to enthesitis as well as the synovitis seen in RA. TNFα is expressed at higher levels than normal, but the relative proportions of other cytokines in psoriatic synovium are different to those seen in RA (Veale & FitzGerald 2002).
Clinical course
The textbook presentation is of a unilateral dactylitis (a ‘sausage digit’), although in reality the initial presentations are diverse. Early axial involvement is found in 40% of people with psoriatic arthritis. Case reports have suggested a temporal association between the onset of psoriatic arthritis and joint trauma in some cases, mimicking the Köebner phenomenon seen in psoriatic skin (Veale & FitzGerald 2002). Distal interphalangeal joint involvement is characteristic, contrasting with the proximal interphalangeal joint presentation seen in RA (Brockbank & Gladman 2002). Metacarpal or metatarsal joints can be affected, and again it is most common for the early presentation to be asymmetrical (Gold et al 1988).
The presence of arthritis in four or more joints is a significant predictor of a worse prognosis (Brockbank & Gladman 2002).
If the disease follows the arthritis mutilans course, the digital deformities tend to occur in haphazard directions, contrasting with the classic lateral drift seen in RA (Gold et al 1988). Ankylosis of affected joints is common and results in rigid deformity. Alternatively, where erosions predominate, flail digits may develop (Brockbank & Gladman 2002).
Medical management
Medical management of psoriatic arthritis is often similar to that of RA, with methotrexate and other DMARDs proving effective in combination with NSAIDs and exercise (Brockbank & Gladman 2002, DeJesus & Tsuchiya 1999). Psoriatic arthritis has proven highly responsive to anti-TNFα therapy in recent trials (Olsen & Stein 2004), and etanercept and infliximab suppress both the joint disease and the skin presentations (Leonardi et al 2003). Local corticosteroid injection into affected joints or tendon sheaths may be helpful (Brockbank & Gladman 2002). Treatment recommendations have recently been published by the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis, which provides guidance on effective treatments for the different disease manifestations (Ritchlin et al 2009).
The foot in psoriatic arthritis
The foot may show the changes typical of psoriatic skin disease, with erythematous patches on the extensor surfaces, or plantar hyperkeratosis or pustule formation on the plantar surfaces. Pitting, discoloration, hyperkeratosis and lysis of the nails may occur, and splinter haemorrhages may be found around the apices of the toes.
Psoriatic arthritis will often present initially in the interphalangeal and then the metatarsophalangeal joints of the feet or hands (Gold et al 1988), although other joints may be affected, sometimes in a single ray pattern (Brockbank & Gladman 2002, Kumar & Madewell 1987).
Inflammation at the entheses results in bony erosions, and on radiographic investigation non-marginal erosions are evident early, with subchondral bone involved soon afterwards (Kumar & Madewell 1987). Reactive new bone formation around affected joints is characteristic, leading to a fuzzy appearance on the radiograph. Endosteal sclerosis will occasionally lead to a substantial increase in density of the shaft, the so-called ‘ivory phalanx’. The highly erosive pathology leads to a whittling of the joint margins and an unusual ‘pencil in cup’ appearance in advanced cases, where the distal end of the affected phalanx is cupped within an excessively concave surface at the proximal end of the more distal phalanx (Gold et al 1988, Kumar & Madewell 1987). Conversely, bony ankylosis can occur instead. Resorption of the distal phalanx occurs in about 5% of patients, resulting in a characteristic, pointed toe pulp (Gold et al 1988, Moll 1987).
Hindfoot involvement mimics that of other seronegative arthropathies. Clinical involvement of the Achilles tendon and plantar fasciitis occurs in about one-third of people with psoriatic arthritis, although pathological signs are observable on ultrasonography in more than three-quarters of patients (Galluzzo et al 2000, Olivieri et al 1998). Bony proliferation of the entheses and exuberant spur formation may be seen on radiography (Gold et al 1988, Kumar & Madewell 1987) (Figure 8.16).
Reactive arthritis
Definition
Reactive arthritis can occur in conjunction with urethritis and conjunctivitis (and other features). Reactive arthritis is an acute inflammatory arthritis arising after a local or systemic infection, typically sexually acquired or postdysenteric. The disease usually affects younger males and is associated with the HLA B27 antigen in more than 90% of cases. The initial presentation is usually in the knee or foot, but spondyloarthropathy is a universal feature as the disease progresses. The disease course is of flares and remissions, which will self-limit in time. A small proportion of cases will persist, but the disease tends to be milder than other inflammatory arthropathies (Kumar & Madewell 1987).
Diagnosis
Reactive arthritis should be suspected where there is an inflammatory arthritis presenting predominantly in the lower limb, especially where the arthritis is accompanied by bony proliferation (Jacobson et al 2008). Multiple joints may be affected, but usually asymmetrically (Moll 1987). The most commonly affected regions in the foot are the metatarsophalangeal joints, the Achilles tendon and its insertion, and the origin of the plantar fascia (Moll 1987).
Keratoderma blenorrhagica can occur in conjunction with the arthritis (Figure 8.17), and it can be difficult to differentiate the skin lesions seen in reactive arthritis from those of psoriasis, leading to confusion over the diagnosis (Kumar & Madewell 1987, Moll 1987).
Medical management
NSAIDs are the first-line treatment, reducing pain and subduing the inflammatory process to some degree (DeJesus & Tsuchiya 1999). Often a course of DMARDs is all that is necessary. DMARDs (typically sulfasalazine and methotrexate) are used for persistent cases. The use of anti-TNF has also been proposed as an approach in reactive arthritis, because early suppression of this relatively short-lived arthritis may result in preservation of maximum joint integrity once the inflammatory stage has passed.
The foot in reactive arthritis
The involvement in the foot largely mirrors that of other seronegative arthropathies, focusing on the hindfoot and, to a lesser extent, the forefoot, but largely sparing the midfoot (Kumar & Madewell 1987). In the hindfoot, the radiological findings of reactive arthritis are similar to those of psoriatic arthritis, with whiskery spurs resulting from enthesopathy at the insertion of the Achilles tendon and plantar fascia. Nodular thickenings may also be found in the tendons (Gerster et al 1977). There is greater differentiation from psoriatic arthritis in the forefoot, however, and reactive arthritis shows less predilection for the lesser interphalangeal joints, with involvement usually confined to the interphalangeal joint of the hallux (Gold et al 1988, Kumar & Madewell 1987). Local corticosteroid injection has been reported to provide symptom relief (Gerster et al 1977).
Connective tissue diseases – scleroderma and lupus
The connective tissue diseases are a mixed group of conditions sharing some clinical features but with differing pathophysiologies. The two most common discrete forms are scleroderma and systemic lupus erythematosus (commonly known as lupus), although there are forms with less clear differentiation (the so-called mixed connective tissue diseases). Scleroderma and lupus are dealt with as separate entities in this chapter for simplicity, but the interested reader is directed to a dedicated rheumatology text for more detail on this complex group of conditions.
Scleroderma
Definition
Scleroderma comes in several forms, including progressive systemic sclerosis, and localised or limited sclerosis. It is an immune-mediated disorder characterised by inflammatory and fibrotic soft-tissue changes. In the systemic form, vascular lesions are also characteristic, affecting the skin and internal structures such as the gastrointestinal system, heart, lungs and kidneys.
Epidemiology
There are around 1500 diagnosed cases of scleroderma in the UK, with a male/female gender split of 1:4 (Arthritis Research Campaign 2002).
Diagnosis
There are long-standing American College of Rheumatology criteria for the classification of systemic sclerosis, which have been updated recently but not well validated (Walker et al 2007). Antinuclear antibodies are usually present in the blood, and extractable nuclear antibodies to SCL-70, centromere and RNP may be found, as may rheumatoid factor. The precise pathology is unknown, although it is well accepted that the underlying aetiology is immune mediated.
Clinical course
The initial presentation of systemic sclerosis may simply be with Raynaud’s phenomenon accompanied by oedema of the hands or feet. The skin in the extremities thickens and tightens, leading to the typical presentation of sclerodactyly. The affected fingers and toes develop flexion contractures that cause limited mobility (Figure 8.18). Fibrosis and resorption of the soft tissue and underlying bony substance of the digital apices can lead to a classical atrophic appearance to the ends of the digits. Changes in the face lead to a mask-like appearance, with puckering of the skin around the mouth. Altered pigmentation is common.
One variant of scleroderma is the CREST syndrome, named after the features Calcinosis, Raynaud’s phenomenon, (o)Esophagitis, Sclerodactyly, and Telangectiasis. In CREST the scleroderma is usually limited to the hands and forearms. The calcinosis may manifest as small subcutaneous nodules on the hands and feet – these resemble gouty tophi clinically but clearly show themselves as containing calcium on plain radiographs. CREST is not a benign condition, having a relatively high prevalence of lung and heart involvement. Indeed, in systemic scleroderma, pulmonary, cardiac and renal involvement leads to shortening of the lifespan (Gold et al 1988), although better modern medical management has improved the prognosis considerably.
Synovitis may be present, but tends to affect large joints rather than the feet (Gold et al 1988). Calcification of peripheral soft tissues may occur in long-standing cases, and calcinosis of the skin may be seen.
Medical management
Much of the literature relating to scleroderma focuses on the management of the vascular complications, such as Raynaud’s phenomenon, and the effects on internal organs. There is little high-quality evidence for most of the current approaches to medical management. The underlying pathology remains poorly understood, and while DMARDs have been shown to be effective in reducing the skin thickening (Lin et al 2003), their effectiveness overall is unclear. Nevertheless, improved medical management of the renal and pulmonary aspects of the disease has led to increased life expectancy in recent years.
The foot in scleroderma
The foot will be involved during the course of the disease in about 90% of patients with systemic sclerosis, but involvement is less common and less severe than in the hands (La Montagna et al 2002). Disorders of the skin and vascular system are the most common (see box 8.4), but the skeletal structures of the foot are involved in three-quarters of patients with progressive systemic sclerosis. Musculoskeletal pain occurs in about one-third of sufferers, even in the absence of arthritis. Radiographic foot involvement is associated with a generally poorer prognosis (La Montagna et al 2002, Ueda et al 1991).
Localised calcinosis in the skin of people with scleroderma has a seed-like appearance similar to that of a seed corn (heloma milliare). No attempt should be made to debride or enucleate areas of calcinosis, as they are extremely painful and there is significant risk of infection and ulceration.
Raynaud’s syndrome is almost universal in the hands, and occurs in the feet of about 90% of sufferers, the incidence increasing with longer disease duration (La Montagna et al 2002). Localised vasculitic lesions and ulceration, typically of the dorsum of the forefoot and the apices of the toes, are also seen in these patients, with a prevalence of 30% and 20–25%, respectively. There is growing evidence for large-vessel involvement in some cases also (La Montagna et al 2002, Sari-Kouzel et al 2001). Subcutaneous calcinosis can be problematic later in the disease, although it occurs in fewer than 20% of feet and less frequently than in the hands. Ulcerative skin lesions are often very painful in patients with connective tissue disease, contrasting with the neuropathic ulceration seen in diabetes. The healing process is typically protracted because of the vasculitis, and when healing does occur it is often through necrosis and fibrosis rather than the granulation and re-epithelialisation process that occurs during the resolution of many other forms of ulceration.
The combination of skin and subcutaneous fibrosis, along with the changes in the underlying skeletal structures lead to difficulties with shoe fitting (Sari-Kouzel et al 2001), and these patients may require assistance with sourcing adequate footwear. It is desirable that all patients with systemic sclerosis undergo regular checks of their foot health and have ready access to foot health services where follow-up is needed.
Lupus
Definition
Systemic lupus erythematosus (SLE) is an immune-mediated connective tissue disease affecting many organ systems and with a variable course and prognosis. In addition to the musculoskeletal effects, renal and cardiopulmonary disease are common features (Maddison 2002). Discoid lupus is a localised form affecting only the skin.
Epidemiology
Estimates of the prevalence of SLE range from 15 per 100 000 to 68 per 100 000 (Bongu et al 2002). SLE is between two and eight times more common in women than in men (McAlindon 2000), and there is a marked variation according to ethnicity (McAlindon 2000) and age (Bongu et al 2002). People of South-Asian or Afro-Caribbean origin are prone to more severe presentations. About two-thirds of patients with SLE will have non-erosive arthritic manifestations, often in the ankle or metatarsophalangeal joints (Gilkes 1987).
Diagnosis
Classically depicted by the characteristic malar ‘butterfly’ rash on the cheeks, the diagnosis of SLE is made on the basis of the 1982 American Rheumatology Association criteria, an 11-item set of criteria including physical characteristics and laboratory investigations such as HLA typing, detection of antinuclear antibodies (McAlindon 2000, Maddison 2002) and antibodies to double-stranded DNA (Maddison 2002). Where the diagnosis is unclear, patients are considered to have ‘undifferentiated connective tissue disease’ (McAlindon 2000). It is common for patients to remain undifferentiated for years before the progress of the disease leads to a clinical picture meeting the criteria for frank SLE.
Pathology
In people with an innate susceptibility, an external trigger is thought to be required to precipitate cases. Infection is a widely noted precipitating factor, with seropositivity for the Epstein-Barr virus associated with a 50-fold increase in risk of developing lupus. Lupus is also thought to be precipitated by exogenous factors in some cases, and a variety of environmental agents such as drugs (antihypertensives, antiarrhythmics) and household chemicals, such as those found in cosmetics and domestic spray cans, have been implicated (McAlindon 2000).
Clinical course
The early clinical course is fairly predictable across the range of lupus-type disorders, with the disease tending to differentiate into one of the more specific forms later (Maddison 2002). The systemic effects result in shortening of the lifespan, and survival rates after 20 years of disease are only 70%, mainly as a consequence of renal disease or increased susceptibility to infection (Bongu et al 2002). There also is increasing evidence of accelerated arthrosclerosis in patients with lupus (Theodoridou et al 2003).
Most patients with SLE will have some musculoskeletal symptoms, and about one-third will develop a non-erosive but rheumatoid-like arthritis (Bongu et al 2002, Reilly et al 1990).
Medical management
There is good recent evidence that early and aggressive treatment improves outcomes for people with lupus (Rao & Hootman 2004). NSAID therapy is used sparingly because of an increased risk of renal dysfunction (Solsky & Wallace 2002). Antimalarial drugs are effective for mild SLE, but the mainstays of therapy are steroids and immunosuppressive drugs.
The foot in lupus
Vascular signs occur in 90% of people with SLE, Raynaud’s disease, with telangectasia and purpura affecting the feet widely. Vasculitis and ulceration are relatively common in this group of patients, affecting between 10% and 20%, often in the lower extremity (Cawley 1987). In addition to the small-vessel vasculitis seen in RA, people with SLE can also develop large-vessel vasculitis (Gladstein et al 1979) and accelerated atherosclerosis (Theodoridou et al 2003), leading to frank gangrene and risk of amputation. Ankle–brachial pressure indices are abnormal in many SLE sufferers and should be used to detect large-vessel disease early in this group (Theodoridou et al 2003).
Patients with SLE may have RA-type presentations in the feet (Mizutani & Quismorio 1984), although there is less joint destruction and erosions are not seen. Tendinopathy can be seen, especially in the Achilles tendon (Bongu et al 2002).
Patients can present with onycholysis and pitting of the nails, similar to that seen in psoriasis. Splinter haemorrhages may also be seen in the nail beds, reflecting the vascular involvement. Skin lesions are common, with discoid or annular erythematous lesions occurring on the legs and feet exacerbated by exposure to sunlight (Callen 2002).
Osteoarthritis
Definition
Osteoarthritis (OA) is characterised by progressive loss of articular cartilage, and remodelling of and change in the structure of subchondral bone.
Epidemiology
OA is the most common form of arthritis and affects between 12% and 35% of the population (Felson et al 2000). The figures vary because of differences in definition, and because of sampling effects as the prevalence rises with age. Osteoarthritic changes based on radiological definitions are very common and lead to higher estimates of prevalence, while prevalence data based on clinical presentations (symptoms/pain) yield lower prevalence rates. The prevalence of OA is known to be higher in women (Arthritis Research Campaign 2002, Hawker 1997) and the most common sites are the hands (1%), hips (0.9%), knees (2.4%) and feet (Hawker 1997). Joint pain is common in the feet of older adults but, importantly, presents most commonly in combination with pain in other joints such as the knees, back, hands and hips (Keenan et al 2006). Subclinical signs are often reported at higher incidence, especially in older patients, with one study of 100 cadaveric lower limbs reporting moderate or severe degeneration in 24% of older hips, 66% of knees and 47% of first metatarsophalangeal joints (Muehleman et al 1997). Apart from the first metatarsophalangeal joint, the joints of the foot and ankle appear fairly resistant to primary OA, and so most cases are secondary to earlier trauma (Thomas & Daniels 2003).
OA leads to the loss of 36 million working days a year, costing the UK economy over £3 billion.
Diagnosis
Diagnostic criteria have been proposed by the American College of Rheumatology and others, although the clinical diagnostic criteria are usually joint specific and therefore too numerous to be listed here. Menz et al (2007) have developed a useful classification system for foot joint OA, based on an atlas of plain film radiographic images. The clinical signs are general stiffness and an aching pain in the affected joint, and there may be tenderness at the joint margins on palpation (Demetriades et al 1998). Crepitus may be felt on passive movement of the body part.
Confirmation of the clinical signs requires radiographic investigation. Radiographic signs featured in the Menz system include narrowing of the joint space on a plain radiograph, bony proliferation around joint margins (osteophyte formation), and sclerosis and cyst formation in the subchondral bone. Periarticular erosions are not seen in OA.
Pathology
OA can be primary (i.e. occurring spontaneously) or may be secondary to trauma or other insult to the joint. OA is no longer considered a simple ‘wear and tear’ arthritis, as it is clear that the process involves elements of repair as well as inflammation and degeneration (Felson et al 2000). Subtle differences in the presentation and local pathology at different sites have led to suggestions that the balance between the biological and mechanical factors is variable, and that OA at such different sites as weight-bearing hip joints and non-weight-bearing finger joints could even be considered separate entities (Felson et al 2000).
Risk factors for OA include inherent factors such as age, ethnicity and gender, combined with acquired factors such as local mechanics, obesity, trauma and deformity (Felson et al 2000).
The pathology involves the whole of the affected joint, as well as focal changes at specific sites. Primary OA can start with a significant inflammatory element, and can cause significant pain, especially early in the disease process. Osteophytes develop at the margins of the articular surfaces near the attachments of the capsule, ligaments or tendons. Endochondral ossification follows, spreading to involve neighbouring soft-tissue structures. In both primary and secondary presentations the cartilage reduces in thickness as the matrix degenerates, especially in regions of higher stresses (Marijnissen et al 2003). Chondrocyte biochemistry leads to a self-promoting degenerative cascade, hastening the process of deterioration (Marijnissen et al 2003). The subchondral bone becomes sclerotic and cysts become evident, again in areas of high stress. The soft-tissue structures such as the synovium and ligaments can be involved, but not to the extent seen in the inflammatory arthritides (Felson et al 2000).
Clinical course
Initial soreness may be accompanied by soft-tissue swelling in primary OA; alternatively, the onset may be quite insidious. Osteophytes form early and are often readily palpable. The initial pain of primary OA may ease as the disease progresses.
Osteoarthritic changes in the hands (Figure 8.19) have been noted to be more severe in those with more physically demanding jobs but OA is not clearly associated with limb dominance (Bergenudd et al 1989). Obesity has been linked to the prevalence of OA in both non-weight-bearing and weight-bearing joints (Hawker 1997). Degenerative joint disease can be superimposed on other forms of arthropathy, and is cumulative and currently irreversible.
Medical management
As noted at the start of this chapter, the medical management of OA still relies on the traditional pyramid approach, in contrast to the paradigm shift for treating immune-mediated inflammatory diseases. In recent years there has been increased emphasis on patient empowerment, and so education and self-management have been adopted as central features of the Arthritis and Musculoskeletal Alliance (ARMA) national standards of care for OA (Arthritis and Musculoskeletal Alliance 2004b). NICE (2008) recommend exercise as the core treatment for early OA, and this can be self-directed. Simple analgesia is NICE’s recommended first line of pharmaceutical therapy, with NSAIDs used if pain persists. Some NSAIDs may themselves be harmful to cartilage and, although there is no strong evidence, the new cox-2 inhibitors have been suggested to be moderately chondroprotective (Marijnissen et al 2003).
As more is known about the cellular and cytokine mechanisms involved in OA, new targeted biological drugs will emerge. Already there are early trials on drugs that modify enzymes involved in the degradation of cartilage. It is possible that future therapies for OA will be just as effective (and costly) as the biological drugs in the inflammatory immune-mediated diseases.
Moderate exercise is known to be effective in reducing pain and the impact of OA on quality of life, and exercise programmes should supplement all medical and podiatric management.
The ideal management of OA is to reverse the degenerative process, although to date there has been very little success with this approach medically. While techniques such as autologous chondrocyte implantation have been used to stimulate small amounts of cartilage regeneration, the technique remains useful only for isolated cartilage defects. Chondrocyte supplementation with mesenchymal stem cells is an avenue receiving some considerable attention in medical research, but any clinical applications remain some way off. The success of replacement arthroplasty is highly joint specific, and implants for foot joints have not been especially successful. A schema for the medical management of OA is presented in Figure 8.20, describing the relationships between the treatments recommended by NICE.
The foot in osteoarthritis
The foot and ankle area has not generally been considered an important anatomical region for OA. Indeed the ankle is notable for being a weight-bearing joint that is particularly resistant to OA (Buckwalter & Saltzman 1999). However, osteoarthritic changes are observable in radiographs of the foot joints in approximately half of people (Lemont & Gibley 1982), and clinical changes are evident in 16% of those aged 55 years and over (Bergenudd et al 1989). The first metatarsophalangeal joint has been proposed to be a highly prevalent site for joint degeneration, second only to the knee (Muehleman et al 1997). Other joints in the foot are affected more sporadically, most often following trauma (Chen 1996). Proximal foot joints in the tarsus and tarsometatarsal region showed degeneration in between 10% and 36% of a cohort of older cadaveric specimens (mean age 76 years).
In one study (Greisberg et al 2003), midfoot OA (Figure 8.21) was considerably more prevalent in patients with severe pes planus than in a control group, but the severity was not related to the degree of deformity.
The most common presentations of OA in the foot are as hallux limitus, hallux rigidus or in association with hallux valgus. In these presentations, degenerative changes at the first metatarsophalangeal joint lead to functional limitation. Pain and stiffness are felt in the affected joint, especially in maximal dorsiflexion, and this may interfere with normal walking (Weinfeld & Schon 1998). Periarticular osteophyte formation leads to thickening of the first metatarsophalangeal joint, and sometimes the formation of an adventitious bursa. The osteophytic change will be evident on the radiograph, as will joint-space narrowing. The limitation of the joint range of movement may be progressive, with decreasing range associated with increasing impairment (Weinfeld & Schon 1998).
OA in the foot is a significant factor in predicting utilisation of healthcare services, and the presence of foot OA doubles the risk of dependence on health support services (Gorter et al 2001). Treatments for OA of the foot vary in their success. NSAIDs have been shown to be beneficial in OA of the foot and may be advised by podiatrists (Jennings 1994), and paracetamol has shown a systematic, if limited, effect on the pain associated with OA generally (Towheed et al 2004). However, long-term oral analgesic or NSAID use should only be undertaken under medical supervision. Weight loss and low-impact exercise are of known benefit, and some effort should be directed towards exercise counselling during the clinical consultation (Rao & Hootman 2004, Thomas & Daniels 2003).
Over-the-counter remedies for osteoarthritic symptoms include a range of pharmaceutical topical agents, and more recently a growing number of alternative treatments, which are used by up to one-third of people with OA (Arthritis Research Campaign 2002). Alternative therapies include fish oil (which is high in omega-3 fatty acids), glucosamine and chondroitin sulfate. Glucosamine has been reported to provide benefit in OA (Matheson & Perry 2003), but NICE (2008) concluded that the evidence was not strong enough to recommend this approach.
Local therapies can be geared toward ameliorating abnormal mechanics or addressing the symptoms, and the following are recommended by NICE. Contoured foot orthoses, provided either to a neutral cast or off the shelf, are effective at reducing foot joint motions and offloading the forefoot and heel regions, and so may help some patients. Similarly, shock-absorbing insoles or footwear can provide symptom relief, and accommodative padding may reduce pressure over prominences resulting from osteoarthritic changes. Footwear adaptations such as rocker-bottom shoes limit the need for movement of degenerate joints while facilitating sagittal plane motion (Thomas & Daniels 2003).
Intra-articular injection of steroid may provide symptom relief for up to 8 weeks (Thomas & Daniels 2003), although in practice the effect rarely lasts longer than 2–4 weeks. Hyaluronate injections have produced moderate benefits in large joints such as the knee, but their use in small joints such as those of the foot is experimental only.
NICE recommended the use of thermal therapies (i.e. application of local heat or cold), and a range of approaches have been described, including direct application of heat, ultrasound and low-level laser therapy. A Cochrane Review of the latter approach concluded that the evidence was equivocal (Brosseau et al 2004), and so ‘low-tech’ solutions are more justifiable.
Surgical intervention is usually definitive, with arthrodesis, or replacement or excision arthroplasty reducing pain significantly. Surgery to the first metatarsophalangeal joint is discussed in detail elsewhere and so is not discussed further here. Less commonly, surgery is required for the subtalar joint, ankle joint and midfoot, where joint degeneration is usually secondary to trauma.
Ankle joint surgeries include arthroscopic and open approaches and are varied. Unless there is advanced degeneration and deformity, arthrodesis of the ankle is considered a salvage procedure, because the significant physical demands on this joint (Marijnissen et al 2003) and the high risk of perioperative infection make this a difficult procedure, with variable intermediate and long-term results (Mann & Chou 1995, Van Eygen et al 1999). More wide-ranging arthrodeses of the ankle and hindfoot, such as the pantalar and triple arthrodeses, are similarly regarded as salvage procedures only (Acosta et al 2000).
Intermediate OA of the ankle may respond positively to a low tibial osteotomy, which redistributes loading stress within the joint (Chen 1996). Surgical joint distraction using external Ilizarov fixation is another experimental technique being used to promote cartilage regeneration, and this has produced some encouraging early results in severe arthritis of the ankle (Marijnissen et al 2003).
Where degenerative changes in the midfoot, hindfoot or ankle joint are severe, arthrodesis is currently the preferred surgical approach. Functionally, neighbouring joints are usually able to accommodate the loss of motion in the fused joint reasonably well, at least initially (Demetriades et al 1998), although ankle arthrodesis leads to premature degeneration in the neighbouring foot joints in the long term (Coester et al 2001). Excision arthroplasty can result in instability, and so the use of this technique is confined to the metatarsophalangeal joints and digits. Joint replacements have a chequered history in the foot because the combination of large forces and small amounts of bone stock leads to prosthetic loosening and tissue reaction to wear debris (Weinfeld & Schon 1998). The proportion of failures associated with foot joint replacement is higher than would be acceptable for larger joints such as the knee and hip. Some success was reported in the early days of total ankle replacement arthroplasty, but follow-up results were poor and the technique fell from favour (Demetriades et al 1998). Several generations of refinement have led to some resurgence in interest, and modern ankle replacements with mobile bearings appear to have better functional outcomes and lower failure rates (Wood et al 2008). Nevertheless, the lifespan of these replacements still falls short of that seen in large-joint implantation (Thomas & Daniels 2003).
Crystal arthropathies (gout and pseudogout)
Definition
The crystal arthropathies, gout and pseudogout, are the clinical manifestations of an inflammatory response to crystal deposition in synovial joints.
Epidemiology
Gout occurs in around 1% of men and 0.3–0.6% of women (Harris et al 1999, McGuire 2003) and the incidence increases with age, being rare in adults under 30 years old. Pseudogout is much less common and is predominantly a disease of the elderly.
Diagnosis
The definitive diagnosis of crystal arthritis is usually simple, and there are long-standing diagnostic criteria (Box 8.5). Crystals may be viewed directly within joint aspirate under polarised light microscopy. The clinical picture in true gout is usually obvious, and microscopy is often only confirmatory, although joint aspiration should always be performed to exclude septic arthritis or coincidental degenerative or inflammatory arthritis (Harris et al 1999). Recent advances in imaging, such as dual-energy computerised tomography, offer a potentially improved method for quantifying the extent of joint and soft-tissue involvement (Choi et al 2008), but these techniques are not yet in widespread use.
Pathology
The underlying pathology of gout is of crystal deposition into the joint space as a result of elevated levels of monosodium urate in the blood stream. Pseudogout occurs when calcium pyrophosphate dihydrate, a normal component of articular cartilage, enters the joint space and is taken up by white cells. A less common form is caused by deposition of calcium hydroxapatite.
The local pathology within the affected joint is of crystal deposition leading to severe synovitis. Synovial proliferation occurs and the inflammatory cascade is instigated and modulated by leucocytes, monocytes and lymphocytes. Tophi are the consequence of phagocytosis of crystals, and are composed of high concentrations of crystals and the detritus of deceased polymorphs.
Clinical course
There are four phases of gout: (1) asymptomatic hyperuricaemia, (2) acute gouty arthritis, (3) intercritical gout and (4) chronic tophaceous gout (Harris et al 1999).
The initial presentation is typically a severe and acute monoarthritis lasting a few days (Figure 8.22). Preceding an acute attack there may be a history of precipitating factors, such as dietary change, an alcohol binge or minor trauma to the joint (Weinfeld & Schon 1998). Onset is often overnight, with a rapid development of a severe monoarthritis manifest as heat, redness and marked swelling. More diffuse swelling can result if more than one joint is involved (Chen & Schumacher 2003). If untreated, episodes may become more frequent and longer in duration. Medical management of the acute phase for all crystal arthropathies is with high therapeutic doses of NSAID. Other therapies, such as oral steroid, are available for patients unable to tolerate high doses of NSAIDs, but are not preferred. Colchicine is only appropriate in the earliest stages of an acute episode of gout. Oral or injected steroid may be of assistance where single or few accessible joints are involved (Harris et al 1999). Local therapies such as the application of ice may be helpful.
The length of the intercritical periods can vary, but will tend to shorten in poorly controlled gout. Three-quarters of patients suffer a second attack within 2 years of the first (Harris et al 1999). Recurrences of acute gouty attacks occur more frequently over time and involve other joints, often symmetrically.
Tophaceous deposits may form in the longer term, initially in joints and tendon sheaths, but as time progresses more extensively in soft tissues (Harris et al 1999, Kerman et al 1993). On plain film radiography joint spaces will be initially preserved, but later marked periarticular erosions will form (Kerr 1998). The tophaceous deposits may be fairly large and, in the foot, can result in problems with shoe fitting (Figure 8.23). Occasionally, large tophi will ulcerate (Weinfeld & Schon 1998).
Preventive regimens of drugs, such as low-dose colchicine, uric clearance promoters, such as Probenecid, and the xanthine oxidase inhibitor allopurinol, can be effective in reducing the frequency of subsequent attacks when combined with dietary modification (Chen & Schumacher 2003, Kerr 1998). For some time patients who were allergic to Allopurinol had no alternatives, but a new inhibitor of xanthine oxidase, Febuxostat, has been developed. Febuxostat is as good as or better than allopurinol in reducing the concentration of uric acid in the blood, and has now been approved by NICE for those people who cannot take allopurinol (Becker et al 2005).
The foot in crystal arthropathies
True gout will onset in the first metatarsophalangeal joint in about half of all cases, although it also has a predilection for other sites in the foot such as the other metatarsophalangeal joints, the first interphalangeal joint, midfoot joints, the subtalar joint and the ankle (Egan et al 1987, Kerr 1998). Pseudogout presents with a less severe synovitis and affects larger joints such as the ankle and knee (Kerr 1998). At onset, crystal arthropathies are characterised by a hyperacute monoarthritis, accompanied by severe swelling erythema and pain. The joint will typically be too painful to move or to touch.
In the chronically gouty joint, there will be considerable joint damage, which in the later stages may require excision or replacement to resolve symptoms (McGuire 2003). Periarticular erosions may have characteristic punched-out or overhanging edges (Egan et al 1987).Two separate case reports have highlighted tophaceous gout affecting the hallucal sesamoids and mimicking tumour or fracture (Reber et al 1997, Liu et al 2003). Other unusual intraosseous presentations reported in the foot include involvement of the navicular and the third metatarsal bone (Surprenant et al 1996, Thomas et al 1998). Tophi may arise extensively in the soft tissues such as tendons, including the Achilles (Kerman et al 1993), and subcutaneous tophi may extrude through the skin (Egan et al 1987).
Infective arthritis
Infective (septic) arthritis arises as a direct response to the presence of infective organisms (usually bacteria) in the joint, and so usually presents as an acute monoarthritis, often with an accompanying systemic presentation of fever. It is uncommon in the foot joints, preferring larger joints such as the knee (Kerr 1998). Infective arthritis is more common in children than adults.
The foot in infective arthritis
Although the foot is rarely affected, infective arthritis can occur secondary to ulceration or following a penetrating injury (Kerr 1998), and so is more prevalent in people with sensory neuropathy (Weinfeld & Schon 1998). Infective arthritis should be considered in the case of an acute monoarthritis in the foot after surgery (Weinfeld & Schon 1998). Infective arthritis requires aspiration of joint fluids and long-term antibiotic therapy, but will usually resolve completely (Kerr 1998).
Other rheumatological conditions
Fibromyalgia
Definition
Fibromyalgia (FM) is a label given to a presentation of chronic, widespread pain in both muscles and joints. It is diagnosed according to criteria published by the American College of Rheumatologists on the basis of 11 or more tender points at specific sites in the body. Prevalence is estimated at 1–2%, the majority of cases arising in females (Linaker et al 1999). It is known that fibromyalgia is associated with higher scores on anxiety and depression scales, and its prevalence increases with age (Linaker et al 1999). Patients report chronic musculoskeletal fatigue and pain, often severe enough to interfere with their ability to work and participate in leisure activities. The clinical course is variable but fairly poor, and fewer than 50% of sufferers are in remission by 10 years. The medical management is directed towards management of chronic pain and its attendant psychological effects. Pain relief is required, usually using analgesics or NSAIDs, although these may not be effective alone. Managing the psychological aspects of fibromyalgia may be helpful, and selective serotonin reuptake inhibitors (SSRIs) or tricyclic antidepressants such as amitriptyline are often prescribed, and may be combined with non-pharmaceutical approaches such as exercise and cognitive–behavioural therapy.
Exercise has been demonstrated to improve outcomes in patients with fibromyalgia in a number of studies (Rao & Hootman 2004). The foot is not an especially common site for involvement in fibromyalgia, but many patients with fibromyalgia will have incidental foot pain, and the general prevalence of foot problems in this group will be at least as high as in the general population. These complex patients will be seen often in foot health clinics.
Polymyalgia rheumatica
Polymyalgia rheumatica is a musculoskeletal pain syndrome usually seen in females over 65 years old. It affects about 7 people per 1000. Musculoskeletal symptoms occur mainly around the upper (in the shoulders and neck) and lower (buttocks and upper thighs) limb girdles. The onset of symptoms may be dramatic over a few days and can lead to severe disability. Systemic symptoms such as fever and night sweats may occur, as may weight loss. The pathology is unknown, although there is a link with giant-cell arteritis, which has provided some clues. This link is vital, however, as new-onset temporal headache with polymyalgic symptoms may herald acute and permanent loss of vision. The clinical course is of diffuse musculoskeletal pain early, and because of its dramatic course patients usually present early. Treatment with NSAIDs may help but low-dose oral steroids have an equally dramatic benefit on the symptoms, this response being part of the criteria for diagnosis. Polymyalgia rheumatica does not affect the feet directly but patients with difficulty bending and with reduced grip strength due to polymyalgia rheumatica may need assistance with basic foot care.
Sjögren’s syndrome
Sjögren’s syndrome is a connective tissue disorder that mainly affects the salivary and tear glands. It is relatively rare and may occur as a primary form (i.e. in the absence of any other disease) or in a secondary form associated with other rheumatic diseases such as RA or lupus. Its main effects are to cause dryness of the mucous membranes, especially the eyes and mouth. The skin can be affected also, and anhidrosis is the most common manifestation in the foot. Patients can benefit from education about the importance of an emollient regimen, including footbaths and creams.
Sjögren’s syndrome can be associated with vasculitis, and the toes can be involved. Management is the same as for the other forms of vasculitis in the rheumatology clinic (Cawley 1987).
Joint hypermobility syndromes
Definition
There are several conditions that are associated with a generalised hypermobility of joints. The most common are the benign familial joint hypermobility syndrome (BFJHS), the nine basic classifications of Ehlers–Danlos syndrome (EDS), Marfan syndrome and osteogenesis imperfecta.
BFJHS and EDS type III are essentially the same condition (Grahame 2000), and in these two presentations the signs are largely confined to joint laxity and skin hyperextensibility. The other disorders present with other systemic effects to a greater or lesser extent. The systemic complications are not detailed in this chapter, and we focus on the musculoskeletal manifestations using BFJHS/EDS type III by way of example.
Epidemiology
The prevalence of hypermobility and related symptoms varies considerably with ethnicity. Inheritance patterns are specific to the disorder (e.g. the EDS classification), with recessive, dominant and X-linked forms all reported. Benign familial joint hypermobility nominally follows an autosomal-dominant pattern of inheritance, although penetrance is variable (Beighton & Horan 1970). The prevalence of hypermobility in Caucasians is reported to be around 5%, while in some racial groups, such as those from the Middle East and the Indian subcontinent, the prevalence is reported to be as a high as 38% (Al-Rawi et al 1985, Biro et al 1983, Larsson et al 1993, Wordsworth et al 1987). BFJHS is more common in women than men (Hudson et al 1995) and joint hypermobility declines with age (Larsson et al 1993). The association between underlying joint hypermobility and symptoms is incomplete, but BFJHS accounts for a large proportion of rheumatology referrals (far more than ankylosing spondylitis or psoriatic arthritis), and so the symptoms apparently have a significant impact in adults (Grahame 1993, Guma et al 2001, Hudson et al 1995, Sacheti et al 1997).
Diagnosis
Diagnosis can be difficult and, because of debate over the definition and importance of hypermobility, the condition is often ignored entirely, especially in mild to moderate cases (Grahame & Bird 2001). Many patients express frustration at the delay in obtaining a diagnosis and a lack of understanding from clinical staff (Gazit et al 2001, Gurley-Green 2001).
The diagnosis of hypermobility as a generic physical presentation has long been made on the basis of the well-known Beighton score (Beighton & Horan 1969), a five-item, nine-point scale recording the physical features associated with systemic joint laxity (Figure 8.24). The physical tests used in the Beighton scoring system are among a number of joint features that patients often use as ‘party tricks’. It is worth noting that repeated performance of these actions can be detrimental to joints, and clinicians should request their demonstration sparingly, and patients should be discouraged from performing them ad hoc (Gurley-Green 2001).
Figure 8.24 The Beighton–Carter–Wilkinson scale for assessing joint hypermobility. (A) Hyperextension of the fingers past 90°. (B) Touch the thumb to the volar aspect of the forearm. (C) Elbow hyperextension >10°. (D) Knee hyperextension >10°. (E) Being able to place the palms flat on the floor with knees extended. One point is given to each positive sign (one point for each positive limb for signs A–D) to yield a score out of 9.
There may be some variation in the anatomical distribution of joint hyperextensibility even within individuals. While overall quantifications are useful, the clinician should pay careful attention to individual joints of interest.
A more specific set of diagnostic criteria was introduced in 2000 to differentiate BFJHS from other sources of hypermobility and idiopathic musculoskeletal pain (Grahame et al 2000). The Revised Brighton Criteria include a number of physical features and historical observations and are specific to benign familial hypermobility syndrome (Box 8.6).
Box 8.6 Revised Brighton (1998) Criteria for the diagnosis of benign familial joint hypermobility syndrome (BFJHS)
BFJHS is diagnosed in the presence of two major criteria, or one major and two minor criteria, or four minor criteria. Two minor criteria will suffice where there is an unequivocally affected first-degree relative.
BFJHS is excluded by presence of Marfan or Ehlers-Danlos syndrome (other than Ehlers-Danlos syndrome type III).
Pathology
The pathology of hypermobility syndrome derives from disordered structure of type I and III collagen (Grahame 2000). Gradation in bundle size is lost, and type I:type III ratios are deranged, leading to decreased tensile strength of connective tissues in the skin and articular structures.
Clinical course
In people with BFJHS and EDS type III the collagen disorder manifests in a tendency to easy bruising and papyraceous scars from impaired healing. Old scars are usually observed readily on the knees of people with BFJHS/EDS type III. More profound manifestations are suggestive of EDS type I. Musculoskeletal symptoms in the hypermobility syndromes are often intermittent, at least at first, and other than long-term and low-grade mechanical joint damage the clinical course is stable. The majority of patients with BFJHS have multiple intermittent joint and soft-tissue pain, with pain in the back, hands, wrists and shoulders being at least as common as lower limb problems (Ainsworth & Aulicino 1993, Gazit et al 2001, Hudson et al 1995, Sacheti et al 1997). Dislocations are common and, while they usually reduce spontaneously, they are painful in the short term and often recur repeatedly, leading to a worsening of the joint stability (Ainsworth & Aulicino 1993).
The clinical rule of thumb is that people with joint hyperlaxity develop similar sorts of overuse-type symptoms as the rest of the population (tenosynovitis, arthralgia etc.) but do so more frequently and more severely.
The link between joint hypermobility and subsequent OA has long been noted (Beighton & Horan 1969, Grahame 2000), although at least one recent study has suggested that generalised joint hypermobility might reduce the risk of arthritis in hand joints (Kraus et al 2004).
Marfan syndrome is associated with joint hypermobility but also with a range of cardiovascular pathologies, and in osteogenesis imperfecta the bony fragility is the primary feature. In both of these conditions the joint laxity is considered a secondary feature and may be of less consequence than the systemic effects.
Medical management
There is little that can be done medically that will directly affect joint mobility. Treatment is aimed at symptom relief, so the mainstays of the management of patients with hypermobility are NSAIDs and selective local corticosteroid in the short term, combined with physical and occupational therapy. Physical therapies must be tailored to the specific needs of patients with joint hypermobility because standard approaches can increase the severity of problems (Gurley-Green 2001). Exercises to strengthen and stabilise affected joints have been demonstrated to reduce joint pain, but the exercise regimens must be continued indefinitely to maintain their effect (Barton & Bird 1996). BFJHS is also noted to be associated with poor proprioception (Grahame 2000), and in our clinic rehabilitation strategies, including proprioception training, are prescribed for hypermobile patients.
Patients with joint hypermobility may develop a chronic pain state, which may be helped by psychological intervention and antidepressant therapy (Grahame 2000, Sacheti et al 1997). There is also a link between BFJHS and fibromyalgia, a recognised chronic pain syndrome (Hudson et al 1995; Karaaslan et al 2002).
The foot in hypermobility syndrome
Hypermobility of large joints in the lower limb is common and leads to musculoskeletal symptoms and reduced quality of life (Redmond et al 2006b). Repeated dislocations can occur, causing altered gait. Ankle and foot hypermobility has been reported to affect as many as 60–94% of adults with hypermobility syndrome (Bulbena et al 1992, Riano et al 2001), but our experience of a large cohort currently enrolled in a clinical trial suggests that, while the prevalence of pes planus in BFJHS is higher than is found in the general population, it might not be as high as others have suggested (Redmond et al 2006a). Certain forms (such as EDS type I) may be more closely associated with pes planus than others.
Where the generalised joint laxity in our BFJHS patients is reflected in the foot, instability appears to be more manifest in the joints of the midfoot than the hindfoot complex (Figure 8.25). It is notable in some of these patients that the instability of the foot joints is more apparent during walking than quiet standing (Redmond et al 2006a), and we consider a dynamic evaluation to be an essential part of the examination of the hypermobile patient.
Joint hypermobility can be associated with some unusual presentations in the feet, including a ‘skewfoot’-type presentation often seen in Marfan syndrome, and talipes-equinovarus-type features (Agnew 1997).
Musculoskeletal pain is common in the feet of people with BFJHS (Redmond et al 2006b), and ankle instability is often troublesome also (Finsterbush & Pogrund 1982).
It can be difficult to achieve satisfactory local analgesia in patients with hypermobility syndrome as the agent diffuses rapidly, preventing adequate nerve blockade (Grahame 2000). Tissue fragility leads to problems with wound closure during surgery, and also contributes to a tendency to wound dehiscence postoperatively. It is common for scar formation following surgery or trauma to be hypertrophic (Tompkins & Bellacosa 1997).
Miscellaneous other conditions
The foot can be involved in a number of other rheumatic conditions. In ulcerative colitis, synovitis occurs in the metatarsophalangeal joints of 2% of sufferers, with ankle involvement in 5–7% (Moll 1987). In Crohn’s disease, the ankle joint is affected in one-third of patients, a similar prevalence to that seen in Whipple’s disease (Moll 1987). Behcet (with cidilla) syndrome usually presents as ulceration in the mouth or genitalia, but can present with ankle synovitis and pustule formation on the feet.
ASSESSING OUTCOMES IN RHEUMATIC DISEASE
Rheumatology as a discipline has been proactive in establishing inventive measures of disease progress and outcome. Outcome measures range from general measures of the impact of musculoskeletal impairment, to particular measures of disease activity and disease-specific impact scales.
For example, the American College of Rheumatology diagnostic criteria for RA have been supplemented by explicit criteria for defining and quantifying improvement or remission (Felson et al 1993, van Gestel et al 1998). This enables clear therapeutic guidelines to be drawn up and has removed much of the ambiguity that hampers other areas of practice. These outcome measures are valuable for evaluating the effect of therapies in clinical trials and in day-to-day clinical practice. Outcomes data can be quick to collect, and provide useful objective information on the merits of a clinical service, as well as helping practitioners reflect on their own practice. We recommend that all practitioners involved in the provision of foot health services in rheumatology include some basic outcome-assessment tools in their standard assessment protocol.
Health outcomes tools can be objective, such as the acute-phase inflammatory markers erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) measured in assessing underlying rheumatoid disease activity, through to more subjective measures, such as pain or impact on quality of life. Some objective measures of function, including basic gait parameters (gait speed, cadence, stride length), are highly correlated with more subjective measures of the impact of disease, such as the Sickness Impact Profile (O’Connell et al 1998). The basic gait parameters are easily and quickly measured without expensive equipment, and are valid and responsive measures of therapeutic change (Fransen & Edmonds 1999, Hamilton et al 2001).
Pain, health status and quality-of-life measures – the easiest way to evaluate the patient-oriented outcomes associated with existing foot health services – can be generic, or disease- or anatomy-specific. Generic measures, such as the Sickness Impact Profile (Bergner et al 1976), SF-36 (Ware et al 1993) and EQ-5D (The Euroqol Group 1990) health assessment questionnaires, measure overall health status and are comparable across diseases.
Disease-specific tools, such as the ASQoL (Doward et al 2003) or the RAQoL (Whalley et al 1997), quality-of-life measures for ankylosing spondylitis and RA, respectively, are intended to pick up features more directly associated with the specific conditions and can be more sensitive.
There are several foot-specific outcomes measures available, the most commonly used being the Foot Function Index (Budiman-Mak et al 1991), the Foot Health Status Questionnaire (Bennett et al 1998) and the Manchester Foot Pain and Disability Questionnaire (Garrow et al 2000), the last of which has recently been revised by Cook et al (2007). These measures are useful, but all have documented shortcomings.
We have developed and validated a foot-specific measure, the Leeds Foot Impact Scale, that is also specific to patients with RA (Helliwell et al 2005). This scale has high validity for foot problems in people with RA, and is the preferred tool for this group. In other rheumatology populations we use the Manchester Foot Pain and Disability Questionnaire and the pain subscale of the Foot Function Index, although in the authors’ opinion some improvements could be made in both these measures, and they need to be used with due consideration of their weaknesses. This is an area where the methodologies informing the development of new instruments is maturing rapidly, and further new tools will doubtless be developed in coming years.
SUMMARY
The foot is often affected in people with rheumatic disorders. The provision of foot health services for this patient group is patchy at best, and frankly inadequate at worst. Foot-related musculoskeletal conditions often coexist with other morbidity, and may even be secondary to impairments associated with systemic effects. Practitioners involved in caring for this group of patients should be aware of current trends in medical management, and should seek to integrate with the rheumatology team. All clinicians engaged in the care of foot-related conditions should remember the high degree of association between the foot and systemic rheumatic disorders, and be mindful of their role in identifying systemic disease if it first presents in the foot.
This is an exciting and developing area of practice in its own right, and more so now that the foot is receiving the recognition is deserves from the rheumatology community.
ACKNOWLEDGEMENTS
We are indebted to Professor Jim Woodburn and Dr Deborah Turner, both of Glasgow Caledonian University, and former members of the FASTER group, and to Heidi Siddle and all the staff in the Academic Unit of Musculoskeletal Disease for their assistance. We would also like to acknowledge the National Institute of Health Research and the Arthritis Research Campaign for funding the programme of foot research in rheumatology at the authors’ institution.
Acosta R, Ushiba J, Cracchiolo A. The results of a primary and staged pantalar arthrodesis and tibiotalocalcaneal arthrodesis in adult patients. Foot & Ankle International. 2000;21(3):182-194.
Agnew P. Evaluation of the child with ligamentous laxity. Clinics in Podiatric Medicine & Surgery. 1997;14(1):117-130.
Ainsworth SR, Aulicino PL. A survey of patients with Ehlers–Danlos syndrome. Clinical Orthopaedics & Related Research. 1993;286:250-256.
Aletaha D, Smolen JS. Advances in anti-inflammatory therapy. Acta Medica Austriaca. 2002;29(1):1-6.
Al-Rawi ZS, Al-Aszawi AJ, Al-Chalabi T. Joint mobility among university students in Iraq. British Journal of Rheumatology. 1985;24(4):326-331.
American College of Rheumatology Subcommittee on Rheumatoid Arthritis. Guidelines for the management of rheumatoid arthritis 2002 Update. Arthritis & Rheumatism. 2002;46(2):328-346.
Arthritis and Musculoskeletal Alliance. Standards of care for people with musculoskeletal conditions – inflammatory arthritis. London. 2004.
Arthritis and Musculoskeletal Alliance. Standards of care for people with musculoskeletal conditions – osteoarthritis. London. 2004.
Arthritis and Musculoskeletal Alliance and Podiatry Rheumatic Care Association. Standards of care for people with musculoskeletal foot health problems. London. 2008.
Arthritis Research Campaign. Arthritis: the big picture. Chesterfield; 2002.
Backman CL, Kennedy SM, Chalmers A, Singer J. Participation in paid and unpaid work by adults with rheumatoid arthritis. Journal of Rheumatology. 2004;31(1):47-56.
Barrett EM, Scott DG, Wiles NJ, Symmons DP. The impact of rheumatoid arthritis on employment status in the early years of disease: a UK community-based study. Rheumatology. 2000;39(12):1403-1409.
Barton LM, Bird HA. Improving pain by the stabilization of hyperlax joints. Journal of Orthopaedic Rheumatology. 1996;9(1):46-51.
Becker MA, Schumacher HRJr, Wortmann RL, et al. Febuxostat compared with allopurinol in patients with hyperuricemia and gout. New England Journal of Medicine. 2005;353(23):2450-2461.
Beighton P, Horan F. Orthopaedic aspects of the Ehlers–Danlos syndrome. Journal of Bone & Joint Surgery – British. 1969;Volume 51(3):444-453.
Beighton PH, Horan FT. Dominant inheritance in familial generalised articular hypermobility. Journal of Bone & Joint Surgery – British. 1970;Volume 52(1):145-147.
Belt EA, Kaarela K, Maenpaa H, et al. Relationship of ankle joint involvement with subtalar destruction in patients with rheumatoid arthritis. A 20-year follow-up study. Joint, Bone, Spine: Revue du Rhumatisme. 2001;68(2):154-157.
Bennett PJ, Patterson C, Wearing S, Baglioni T. Development and validation of a questionnaire designed to measure foot-health status. Journal of the American Podiatric Medical Association. 1998;88(9):419-428.
Benvenuti F, Ferrucci L, Guralnik JM, et al. Foot pain and disability in older persons: an epidemiologic survey. Journal of the American Geriatrics Society. 1995;43(5):479-484.
Bergenudd H, Lindgarde F, Nilsson B. Prevalence and coincidence of degenerative changes of the hands and feet in middle age and their relationship to occupational work load, intelligence, and social background. Clinical Orthopaedics & Related Research. 1989;239:306-310.
Berger I, Martens K, Meyer-Scholten C. Rheumatoid necroses in the forefoot. Foot & Ankle International. 2004;25(5):336-339.
Bergner M, Bobbitt RA, Pollard WE, et al. The Sickness Impact Profile: validation of a health status measure. Medical Care. 1976;19(1):57-67.
Biro F, Gewanter HL, Baum J. The hypermobility syndrome. Pediatrics. 1983;72(5):701-706.
Bitzan P, Giurea A, Wanivenhaus A. Plantar pressure distribution after resection of the metatarsal heads in rheumatoid arthritis. Foot & Ankle International. 1997;18(7):391-397.
Black JR, Hale WE. Prevalence of foot complaints in the elderly. Journal of the American Podiatric Medical Association. 1987;77(6):308-311.
Bongu A, Chang E, Ramsey-Goldman R. Can morbidity and mortality of SLE be improved. Clinical Rheumatology. 2002;16(2):313-332.
Bouysset M, Bonvoisin B, Lejeune E, Bouvier M. Flattening of the rheumatoid foot in tarsal arthritis on X-ray. Scandinavian Journal of Rheumatology. 1987;16(2):127-133.
Bouysset M, Tebib J, Weil G, et al. The rheumatoid heel: its relationship to other disorders in the rheumatoid foot. Clinical Rheumatology. 1989;8(2):208-214.
Bouysset M, Tavernier T, Tebib J, et al. CT and MRI evaluation of tenosynovitis of the rheumatoid hindfoot. Clinical Rheumatology. 1995;14(3):303-307.
Bouysset M, Tebib J, Tavernier T, et al. Posterior tibial tendon and subtalar joint complex in rheumatoid arthritis: magnetic resonance imaging study. Journal of Rheumatology. 2003;30(9):1951-1954.
Braun J, Sieper J. Ankylosing spondylitis. The Lancet. 2007;369(9570):1379-1390.
Breedveld FC, Emery P, Keystone E, et al. Infliximab in active early rheumatoid arthritis. Annals of the Rheumatic Diseases. 2004;63(2):149-155.
Brockbank J, Gladman D. Diagnosis and management of psoriatic arthritis. Drugs. 2002;62(17):2447-2457.
Brosseau L, Welch V, Wells G, et al. Low level laser therapy (classes I, II and III) for treating rheumatoid arthritis. Cochrane Database of Systematic Reviews. 2004;2:2.
Buckwalter JA, Saltzman CL. Ankle osteoarthritis: distinctive characteristics. Instructional Course Lectures. 1999;48:233-241.
Budiman-Mak E, Conrad KJ, Roach KE, et al. The Foot Function Index: a measure of foot pain and disability. Journal of Clinical Epidemiology. 1991;44(6):561-570.
Budiman-Mak E, Conrad KJ, Roach KE, et al. Can foot orthoses prevent hallux valgus deformity in rheumatoid arthritis? A randomized clinical trial. Journal of Clinical Rheumatology. 1995;1(6):313-321.
Bukhari MA, Wiles NJ, Lunt M, et al. Influence of disease-modifying therapy on radiographic outcome in inflammatory polyarthritis at five years: results from a large observational inception study. Arthritis & Rheumatism. 2003;48(1):46-53.
Bulbena A, Duro JC, Porta M, et al. Clinical assessment of hypermobility of joints: assembling criteria. Journal of Rheumatology. 1992;19(1):115-122.
Callen JP. Management of skin disease in patients with lupus erythematosus. Clinical Rheumatology. 2002;16(2):245-264.
Cannella AC, O’Dell JR. Is there still a role for traditional disease-modifying antirheumatic drugs (DMARDs) in rheumatoid arthritis? Current Opinion in Rheumatology. 2003;15(3):185-192.
Cardone DA, Tallia AF. Joint and soft tissue injection. American Family Physician. 2002;66(2):283-288.
Cawley MI. Vasculitis and ulceration in rheumatic diseases of the foot. Baillière’s Clinical Rheumatology. 1987;1(2):315-333.
Chalmers AC, Busby C, Goyert J, et al. Metatarsalgia and rheumatoid arthritis – a randomized, single blind, sequential trial comparing 2 types of foot orthoses and supportive shoes. Journal of Rheumatology. 2000;27(7):1643-1647.
Chen BX. Arthritis of the foot and ankle. Current Opinion in Orthopedics. 1996;7(3):87-91.
Chen J, Devine A, Dick IM, et al. Prevalence of lower extremity pain and its association with functionality and quality of life in elderly women in Australia. Journal of Rheumatology. 2003;30(12):2689-2693.
Chen LX, Schumacher HR. Gout and gout mimickers: 20 clinical pearls: a bigger diagnostic and management challenge than it may seem. Journal of Musculoskeletal Medicine. 2003;20(5):254-258.
Choi HK, Al-Arfaj A, Eftekhari A, et al 2008 Dual energy computed tomography in tophaceous gout. Annals of the Rheumatic Diseases ard.2008.099713 (Epub).
Choy EH, Panayi GS. Cytokine pathways and joint inflammation in rheumatoid arthritis. New England Journal of Medicine. 2001;344(12):907-916.
Choy EHS, Scott DL, Kingsley GH, et al. Treating rheumatoid arthritis early with disease modifying drugs reduces joint damage: a randomised double blind trial of sulphasalazine vs diclofenac sodium. Clinical & Experimental Rheumatology. 2002;20(3):351-358.
Cimino WG, O’Malley MJ. Rheumatoid arthritis of the ankle and the hindfoot. Clinics in Podiatric Medicine & Surgery. 1999;16(2):373-389.
Coady D, Walker D, Kay L. Regional Examination of the Musculoskeletal System (REMS): a core set of clinical skills for medical students. Rheumatology. 2004;43(5):633-639.
Coester LM, Saltzman CL, Leupold J, Pontarelli W. Long-term results following ankle arthrodesis for post-traumatic arthritis. Journal of Bone & Joint Surgery American. 2001;83(2):219-228.
Cook CE, Cleland J, Pietrobon R, et al. Calibration of an item pool for assessing the disability associated with foot pain: an application of item response theory to the Manchester Foot Pain and Disability Index. Physiotherapy. 2007;93(2):89-95.
Cracchiolo IA. The rheumatoid foot and ankle: pathology and treatment. Foot. 1993;3(3):126-134.
Crawford F, Atkins D, Young P, Edwards J. Steroid injection for heel pain: evidence of short-term effectiveness. A randomized controlled trial. Rheumatology. 1999;38(10):974-977.
Cunningham LS, Kelsey JL. Epidemiology of musculoskeletal impairments and associated disability. American Journal of Public Health. 1984;74(6):574-579.
Davys H, Turner D, Emery P, Woodburn J. A comparison of scalpel debridement versus sham procedure for painful forefoot callosities in rheumatoid arthritis. Annals of the Rheumatic Diseases. 2004;63(Suppl 1):427.
de Boer IG, Peeters AJ, Ronday HK, et al. Assistive devices: usage in patients with rheumatoid arthritis. Clinical Rheumatology. 2009;28(2):119-128.
DeJesus JM, Tsuchiya C. Pharmacologic management of the arthritic foot and ankle. Clinics in Podiatric Medicine & Surgery. 1999;16(2):271-284.
Demetriades L, Strauss E, Gallina J. Osteoarthritis of the ankle. Clinical Orthopaedics & Related Research. 1998;349:28-42.
Devauchelle-Pensec V, Saraux A, Alapetite S, et al. Diagnostic value of radiographs of the hands and feet in early rheumatoid arthritis. Joint Bone Spine. 2002;69(5):434-441.
Doward LC, Spoorenberg A, Cook SA, et al. Development of the ASQoL: a quality of life instrument specific to ankylosing spondylitis. Annals of the Rheumatic Diseases. 2003;62(1):20-26.
Dunn JE, Link CL, Felson DT, et al. Prevalence of foot and ankle conditions in a multiethnic community sample of older adults. American Journal of Epidemiology. 2004;159(5):491-498.
Edwards JCW, Szczepanski L, Szechinski J, et al. Efficacy of B-cell-targeted therapy with Rituximab in patients with rheumatoid arthritis. New England Journal of Medicine. 2004;350:2572-2581.
Egan M, Brosseau L, Farmer M, et al 2003 Splints/orthoses in the treatment of rheumatoid arthritis. Cochrane Database of Systematic Reviews 1:CD004018.
Egan R, Sartoris J, Dresnick D, et al. Radiographic features of gout in the foot. Journal of Foot Surgery. 1987;26(5):434-439.
Emery P, Breedveld FC, Dougados D, et al. Early referral recommendation for newly diagnosed rheumatoid arthritis: evidence based development of a clinical guide. Annals of the Rheumatic Diseases. 2002;61(4):290-297.
Emery P, Seto Y. Role of biologics in early arthritis. Clinical & Experimental Rheumatology. 2003;21(5 Suppl 31):S191-S194.
Escalante A, Del Rincon I. How much disability in rheumatoid arthritis is explained by rheumatoid arthritis? Arthritis & Rheumatism. 1999;42(8):1712-1721.
Escalante A, Del Rincon I. The disablement process in rheumatoid arthritis. Arthritis & Rheumatism. 2002;47(3):333-342.
Feldmann M. Development of anti-TNF therapy for rheumatoid arthritis. Nature Reviews. Immunology. 2002;2(5):364-371.
Feldmann M, Maini R. Role of cytokines in rheumatoid arthritis: an education in pathophysiology and therapeutics. Immunological Reviews. 2008;223(1):7-19.
Felson DT, Anderson JJ, Boers M, et al. The American College of Rheumatology preliminary core set of disease activity measures for rheumatoid arthritis clinical trials. The Committee on Outcome Measures in Rheumatoid Arthritis Clinical Trials. Arthritis & Rheumatism. 1993;36(6):729-740.
Felson DT, Lawrence RC, Dieppe PA, et al. Osteoarthritis: new insights. Part 1: The disease and its risk factors. Annals of Internal Medicine. 2000;133(8):635-646.
Finsterbush A, Pogrund H. The hypermobility syndrome. Musculoskeletal complaints in 100 consecutive cases of generalized joint hypermobility. Clinical Orthopaedics & Related Research. 1982;168:124-127.
Firth J, Hale C, et al. The prevalence of foot ulceration in patients with rheumatoid arthritis. Arthritis & Rheumatism (Arthritis Care and Research). 2008;59(2):200-205.
Fitzgerald GA. Coxibs and cardiovascular disease. New England Journal of Medicine. 2004;351:1709-1711.
Fransen M, Edmonds J. Off-the-shelf orthopedic footwear for people with rheumatoid arthritis. Arthritis Care & Research. 1997;10(4):250-256.
Fransen M, Edmonds J. Gait variables: appropriate objective outcome measures in rheumatoid arthritis. Rheumatology. 1999;38(7):663-667.
Fries JF, Williams CA, Morfeld D, et al. Reduction in long-term disability in patients with rheumatoid arthritis by disease-modifying antirheumatic drug-based treatment strategies. Arthritis & Rheumatism. 1996;39(4):616-622.
Galluzzo E, Lischi D, Taglione E, et al. Sonographic analysis of the ankle in patients with psoriatic arthritis. Scandinavian Journal of Rheumatology. 2000;29(1):52-55.
Garner S, Fidan D, Frankish R, et al. Rofecoxib for rheumatoid arthritis. Cochrane Database of Systematic Reviews. 2004;2:2.
Garner S, Fidan D, Frankish R, et al. Celecoxib for rheumatoid arthritis. Cochrane Database of Systematic Reviews. 2004;2:2.
Garrow AP, Papageorgiou AC, Silman AJ, et al. Development and validation of a questionnaire to assess disabling foot pain. Pain. 2000;85(1–2):107-113.
Garrow AP, Silman AJ, Macfarlane GJ. The Cheshire Foot Pain and Disability Survey: a population survey assessing prevalence and associations. Pain. 2004;110(1–2):378-384.
Gazit Y, Nahir AM. Hypermobility syndrome – a disease or not a disease? Annals of the Rheumatic Diseases. 60(Suppl 1), 2001.
Genta MS, Genta RM, Gabay C. systemic rheumatoid vasculitis: a review. Seminars in Arthritis and Rheumatism. 2006;36(2):88-98.
Gerster JC, Vischer TL, Bennani A, Fallet GH. The painful heel. Comparative study in rheumatoid arthritis, ankylosing spondylitis, Reiter’s syndrome, and generalized osteoarthrosis. Annals of the Rheumatic Diseases. 1977;36(4):343-348.
Gilkes JJ. Skin and nail changes in the arthritic foot. Baillière’s Clinical Rheumatology. 1987;1(2):335-354.
Gladstein GS, Rynes RI, Parhami M, Bartholomew LE. Gangrene of a foot secondary to systemic lupus erythematosus with large vessel vasculitis. Journal of Rheumatology. 1979;6(5):549-553.
Gold RH, Bassett LW, Seeger LL. The other arthritides. Roentgenologic features of osteoarthritis, erosive osteoarthritis, ankylosing spondylitis, psoriatic arthritis, Reiter’s disease, multicentric reticulohistiocytosis, and progressive systemic sclerosis. Radiologic Clinics of North America. 1988;26(6):1195-1212.
Gordon P, West J, Jones H, Gibson T. A 10 year prospective followup of patients with rheumatoid arthritis: 1986–96. Journal of Rheumatology. 2001;28(11):2409-2415.
Gorter K, Kuyvenhoven M, de Melker R. Health care utilisation by older people with non-traumatic foot complaints. What makes the difference? Scandinavian Journal of Primary Health Care. 2001;19(3):191-193.
Gorter KJ, Kuyvenhoven MM, de Melker RA. Nontraumatic foot complaints in older people. A population-based survey of risk factors, mobility, and well-being. Journal of the American Podiatric Medical Association. 2000;90(8):397-402.
Grahame R. Topical reviews: hypermobility syndrome. ARC Reports on Rheumatic Diseases Series. 2(25), 1993.
Grahame R. Pain, distress and joint hyperlaxity. Joint, Bone, Spine: Revue du Rhumatisme. 2000;67(3):157-163.
Grahame R, Bird H. British consultant rheumatologists perceptions about the hypermobility syndrome: a national survey. Rheumatology. 2001;40(5):559-562.
Grahame R, Bird HA, Child A. The revised (Brighton: 1998) criteria for the diagnosis of benign joint hypermobility syndrome (BJHS). Journal of Rheumatology. 2000;27(7):1777-1779.
Greisberg J, Hansen STJr, Sangeorzan B. Deformity and degeneration in the hindfoot and midfoot joints of the adult acquired flatfoot. Foot & Ankle International. 2003;24(7):530-534.
Guma M, Roca J, Holgado S, et al. An estimation of the prevalence of hypermobility. Annals of the Rheumatic Diseases. 60(Suppl 1), 2001. Thu: 0220
Gurley-Green S. Living with the hypermobility syndrome. Rheumatology. 2001;40(5):487-489.
Hamalainen M, Raunio P. Long term followup of rheumatoid forefoot surgery. Clinical Orthopaedics & Related Research. 1997;340:34-38.
Hamilton J, Brydson G, Fraser G, Grant M. Walking ability as a measure of treatment effect in early rheumatoid arthritis. Clinical Rehabilitation. 2001;15(2):142-147.
Harris MD, Siegel LB, Alloway JA. Gout hyperuricemia. American Family Physician. 1999;59(4):925-934.
Harvey I, Frankel S, Marks R, et al. Foot morbidity and exposure to chiropody: population based study. BMJ. 1997;315(7115):1054-1055.
Hawker G. Update on the epidemiology of the rheumatic diseases. Current Opinion in Rheumatology. 1997;9(2):90-94.
Hazes JM. Determinants of physical function in rheumatoid arthritis: association with the disease process. Rheumatology. 2003;42(Suppl 2):ii17-ii21.
Helliwell PS. Lessons to be learned: review of a multidisciplinary foot clinic in rheumatology. Rheumatology. 2003;42(11):1426-1427.
Helliwell PS, Allen N, Gilworth G, et al. Development of a foot impact scale for rheumatoid arthritis. Rheumatology. 2005;47:ii27-ii28.
Helliwell PS, O’Hara M, Holdsworth J, et al. A 12-month randomized controlled trial of patient education on radiographic changes and quality of life in early rheumatoid arthritis. Rheumatology. 1999;38(4):303-308.
Hendry G, Gardner-Medwin J, Watt GF, Woodburn J. A survey of foot problems in juvenile idiopathic arthritis. Musculoskeletal Care. 2008;6:221-232.
Herold DC, Palmer RG. Questionnaire study of the use of surgical shoes prescribed in a rheumatology outpatient clinic. Journal of Rheumatology. 1992;19(10):1542-1544.
Hodge MC, Bach TM, Carter GM, et al. Novel Award First Prize Paper. Orthotic management of plantar pressure and pain in rheumatoid arthritis. Clinical Biomechanics. 1999;14(8):567-575.
Hudson N, Starr MR, Esdaile JM, Fitzcharles MA. Diagnostic associations with hypermobility in rheumatology patients. British Journal of Rheumatology. 1995;34(12):1157-1161.
Hughes J, Grace D, Clark P, Klenerman L. Metatarsal head excision for rheumatoid arthritis. 4-year follow-up of 68 feet with and without hallux fusion. Acta Orthopaedica Scandinavica. 1991;62(1):63-66.
Jacobson JA, Girish G, Jiang Y, Resnick D. Radiographic evaluation of arthritis: inflammatory conditions. Radiology. 2008;248(2):378-389.
Jennings MB. Comparison of piroxicam and naproxen in osteoarthritis of the foot. Journal of the American Podiatric Medical Association. 1994;84(7):348-354.
Jernberg ET, Simkin P, Kravette M, et al. The posterior tibial tendon and the tarsal sinus in rheumatoid flat foot: magnetic resonance imaging of 40 feet. Journal of Rheumatology. 1999;26(2):289-293.
Juni P, Nartey L, Reichenbaum S, et al. Risk of cardiovascular events and rofecoxib: cumulative meta-analysis. Lancet. 2004;364:2021-2029.
Karaaslan Y, Haznedaroglu S, Ozturk M. Joint hypermobility and primary fibromyalgia: a clinical enigma. Journal of Rheumatology. 2002;27(7):1774-1776.
Kavlak Y, Uygur F, Korkmaz C, Bek N. Outcome of orthoses intervention in the rheumatoid foot. Foot & Ankle International. 2003;24(6):494-499.
Kay S, Nancarrow JD. Spontaneous healing and relief of pain in a patient with intractable vasculitic ulceration of the lower limb following an intravenous infusion of prostacyclin: a case report. British Journal of Plastic Surgery. 1984;37(2):175-178.
Keenan MA, Peabody TD, Gronley JK, Perry J. Valgus deformities of the feet and characteristics of gait in patients who have rheumatoid arthritis. Journal of Bone & Joint Surgery. 1991;73(2):237-247.
Keenan A-M, Tennant A, Fear J, Emery P, Conaghan PG. Impact of multiple joint problems on daily living tasks in people in the community over age fifty-five. Arthritis and Rheumatism. 2006;55:757-764.
Kerman BL, Mack G, Moshirfar MM. Tophaceous gout of the foot: an unusual presentation of severe chronic gout in an undiagnosed patient. Journal of Foot & Ankle Surgery. 1993;32(2):167-170.
Kerr LD. Arthritis of the forefoot. A review from a rheumatologic and medical perspective. Clinical Orthopaedics & Related Research. 1998;349:20-27.
Kerry RM, Holt GM, Stockley I. The foot in chronic rheumatoid arthritis: a continuing problem. Foot. 1994;4(4):201-203.
Korda J, Balint GP. When to consult the podiatrist. Best Practice & Research in Clinical Rheumatology. 2004;18(4):587-611.
Kraus VB, Li YJ, Martin ER, et al. Articular hypermobility is a protective factor for hand osteoarthritis. Arthritis & Rheumatism. 2004;50(7):2178-2183.
Kumar R, Madewell JE. Rheumatoid and seronegative arthropathies of the foot. Radiologic Clinics of North America. 1987;25(6):1263-1288.
La Montagna G, Baruffo A, Tirri R, et al. Foot involvement in systemic sclerosis: a longitudinal study of 100 patients. Seminars in Arthritis & Rheumatism. 2002;31(4):248-255.
Lard LR, Visser H, Speyer I, et al. Early versus delayed treatment in patients with recent-onset rheumatoid arthritis: comparison of two cohorts who received different treatment strategies. American Journal of Medicine. 2001;111(6):446-451.
Larsson LG, Baum J, Mudholkar GS, Srivastava DK. Hypermobility: prevalence and features in a Swedish population. British Journal of Rheumatology. 1993;32(2):116-119.
Lemont H, Gibley CWJr. Prevalence of osteoarthritis of the foot. Journal of the American Podiatry Association. 1982;72(5):214-216.
Leonardi CL, Powers JL, Matheson RT, et al. Etanercept as monotherapy in patients with psoriasis. New England Journal of Medicine. 2003;349(21):2014-2022.
Leveille SG, Guralnik JM, Ferrucci L, et al. Foot pain and disability in older women. American Journal of Epidemiology. 1998;148(7):657-665.
Lin AT, Clements PJ, Furst DE. Update on disease-modifying antirheumatic drugs in the treatment of systemic sclerosis. Rheumatic Diseases Clinics of North America. 2003;29(2):409-426.
Linaker CH, Walker-Bone K, Palmer K, Cooper C. Frequency and impact of regional musculoskeletal disorders. Baillière’s Clinical Rheumatology. 1999;13(2):197-215.
Liu SZ, Yeh LR, Chou YJ, et al. Isolated intraosseous gout in hallux sesamoid mimicking a bone tumor in a teenaged patient. Skeletal Radiology. 2003;32(11):647-650.
Lord M, Foulston J. Surgical footwear: a survey of prescribing consultants. BMJ. 1989;299(6700):9.
Lukas C, Landewe R, Sieper J, et al. Development of an ASAS-endorsed disease activity score (ASDAS) in patients with ankylosing spondylitis. Annals of the Rheumatic Diseases. 2009;68(1):18-24.
Maddison PJ. Is it SLE? Clinical Rheumatology. 2002;16(2):167-180.
Mann RA, Chou LB. Tibiocalcaneal arthrodesis. Foot & Ankle International. 1995;16(7):401-405.
March L, Lapsley H. What are the costs to society and the potential benefits from the effective management of early rheumatoid arthritis? Best Practice & Research in Clinical Rheumatology. 2001;15(1):171-185.
Marijnissen AC, van Roermund PM, van Melkebeek J, Lafeber FP. Clinical benefit of joint distraction in the treatment of ankle osteoarthritis. Foot & Ankle Clinics. 2003;8(2):335-346.
Marzo-Ortega H, McGonagle D, O’Connor P, Emery P. Efficacy of etanercept in the treatment of the entheseal pathology in resistant spondylarthropathy: a clinical and magnetic resonance imaging study. Arthritis & Rheumatism. 2001;44(9):2112-2117.
Masterton E, Mulcahy DMC, Elwain J, McInerney D. The plano valgus rheumatoid foot – is tibialis posterior tendon rupture a factor? British Journal of Rheumatology. 1995;34(7):645-646.
Matheson AJ, Perry CM. Glucosamine: a review of its use in the management of osteoarthritis. Drugs & Aging. 2003;20(14):1041-1060.
McAlindon T. Update on the epidemiology of systemic lupus erythematosus: new spins on old ideas. Current Opinion in Rheumatology. 2000;12(2):104-112.
McGonagle D, Marzo-Ortega H, O’Connor P, et al. The role of biomechanical factors and HLA-B27 in magnetic resonance imaging-determined bone changes in plantar fascia enthesopathy. Arthritis & Rheumatism. 2002;46(2):489-493.
McGuire JB. Arthritis and related diseases of the foot and ankle: rehabilitation and biomechanical considerations. Clinics in Podiatric Medicine & Surgery. 2003;20(3):469-485. ix
Menz HB, Munteanu SE, Landorf KB, et al. Radiographic classification of osteoarthritis in commonly affected joints of the foot. Osteoarthritis and Cartilage. 2007;15(11):1333-1338.
Michelson J, Easley M, Wigley FM, Hellmann D. Foot and ankle problems in rheumatoid arthritis. Foot & Ankle International. 1994;15(11):608-613.
Mizutani W, Quismorio FPJr. Lupus foot: deforming arthropathy of the feet in systemic lupus erythematosus. Journal of Rheumatology. 1984;11(1):80-82.
Moll JMH. Seronegative arthropathies in the foot. Baillière’s Clinical Rheumatology. 1987;1(2):289-314.
Muehleman C, Bareither D, Huch K, et al. Prevalence of degenerative morphological changes in the joints of the lower extremity. Osteoarthritis & Cartilage. 1997;5(1):23-37.
Mulherin D, Price M. Efficacy of tibial nerve block, local steroid injection or both in the treatment of plantar heel pain syndrome. The Foot. 2009;19(2):98-100.
Munro BJ, Steele JR. Foot-care awareness. A survey of persons aged 65 years and older. Journal of the American Podiatric Medical Association. 1998;88(5):242-248.
NICE. Guidance on the use of etanercept and inflixmab for the treatment of rheumatoid arthritis. Technology Appraisal Guidance No. 36. London: National Institute for Clinical Excellence; 2002.
NICE. Clinical Guideline CG 59:The care and management of osteoarthritis in adults. NICE Clinical Guidelines. London: National Institute for Health and Clinical Excellence; 2008.
NICE. Clinical Guideline CG 79: The care and management of rheumatoid arthritis in adults. NICE Clinical Guidelines. London: National Institute for Health and Clinical Excellence; 2009.
O’Brien TS, Hart TS, Gould JS. Extraosseous manifestations of rheumatoid arthritis in the foot and ankle. Clinical Orthopaedics & Related Research. 1997;340:26-33.
O’Connell PG, Lohmann Siegel K, Kepple TM, et al. Forefoot deformity, pain, and mobility in rheumatoid and nonarthritic subjects. Journal of Rheumatology. 1998;25(9):1681-1686.
O’Dell JR. The therapeutic pyramid: a work in progress. Journal of Rheumatology. 1997;24(6):1028-1030.
O’Dell JR. Therapeutic strategies for rheumatoid arthritis. New England Journal of Medicine. 2004;350:2591-2602.
Odding E, Valkenburg HA, Algra D, et al. Association of locomotor complaints and disability in the Rotterdam study. Annals of the Rheumatic Diseases. 1995;54(9):721-725.
Olivieri I, Barozzi L, Padula A. Enthesiopathy: clinical manifestations, imaging and treatment. Baillière’s Clinical Rheumatology. 1998;12(4):665-681.
Olivieri I, Scarano E, Padula A, et al. Switching tumor necrosis factor alpha inhibitors in HLA-B27-associated severe heel enthesitis. Arthritis & Rheumatism. 2007;57(8):1572-1574.
Olsen N, Stein M. New drugs for rheumatoid arthritis. New England Journal of Medicine. 2004;350:2567-2579.
Osiri M, Shea B, Robinson V, et al. Leflunomide for treating rheumatoid arthritis. Cochrane Database of Systematic Reviews. 2004;2:2.
Ostergaard M, Szkudlarek M. Imaging in rheumatoid arthritis – why MRI and ultrasonography can no longer be ignored. Scandinavian Journal of Rheumatology. 2003;32(2):63-73.
Otter SY, Cryer A. Biologic agents used to treat rheumatoid arthritis and their relevance to podiatrists: a practice update. Musculoskeletal Care. 2004;2(1):51-59.
Pensec VD, Saraux A, Berthelot JM, et al. Ability of foot radiographs to predict rheumatoid arthritis in patients with early arthritis. Journal of Rheumatology. 2004;31(1):66-70.
Petty RE, Southwood TR, Baum J, et al. Revision of the proposed classification criteria for juvenile idiopathic arthritis: Durban, 1997. Journal of Rheumatology. 1991;25(10):1991-1994.
Pincus T, O’Dell JR, Kremer JM. Combination therapy with multiple disease-modifying antirheumatic drugs in rheumatoid arthritis: a preventive strategy. Annals of Internal Medicine. 1999;131(10):768-774.
Platto MJ, O’Connell PG, Hicks JE, Gerber LH. The relationship of pain and deformity of the rheumatoid foot to gait and an index of functional ambulation. Journal of Rheumatology. 1991;18(1):38-43.
Port M, McCarthy DJ, Chu S. The foot and systemic disease in the Veterans Administration: a quantitation of the relationship between systemic disease and pedal problems. Journal of the American Podiatry Association. 1980;70(8):397-404.
Prier A, Berenbaum F, Karneff A, et al. Multidisciplinary day hospital treatment of rheumatoid arthritis patients. Evaluation after two years. Revue du Rhumatisme. 1997;64(7–9):443-450. (English Edition)
Priolo F, Bacarini L, Cannista M, et al. Radiographic changes in the feet of patients with early rheumatoid arthritis. Journal of Rheumatology. 1997;24(11):2113-2118.
Puolakka K, Kautiainen H, Mottonen T, et al. Impact of initial aggressive drug treatment with a combination of disease-modifying antirheumatic drugs on the development of work disability in early rheumatoid arthritis: a five-year randomized followup trial. Arthritis & Rheumatism. 2004;50(1):55-62.
Rao JK, Hootman JM. Prevention research and rheumatic disease. Current Opinion in Rheumatology. 2004;16(2):119-124.
Rau R, Schleusser B, Herborn G, Karger T. Long-term treatment of destructive rheumatoid arthritis with methotrexate. Journal of Rheumatology. 1997;24(10):1881-1889.
Reber PU, Patel AG, Noesberger B. Gout: rare cause of hallucal sesamoid pain: a case report. Foot & Ankle International. 1997;18(12):818-820.
Redmond A, Allen N, Vernon W. Effect of scalpel debridement on the pain associated with plantar hyperkeratosis. Journal of the American Podiatric Medical Association. 1999;89(10):515-519.
Redmond AC, Helliwell PS 2005 A national survey of foot health services in UK rheumatology practice. Unpublished.
Redmond AC, Keenan A, Landorf KB, Emery P. Off-the-shelf contoured orthoses demonstrate comparable mechanical properties to custom-made foot orthoses at less cost. Rheumatology. 2004;43(4):ii149.
Redmond AC, Hain J, Bird HA, et al. The distribution and prevalence of symptoms associated with hypermobility syndrome. Annals of the Rheumatic Diseases. 2006;65(Suppl II):241.
Redmond AC, Helliwell PS, Bird HA, et al. Pain and health status in people with hypermobility syndrome are associated with overall joint mobility and selected local mechanical factors. Rheumatology. 2006;45(1):108.
Reilly PA, Evison G, McHugh NJ, Maddison PJ. Arthropathy of hands and feet in systemic lupus erythematosus. Journal of Rheumatology. 1990;17(6):777-784.
Riano F, Sanchez O, Pena NZT. Joint hypermobility syndrome: a prospective study of articular and non-rheumatic manifestation in a Venezuelan population. Annals of the Rheumatic Diseases. 60(Suppl 1), 2001. Thu: 0217
Riemsma RP, Kirwan JR, Taal E, Rasker JJ. Patient education for adults with rheumatoid arthritis. Cochrane Database of Systematic Reviews. 2004;2:2.
Riente L, Delle Sedie A, Iagnocco A, et al. Ultrasound imaging for the rheumatologist. V. Ultrasonography of the ankle and foot. Clinical and Experimental Rheumatology. 2006;24:493-498.
Ritchlin CT, Kavanaugh A, Gladman DD, et al. Treatment recommendations for psoriatic arthritis. Annals of the Rheumatic Diseases. 2009;68:1387-1394.
Rosenberg WWJ, De Waal Malefijt MC, Laan RFJM, Go SL. Forefoot reconstruction with combined first metatarsus osteotomy, metatarsophalangeal fusion and resection of the lesser metatarsal heads in rheumatoid patients. Foot & Ankle Surgery. 2000;6(2):99-104.
Sacheti A, Szemere J, Bernstein B, et al. Chronic pain is a manifestation of the Ehlers–Danlos syndrome. Journal of Pain & Symptom Management. 1997;14(2):88-93.
Sanmarti R, Gomez A, Ercilla G, et al. Radiological progression in early rheumatoid arthritis after DMARDS: a one-year follow-up study in a clinical setting. Rheumatology. 2003;42(9):1044-1049.
Sari-Kouzel H, Hutchinson CE, Middleton A, et al. Foot problems in patients with systemic sclerosis. Rheumatology. 2001;40(4):410-413.
Schmiegel A, Rosenbaum D, Schorat A, et al. Assessment of foot impairment in rheumatoid arthritis patients by dynamic pedobarography. Gait & Posture. 2008;27(1):110-114.
Solsky MA, Wallace DJ. New therapies in systemic lupus erythematosus. Clinical Rheumatology. 2002;16(2):293-312.
Steed DL, Edington H, Moosa HH, Webster MW. Organization and development of a university multidisciplinary wound care clinic. Surgery. 1993;114(4):775-778. discussion 778–779
Steultjens EMJ, Dekker J, Bouter LM, et al. Occupational therapy for rheumatoid arthritis. Cochrane Database of Systematic Reviews. 2004;2:2.
Stiskal M, Szolar DH, Stenzel I, et al. Magnetic resonance imaging of Achilles tendon in patients with rheumatoid arthritis. Investigative Radiology. 1997;32(10):602-608.
Surprenant MS, Levy AI, Hanfit JR. Intraosseous gout of the foot: an unusual case report. Journal of Foot & Ankle Surgery. 1996;35(3):237-243.
Symmons D, Asten P, McNally R, Webb R. Healthcare needs assessment for musculoskeletal diseases: the first step – Estimating the number of incidents and prevalent cases. Manchester: arc Epidemiology Unit, University of Manchester; 2002.
Szkudlarek M, Court-Payen M, Jacobsen S, et al. Interobserver agreement in ultrasonography of the finger and toe joints in rheumatoid arthritis. Arthritis & Rheumatism. 2003;48(4):955-962.
Taylor W, Gladman D, Helliwell P, et al. Classification criteria for psoriatic arthritis: development of new criteria from a large international study. Arthritis and Rheumatism. 2006;54:2665-2673.
The Euroqol Group. EuroQol: a new facility for the measurement of health related quality of life. Health Policy. 1990;16(3):199-208.
Theodoridou A, Bento L, D’Cruz DP, et al. Prevalence and associations of an abnormal ankle–brachial index in systemic lupus erythematosus: a pilot study. Annals of the Rheumatic Diseases. 2003;62:1199-1203.
Thomas E, Olive P, Canovas F, et al. Tophaceous gout of the navicular bone as a cause of medial inflammatory tumor of the foot. Foot & Ankle International. 1998;19(1):48-51.
Thomas E, Peat G, Harris L, et al. The prevalence of pain and pain interference in a general population of older adults: cross-sectional findings from the North Staffordshire Osteoarthritis Project (NorStOP). Pain. 2004;110:361-368.
Thomas RH, Daniels TR. Current concepts review. Ankle arthritis. Journal of Bone & Joint Surgery – American. 2003;Volume 85A(5):923-936.
Tompkins MH, Bellacosa RA. Podiatric surgical considerations in the Ehlers–Danlos patient. Journal of Foot & Ankle Surgery. 1997;36(5):381-387.
Toolan BC, Hansen STJr. Surgery of the rheumatoid foot and ankle. Current Opinion in Rheumatology. 1998;10(2):116-119.
Towheed TE, Judd MJ, Hochberg MC, Wells G. Acetaminophen for osteoarthritis. Cochrane Database of Systematic Reviews. 2004;2:2.
Tsokos G. B-Cells be gone – B-cell depletion in the treatment of rheumatoid arthritis. New England Journal of Medicine. 2004;350:2546-2548.
Turesson C, Matteson EL. Vasculitis in rheumatoid arthritis. Current Opinion in Rheumatology. 2009;21(1):35-40.
Turner DW, Helliwell P. Pes plano valgus in RA: a descriptive and analytical study of foot function determined by gait analysis. Musculoskeletal Care. 2003;1(1):23-33.
Turner DE, Helliwell PS, Lohmann Siegel K, Woodburn J. Biomechanics of the foot in rheumatoid arthritis: identifying abnormal function and the factors associated with localised disease impact. Clinical Biomechanics. 2008;23(1):93-100.
Ueda H, Akahoshi T, Kashiwazaki S. Radiological changes in feet of patients with progressive systemic sclerosis. Japanese Journal of Rheumatology. 1991;3(1):73-78.
Urwin M, Symmons D, Allison T, et al. Estimating the burden of musculoskeletal disorders in the community: the comparative prevalence of symptoms at different anatomical sites, and the relation to social deprivation. Annals of the Rheumatic Diseases. 1998;57(11):649-655.
van der Heijde D. New directions in classification and outcome assessment in ankylosing spondylitis. Current Rheumatology Reports. 2004;6(2):98-101.
van der Leeden M, Steultjens M, Dekker JHM, et al. Is disease duration related to foot function, pain and disability in rheumatoid arthritis patients with foot complaints? Clinical Biomechanics. 2008;23(5):693.
van der Leeden M, Steultjens MPM, et al. Prevalence and course of forefoot impairments and walking disability in the first eight years of rheumatoid arthritis. Arthritis & Rheumatism (Arthritis Care and Research). 2008;59(11):1596-1602.
Van Eygen P, Dereymaeker G, Driesen R, De Ferm A. Long-term follow-up of open ankle arthrodesis. Foot & Ankle Surgery. 1999;5(4):271-275.
van Gestel AM, Prevoo ML, van’t Hof MA, et al. Development and validation of the European League Against Rheumatism response criteria for rheumatoid arthritis. Comparison with the preliminary American College of Rheumatology and the World Health Organization/International League Against Rheumatism Criteria. Arthritis & Rheumatism. 1996;39(1):34-40.
van Gestel AM, Haagsma CJ, van Riel PL. Validation of rheumatoid arthritis improvement criteria that include simplified joint counts. Arthritis & Rheumatism. 1998;41(10):1845-1850.
van Venrooij WJ, van Beers JJBC, Pruijn GJM. Anti-CCP antibody, a marker for the early detection of rheumatoid arthritis. The Year in Immunology. 2008;1143:268-285.
Veale DJ, FitzGerald O. Psoriatic arthritis – pathogenesis and epidemiology. Clinical & Experimental Rheumatology. 2002;20(6 Suppl 28):S27-S33.
Verstappen SM, Jacobs JW, Bijlsma JW, et al. Five-year followup of rheumatoid arthritis patients after early treatment with disease-modifying antirheumatic drugs versus treatment according to the pyramid approach in the first year. Arthritis & Rheumatism. 2003;48(7):1797-1807.
Verstappen SMM, Jacobs JWG, Bijlsma JWJ, et al. The Utrecht experience with different treatment strategies in early rheumatoid arthritis. Clinical & Experimental Rheumatology. 2003;21(5 Suppl 31):S165-S168.
Vidigal E, Jacoby RK, Dixon AS, et al. The foot in chronic rheumatoid arthritis. Annals of the Rheumatic Diseases. 1975;34(4):292-297.
Wakefield RJ, Gibbon WW, Conaghan PG, et al. The value of sonography in the detection of bone erosions in patients with rheumatoid arthritis: A comparison with conventional radiography. Arthritis & Rheumatism. 2000;43(12):2762-2770.
Ward MM. Health services in rheumatology. Current Opinion in Rheumatology. 2000;12(2):99-103.
Walker JG, Pope J, Baron M, et al. The development of systemic sclerosis classification criteria. Clinical Rheumatology. 2007;26(9):1401-1409.
Ware J, Snow KK, Kosinski M, Gandek B. SF-36 Health Survey. Manual and interpretation guide. Boston, MA: Boston Health Institute, New England Medical Center; 1993.
Weinfeld SB, Schon LC. Hallux metatarsophalangeal arthritis. Clinical Orthopaedics & Related Research. 1998;349:9-19.
Whalley D, McKenna SP, de Jong Z, van der Heijde D. Quality of life in rheumatoid arthritis. British Journal of Rheumatology. 1997;36(8):884-888.
White EG, Mulley GP. Footcare for very elderly people: a community survey. Age & Ageing. 1989;18(4):276-278.
Wickman AM, Pinzur MS, Kadanoff R, Juknelis D. Health-related quality of life for patients with rheumatoid arthritis foot involvement. Foot & Ankle International. 2004;25(1):19-26.
Williams A, Bowden A. An audit of foot problems in rheumatic disease. Nottingham: Society of Chiropodists and Podiatrists National Conference; 2002.
Williams AE, Rome K, et al. A clinical trial of specialist footwear for patients with rheumatoid arthritis. Rheumatology. 2007;46(2):302-307.
Wolfe F, Rehman Q, Lane NE, Kremer J. Starting a disease modifying antirheumatic drug or a biologic agent in rheumatoid arthritis: standards of practice for RA treatment. Journal of Rheumatology. 2001;28(7):1704-1711.
Wood PLR, Clough TM, Smith R. The present state of ankle arthroplasty. Foot and Ankle Surgery. 2008;14(3):115-119.
Woodburn J, Turner DE, Helliwell PS, Barker S. A preliminary study determining the feasibility of electromagnetic tracking for kinematics at the ankle joint complex. Rheumatology. 1999;38(12):1260-1268.
Woodburn J, Stableford Z, Helliwell PS. Preliminary investigation of debridement of plantar callosities in rheumatoid arthritis. Rheumatology. 2000;39(6):652-654.
Woodburn J, Barker S, Helliwell PS. A randomized controlled trial of foot orthoses in rheumatoid arthritis. Journal of Rheumatology. 2002;29(7):1377-1383.
Woodburn J, Helliwell PS, Barker S. Three-dimensional kinematics at the ankle joint complex in rheumatoid arthritis patients with painful valgus deformity of the rearfoot. Rheumatology. 2002;41(12):1406-1412.
Woodburn J, Helliwell PS, Barker S. Changes in 3D joint kinematics support the continuous use of orthoses in the management of painful rearfoot deformity in rheumatoid arthritis. Journal of Rheumatology. 2003;30(11):2356-2364.
Woodburn J, Udupa JK, Hirsch BE, et al. The geometric architecture of the subtalar and midtarsal joints in rheumatoid arthritis based on magnetic resonance imaging. Arthritis & Rheumatism. 2002;46(12):3168-3177.
Wordsworth P, Ogilvie D, Smith R, Sykes B. Joint mobility with particular reference to racial variation and inherited connective tissue disorders. British Journal of Rheumatology. 1987;26(1):9-12.
Young A, Dixey J, Cox N, et al. How does functional disability in early rheumatoid arthritis (RA) affect patients and their lives? Results of 5 years of follow-up in 732 patients from the Early RA Study (ERAS). Rheumatology. 2000;39(6):603-611.