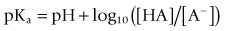Chapter 20 Local anaesthesia
INTRODUCTION AND BACKGROUND
While it is appreciated that individual countries will have legislation pertaining to the access to and use of local anaesthetic agents, this text, in terms of a legal context, is directed to those who practise in the UK.
The purification and synthesis of cocaine by Niemann in 1860 led to its subsequent use by Köller for anaesthesia of the eye, and the first nerve block was carried out by Halsted in 1884. In 1904, Einhorn synthesised procaine, which was introduced to clinical practice by Braun in 1905, and the rest, as they say, is history. The following century saw the introduction of a number of new local anaesthetic agents, with the most recent being ropivacaine, which was available for clinical use from 1996 (Ruetsch et al 2001).
Access to local anaesthetic for podiatrists in the UK was first approved by the Chiropodists Board in 1972 (Dagnall 1995) to include specifically lidocaine, mepivacaine, bupivacaine and prilocaine. Subsequent amendments to the 1968 Medicines Act by Statutory Instruments has seen the addition of lidocaine and bupivacaine with 1 : 200 000 epinephrine in 1997 (Order 1830 Department of Health), and most recently the addition in 2006 of levobupivacaine and ropivacaine (Order 2807 Department of Health). Dosage calculation is incumbent on the practitioner and is variable up to the maximum safe dose (MSD). Local anaesthetics are available as amino amide and amino ester solutions, but the esters, while available for medical and dental use, are not normally accessible for podiatric use in the UK.
BASIC CHEMISTRY AND PHARMACOLOGY
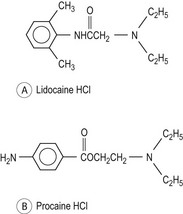
Local anaesthetics are weak bases that are insoluble in water, which are combined with a strong acid to provide a water-soluble salt. They have a lipid-soluble hydrophobic aromatic group linked by an intermediate chain (which determines the class of drug) to a charged amine lipophilic group (Fig. 20.1).
In practice, they are all supplied as hydrochloride solutions. At normal body pH of 7.4 they exist as both ionised and unionised forms. The proportions of each are dependent on the pKa (the acid dissociation constant) of the drug molecule, which is the pH at which the drug is 50% ionised. This is given by the Henderson–Hasselbach equation
where the square brackets denote concentration. The pKa determines the onset of action. Ionisation of the drug is in reference to the fact that most drugs have a mixture of molecules, some of which are uncharged, or unionised, and some of which have a negative or positive charge (i.e. are ionised). Only the unionised form of the drug is able to penetrate the membrane barriers, but it will revert in the lower pH of the axoplasm to an ionised form (Tetztaff 2000).
Amino amides have significant advantages over the amino esters, because they are more stable than esters. Esters are more easily hydrolysed than amides, particularly if solutions are autoclaved or exposed to alkaline solutions. Consequently, they are prepared in acidic solutions and so they are more stable in an ionised soluble form; however, they do not store as well and cannot be autoclaved. The pH of esters is 2.7–5.5 and that of amides 4.1–6.5. With a higher pH there is more rapid penetration at sites of action. Esters are far more likely to cause a sensitivity, or allergic reaction, because in their metabolism they produce para-aminobenzoate (PABA). The onset and duration of action of amides is also superior.
The molecular weights of local anaesthetic agents vary and there is some evidence to indicate that this may affect the absorption of the drug (Wildsmith et al 1987).
Lipid solubility is the primary determinant of the potency of a local anaesthetic. Higher lipid solubility is accompanied by a greater degree of protein binding, with similar implications for potency and duration of action of the anaesthetic effect. Aqueous solubility is another important physiochemical property, and is directly related to the extent of ionisation (and therefore decreases as the pH is raised) and inversely related to its lipid solubility.
Stereoisomerism describes the existence of molecules that have the same structure but different spatial arrangements around an atom. This becomes relevant when one considers that bupivacaine has two stereoisomers, known as the R and S forms. Bupivacaine has been developed into a single stereoisomer, known as levobupivacaine (Calvey & Williams 2008).
Membrane electrophysiology
Local anaesthetic solutions exert their primary pharmacological action by interfering with the excitation–conduction process of peripheral nerve fibres and endings, decreasing the rate and degree of depolarisation of the nerve membrane such that the threshold potential for transmission is not achieved and the nerve ceases to conduct (de Jong 1994). There is no effect on the resting or threshold potentials, but prolongation of the repolarisation phase and refractory period may also play a role in anaesthetic action. All excitable tissues possess special voltage-sensitive channels (Morgan & Johnson 2000).
Most of the clinically useful anaesthetic is likely to act by displacement of the calcium ions (Ca2+) from a lipoprotein receptor site on the interior of the nerve cell membrane. This will block the sodium ion (Na+) channels. Potassium ion (K+) channels are also blockable, but are less sensitive than the sodium channels. However, potassium channels are important in regulating the resting potential. The voltage-sensitive channels can be open, closed or inactivated. It is also possible that the local anaesthetic binds at some receptor sites intracellularly, called G-protein receptors (Scholz 2002).
Local anaesthetics are use or state dependent; that is, the degree of block is proportional to the rate of nerve stimulation (i.e. the more rapidly firing neurons are more susceptible than the slower ones) (Wildsmith 2007). Nerve fibres may be of one type or mixed; however, it is generally the smaller C diameter fibres which are more sensitive than larger myelinated A fibres. Thus, a differential block can be achieved where the smaller pain and autonomic fibres are blocked while coarse touch and movement fibres are spared (Strichartz & Ritchie 1987).
CHOICE OF LOCAL ANAESTHETIC AND DOSAGE
The choice of local anaesthetic is primarily determined by the practitioner’s preference, experience, the characteristics of the drug (e.g. protein binding and metabolism) and the procedure to be carried out. Minor procedures, such as toenail avulsion with phenolisation, that are quickly completed and which are associated with little postoperative pain can be successfully undertaken with lidocaine. If there is a known allergy or sensitivity to a local anaesthetic, then choosing the least chemically similar local anaesthetic is the best alternative. Longer and more postoperatively painful procedures can be best dealt with using a longer acting anaesthetic such as bupivacaine or levobupivacaine (Thompson & Lalonde 2006). However, the availability of local anaesthetic agents may not always simply be a matter of practitioner choice, but may be influenced by the access that the individual practitioner has to these agents. For example, as previously mentioned, in the UK esters are not available to podiatrists. There may be a Health Trust protocol/policy that limits ‘open’ access to all the amide agents. Hospital and Trust chief pharmacists may not permit access to 4% Citanest® as this is only licensed for dental use (see below). Etidocaine hydrochloride, while available in the USA, is not currently available to UK podiatrists. Whichever agent is chosen, it should conform to what are often regarded as the ideal characteristics (Uddin & Reilly 2008) and these are summarised below:
Figures for the MSD are not universally accepted, and variation occurs between countries and formularies. Indeed, the traditional methods of dose calculation are sometimes challenged:
In most cases there is no scientific justification for presenting exact milligram doses or mg/kg doses as maximum dose recommendations. Instead, only clinically adequate and safe doses (ranges) that are block specific are justified, taking into consideration the site of local anesthetic injection, and patient related factors such as age, organ dysfunctions, and pregnancy, which may influence the effect and pharmacokinetics of the local anesthetic.
Readers are advised to consult their home formularies (e.g. the British National Formulary and Martindale’s). Whatever the suggested safe dosage, it is incumbent on the practitioner to consider factors such as the patient’s general health and age, the integrity of their vasculature, the type of procedure to be undertaken and the anatomical site, as the effect of large doses is fairly meaningless unless the site of injection is considered, with the more peripheral injection sites being less of a risk than core areas such as the head and neck (Lagan & McClure 2004).
In general in the UK, the podiatrist calculates the MSD based on body weight, by multiplying the agent in milligrams (mg) by the body weight in kilograms (kg). Many drug calculations can be based on surface area, and the British National Formulary has an online calculator to enable this. It is deemed prudent to consider this only to a maximum body weight of 70 kg and as a ‘24-hour’ dose (Hardie & Lorimer 2006).
A simple way to arrive at a MSD dose is as follows. First, calculate the MSDs:
It is not unusual in podiatric surgery to use more than one local anaesthetic agent, whereby a fast-acting drug such as lidocaine is followed by a longer acting agent such as bupivacaine. This will sustain the anaesthesia throughout the procedure and possibly extend postoperative pain relief. The maths is a little more involved, but not difficult. For example, if your 65-kg patient has received 5 ml of 1% lidocaine and now requires levobupivacaine 0.5% to complete the procedure:
The patient could therefore receive 19.3 ml of 0.5% levobupivacaine in addition to the 5 ml of 1% lidocaine before being considered as over the MSD. Alternative ways to calculate the MSD are available, and some podiatrists refer to a chart of MSD values (Butterworth & Dockery 1992, Hardie & Lorimer 2006, Uddin & Reilly 2008) (Table 20.1).
Table 20.1 The maximum safe dose (MSD) of amide local anaesthetic agents
| Generic name | Common brand names: | MSD in plain solution |
|---|---|---|
| Bupivacaine hydrochloride | 150 mg (2 mg/kg) | |
| Lidocaine hydrochloride | Xylocaine | 200 mg (3 mg/kg) |
| Levobupivacaine hydrochloride | Chirocaine | 150 mg (2 mg/kg) |
| Mepivacaine hydrochloride | 400 mg (6 mg/kg) | |
| Prilocaine hydrochloride | Citanest | 400 mg (6 mg/kg) |
| Ropivacaine hydrochloride | Naropin | 200 mg (4 mg/kg) |
| Etidocaine hydrochloride (USA) | Duranest | 300 mg (6 mg/kg) |
Note: in general, the MSD is increased in solutions that have vasoconstrictors added.
Bupivacaine
Bupivacaine, a homologue of mepivacaine and ropivacaine, is a very stable hydrochloride and has been used for its long duration, which may be up to four times that of lidocaine (McClure & Rubin 2005). It is reported to be four times as potent as lidocaine and mepivacaine – a 0.5% solution is approximately equivalent to a 2% solution of lidocaine. It is available in concentrations of 0.25–0.75% and can be acquired with 1 : 200 000 adrenaline added. If the latter agent is used, it would be prudent to retain the same MSD of 150 mg (Mather et al 2005). A disadvantage of bupivacaine is that it does have a slow onset time. There is more likelihood of cardiac toxicity with this drug; for example, cardiac arrhythmias and reduced myocardial contractility, often resulting in a resistant ventricular fibrillation (Royse & Royse 2005), and patients who suffered this were difficult to resuscitate. Bupivacaine may also increase the toxicity of certain drugs that are metabolised by plasma cholinesterases. In podiatry it is more often used in the surgical field.
Levobupivacaine
The great advantage of levobupivacaine over bupivacaine is its reduced cardiotoxicity, while it retains the desirable qualities of being long acting and virtually as potent. Levobupivacaine is the S-enantiomer of racemic bupivacaine (McLeod & Burke 2001). It is available as a 0.25%, 0.5% or 0.75% solution. It is not available in cartridges for dental syringes. The MSD is the same as for bupivacaine (i.e. 150 mg; 2 mg/kg). Levobupivacaine is contraindicated for a Bier’s block in those with severe hypotension, as it has been associated with cardiac shock and serious cardiac arrhythmias. There is little research to show that it is unsafe in other situations or with certain drugs, but it is best avoided in those taking antiarrhythmic drugs such as mexiletine (Burlacu & Bugg 2008). Patients given levobupivacaine should be warned not to drive and/or operate machinery afterwards, and while no time is specified on the data sheet 12 hours seems a sensible recommendation (Foster & Markham 2000).
Etidocaine
This agent is used predominantly in the USA, particularly by the dental profession. It is has a rapid onset and medium to long duration of action of approximately 4 hours. Generally available in concentrations of 1% and 1.5%, it is also available with 1:200 000 adrenaline added (Wynn 1995). Currently, this agent is not available to UK podiatrists, but it has been used for a long time by some American podiatrists (Caputo et al 1982).
Lidocaine
Since its introduction to clinical practice in 1948, lidocaine has become one of the most widely used local anaesthetics (Winstanley & Walley 2002). It is a fairly fast-acting local anaesthetic, with a variable duration of action, which may be as long as 3 hours. It is usually used in plain solutions by podiatrists, but from 1998 was available with adrenaline 1:200 000 added as a vasoconstrictor to certified podiatrists in the UK. The MSD is 200 mg (3 mg/kg). In solutions with adrenaline, the MSD is increased to 7 mg/kg to a maximum of approximately 490 mg. It is available in a number of concentrations from 0.5% to 4%; however, the higher concentrations are usually for intrathecal injection, while the 1% and 2% solutions are the favoured strengths for peripheral nerve block anaesthesia. It is usually supplied in ampoules, but may sometimes be available in a cartridge format that fits into the dental syringes much favoured in podiatry.
There are other medical uses for lidocaine, such as in the management of cardiac ventricular arrhythmia, in this case marketed as Xylogard, this use being due to its marked membrane-stabilising properties. However, for this reason high doses should be avoided in patients with hypovolaemia or heart block problems (Smith, 2007). There are also some pharmacological interactions to be considered, for example: propranolol (Inderal), a beta blocker used in patients with angina, which slows hepatic clearance; cimetidine, which is widely used for peptic ulceration, also interferes with hepatic clearance; and ranitidine, also an ulcer treatment, requires the same consideration. However, these are all considered relative contraindications, and in the main will not be a problem with small dosages (www.astrazeneca.co.uk).
Lidocaine is also used in topical creams, the most relevant of which is EMLA® (eutectic mixture of lignocaine and prilocaine), a cutaneous gel applied to the skin under a dressing for up to an hour to facilitate pain-free injections. This is particularly useful with nervous children.
Mepivacaine
Known under the brand names of Carbocaine, Scandonest and Scandicaine, mepivacaine is related to bupivacaine and ropivacaine and was introduced into clinical practice in 1957 (Ruetsch et al 2001). It is less toxic than lidocaine, particularly to neural tissues. Mepivacaine has a less pronounced vasodilator effect than lidocaine, probably because of its slower clearance. It has a longer duration and quicker onset of action than lidocaine. Because of its slower clearance its use is not advised in obstetric anaesthesia (Kuczkowski 2004). The MSD is 400 mg based on 6 mg/kg, thus enabling a larger dose to be given than with lidocaine. It is available in cartridges for use with dental or disposable syringes. It is available in concentrations of 0.5–4%. In podiatry, solutions up to 3% are used, with Scandonest 3% (plain solution) being the choice in the UK. While this drug is not licensed for the treatment of children, its use in podiatry is indemnified by the Society of Chiropodists & Podiatrists. The law allows podiatrists who hold a certificate of competence in local anaesthesia to administer the substance without any requirement that such administration must be in accordance with the terms of the licence.
The Society would consider the administration of Scandonest, suitably dose adjusted for children, to be acceptable professional practice. This would not of course preclude any civil liability should something go wrong, but this would be the situation whatever treatment was being administered.
(Ashcroft 2003, p. 6)
Prilocaine
Prilocaine was introduced into clinical practice in 1960, after being synthesised by Löfgren and Tegnér in 1959 (Ruetsch et al 2001). This agent has an MSD of 400 mg, and is most commonly available in concentrations of 1–4%. It is also available with the addition of octapressin or adrenaline for use in dentistry. It is available in ampoules or in dental cartridges in the higher concentrations. While the effectiveness and duration of action are only slightly greater than those of lidocaine or mepivacaine, its safety profile is superior to both due to its rapid clearance (Arthur et al 1987). Being a toluidine derivative its metabolism will produce ortho-toluidine, and in large doses (usually exceeding 600 mg) it may produce methaemoglobinaemia. This is a cyanotic condition and the patient will develop a bluish hue. It occurs because there are ferric rather than ferrous ions in the haematin in the haemoglobin, so the oxygen is unable to combine with ferric ions and transport the oxygen. It is not generally considered a serious condition in otherwise healthy individuals, but some authorities recommend not using prilocaine in the early stages of pregnancy, or in cases where there is severe anaemia or circulatory impairment (Haas & Carmichael 2007).
Citanest 4% has been a very popular with British podiatrists for many years, but can sometimes be difficult to obtain because of its license status. The producer, Astra-Zeneca, has advised against its use at the 4% strength in digital nerve blocks. The company advise the lower concentration of 1% or 2%, as they feel the 4% strength has led to injection-site necrosis on a number of occasions (Jones, 22 December 2000, personal correspondence). They emphasise that its licence is specifically for dentistry (Spires-Lane, 11 December 2000, personal correspondence ). The fact that the drug is licensed specifically for dentistry does not make it illegal for the podiatrist to use it; however, it does mean that the practitioner is willing to take responsibility for its use off licence, and should any mishap occur they would have to defend their use of that agent (Ashcroft, 11 January 2001, personal correspondence). The final decision remains with the podiatrist.
Ropivacaine
Although synthesised in the late 1950s by Ekenstam, Naropin was not used clinically until 1996 (Whiteside & Wildsmith 2001). It was made available to appropriately qualified UK podiatrists in November 2006 (Order 2807 Department of Health). It is produced as a single-enantiomer drug, unlike bupivacaine and mepivacaine, to which it is closely related. Bupivacaine and mepivacaine are analogues of ropivacaine. However, ropivacaine is marketed as a single stereoisomer, while bupivacaine and mepivacaine are sold as equal mixtures of the two possible stereoisomers. Ropivacaine was created for its long-term efficacy, having a duration of action about 10% less than that of bupivacaine, but with a much safer profile. It also has a more discrete separation between motor and sensory block (McClure 1996). The evidence for the side-effects of and contraindications to ropivacaine is sparse. There is no evidence for dangers in pregnancy and it is used for epidural deliveries. Its use on patients with hypotension should be avoided. There is no direct evidence to indicate that patients should not drive after receiving the drug, but good sense should prevail. Prolonged administration in patients on Faverin (a serotonin reuptake inhibitor/antidepressant) should be avoided (Hansen 2004). It is available in 10 ml polypropylene blister packs, in concentrations of 0.25%, 0.5% and 0.75%. The MSD is not clearly defined by the manufacturer, but the suggested volumes would indicate approximately 4 mg/kg with a maximum dose of 300 mg (Astra-Zeneca 2001). Unlike most other anaesthetic agents, the addition of adrenaline has little effect on the drug’s onset or duration.
LOCAL AND SYSTEMIC COMPLICATIONS AND TOXICITY
Local effects
The following is not exhaustive list, and in most circumstances the risk should be minimised by best clinical practice and attention to detail.
Systemic effects
Local anaesthetics are very safe, and in the doses used in standard podiatric practice are unlikely to cause problems. However, when larger doses are used, as in podiatric surgery, the risks increase (Maher et al 2008). Even larger volumes are used in orthopaedics and other disciplines, where patients may have a line access for continuous input of the anaesthetic (Wiegel et al 2007).
All local anaesthetics can cause systemic toxicity but, as mentioned above, some have a greater potential for specific problems (e.g. bupivacaine and cardiotoxicity). The patient’s ability for hepatic metabolism and renal clearance of amide anaesthetic agents has to be considered. Those agents that have a more rapid clearance will be safer than those that have a longer half-life and slower clearance. Acute toxicity following administration of anaesthetic solution is very rare, but recent advances have seen the introduction of Intralipid as an intravenous treatment for cardiac arrest following local anaesthetic reaction (Clark 2008). This has prompted the suggestion that patients undergoing foot surgery under popliteal blocks and large volumes of local anaesthetic should have an indwelling cannula in case of emergencies arising during the procedure (Maher et al 2008).
It is incumbent on the practitioner to assess the patient’s suitability for the anaesthetic and subsequent procedure (Crausman & Glod 2004). A full medical, medication and social history should be completed, and if there are doubts about the patient’s suitability for local anaesthesia the best practice would be to consult the patient’s medical practitioner or medical consultant.
The following areas are highlighted for particular consideration:
Central nervous system (CNS)
Local anaesthetics have an intrinsic ability to cause irritation of the CNS as they readily cross the blood–brain barrier. They stimulate the cerebral cortex but will then depress the medulla, particularly the vasomotor and respiratory centres (Finucane 2007). It would be considered very bad practice to administer excessively high doses of local anaesthetic to a patient, but if this occurred the patient may show one or more of the following signs/symptoms: restlessness, or general irritability, dizziness/blurred vision, and possibly tinnitus and numbness in the mouth region. It is then possible that they may feel nauseous and begin trembling and twitching, and possibly convulsing. Drowsiness, leading to unconsciousness may follow and, finally, this could lead respiratory failure. This could ultimately impact on the cardiovascular system, which in turn would also collapse. Specific conditions, such as patients with epilepsy, may need special consideration, especially if they are not stabilised well by their medication. However, it is often the ‘situation’, rather than the local anaesthetic that heightens tension and could precipitate a convulsion.
Cardiovascular system
Although higher doses of local anaesthetic are required to cause toxicity in the cardiovascular system than in the CNS, an initial increase in peripheral vascular resistance has been noted, probably because of the effects on vascular smooth muscle (Rang et al 2003). The main cardiovascular effects in very high doses would be depression of the myocardium, systemic hypotension due to a generalised vasodilatation and a decrease in myocardial contractibility. This would lead to a fall in cardiac output. Bradycardia and arrhythmias may then occur, ultimately leading to ventricular fibrillation and cardiac arrest. Bupivacaine has been noted for its cardiac toxicity (Casati & Putzu 2005) but the newer pure S-isomers levobupivacaine and ropivacaine have a much better safety profile with regard to cardiotoxicity.
Allergic reaction
A patient having a known allergy or hypersensitivity to a local anaesthetic will constitute an absolute contraindication. While fatalities due to an allergic reaction (e.g. due to a full-blown anaphylactic reaction) are extremely rare, a number of non-life-threatening situations may arise. For example, there may be an urticarial reaction or difficulty in breathing due to oedema in the laryngeal pathway. The number of cases of allergy, and in particular anaphylactic shock and death, are sometimes quoted as approximately 1 in 200 000, but the reliability of these statistics may be dubious (Finucane 2003).
Systemic toxicity is far more likely to occur when the anaesthetic is injected intravenously and in large doses. Good practice reduces these chances and leaves one time to deal with the ‘lesser emergencies’ caused by psychogenic reactions.
Hepatic and renal function
The metabolism, by hydroxylation and conjugation within the liver, of amide local anaesthetics relies on an adequately functioning hepatic system. In those patients suffering from any liver disorder, such as cirrhosis or hepatitis, care will be needed. A history of alcohol abuse may be an underlying factor. The clearance of the drug relies on kidney function, with up to 86% of drug being excreted via this channel (Malamed 2004).
Pregnancy
There is a dearth of research and literature pertaining to the use of local anaesthetics in pregnancy. There also appears to be a divergence of opinion on whether these drugs are safe to use in non-obstetric surgery, although many feel that their safety is established (Kuczkowski 2004). Known adverse affects, such as fetal bradycardia, have been documented in the literature. However, the caution that applies to the use during pregnancy of mepivacaine and bupivacaine in particular refers to data collected in animal teratogenic studies (Briggs et al 1990). That said, as it is known that these agents cross the placenta it seems sensible to avoid local anaesthetics in the first trimester, and as most foot surgery is elective the procedure may be delayed until after delivery. In emergency situations this debate may not arise.
Drug interactions
Individual interactions have been noted above under each of the local anaesthetic agents. However, it is worth emphasising that any drugs that compromise the liver’s ability to carry out detoxification by inhibiting the action of the hepatic microsomes, in particular the microsomal cytochrome P450 enzymes, may present a clinical problem (Smith 2007). For this reason, drugs such as the monoamine oxidase inhibitors and the procarbazines will require noting. The action of some drugs may be affected by the systemic action of the local anaesthetic rather than by a true pharmacological interaction; for example, by slowing the patient’s heart rate and with a generalised vasodilatation, patients taking diuretics and other antihypertensive agents may experience hypotension. Furthermore, one should be aware that summation interactions may occur where drugs have similar receptor sites. However, in the main, and particularly in podiatric practice, local anaesthetics have proven to be extremely safe.
LOCAL ANAESTHETICS IN PRACTICE
Safe use of local anaesthetics and the associated equipment is paramount. Not only has the patient’s health to be assessed, but also the safety of the practitioner.
Any injectable anaesthetic being used should be confirmed as the agent of choice and have its expiry date and batch number noted. These details should be entered into the patient’s record and kept with the consent forms for the administration of the agent and the subsequent procedure.
From the practitioner’s perspective, a needlestick injury poses the primary risk. Resheathing needles constitutes over 50% of needlestick incidents in the UK (Royal College of Nursing 2003). Other incidents occur during disposal, suturing and the procedure itself. The risk of associated blood-borne viruses is obvious (Gabriel 2009), and these injuries are preventable. A number of strategies may be employed to reduce this risk (Raghavendran et al 2006). Firstly, never resheath needles. For disposable systems, a needle guard may be used (Fig. 20.2). However, disposing of the whole system into a sharps container would be the best option. If reusable dental cartridge syringes are being used it is possible to use the Safe-Point system (Fig. 20.3), or to remove the needle using locking forceps. Several dental safety systems are available that can accept an anaesthetic cartridge and are ready fitted with a 27- or 30-gauge needle in either long or short format. On completion of the injection the needle is not disconnected, but a sliding protector sleeve is moved forward and covers the needle entirely; the whole apparatus is then disposed of.
To administer local anaesthetics successfully it is necessary to consider the anatomy of the area and the site of injection. Note any physical/anatomical barriers, such as scar tissue, skin lesions, superficial blood vessels and infected areas, that might impede the movement of the needle or cause damage to the tissues. Histologically, the myelin sheath will absorb varying amounts of the anaesthetic, and the diameter of the fibres in the area will also influence the outcome. There will often be a need to redirect the needle. Some 8–10 mm of nerve fibre will need to be affected by the local anaesthetic for anaesthesia to occur (de Jong 1994). Even with meticulous planning and procedure, anaesthetic failure can occur. This may be due in some cases to poor anatomical knowledge, anatomical variation or the patient’s psychological state, and, rarely, to specific conditions such as hypermobility syndromes (Hakim 2005).
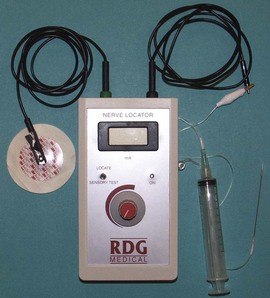
The scope of anaesthetic practice varies within podiatry. All UK podiatry students are trained in infiltration, and digital and ankle block techniques, but there is a wide variation in the actual use of these skills in practice. The technique most frequently used by UK podiatrists is the digital block, facilitating nail surgery. In the surgical field, podiatrists routinely perform ankle and popliteal blocks. With the larger and more proximal nerve blocks accurate identification of the site of the nerve may be difficult. This technique is augmented by the use of a nerve locator/stimulator (Fig. 20.4) or, increasingly, utilisation of ultrasound-guided injections. The use of the nerve stimulator requires insertion of a relatively blunt insulated needle deep into the tissues in the proximity of the nerve. The needle is supported by the use of the nerve stimulator, which emits an audible signal, allowing for adjustment of current on the stimulator. The muscle will twitch on stimulation and the current is then reduced until twitching stops. The process can be repeated until the operator is confident that the needle is in close proximity to the nerve, and the current is reduced to approximately 0.5 on the readout (Hardie & Lorimer 2006). The anaesthetic can then be introduced from the syringe via the cannula. The use of the stimulator is an efficient way of introducing relatively large amounts of anaesthetic into the popliteal fossa and, while there are some limitations to the process, the use of the nerve stimulator has been described as the gold standard for this type of anaesthesia (Jochim et al 2006). The use of the ultrasound-guided anaesthetic injection is developing and has been used in more proximal lower limb blocks (Peterson et al 2002). Furthermore, the combination of a nerve stimulator and ultrasound guidance seems to offer the least traumatic approach, and may assist in avoiding nerve damage (Moore et al 1994).
SPECIFIC SITES
Local infiltration
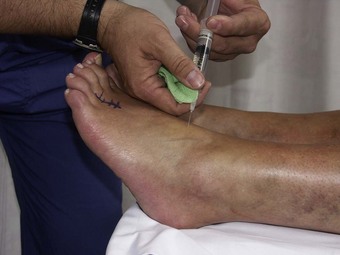
This is the simplest of the techniques and requires the deposition of the anaesthetic around and subcutaneously beneath the lesion (Fig. 20.5). It may be used for a variety of skin lesions, such as warts, basal cell carcinoma and various minor excrescences, and is ideal where electrocautery, curettage or scalpel excision is to be used (Koay & Orengo 2002).
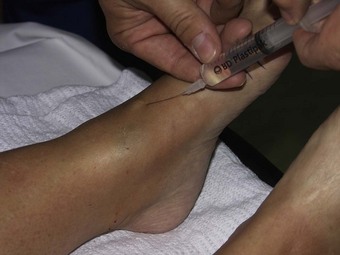
The digital block
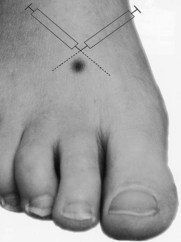
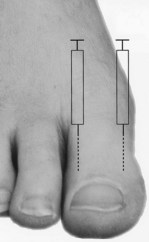
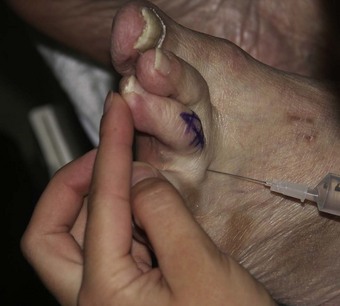
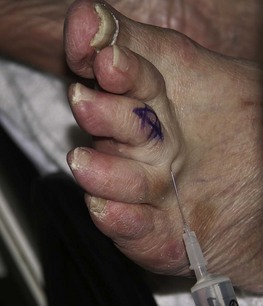
Perhaps the most widely used local anaesthetic procedure in podiatry, the digital block is administered to block the hallux and any of the lesser digits (Fig. 20.6). There are a number of variations to this technique, with each practitioner favouring their own. In its basic form the needle is directed from dorsal to plantar at an angle of 80–90° to the skin surface and slightly distal to the webbing line. The needle is advanced and local anaesthetic deposited en route and on the plantar side of the digit. The needle may be withdrawn slightly, without leaving the skin, and redirected so that the points of the needle converge towards the plantar surface, forming a ‘triangular approach’ (Figs 20.7 and 20.8). It is the authors’ practice to keep injecting the solution as the needle advances. This technique does not require aspiration, as the needle is moving and there are no large vessels in this area; however, this remains a decision for the individual practitioner to make. Some podiatrists will also direct the needle laterally across the dorsum of the toe (Uddin & Reilly 2008). In the hallux, the four digital nerves will be anaesthetised. The plantar nerves arise from the medial plantar nerve and these traverse the apex of the toe. Dorsally, the deep peroneal (fibular) will supply the medial border of the hallux and the medial dorsal cutaneous branch of the superficial peroneal (fibular) nerve will have some input along the lateral side. Anaesthesia can normally be achieved with approximately 2–4 ml of solution; occasionally there may be a need to use rather more. Lesser toes rarely require more than 1–2 ml. The main problems with this procedure arise if excessive amounts of solution are forced into an area where distension cannot occur (see earlier reference on personal communication). Digital blocks can be uncomfortable, especially in children or those with low pain thresholds. The use of a needleless system has been considered (Dialynas et al 2003), but has not found widespread use in podiatric practice. Furthermore, each practitioner has their own preferred delivery systems and technique, and novel methods are sometimes used as they may be less painful or use less local anaesthetic (Ouzounov 2005). The use of vasoconstrictors (e.g. adrenaline 1 : 200 000) in end organs and digits is generally advised against (Dollery 1999). However, there is little evidence to support its avoidance when using the amide anaesthetics (Wilhelmi et al 2001), and extensive literature searches have highlighted only 50 cases of digital gangrene since the turn of the last century, and none involving lidocaine with adrenaline (Denkler 2001). It might be that other factors, such as the use of digital tourniquets or infection, heighten the risk of necrosis. It should also be noted that much of the evidence pertains to the fingers, not the toes. Vasoconstrictors may also have adverse systemic effects, such as increased heart rate, tremors and palpitations.
The ray block
This is usually associated with the first (Mayo block) and fifth rays and is a highly effective technique for anaesthesia of the forefoot, for example for bunion, lesser ray procedures and neuroma. The needle is advanced at a proximal point in the metatarsal interspace and from a dorsal start and is then directed plantarly, where additional anaesthetic is deposited. The needle is then withdrawn and redirected medially and, if required, laterally so that there is a ‘collaring’ of the ray (Fig. 20.9). Anaesthesia of the other rays follows a similar pattern to the Mayo block. This can be a very effective technique, with a low failure rate of less than 1% (Worrell & Barbour 1996).
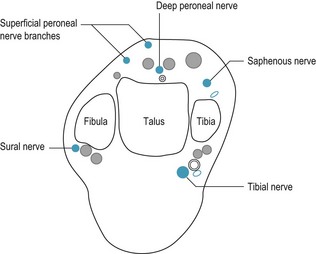
More extensive anaesthesia is achieved by blocking the nerves around the ankle. Only rarely is it necessary to block all the nerves at the same time. The nerves involved are the posterior tibial, saphenous, sural, deep and superficial peroneal (Fig. 20.10). All, with the exception of the saphenous nerve, which is a terminal branch of the femoral nerve, are branches of the sciatic nerve. The practicality of these injections is aided by having a relaxed and comfortable patient. All injections may be done with the patient supine, and rotating the leg into an accessible position. However, some practitioners would have the patient in a prone position for the posterior tibial and the sural blocks; it really depends on operator preference and to some extent the patient’s comfort.
Posterior tibial nerve
This is the larger of the two branches of the sciatic nerve (root index L4, L5 and S1, S2, S3). At the ankle it runs medially in the neurovascular bundle behind the posterior tibial artery. It passes behind the medial malleolus and flexor retinaculum, and then gives off calcaneal branches before dividing into the medial and lateral plantar nerves. As with all anatomy, there may be individual variations. This may be the reason for failure to achieve anaesthesia in some patients. The most useful indicator for finding the nerve is to palpate the posterior tibial pulse (McGlamry et al 2001). The usual way to approach this injection is to palpate the posterior tibial artery and introduce the needle at the medial aspect of the Achilles tendon. Advance the needle in an anterior direction to the posterior surface of the tibia, and then withdraw very slightly and aspirate before injecting. Whether you choose to actively cause a paraesthesia is again a matter for debate. It is advisable to inform the patient of possible paraesthesia. You should then withdraw slightly and aspirate, and this should result in a good anaesthesia. An alternative method is to go about 4 cm above the medial malleolus, direct the needle deeply to the medial border of the Achilles tendon and then inject into the neurovascular channel. A nerve stimulator may be used to locate the nerve. Although unlikely, putting too much local anaesthetic into the bundle could cause a pressure necrosis, as the nerve lies between the tibial and flexor retinaculum. Aspiration avoids intravascular injection. Approximately 5–10 ml of anaesthetic will be required, and there may be a time lag before full anaesthesia is achieved.
The sural nerve (root index S1/S2)
This nerve, also known as the lateral cutaneous nerve, continues down the posterior calf and runs behind the lateral malleolus with the short saphenous vein. It is a cutaneous nerve supplying the lateral aspect of the foot and the fifth toe. While not particularly difficult to anaesthetise, ultrasound guidance has been used to improve the success rate (MacFarlane & Brull 2009, Redborg 2009).
Insert the needle lateral to the Achilles tendon and at a right angle to the lateral malleolus (Fig. 20.11). Advance needle to lateral malleolus and then withdraw slightly. Aspirate and inject. It is possible to inject, with a fanning action, about 1 cm above the lateral malleolus and this tends to take out the small branches or one can raise a wheal from the Achilles tendon to the lateral malleolus.
Saphenous nerve (root index L3/L4)
This nerve, which is the sensory terminal branch of the femoral nerve, passes down the anteromedial aspect of the leg and enters the dorsum of the foot (accompanying the long saphenous vein) about one finger’s width from the medial malleolus (Fig. 20.12). Enter the needle between the tendon of the tibialis anterior and the long saphenous vein. Advance to the medial malleolus, aspirate and inject. Aspiration is essential due to the close proximity of the vein.
The superficial peroneal (fibular) nerves (root index L4/L5, S1)
One of two terminal branches of the common peroneal (fibular) nerve. It becomes cutaneous between the middle and distal thirds of the leg and divides into two branches, medial and lateral although the point of division varies from person to person. The medial branch supplies the skin of the mediodorsal aspect of the foot, medial aspect of the hallux and adjacent sides of the second, third and fourth toes (Dhukaram & Senthil 2004). The lateral branch supplies the intermediate and lateral skin on the dorsum of the foot, the adjacent sides of the third and fourth toes and the medial aspect of the fifth toe.
The medial branch is centrally located between the malleoli as it crosses the extensor digitorum longus (EDL) tendons. If you ask the patient to dorsiflex the foot, and then palpate the EDL tendons, you should be able to roll the nerve with your index finger. Insert the needle on either side of the nerve. It is not vital to aspirate for this procedure (Fig. 20.13).
The lateral branch can be palpated lateral to the peroneus (fibularis) tertius muscle between the malleoli. Ask the patient to dorsiflex the foot, and then palpate the peroneus tertius or lateral edge of the EDL muscles. Rolling the nerve will elicit a slight paraesthesia. Inject slowly, there is no need to aspirate. Alternatively, a wheal of anaesthetic may be deposited subcutaneously across the anterior aspect of the ankle.
The deep peroneal (fibular) nerve (root index L4/L5, S1/S2)
This enters the dorsum of the foot between the tendons of the tibialis anterior and the extensor hallucis longus (Kopka & Serpell 2005). It is covered by the superior and inferior retinaculum and runs deep, roughly following the course of the anterior tibial artery. It supplies opposing sides of the hallux and the second toe. Insert the needle between the two tendons and advance until you make contact with the bone, aspirate and inject. Anaesthesia will be over the superolateral foot (Fig. 20.14).
The popliteal block
The use of this block is expanding in podiatric surgery (Rees & Tagoe 2002). The technique is suitable for more extensive, long-duration forefoot and midfoot operations and for more involved rearfoot procedures where general and spinal anaesthesia is best avoided. In practical terms, both the saphenous nerve on the medial aspect of the knee and terminal portion of the sciatic nerve in the popliteal fossa will need to be blocked (Donohue et al 2004). In an anatomical sense this means that the tibial and common peroneal nerves, which share a common epineural sheath, will be anaesthetised.
This technique does mean that the patient is less mobile for some time following the procedure until motor function returns. However, this block allows a long postoperative anaesthesia, which helps with pain management and allows for a painless application of a midcalf or ankle tourniquet (Reilley et al 2002).
Because it is not possible to palpate these nerves, the use of a nerve stimulator/locator is required. In addition, a Doppler flowmeter may be used to identify the position of the popliteal artery.
Various techniques have been developed to approach the popliteal fossa. Donohue et al (2004) refer to the ‘classic’ approach, which is a posterior injection where the needle is introduced 1 cm lateral to a line drawn from the borders of the semimembranosus medially, the biceps femoris laterally and a line about 5 cm above the popliteal crease. However, they say that this has been superseded by an intertendinous approach that ‘calls for needle insertion midway between the semimembranosus and biceps femoris tendons and 5 cm above the popliteal crease’ (Donohue et al 2004, p. 370) (Fig. 20.15).
An alternative lateral approach may be used with the patient either sitting with the knee flexed or in a supine position. In this technique, the needle is passed from the lateral side of the thigh along the anterior border of the biceps femoris tendon, about 5 cm proximal to the popliteal crease. The saphenous nerve may also be anaesthetised by injecting along a line drawn from the tuberosity of the tibia to an intersection of the anterior and medial borders of the gastrocnemius muscle. Fairly large volumes (10–20 ml) of 0.5% levobupivacaine, 0.5% bupivacaine or 0.75% ropivacaine may need to be used.
The success of these procedures is likely to see their increased usage in podiatric surgery. However, while the procedure does have significant advantages in some scenarios, as highlighted above, the ankle block remains a very effective technique for most forefoot surgery (Miques et al 2005).
Arthur BR, Wildsmith JAW, Tucker GT. Pharmacology of local anaesthetic drugs. In: Wildsmith JAW, Armitage EN, editors. Principles and practice of regional anaesthesia. London: Churchill Livingstone, 1987.
Ashcroft D 2003 Statement of indemnity for Mepivacaine. Podiatry Now 6.
Astra-Zeneca 2001 Naropin (ropivacaine hydrochloride). Product monograph/data sheet.
Briggs GG, Freeman RK, Yaffe SJ. Drugs in pregnancy and lactation: a reference guide to fetal and neonatal risk, 3rd edn. Baltimore, OH: Williams & Wilkins; 1990.
Burlacu CL, Bugg DJ. Update on local anesthetics: focus on levobupivacaine. Therapeutic Clinical Risk Management. 2008;4(2):381-392.
Butterworth RF, Dockery GL. Forefoot Surgery. St Louis, MI: Mosby; 1992.
Calvey N, Williams NE. Principles and practice of pharmacology for anaesthetists, 5th ed. London: Wiley-Blackwell; 2008.
Caputo LJ, Hembree JL, Dobbs BM. Two long acting anesthetics: etidocaine HCL, bupivacaine HCL. Journal of the American Podiatry Association. 1982;72(4):186-190.
Casati A, Putzu M. Bupivacaine, levobupivacaine and ropivacaine: are they clinically different? Clinical Anaesthesiology. 2005;19(2):247-268.
Clark MK. Lipid emulsion as a rescue for local anaesthetic related cardiotoxicity. Journal of Perianesthesia Nursing. 2008;23(2):111-121.
Crausman RS, Glod DJ. Perioperative medical assessment of the podiatric surgical patient. Journal of the American Podiatric Medical Association. 2004;94(2):86-89.
Cavaliere RG, Pappas E. Anesthesia. 3rd ed. Banks AS, Downey MS, Martin DE, Miller SJ, editors. McGlamrys’ Comprehensive Textbook of Foot and Ankle Surgery. vol 1. Lippincott Williams and Wilkins, Philadelphia. 2001.
de Jong RH. Local anesthetics. St Louis, MI: Mosby; 1994.
Dagnall C. History of the Society 1945–1995. Journal of British Podiatric Medicine. 1995;50:21-27.
Denkler K. A comprehensive review of epinephrine in the finger: to do or not to do? Plastic & Reconstructive Surgery. 2001;108(1):114-124.
Dhukaram V, Senthil KC. Nerve blocks in foot and ankle surgery. Foot and Ankle Surgery. 2004;10(1):1-3.
Dialynas M, Hollingsworth SJ, Cooper D, Barker SGE. Use of a needleless injection system for digital ring block anesthesia. Journal of the American Podiatric Medical Association. 2003;93(1):23-26.
Dollery C. Therapeutic drugs, 2nd edn. Edinburgh: Churchill Livingstone; 1999.
Donohue CM, Goss LR, Metz S, et al. Combined popliteal and saphenous nerve blocks at the knee. Journal of the American Podiatric Medical Association. 2004;94(4):368-374.
Finucane B. Allergies to local anesthetics: the real truth. Canadian Journal of Anesthesia. 2003;5:869-874.
Finucane B. Complications of regional anesthesia, 2nd edn. Berlin: Springer-Verlag; 2007.
Foster RH, Markham A. Levobupivacaine: a review of its pharmacology and use as a local anaesthetic. Drugs. 2000;59(3):551-579.
Gabriel J. Reducing needlestick and sharps injuries among healthcare workers. Nursing Standard. 2009;23(22):41-44.
Hakim AJ. Local anaesthetic failure in joint hypermobility syndrome. Journal of the Royal Society of Medicine. 2005;98(2):84.
Hardie RJ, Lorimer DL. Local anaesthesia. In Lorimer DL, French GJ, O’Donnell M, Burrow JG, Wall B, editors: Neale’s disorders of the foot, 7th edn, Edinburgh: Churchill-Livingstone, 2006.
Haas DA, Carmichael FJL. Local anaesthetics. In: Kalant H, Roschalu WH, editors. Principles of medical pharmacology. London: Churchill Livingstone, 2007.
Hansen TG. Ropivacaine: a pharmacologic review. Expert review. Neurotherapeutics. 2004;4(5):781-791.
Jochim D, Iohom G, Diarra DP, et al. An objective assessment of nerve stimulators used for popliteal nerve blocks. Anaesthesia. 2006;61(6):557-564.
Koay J, Orengo L. Application of local anaesthesia in dermatologic surgery. Dermatological Surgery. 2002;28:143.
Kopka A, Serpell MG. Distal nerve blocks of the lower limb. Continuing Education in Anaesthesia, Critical Care and Pain. 2005;5(5):166-170.
Kuczkowski KM. Non-obstetric surgery during pregnancy: what are the risks of anesthesia? Obstetrical & Gynecological Survey. 2004;59(1):52-56.
Lagan G, McClure HA. Review of local anaesthetic agents. Current Anaesthesia & Critical Care. 2004;15:247-254.
MacFarlane A, Brull R. Ultrasound guided ankle block. The Journal of the New York School of Regional Anaesthesia (NYSORA). 2009;12:1-5.
McClure JH. Ropivacaine. British Journal of Anaesthesia. 1996;76:300-307.
McClure HA, Rubin AP. Review of local anaesthetic agents. Minerva Anestesiol. 2005;71:59-74.
McLeod GA, Burke D. Levobupivacaine. Anaesthesia. 2001;56:331-341.
Maher AJ, Metcalfe SA, Parr S. Local anaesthetic toxicity. The Foot. 2008;18(4):192-197.
Malamed SF. Handbook of local anesthesia, 5th edn. New York: Mosby Year Book; 2004.
Mather E, Copeland S, Ladd L. Acute toxicity of local anaesthetics: underlying pharmacokinetic and pharmacodynamic concepts. Regional Anaesthesia and Pain Medicine. 2005;30:553-566.
Miques A, Slullitel G, Vescovo A, et al. Peripheral foot blockade versus popliteal fossa nerve block: a prospective randomised trial in 51 patients. Journal of Foot & Ankle Surgery. 2005;44(5):354-357.
Moore DC, Mulroy MF, Thompson GE. Peripheral nerve damage and regional anaesthesia (editorial). British Journal of Anaesthesia. 1994;73:435-436.
Morgan R, Johnson M. Pharmacology for podiatrists. Oxford: Blackwell-Science; 2000.
Mulroy M. Systemic toxicity and cardiotoxicity from local anaesthetics: incidence and preventative measures. Regional Anesthesia & Pain Medicine. 2002;27:556-561.
Ouzounov KG. New nail block technique. Journal of the American Podiatric Medicine Association. 2005;95(6):589-592.
Peterson MK, Millar FA, Sheppard DG. Ultrasound guided nerve blocks (editorial). British Journal of Anaesthesia. 2002;88(5):621-623.
Raghavendran S, Bagry HS, Leith S, Budd JM. Needlestick injuries: a comparison of practice and attitudes in two UK district General Hospitals. Anaesthesia. 2006;6(9):867-872.
Rang HP, Dale MM, Ritter JM, Moore PK. Pharmacology. Edinburgh: Churchill-Livingstone; 2003.
Redborg KE, Sites BD, Chinn CD, et al. Ultrasound improves the success rate of a sural nerve at the ankle. Regional Anesthesia and Pain Medicine. 2009;34(1):24-28.
Rees S, Tagoe M. The efficacy and tolerance of local anaesthesia without sedation for foot surgery. The Foot. 2002;12:188-192.
Reilley TE, Gerhardt MA. Anaesthesia for foot and ankle surgery. Clinics in Podiatric Medicine and Surgery. 2002;19(1):125-147.
Rosenberg P, Veering B, Urmey W. Maximum recommended doses of local anesthetics: a multifactorial concept. Regional Anesthesia and Pain Medicine. 2004;29(6):564-575.
Royal College of Nursing. Monitoring sharps injuries: what can the RCN EPInet surveillance study tell us?. London: RCN; 2003.
Royse CT, Royse AG. The myocardial and vascular effects of bupivacaine, levobupivacaine and ropivacaine using pressure volume loops. Anesthesia and Analgesia. 2005;101:679-687.
Ruetsch YA, Boni T, Borgeat A. From cocaine to ropivacaine: the history of local anaesthetic drugs. Current Topics in Medicinal Chemistry. 2001;1(3):175-182.
Safe-Point Healthcare Ltd. Safe-Point – the automatic needle remover for dentists and podiatrists. Available at http:www.safe-point.co.uk. (18 September 2009)
Scholz A. Mechanisms of (local) anaesthetics on voltage gated sodium and other ion channels. British Journal of Anaesthesia. 2002;89:52-61.
Smith T. Systemic effects of local anaesthetics. Anaesthesia and Intensive Care Medicine. 2007;8(4):155-158.
Strichartz GR, Ritchie JM. The action of local anaesthetics on ion channels of excitable tissues. In: Strichartz GR, editor. Local anaesthetics: handbook of experimental pharmacology. Berlin: Springer-Verlag; 1987:21-52.
Tetztaff J. The Pharmacology of local anesthetics. Anesthesiology Clinics of North America. 2000;18(2):217-233.
Thompson CJ, Lalonde DH. Randomised double blind comparison of duration of anesthesia among three commonly used agents. Plastic and Reconstructive Surgery. 2006;118(2):429-432.
Uddin A, Reilly I. Ropivacaine and levobupivacaine: potential uses in podiatric medicine and surgery. Podiatry Now. 2008;11(4):22-28.
Whiteside JB, Wildsmith JAW. Developments in local anaesthetic drugs. British Journal of Anaesthesia. 2001;87(1):27-35.
Wiegel M, Gottschalt U, Hennenbach R, et al. Complications and adverse effects associated with continuous peripheral nerve blocks in orthopaedic patients. Anesthesia & Analgesia. 2007;104(6):1578-1582.
Wildsmith AW. Local anaesthetic agents. In: Aitkenhead AR, Smith G, David J, et al, editors. Textbook of anaesthesia. Edinburgh: Churchill Livingstone, 2007.
Wildsmith JA, Gissen AJ, Takman B, et al. Differential nerve blockade: esters versus amides and the influence of pKa. British Journal of Anaesthesia. 1987;59:379-384.
Wilhelmi BJ, Blackwell SJ, Miller JH, et al. Do not use epinephrine in digital blocks: myth or truth? Plastic & Reconstructive Surgery. 2001;107(2):393-397.
Winstanley P, Walley T. Medical pharmacology, 2nd edn. Edinburgh: Churchill Livingstone; 2002.
Worrell JB, Barbour G. The Mayo block: an efficacious block for hallux and first metatarsal surgery. American Association of Nurse Anesthetics. 1996;64(2):146-152.
Wynn R L. Recent research on mechanisms of local anesthetics. General Dentistry. 1995;43(4):361-368.
British Medical Association. http://www.bma.org.uk (19 September 2009)
British National Formulary. www.bnf.org. (19 September 2009)
Martindale’s: The ‘Virtual’ Pharmacy, Pharmacology & Toxicology Center. www.martindalecenter.com. (19 September 2009)
New York School of Regional Anesthesia (NYSORA). http://www.nysora.com. (19 September 2009)
Ashcroft D Personal correspondence by email, 11 January 2001, SOCAP.
Jones G Personal correspondence by email, 11 December 2000, Astra-Zeneca.
Spires-Lane V Personal correspondence by email, 22 December 2000, 361–368, Astra-Zeneca.