CHAPTER 139 Cardiovascular System Assessment
CHAPTER 139 Cardiovascular System Assessment
HISTORY
The focus of the cardiovascular history depends on patient’s age and is directed by the chief complaint. The prenatal history may reveal a maternal infection early in pregnancy (possibly teratogenic) or later in pregnancy (causing myocarditis or myocardial dysfunction in infants). A maternal history of medication, drug, or alcohol use or excessive smoking may contribute to cardiac and other systemic findings. While growth is a valuable sign of cardiovascular health, the birth weight is an indicator of the prenatal health of the fetus and the mother. Infants with heart failure grow poorly, with weight being more significantly affected than height and head circumference.
Heart failure may present with fatigue or diaphoresis with feeds or fussiness. Feeding may be difficult and prolonged because of tachypnea. Tachypnea without significant dyspnea may be present. Older children with heart failure may have easy fatigability, shortness of breath on exertion, and sometimes orthopnea. Exercise intolerance may be determined by asking how well children keep up while playing or exercising. Patients may have been misdiagnosed with recurrent pneumonia, bronchitis, wheezing, or asthma before heart failure is recognized.
A history of a heart murmur is important, but many well children have an innocent murmur at some time in their lives. Other cardiac symptoms include cyanosis, palpitations, chest pain, syncope, and near-syncope. A review of systems assesses for possible systemic diseases or congenital malformation syndromes that may cause cardiac abnormalities (Tables 139-1 and 139-2). Current and past medication use as well as history of drug use, is important. Family history should be reviewed for hereditary diseases, early atherosclerotic heart disease, congenital heart disease, sudden unexplained deaths, thrombophilia, rheumatic fever, hypertension, and hypercholesterolemia.
TABLE 139-1 Cardiac Manifestations of Systemic Diseases
| Systemic Disease | Cardiac Complications |
|---|---|
| Hunter-Hurler syndrome | Valvular insufficiency, heart failure, hypertension |
| Fabry disease | Mitral insufficiency, coronary artery disease with myocardial infarction |
| Pompe disease | Short PR interval, cardiomegaly, heart failure, arrhythmias |
| Friedreich ataxia | Cardiomyopathy, arrhythmias |
| Duchenne dystrophy | Cardiomyopathy, heart failure |
| Juvenile rheumatoid arthritis | Pericarditis |
| Systemic lupus erythematosus | Pericarditis, Libman-Sacks endocarditis, congenital AV block |
| Marfan syndrome | Aortic and mitral insufficiency, dissecting aortic aneurysm |
| Homocystinuria | Coronary thrombosis |
| Kawasaki disease | Coronary artery aneurysm, thrombosis, myocardial infarction, myocarditis |
| Lyme disease | Arrhythmias, myocarditis, heart failure |
| Graves disease (hyperthyroidism) | Tachycardia, arrhythmias, heart failure |
| Tuberous sclerosis | Cardiac rhabdomyoma |
| Neurofibromatosis | Pulmonic stenosis, coarctation of aorta |
AV, atrioventricular.
PHYSICAL EXAMINATION
A complete cardiovascular examination starts in the supine position and includes evaluation in sitting and standing positions when possible. Much information regarding the cardiovascular status can be gained by inspection, which is supplemented by palpation and auscultation.
The examination starts with vital signs. The normal heart rate varies with age and activity. Newborn resting heart rates are approximately 120 beats/minute. It is slightly higher in infants 3 to 6 months of age and then gradually declines through adolescence when the average resting rate is near 80 beats/minute. The range of normal for any given age is relatively wide, approximately 30 beats/minute above or below the average. Tachycardia may be a manifestation of anemia, dehydration, shock, heart failure, or dysrhythmia. Bradycardia can be a normal finding in patient with high vagal tone (athletes), but may be a manifestation of atrioventricular block. The respiratory rate of infants is best assessed while observing the infant sitting quietly with the parent. Respiratory rate may be increased when there is a left-to-right shunt or pulmonary venous congestion.
Normal blood pressure also varies with age. A properly sized cuff, needed to obtain reliable information, should have a bladder width that is at least 90% of the arm circumference and a length that is 80% to 100% of the arm circumference. Initially, blood pressure in the right arm is measured. If elevated, measurements in the left arm and legs are indicated to evaluate for possible coarctation of the aorta. The pulse pressure is determined by subtracting the diastolic pressure from the systolic pressure. It is normally below 50 mm Hg or half the systolic pressure, whichever is less. A wide pulse pressure may be seen with aortopulmonary connections (patent ductus arteriosus [PDA], truncus arteriosus, arteriovenous malformations), aortic insufficiency, or relative intravascular volume depletion (anemia, vasodilation with fever or sepsis). A narrow pulse pressure is seen with pericardial tamponade, aortic stenosis, and heart failure.
Inspection includes general appearance, nutritional status, circulation, and respiratory effort. Many chromosomal abnormalities and syndromes associated with cardiac defects have dysmorphic features or failure to thrive (see Table 139-2). Skin color must be assessed for cyanosis and pallor. Central cyanosis (tongue, lips) is associated with arterial desaturation; isolated peripheral cyanosis (hands, feet) is associated with normal arterial saturation and increased peripheral extraction of oxygen. Perioral cyanosis is a common finding, especially in pale infants or when infants and toddlers become cold. Chronic arterial desaturation results in clubbing of the fingernails and toenails. Inspection of the chest may reveal asymmetry or a prominent left precordium suggesting chronic cardiac enlargement.
After inspection, palpation of pulses in all four extremities, the precordial activity, and the abdomen is performed. Pulses are assessed for rate, regularity, intensity, symmetry, and timing between upper and lower extremities. A good pedal pulse with normal right arm blood pressure effectively rules out coarctation of the aorta. Precordial palpation may suggest significant cardiovascular disease in the absence of obvious auscultatory findings. The precordium should be assessed for apical impulse, point of maximum impulse, hyperactivity, and presence of a thrill. Abdominal palpation assesses liver and spleen size. The liver size provides an assessment of intravascular volume and is enlarged with systemic venous congestion. The spleen may be enlarged with infective endocarditis.
Auscultation is the most important part of the cardiovascular examination, but should supplement what already has been found by inspection and palpation. Systematic listening in a quiet room allows assessment of each portion of the cardiac cycle. In addition to heart rate and regularity, the heart sounds, clicks, and murmurs need to be timed and characterized.
Heart Sounds
S1 is associated with closure of the mitral and tricuspid valves, is usually single, and is best heard at the lower left sternal border (LLSB) or apex (Fig. 139-1). Although it can normally be split, if a split S1 is heard, the possibility of an ejection click or, much less commonly, an S4 should be considered. S2 is associated with closure of the aortic and pulmonary valves. It should normally split with inspiration and be single with exhalation. Abnormalities of splitting and intensity of the pulmonary component are associated with significant anatomic and physiologic abnormalities (Table 139-3). S3 is heard in early diastole and is related to rapid ventricular filling. It is best heard at the LLSB or apex and may be a normal sound. A loud S3 is abnormal and heard in conditions with dilated ventricles. S4 occurs late in diastole just before S1, is best heard at the LLSB/apex, and is associated with decreased ventricular compliance. It is rare and is always abnormal.
TABLE 139-3 Abnormal Second Heart Sound
SINGLE S2
WIDELY SPLIT S2
PARADOXICALLY SPLIT S2
ABNORMAL INTENSITY OF P2
ASD, atrial septal defect; d-TGA, dextro-transposition of the great arteries; l-TGA, levo-transposition of the great arteries; PAPVR, partial anomalous pulmonary venous return.
Clicks
A click implies a valvular abnormality or dilated great artery. It may be ejection or midsystolic in timing and may or may not be associated with a murmur. A midsystolic click is associated with mitral valve prolapse. Ejection clicks occur early in systole. Pulmonary ejection clicks are best heard at the left upper sternal border and vary in intensity with respiration. Aortic clicks are often louder at the apex, left midsternal border, or right upper sternal border and do not vary with respiration.
Murmurs
Murmur evaluation should determine timing, duration, location, intensity, radiation, and frequency or pitch of the murmur. The timing is most important in determining a murmur’s significance and can be used to develop a differential diagnosis and determine the need for further evaluation (see Fig. 139-1). Murmurs should be classified as systolic, diastolic, or continuous. Most murmurs are systolic and can be divided further into systolic ejection murmurs or holosystolic (also called pansystolic or regurgitant) murmurs. Ejection murmurs are crescendo-decrescendo with a short time between S1 and the onset of the murmur (isovolumic contraction). Systolic ejection murmurs require the ejection of blood from the ventricle and may occur with aortic stenosis, pulmonary stenosis, atrial septal defects (ASDs), and coarctation of the aorta. Holosystolic murmurs have their onset with S1. The murmur has a plateau quality and may be heard with ventricular septal defects (VSDs) and mitral or tricuspid regurgitation. A late regurgitant murmur may be heard after a midsystolic click in mitral valve prolapse.
Murmurs are often heard along the path of blood flow. Ejection murmurs usually are best heard at the base of the heart, whereas holosystolic murmurs are louder at the LLSB and apex. Pulmonary ejection murmurs radiate to the back and axilla. Aortic ejection murmurs radiate to the neck. The intensity or loudness of a heart murmur is assessed as grade I through VI (Table 139-4). The frequency or pitch of a murmur provides information regarding the pressure gradient. The higher the pressure gradient across a narrowed area (valve, vessel, or defect), the faster the flow and higher the frequency of the murmur. Low-frequency murmurs imply low pressure gradients and mild obstruction or less restriction to flow.
TABLE 139-4 Heart Murmur Intensity
| Grade | Description |
|---|---|
| Grade I | Very soft, heard in quiet room with cooperative patient |
| Grade II | Easily heard but not loud |
| Grade III | Loud but no thrill |
| Grade IV | Loud with palpable thrill |
| Grade V | Loud with thrill, audible with stethoscope at 45-degree angle |
| Grade VI | Loud with thrill, audible with stethoscope off chest 1 cm |
Diastolic murmurs are much less common than systolic murmurs, and should be considered abnormal. Early diastolic murmurs occur when there is regurgitation through the aortic or pulmonary valve. Mid-diastolic murmurs are caused by increased flow (ASD, VSD) or anatomic stenosis across the mitral or tricuspid valves.
Continuous murmurs are heard when there is flow through the entire cardiac cycle and are abnormal with one common exception, the venous hum. A PDA is the most common abnormal continuous murmur. Continuous murmurs can also be heard with coarctation of the aorta when collateral vessels are present.
Normal physiologic or innocent murmurs are common, occurring in at least 80% of normal infants and children. They have also been called benign, functional, vibratory, and flow murmurs. These normal murmurs are heard most often during the first 6 months of life, from 3 to 6 years of age, and in early adolescence. Characteristic findings of innocent murmurs include the quality of the sound, lack of significant radiation, and significant alteration in the intensity of the murmur with positional changes (Table 139-5). Most important, the cardiovascular history and examination are otherwise normal. The presence of symptoms, including failure to thrive or dysmorphic features should make one more cautious about diagnosing a normal murmur. Diastolic, holosystolic, late systolic, and continuous (except for the venous hum) murmurs and the presence of a thrill are not normal.
TABLE 139-5 Normal or Innocent Heart Murmurs
| Murmur | Timing/Location/Quality | Usual Age at Diagnosis |
|---|---|---|
| Still’s murmur/vibratory murmur | 3–6 yr | |
| Venous hum | 3–6 yr | |
| Carotid bruit | Any age | |
| Adolescent ejection murmur | 8–14 yr | |
| Peripheral pulmonary stenosis | Newborn–6 mo |
LLSB, left lower scapular border; LUSB, left upper scapular border; RUSB, right upper scapular border.
LABORATORY AND IMAGING TESTS
Pulse Oximetry
Recognizing cyanosis depends on experience and the patient’s hemoglobin. Mild desaturation that is not clinically apparent may be the only early finding in complex congenital heart defects. Comparing pulse oximetry between the right arm and a lower extremity may allow diagnosis of a ductal dependent lesion in which desaturated blood flows right to left across a PDA to perfuse the lower body.
Electrocardiography
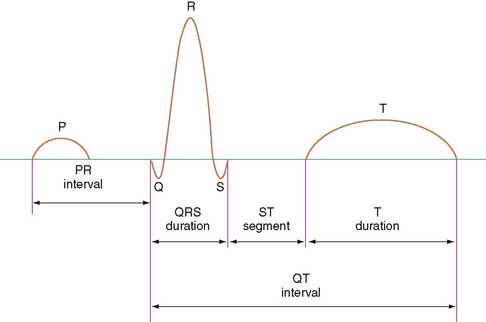
The 12-lead electrocardiogram (ECG) provides information about the rate, rhythm, depolarization, and repolarization of the cardiac cells and the size and wall thickness of the chambers. It should be assessed for rate, rhythm, axis (P wave, QRS, and T wave), intervals (PR, QRS, QTc) (Fig. 139-2), and voltages (left atrial, right atrial, left ventricular, right ventricular) adjusted for the child’s age.
The P wave represents atrial depolarization. A criterion for right atrial enlargement is an increase of the amplitude of the P wave, reflected best in lead II. The diagnosis of left atrial enlargement is made by prolongation of the second portion of the P wave, exhibited best in the chest leads.
The PR interval, measured from the beginning of the P wave to the beginning of the QRS complex, represents the time it takes for electricity to travel from the high right atrium to the ventricular myocardium. The PR interval increases with age. Conduction time is shortened when the conduction velocity is increased (glycogen storage disease) or when the atrioventricular node is bypassed (Wolff-Parkinson-White syndrome). A prolonged PR interval usually indicates slow conduction through the atrioventricular node. Diseases in the atrial myocardium, bundle of His, or Purkinje system may also contribute to prolonged PR intervals.
The QRS complex represents ventricular depolarization. A specific sequence of activation can be observed on the ECG. A greater ventricular volume or mass causes a greater magnitude of the complex. The proximity of the right ventricle to the chest surface accentuates that ventricle’s contribution to the complex. Changes in the normal ECG occur with age. Normative data for each age group must be known to make a diagnosis from the ECG.
The QT interval is measured from the beginning of the QRS complex to the end of the T wave. The corrected QT interval (corrected for rate) should be less than 0.45 second  . The interval may be prolonged in children with hypocalcemia or severe hypokalemia. It is also prolonged in a group of children at risk for severe ventricular arrhythmias and sudden death (prolonged QT syndrome). Drugs such as quinidine and erythromycin may prolong the QT interval.
. The interval may be prolonged in children with hypocalcemia or severe hypokalemia. It is also prolonged in a group of children at risk for severe ventricular arrhythmias and sudden death (prolonged QT syndrome). Drugs such as quinidine and erythromycin may prolong the QT interval.
Chest Radiography
A systematic approach to reading a chest radiograph includes assessment of extracardiac structures, the shape and size of the heart, and the size and position of the pulmonary artery and aorta (Fig. 139-3). Abnormalities of the thoracic skeleton, diaphragms, lungs, or upper abdomen may be associated with congenital heart defects. Assessment of the location and size of the heart and cardiac silhouette may suggest a cardiac defect. On a good inspiratory film, the cardiothoracic ratio should be less than 55% in infants under 1 year of age and less than 50% in older children and adolescents. An enlarged heart may be due to an increased volume load (left-right shunt) or due to myocardial dysfunction (dilated cardiomyopathy). A normal heart size virtually rules out heart failure; however, a large heart is not diagnostic of heart failure. The shape of the heart may suggest specific congenital heart defects. The most common examples are the boot-shaped heart seen with tetralogy of Fallot, the egg-on-a-string seen with dextroposed transposition of the great arteries, and the “snowman” seen with supracardiac total anomalous pulmonary venous return. The chest x-ray can aid in the assessment of pulmonary blood flow. Defects associated with left-to-right shunting have increased pulmonary blood flow (shunt vascularity) on x-ray. Right-to-left shunts have decreased pulmonary blood flow.
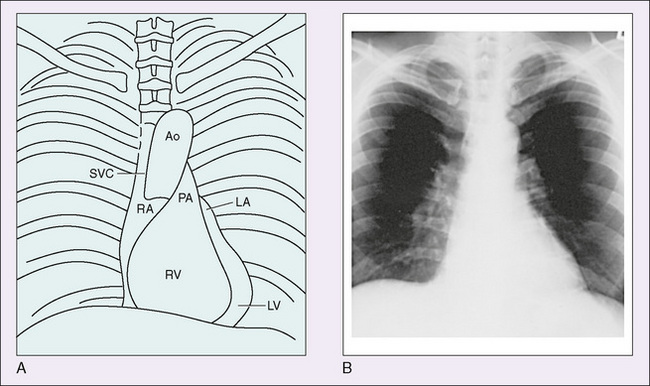
FIGURE 139-3 A, Parts of the heart whose outlines can be identified on a routine chest x-ray. B, Routine posteroanterior x-ray of the normal cardiac silhouette. Ao, aorta; LA, left atrium; LV, left ventricle; PA, pulmonary artery; RA, right atrium; RV, right ventricle; SVC, superior vena cava.
(From Andreoli TE, Carpenter CCJ, Plum F, et al [eds]: Cecil Essentials of Medicine, 2nd ed. Philadelphia, WB Saunders, 1990.)
Echocardiography
Echocardiography has become the most important noninvasive tool in the diagnosis and management of cardiac disease. Using ultrasound, two-dimensional echocardiography provides a full anatomic evaluation in most congenital heart defects (Fig. 139-4). Physiologic data on the direction of blood flow can be obtained with the use of pulsed, continuous wave, and color flow Doppler. Imaging from multiple views provides an assessment of spatial relationships. Three-dimensional (3D) and four-dimensional (4D) imaging now allows reconstruction of the heart and manipulation of the images to provide more detail, especially with regard to valve structure and function. Prenatal or fetal echocardiography can diagnose congenital heart disease by 18 weeks of gestation and allows for delivery of the infant at a tertiary care hospital, improving the timeliness of therapy. Many congenital heart defects now are surgically repaired based on the echocardiogram without need for cardiac catheterization.
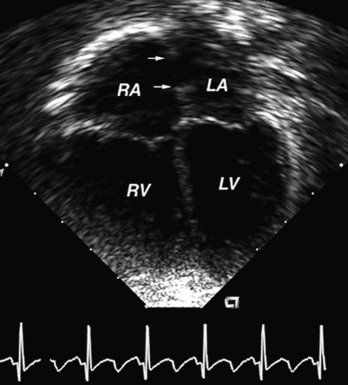
FIGURE 139-4 Four-chamber echocardiogram of an atrial septal defect. The defect margins are identified by the two arrows. LA, left atrium; LV, left ventricle; RA, right atrium; RV, right ventricle.
Transesophageal echocardiography (TEE) provides better imaging when transthoracic imaging is inadequate. It is used intraoperatively to assess results and cardiac function after surgery. TEE and intracardiac echocardiography are used to guide interventional catheterization and radiofrequency ablation of dysrhythmias.
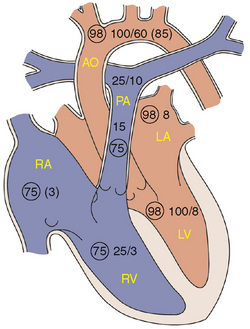
Cardiac Catheterization
Cardiac catheterization is performed in patients who need additional anatomic information or precise hemodynamic information before operating or establishing a management plan. Pressures, oxygen saturations, and oxygen content are measured in each chamber and blood vessel entered (Fig. 139-5). This information is used to calculate systemic and pulmonary blood flow and systemic and pulmonary vascular resistance. Angiography is performed by injecting contrast material into selected sites to define anatomy and supplement noninvasive information. An increasing percentage of cardiac catheterizations are done to perform an intervention including balloon dilation of stenotic valves and vessels, ballooning and stenting of stenotic lesions, closure of collateral vessels by coil embolization, and device closure of PDAs, secundum ASDs, patent foramen ovales, and muscular VSDs. Catheterization with electrophysiologic studies allows for precise mapping of the electrical activity, can assess the risk of abnormal heart rhythms, and often are done in anticipation of radiofrequency ablation. Radiofrequency ablation alters the site of a dysrhythmia so that it no longer can cause the dysrhythmia.