CHAPTER 149 Hematology Assessment
CHAPTER 149 Hematology Assessment
HISTORY
A detailed, careful history of the onset, severity, progression, associated symptoms, presence of systemic complaints, and exacerbating factors is crucial to the diagnosis of a blood disorder. In many blood disorders, a detailed pedigree is essential because a pattern of inheritance can point to the diagnosis.
PHYSICAL EXAMINATION AND COMMON MANIFESTATIONS
The physical examination of patients with blood disorders first focuses on hemodynamic stability. Acute episodes of anemia may be life-threatening, presenting with impairment of perfusion and cognitive status. The two most common findings of anemia include pallor and jaundice. The presence of petechiae, purpura, or deeper sites of bleeding, including generalized hemorrhage, indicates abnormalities of platelets, coagulation factors, or a consumptive coagulopathy. Growth parameters point to whether anemia is an acute or chronic process. Severe types of anemia, thrombocytopenia, and pancytopenia are associated with congenital anomalies and a pattern of growth delay. The presence or absence of other organ system involvement or systemic illness (especially hepatosplenomegaly and lymphadenopathy), point to a generalized illness as the cause for hematologic abnormalities (Table 149-1).
TABLE 149-1 Presentation of Hematologic Disorders
| Condition | Symptoms and Signs | Common Examples |
|---|---|---|
| Anemia | Pallor, fatigue, heart failure, jaundice | Iron deficiency, hemolytic anemia |
| Polycythemia | Irritability, cyanosis, seizures, jaundice, stroke, headache | Cyanotic heart disease, infant of diabetic mother, cystic fibrosis |
| Neutropenia | Fever, pharyngitis, oral ulceration, cellulitis, lymphadenopathy, bacteremia, gingivitis | Congenital or drug-induced agranulocytosis, leukemia |
| Thrombocytopenia | Petechiae, ecchymosis, gastrointestinal hemorrhage, epistaxis | ITP, leukemia |
| Coagulopathy | Bruising, hemarthrosis, mucosal bleeding | von Willebrand disease, hemophilia, DIC |
| Thrombosis | Pulmonary embolism, deep vein thrombosis | Lupus anticoagulant; protein C, protein S, or antithrombin III deficiency; factor V Leiden, prothrombin 20210 |
DIC, disseminated intravascular coagulation; ITP, idiopathic thrombocytopenic purpura.
INITIAL DIAGNOSTIC EVALUATION
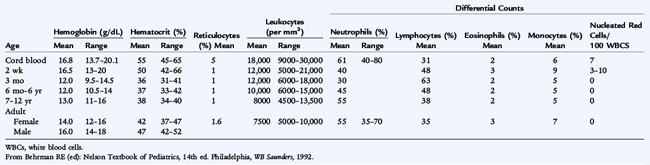
The basis for the diagnosis of blood disorders is laboratory testing. Diagnosis of pediatric blood disorders requires a detailed knowledge of normal hematologic values during infancy and childhood which varies according to age and, after puberty, according to sex (Table 149-2). From the history, physical examination, and screening laboratory studies, the astute clinician can use specific diagnostic testing to confirm the diagnosis.
DEVELOPMENTAL HEMATOLOGY
Hematopoiesis begins by 3 weeks of gestation with erythropoiesis in the yolk sac. By 2 months’ gestation, the primary site of hematopoiesis has migrated to the liver. By 5 to 6 months’ gestation, the process of hematopoiesis shifts from the liver to the bone marrow. An extremely premature infant may have significant extramedullary hematopoiesis due to limited bone marrow hematopoiesis. During infancy, virtually all marrow cavities are actively hematopoietic and the proportion of hematopoietic to stromal elements is quite high. As the child grows, hematopoiesis moves to the central bones of the body (vertebrae, sternum, ribs, and pelvis), and the marrow is gradually replaced with fat. This replacement is partially reversible. Hemolysis or marrow damage may lead to marrow repopulation of cavities where hematopoiesis previously had ceased or may cause a delay in the shift of hematopoiesis. Children with thalassemia and other chronic hemolytic diseases may have large head circumferences and prominent skull bones as a result of increased erythropoiesis within the medullary cavities of the skull. Hepatosplenomegaly in patients with chronic hemolysis may signify extramedullary hematopoiesis. When a patient with cytopenia is being evaluated, a bone marrow examination provides valuable information about processes that lead to underproduction of circulating cells. Additionally, bone marrow infiltration by neoplastic elements or storage cells often occurs in concert with infiltration in the spleen, liver, and lymph nodes.
The hematopoietic cells consist of the following:
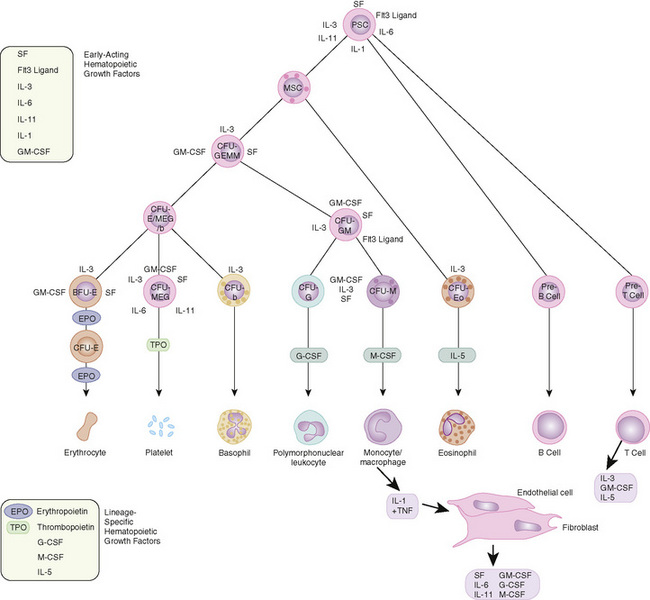
FIGURE 149-1 Major cytokine sources and actions to promote hematopoiesis. Cells of the bone marrow microenvironment, such as macrophages, endothelial cells, and reticular fibroblasts, produce macrophage colony-stimulating factor (M-CSF), granulocyte-macrophage colony-stimulating factor (GM-CSF), and granulocyte colony-stimulating factor (G-CSF) after stimulation. These cytokines and others as listed in the text have overlapping interactions during hematopoietic differentiation, as indicated; for all lineages, optimal development requires a combination of early and late acting factors. BFU, burst-forming unit; CFU, colony-forming unit; EPO, erythropoietin; IL, interleukin; MSC, myeloid stem cells; PSC, pluripotent stem cells; TNF, tumor necrosis factor; TPO, thrombopoietin.
(From Sieff CA, Nathan DG, Clark SC: The anatomy and physiology of hematopoiesis. In Orkin SH, Nathan DG [eds]: Hematology of Infancy and Childhood, 5th ed. Philadelphia, WB Saunders, 1998, p 168.)
Hematopoiesis is controlled by numerous cytokines. The bone marrow is the major storage organ for mature neutrophils and contains about seven times the intravascular pool of neutrophils. It contains 2.5 to 5 times as many cells of myeloid lineage as cells of erythroid lineage. Smaller numbers of megakaryocytes, plasma cells, histiocytes, lymphocytes, and stromal cells are also stored in the marrow.
Erythropoiesis (red blood cell [RBC] production) is controlled by erythropoietin, a glycoprotein that stimulates primitive pluripotential stem cells to differentiate along the erythroid line and is made by the juxtaglomerular apparatus of the kidney in response to local tissue hypoxia. The normally high hemoglobin level of the fetus is a result of fetal erythropoietin production in the liver in response to low PO2 in utero. Control of erythropoiesis by erythropoietin begins at the time of hepatic hematopoiesis in early gestation. Erythropoietin leads to production of the erythroid colony-forming unit. The earliest recognizable erythroid cell in vivo is the erythroblast, which forms eight or more daughter cells. The immature RBC nucleus becomes gradually pyknotic as the cell matures and eventually is extruded before being released from the marrow as a reticulocyte. The reticulocyte maintains residual mitochondrial and protein synthetic capacity. These highly specialized RBC precursors are engaged primarily in the production of globin chains, glycolytic enzymes, and heme. Iron is taken up via transferrin receptors and incorporated into the heme ring, which combines with globin chains synthesized within the immature RBC. When the messenger RNA and mitochondria are gone from the RBC, heme or protein synthesis is no longer possible; however, the RBC continues to function for its normal life span of about 120 days in older children and adults.
During embryonic and fetal life, the globin genes are sequentially activated and inactivated. Embryonic hemoglobins are produced during yolk sac erythropoiesis, then replaced by fetal hemoglobin (hemoglobin F, α2γ2) during the hepatic phase. During the third trimester, gamma chain production gradually diminishes, and gamma chains are replaced by beta chains, resulting in hemoglobin A (α2β2). Some fetal factors (e.g., infant of a diabetic mother) delay onset of beta chain production, but premature birth does not. Just after birth, with the expansion of the lungs and rapid increases in oxygen saturation, erythropoietin production stops and, thus, erythropoiesis ceases. Fetal RBCs have a shorter survival time (60 days).
Fetal RBCs have less deformable membranes as well as enzymatic differences from the red blood cells of older children. Senescent RBCs are destroyed in the liver and spleen due to changes in their membrane sialic acid content and the metabolic depletion that occurs as they age. During the first few months of postnatal life, rapid growth, shortened RBC survival, and cessation of erythropoiesis cause a gradual decline in hemoglobin levels, with a nadir at 8 to 10 weeks of life. This so-called physiologic nadir is accentuated in premature infants. Erythropoietin is produced in response to the decline in hemoglobin and decreased oxygen delivery. Erythropoiesis subsequently resumes with an increase in the reticulocyte count. The hemoglobin level gradually increases, accompanied by synthesis of increasing amounts of hemoglobin A. By 6 months of age in healthy infants, only trace gamma chain synthesis occurs.
Production of neutrophil precursors is controlled predominantly by two different colony-stimulating factors (see Fig. 149-1). The most immature neutrophil precursors are controlled by granulocyte-monocyte colony-stimulating factor (GM-CSF), produced by monocytes and lymphocytes. GM-CSF increases the entry of primitive precursor cells into the myeloid line of differentiation. Granulocyte colony-stimulating factor (G-CSF) augments the production of more mature granulocyte precursors. GM-CSF and G-CSF, working in concert, can augment production of neutrophils, shorten the usual baseline 10- to 14-day production time from stem cell to mature neutrophil, and stimulate functional activity. The rapid increase in neutrophil count that occurs with infection is caused by release of stored neutrophils from the bone marrow. This activity also is under the control of GM-CSF. During maturation, a mitotic pool of neutrophil precursors exists—myeloblasts, promyelocytes, and myelocytes possessing primary granules. The postmitotic pool consists of metamyelocytes, bands, and mature polymorphonuclear leukocytes containing secondary or specific granules that define the cell type. Only bands and mature neutrophils are fully functional with regard to phagocytosis, chemotaxis, and bacterial killing. Neutrophils migrate from the bone marrow, circulate for 6 to 7 hours, and enter the tissues, where they become end-stage cells that do not recirculate. Eosinophil production is under the control of a related glycoprotein hormone, interleukin 3. Eosinophils, which play a role in host defense against parasites, also are capable of living in tissues for prolonged periods.
Megakaryocytes are giant, multinucleated cells that derive from the primitive stem cell and are polyploid (16–32 times the normal DNA content) because of nuclear but not cytoplasmic cell division. Platelets form by invagination of the megakaryocytic cell membrane and bud off from the periphery. Thrombopoietin is the primary regulator of platelet production. Platelets adhere to damaged endothelium and subendothelial surfaces via specific receptors for the adhesive proteins, von Willebrand factor (vWF), and fibrinogen. Platelets also have specific granules that readily release their contents after stimulation and trigger the process of platelet aggregation. Platelets circulate for 7 to 10 days and have no nucleus.
Lymphocytes are particularly abundant in the bone marrow of young children, although they are a significant component of normal bone marrow at all ages. These are primarily B lymphocytes arising in the spleen and lymph nodes, but T lymphocytes also are present.