CHAPTER 154 Principles of Cancer Treatment
CHAPTER 154 Principles of Cancer Treatment
The overall goal of pediatric oncology is to cure all patients with minimal toxicity. Overall outcomes have been shown to be superior for patients treated in clinical trials compared to those not treated in clinical trials. Treatment for children with cancer is often multimodal and may involve surgery, radiation therapy, and chemotherapy. Surgery and radiation are generally local treatment modalities (an exception is total body irradiation as part of a bone marrow or stem cell transplant), whereas chemotherapy has both local and systemic effects.
Primary prevention strategies for most pediatric malignancies are unknown. Two exceptions are the use of hepatitis B vaccine to lower the rates of hepatocellular carcinoma and the use of human papillomavirus vaccine to reduce the risk of cervical, vulvar, and vaginal cancers. Childhood malignancies are not associated with tobacco or alcohol use, dietary factors, or sun exposure. Treatment with certain chemotherapy agents and radiation therapy increases the rate of second malignancies. Secondary prevention may be accomplished by screening the at-risk child (e.g., a child with Beckwith-Wiedemann syndrome or twin of a leukemia patient) but these opportunities are rare.
ONCOLOGIC EMERGENCIES
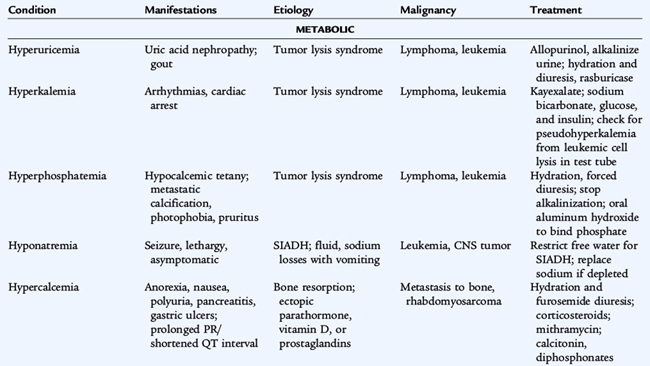
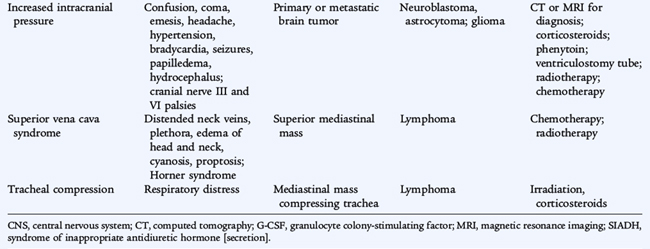
Adverse effects of tumors and treatments may result in oncologic emergencies in children and adolescents (Table 154-1). Mediastinal masses from lymphoma can cause life-threatening airway obstruction. Tumors with a large tumor burden (e.g., Burkitt lymphoma, germ cell tumors) may affect renal function adversely from tubular deposition of uric acid crystals. Allopurinol or rasburicase can be administered before chemotherapy to minimize this effect.
A common metabolic emergency is tumor lysis syndrome, often seen in treatment of leukemia and lymphoma. Large amounts of phosphate, potassium, and uric acid are released into the circulation from lysed cells. Overwhelming infection and spinal cord compression with neurologic compromise are other oncologic emergencies.
SURGERY
Appropriate imaging (usually with computed tomography [CT] or magnetic resonance imaging [MRI]) for solid tumors presenting as a palpable mass or mass-related symptoms (pain, respiratory distress, intestinal obstruction) should be obtained and followed by resection (when possible) or a biopsy (if complete resection is not feasible). An exception to this is a suspected lymphoma, for which a biopsy may be needed. However, almost all pediatric lymphomas are chemosensitive and do not require surgical resection.
Many pediatric solid tumors (non-Hodgkin lymphoma, neuroblastoma, Ewing sarcoma, and rhabdomyosarcoma) may have very similar appearances on light microscopic examination. This group of tumors is referred to as small, round blue cell tumors. To diagnose the particular tumor type adequately, surface marker analysis, immunohistochemistry, and electron microscopy may be required. Cytogenetic evaluations should be carried out, because many types of leukemia and certain solid tumors show either specific chromosomal translocations or other recurring cytogenetic abnormalities that can lead to the correct diagnosis. It is crucial to determine the amount of tissue required and the appropriate distribution of the tissue for testing so that all necessary studies are performed. A general oncologic surgery principle is to resect not just the tumor, but in most cases a surrounding margin of normal tissue to ensure the entire tumor has been resected.
CHEMOTHERAPY
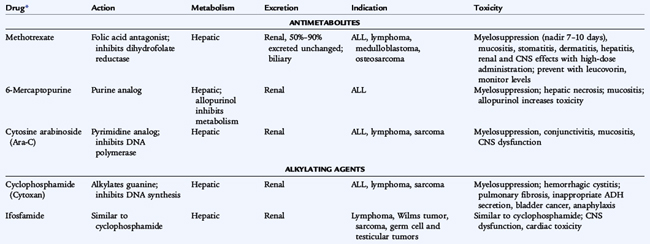
Because most pediatric solid tumors have a high risk for micrometastatic disease at the time of diagnosis, chemotherapy is used in almost all cases (Table 154-2). Exceptions include low-stage neuroblastoma (particularly in infants) and low-grade central nervous system (CNS) tumors. For patients with localized solid tumors, chemotherapy administered after removal of the primary tumor is referred to as adjuvant therapy. Chemotherapy administered while the primary tumor is still present is referred to as neoadjuvant chemotherapy. Neoadjuvant chemotherapy has a number of potential benefits, including an early attack on presumed micrometastatic disease, shrinkage of the primary tumor to facilitate local control, and additional time to plan for definitive surgery. In patients with osteosarcoma and Ewing sarcoma who undergo surgical removal of the primary tumor following neoadjuvant chemotherapy, a higher degree of necrosis following chemotherapy correlates with better prognosis.
Resistance to a particular chemotherapeutic agent can develop in a number of ways: decreased influx or increased efflux of the chemotherapeutic agent from the malignant cell; mutation in the target tissue so that it cannot be inhibited by the drug; amplification of a drug target to overcome inhibition; and blockade of normal cellular processes, which leads to programmed cell death or apoptosis. Because mutation is an ongoing process in malignant tumors, it follows that certain subpopulations of tumor cells within a tumor may be more or less sensitive to any particular chemotherapy drug. Given this fact, combinations of chemotherapy drugs are used, as opposed to sequential single agents, to treat the various forms of childhood cancer.
The blood-brain barrier prevents the penetration of chemotherapeutic drugs into the CNS; instillation of the chemotherapeutic agent directly into the cerebrospinal fluid (by lumbar puncture) may be necessary. Radiation therapy also circumvents the blood-brain barrier.
RADIATION THERAPY
Radiation therapy is the process of delivering ionizing radiation to malignant cells in order to kill them directly or, more commonly, prevent them from dividing by interfering with DNA replication. Conventional radiation therapy uses photons, but atomic particles such as electrons, protons, and neutrons can also be used. Not all tumors are radiosensitive, and radiation therapy is not necessary in all tumors that are radiosensitive.
OTHER THERAPIES
Certain cancers have been treated with cytokines, biologic response modifiers, or monoclonal antibodies in addition to standard treatments. Targeted therapies specifically target the tumor cells, sparing normal host cells. Imatinib mesylate is a protein kinase inhibitor that targets the effects of the t(9;22) translocation of chronic myeloid leukemia and acute lymphoblastic leukemia.
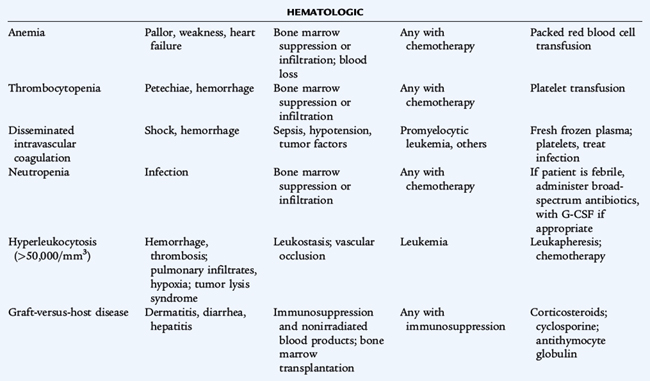
Supportive care also plays an important role in pediatric oncology, including the use of appropriate antimicrobial agents, blood products, nutritional support, intensive care, and complementary and alternative therapies.
ADVERSE EFFECTS
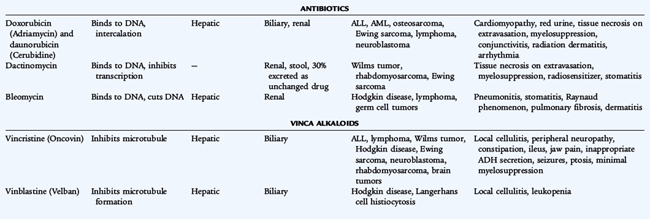
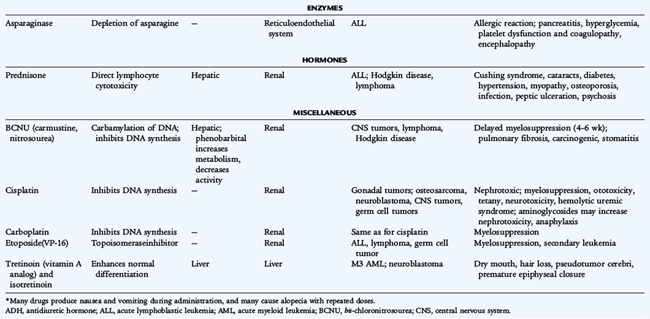
Because chemotherapy agents are cellular toxins, numerous adverse effects are associated with their use. Bone marrow suppression, immunosuppression, nausea, vomiting, and alopecia are general adverse effects of commonly used chemotherapy drugs. Each chemotherapy drug also has specific toxicities. Doxorubicin can cause cardiac damage; cisplatin can cause renal damage and ototoxicity; bleomycin can cause pulmonary fibrosis; cyclophosphamide and ifosfamide can cause hemorrhagic cystitis; and vincristine can cause peripheral neuropathy. Radiation therapy produces many adverse effects such as mucositis, growth retardation, organ dysfunction, and the later development of secondary cancers. Significant therapy-related late effects may develop in pediatric cancer patients (Table 154-3).
TABLE 154-3 Long-Term Sequelae of Cancer Therapy
| Problem | Etiologic Agent(s) |
|---|---|
| Infertility | Alkylating agents; irradiation |
| Second cancers | Genetic predisposition; irradiation; alkylating agents, VP-16, topoisomerase II inhibitors |
| Sepsis | Splenectomy |
| Hepatotoxicity | Methotrexate, 6-mercaptopurine; radiation |
| Hepatic veno-occlusive disease | High-dose, intensive chemotherapy (busulfan, cyclophosphamide) ± bone marrow transplant |
| Scoliosis | Radiation |
| Pulmonary (pneumonia, fibrosis) | Radiation; bleomycin, busulfan |
| Cardiomyopathy, pericarditis | Doxorubicin (Adriamycin), daunomycin; radiation |
| Leukoencephalopathy | Cranial irradiation ± methotrexate |
| Cognition/intelligence | Cranial irradiation ± methotrexate |
| Pituitary dysfunction (isolated growth hormone deficiency, panhypopituitary) | Cranial irradiation |
| Psychosocial | Stress, anxiety, death of peers; conditioned responses to chemotherapy |
| Thyroid dysfunction | Radiation |
| Osteonecrosis | Corticosteroids |