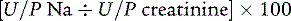CHAPTER 165 Acute and Chronic Renal Failure
CHAPTER 165 Acute and Chronic Renal Failure
ACUTE RENAL FAILURE
Etiology and Pathophysiology
Acute renal failure (ARF) is defined as an abrupt and significant decrease in glomerular filtration rate (GFR) and tubular function. This may lead to decreased excretion of waste products (creatinine, urea, phosphate) and water, resulting in azotemia and altered body fluid homeostasis. Urine output may be low, normal, or high. Early recognition and management are crucial.
ARF may be oliguric (<1 mL/kg/hour in neonates and infants, <0.5 mL/kg/hour in children) or nonoliguric. Nonoliguric ARF can be easily missed. Despite normal urine output, electrolyte disturbances and uremia may become significant. Urine osmolality is typically similar to serum osmolality in such patients.
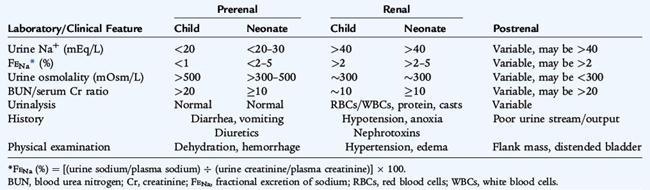
The major causes of ARF may be divided into prerenal (renal underperfusion); intrinsic renal (vascular, immunologic, ischemic, or toxic kidney injury); and postrenal (urinary tract obstruction or stasis) (Table 165-1). This classification is useful to apply to the differential diagnosis of oliguric states. If a child has an acute elevation in serum creatinine and no recent oliguria, the likelihood of a prerenal cause for ARF is low. Patients may migrate from one category to another; renal underperfusion (prerenal) or obstruction (postrenal) for an extended period may result in intrinsic renal damage and ARF.
Acute tubular necrosis (ATN) is the most common cause of ARF in children and is usually the consequence of renal underperfusion. Hypoxia-ischemia resulting from poor perfusion leads to early renal vasoconstriction and eventual tubular injury. Toxic injury secondary to drugs, exogenous toxins (ethylene glycol, methanol), or endogenous toxins (myoglobin, hemoglobin) also result in ATN. Severe vascular compromise with or without secondary arterial or venous thrombosis may result in acute cortical necrosis.
Clinical Manifestations
The history, physical examination, and laboratory data usually allow proper classification of the child with ARF (Table 165-2). A precipitating illness associated with vomiting and diarrhea or inadequate oral intake resulting in oliguria are consistent with prerenal causes. Postrenal causes may be occult and are not associated with hypoperfusion or dehydration. With obstructive causes, flank masses or a distended bladder may be present on examination.
Intrinsic renal failure can be associated with signs of volume overload (hypertension, cardiac enlargement, a gallop rhythm). Urine output characteristically is decreased, and signs of systemic involvement may be evident. Urinalysis usually reveals red blood cells (RBCs) and granular casts, with mild to moderate proteinuria.
Diagnostic Studies
In oliguric states, differentiation between prerenal azotemia and intrinsic renal disease is aided by determining the fractional excretion of sodium (FENa). The FENa is the percentage of sodium filtered by the glomeruli that is reabsorbed by the tubules and is calculated by the following equation:
where U and P are the urine and plasma concentrations. Values under 1% are consistent with prerenal azotemia (as the kidney maximizes water and salt reabsorption). Values greater than 2% are consistent with intrinsic renal dysfunction. A ratio of serum blood urea nitrogen (BUN) and creatinine greater than 20:1 is typical of prerenal states. Urinalysis may identify hematuria, proteinuria, or casts, which further support intrinsic or postrenal causes of ARF.
Hyperkalemia can be seen in patients with ARF as a result of decreased potassium excretion. Acidosis is due to impaired secretion of hydrogen ions and catabolic waste products. Hypocalcemia in ARF is often exacerbated by hyperphosphatemia and, if untreated, can lead to tetany or seizures.
Ultrasound imaging may reveal increased echogenicity with ATN and loss of corticomedullary differentiation with more severe involvement. Radiologic studies (ultrasound, voiding cystourethrogram [VCUG], computed tomography [CT], nuclear imaging) are often helpful in determining the cause of obstruction in postrenal cases.
Renal biopsy, usually performed percutaneously, may be indicated if the presentation of ARF is atypical to assess the severity of systemic disease involvement, to guide therapy, or establish a prognosis. Light microscopy should be augmented by immunofluorescence and electron microscopy.
Treatment
Careful assessment of intake and output should be augmented with serial measurements of body weight. Initial fluid and electrolyte therapy provides water to equal insensible losses; water and electrolytes are then added to replace ongoing losses. If hypovolemia is present, intravascular volume should be expanded by intravenous (IV) administration of physiologic saline. If hypervolemia is present, 1 to 2 mg/kg of furosemide may be attempted. Severe fluid overload in the presence of marked oliguria or anuria is one indication for dialysis. Serum electrolyte levels should be determined frequently during the acute phase of ARF.
Foods, fluids, and medications that contain potassium should be restricted until renal function is re-established or dialysis initiated. The major risk of hyperkalemia is arrhythmias (see Chapter 142). Intravenous calcium will block the acute cardiac toxicity of hyperkalemia while measures to shift potassium onto cells (bicarbonate, beta-agonists, glucose/insulin) and hasten removal (diuretics, sodium-potassium exchange resins, dialysis) are initiated. Although sodium bicarbonate counteracts acidosis, it engenders a risk of fluid overload, hypernatremia, and hypertension.
Treatment of hypocalcemia and hyperphosphatemia primarily involves efforts to lower the serum phosphorus by dietary phosphorus restriction and administration of phosphate binders (calcium acetate and calcium carbonate). Symptomatic hypocalcemia can be treated with IV calcium; it must be given cautiously to avoid precipitation with circulating phosphorus.
In children with ARF, dialysis has three major indications:
Renal replacement therapies in children include peritoneal dialysis, hemodialysis, and continuous renal replacement therapy (continuous venovenous hemofiltration). Careful monitoring of blood levels of drugs excreted by the kidney and appropriate adjustment of either the total dose or dosing intervals are necessary to prevent complications or further renal injury.
CHRONIC KIDNEY DISEASE
Etiology and Epidemiology
Congenital and obstructive abnormalities are the most common causes of chronic kidney disease (CKD) that present between birth and 10 years of age. After age 10, acquired diseases, such as FSGS and chronic GN, are more common causes of CKD. The risk of progression to ESRD is related to the cause of CKD and modifiable factors, such as UTI and hypertension. During puberty, renal function may deteriorate if the damaged kidneys are not able to grow and adapt to the increased demands of larger body size. CKD is staged to facilitate appropriate evaluation and monitoring (Table 165-3).
TABLE 165-3 Classification of the Stages of Chronic Kidney Disease
| Stage | GFR (mL/min/1.73 m2)* | Description |
|---|---|---|
| 1 | ≥90 | Minimal kidney damage |
| 2 | 60–89 | Kidney damage with mild reduction of GFR |
| 3 | 30–59 | Moderate reduction of GFR |
| 4 | 15–29 | Severe reduction of GFR |
| 5 | <15 (or dialysis) | Kidney failure |
GFR, glomerular filtration rate.
* GFR ranges apply for children 2 years of age and older.
From National Kidney Foundation Kidney Disease Outcomes Quality Initiative.
Clinical Manifestations
The factors associated with growth failure in children with CKD include poor nutrition, renal osteodystrophy (ROD), acidosis, anemia, hormonal abnormalities, medication toxicity (steroids), and the growth hormone/IGF-1 resistance seen in uremia. Anemia results primarily from a failure of the kidney to produce adequate erythropoietin and an impaired response to erythropoietin due to uremia. Renal osteodystrophy is common and is related to phosphate retention from low GFR and diminished 1,25-dihydroxyvitamin D production in the kidney. Secondary hyperparathyroidism and poor bone mineralization may ensue and lead to fractures and bone deformities. Delayed puberty may be due to altered gonadotropin secretion and feedback patterns. Hypertension is relatively common in CKD and may be asymptomatic or associated with headaches, left ventricular hypertrophy (LVH), and heart failure. Learning and school performance may also be impaired in CKD.
Treatment
The management of children with CKD requires a multidisciplinary team of pediatric practitioners to address their growth and development. Adequate nutrition should be provided even if this requires dietary supplements and tube feedings. With very low GFR, maintenance dialysis may be needed for growing children to allow adequate fluid and recommended daily allowance of protein and calories. In infants with CKD, a low-solute formula with a phosphate binder may be indicated.
When acidosis develops, sodium bicarbonate or sodium citrate is indicated. Unless a child is oliguric, fluid restriction is not necessary. Sodium intake depends on the particular sodium handling aspects of the renal disease. Many children with congenital renal disorders waste sodium in their urine and require supplemental salt. Conversely, children with GN tend to retain sodium and may become hypertensive or edematous if given excess salt. High-potassium foods should be avoided in advanced CKD.
The initial therapy for ROD is to restrict phosphate in the diet. Oral phosphate binders are used if this is not sufficient. Supplemental calcium may be needed. Parathyroid hormone levels should be kept in the normal range and this may require supplemental vitamin D analog (calcitriol, paricalcital) therapy.
Recombinant-produced erythropoietin is used to maintain near normal hemoglobin levels in children with CKD. Effective erythropoiesis requires iron and typically depletes iron stores. Parenteral iron is often needed for children on erythropoietin. Growth failure is more common with advanced stages of CKD. Recombinant-produced growth hormone is useful in children with CKD who are not growing well despite proper management.
The optimal treatment of ESRD is renal transplantation. Identifying potential live donors should occur as the child reaches CKD stage 3 to 4. For children with slowly progressing CKD, preemptive renal transplantation can be used to avoid the stress of dialysis. Deceased and living donors can be used for renal transplantation. Live donor transplantation has several advantages and is often the first choice for children, when available. Living donors can be scheduled at the convenience of the donor and the recipient. Additionally, there is slightly better renal function and graft survival with live donor kidneys compared with cadaveric kidneys.
Maintenance dialysis is effective for a child awaiting renal transplantation or for whom renal transplantation is not possible. Peritoneal dialysis (PD) is effective in even small infants, and about 40% of children who require chronic dialysis use this modality in the United States. Hemodialysis is used more often for older and larger children, when intravascular access is less of a concern, and for children whose parents cannot provide home PD.
Prognosis
Infants and children with ESRD have a good prognosis, given the effectiveness of dialysis and transplantation. Kidney transplants have an excellent success rate. More than 90% of transplants in children (living or deceased donor) function 1 year after transplant; more than 50% still function 20 years later. Lifelong immunosuppressive medications are necessary. The major complications relate to side effects of medications: infections, cardiac complications (left ventricular hypertrophy [LVH], atherosclerosis, arrhythmias), and increase in malignancies. Children after renal transplant who have not had prior Epstein-Barr virus (EBV) infection are more susceptible to develop an EBV-mediated post-transplant lymphoproliferative disorder (PTLD) that resembles malignant lymphoma. With early detection, PTLD and other malignancies can usually be treated successfully.