CHAPTER 190 Atopic Dermatitis
CHAPTER 190 Atopic Dermatitis
ETIOLOGY
Atopic dermatitis is a chronic inflammatory disease with no known cure. It is a form of eczema associated with significant psychosocial morbidity and decreased health-related quality of life. For many affected individuals, atopic dermatitis is the skin manifestation of atopy accompanied by asthma and allergic rhinitis. Affected individuals may have only one or two manifestations of atopy; up to one quarter of affected individuals have no other forms of atopy. Genetics and environmental factors play a role. Genes for atopic dermatitis have been linked to many chromosomes including loci that control inflammation, as well as to single gene disorders, suggesting genetic polymorphism.
Inflammatory mediators involved include predominantly T helper cells, with the TH2 pathway implicated early in acute lesions and a TH1 predominance found in chronic lesions. Langerhans cells, IgE, and eosinophils play a prominent role, as well as many other inflammatory mediators. Two forms of atopic dermatitis have been proposed recently: an atopic form (also called extrinsic) and a nonatopic (intrinsic) form, because some individuals show no sensitizations to common allergens. The conditions behave similarly.
EPIDEMIOLOGY
Atopic dermatitis is the most common skin disease in children, with an estimated prevalence of up to 20% of children. Only 1% to 2% of adults manifest disease. In addition to genetic factors, an environmental influence contributes. Atopic dermatitis occurs more frequently in urban areas and in higher socioeconomic classes and less frequently in rural areas. Prevalence is lower in areas where industrial pollution is less and where eosinophil-mediated infections such as helminthic infections are endemic.
Patients generally have a family history of atopy. Children with atopic dermatitis are predisposed to the development of allergy and allergic rhinitis, referred to as the atopic march. Asthma develops in up to half of children with atopic dermatitis, and allergic rhinitis even more frequently. Food sensitivities are commonly associated with atopic dermatitis.
CLINICAL MANIFESTATIONS
Atopic dermatitis manifests with a defective skin barrier, reduced innate immune responses, and exaggerated immune responses to allergens and microbes. Clinically, this leads to characteristic skin lesions with inflammation and increased skin infections. A chronic, relapsing course is typical. Pruritus and skin hyper-reactivity manifest with characteristic erythematous skin lesions. Foods, allergens, infections, irritants, extremes of temperature, and lack of humidity can exacerbate the condition as well as scratching or rubbing. Triggers are somewhat variable from individual to individual.
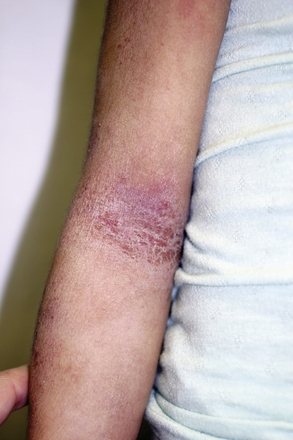
Characteristic locations vary with the age of the patient. Infantile eczema typically affects the face and extensor surfaces of the extremities and is often generalized. Childhood lesions predominate in flexural surfaces (antecubital and popliteal fossae), wrists, ankles, hands, and feet (Fig. 190-1). The adult phase occurs after puberty and manifests in the flexural areas including the neck as well as predominant involvement on the face, dorsa of the hands, fingers and toes, and the upper arms and back. Generalized xerosis is commonly found in all stages. The condition generally remits in adulthood.
Characteristic lesions of atopic dermatitis are erythematous plaques with ill-defined borders and overlying hyperkeratosis. Lesions can be secondarily excoriated or have an overlying crusting that is yellow or hemorrhagic. Weeping may be present in acute stages. Lichenification is found in older lesions. Formation of fissures is common in both acute and chronic lesions. Papular lesions may also occur. The formation of vesicles is associated with intensely inflammatory lesions. Regardless of the stage, hypo- and hyperpigmentation can be seen, representing transient changes. Atopic dermatitis is not usually scarring unless secondary features become severe (e.g., infection or physical manipulation [scratching]).
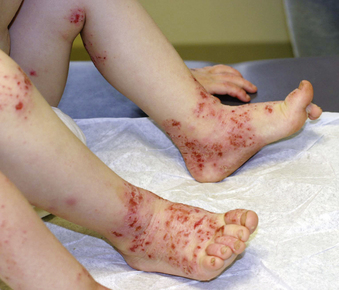
Secondary bacterial infection, most commonly with Staphylococcus aureus or less commonly with Streptococcus pyogenes, is frequently present. Patients are at increased risk for infections with cutaneous viruses, such as herpes simplex virus or varicella virus, and can develop disseminated skin infections with viruses such as herpes simplex virus (eczema herpeticum), varicella-zoster virus, smallpox virus (eczema vaccinatum), and Molluscum contagiosum. Atopic skin is more susceptible to fungal infections as well. Signs of concomitant infection include acute worsening of disease in an otherwise well-controlled patient, resistance to standard therapy, fever, and presence of pustules, fissures, or exudative or crusted lesions (Fig. 190-2). Eczema herpeticum can be life threatening.
LABORATORY AND IMAGING STUDIES
Diagnosis of atopic dermatitis is based on clinical signs and symptoms. Skin biopsy findings are generally characteristic but not exclusively diagnostic and can overlap with other skin conditions. Peripheral blood eosinophilia and elevated IgE levels can be found but are not specific. Skin testing or measurement of specific IgE antibody levels can detect sensitization to inhalant allergens. Skin tests should be interpreted with caution in patients with active skin lesions as false positive findings occur.
DIFFERENTIAL DIAGNOSIS
The differential diagnosis of atopic dermatitis is extensive, but the history of a relapsing pruritic condition in the setting of atopy and skin lesions in a characteristic distribution is typical.
The lesions of seborrheic dermatitis have circumscribed and well-defined borders, and the scale or hyperkeratosis, when present, is thicker, greasy, and yellowish. Their distribution is different than that of atopic dermatitis. Occasionally the two conditions coexist.
Psoriasis tends to localize on the elbows and knees, the pretibial areas, and scalp. The lesions of psoriasis on exposed surfaces are salmon color at the base with an overlying hyperkeratosis that is much thicker with silver coloration. They are generally very well demarcated, oval or round, thick plaques.
Contact dermatitis has a distribution limited to one area of the body corresponding to the contact with the allergen. The lesions generally make bizarre, linear, square, or angulated shapes corresponding to the source. Nickel dermatitis is common and results from contact sensitization to nickel in metals. It occurs in characteristic locations, such as the periumbilical area (where metal from pants snaps rubs against skin); on the ear lobule or inferior to the auricle on the neck (where earrings contact skin); circumferentially around the neck (necklaces); and under rings or wristbands. Patients with atopic dermatitis can have concomitant contact dermatitis as well.
TREATMENT
Ideal therapy for atopic dermatitis includes three main components: avoidance of triggers of inflammation, use of topical anti-inflammatory medication to affected areas of skin when needed, and frequent, liberal use of bland emollients to restore the skin barrier. Control of pruritus and infection should be considered on an individual basis. If topical therapy and these measures are inadequate, systemic therapy with immunosuppressive agents or ultraviolet light therapy may be indicated.
Common triggers of inflammation in atopic dermatitis include rubbing or scratching, contact with saliva or foods that are acidic, soaps and detergents, fabric softeners, wool or other harsh materials, bubble baths and other products with fragrances that contact the skin, sweat, highly chlorinated pools, low humidity, tobacco smoke, dust mites, animal dander, grass pollens, and molds. Exposure to these triggers should be limited whenever possible. Infections that are unrelated to skin disease can also exacerbate atopic dermatitis.
A daily short bath with warm but not hot water is generally advisable followed by immediate application of emollients and medications before evaporative loss is allowed to occur. Some controversy surrounds the frequency of bathing as there are some advocates of limiting bathing for these patients to less than once daily. Additional information for patients and families can be found at the website of the National Eczema Association for Science and Education (http://www.nationaleczema.org).
Food allergy is commonly seen in patients with atopic dermatitis and can contribute to clinical exacerbations of disease. More frequently, skin manifestations occur independent of the food allergy exacerbation. Eggs, milk, nuts, wheat, and fish are the most commonly implicated food allergens. Egg allergy may be of particular relevance. Excessively restrictive diets should be avoided.
Topical corticosteroids are the mainstay of anti-inflammatory therapy for atopic dermatitis and are used intermittently as needed on affected areas. Only mild to moderately potent preparations should be used in children and on areas of thinner skin such as the face, genital, and intertriginous areas. Topical corticosteroids should be used in conjunction with adequate skin care, such as avoiding triggers of inflammation and frequent application of emollients. The goal is to limit the need for anti-inflammatory medications and thereby avoid potential for adverse effects with prolonged use or frequent application to large body surface areas. Different therapeutic schemes have been recommended, including monotherapy or combination therapy with nonsteroidal anti-inflammatory medications (topical calcineurin inhibitors). Monotherapy schemes with topical corticosteroids may involve early use of moderate potency corticosteroids for more rapid improvement followed by tapering to less potent corticosteroids. Early lesions and lesions in anatomic areas with thinner skin (face, genitals, axillae, antecubital and popliteal fossae) respond more readily to lower potency topical corticosteroid preparations than do chronic, thicker lesions and lesions in areas of thicker skin (hands and feet). It is important to individualize the topical corticosteroid treatment regimen.
Hundreds of topical corticosteroids are available and are classified according to strength from I to VII. Class I is the highest potency and class VII is the lowest potency. Potency varies according to the steroid molecule (active ingredient) and, for a given ingredient, strength can vary according to relative concentration and vehicle base. Class I steroids are generally avoided in young children. Classes I and II steroids are avoided in areas of thinner skin or enhanced penetration. Inadvertent or purposeful enhanced penetration occurs in areas of natural occlusion (flexures such as axillae and groin), with external occlusion (diapers or bandages), in areas of open skin (excoriations), and with heat or hydration. Use of wet wraps with lower strength topical corticosteroid application takes advantage of this principle of heat and hydration for enhanced penetration for recalcitrant lesions.
Corticosteroids are available in different vehicles. In general, ointments are very effective because of their occlusive nature and they are very well tolerated. Creams may be slightly less effective for a given steroid ingredient, but may be more cosmetically acceptable for older patients or in warmer climates. Lotions may have more preservatives that can cause irritation and are generally less potent. Sprays, foams, solutions, and gels can be especially useful for hair-bearing areas, and can be very potent. Sprays, solutions, and gels can be particularly irritating when applied to atopic skin and should generally be avoided on areas of open skin. Twice-daily application of corticosteroids is recommended.
Topical calcineurin inhibitors (also referred to as topical immune modulators) have been recently introduced in the therapy for atopic dermatitis and include topical tacrolimus and pimecrolimus. They can be effective for mild to moderate atopic dermatitis, and can be helpful adjunctive agents in severe atopic dermatitis. These agents selectively inhibit T-cell proliferation by inhibiting calcineurin and subsequent phosphatase activity, which normally lead to production of inflammatory mediators and T-cell proliferation. There is no potential for skin atrophy. Thus, these agents are particularly useful in face or neck or genital lesions. These agents can be particularly helpful as monotherapy or, anecdotally, when used in combination with corticosteroid therapy. Many different application schemes have been advocated. They are currently approved for intermittent therapy as second-line treatments for mild to moderate atopic dermatitis. Long-term studies, combined with other modalities for treatment, are under way.
Topical antibiotic therapy as monotherapy for atopic dermatitis is ineffective. Some improvement can be seen because of the natural moisturizing properties of topical antibiotics and treatment of the exacerbating infection. They are more effective when combined with topical anti-inflammatory treatment.
Topical extract of coal tar can be used as an effective anti-inflammatory medication for atopic dermatitis and can be compounded in low concentrations with an emollient vehicle base. Cosmetic inelegance with staining and odor, as well as potential for irritation, limits its use. Tar is also mutagenic.
Antihistamines are useful adjunctive therapy, especially during flares. Sedating antihistamines are generally prescribed in low doses for nighttime use (one fourth of the total dose), improving itching and the sleeplessness that can be related to scratching during the night. Additional daytime doses can be added on an individual basis when needed, but are infrequently necessary. Nighttime dosing of nonsedating oral antihistamines may be more advantageous for school-age children when drowsiness is not desirable.
Systemic corticosteroids are indicated for short-term administration for cases of severe disease and may be appropriate in selected rare cases when adequate topical therapy failed or is being instituted. Systemic corticosteroid courses should be adequately tapered and used in conjunction with an appropriate atopic skin care regimen. Rebound flare of atopic dermatitis is common following withdrawal of corticosteroids and should be anticipated to avoid misinterpretation of the natural disease severity. Long-term and frequent repeated courses should be avoided to prevent adverse effects.
Systemic cyclosporine (up to 5 mg/kg/day) can be effective therapy for atopic dermatitis in severe cases. It can be tapered once disease is controlled and is typically used on a longer-term basis (5–12 months) to produce remission. Ultraviolet light therapy (UVB, narrow band UVB, UVA, or UVA1) can be used alternatively in moderate to severe cases in older children. Typically, light therapy is administered two to three times weekly until improvement is seen, and then is tapered or discontinued once the acute flare has resolved. Requirements for frequent office visits, ability to cooperate with standing in a light box while wearing protective goggles, and risk of long-term skin damage, including the potential for skin cancer development with excessive UV light exposure, prevent more frequent use of light therapy in children.
COMPLICATIONS
An increased tendency toward bacterial, viral, and fungal skin infections is due to an impaired skin barrier and results from changes in the stratum corneum, lipid metabolism in the epidermis, a decrease in innate immune proteins in the skin, as well as maladaptive secondary immune responses.
Superinfection with S. aureus is the most common secondary skin infection found in atopic dermatitis. Group A streptococcus infection is also common. Infection manifests with pustules, erythema, crusting, scabbing, flare of disease, or lack of response to adequate anti-inflammatory therapy. Localized lesions can be treated with topical mupirocin. Widespread and generalized lesions require systemic therapy, most commonly with a first-generation cephalosporin, such as cephalexin. Erythromycin and azithromycin can also be effective unless patients are colonized with a macrolide-resistant strain of S. aureus. Methicillin-resistant S. aureus infection manifests with deeper, more inflammatory skin lesions. Diagnosis is confirmed by culture and treatment guided by antimicrobial susceptibilities. Treatment should include concomitant atopic skin care routine to treat active areas of inflammation, restore the skin barrier, and avoid triggers of inflammation. Although secondary skin infection with S. aureus is common, progression to cellulitis or septicemia is unusual. Colonization and infection worsens inflammation in atopic dermatitis by promoting resistance to topical corticosteroids, superantigen activation in susceptible individuals, and production of IgE antistaphylococcal antibodies.
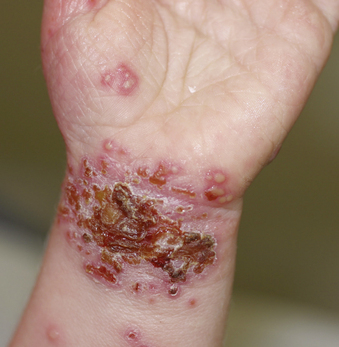
Eczema herpeticum (Kaposi’s varicelliform eruption) is one of the potentially serious infectious complications in atopic dermatitis. After herpes simplex virus (HSV) infection and an incubation period of 5 to 12 days, an eruption of multiple, pruritic vesiculopustular lesions occurs in a disseminated pattern. Characteristic lesions are umbilicated vesicles and pustules and punctuate hemorrhagic crusts that coalesce in groups that are discontinuously distributed over the skin and surrounded by inflammation (Fig. 190-3). Irritability, anorexia, and fever can also be seen. Systemic and central nervous system disease have been reported. Bacterial superinfection of eroded areas of the skin often occurs. Diagnosis can be made rapidly from a scraping from the skin lesion stained with Giemsa or Wright’s stain (Tzanck test), though these are not highly sensitive. These stains allow microscopic visualization of the presence of multinucleated giant cells indicative of herpes simplex virus or varicella-zoster virus infection. Vesicle fluid can also be sent for rapid direct fluorescent antibody testing or for culture for virus confirmation. Polymerase chain reaction (PCR) detection for herpes simplex virus DNA from fluid is also available. Laboratory confirmation of infection is important because similar clinical manifestations can occur with bacterial infections.
Complications from topical corticosteroid therapy include local development of acne, hypopigmentation, and hypertrichosis. Atrophy, telangiectasia, or striae can result from misuse of corticosteroids (too high potency or application for longer than appropriate). Glaucoma and cataract formation have been reported in association with chronic topical corticosteroid use. Hypothalamic-pituitary adrenal axis suppression can occur with high potency steroid use or chronic use of low potency topical corticosteroids to large body surface areas. In general, topical corticosteroids should be applied only to affected areas, discontinued when no longer needed, and used in conjunction with appropriate atopic skin care regimens that lessen the need for topical steroid therapy. Rotational or combination therapy with nonsteroidal anti-inflammatory agents can limit application of corticosteroids preventing adverse effects.
The psychosocial impact of the condition can be significant. There is often disfigurement, lack of sleep from restlessness and pruritus resulting in irritability and fatigue, and limitations on participations in sports. Significant time, as well as financial strain, are involved in caring for a child with atopic dermatitis. Thus, management should address these potential issues and provide adequate anticipatory guidance.
PROGNOSIS
Atopic dermatitis frequently remits during childhood and is much less common after puberty. The condition is generally most severe and widespread in infancy and early childhood. Relapse of disease in adults can occur and commonly manifests as face or hand dermatitis. Frequently adults have generalized dry skin and are aware that their skin is sensitive to many over-the-counter preparations. Patients with atopic dermatitis can also develop asthma and allergic rhinitis. Asthma is more commonly associated with more severe skin disease.
PREVENTION
Individual flares of atopic dermatitis can be prevented by avoiding triggers of inflammation and can be lessened with frequent emollient application. Recent evidence shows that breastfeeding for at least 4 months, compared with feeding formula made with intact cow’s milk protein, prevents or delays the occurrence of atopic dermatitis (as well as cow’s milk allergy and wheezing) in early childhood. For infants with a parent or sibling with atopic disease and who are not exclusively breastfed for 4 to 6 months, there is modest evidence that the onset of atopic disease, and especially atopic dermatitis, may be delayed or prevented by the use of extensively hydrolyzed casein-based formulas. There is insufficient evidence that soy-based formulas, delaying the introduction of complementary foods beyond 4 to 6 months of age, or other dietary intervention prevents the development of atopic disease. There is no convincing evidence that women who avoid peanuts or other foods during pregnancy or while breastfeeding lower their child’s risk of allergies.