105 Neonatal Infections
Neonates are uniquely susceptible to bacterial and viral infections because of deficiencies in immune function, including neutrophil function and humoral and cellular immunity. Before the introduction of antibiotics, mortality from neonatal sepsis approached 90%; however, even with the advent of antibiotics and identification of common causative organisms, mortality remains 20% to 50%. With continually updated guidelines for maternal screening, the rates of maternally transmitted infections also continue to fall; however, because of significant morbidity, pediatricians must continue to have a high index of suspicion for these infections.
Neonatal Sepsis
Etiology and Pathogenesis
Overall, the incidence of neonatal sepsis is 3 to 4 per 1000 births; it accounts for 25 of 1000 preterm live births. The overall mortality rate is approximately 25%. To aid in identification of likely pathogens, neonatal sepsis is divided into two classifications, early- and late-onset sepsis.
Early-onset sepsis represents infection acquired from the maternal genital tract, whether via ascension into the uterus following rupture of membranes or during passage through the vaginal canal during the birth process. As such, it generally presents within the first five to seven days of life with a sudden onset and fulminant course. Ports of entry include the conjunctivae, respiratory tract, umbilicus, skin, and oropharynx. Implicated organisms include group B β-hemolytic streptococci (GBS), Escherichia coli, and less commonly other gram-negative enteric organisms and Listeria monocytogenes.
GBS infections account for a large number of cases of early-onset sepsis and have been a focus of Centers for Disease Control and Prevention guidelines because of significant mortality. GBS colonizes the genital tract of 30% of women, and all pregnant women are screened for vaginal and rectal colonization at 35 to 37 weeks of gestation. Colonized women are treated with intrapartum penicillin chemoprophylaxis when delivering vaginally; since the institution of these guidelines, mortality from GBS disease has declined by 80%.
Late-onset sepsis may represent organisms acquired from the maternal genital tract but more often are transmitted horizontally by caregivers. Late-onset disease is more common after the first week of life and is more likely to present with urinary tract infection or meningitis caused by hematogenous seeding of end organs. The most common organisms are gram-positive organisms, such as Staphylococcus aureus and GBS, in addition to E. coli and other gram negative organisms. In the community, viral infection should also be included in the differential diagnosis. For hospitalized infants, the most common pathogen is Staphylococcus epidermidis; other organisms to consider include Pseudomonas aeruginosa, Candida spp., anaerobes, and methicillin-resistant S. aureus.
Clinical Presentation
Neonatal sepsis may present as a constellation of nonspecific symptoms including one or more of the following: progressive lethargy or irritability, apnea, temperature instability, poor feeding, changes in tone, abdominal distension, vomiting or loose stools, or decreased urine output. Fever in a neonate is defined as a temperature of 38.0°C or greater. Clinicians should have a very high index of suspicion for sepsis in an ill-appearing or febrile infant.
Evaluation and Management
A thorough birth history should be obtained including the following: gestational age (prematurity is the most significant risk factor in the evaluation of neonatal sepsis); duration of rupture of membranes; presence of meconium stained amniotic fluid; indicators of chorioamnionitis, including maternal fever in the peripartum period, fundal tenderness, and foul-smelling amniotic fluid; and invasive procedures such as fetal scalp electrodes. For reasons that are unclear, male infants are four times as likely as female infants to develop early-onset sepsis. The antepartum history is equally as important and should include maternal infections or exposures during pregnancy, the duration of prenatal care, and results of prenatal testing if available. The U.S. Preventative Services Task Force recommends routine screening of mothers for the following: syphilis (rapid plasma reagin [RPR]), hepatitis B (hepatitis B surface antigen [HBsAg]), rubella (titers), HIV, gonorrhea, chlamydia, and GBS.
A thorough physical examination should be performed with special attention to the following findings: growth parameters including head circumference, overall appearance of the infant, presence of a bulging fontanel, eye discharge and ophthalmologic examination, nasal discharge, hydration status, respiratory symptoms, perfusion and cardiac examination, abdominal distension, organomegaly, erythema or drainage from the umbilicus, skin lesions including petechiae and vesicles, jaundice, and the overall neurologic examination, including tone.
Newborns with historical factors putting them at significant risk for early-onset sepsis should receive serial screening complete blood counts (CBCs) and C-reactive protein levels in the newborn nursery, regardless of their clinical appearance. For infants who are septic appearing in the first days of life, a complete evaluation, including blood culture and lumbar puncture with cerebrospinal fluid (CSF) culture, should be obtained and the infant initiated on appropriate antibiotic coverage until culture results are negative for at least 48 hours. GBS and Listeria coverage with a β-lactam and gram-negative coverage with an aminoglycoside (usually ampicillin and gentamicin) is recommended. Antibiotic therapy may be tailored to cover organisms common to a particular nursery, as indicated.
Infants who present after 5 to 7 days of life with any of the symptoms detailed above should be evaluated for late-onset sepsis. Blood, urine, and CSF studies, including cultures before the initiation of antibiotics, should be obtained. Exact laboratory criteria for the diagnosis of late-onset sepsis is somewhat controversial; however, an elevated white blood cell count on an enhanced urinalysis specimen, CSF specimen, or peripheral blood sample is concerning for serious bacterial infection. As in early-onset sepsis, infants should be started on appropriate antibiotics until all culture results are negative. Because meningitis features more prominently in late-onset sepsis, a third-generation cephalosporin (usually cefotaxime) may be substituted for gentamicin if concern for meningitis is high based on clinical examination or lumbar puncture results.
Torch Infections
Etiology and Pathogenesis
The TORCH (toxoplasmosis or Toxoplasma gondii, other infections, rubella, cytomegalovirus [CMV], and herpes simplex virus [HSV]) infections are congenital infections that are transmitted transplacentally from mother to fetus. As a rule, the likelihood of transmission to a fetus is greatest when primary maternal infection occurs. Gestational age at the time of exposure determines the severity of the disease. Neonates who appear most severely infected at birth have most likely been infected during the first trimester; exposure during the first few weeks of gestation may also result in intrauterine demise. TORCH is an acronym that represents the most common pathogens responsible for transplacentally transmitted infections. Historically, these include toxoplasmosis or T. gondii, other infections, rubella, CMV, and HSV.
Toxoplasma gondii
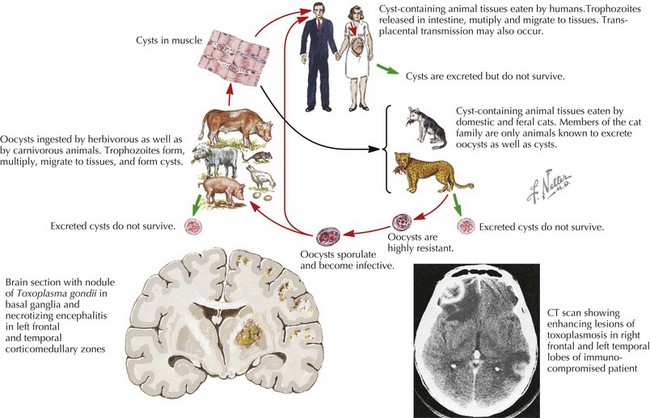
This is an obligate intracellular protozoan of which cats are the primary host. Maternal infection occurs by ingestion of oocytes via contaminated food or water, and infection persists in the tissues of the human host. Thirty percent of fetuses acquire infection at the time of primary maternal acquisition, resulting in 3500 newly infected infants per year.
Other: Syphilis, Hepatitis B, and HIV
Neonatal HIV infection occurs transplacentally (≤40% of cases), during delivery, and via breastfeeding postnatally. The risk of transmission increases with high maternal viral load. In the United States, 150 to 300 infants with HIV are born yearly, representing a significant decrease over the past 10 years because of the availability of antiretroviral therapy for pregnant women.
Hepatitis B is a DNA-containing hepadnavirus that is transmitted vertically during delivery in almost all cases, and risk is highest with high circulating viral titers.
The spirochete Treponema pallidum is the causative agent of syphilis. Rates of primary and secondary syphilis infection continue to decrease within the United States. Maternal factors associated with infection include lack of prenatal care and cocaine use.
Rubella
Rubella is an RNA virus in the Togaviridae family. The incidence of neonatal rubella has declined 99% since the immunization program began in the 1960s and includes 400 new cases per year.
Clinical Presentation
TORCH infections are often part of a large differential diagnosis and share many overlapping features. These infections may result in dysmorphic facies, developmental delay, or symmetric intrauterine growth retardation. Other signs and symptoms common to vertically transmitted infections are hepatosplenomegaly, micro- or macrocephaly, and abnormal tone.
Toxoplasmosis generally presents within the second or third decade of life with visual and learning difficulties. Eighty-five percent of affected infants appear normal at birth. The most severe form is noted in the first month of life with intrauterine growth restriction, hepatosplenomegaly, micro- or macrocephaly, abnormal tone, or seizures. The classic triad of chorioretinitis, intracranial calcifications, and hydrocephalus presents in fewer than 10% of infants.
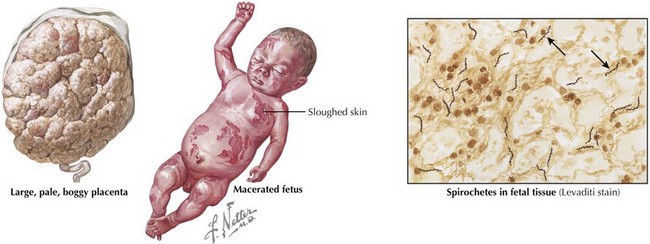
Congenital syphilis is similar to the acquired form of syphilis in that it presents with early and late manifestations. Most cases are detected by routine maternal screening. Early syphilis presents within the first 2 years of life, with one-third of patients symptomatic at birth. Symptoms can affect almost any organ system and include the following: hepatosplenomegaly, jaundice, Coombs’-negative hemolytic anemia, thrombocytopenia, rhinitis (snuffles), condylomatous skin or mucous membrane lesions, diffuse mucocutaneous rash involving the palms and soles, osteochondritis presenting as pain and refusal to move the affected limb (pseudoparalysis of Parrot), periosteitis, chorioretinitis, failure to thrive, or renal disease (Figure 105-1). Late manifestations are not detailed here but result from chronic tissue inflammation, most commonly involving the central nervous system (CNS), bones, and teeth, and present within the first 2 decades of life.
With an incubation of 2 to 6 months, hepatitis B is an unlikely cause of neonatal cholestasis and can remain asymptomatic for years. After 2 months of age, hepatitis B presents with self-limited hepatitis, fulminant hepatitis, or insidious onset of cirrhosis and progression to liver failure.
HIV screening is recommended for all pregnant women because of advances in antiretroviral prophylaxis. The median age of onset in infants who acquire the virus perinatally is 12 to 18 months if left untreated, and it carries a 20% mortality rate in the first 4 years of life. Briefly, the clinical presentation can vary from mild systemic symptoms with recurrent fever or failure to thrive, recurrent mild infections, to serious bacterial infections and AIDS-defining illnesses.
Congenital rubella presents with many of the common symptoms of TORCH infections. Unique features include patent ductus arteriosus and extramedullary hematopoiesis, resulting in the characteristic rash known as “blueberry muffin baby.”
Cytomegalic inclusion disease is apparent at birth and is the most severe form of CMV disease, representing 10% of all cases. Other features of TORCH infections are also present, including intrauterine growth restriction, hepatosplenomegaly, thrombocytopenia, and dermal erythropoiesis. CNS manifestations include ventriculomegaly, microcephaly, chorioretinitis, and sensorineural hearing loss.
Infection with HSV acquired in the perinatal period presents within the first 4 weeks of life with one of three presentations. Approximately 45% of patients present with skin, eye, and mouth disease; other presentations include CNS involvement only (seizures, hypotonia) and disseminated disease (CNS, liver, adrenal, and pulmonary disease). Congenital HSV represents intrauterine infection and presents at birth with microcephaly, vesicular lesions, and micro-ophthalmia.
Evaluation and Management
Toxoplasmosis
If in utero infection is suspected based on prenatal ultrasonographic findings, amniotic fluid can be sent for Toxoplasmosis polymerase chain reaction (PCR). Postnatally, the recommended workup includes ophthalmologic, neurologic, and audiologic evaluations, as well as CSF PCR testing for Toxoplasma and head computed tomography (CT). For infected newborns, treatment with pyrimethamine, sulfadiazine, and leucovorin is the most widely accepted regimen and results in improved ocular outcomes. All pregnant women should be counseled to avoid changing cats’ litter boxes and to wear gloves when in contact with soil (Figure 105-2).
Hepatitis B
Prevention is the most important aspect of disease management because 95% of vertically transmitted cases can be prevented. Management includes both active and passive immunoprophylaxis. Infants born to mothers with positive or unknown HBsAg status should receive the hepatitis B vaccine within 12 hours of life. Hepatitis B vaccination should then proceed as recommended by the current immunization schedule. If the mother is positive, hepatitis B immunoglobulin (HBIG) should be administered with vaccine. If maternal status is unknown and the infant is born at term, HBIG should be administered within the first 7 days of life pending results of maternal testing. If the infant weighs less than 2 kg, HBIG should be administered within the first 12 hours of life even if maternal testing is still pending, and these infants should receive 4 total doses of hepatitis B vaccine.
Syphilis
All pregnant women should be screened with a serum RPR; positive test results should be confirmed with antitreponemal testing with either the fluorescent antitreponemal antibody absorption test (FTA-ABS) or microhemagglutination assay for antibodies to T. pallidum (MHA-TP). RPR titers can be used to follow disease activity. Serum RPR or Venereal Disease Research Laboratory test (VDRL) should be done on all infants of mothers with a positive screen, and full evaluation should be performed on infants with abnormal examination results, whose RPR titers are fourfold greater than maternal titers, or if maternal treatment is unknown, with an antibiotic other than penicillin or given within 1 month of delivery or if maternal titers have not fallen before delivery. Full evaluation includes a CBC, CSF for cell count, protein and VDRL, and long bone radiographs. Standard treatment is with penicillin for 10 days.
HIV
To decrease the rate of vertical transmission of HIV, delivery via caesarian section before rupture of membranes is recommended, with complete avoidance of breastfeeding. Maternal therapy with zidovudine (AZT) should begin during the first trimester, with intravenous (IV) therapy during delivery and administration of AZT to the newborn as soon as possible (within hours) after birth and continuing until at least 6 weeks of age. In neonates with suspected HIV infection, HIV PCR testing is recommended because of transplacental transmission of anti-HIV antibodies to the infant, which may result in false-positive test results. Thirty percent of infants tested using detection of nucleic acid will have a positive test result within the first 48 hours of life, and almost all have detectable viral load by 1 month of age. If both test results are negative, the absence of HIV infection can be confirmed between 12 and 18 months of age by HIV antibody testing.
Rubella
No specific treatment for rubella is available; however, infection is ongoing, and infants should be considered infectious until 1 year of age. Current guidelines recommend serum immunoglobulin M (IgM) in neonates and serial measurements of rubella-specific IgG at 3, 6, and 12 months. Increasing titers are indicative of ongoing infection.
CMV
The diagnosis of CMV requires isolation of virus (PCR testing) from urine, respiratory secretions, stool, or CSF obtained within 3 weeks of birth. Head imaging with head ultrasound or CT scan should be performed in suspected cases, and periventricular calcifications are predictive of later neurologic outcomes. Hearing screening may not identify associated sensorineural hearing loss until several months after birth. Ganciclovir may be used in infants with demonstrated CNS involvement and has been shown to decrease sensorineural hearing loss in this specific population.
Herpes Simplex Virus
If present, vesicles should be unroofed and fluid sent for HSV PCR and culture. Suspicion for disseminated HSV should be high in all ill-appearing neonates younger than 1 month of age, and serum and CSF HSV PCR testing should be considered. IV acyclovir is the treatment of choice and should be continued for 14 to 21 days depending on the extent of infection.
Other Infections
Etiology and Pathogenesis
Neisseria gonorrhoeae is a gram-negative diplococcus that resides intracellularly. The gonococcus is transmitted when the neonate passes through the vaginal canal. Chlamydia trachomatis is an obligate intracellular bacteria with 18 serologic variants. Neonatal acquisition from the vaginal canal is approximately 50%, and the nasopharynx is the most commonly primarily infected site.
Clinical Presentation
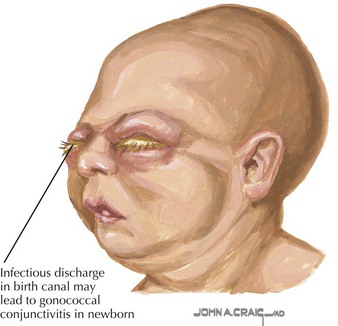
Gonococcal infection generally presents as purulent unilateral or bilateral eye discharge and conjunctival erythema, termed ophthalmia neonatorum (Figure 105-3). Scalp abscess after fetal monitoring has also been noted. Less commonly, meningitis or disseminated disease can present and should be included in the differential diagnosis of a septic-appearing infant. Similarly, chlamydial disease presents as purulent eye discharge and may form a membrane on the conjunctivae. Neonatal pneumonia is another classic presentation of neonatal chlamydial infection, presenting with staccato cough, tachypnea, and occasionally nasal congestion within the first 2 to 3 months of life.
Etiology and Management
Neisseria gonorrhoeae
Gram-negative diplococci may be visualized on Gram stain from samples taken from eye drainage, blood, or CSF. Nuclear acid amplification is the most sensitive mode of testing and can be used on a variety of body fluid samples. Prophylaxis with erythromycin or tetracycline eye ointment within 1 hour of delivery is effective at preventing neonatal ophthalmia. Treatment with an extended-spectrum cephalosporin (ceftriaxone or cefotaxime) in addition to frequent eye irrigation is recommended.
Chlamydia trachomatis
Birth prophylaxis with eye ointment as above is not as effective in preventing Chlamydia ophthalmia. Nuclear acid amplification is also a sensitive method of testing for chlamydial infection from body fluids. including eye discharge. If an infant is positively diagnosed with chlamydial infection of the eye, the infant should receive a 14-day course of oral erythromycin for presumed chlamydial pneumonia, which may present days later.
Future Directions
The laboratory criteria for diagnosis, hospital admission, and antibiotic administration in the setting of suspected neonatal sepsis continue to undergo rigorous evaluation, as are new laboratory markers for sepsis. Clinicians should maintain a high suspicion in all infants presenting with parental concerns as above because neonatal sepsis can have devastating consequences.
Baker MD, Bell LM, Avner JR. Outpatient management without antibiotics of fever in selected infants. N Engl J Med. 1993;329(20):1437-1441.
Gordon A, Jeffery HE. Antibiotic regimens for suspected late onset sepsis in newborn infants. Cochrane Database Syst Rev (3):CD004501, 2005.
Hammerschlag MR, Cummings C, Roblin PM, et al. Efficacy of neonatal ocular prophylaxis for the prevention of chlamydial and gonococcal conjunctivitis. N Engl J Med. 1989;320(12):769-772.
Kimberlin DW, Lin CY, Jacobs RF, et al. Natural history of neonatal herpes simplex virus infections in the acyclovir era. Pediatrics. 2001;108(2):223-229.
Lee C, Gong Y, Brok J, Boxall EH, Gluud C. Effect of hepatitis B immunisation in newborn infants of mothers positive for hepatitis B surface antigen: systematic review and meta-analysis. BMJ. 2006;332(7537):328-336.
Lopez A, Dietz V, Wilson M, Narvin T, Jones JL. Preventing congenital toxoplasmosis. MMWR. Morbid Mortal Wkly Rep. 2000;49(RR02):57-75.
Mofenson LM. U.S. Public Health Service Task Force recommendations for use of antiretroviral drugs in pregnant HIV-1 infected women for maternal health and for interventions to reduce perinatal HIV-1 transmission in the United States. MMWR Morbid Mortal Wkly Rep. 2002;51(RR18):1-38.
Pickering LK, editor. Red Book: 2009 Report of the Committee on Infectious Diseases, ed 27, Elk Grove Village, IL: American Academy of Pediatrics, 2009.
Schrag SJ, Gorwitz R, Fultz-Butts K, Schuchat A. Prevention of perinatal group B streptococcal disease. Revised guidelines from CDC. MMWR. Morbid Mortal Wkly Rep. 2002;51(RR-11):1-22.
Schrag SJ, Zell ER, Lynfield R, et al. A population-based comparison of strategies to prevent early-onset group B streptococcal disease in neonates. N Engl J Med. 2002;347(4):233-239.