Motor Learning
After reviewing this chapter, the student or practitioner will be able to do the following:
1 Describe how motor control affects occupational performance.
2 Describe dynamic systems theory and how it explains motor control.
3 Describe the task-oriented approach to motor learning.
4 Describe constraint-induced movement therapy as an intervention targeting functional use of a hemiparetic upper extremity following a neurologic insult to the brain.
5 Describe the use of robotics in the use of upper extremity motor control.
6 Describe the use of virtual reality technology in the use of upper extremity motor control.
7 Describe the use of bilateral training techniques for improving upper extremity motor control.
8 Develop client-centered and occupation-based treatment programs to facilitate motor learning.
Theoretic Foundations of Motor Learning
Motor learning is the acquisition and modification of learned movement patterns over time.51 It involves cognitive and perceptual processes to code various motor programs. Motor learning requires practice and experience, which leads to permanent changes in the person’s ability to produce movement sufficient to meet the demands of occupational performance. Motor control is the outcome of motor learning and involves the ability to produce purposeful movements of the extremities and postural adjustments in response to activity and environmental demands.5
The process of motor learning after a CVA, traumatic brain injury, cerebral palsy, and other neurologic insults to the brain has received a great deal of attention in research and occupational therapy (OT) practice. Researchers have found that 5 years after the onset of a stroke, approximately 56% of clients continue to have severe hemiparesis.11 The initial degree of motor impairment is a predictor of motor recovery. Dominkus and colleagues9 assessed motor recovery in the upper extremity with the Motricity Index8 and found that a client with initial paresis was 4.58 times more likely to achieve motor recovery than a client with initial paralysis. Furthermore, nearly 400,000 people survive with some level of neurologic impairment and disability.18 The explosion of new research on brain plasticity, or the ability of the brain to reorganize and develop new pathways, has paved the way for evolving approaches that target the ability of the individual to regain movement that enhances occupational performance, participation, and quality of life.51 Recent studies support the theory that a form of brain plasticity known as cortical map reorganization plays a major role in regaining functional use of a hemiparetic upper extremity after a CVA.6,25
Dynamic Systems Theory
Modern motor control approaches are based on dynamic systems theory, which views motor behavior as a dynamic interaction between client factors (e.g., sensorimotor, cognitive, perceptual, and psychosocial), the context (e.g., physical, cultural, spiritual, social, temporal, personal, and virtual), and the occupations that must be performed to enact the client’s roles.30,31 Dynamic systems theory is based on a heterarchic model in which each component (e.g., client, environment, and occupational performance) is viewed as being critical in a dynamic interaction to support the client’s ability to engage in occupation.5,51 Thus, movement and motor control are the result of a dynamic interaction between each of the subsystems. In addition, any change in the system has an effect on all the subsystems. For example, a CVA can lead to changes in the client’s sensorimotor, cognitive, and perceptual skills, which affects motor control, engagement in occupation, and the person’s ability to master the environment.
The heterarchic model is contrasted to the hierarchic model, which views higher centers in the central nervous system as having control over the subordinate lower centers.31 The traditional sensorimotor approaches, such as neurodevelopmental treatment, proprioceptive neuromuscular facilitation, and the theories developed by Rood and Brunnstom, are based on a hierarchic model. Recent research supports a dynamic systems theory approach in which motor learning plus the development of motor control is a dynamic process that involves interaction of the person, environment, and occupations that the client needs to perform or wants to perform.
Task-Oriented Approach
The task-oriented approach to motor recovery is based on dynamic systems principles in which occupational performance and motor recovery are achieved by a dynamic interaction of the person, the environment, and the occupations that the person is performing.30,31 In OT, this approach is occupation based and client centered and focuses on enabling the client to achieve motor recovery through occupational performance using real objects, environments, and meaningful occupations.47,49 Research shows that the use of real objects from the environment versus simulated objects produces better functional movement.71 The client must also have the opportunity to attempt to solve motor problems in the context of multiple environments by using a variety of strategies. For example, Richard (see the case study) would need to be able to turn the pages in the prayer book in the spiritual context of his place of worship, in addition to using motor strategies that allow him to move from sitting to standing to kneeling. Research has shown that the environmental context plays a key role in the transfer of motor skill acquisition.13,29
Occupations that the client has identified as being important through the COPM23 can be used to motivate the client to problem-solve various motor strategies. An intervention plan that is developed collaboratively between the client and the therapist can aid the client in taking a more proactive role in making progress toward his or her outcomes and can facilitate more effective follow-through. Research has shown that a client-centered and occupation-based approach can assist clients in attaining their personal goals.61,62 Emerging research is also demonstrating that an occupation-based approach is more effective than rote exercise in remediating motor control impairments.7,14,40,41,50,60,63,72 In addition, engaging in an occupation or activity from start to finish has been shown to elicit a more efficient, forceful, and coordinated motor response than has performing only small portions of an activity.28
Activity analysis must be used to analyze the necessary movements that the client must perform to complete the task successfully. Effective upgrading and downgrading of the activity must be incorporated for clients to feel that they are successfully moving toward their goals. In addition, motor learning can take place only if the client has multiple practice opportunities across several real environmental contexts.13,24
Qualitative (e.g., verbal encouragement) and quantitative (e.g., concrete measures of success or failure during a motor task) analyses have been shown to produce knowledge of results, which enables the client to cognitively reflect on the strengths and weaknesses of a particular motor strategy and implement a more effective strategy for completing an activity.19,65,67 Through this dialogue, the client can learn to transfer various motor skills across multiple contexts.
Constraint-Induced Movement Therapy
Constraint-induced movement therapy (CIMT), or forced use, is a therapeutic strategy designed to promote functional use of a hemiparetic upper extremity and has been credited with speeding up the cortical map reorganization process in nonhuman primates57 and in humans.22 CIMT is also based on the principles of dynamic systems theory and a task-oriented approach to the acquisition of motor control. In other methods of stroke rehabilitation, clients learn to use the more functional or less involved upper extremity for daily activities. This treatment approach may foster learned nonuse of the more involved upper extremity. Learned nonuse is a phenomenon in which the individual neglects to use the affected or more involved extremity because of the extreme difficulty coordinating movement after the onset of a stroke, brain injury, or other neurologic condition.22
As a person or animal moves through its environment and manipulates objects, it receives sensory feedback from various sources simultaneously. Vision, audition, proprioception, and kinesthesia provide important sensory information for skilled movement. The importance of sensory information on motor action was demonstrated in the classic experiment carried out by Mott and Sherrington.39 The afferent (sensory) input enters the spinal cord through the dorsal roots of the spinal nerves. By selectively severing the dorsal roots, sensory input was effectively eliminated while leaving motor innervation basically intact. Experiments conducted before 1955 demonstrated that deafferentation of a single forelimb in rhesus monkeys resulted in an unused extremity when the animal is unrestricted.
Animal research has led to the discovery that cortical reorganization occurs after injury to the nervous system.25 Research on somatosensory deafferentation in monkeys has shown that if a single monkey forelimb is deafferented, the monkey will not use that extremity.20,21 In other words, the procedure left the monkeys with intact motor nerves but no sensation in the affected upper extremity. The initial shock to the nervous system prevented movement. Even after the nervous system recovered, the monkeys failed to use their affected limb. Taub54 theorized that use of the deafferented extremity could be retrained if the intact extremity is restrained and the monkey is forced to use the affected extremity. If the restraint was used for a specific time, between 1 and 2 weeks, the functional improvement could be permanent. These studies have shown that certain training procedures can be used to enable monkeys to regain use of their deafferented extremity.20,21,55,56 Experimental evidence indicates that the persistent loss of motor function caused by deafferentation was due to learned behavioral suppression, a phenomenon called learned nonuse.54 Conditioned response techniques did not show promise in restoring use of the extremity for daily activities.
Another therapeutic strategy, shaping, did result in significant improvements in motor function during ADLs. Shaping procedures are behavioral techniques that approach a desired motor outcome in small, successive increments.36,44,58 Shaping strategies allow subjects to experience successful gains in performance with relatively small amounts of motor improvement. Explanations from several studies have led to the development of a hypothesis that explains why the constraint and training procedures improve motor recovery after deafferentation.
The theory of learned nonuse was first described by Taub and is thought to extend to humans after central nervous system damage (Figure 32-1).54–56,68 Animal research has demonstrated that when the forelimb is not functional, the animal no longer uses the affected upper extremity for everyday tasks. This tendency has led to reinforcement and persistence of nonuse of the affected limb. Constraining the unaffected limb of the monkeys provided the early evidence for reversing learned nonuse.

FIGURE 32-1 Client with a left cerebrovascular accident and right hemiparesis before initiation of the constraint-induced movement therapy (CIMT) program. The client demonstrates learned nonuse by using her stronger, uninvolved left upper extremity during self-feeding. (Courtesy Remy Chu, OTR/L.)
To date, rehabilitation methods for those who have sustained a CVA or other neurologic insult to the brain have included, but are not limited to biofeedback, neuromuscular facilitation, and operant conditioning. These various forms of rehabilitation are often used in conjunction with methods that teach clients to compensate by using their intact upper extremity to perform their day-to-day functional activities. Compensatory rehabilitation strategies may improve the efficiency of the intact upper extremity, but at the same time they encourage learned nonuse of the affected upper extremity. At present, there is minimal experimental evidence demonstrating the positive benefits of these forms of therapy.
An alternative therapeutic approach, CIMT, has been used extensively for rehabilitation. Unlike more traditional rehabilitation therapies, CIMT forces the client to use the more involved upper extremity by immobilizing the less involved or unaffected upper extremity in a sling, mitt, or a combination of both (Figure 32-2). Clients then practice using their affected upper extremity on an intensive basis for several consecutive weeks by using shaping movements with the affected upper extremity. The improvements noted in the more involved extremity after this program were attributed to the learned nonuse phenomenon described by Taub54; thus, part of the theoretic framework of CIMT is taken from the neurophysiologic and behavioral studies of the learned nonuse of the more affected limb seen in animal experiments. Constraining the intact forelimb of these monkeys provided the first evidence of the ability to overcome the learned nonuse phenomenon. This success led to further studies involving humans with hemiparesis that developed after a stroke, brain injury, and other neurologic conditions.

FIGURE 32-2 Constraint-induced movement therapy forces the individual to use the affected upper extremity by immobilizing the unaffected upper extremity in a mitt.
Wolf68,69 and Miltner and associates35 demonstrated the positive effects of using CIMT in individuals with stroke. Liepert and coworkers25 demonstrated cortical reorganization in humans undergoing CIMT. In 1993, Taub and colleagues59 found significant results when using CIMT in a randomized clinical trial of nine individuals after stroke. These subjects had experienced strokes from 1 to 18 years before participation in CIMT and were required to meet inclusion criteria similar to those used by Wolf and coworkers8 (e.g., possessing some wrist and finger extension while still demonstrating significant disability). Additionally, the subjects had to demonstrate good balance because they would be wearing a sling and would therefore be unable to use their less affected or unaffected upper extremity to protect themselves in the event of a fall. The subjects were randomly assigned to either an experimental group or a control group. The subjects assigned to the experimental group wore a sling on the less involved upper extremity for a period of 12 days. During the 12 days the sling was worn throughout all waking hours except when specific activities were being carried out, such as when it was unsafe or extremely difficult to use the more affected arm exclusively. The study also used a behavioral contract that included an agreement from the subjects to wear the restraint device for at least 90% of their waking hours during the 12-day intervention period. The behavioral contract specifically identified activities during which the subject was to use the more involved arm exclusively, to use both arms, and/or to use the less involved arm (for safety reasons). Participants in the control group were instructed to focus attention on the more involved upper extremity and were encouraged to attempt to use the more involved upper extremity for as many new functional activities as possible at home. Activities were recorded throughout the 2-week period. Those in the control group also received two sessions involving activities requiring active movement of the more involved extremity and were provided an individualized range-of-motion exercise program. The effectiveness of treatment was measured with the Wolf Motor Function Test (WMFT) and the Motor Activity Log (MAL), a structured interview exploring functional use in the life setting. Speed, quality of movement, and functional use of the affected upper extremity were significantly improved in the research group in comparison to the control group, and functional movement was maintained over a 2-year period. In 1999, Kunkel and associates22 also demonstrated a greater than 100% increase in the amount of use of the affected upper extremity in real-world environments. The effective factor in all forms of CIMT appears to be intensive practice and functional use of the affected upper extremity repeatedly across multiple contexts for many hours a day for a period of consecutive days.70
Over the past 30 years, numerous studies have further confirmed the effectiveness of CIMT versus traditional rehabilitation therapy in improving the client’s functional motor control after stroke.50 More recently, however, research involving CIMT has used various populations, altered the inclusion criteria, and modified the intensity of treatment and the length of the therapy program from the original research protocol. For example, Pierce and colleagues46 found that the forced-use component of CIMT in conjunction with a home program may be effectively incorporated into the traditional outpatient setting. Furthermore, Page and associates43 found that repeated task-specific practice is more critical than intensity in improving upper extremity function.
In the Very Early Constraint-Induced Movement during Stroke Rehabilitation (VECTORS) study, 52 subjects were randomized to one of three groups an average of 9.7 days after stroke. The three groups included the following: (1) standard CIMT in which subjects received 2 hours of shaping therapy and wore a mitt for 6 hours per day, (2) high-intensity CIMT in which clients underwent 3 hours of shaping exercise per day and wore a mitt for 90% of waking hours, and (3) the control treatment consisting of 1 hour of ADL training and 1 hour of upper extremity bilateral training exercises. All treatment was provided for 2 weeks. The primary end point measure was the total Action Research Arm Test (ARAT) score on the more affected side at 90 days after stroke onset. In all groups the total ARAT score improved with time. Subjects in the standard CIMT and control treatment groups achieved similar gains in total ARAT score (24.2 and 25.7, respectively), whereas subjects in the high-intensity CIMT group exhibited an average gain of just 12.6 points.10 Overall, there is conflicting evidence of the benefit of CMIT over traditional therapies in the acute stage of stroke; however, there is strong evidence of the benefit of CIMT and modified CIMT over traditional therapies in the chronic stage of stroke. The benefits appear to be confined to stroke patients with some active wrist and hand movements, particularly those with sensory loss and neglect. These findings have broadened the scope and applicability of CIMT to various populations.
Although recent CIMT research has expanded the scope to include different diagnostic criteria, different treatment regimens, and different inclusion criteria, little of the current CIMT research has addressed the participant’s self-reported satisfaction in life roles after completing a CIMT program. After individuals have a stroke or other neurologic condition, they experience a disruption in their life roles. This disruption can lead to feelings of ineffectiveness, incompetence, and helplessness. To restore health and quality of life, one must identify and alter one’s lifestyle to improve the fit between oneself and the environment. CIMT has been proved to be effective in improving motor control in individuals who have experienced a stroke or other neurologic condition. Functional carryover of CIMT from the clinic to the natural environment has been demonstrated. Research has shown significant improvement in daily use of the affected upper extremity and an increase in the speed at which the individual carries out activities after participating in a CIMT program. Increased life satisfaction resulting from an increased ability to use the affected upper extremity has been noted in individuals who reported satisfaction in performance of meaningful daily activities and life roles.42,48
To determine whether a subject meets the inclusion criteria for CIMT, a telephone screening protocol is often administered.37 Many research studies use a CIMT protocol that contains typical inclusion criteria for use of this therapeutic strategy. These criteria can include (1) a first-time CVA that occurred more than 1 year earlier; (2) not currently receiving any therapeutic intervention; (3) a score of higher than 44 on the Berg Balance Test2 or limited balance problems requiring an assistive device for mobility in clients who had a full-time caregiver to assist in any balance issues; (4) ability to move the affected arm in 45 degrees of shoulder flexion and abduction, 90 degrees of elbow flexion and extension, 20 degrees of wrist extension, and 10 degrees of extension at the metacarpal phalanges and interphalanges as determined by the client’s available active range of motion (Figure 32-3); (5) no significant cognitive impairments as demonstrated by a Mini-Mental State Examination score of at least 2215 or other type of cognitive test; (6) no pre-existing comorbid conditions that might interfere with mobility or function; (7) limited spasticity (score of 0 or 1) as measured by the Modified Ashworth Scale3; and (8) the ability to identify an individual or caregiver who could assist in the home program.37,48 In summary, potential reasons for a person to be excluded may include motor ability that is too high or too low, cognitive deficits that prevent adequate participation, and an existing high degree of functional use of the affected upper extremity.
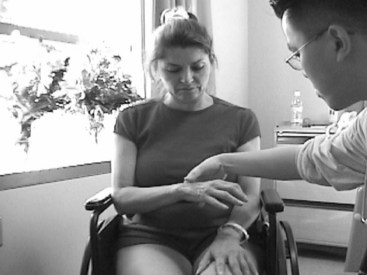
FIGURE 32-3 The client is asked to demonstrate active wrist and finger extension in her dominant right hand. The client is able to achieve 20 degrees of wrist extension and 10 degrees of finger extension (at the metacarpal phalanges) from a flexed position. (Courtesy Remy Chu, OTR/L.)
A battery of assessments are typically administered to all clients included in a CIMT treatment program.37 Results from these assessments are used to test certain research hypotheses, whereas other assessments are used for diagnostic purposes or to generate new hypotheses. Some typical assessments include the WMFT and MAL.
The WMFT consists of 15 motor items that examine contributions from the distal and proximal muscles of the arm. The tasks in this assessment are sequenced from proximal to distal and gross to fine motor. The majority of tasks are completed with the subject seated in a chair. Standard tasks such as lifting the forearm to the table, reaching for an object, or lifting a pencil are rated on a scale from 0 (does not attempt with the weaker arm) to 5 (movement appears normal), and the time to complete the task is measured (Figure 32-4). The WMFT uses a grid or template that is taped to the desk to specify standardized measurements. The WMFT is administered before the intervention, immediately after the intervention, and at a designated follow-up time.
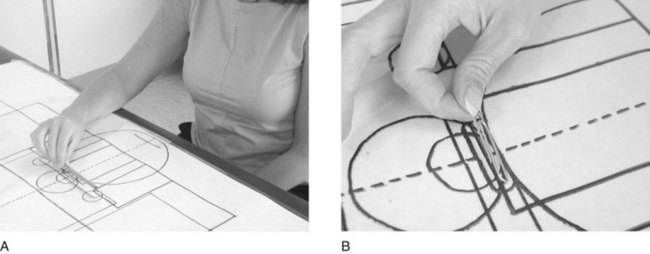
FIGURE 32-4 The Wolf Motor Function Test includes standard tasks such as lifting the forearm to the table and reaching for an object.
The MAL was developed for the purpose of assessing activities attempted outside the clinical setting. The MAL is a self-reported, 30-item instrument administered in an interview format. Subjects are asked to rate their performance on each activity reported, and emphasis is placed on clients’ functional use of the hemiparetic upper extremity in their home environment. The assessment is administered approximately 10 times throughout the course of CIMT intervention. The instrument consists of specific functional activities, such as turning on a light switch or opening a drawer. The amount of functional use of the more involved upper extremity is rated by the participant from 0 (never used) to 5 (involved arm used the same as it was before the stroke). Quality of movement (how well) is also self-rated from 0 (not used) to 5 (normal movement).
Therapeutic procedures using CIMT in the clinic are performed under the supervision of an OT practitioner (Figure 32-5). The procedures are effective only if use of the affected upper extremity is carried over to the client’s home and community environment. Clients are asked about their compliance in incorporating their affected upper extremity into functional activities as noted in a log or personal diary. The home diary is used to outline the clients’ activities from the time at which they are discharged from the clinic until they return for their CIMT sessions. A typical daily schedule is often used. The schedule includes the time and length of rest periods. Furthermore, specific shaping task practice is also listed on the daily schedule. A desired motor or behavioral objective is approached in small steps with an individualized shaping plan. During shaping, explicit feedback is provided to identify small improvements in motor or functional performance. The shaping task selected depends on the specific joint movements exhibiting impairment that have the greatest chance of improving and the client’s preference among tasks that have a similar potential for producing specific improvements. Applying CIMT and shaping requires repetitive, supervised, constant practice. CIMT study protocols call for 6 hours of continuous task practice per day (Figures 32-6 and 32-7).

FIGURE 32-5 A, Initiation of the constraint-induced movement therapy (CIMT) program during a self-feeding activity. The client requires minimal hand-over-hand physical assistance to take her first bite with her weaker right hand. Hand-over-hand physical assistance focuses on eliminating gravity as the client brings the spoon to her mouth. B and C, After 10 CIMT trials, the client is attempting to feed herself without physical assistance from the occupational therapist. (Courtesy Remy Chu, OTR/L.)
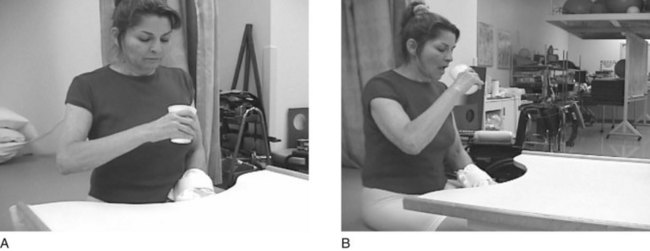
FIGURE 32-6 The client is performing a block trial (cup-to-mouth trial). The client demonstrates less strain in her neck and shoulder while bringing the cup to her mouth. (Courtesy Remy Chu, OTR/L.)
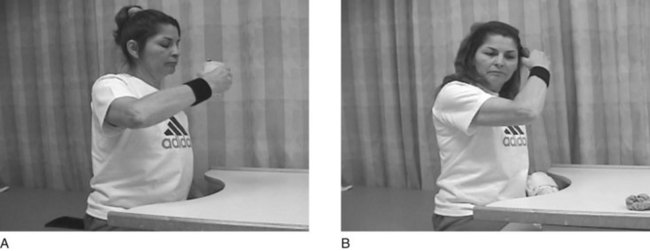
FIGURE 32-7 A, After 1.5 weeks of constraint-induced movement therapy (CIMT), the client is able to bring a cup to her mouth independently with the weaker right upper extremity. B, She is also able to bring her right hand to her head to brush her hair. (Courtesy Remy Chu, OTR/L.)
An important component of interventions designed to enhance motor learning is the feedback received by the client while attempting a movement strategy and the results of the movement action.51 Feedback refers to the sensory experiences intrinsic to the client and the external information provided by the environment, which includes verbal comments from the OT practitioner. This combined type of feedback can be seen when a client attempts to place a hemiparetic arm into the wrong sleeve of a button front shirt and receives the visual information that something does not “look correct” along with verbal directions by the OT practitioner that placing the arm in the other sleeve will make the task easier. Knowledge of the results is a form of external feedback in which the client assesses whether the correct results were achieved after completion of the motor action. The OT practitioner assists the client by providing feedback during performance of the motor task and on completion of the motor task to assess the results. These strategies are combined with shaping to foster a higher frequency of successful motor practice for functional tasks.
A more recent study that evaluated the immediate and long-term effects of upper extremity rehabilitation approaches for stroke compared functional task practice and strength training with standard care and found that task specificity and stroke severity are important factors in rehabilitation of arm use after an acute stroke.66 Another recent approach that involves repetitive training of the paretic upper extremity on task-oriented activities has provided evidence of efficacy in stroke survivors. The Effect of Constraint-Induced Movement Therapy on Upper Extremity Function (EXCITE) trial of individuals 3 to 9 months after stroke found that CMIT produced statistically and clinically relevant improvements in arm motor function that persisted for at least 1 year.69
Robotics
Robot-assisted therapies involve the use of a robot to assist in initiation of movement, guidance, and resistance to movement, as well as to provide feedback. Mirror-image motion enabler (MIME) robotic devices were developed to enable unrestricted unilateral or bilateral shoulder or elbow movement. The robotic unit applies force to the more affected forearm during goal-directed movements. A randomized study involving the use of a robotic device and a control group found that there were no significant differences between the groups on either of the ADL assessments but that the clients in the robotic group exhibited a trend toward greater improvement in Fugl-Meyer scores. These differences achieved statistical significance only if the shoulder and elbow portions of the Fugl-Meyer test were considered.4 Another study examined robot-assisted movement therapy versus conventional therapy in individuals with chronic hemiparesis. This study found that after the first and second months of treatment, the group using robot-assisted therapy had significantly greater improvements in the proximal movement portion of the Fugl-Meyer test. The clients using the robots also had larger gains in strength and larger increases in reach extent after 2 months of treatment. At 6 months, no significant differences were seen between the two groups on the Fugl-Meyer test, although the robot group did have significantly greater improvements on the Functional Independence Measure.26 In two other studies using robotic therapy, one found that significantly greater gains were attained after treatment in the robot-combined group than in the control group; however, the gains were not maintained at 6 months’ follow-up.27 The other study examined 19 clients following stroke with resultant hemiplegia who underwent a standardized passive exercise program using a robotic arm targeting the upper extremity. This study found no significant changes in pre-exercise and post-exercise responses.45 The conclusions regarding the use of robotics for rehabilitation of the upper extremity show that sensorimotor training with robotic devices improves upper extremity functional outcomes and motor outcomes of the shoulder and elbow. Overall, robot-assisted upper extremity therapy may assist in improving motor function during the inpatient period after a stroke.1,12,64
Virtual Reality Technology
Virtual reality technology allows individuals to experience and interact with three-dimensional environments. Merians and colleagues33,34 found that computerized virtual environments have opened the doors to an “exercise environment where the intensity of practice and positive feedback can be consistently and systematically manipulated and enhanced to create the most appropriate, individualized motor learning approach. Adding computerized virtual reality to computerized motor learning activities provides a three-dimensional spatial correspondence between the amount of movement in the real world and the amount of movement seen on the computer screen. This exact representation allows for visual feedback and guidance for the patient.” Virtual reality is an innovative treatment approach that is in its infancy, but it has shown promise in improving motor function in clients following a stroke and experiencing chronic hemiparesis.
Bilateral Training Techniques
As new theories of neural plasticity have developed, the use of bilateral training techniques for the upper limb following stroke have been discussed. Bilateral upper limb training is a technique in which clients practice the same activities with both upper limbs simultaneously. Theoretically, use of the intact limb assists in promoting functional recovery of the impaired limb through facilitative coupling effects between the upper limbs. Practicing bilateral movements allows activation of the intact hemisphere to facilitate activation of the damaged hemisphere through neural networks linked via the corpus callosum.38,53 In a systematic review that examined 11 clinical trials, Stewart and colleagues52 found that bilateral movement training alone or in combination with sensory feedback is an effective stroke rehabilitation protocol during the subacute and chronic phases of motor recovery.
Summary
Dynamic systems theory supports a heterarchic model of motor control in which motor acquisition is influenced by the client, the environment, and the occupations that the client needs to perform or wants to perform. A task-oriented approach supports the use of occupation-based and client-centered interventions to assist clients in problem solving (e.g., learning how they will perform their desired occupations in a variety of contexts to support transfer of motor learning). CIMT is a task-oriented approach that focuses on constraining use of the unaffected upper extremity to facilitate motor recovery in the affected upper extremity during occupational performance. More research is needed in OT to support the use of a task-oriented CIMT approach to motor recovery to improve participation in occupation. Sensorimotor training with the use of robotic devices improves functional and motor outcomes of the shoulder and elbow; however, it does not improve functional and motor outcomes of the wrist and hand. In virtual reality technology, preliminary evidence has shown that this form of intervention may improve motor outcomes. Finally, bilateral arm training uses the intact extremity to assist the impaired extremity through facilitative coupling effects between both upper extremities.
1. How does motor control affect occupational performance?
2. What is the task-oriented approach to motor learning?
3. What is dynamic systems theory and how does it explain motor control?
4. What is constraint-induced movement therapy and how does this approach increase functional use of a hemiparetic upper extremity after a neurologic insult to the brain?
5. What is robot-assisted therapy and how does this approach improve motor control of the upper extremity?
6. What is virtual reality technology and how is this used to improve upper extremity motor control?
7. How are bilateral training techniques used to improve upper extremity motor control?
8. How would you develop a client-centered and occupation-based treatment program to facilitate motor learning?
References
1. Aisen, ML, Krebs, HI, Hogan, N, et al. The effect of robot-assisted therapy and rehabilitative training on motor recovery following stroke. Arch Neurol. 1997;54:443–446.
2. Berg, KO, Wood-Dauphinee, SL, Williams, JI, Maki, B. Measuring balance in the elderly: validation of an instrument. Can J Public Health. 1992;83(Suppl 2):S7.
3. Bohannon, RW, Smith, MB. Interrater of a Modified Ashworth Scale of muscle spasticity. Phys Ther. 1986;67:206.
4. Burgar, CG, Lum, PS, Shor, PC, et al. Development of robots for rehabilitation therapy: the Palo Alto VA/Stanford experience. J Rehabil Res Dev. 2000;37:663–673.
5. Carr, JH, Shepherd, RB. Neurological rehabilitation: optimizing motor performance. Oxford: Butterworth-Heinemann; 1998.
6. Cramer, SC, Moore, CI, Finklestein, SP, Rosen, BR. A pilot study of somatotopic mapping after cortical infarct. Stroke. 2000;31:668.
7. Dearn, CM, Shepherd, RB. Task-related training improves performance of seated reaching tasks after stroke: a randomized controlled trial. Stroke. 1997;28:722.
8. Demeurisse, G, Gemol, O, Robaye, E. Motor evaluation in vascular hemiplegia. Eur Neurol. 1980;19:382–389.
9. Dominkus, M, Grisold, W, Jelinek, V. Transcranial electrical motor evoked potentials as a prognostic indicator for motor recovery in stroke patients. J Neurol Neurosurg Psychiatry. 1990;53:745–748.
10. Dromerick, AW, Lang, CE, Birkenmeier, RL, et al. Very Early Constraint-Induced Movement during Stroke Rehabilitation (VECTORS): a single-center RCT. Neurology. 2009;73:195–201.
11. Duncan, PW, Goldstein, LB, Matchar, D, et al. Measurement of motor recovery after stroke. Outcome assessment and sample size requirements. Stroke. 1992;23:1084.
12. Fasoli, SE, Krebs, HI, Ferraro, M, et al. Does shorter rehabilitation limit potential recovery poststroke? Neurorehabil Neural Repair. 2004;18:88–94.
13. Ferguson, MC, Rice, MS. The effect of contextual relevance on motor skills transfer. Am J Occup Ther. 2001;55:558.
14. Flinn, NA. Clinical interpretation of effect of rehabilitation tasks on organization of movement after stroke. Am J Occup Ther. 1999;53:345.
15. Folstein, MF, Folstein, SE, McHugh, PR. “Mini-mental state.” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189.
16. Jarus, T. Is more always better? Optimal amounts of feedback in learning to calibrate sensory awareness. Occup Ther J Res. 1995;15:181.
17. Jarus, T. Motor learning and occupational therapy: the organization of practice. Am J Occup Ther. 1994;48:810.
18. Kelly-Hayes, M, Robertson, JT, Broderick, JP, et al. The American Heart Association Stroke Outcome Classification. Stroke. 1998;29:1274–1280.
19. Kilduski, NC, Rice, MS. Qualitative and quantitative knowledge of results: effects on motor learning. Am J Occup Ther. 2003;57:329.
20. Knapp, HD, Taub, E, Berman, AJ. Effect of deafferentation on a conditioned avoidance response. Science. 1958;128:842.
21. Knapp, HD, Taub, E, Berman, AJ. Movement in monkeys with deafferented forelimbs. Exp Neurol. 1963;7:305.
22. Kunkel, A, Kopp, B, Müller, G, et al. Constraint-induced movement therapy for motor recovery in chronic stroke patients. Arch Phys Med Rehabil. 1999;80:624.
23. Law, M, Baptiste, S, Carswell, A, et al. The Canadian occupational performance measure, ed 4. Ottawa, Canada: CAOT Publications; 2005.
24. Lee, TD, Swanson, LR, Hall, AL. What is repeated in a repetition? Effects of practice conditions on motor skills acquisition. Phys Ther. 1991;71:150.
25. Liepert, J, Bauder, H, Wolfgang, HR, et al. Treatment-induced cortical reorganization after stroke in humans. Stroke. 2000;31:1210.
26. Lum, PS, Burgar, CG, Shor, PC, et al. Robot-assisted movement training compared with conventional therapy techniques for the rehabilitation of upper-limb motor function after stroke. Arch Phys Med Rehabil. 2002;83:952–959.
27. Lum, PS, Burgar, CG, Loos, MV, et al. MIME robotic device for upper limb neurorehabilitation in subacute stroke subjects: a follow-up study. J Rehabil Res Dev. 2006;43:631–642.
28. Ma, HI, Trombly, CA. The comparison of motor performance between part and whole tasks in elderly persons. Am J Occup Ther. 2001;55:62.
29. Ma, HI, Trombly, CA, Robinson-Podolski, C. The effect of context on skill acquisition and transfer. Am J Occup Ther. 1999;53:138.
30. Mathiowetz, V. OT task-oriented approach to person post-stroke. In Gillen G, Burkhardt A, eds.: Stroke rehabilitation: a function-based approach, ed 2, St Louis, Mo: Mosby, 2004.
31. Mathiowetz, V, Bass Haugen, J. Motor behavior research: implications for therapeutic approaches to CNS dysfunction. Am J Occup Ther. 1994;47:733.
32. Merians, A, Winstein, CJ, Sullivan, K, Pohl, PS. Effects of feedback for motor skills learning in older healthy subjects and individuals post-stroke. Neurol Rep. 1995;19:23.
33. Merians, AS, Jack, D, Boian, R, et al. Virtual reality–augmented rehabilitation for patients following stroke. Phys Ther. 2002;82:898–915.
34. Merians, AS, Poizner, H, Boian, R, et al. Sensorimotor training in a virtual reality environment: does it improve functional recovery poststroke? Neurorehabil Neural Repair. 2006;20:252–267.
35. Miltner, WH, Bauder, H, Sommer, M, et al. Effects of constraint-induced movement therapy on patients with chronic motor deficits after stroke: a replication. Stroke. 1999;30:586.
36. Morgan, GW. The shaping game: a technique. Behav Ther. 1974;5:481.
37. Morris, DM, Crago, JE, DeLuca, SC, et al. Constraint-induced movement therapy for motor recovery after stroke. NeuroRehabilitation. 1997;9:29.
38. Morris, JH, van, WF, Joice, S, Ogston, SA, et al. A comparison of bilateral and unilateral upper-limb task training in early poststroke rehabilitation: a randomized controlled trial. Arch Phys Med Rehabil. 2008;89:1237–1245.
39. Mott, FW, Sherrington, CS. Experiments upon the influence of sensory nerves upon movement and nutrition of the limbs. Proc R Soc Lond. 1895;57:481.
40. Nagel, MJ, Rice, MS. Cross-transfer effects in the upper extremity during an occupationally embedded exercise. Am J Occup Ther. 2001;55:317.
41. Neistadt, M. The effects of different treatment activities on functional fine motor coordination in adults with brain injury. Am J Occup Ther. 1994;48:877.
42. Ostendorf, CG, Wolf, SL. Effect of forced use of the upper extremity of a hemiplegic patient on changes in function: a single-case design. Phys Ther. 1981;61:1022.
43. Page, SJ, Sisto, S, Levine, P, McGrath, RE. Efficacy of modified constraint-induced movement therapy in chronic stroke: a single-blinded randomized controlled trial. Arch Phys Med Rehabil. 2004;85:14.
44. Panyan, MV. How to use shaping. Lawrence, Kan: H & H Enterprises; 1980.
45. Patel, S, Ho, JT, Kumar, R, et al. Changes in motor neuron excitability in hemiplegic subjects after passive exercise when using a robotic arm. Arch Phys Med Rehabil. 2006;87:1257–1261.
46. Pierce, SR, Gallagher, KG, Schaumburg, SW, et al. Home forced use in an outpatient rehabilitation program for adults with hemiplegia: a pilot study. Neurorehabil Neural Repair. 2003;17:214.
47. Poole, JL. Application of motor learning principles in occupational therapy. Am J Occup Ther. 1991;45:531.
48. Roberts, PS, Vegher, JA, Gilewski, M, et al. Client-centered occupational therapy using constraint-induced therapy. J Stroke Cerebrovasc Dis. 2005;14:115.
49. Sabari, JS. Motor learning concepts applied to activity-based intervention with adults with hemiplegia. Am J Occup Ther. 1991;45:523.
50. Shepherd, RB. Exercise and training to optimize functional motor performance in stroke: driving neural reorganization? Neural Plast. 2001;2:121.
51. Shumway-Cook, A, Woollacott, M. Motor control: theory and practical applications, ed 3. Philadelphia, Pa: Lippincott Williams & Wilkins; 2007.
52. Stewart, KC, Cauraugh, JH, Summers, JJ. Bilateral movement training and stroke rehabilitation: a systematic review and meta-analysis. J Neurol. 2006;244:89–95.
53. Summers, JJ, Kagerer, FA, Garry, MI, et al. Bilateral and unilateral movement training on upper limb function in chronic stroke patients. A TMS study. J Neurol Sci. 2007;252:76–82.
54. Taub, E. Movement in nonhuman primates deprived of somatosensory feedback. Exerc Sport Sci Rev. 1977;4:335.
55. Taub, E, Bacon, R, Berman, AJ. The acquisition of a trace-conditioned avoidance response after deafferentation of the responding limb. J Comp Physiol Psychol. 1965;58:275.
56. Taub, E, Berman, AJ. Avoidance conditioning in the absence of relevant proprioceptive and exteroceptive feedback. J Comp Physiol Psychol. 1963;56:1012.
57. Taub, E, Crago, JE, Burgio, LD, et al. An operant approach to rehabilitation medicine: overcoming learned nonuse by shaping. J Exp Anal Behav. 1994;61:281.
58. Taub, E, Goldberg, IA, Taub, PB. Deafferentation in monkeys: pointing at a target without visual feedback. Exp Neurol. 1975;46:178.
59. Taub, E, Miller, NE, Novack, TA, et al. Technique to improve chronic motor deficit after stroke. Arch Phys Med Rehabil. 1993;74:347.
60. Thielman, GT, Dean, CM, Gentile, AM. Rehabilitation of reaching after stroke: task-related training versus progressive resistive exercise. Arch Phys Med Rehabil. 2004;85:1613.
61. Trombly, CA, Radomski, MV, Davis, ES. Achievement of self-identified goals by adults with traumatic brain injury: phase I. Am J Occup Ther. 1998;52:810.
62. Trombly, CA, Radomski, MV, Trexel, C, et al. Occupational therapy and achievement of self-identified goals by adults with acquired brain injury: phase II. Am J Occup Ther. 2002;56:489.
63. Trombly, C, Wu, CY. Effect of rehabilitation tasks on organization of movement after stroke. Am J Occup Ther. 1998;53:333.
64. Volpe, BIT, Krebs, HI, Hogan, N, et al. Robot training enhanced motor outcome in patients with stroke maintained over three years. Neurology. 1999;53:1874–1876.
65. Winstein, CJ. Knowledge of results and motor learning: implications for physical therapy. Phys Ther. 1991;71:140.
66. Winstein, CJ, Rose, DK, Tan, SM, et al. A randomized controlled comparison of upper extremity rehabilitation strategies in acute stroke: a pilot study of immediate and long term outcomes. Arch Phys Med Rehabil. 2004;85:620–628.
67. Winstein, CJ, Schmidt, RA. Reduced frequency of knowledge of results enhances motor skills learning. J Exp Psychol Learn Mem Cogn. 1990;16:677.
68. Wolf, SL, Lecraw, DE, Barton, LA, Jann, BB. Forced use in hemiplegic upper extremities to reverse the effect of learned non-use among chronic stroke and head injured patients. Exp Neurol. 1989;104:125.
69. Wolf, SL, Winstein, CJ, Miller, JP, et al. Effect of constraint-induced movement therapy on upper extremity function 3 to 9 months after stroke. The EXCITE randomized clinical trial. JAMA. 2006;296:2095–2104.
70. Wolfgang, HR, Miltner, W, Bauder, H, et al. Effects of constraint-induced movement therapy on patients with chronic motor deficits after stroke: a replication. Stroke. 1999;30:586.
71. Wu, C-Y, Trombly, CA, Lin, K-C, Tickle-Degnen, L. A kinematic study of contextual effects on reaching performance in persons with and without stroke: influences of object availability. Arch Phys Med Rehabil. 2000;81:95.
72. Wu, CY, Wong, MK, Lin, KC, Chen, HC. Effects of task goal and personal preference on seated reaching kinematics after stroke. Stroke. 2001;32:70.