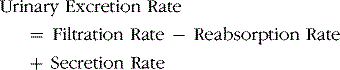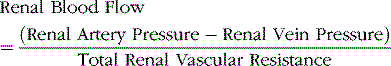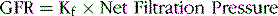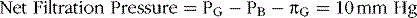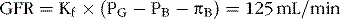CHAPTER 26 Urine Formation by the Kidneys
I. Glomerular Filtration, Renal Blood Flow, and Their Control
The multiple functions of the kidney in the maintenance of homeostasis include the following:
Urine Formation Results from Glomerular Filtration, Tubular Reabsorption, and Tubular Secretion (p. 310)
A primary function of the kidney is to “clear” unneeded substances from the blood and excrete them in the urine and to return needed substances to the blood. The first step in the performance of this function is filtration of fluid from the glomerular capillaries into the renal tubules, a process called glomerular filtration. As the glomerular filtrate flows through the tubules, the volume of filtrate is reduced, and its composition is altered by tubular reabsorption (the return of water and solutes from the tubules back into the blood) and by tubular secretion (the net movement of water and solutes into the tubules), each of which is highly variable depending on the body’s needs. Thus excretion of each substance in the urine involves a specific combination of filtration, reabsorption, and secretion (Fig. 26–1), as expressed by the following relation.
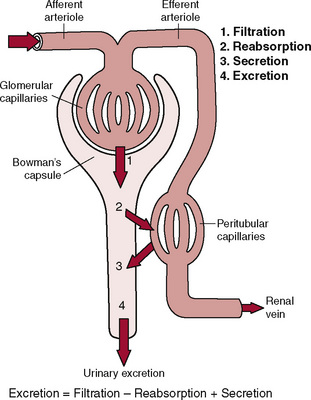
Figure 26–1 Basic kidney processes that determine the composition of the urine. The urinary excretion rate of a substance is equal to the rate at which the substance is filtered minus its reabsorption rate plus the rate at which it is secreted from the peritubular capillary blood into the tubules.
Each of these processes is physiologically controlled, and changes in the excretion rate can obviously occur via changes in glomerular filtration, tubular reabsorption, or tubular secretion.
Renal Blood Flow Constitutes about 22% of the Cardiac Output
Blood flows to each kidney through a renal artery, which branches progressively to form the interlobar arteries, arcuate arteries, interlobular arteries, and afferent arterioles, which lead to the glomerular capillaries, where filtration of fluid and solutes begins. The capillaries of each glomerulus coalesce to form an efferent arteriole, which leads to a second capillary network, the peritubular capillaries, which surround the tubules. The peritubular capillaries empty into the vessels of the venous system, which run parallel to the arteriolar vessels, and progressively form the interlobular vein, arcuate vein, interlobar vein, and renal vein, which leaves the kidney along the renal artery and ureter. The vasa recta are specialized peritubular capillaries that dip into the renal medulla and run parallel to the loops of Henle. The outer portion of the kidney, the renal cortex, receives most of the blood flow of the kidney; only 1% to 2% of the total renal blood flow passes through the vasa recta, which supply the renal medulla.
Two distinguishing features of the renal circulation are (1) the high rate of blood flow (about 1100 mL/min for a 70-kg man) relative to the tissue weight (about 300 g for the two kidneys) and (2) the presence of two capillary beds, the glomerular and peritubular capillaries, which are arranged in series and separated by efferent arterioles. The glomerular capillaries filter large amounts of fluid and solutes, most of which are reabsorbed from the renal tubules into the peritubular capillaries.
Renal blood flow is determined according to the pressure gradient across the renal vasculature and the total renal vascular resistance, as expressed by the following relation:
The total renal vascular resistance is the sum of the resistances of the individual vascular segments, including the arteries, arterioles, capillaries, and veins. Most of the renal vascular resistance resides in three major segments: interlobular arteries, afferent arterioles, and efferent arterioles.
The Nephron Is the Structural and Functional Unit of the Kidney
Each kidney has about 800,000 to 1,000,000 nephrons, each of which is capable of forming urine. A nephron is comprised of a tuft of glomerular capillaries called the glomerulus in which large amounts of fluid are filtered from the blood, a capsule around the glomerulus called Bowman’s capsule, and a long tubule in which the filtered fluid is converted to urine on its way to the renal pelvis, which receives urine from all of the nephrons.
The renal tubule is subdivided into the following major sections, each of which has different structural and functional characteristics: (1) the proximal tubule, which lies in the outer portion of the kidney (cortex); (2) the loop of Henle, which includes descending and ascending limbs that dip into the inner part of the kidney (medulla); (3) the distal tubule, which lies in the renal cortex; and (4) the connecting tubule, the cortical collecting tubule, and the cortical collecting duct, which begin in the cortex and run downward into the medulla to become (5) the medullary collecting duct. Urine passes from the renal pelvis to the bladder, where it is stored until it is eventually expelled from the body through the process of micturition, or urination.
Micturition (p. 307)
Micturition is the process by which the urinary bladder empties when it becomes filled and involves two main steps: (1) the bladder fills progressively until the tension in its walls rises above a threshold level, which elicits the second step, and (2) a nervous reflex, called the micturition reflex, is activated and empties the bladder or, if this fails, at least causes a conscious desire to urinate.
Physiologic Anatomy and Nervous Connections of the Bladder
The ureters carry the urine from the renal pelvis to the bladder, where they pass obliquely through the bladder wall before emptying into the bladder chamber. There are no major changes in the composition of the urine as it flows through the ureters into the bladder. Peristaltic contractions of the ureter, which are enhanced by parasympathetic stimulation, force the urine from the renal pelvis toward the bladder.
The urinary bladder is a smooth muscle chamber composed of two main parts: (1) the body, which is the major portion of the bladder in which urine collects, and (2) the neck, which is a funnel-shaped extension of the body that connects with the urethra.
The smooth muscle of the bladder is called the detrusor muscle. When the fibers contract, they can increase the pressure of the bladder to 40 to 60 mm Hg and therefore play a major role in emptying the bladder.
The bladder neck (posterior urethra) is composed of detrusor muscle interlaced with a large amount of elastic tissue. The muscle in this area is called the internal sphincter; its natural tone keeps the bladder from emptying until the pressure in the main part of the bladder rises above a critical threshold.
Beyond the posterior urethra, the urethra passes through the urogenital diaphragm, which contains a layer of muscle called the external sphincter of the bladder. This muscle is a voluntary skeletal muscle and can be used consciously to prevent urination even when involuntary controls are attempting to empty the bladder.
Pelvic Nerves Provide the Principal Nervous Supply of the Bladder
Coursing through the pelvic nerves, which connect with the spinal cord through the sacral plexus, are both sensory nerve fibers and motor nerve fibers. The sensory nerve fibers detect the stretch of the bladder wall and initiate reflexes that cause bladder emptying. The motor nerves transmitted to the pelvic nerves are parasympathetic fibers.
Micturition Reflex Is a Spinal Cord Reflex
The micturition reflex is a single complete cycle of (1) a progressive and rapid increase in bladder pressure, (2) a period of sustained increase in bladder pressure, and (3) a return of the pressure to the basal tone of the bladder, as follows:
Glomerular Filtration Is the First Step in Urine Formation (p. 312)
The composition of the glomerular filtrate is almost identical to that of plasma except that it has virtually no protein (only about 0.03%). The glomerular filtration rate (GFR) is normally about 125 mL/min, or about 20% of the renal plasma flow; thus the fraction of renal plasma flow that is filtered (filtration fraction) averages about 0.2.
The GFR is determined according to the net filtration pressure across the glomerular capillaries and the glomerular capillary filtration coefficient (Kf), which is the product of the permeability and surface area of the capillaries.
The net filtration pressure is the sum of hydrostatic and colloid osmotic forces acting across the glomerular capillaries and includes (1) the hydrostatic pressure inside the capillaries—the glomerular hydrostatic pressure (PG), which is normally about 60 mm Hg and promotes filtration; (2) the hydrostatic pressure in Bowman’s capsule outside the capillaries (PB), which is normally 18 mm Hg and opposes filtration; (3) the colloid osmotic pressure of the glomerular capillary plasma proteins (πG), which averages 32 mm Hg and opposes filtration; and (4) the colloid osmotic pressure of proteins in Bowman’s capsule (πB), which is near zero and therefore under normal conditions has little effect on filtration.
Decreased Glomerular Capillary Filtration Coefficient (Kf) Decreases the GFR
Although changes in Kf have a proportional effect on the GFR, this is not a primary mechanism for physiologic control of the GFR. Nevertheless, in some diseases, such as uncontrolled hypertension and diabetes mellitus, the GFR is reduced because of increased thickness of the glomerular capillary membrane, which reduces the Kf, or because of severe damage to the capillaries and loss of capillary filtration surface area.
Increased Bowman’s Capsule Pressure Decreases the GFR
Changes in Bowman’s capsule pressure normally do not control the GFR; however, in certain pathologic states, such as urinary tract obstruction, Bowman’s capsule pressure may increase to such a high level that the GFR is reduced. For example, precipitation of calcium or uric acid may lead to “stones” that lodge in the urinary tract, often in the ureter, thereby obstructing urine flow and increasing Bowman’s capsule pressure.
Increased Glomerular Capillary Colloid Osmotic Pressure Decreases the GFR
The two factors that influence glomerular capillary colloid osmotic pressure are (1) the arterial colloid osmotic pressure and (2) the fraction of plasma filtered by the glomerular capillaries (filtration fraction). An increase in either the arterial colloid osmotic pressure or the filtration fraction increases the glomerular capillary colloid osmotic pressure. Conversely, a decrease in the arterial plasma colloid osmotic pressure or the filtration fraction reduces the glomerular colloid osmotic pressure. Because the filtration fraction is the GFR/renal plasma flow ratio, a decrease in renal plasma flow increases the filtration fraction. Therefore even with constant glomerular hydrostatic pressure, decreased renal blood flow tends to increase the glomerular colloid osmotic pressure and decrease the GFR.
Increased Glomerular Capillary Hydrostatic Pressure Increases the GFR
Glomerular hydrostatic pressure is determined by three variables, each of which is physiologically regulated:
Glomerular Filtration and Renal Blood Flow Are Controlled by Neurohumoral Systems and Intrarenal Mechanisms (p. 317)
The determinants of the GFR that are most variable and subject to physiologic control include the glomerular hydrostatic pressure and glomerular capillary colloid osmotic pressure. These pressures, in turn, are influenced by the sympathetic nervous system, hormones, autacoids (vasoactive substances released in the kidney), and other intrarenal feedback control mechanisms.
Strong Sympathetic Nervous System Activation Decreases GFR
Strong activation of the sympathetic nervous system constricts the renal arterioles and decreases renal blood flow and the GFR. This effect is most important in reducing the GFR during severe, acute disturbances such as those elicited by the defense reaction, brain ischemia, or severe hemorrhage.
Hormones and Autacoids Control the GFR and Renal Blood Flow
Several hormones and autacoids can also influence the GFR and renal blood flow.
GFR and Renal Blood Flow Are Autoregulated During Changes in Arterial Pressure (p. 319)
In normal kidneys, a fall in arterial pressure to as low as 75 mm Hg or a rise to as high as 160 mm Hg changes the GFR by only a few percentage points; this relative constancy of the GFR and renal blood flow is referred to as autoregulation. Although autoregulation of GFR and renal blood flow is not perfect it prevents potentially marked changes in the GFR and therefore in renal excretion of water and solutes that would otherwise occur with changes in blood pressure.
Tubuloglomerular Feedback Is a Key Component of Renal Autoregulation
This feedback has two parts—an afferent arteriolar mechanism and an efferent arteriolar mechanism—both of which depend on the special anatomic arrangement of the juxtaglomerular complex. The juxtaglomerular complex consists of macula densa cells in the initial portion of the distal tubule and juxtaglomerular cells in the walls of the afferent and efferent arterioles. When blood pressure is decreased, delivery of sodium chloride is decreased to the macula densa cells, which are capable of sensing this change. The decrease in sodium chloride concentration at the macula densa, in turn, causes two main effects: (1) a decrease in the resistance of the afferent arterioles, which increases glomerular hydrostatic pressure and the GFR toward normal levels, and (2) an increase in renin release from the juxtaglomerular cells of the afferent and efferent arterioles, which causes increased angiotensin II formation. Angiotensin II then constricts efferent arterioles, increases arterial pressure, and increases glomerular hydrostatic pressure and the GFR toward normal levels.
Myogenic Mechanism Contributes to Autoregulation of Renal Blood Flow and the GFR
This mechanism refers to the intrinsic capability of blood vessels to constrict when blood pressure is increased. The constriction prevents the vessel from being overstretched and, by increasing vascular resistance, helps prevent excessive increases in renal blood flow and the GFR when blood pressure rises. Conversely, with decreased blood pressure, the myogenic mechanism contributes to decreased vascular resistance.