CHAPTER 32 Red Blood Cells, Anemia, and Polycythemia
A major function of red blood cells is to transport hemoglobin, which in turn carries oxygen from the lungs to the tissues. Normal red blood cells are biconcave discs, although the shapes can change markedly as the cells pass through the capillaries. The normal red blood cell has a great excess of cell membrane relative to the quantity of material it contains. Deformation of the cell does not stretch the membrane and consequently does not rupture the cell. The average number of red blood cells per cubic millimeter is 5,200,000 ± 300,000 in men and 4,700,000 ± 300,000 in women.
Red Blood Cells Have the Ability to Concentrate Hemoglobin
In normal individuals, the percentage of hemoglobin is almost always near the maximum level in each cell (about 34 g/dL). The blood contains an average of 15 g of hemoglobin per 100 mL (16 g in men and 14 g in women). Each gram of pure hemoglobin is capable of combining with approximately 1.34 mL of oxygen. In a healthy person, more than 20 mL of oxygen can be carried in combination with the hemoglobin in each 100 mL of blood.
Genesis of Blood Cells
All circulating blood cells are derived from pluripotential hemopoietic stem cells. The pluripotential cells differentiate to form the peripheral blood cells. As these cells reproduce, a portion is exactly like the original pluripotential cells. These cells are retained in the bone marrow to maintain a constant supply. The early offspring of the stem cells cannot be recognized as different types of blood cell even though they already have been committed to a particular cell line; these cells are called committed stem cells. Different committed stem cells produce different colonies of specific types of blood cells.
The growth and reproduction of the various stem cells are controlled by multiple proteins called growth inducers, which promote growth, but not differentiation, of the cells. This is the function of another set of proteins, called differentiation inducers. Each of these inducers causes one type of stem cell to differentiate one or more steps toward the final type of adult blood cell. The formation of growth inducers and differentiation inducers is controlled by factors outside the bone marrow. In the case of red blood cells, exposure of the body to a low level of oxygen for a long period induces growth, differentiation, and production of greatly increased numbers of erythrocytes.
Regulation of Red Blood Cell Production—The Role of Erythropoietin (p. 416)
The total mass of red blood cells in the circulatory system is regulated within narrow limits. Any condition that causes the quantity of oxygen that is transported in the tissues to decrease ordinarily increases the rate of red blood cell production. The principal factor that stimulates red blood cell production is the circulating hormone erythropoietin. In a normal individual, about 90% of erythropoietin is formed in the kidneys, with the remainder formed mainly in the liver. The structure in the kidney in which the erythropoietin is formed is not known. Some studies suggest that erythropoietin is secreted by fibroblast-like interstitial cells surrounding the tubules in the cortex and outer medulla where much of the kidney’s oxygen consumption occurs. Other cells including the renal epithelial cells themselves also secrete erythropoietin in response to hypoxia (Fig. 32–1).
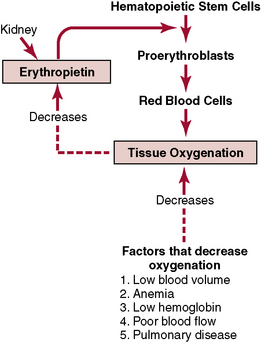
Figure 32–1 Function of the erythropoietin mechanism to increase production of red blood cells when tissue oxygenation decreases.
When both kidneys are surgically removed or destroyed by renal disease, the individual invariably becomes extremely anemic because the amount of erythropoietin formed in nonrenal tissues is sufficient to cause only one third to one half as many red blood cells to be formed as are needed by the body.
Vitamin B12 and Folic Acid Are Important for the Final Maturation of Red Blood Cells
Both vitamin B12 and folic acid are essential to the synthesis of DNA. The lack of either of these vitamins results in a diminished quantity of DNA and, consequently, failure of nuclear maturation and division. In addition to failure to proliferate, the red blood cells become larger than normal, developing into megaloblasts. These cells have irregular shapes and flimsy cell membranes; they are capable of carrying oxygen normally, but their fragility causes them to have a short life span—one half to one third that of normal. Vitamin B12 or folic acid deficiency therefore causes maturation failure during the process of erythropoiesis.
A common cause of maturation failure is an inability to absorb vitamin B12 from the gastrointestinal tract. This often occurs in persons with pernicious anemia, a disease in which the basic abnormality is atrophic gastric mucosa. The parietal cells of the gastric gland secrete a glycoprotein called intrinsic factor, which combines with vitamin B12 to make it available for absorption by the gut. The intrinsic factor binds tightly with vitamin B12 and protects the vitamin from digestion by the gastrointestinal enzymes. The intrinsic factor-vitamin B12 complex binds to specific receptor sites on the brush border membranes of mucosal cells of the ileum. Vitamin B12 is then transported into the blood via the process of pinocytosis. A lack of intrinsic factor causes loss of much of the vitamin resulting from enzyme action in the gut and failure of absorption.
Formation of Hemoglobin (p. 417)
Synthesis of hemoglobin begins when the red blood cell is in the proerythroblast stage and continues into the reticulocyte stage, at which point the cell leaves the bone marrow and passes into the bloodstream. During the formation of hemoglobin, the heme molecule combines with a long polypeptide chain called a globin to form a subunit of hemoglobin called a hemoglobin chain. Four hemoglobin chains bind together loosely to form the entire hemoglobin molecule.
The most important feature of the hemoglobin molecule is its ability to bind loosely and reversibly with oxygen. The oxygen atom binds loosely with one of the so-called coordination bonds of the iron atom in hemoglobin. When bound to the iron heme, oxygen is carried as molecular oxygen, composed of two oxygen atoms. Oxygen is released into the tissue fluids in the form of dissolved molecular oxygen rather than as ionic oxygen.
Iron Metabolism (p. 418)
Iron is important for the formation of hemoglobin, myoglobin, and other substances, such as the cytochromes, cytochrome oxidase, peroxidase, and catalase. The total average quantity of iron in the body is about 4 to 5 g. About 65% of this amount is in the form of hemoglobin. About 4% is in the form of myoglobin, 1% is in the form of the various heme compounds that promote intracellular oxidation, 0.1% is combined with the protein transferrin in the blood plasma, and 15% to 30% is stored mainly in the reticuloendothelial system and liver parenchymal cells, principally in the form of ferritin.
Iron Is Transported and Stored
When iron is absorbed from the small intestine it immediately combines with a β-globulin called apotransferrin, to form transferrin, which is transported in the plasma. This iron is loosely bound. Excess iron in the blood is deposited in liver hepatocytes and in reticuloendothelial cells of the bone marrow. Once inside the cell’s cytoplasm, iron combines with the protein apoferritin to form ferritin. Varying quantities of iron can combine in clusters of iron radicals in the ferritin.
When the quantity of iron in the plasma decreases to less than normal, iron is removed from ferritin quite easily and transported by transferrin in the plasma to the portions of the body where it is needed. A unique characteristic of the transferrin molecule is its ability to bind strongly with receptors in the cell membranes of the erythroblasts and bone marrow. Transferrin is ingested via endocytosis into the erythroblasts along with the bound iron. Transferrin delivers the iron directly to the mitochondria, where heme is synthesized.
When red blood cells have reached the end of their life span and are destroyed, the hemoglobin released is ingested by cells of the monocyte-macrophage system. The free iron that is liberated can be stored in the ferritin pool or reused for formation of hemoglobin.
Anemias (p. 420)
Anemia means a deficiency of red blood cells and can be caused by rapid loss of red blood cells or slow production of red blood cells.
Polycythemia (p. 421)
Polycythemia is a condition in which the number of red blood cells in the circulation increases owing to hypoxia or genetic aberration. Individuals who live at high altitudes have physiologic polycythemia as a result of the thin atmosphere. Polycythemia can also occur in individuals with cardiac failure because of decreased delivery of oxygen to the tissues.
Polycythemia vera is a genetic aberration in the hemocytoblastic cell line. The blast cells continue to produce red blood cells even though too many blood cells are present in the circulation. The hematocrit can rise to 60% to 70%.
Polycythemia greatly increases the viscosity of the blood; as a result, blood flow through the vessels is often sluggish.