CHAPTER 79 Parathyroid Hormone, Calcitonin, Calcium and Phosphate Metabolism, Vitamin D, Bone, and Teeth
The physiology of calcium and phosphate metabolism, the function of vitamin D, and the formation of bone and teeth are all tied together in a common system with the two main regulatory hormones parathyroid hormone (PTH) and calcitonin.
Calcium and Phosphate in the Extracellular Fluid and Plasma—Function of Vitamin D (p. 960)
Control of Vitamin D Formation
The active form of vitamin D, 1,25-dihydroxycholecalciferol, is carefully regulated via the following steps:
Because PTH formation is stimulated by reduction in the extracellular fluid (ECF) concentration of calcium, formation of 1,25-dihydroxycholecalciferol also increases when the calcium concentration in the ECF falls.
Gastrointestinal Calcium Absorption—1,25-Dihydroxycholecalciferol
In the epithelial cells of the small intestine, 1,25-dihydroxycholecalciferol stimulates formation of calcium binding protein, calcium-stimulated ATPase, and alkaline phosphatase, all of which promote absorption of calcium ions out of the lumen of the intestine.
Being a divalent cation, Ca2+ cannot cross the cell membrane of the epithelial cells without the mechanisms activated by 1,25-dihydroxycholecalciferol; therefore, calcium absorption will occur at a rate determined specifically by the activity of the mechanisms regulated by 1,25-dihydroxycholecalciferol.
Phosphate ions are absorbed in a relatively unregulated manner, although the rate of absorption is increased by administration of 1,25-dihydroxycholecalciferol.
Calcium and Phosphate in the Extracellular Fluid and Plasma (p. 955)
Accurate Regulation of Calcium Ion Concentration Is Essential to Normal Function of the Neuromuscular System and the Skeletal System
If the concentration of calcium in the ECF falls to less than 50% of normal for even brief periods, neuromuscular dysfunction of the skeletal muscles results, initially in the form of hyper-reflexivity and finally as tetanic contractions. If calcium ion concentration increases to 50% greater than normal, central nervous system depression occurs, along with slowing of the contractions of the smooth muscle of the gastrointestinal tract.
Calcium is found normally in the ECF in a total concentration of 2.4 mmol/L, or 9.4 mg/dL. In the ECF, 50% of the calcium is in the free divalent cation form, 40% is loosely bound to proteins, and 10% is in the nonionized form.
Phosphate ion concentration in the ECF can vary rather widely without physiologic impact. Phosphate in the ECF can be either monobasic (H2PO4−) or dibasic (HPO42−). The normal concentration of H2PO4− is 0.26 mmol/L, whereas HPO42− is found at a concentration of 1.05 mmol/L. The relative concentrations of the two are affected by the pH of the ECF, with a reduction in pH increasing the amount of H2PO4− and decreasing the concentration of HPO42−. Clinically, total phosphate concentration is usually expressed in milligrams per deciliter and is normally 3 to 4 mg/dL.
Bone and Its Relation to Extracellular Calcium and Phosphates (p. 957)
Bone Is Composed Mostly of Calcium and Phosphate Salts along with Organic Matrix
Approximately 70% of bone is calcium salts; most is in the form of large crystals of hydroxyapatite, Ca10(PO4)6(OH)2. Bone is about 30% organic matrix, made up of collagen fibers and cells. Some calcium in bone is not in crystalline form and therefore is rapidly exchangeable with calcium in the ECF.
Bone Calcification
Bone formation begins with secretion of collagen fibers by osteoblast cells; the uncalcified collagen structure is referred to as osteoid. Calcification of the osteoid takes place over a period of weeks.
Bone Is Continually Deposited by Osteoblasts and Absorbed by Osteoclasts, a Dynamic Process Referred to as Remodeling
Bone has the capacity to undergo remodeling throughout life, although the process takes place much more rapidly in children and young adults than in the elderly. Osteoclast cells digest bone, after which osteoblasts deposit new bone. The balance between the two processes is affected by the following:
The Calcium and Phosphate Present in Bone Serve as Reservoirs for the Ions in the ECF
About 99% of the total body calcium is in bone, whereas less than 1% is in the ECF. If the calcium ion concentration in the ECF falls below normal, calcium ions move from bone into the ECF. The calcium and phosphate distribution in bone and ECF is affected by PTH and 1,25-dihydroxycholecalciferol, which stimulate movement of calcium and phosphate from bone to the ECF, and by calcitonin, which has the opposite effect. Conversely, when the calcium concentration in the ECF increases above normal, calcium can be deposited in the bone.
Parathyroid Hormone (p. 962)
Parathyroid Hormone Secretion Increases in Response to Reduction in Extracellular Calcium Concentration
The hormone is formed in the chief cells of the parathyroid glands located immediately behind the thyroid gland. The rate of formation of PTH is strongly regulated by the ECF calcium ion concentration; small decreases in the concentration of the ion result in large increases in the rate of PTH formation. If the reduction below the normal level of calcium concentration persists, the parathyroid glands hypertrophy, as occurs with pregnancy and disease states such as rickets that are characterized by inadequate calcium absorption from the gastrointestinal tract.
Increases in PTH Concentration Decrease Renal Calcium Excretion
Normally, more than 99% of calcium filtered at the glomerulus is reabsorbed along the tubule. Approximately 5% of the filtered calcium is reabsorbed in the collecting tubules, and it is calcium transport in this segment that is stimulated by PTH. Other factors that affect calcium excretion include the following.
| Increase Calcium Excretion | Decrease Calcium Excretion |
|---|---|
| Decreased [PTH] | Increased [PTH] |
| Increased ECFV | Decreased ECFV |
| Decreased [HPO42−] | Increased [HPO42−] |
| Metabolic acidosis | Metabolic alkalosis |
Increases in PTH Concentration Elevate Phosphate Excretion
Phosphate excretion is regulated as a tubular maximum (Tm) system (see Chapter 29). Approximately 80% is reabsorbed in the proximal tubule, with additional absorption taking place at more distal sites in the nephron. PTH inhibits phosphate reabsorption in the proximal tubule; other factors that affect phosphate excretion include the following.
| Increase HPO42− Excretion | Decrease HPO42− Excretion |
|---|---|
| Increased [PTH] | Decreased [PTH] |
| Increased ECF volume | Decreased ECF volume |
| Increased [HPO42−] | Decreased [HPO42−] |
| Metabolic acidosis | Metabolic alkalosis |
Calcitonin (p. 966)
Calcitonin Secretion Increases in Response to Elevation of the Extracellular Calcium Concentration
The hormone is a polypeptide with 32 amino acids secreted from the parafollicular cells found in the interstitial tissue of the thyroid gland. In general, its effects are opposite those of PTH in the bone and renal tubule, and the magnitude of its effects is much less than that of PTH.
Overall Control of Calcium Ion Concentration (p. 966)
Calcium concentration in the ECF is controlled by a system that affects the distribution between the calcium stored in bone and the ECF, the rate of intake from the gastrointestinal tract, and the rate of excretion by the kidneys (Fig. 79–1).
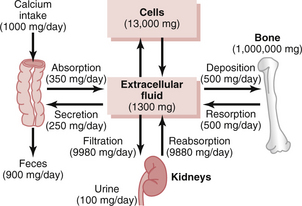
Figure 79–1 Overview of calcium exchange between different tissue compartments in a person ingesting 1000 mg of calcium per day. Note that most of the ingested calcium is normally eliminated in the feces, although the kidneys have the capacity to excrete large amounts by reducing tabular reabsorption of calcium
Regulation of Calcium Distribution between Bone and Extracellular Fluid
When ECF calcium concentration falls, the following changes take place:
Regulation of Absorption from the Gastrointestinal Tract
When calcium concentration in the ECF falls, the following changes take place:
Regulation of Renal Calcium and Phosphate Excretion
When calcium concentration in the ECF falls, PTH formation increases and the following changes occur:
In humans, the most important feedback control mechanism is the effect of a reduction in ECF calcium concentration to increase the PTH formation. The involvement of calcitonin in the control system is of minor importance in adults.
Pathophysiology of Parathyroid and Bone Diseases (p. 967)
Hypoparathyroidism Decreases Extracellular Calcium Concentration
With inadequate formation of PTH, osteoclasts become inactive and the formation of 1,25-dihydroxycholecalciferol declines to low levels. Transfer of calcium from bone to the ECF decreases, calcium absorption from the gut decreases to low levels, and calcium excretion by the kidneys is greater than the rate of absorption from the gut. As a result, the calcium concentration in the ECF falls below normal levels, and the phosphate concentration remains normal or is elevated. The condition can be treated with very large doses of vitamin D, which have the effect of stimulating gastrointestinal calcium absorption, or by the administration of 1,25-dihydroxycholecalciferol.
Excessive Formation of PTH by the Parathyroid Gland (Hyperparathyroidism) Causes Loss of Calcium from Bone and Increased Extracellular Calcium Concentration
Excessive PTH levels stimulate osteoclastic activity, renal retention of calcium and excretion of phosphate, and increased formation of 1,25-dihydroxycholecalciferol. Calcium concentration in the ECF is greater than normal, and phosphate levels are below normal. The most serious consequences are related to the damage done by excessive osteoclastic absorption of bone, which results in weakening of the bone.
Rickets Is Caused by Inadequate Absorption of Calcium from the Gastrointestinal Tract
This can be due to inadequate calcium in the diet or failure to form adequate amounts of 1,25-dihydroxycholecalciferol. If the kidneys are damaged or absent, 1,25-dihydroxycholecalciferol cannot be formed. Because of inadequate absorption of calcium, PTH levels are elevated, which stimulates osteoclastic resorption of bone and release of calcium to the ECF. In addition, the elevated PTH levels exert renal effects, causing retention of calcium and excretion of phosphate. The net results of these effects are weakening of the bones, a below-normal phosphate concentration, and for periods of months an only slightly below normal calcium concentration resulting from the transfer of calcium from bone to the ECF.
Osteoporosis Is Caused by Depressed Deposition of New Bone by the Osteoblasts
As a result, the rate of osteoclastic resorption of bone exceeds the rate of deposition of new bone.
The most common causes of the condition are (1) lack of physical stress on the bones because of insufficient physical activity; (2) postmenopausal lack of estrogen, because estrogen normally decreases the number and activity of osteoclasts; and (3) old age, in which growth hormone and other factors that contribute to bone formation diminish greatly.
In men, testosterone levels decline gradually but continue to provide a significant anabolic effect into the seventh and eighth decades of life. In women, estrogen formation falls to near zero at menopause, usually at about 50 years of age. The decline in estrogen concentration shifts the balance between deposition and resorption of bone, although no symptoms are apparent for many years. Starting even before menopause, calcium is continually lost from the skeleton. After years of the gradual wasting of calcium, the bones become weakened to the point that symptoms appear, such as vertebral compression and brittleness of the long bones and pelvis. The condition can be prevented with estrogen replacement therapy beginning at menopause. Calcium supplements after menopause are not effective because the condition is not characterized by inadequate calcium in the ECF.
Physiology of the Teeth (p. 969)
Teeth are composed of four parts: enamel, dentine, cementum, and pulp.
Enamel Makes Up the Outer Layer of the Crown of the Tooth
It is composed of very large, dense crystals of hydroxyapatite embedded in a tight meshwork of protein fibers similar to keratin in hair. The crystalline structure makes the enamel extremely hard, whereas the protein, which is completely insoluble, provides resistance to enzymes, acids, and other corrosive substances.
Dentine Makes Up the Main Body of the Tooth
It is composed of hydroxyapatite crystals embedded in a strong meshwork of collagen fibers, a structure similar to bone. Dentine has no cellular components; all of the nourishment of the structure is provided from odontoblast cells, which line the inner surface of the dentine along the wall of the pulp cavity.