22 Hypothyroidism
INTRODUCTION
Despite being the most common endocrinopathy, hypothyroidism can be a challenging disease to diagnose. Although there are many diagnostic tests, they all have their limitations, as thyroid function can be influenced by many different intrinsic and extrinsic factors. Hypothyroidism can cause a wide variety of symptoms involving almost any organ system; however, dermatological signs are the most common. This report describes a case where the cutaneous signs were prominent.
CASE HISTORY
The condition most commonly affects mainly middle-aged to older dogs and certain breeds are predisposed (see ‘Epidemiology’ section). Cutaneous signs are gradual in onset and generally non-pruritic, unless there is a concurrent secondary infection. Most clients are unaware of systemic signs such as lethargy, exercise intolerance, heat seeking and weight gain. Close questioning on changes in demeanour and general health is therefore essential during the history taking.
The relevant history in this case was:
CLINICAL EXAMINATION
Physical and skin examinations may reveal a great variety of systemic and cutaneous symptoms that vary from case to case (Tables 22.1 and 22.2). The relevant findings in this case were:
Table 22.1 Systemic signs associated with hypothyroidism
| Common signs | Uncommon or rare signs |
|---|---|
Table 22.2 Dermatological abnormalities in canine hypothyroidism
| Common | Uncommon or rare |
|---|---|
| Thin, easily epilated hair coat | Pyoderma |
| Symmetrical alopecia | Ceruminous otitis externa |
| Failure of hair growth after clipping | Demodicosis |
| Scaling | Myxoedema |
| Hyperpigmentation | Lightening of the hair coat colour |
| Comedone formation |
CASE WORK-UP
The following diagnostic tests were performed:
At this stage, given the short duration of the disease, cyclical flank alopecia could not be ruled out, but in such cases thyroid function is unaffected. Because there was no history of polyuria, polydipsia or polyphagia, hyperadrenocorticism was unlikely.
Blood tests for hypothyroidism
Making a definitive diagnosis of hypothyroidism can prove challenging in some cases. The diagnosis is based on history, clinical signs and the demonstration of suppressed thyroid function. Because there are so many factors affecting thyroid hormone concentrations including age, breed, concurrent disease and drug therapy (see ‘Anatomy and Physiology Refresher’), the diagnosis is not always easy to confirm. At the time of writing, there are no completely reliable tests that can distinguish euthyroid from hypothyroid dogs. A single thyroid assay is almost invariably misleading and multiple tests are recommended to try and confirm the diagnosis.
Haematology and biochemistry: If the history or clinical examination suggests hypothyroidism, routine haematological and biochemical examinations should be performed. This helps to exclude intercurrent disease leading to the euthyroid sick syndrome (see below). A mild, normochromic normocytic anaemia may be present in up to half of all cases of hypothyroidism. Hypercholesterolaemia is another frequent, but non-specific, finding in up to 70% of cases.
Thyroid function tests: Thyroid function tests should be performed where there are clinical signs suggestive of hypothyroidism. The clinician should rule out or treat concurrent diseases before evaluating thyroid function and, wherever possible, stop any drug treatment at least 4 weeks prior to testing. Where this is not possible, any results should be interpreted in the knowledge that drugs which could affect thyroid function have been administered. The following is a summary of the thyroid function tests currently available and their application in the diagnosis of hypothyroidism. Always use a combination of thyroid function tests to support the diagnosis.
TT4: Basal serum total thyroxine (TT4) measures protein- and non-protein-bound ‘free’ serum thyroxine. The concentration decreases in hypothyroidism, but also decreases with euthyroid sick syndrome and drug administration. There is a large overlap between euthyroid and hypothyroid dogs, and up to 25% of euthyroid dogs will have suppressed TT4 values; however, as a general rule, 99% of dogs with TT4 > 15 nmol/l will be euthyroid and >95% of dogs with TT4 < 5 nmol/l will be hypothyroid. Concentrations will be artificially (markedly) elevated in the presence of anti-T4 autoantibodies (AT4A), although this is an uncommon situation.
fT4ED: Basal free T4 by equilibrium dialysis (fT4ED) measures non-protein-bound circulating T4. Concentrations of fT4 will decrease in hypothyroidism, but may be maintained in early hypothyroidism, reducing the sensitivity of this test. It is expensive in comparison to TT4, but fT4 should be less affected by non-thyroidal illness than TT4 and is unaffected by the presence of AT4A. Note: some laboratories may measure free T4 using ‘analogue’ assay techniques, which is no more use than measuring TT4 alone.
cTSH: Canine thyroid-stimulating hormone (cTSH) measures circulating TSH. It is often used in conjunction with TT4 or fT4 as a screening test for hypothyroidism. Concentrations of cTSH increase in hypothyroidism, due to the decreased negative feedback effect of T4 and T3 on the anterior pituitary gland. This test may be affected by drug administration and by non-thyroidal illness. Between 13% and 38% of hypothyroid dogs have cTSH in the normal range (usually 0.02–0.68 ng/ml) and 7–18% of euthyroid dogs have cTSH in the hypothyroid range.
TT3 and fT3: Total and free triiodothyronine (TT3 and fT3) measurements are of little value in the diagno-sis of most cases of hypothyroidism. TT3 concentrations tend to be preferentially maintained during early hypothyroidism.
TSH stim: Thyroid-stimulating hormone stimulation test (TSH stim) is considered the gold standard test in the diagnosis of canine hypothyroidism. Serum TT4 is measured before and 6 hours after intravenous injection of 0.1 IU/kg of thyrotropin (TSH). Thyroxine response to TSH is small to non-existent in hypothyroid dogs; however, a normal response may be seen in very early cases of hypothyroidism. Some drug treatments and severe non-thyroidal illness can also interfere with this test. As bovine TSH is no longer available, the use of recombinant human TSH (rhTSH) has been validated for this test.
TRH stim: In the thyrotropin-releasing hormone stimulation test (TRH stim), TT4 is measured before and 4 hours after intravenous injection of TRH. At least one study has concluded that this is not a useful test in the diagnosis of hypothyroidism, although the debate continues. Up to 25% of euthyroid dogs will fail to stimulate at all.
Thyroglobulin autoantibodies (TGAA): Thyroglobulin is a protein involved in the production and storage of thyroid hormones within the thyroid gland. The presence of TGAA is an indicator of lymphocytic thyroiditis, but does not diagnose clinical hypothyroidism.
Anti-thyroid hormone antibodies AT3A/AT4A: These antibodies are occasionally found in canine sera and can interfere with the assays for TT4, fT4, TT3 and fT3, producing spuriously increased concentrations. They are not used as a diagnostic test for hypothyroidism.
In this case, the following tests were performed:
Skin biopsy for histopathology was not considered necessary in this case.
DIAGNOSIS
The diagnosis of primary hypothyroidism in this case was made on a combination of history, clinical signs and laboratory tests. The increase in thyroglobulin autoantibodies supported lymphocytic thyroiditis as the cause of hypothyroidism.
PROGNOSIS
The prognosis is generally good for most hypothyroid dogs with resolution of clinical signs on thyroid hormone supplementation. Lifelong treatment is required. There is usually improved demeanour and activity levels within a few weeks of initiation of therapy but hair regrowth may take many months.
ANATOMY AND PHYSIOLOGY REFRESHER
The thyroid gland in the dog consists of two lobes, each of which lies laterally to the fifth to eighth tracheal rings. A fibrous capsule covers the external surface and intersperses into the parenchyma, dividing it into lobules. Microscopically, the parenchyma is composed of lobules, which in turn are made up of a number of hollow, spherical epithelium-lined follicles. The follicle is the basic functional unit of the thyroid gland. The centre of each follicle is composed of a viscous homogeneous substance, the colloid, that is rich in thyroglobulin.
The process of formation and secretion of thyroid hormones is complex. Iodide, the main building block of the thyroid hormones, is actively transported from the extracellular fluid (capillaries) into the thyroid follicular cells. Within the cell, inorganic iodide is oxidized by thyroid peroxidase in the presence of H2O2 into a reactive intermediate that is incorporated into tyrosine residues of thyroglobulin to make monoiodotyrosine (MIT) or diiodotyrosine (DIT). This iodination of thyroglobulin takes place in the follicular (apical) border of the cell, and MIT and DIT are transported into the colloid by exocytosis.
The process of excretion of thyroid hormone involves MIT- and DIT-bound thyroglobulin being taken up by the epithelial cells by endocytosis of colloid droplets, which are processed and degraded by proteolytic enzymes into the thyroid hormones thyroxine (T4) and triiodothyronine (T3). T4 is formed by coupling two DIT molecules, T3 by coupling one DIT with one MIT molecule. The thyroid favours the production of thyroxine; however, in an iodine-deficient state, or during thyroid failure, T3 is produced in preference.
The circulatory levels of thyroxine regulate thyroid function by a negative feedback mechanism, involving the hypothalamus and the pars distalis of the pituitary gland. Thyroid-releasing hormone (TRH), produced by the hypothalamus in a tonic fashion, induces the transcription and secretion of thyroid-stimulating hormone (TSH) by the pars distalis. In addition, TSH is also secreted by a direct negative feedback, in response to reduced circulatory levels of T4. TSH stimulates the synthesis and secretion of thyroid hormone. It also promotes the growth of thyrocytes and deiodination of T4 to T3. T3 has three to four times the metabolic potency of T4 and 80% of secreted T4 is deiodinated to form T3 and rT3 (metabolically inactive form) in the peripheral tissues.
Maintaining circulatory levels of thyroid hormone involves complex interactions between the production, secretion, distribution, metabolism and excretion of the hormone. Thus, any factors influencing any of these processes will eventually affect the serum concentration of thyroxine. Ninety-nine per cent of thyroid hormone (both T4 and T3) in serum is protein bound to thyroxine-binding globulin (TBG) and other proteins. The factors that influence the concentration of TBG will also influence the total serum concentration of thyroid hormone. These factors include a variety of diseases and pharmacological agents. Only unbound thyroid hormone is able to enter the cells and undergo excretion; therefore, for assessment of thyroid function, measurement of unbound thyroid hormones may provide a more accurate indication of thyroid status (see above section on thyroid tests).
Factors affecting thyroid function
As previously stated, age, breed, diurnal variation, drug administration and concurrent illness all affect serum thyroid hormone concentrations.
Age: Puppies in the first 3–4 months of life have serum total T4 concentrations two to five times higher than those of healthy adults, and healthy sighthounds have lower resting serum T4 concentrations than do mixed breed dogs.
Drugs: Drugs known to affect serum thyroid hormone concentrations include phenobarbitol, phenytoin, diazepam, glucocorticoids, sulphonamides, amitriptyline, clomipramine and some (although not all) non-steroidal anti-inflammatory drugs.
Illness: Illness of any type can alter hormone secretion, serum protein binding, distribution, metabolism and excretion, and the effect varies depending on the disease involved.
Euthyroid sick syndrome: Euthyroid sick syndrome is thought to be a protective mechanism in sick animals to prevent the catabolic effects of the metabolically active thyroid hormones T4 and, in particular, T3. The patients with this syndrome are euthyroid and will not benefit from thyroid hormone supplementation. In this syndrome total thyroid hormone concentrations tend to be decreased, but the free hormone fraction remains within reference ranges, thus the diagnosis of hypothyroidism based on basal total T4 serum concentrations is inherently unreliable because of the large overlap between concentrations in healthy, euthyroid sick and hypothyroid dogs.
AETIOPATHOGENESIS OF HYPOTHYROIDISM
Hypothyroidism results from decreased concentrations of circulating and tissue triiodothyronine (T3) and thyroxine (T4). More than 95% of cases of hypothyroidism in adult dogs are due to the destruction of the thyroid gland, as a result of a lymphocytic thyroiditis or idiopathic thyroid atrophy (thought to be the end stage of lymphocytic thyroiditis). Very rarely, a failure in the production of thyroid-stimulating hormone (TSH) from the anterior pituitary or thyrotropin-releasing hormone (TRH) from the hypothalamus can result in secondary or tertiary hypothyroidism respectively.
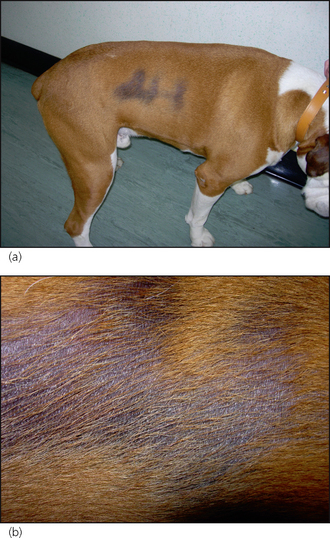
There are thyroid hormone receptors in cells of all organ systems, and therefore hypothyroidism causes a wide variety of systemic (Table 22.1) and dermatological (Table 22.2) signs. Dermatological signs are seen in around 80% of cases of canine hypothyroidism. Lack of thyroid hormone results in the inhibition of hair growth (anagen) and the majority of hair follicles remain in the resting phase (telogen). Hairs are either retained for long periods, leading to bleaching, or shed and not replaced, leading to alopecia. Alopecia over the tail, pinnae and bridge of the nose, as well as the flanks, are commonly seen in hypothyroidism. Hypothyroidism causes alterations in epidermal fatty acid composition, which in turn results in defective barrier function, as well as defects in humoral and cellular immune responses, both of which predispose to pyoderma. Scaling and pigmentary changes are also quite frequent findings in hypothyroidism.
EPIDEMIOLOGY
Hypothyroidism is the commonest of the endocrine diseases. Breed predispositions are reported in Airedales, boxers, dachshunds, Dobermanns, golden retrievers, miniature schnauzers, Irish setters and Great Danes. In general, hypothyroidism is seen in middle-aged and older dogs, but can occur at a younger age, particularly in the predisposed giant breeds. However, it is very rare to see hypothyroidism in a dog less than 2 years old.
TREATMENT
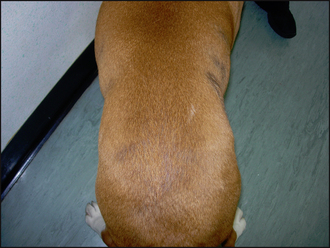
Lifelong treatment is required and therefore a firm diagnosis should be made prior to starting thyroid supplementation. The use of therapeutic trials is not recommended. Treatment in this case was an oral supplementation of levothyroxine administered at 0.02 mg/kg twice daily. The majority of cases of canine hypothyroidism, however, are reported to respond satisfactorily to once-daily dosage. When treating dogs suffering from congestive cardiac failure, renal failure, hepatic disease or diabetes mellitus, the starting dose should be 0.005 mg/kg and increased gradually over 3–4 weeks. Lethargy and mental demeanour will be the first symptoms to improve, generally within 2 weeks, but hair coat changes frequently take months to resolve. In this case the demeanour improved within 3 weeks and complete hair regrowth was seen within 3 months (Fig. 22.5).
THERAPEUTIC MONITORING
Post-pill monitoring of serum thyroxine concentrations is advisable, to ensure adequate supplementation and avoid the potentially harmful effects of thyrotoxicosis. Two to four weeks after commencement of therapy, serum TT4 concentration should be measured 4–6 hours after levothyroxine administration. Post-pill serum TT4 levels of between 30 and 65 nmol/l indicate that there is good absorption of T4. However, if the individual shows signs of thyrotoxicosis (weight loss, tachycardia, panting, anxiousness, polydipsia, polyphagia, polyuria, gastrointestinal signs) at the higher level, consider reducing the dosage slightly. Dogs with post-pill TT4 of less than 35 nmol/l usually require an increase in levothyroxine dosage to achieve clinical improvement. Post-pill TT4 measured at 6 hours in this case was 64 mmol/l. Post-pill cTSH was not measured in this case, but it may be of value to identify dogs which have been undertreated but will not distinguish between those that are adequately or oversupplemented.
Most nurses will be involved in sample collection, storage and transportation, and therefore should be aware of the factors that may influence the assay:
FOLLOW-UP
In this case the dog had a good response to therapy and the owner reported that, within 2 weeks, the dog was obviously more energetic. Full hair regrowth took several months. Over a 2-year period, the dog has been maintained on twice-daily levothyroxine treatment at the same dosage and is having 6-monthly post-pill monitoring of TT4.