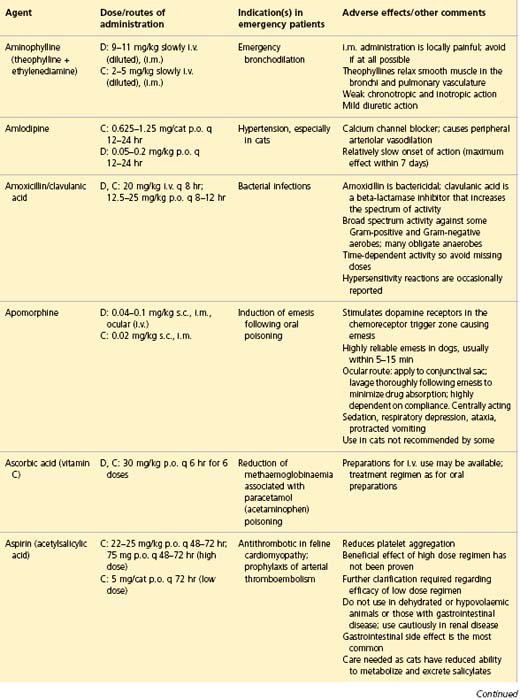
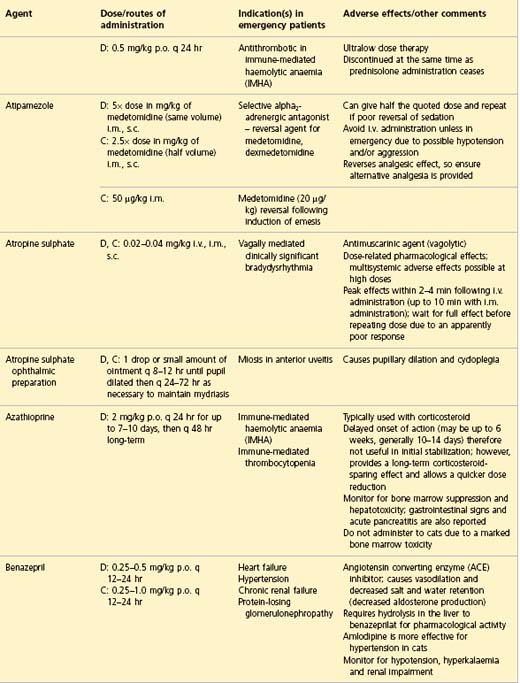
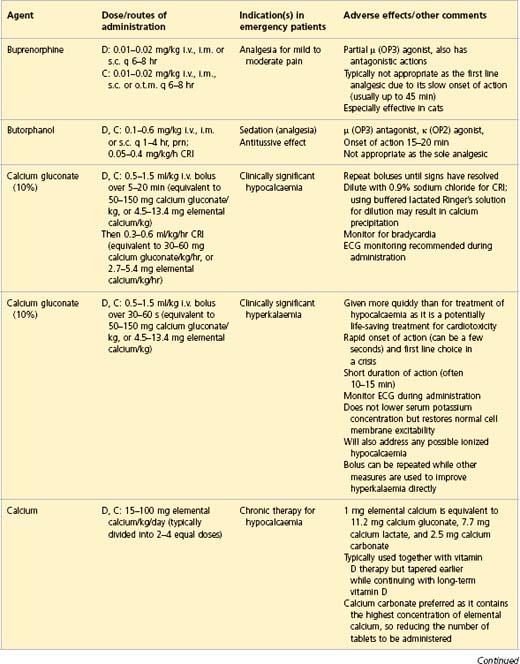
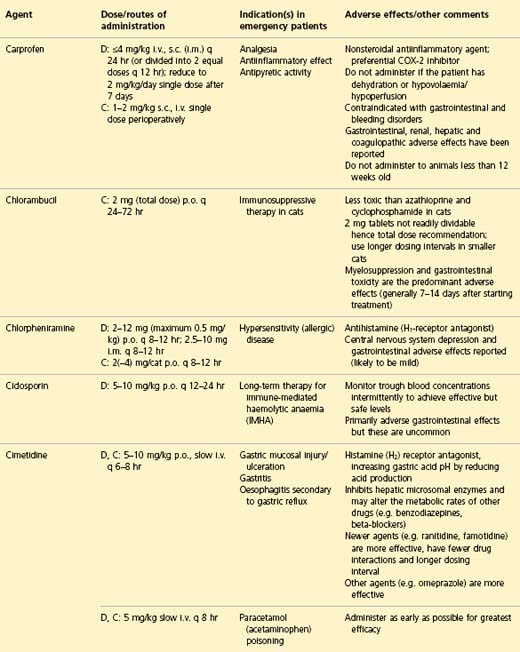
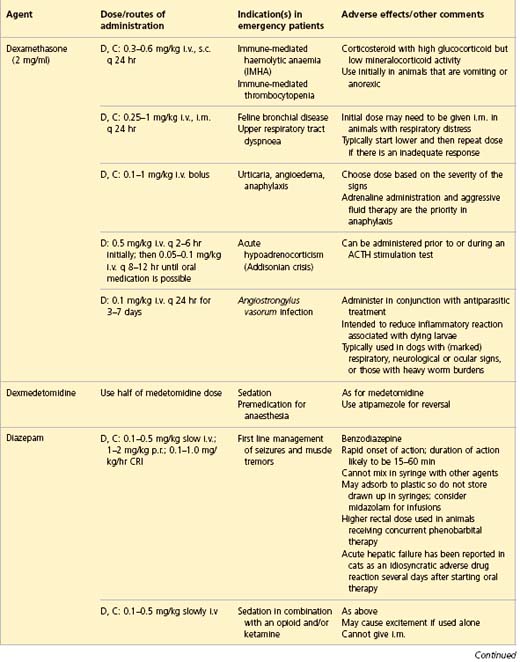
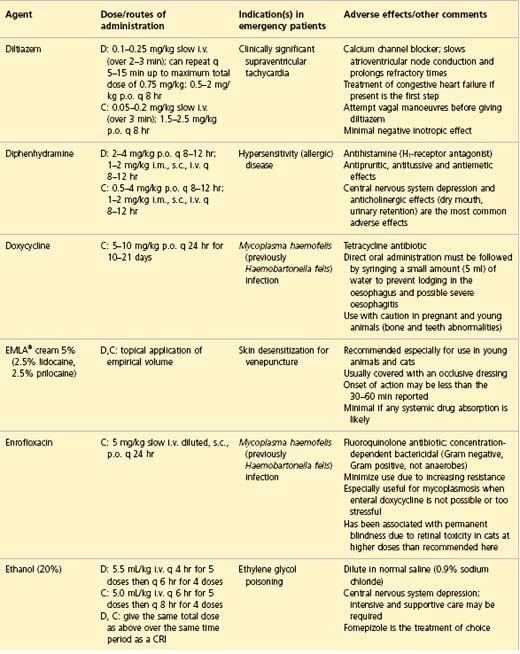
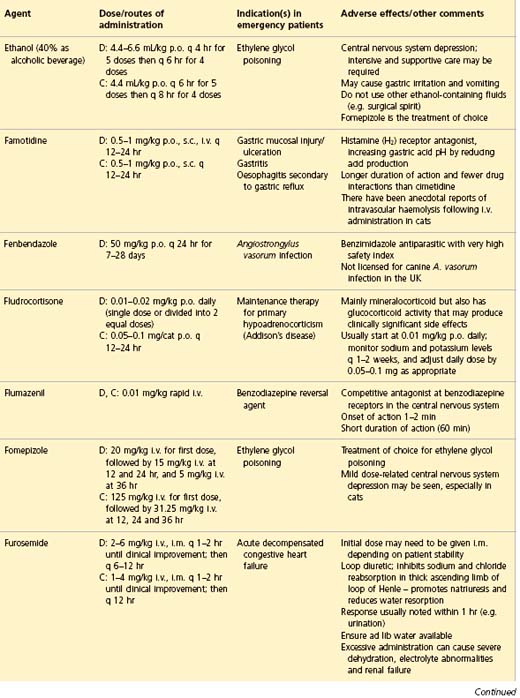
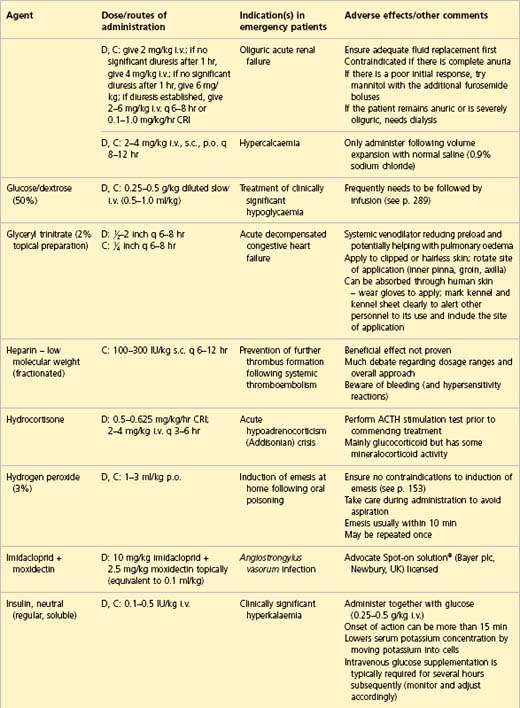
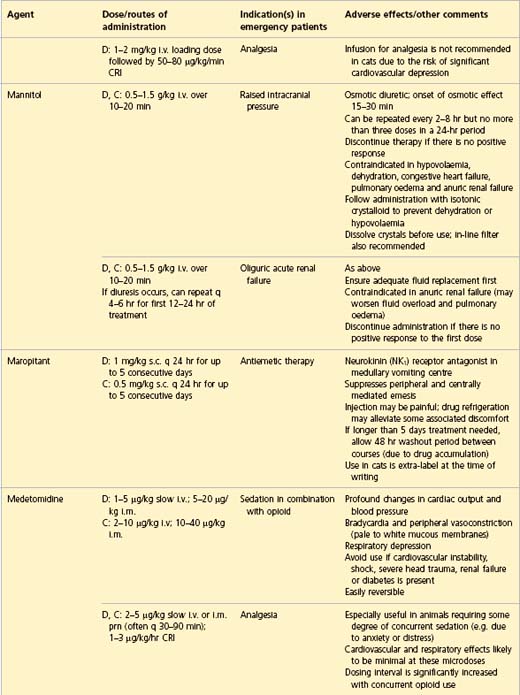
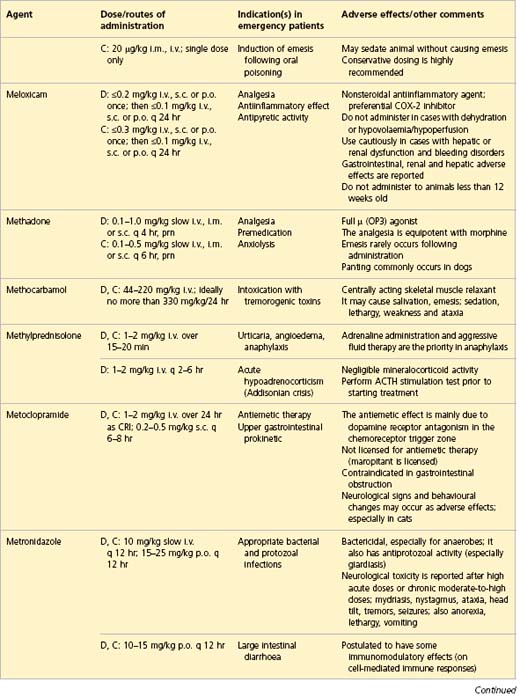
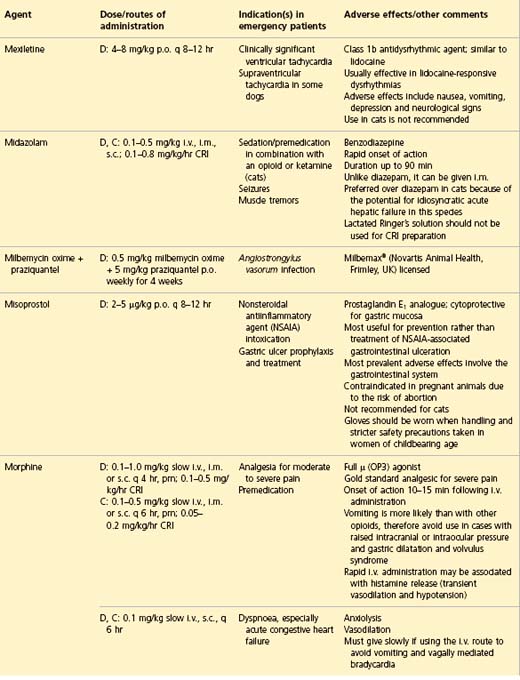
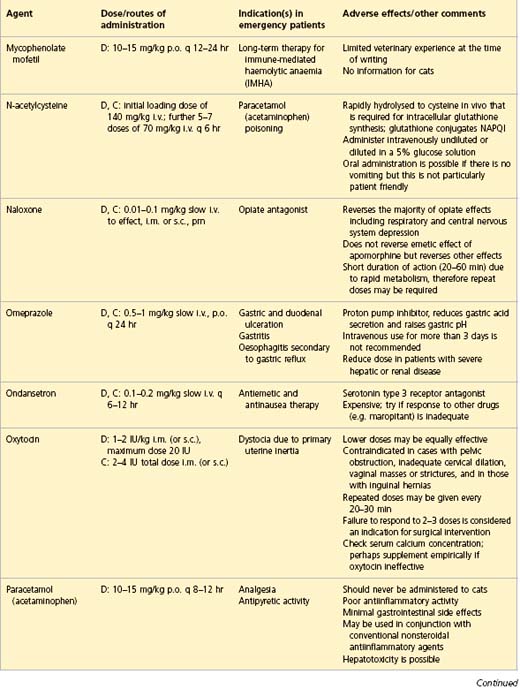
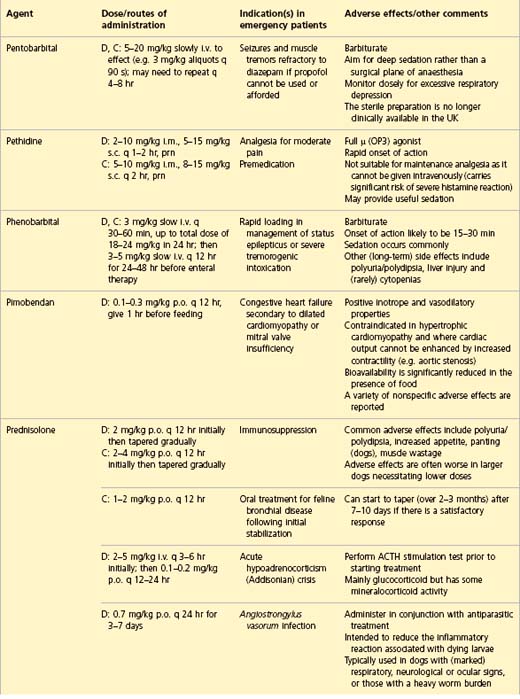
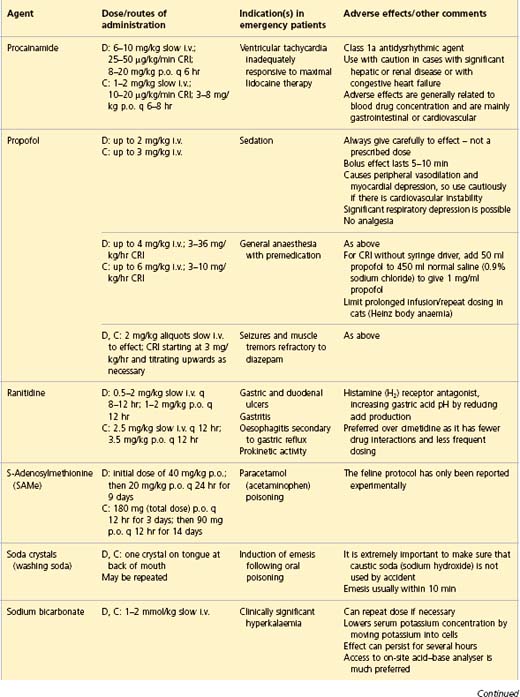
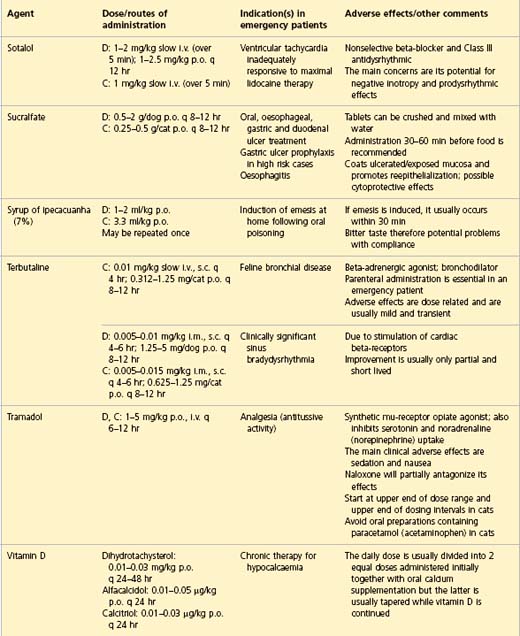
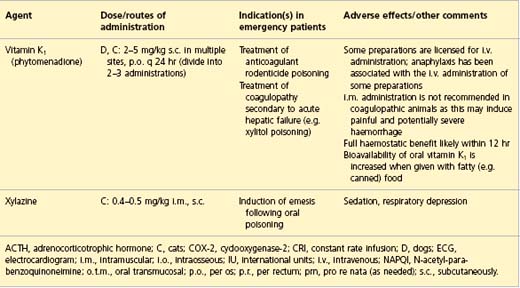
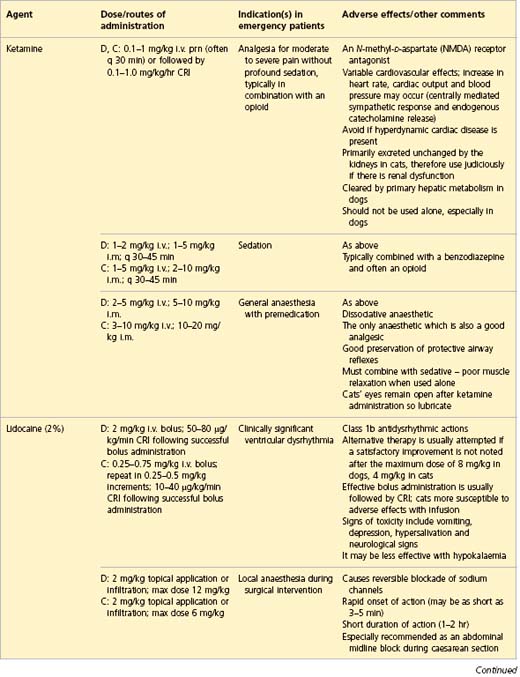
APPENDIX 1 Drug formulary
Additional Useful Information
Intravenous glucose supplementation
| Glucose solution required | SIZE OF ISOTONIC CRYSTALLOID FLUID BAG | |
|---|---|---|
| 500 ml | 1000 ml | |
| Volume to be removed and added | ||
| 1.25% (12.5 mg/ml) | 12.5 ml | 25 ml |
| 2.5% (25 mg/ml) | 25 ml | 50 ml |
| 5% (50 mg/ml) | 50 ml | 100 ml |
| 7.5% (75 mg/ml) | 75 ml | 150 ml |
| 10% (100 mg/ml) | 100 ml | 200 ml |
This table presumes the use of a 50% (500 mg/ml) glucose (dextrose) solution and either 0.9% sodium chloride (normal saline) or buffered lactated Ringer’s solution (Hartmann’s solution, compound sodium lactate).
The volumes listed are the amount of fluid that should be removed from the isotonic crystalloid bag, and then replaced by an equal volume of 50% glucose solution in order to make a glucose solution of the desired concentration.
For example, to make a 5% glucose saline solution, remove 50 ml from a 500 ml bag of 0.9% sodium chloride and then add 50 ml of 50% glucose solution to the bag.
Intravenous potassium chloride supplementation
| Serum potassium concentration (mmol/l) | AMOUNT OF POTASSIUM CHLORIDE REQUIRED (mEq) | |
|---|---|---|
| Per 500 ml fluid | Per 1000 ml fluid | |
| 3.5–5.5 | 10 | 20 |
| 3.0–3.5 | 15 | 30 |
| 2.5–3.0 | 20 | 40 |
| 2.0–2.5 | 30 | 60 |
| < 2.0 | 40 | 80 |
Note: 1 mEq of potassium is equivalent to 1 mmol of potassium.
This table is one example of a number of similar tables presenting comparable guidelines. These guidelines are very much empirically derived and clearly the amount of potassium delivered to the patient depends on the fluid rate as much as the potassium concentration. If a satisfactory response to empirical supplementation is not achieved, the actual rate of potassium administration should be calculated and used to guide subsequent therapy.
Potassium supplementation should not exceed a rate of 0.5 mEq/kg/h and the use of enteral supplementation should not be overlooked. In the exceptional circumstance where it is necessary to exceed this rate, continuous electrocardiogram monitoring should be used if available, and great care must be taken to alert all staff to the use of such a high potassium supplementation in order to avoid inadvertent bolus administration that could well be fatal. This rate of potassium supplementation must only be administered using an infusion pump or syringe driver, and the fluid bag and infusion device must be clearly labelled, e.g. ‘high potassium concentration, do not bolus!’.