Methods of respiratory muscle training
GENERAL TRAINING PRINCIPLES
The respiratory muscles are unique amongst skeletal muscles because of their continuous activity throughout life. For many years it was assumed that this resulted in a state of optimal training adaptation. As was described in Chapter 1, the structural and metabolic properties of the respiratory muscles would seem to support this notion, as they have properties that suit them ideally to their continuous activity. However, it became apparent from research conducted during the 1990s on young athletes that the respiratory muscles, specifically the diaphragm, exhibit fatigue following strenuous exercise (Johnson et al, 1993). The presence of fatigue is an indication that muscles are working at the limits of their capacity. These data therefore provided the first evidence that the respiratory muscles were not immune to fatigue. The data also raised the question of whether the respiratory muscles were capable of responding to training stimuli and displaying improvements in function in the same way that, say, leg muscles respond to running or weight training.
This chapter will describe the generic principles that underpin muscle training, and consider how these can be applied to the respiratory muscles. It will further consider the methods that are available to train the respiratory muscles, and the changes in respiratory muscle function that each method induces. Consideration will also be given to the relative merits of commercially available respiratory muscle training equipment.
There are three training principles that are well established for skeletal muscles – namely ‘overload’, ‘specificity’ and ‘reversibility’ (Pardy & Leith, 1995). The following section provides an overview of the evidence that respiratory muscles respond to these principles in the same manner as other muscles have been shown to (Romer & McConnell, 2003).
Overload
To obtain a training response, muscle fibres must be overloaded. Implicit within this principle is the concept of training duration, intensity and frequency. In other words, muscles can be overloaded by requiring them to work for longer, at higher intensity and / or more frequently than they are accustomed to. Most training regimens combine two or three of these factors in order to achieve overload. The overload principle will be considered first for healthy people, and then for patients.
Healthy people
In healthy people, two main forms of overload have been imposed upon the respiratory muscles: (1) external loads at the mouth (intensity), and (2) voluntary hyperpnoea (increased breathing volume and flow rate) for extended periods (intensity and duration). In both cases, the training takes place daily, or at least three times per week (frequency).
Studies employing external loading have typically used load intensities in excess of 50% of inspiratory muscle strength (maximal inspiratory pressure: MIP), at a frequency of once or twice per day, for 5–7 days per week (McConnell & Romer, 2004). Loading at 50–70% of MIP typically yields task failure (see Ch. 6) within a duration of 30 breaths, or 2–3 minutes (intensity = 50–70%; duration = 30 breaths; frequency = twice daily). Statistically significant changes in muscle function have been measured within 3 weeks (Romer & McConnell, 2003), with a plateau in improvement occurring after around 6 weeks of training, despite continuous increases of the training load (Volianitis et al, 2001; Romer & McConnell, 2003). Changes in strength occurring within the first 2 weeks of strength training have traditionally been attributed to a neural adaptation process (Jones et al, 1989), i.e., improving the coordinated activation of synergistic muscles. Although this adaptation undoubtedly makes a contribution to the immediate short-term improvements seen in respiratory muscles, evidence from animal studies suggests that structural adaptation occurs within days of overload (Gea et al, 2000). Furthermore, in human beings, improvements in diaphragm thickness (8–12%) have been reported following just 4 weeks of inspiratory muscle training (IMT) (Downey et al, 2007) confirming the presence of rapid fibre hypertrophy in response to loading. These changes in diaphragm thickness were parallelled by improvements in maximal inspiratory pressure (MIP) (Downey et al, 2007). In placebo-controlled trials of IMT in healthy people, loads of 15% of MIP have been used as the placebo condition. When a 15% load is implemented with 30–60 repetitions it does not provide sufficient overload, as it fails to elicit changes in MIP (see (McConnell & Romer, 2004). Thus research suggests that inspiratory muscle overload in healthy people requires loads of 50–70% of MIP, eliciting muscle adaptations within 3–4 weeks.
Studies using hyperpnoea training have typically induced overload at intensities corresponding to 70% of maximum voluntary ventilation (MVV), for a duration of 15–40 minutes per day, at a frequency of once per day, for 4–5 days per week (Verges et al, 2008a). In the case of hyperpnoea training, overload is achieved by increasing the rate of air flow, with the inspiratory muscles working against the inherent resistance and elastance of the respiratory system (intensity = 70%; duration = 15–40 minutes; frequency = 4–5 days per week). Improvements in muscle function (endurance) are evident within 4 weeks (Verges et al, 2008a). There are currently no data to indicate the point at which functional improvements plateau after commencing of training.
The intensity and frequency dimensions of overload warrant specific mention, as they need to be balanced carefully so as not to tip the respiratory muscles into a state of ‘overtraining’. Most studies have implemented moderate-intensity IMT (50–70%) daily, but the intensity and frequency balance has yet to be studied systematically, or with consideration for other stimuli that overload the respiratory muscles. In the case of athletes this might be concurrent athletic training, and / or exposure to altitude, but in the case of patients it might be exercise training and / or the effects of an exacerbation. Preliminary unpublished data from healthy young soccer and rugby players (McConnell, unpublished observations) suggests that twice-daily high-intensity IMT (70–80% of MIP) may induce a state of chronic inspiratory muscle fatigue in athletes who are undergoing concurrent whole-body training. These training conditions appear to elicit suboptimal improvements in function. Thus, present evidence suggests that low-to-moderate-intensity loading (30–60% MIP) can be implemented daily, whereas high-intensity loading (>70% MIP) should be implemented no more than once every other day.
Patients
In the majority of reported studies in patients with respiratory disease, training has been undertaken by imposing an external load at the mouth (intensity) for 10–30 minutes (duration). Typically, training has been undertaken for 2 to 3 months, but structural and biochemical adaptations to the inspiratory muscles are evident within 6 weeks (Ramirez-Sarmiento et al, 2002). A study in patients with chronic obstructive pulmonary disease (COPD) has demonstrated the time course of changes in strength over a 12-month intervention (Weiner et al, 2004). Weiner and colleagues (Weiner et al, 2004) noted the largest improvement in MIP during the first 3 months of their study (32%), followed by smaller increases (~ 6%) for the four subsequent 3-month blocks of IMT. Training sessions have typically been conducted in continuous bouts lasting 10–30 minutes, 1–2 times a day, for 5–7 days per week.
The intensity dimension has not been studied extensively in patients, but data from seven studies of patients with COPD collated by Pardy & Rochester (1992) suggested a significant positive relationship between the percentage increase in MIP and the relative magnitude of the inspiratory training load. In other words, the higher the load relative to the subject's MIP, the greater was the increase in MIP induced by training. The collated data suggest that, to achieve a 20% increase in MIP, a load of at least 30% of maximum strength is required. This suggestion is also supported by data from a 2002 meta-analysis of IMT (Lotters et al, 2002), and in a study that compared the efficacy of high- (52% MIP) and low-intensity (22%) IMT (Preusser et al, 1994); high-intensity IMT increased MIP by 35% (p < 0.05), whereas low-intensity IMT increased MIP by only 10% (p > 0.05) (Preusser et al, 1994). Collectively, the literature supports the need for training loads to exceed 30% MIP, but the question of whether more is better when it comes to the magnitude of inspiratory loading has not been examined systematically. A handful of studies have reported the effects of high-intensity training in patients with COPD, and these suggest that when loads are 68% of MIP (Sturdy et al, 2003), ‘the highest tolerable inspiratory threshold load’ (Hill et al, 2006) or ‘80% of maximal effort’ (Enright et al, 2006), then greater increases in MIP are achieved (29–41%) compared with low-to-moderate intensities (15–23%; Geddes et al, 2008).
However, it is important to note that in all of the studies employing high-intensity IMT the frequency of training has been only 3 days per week, compared with twice daily IMT in the studies using low to moderate loads (i.e., three sessions per week compared with 14). This is potentially very important from a practical point of view, as it suggests that high-intensity training may be far more time efficient, as well as more effective. However, a note of caution should also be expressed, as daily high-intensity IMT may overload the muscles to the extent that chronic inspiratory muscle fatigue and suboptimal adaptations are elicited (see above). Practical suggestions regarding load setting can be found in Chapter 6.
Studies of IMT in patients have typically been of much longer duration than those in healthy young athletes (3 months vs 4–6 weeks). As mentioned previously, Weiner and colleagues noted the largest improvement in MIP during the first 3 months of their study, followed by a gradual plateau of improvement (Weiner et al, 2004). Similar observations were made by Larson et al (1988) after 1 month of training, and by Lisboa et al (1997). This ‘plateau’ effect is also apparent in studies on healthy people, where MIP increases most rapidly during the first 3 weeks, then rising more slowly to a plateau by around 6 weeks (Volianitis et al, 2001; Romer & McConnell, 2003). The development of a plateau cannot be ascribed to a lack of load progression (increasing the training load to accommodate increases in MIP) as it occurs regardless of this measure. Instead, it is a reflection of a basic property of muscle adaptation to strength-training stimuli (Moritani & deVries, 1979; Hakkinen et al, 1987), which necessitates periodic changes in the nature of the training stimulus in order to maintain the adaptation process; this is one of the reasons why athletes periodize their training.
Specificity
The adaptations elicited by training depend upon the type of stimulus to which the muscle is subjected. This is best illustrated by considering the polar opposites of strength and endurance training: muscles tend to respond to strength-training stimuli (high intensity and short duration) by improving strength, and to endurance-training stimuli (low intensity and long duration) by improving endurance.
Training for strength
Generally, respiratory muscles respond to high-load–low-frequency loading with a strength-training response (Pardy & Rochester, 1992; Tzelepis et al, 1994a; Romer & McConnell, 2003). However, as well as load specificity, there is also an element of flow specificity that must be borne in mind as the two are interrelated (Tzelepis et al, 1994a; Romer & McConnell, 2003). This is because of the limitations imposed by the force–velocity relationship of muscles (see Ch. 1); high loads cannot be overcome at high velocities of muscle shortening. Training stimuli with high loads and low velocities (e.g., a Mueller manoeuvre) elicit increases in MIP, but do not elicit increases in maximal shortening velocity (peak inspiratory flow rate) (see Ch. 4, Fig. 4.2). Conversely, training with low loads and high velocities of shortening (e.g., unloaded hyperpnoea) elicit increases in maximal shortening velocity, but not MIP (see Ch. 4, Fig. 4.2) (Tzelepis et al, 1994a; Romer & McConnell, 2003). Interestingly, training stimuli with intermediate loads and shortening velocities elicit improvements in both qualities (Tzelepis et al, 1994a; Romer & McConnell, 2003), which arguably provides the ‘best of both worlds’ (see Ch. 4, Fig. 4.2).
A number of studies have now demonstrated in healthy people (Enright et al, 2006; Downey et al, 2007) and patients (Ramirez-Sarmiento et al, 2002; Enright et al, 2004; Chiappa et al, 2008; West et al, 2009) that the increase in MIP that follows strength training of the inspiratory muscles is secondary to hypertrophy.
Training for endurance
An endurance-conditioning response can be elicited with prolonged low-load–high-frequency contractions, which have typically been imposed upon the respiratory muscles using prolonged voluntary hyperpnoea (Boutellier & Piwko, 1992), but endurance can also be improved through strength training (Belman & Shadmehr, 1988; Harver et al, 1989). There is a common misconception that muscle endurance can be improved only using a specific endurance-training stimulus. However, stronger muscles perform a given task at a lower percentage of their maximum capacity than weaker muscles, which has beneficial consequences for fatigue resistance (endurance) (Belman & Shadmehr, 1988). Thus, inspiratory muscle strength training provides a ‘dual-conditioning’ response. There is no evidence that a specific endurance-training stimulus, such as hyperpnoea, improves MIP (Leith & Bradley, 1976; O'Kroy & Coast, 1993); indeed, this would not be expected as strength improves only when the tension within muscles is increased by the imposition of an external load. Collectively, the data suggest that training regimens with a moderate strength bias have the capacity to improve maximal strength, velocity of shortening and power output (Romer & McConnell, 2003) as well as endurance (Romer & McConnell, unpublished observations). This versatility supports the implementation of training with a bias towards strength at a moderate intensity.
The effect of lung volume (muscle length)
To date, only one study has examined whether the lung volume at which IMT occurs has any influence upon training outcomes (Tzelepis et al, 1994b). The data indicate that improvements in inspiratory muscle strength are specific to the lung volume at which training occurs (Tzelepis et al, 1994b). When three groups of healthy participants performed 6 weeks of repeated static maximum inspiratory manoeuvres at one of three lung volumes (residual volume, functional residual capacity (FRC), or FRC plus one-half of inspiratory capacity), the greatest improvements in strength occurred at the volume at which the participants trained. In addition, the improvements were significantly greater for those who trained at low lung volumes. Furthermore, the range of lung volume over which strength was increased was also greatest for those who trained at low lung volumes. These data suggest that IMT should be conducted over the greatest range of lung volumes possible, commencing as close as possible to residual volume.
A caveat that must be borne in mind in the context of lung volume specificity is the volume–pressure relationship of the inspiratory muscles (see Ch. 1). The inspiratory muscles become progressively weaker as the lungs inflate, such that under conditions of inspiratory loading the breath may be curtailed before the lungs are completely full. This ‘clipping’ of inspired volume is influenced by: (1) the magnitude of the load (occurring earlier with heavier loads), and (2) the fatigue state of the inspiratory muscles (occurring earlier in the presence of fatigue) (see also Ch. 6, Fig. 6.2). This means that, for most resistance IMT devices, a compromise must be struck between the magnitude of the load and the volume of the breath, as it is impossible to achieve high loads and high volumes simultaneously. The impact of these issues upon training load selection is considered in Chapter 6. The influence upon the design of IMT products will be considered at the end of this chapter.
Reversibility
The phenomenon of ‘use it or lose it’ describes the reversibility of training benefits. Despite the continuous activity of the respiratory muscles, even under resting conditions, this is insufficient to protect them against detraining. Sensitivity to prevailing levels of work is illustrated by the dose-response relationship between levels of physical activity and inspiratory muscle function that has been identified in elderly people (Buchman et al, 2008). Furthermore, in circumstances such as mechanical ventilation, where complete inactivity is imposed, inspiratory muscle function deteriorates precipitously;); 18 to 69 hours of complete diaphragmatic inactivity due to mechanical ventilation decreased the cross-sectional areas of diaphragmatic fibres by at least 50% (Tobin et al, 2010).
Detraining
Unfortunately, the extent and time course of inspiratory muscle detraining are not well documented, but two studies of resistance IMT do shed some light on these issues. In healthy young adults, Romer & McConnell (2003) documented regression of IMT-induced changes in inspiratory muscle function (9 weeks of three differing IMT regimens) over an 18-week period of detraining. Decrements were observed at 9 weeks, with no further changes in strength-related measures at 18 weeks post-IMT. In contrast, endurance continued to decline between 9 and 18 weeks of detraining (Romer & McConnell, unpublished observations). Inspiratory muscle function remained significantly above baseline at 18 weeks, with a loss of 32% of the improvement in strength, 65% of the improvement in maximum shortening velocity and 75% of the improvement in inspiratory muscle endurance.
In patients with COPD, Weiner et al (2004) observed the detraining response of a group of COPD patients who had completed a 3-month intensive IMT programme. The detraining group undertook sham training (inspiratory load of 7 cmH2O) for the next 12 months and were reassessed at 3-month intervals. After 3 months of detraining, both MIP and inspiratory muscle endurance remained elevated compared with baseline (MIP 19%, endurance 22%), but after 12 months they were not significantly different from baseline. Collectively, these data suggest that inspiratory muscles respond in a similar manner to other muscles when a training stimulus is removed (Mujika & Padilla, 2000a, b) and that most of the losses of function occur within 2 to 3 months of the cessation of training.
Maintenance
On a more positive note, the two detraining studies described above (Romer & McConnell, 2003; Weiner et al, 2004) also demonstrated that IMT-induced improvements in inspiratory muscle function can be sustained with maintenance training programmes in which training frequency is reduced. Training frequency can be reduced by as much as two-thirds without loss of function, i.e., to 2 days per week in healthy adults (Romer & McConnell, 2003) and to 3 days per week in patients with COPD (Weiner et al, 2004).
In summary, the literature supports the notion that the general training principles of overload, specificity and reversibility apply as much to the training of respiratory muscles as they do to limb muscles. This means that respiratory training interventions should apply these principles in order to obtain specific functional outcomes (see Ch. 6).
DIFFERENT FORMS OF RMT AND THEIR OUTCOMES
Training methods can be subdivided broadly into two types: (1) resistance training in which the muscles are subjected to external loading that is akin to lifting a weight, and (2) endurance training in which the respiratory muscles are required to work at high shortening velocities for prolonged periods of time. In the case of the latter, the only load imposed upon the respiratory muscles is that of the inherent flow resistance and elastance of the respiratory system.
Resistance training
Conflict of interest statement:
The author is an inventor of two inspiratory muscle training products: (POWERbreathe and POWERbreathe K-Series). In the interests of complete transparency, the author declares a beneficial interest in these products in the form of a share of licence income to the University of Birmingham and Brunel University. The author also acts as a consultant to POWERbreathe International Ltd.
Inspiratory flow resistive loading
Inspiratory flow resistive loading (IFRL) requires inhalation via a variable diameter orifice whereby, for a given flow, the smaller the orifice the greater the resistive load. Studies utilizing IFRL have reported increases in inspiratory muscle strength in the range of 18% to 54% (Leith & Bradley, 1976; Hanel & Secher, 1991). However, an inherent limitation of IFRL is that inspiratory pressure, and thus training load, varies with flow (according to a power function) and not just to orifice size. Therefore, it is vitally important that breathing pattern is monitored during IFRL if a quantifiable training stimulus is to be provided. In a 1992 meta-analysis of respiratory muscle training (RMT) in patients with chronic obstructive pulmonary disease (COPD), it was concluded that studies employing IFRL in which inspiratory flow was not controlled failed to elicit improvements in inspiratory muscle function (Smith et al, 1992). Although modified flow resistive loading devices can be used to control flow (Belman & Shadmehr, 1991), such modifications require complex and expensive hardware making IFRL impractical for routine use.
A novel approach to IFRL, based on the Test of Incremental Respiratory Endurance (TIRE) technique (Chatham et al, 1995), has been used by some investigators to train the inspiratory muscles (Chatham et al, 1996) and to test the effects of this training upon exercise performance (Chatham et al, 1999; Enright et al, 2006; Mickleborough et al, 2008). The TIRE system uses a flow resistive load (2 mm diameter orifice), an electronic manometer attached via a serial interface to a computer, and dedicated software. Initially, several sustained maximal inspiratory efforts through the orifice are performed to provide a baseline pressure–time profile. A target pressure–time profile is then presented, typically set at 80% of the maximal effort. The manoeuvre is then repeated six times with 60 seconds recovery between efforts before the resting time is reduced to 45 seconds. A further six efforts are then completed, whereby the recovery time is reduced to 30 seconds and the user repeats the exercise. There are six different levels in all, with diminishing recovery times down to 5 seconds between breaths (incremental IFRL). The exercise is terminated when the participant either completes the prescribed number of breathing manoeuvres, or the pressure generated falls beneath the reference pressure–time profile.
Incremental IFRL has been shown to increase inspiratory muscle strength in healthy people (Enright et al, 2006; Mickleborough et al, 2008; Mickleborough et al, 2009) and patients with cystic fibrosis (Enright et al, 2004). Although the technique appears to overcome the primary limitations of flow resistive loading, the functional relevance of incremental IFRL is questionable. This type of sustained maximal inspiratory effort bears no relation to the dynamic function of inspiratory muscles during whole-body endurance exercise. The influence of incremental IFRL is therefore likely to be confined to the force (pressure) axis of the force–velocity relationship of the inspiratory muscles (Romer & McConnell, 2003). Furthermore, training sessions are physically demanding and time consuming (a complete training session takes ~ 30 minutes).
Dynamic inspiratory flow resistive loading
Most recently, an electronic product has been launched with a dynamically adjusting inspiratory flow resistor (dynamic IFRL). The magnitude of the inspiratory load can be manipulated within and between breaths to create an extremely versatile loading system. The device tapers the inspiratory load in such a way that the load is maintained at any pre-set percentage of MIP throughout the breath; in other words, the absolute load declines as the inspiratory muscles weaken during lung inflation. This eliminates the breath ‘clipping’ that arises with high inspiratory loads and / or inspiratory muscle fatigue. Unlike incremental IFRL, training can be achieved at physiologically relevant inspiratory flow rates. Preliminary data from a pilot study in patients with COPD comparing dynamic IFRL to IPTL (see below) suggests that dynamic IFRL elicits superior improvements in a wide range of functional measures of inspiratory muscle performance (Langer et al, unpublished observations). For example, the patients undertaking dynamic IFRL were able to sustain higher intensity training loads, resulting in superior improvement in MIP after 8 weeks of training (23% vs 15%, p<0.03).
Inspiratory pressure threshold loading
Inspiratory pressure threshold loading (IPTL) requires individuals to produce an inspiratory pressure sufficient to overcome a negative pressure load and thereby initiate inhalation. Threshold loading permits loading at a quantifiable, variable intensity by providing near-flow-independent resistance to inspiration. This can be achieved in several ways, e.g., with a weighted plunger (Nickerson & Keens, 1982), a solenoid valve (Bardsley et al, 1993), a constant negative pressure system (Chen et al, 1998), or a spring-loaded poppet valve (Caine & McConnell, 2000) (Fig. 5.1). Threshold loading has been shown to induce improvements in inspiratory muscle strength in healthy young adults (Inbar et al, 2000; Volianitis et al, 2001; Romer et al, 2002b, c; Romer & McConnell, 2003; Edwards & Cooke, 2004; McConnell & Sharpe, 2005; McConnell & Lomax, 2006; Downey et al, 2007; Griffiths & McConnell, 2007; How et al, 2007; Johnson et al, 2007; Witt et al, 2007; Brown et al, 2008, 2010, 2011; Edwards et al, 2008; Klusiewicz et al, 2008; Tong et al, 2008; Kilding et al, 2009; Lomax & McConnell, 2009; Nicks et al, 2009; Verges et al, 2009; Bailey et al, 2010; Lomax, 2010; Turner et al, 2012), as well as in patients with COPD (see Gosselink et al, 2011), heart failure (see Smart et al, 2012) and neuromuscular disease (Topin et al, 2002). Maximum rate of muscle shortening (peak inspiratory flow rate) is also improved (Villafranca et al, 1998; Romer et al, 2002a; Romer & McConnell, 2003; Weiner & Weiner, 2006), as are maximal power output (Lisboa et al, 1994; Villafranca et al, 1998; Romer & McConnell, 2003) and inspiratory muscle endurance (Lisboa et al, 1994; Ramirez-Sarmiento et al, 2002; Weiner et al, 2004).
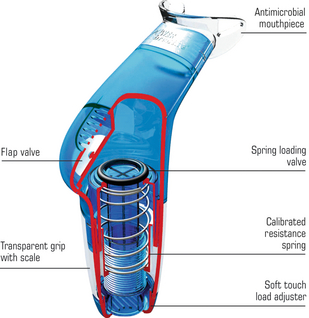
Figure 5.1 A pressure threshold inspiratory muscle trainer illustrating the spring-loaded poppet valve that provides the training load to the inspiratory muscles. (From POWERbreathe International Ltd, with permission.)
Because of its flow independence, training using IPTL can be undertaken effectively without the need to regulate breathing pattern. In addition, IPTL using a device with a mechanical poppet valve is both portable and easy to use.
Expiratory pressure threshold loading
Expiratory pressure threshold loading (EPTL) requires individuals to produce an expiratory pressure sufficient to overcome a positive pressure load and thereby initiate expiration. As is the case with IPTL, loading can be imposed at a quantifiable intensity by providing near-flow-independent resistance to expiration. This type of training has been far less extensively studied than IPTL, but EPTL has been shown to generate increases in expiratory muscle strength in both healthy people (Suzuki et al, 1995; Baker et al, 2005; Griffiths & McConnell, 2007) and patients with COPD (Weiner et al, 2003b; Mota et al, 2007) and multiple sclerosis (Smeltzer et al, 1996). Weiner and colleagues also demonstrated an improvement in the endurance performance of the expiratory muscles following EPTL (Weiner et al, 2003a).
Concurrent IPTL and EPTL
The results of studies that have imposed pressure threshold loads upon the inspiratory and expiratory muscles within the same breath cycle suggest that this type of concurrent loading may be counter-productive. For example, when IPTL was added to EPTL after a period of 4 weeks in well-trained rowers, the increase in inspiratory muscle strength over the subsequent 6 weeks of concurrent training was just 13%, which is less than half the value typically attainable when IPTL is implemented without concurrent EPTL (Griffiths & McConnell, 2007). Similar findings were made in young swimmers who undertook simultaneous IPTL / EPTL (Wells et al, 2005). After 12 weeks of training with incrementally increasing inspiratory and expiratory loads, inspiratory and expiratory muscle strength improved by only 8%. This was identical to the change shown over the same period in the sham-training control group. However, one study in healthy elderly people demonstrated improvements in both MIP and MEP following concurrent IPTL / EPTL (Watsford & Murphy, 2008). In this study, the selection of the training load was dictated objectively for the inspiratory load, with the expiratory load being adjusted arbitrarily to a level that was ‘tolerable’. This may explain the fact that significant improvements in MIP and MEP were observed in this study, but not in the one by Wells et al (2005). Generally, these data suggest that care is required when using concurrent IPTL and EPTL, as loading both phases of the breathing cycle simultaneously can generate suboptimal improvements in respiratory muscle strength.
Endurance training
Voluntary isocapnic hyperpnoea training
Voluntary isocapnic hyperpnoea (VIH) training requires individuals to maintain high target levels of ventilation for up to 30 minutes. To prevent hypocapnia, participants may simply rebreathe through a dead space. However, most studies have used more elaborate apparatus that supplies supplemental oxygen to avoid hypoxaemia while maintaining isocapnia. Training sessions are typically conducted 3 to 5 times per week at ~ 60–90% of maximum voluntary ventilation (MVV). Using VIH in healthy people, several investigators have shown increases in endurance during sustained isocapnic ventilation (Boutellier et al, 1992; Boutellier & Piwko, 1992; Markov et al, 1996; Spengler et al, 1999; Stuessi et al, 2001; Leddy et al, 2007; Wylegala et al, 2007; Verges et al, 2008b; Verges et al, 2009), maximum sustainable ventilation (MSV) (Leith & Bradley, 1976; Belman & Gaesser, 1988) and MVV (Leith & Bradley, 1976; Belman & Gaesser, 1988; Spengler et al, 1999; Leddy et al, 2007; Wylegala et al, 2007; Verges et al, 2009). The latter is consistent with improvements in peak velocity of muscle shortening. Typically, pulmonary function indices such as vital capacity and forced expiratory volume in 1 second remain unaffected by VIH (Spengler et al, 1999; Stuessi et al, 2001; Leddy et al, 2007; Wylegala et al, 2007). However, one recent study showed vital capacity increased significantly after VIH (Verges et al, 2009). Studies in patients are less numerous, but VIH appears to elicit similar changes to those observed in healthy people, when implemented in patients with neuromuscular disease (Rassler et al, 2007) and COPD (Mador et al, 2005).
Voluntary isocapnic hyperpnoea is a relatively time-consuming (typically 30 minutes per session) and physically demanding mode of RMT requiring a high degree of motivation. It also requires supplemental carbon dioxide or partial rebreathing in order to prevent hypocapnia (low carbon dioxide levels). Although VIH improves indices of respiratory muscle endurance, it does not improve the maximal pressure-generating capacity of the respiratory muscles (Leith & Bradley, 1976; Verges et al, 2008a). The influence of VIH is thus confined to the velocity (flow) axis of the force–velocity relationship of the inspiratory muscles (Romer & McConnell, 2003).
Summary
The preceding evidence points to resistance training as providing the most versatile training stimulus, as judged by the range of adaptions elicited (strength, power, shortening velocity and endurance). It is also the most time efficient and least arduous method of improving respiratory muscle function. The following section will consider commercially available training equipment, which will provide the final pieces of information required to select a suitable training method i.e., those of portability and relative cost.
PROPRIETARY TRAINING EQUIPMENT
The first part of this section will describe the range of mechanical principles that have been exploited to load the respiratory muscles. This will be followed by a description of the commercial products that utilize these basic principles. However, the latter is limited to those products that have been tested in placebo-controlled trials on patients and / or healthy young people, and shown to generate statistically significant improvements in at least one aspect of respiratory muscle function, as well as being published in peer-reviewed professional journals. The merits and limitations of each device will be summarized in order to assist the selection of the most appropriate device(s) for a given application.
Respiratory training equipment falls into two main categories: devices that impose a resistance-training stimulus and devices that impose an endurance-training stimulus.
Resistance training
These products fall into three main classes on the basis of how the load is generated:
Passive flow resistance devices
The mechanical products in this class employ simple dials that allow the user to select inspired airway orifices with differing surface area; the smaller the surface area, the larger is the inspired resistance. However, because these loads are passive, and generated by the inspired air flow (no flow = no load), they are highly sensitive to the influence of inspiratory flow rate, which makes loading unreliable. Because of their simplicity and cheapness of manufacture, the mechanical versions of these products are the most abundant proprietary devices on the market. However, because of their inherent limitations this class contains only one product that meets the requirements for inclusion in this summary, viz., the Pflex® (Respironics Inc. USA; Table 5.1). The main advantages of the Pflex® are its price (less than £15, at the time of writing) and convenience. However, training load and progression are impossible to quantify without providing simultaneous feedback of inspiratory flow rate using another piece of equipment. When used in this way, so-called ‘targeted flow resistive training’, there is no difference in the quality of the improvement in strength that can be achieved compared with pressure threshold loading products (Hsiao et al, 2003).
Table 5.1
Comparison of evidence-based proprietary respiratory muscle training equipment*
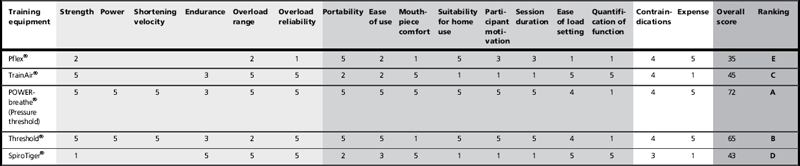
1 = poor; 5 = excellent. A = top ranked; E = bottom ranked. No score indicates no evidence.
Training quality and versatility
Participant comfort and convenience
*Please refer to the Author's Conflict of Interest Statement on p. 138.
The addition of pressure measurement, other electronics and software to a simple flow resistor make load setting reliable and quantifiable, but also adds considerably to the cost and bulk of the equipment. The only product of this type to be supported by published data is the TrainAir® (Project Electronics Ltd., UK), which is based on the Test of Incremental Respiratory Endurance (TIRE) technique (Chatham et al, 1995) described above. The equipment requires interface to a laptop, and its cost (about £500) makes it the preserve of specialist clinics. The training is also very time consuming and strenuous. In its favour are the continuous biofeedback of training intensity, and built-in assessment of inspiratory muscle function.
Dynamically adjusted flow resistance devices
This is a new approach to respiratory training that, at the time of writing, is supported only by unpublished pilot data. However, a description is included here because this method of loading will become more widely used in the future. As described above, the inherent limitation of a passive flow resistor is that variations in flow rate result in variations in load; this has been overcome in the new generation of flow resistance devices by using continuous, dynamic adjustment of the flow resistor. In essence, the surface area of a variable flow orifice is varied within a breath according to the prevailing respiratory flow rate. The controlled variable can be either the pressure load or the respired flow rate. Currently the only product of this type available is the POWERbreathe® K-Series (POWERbreathe International Ltd.), which was launched in 2010. The product ranges in price from £250 to £450, with the higher-priced products being downloadable and programmable by the user, as well as providing real-time computer-based biofeedback during training. The ability to make within- and between-breath adjustments makes this type of device extremely versatile from the loading perspective. Of the many unique features offered by this method is the ability to taper the load according to the prevailing strength of the muscles. In the case of inspiratory loading, for example, the load can be profiled to maintain it at the same percentage of MIP irrespective of lung volume. This means that the premature curtailment of inspiration (breath ‘clipping’) that occurs with heavy-pressure threshold loading does not arise; thus, higher loads and higher tidal volumes can be achieved. As with other electronic devices, price makes this method the preserve of specialist clinics.
Pressure threshold devices
There are two products of this type that meet the requirements for inclusion in this summary. One has been applied to both the medical and sports settings (POWERbreathe®, POWERbreathe International Ltd.), the other to just the clinical environment (Threshold®, Respironics Inc.). Both products are supported by extensive, high-quality published research. The principal differences between the products are their loading ranges (see Table 5.1), mouthpiece, separation of inspiratory and expiratory flow paths and price (Threshold about £14 vs POWERbreathe® about £30). The loading range of the Threshold® renders it unusable by anyone whose baseline maximal inspiratory pressure (MIP) exceeds ~ 60 cmH2O. This is because its load-setting range of 9 to 41 cmH2O is unable to accommodate an adequate training load (~ 50% MIP) for individuals with a MIP of > 80 cmH2O. Thus, someone starting training with a MIP of 60 cmH2O, and improving by 30%, will rapidly reach the limits of the spring to provide an adequate training stimulus. The other product (POWERbreathe®) is supplied in a range of models with load settings spanning 17–98 cmH2O, 23–186 cmH2O and 29–274 cmH2O; there is also a model that is approved for UK National Health Service prescription (POWERbreathe® Medic). The POWERbreathe® product also separates inspiratory and expiratory flow paths such that the inspiratory valve is protected from expirate. Finally, the Threshold® (like the Pflex®) provides only a hard plastic tube mouthpiece that makes it challenging for some users to maintain an airtight seal, whereas the POWERbreathe® has a flexible flanged mouthpiece that is both comfortable and airtight; it can also be interfaced via a facemask. The low price of these devices make them ideal for use by patients in a domiciliary setting.
Endurance training
There is only one commercial product providing this type of respiratory training (SpiroTiger®, Idag AG, Switzerland). Like the two pressure threshold trainers, the efficacy of the SpiroTiger is supported by an extensive number of published research papers, primarily in a sports setting. As described in the section above, the overloading stimulus is provided by vigorous hyperventilation for periods of around 30 minutes. Because the product requires a rebreathing circuit and a method to monitor the training intensity, it is bulky and expensive (about £500), which renders it primarily a clinic- or sports-based training system. The training is also time consuming and extremely strenuous, requiring a very high level of user commitment in order to achieve and sustain the prescribed training intensity of ~ 60–90% of maximum voluntary ventilation.
MERITS AND LIMITATIONS OF DIFFERENT TRAINING METHODS AND EQUIPMENT
Table 5.1 summarizes the merits and limitations of the various training equipment, taking into consideration factors such as reliability, range of functional outcomes, price, ease of use, training session duration, etc. By referring to this table, it is possible to highlight the factors that are important for a given application, and to select the most appropriate form of apparatus. The table also provides an overall score for each piece of equipment.
Table 5.1 suggests that the most versatile, cost-effective, convenient and time-efficient method of respiratory training is provided by inspiratory pressure threshold loading, which is supported by the overall score received for these products. This method is also the most widely used, and best supported by research evidence. Accordingly, all subsequent advice and guidance in this book will be provided for pressure threshold IMT.
For the latest information on equipment and accessories for RMT, visit www.physiobreathe.com.
References
Bailey, S.J., Romer, L.M., Kelly, J., et al. Inspiratory muscle training enhances pulmonary O(2) uptake kinetics and high-intensity exercise tolerance in humans. J. Appl. Physiol. 2010;109:457–468.
Baker, S., Davenport, P., Sapienza, C. Examination of strength training and detraining effects in expiratory muscles. J. Speech Lang. Hear. Res. 2005;48:1325–1333.
Bardsley, P.A., Bentley, S., Hall, H.S., et al. Measurement of inspiratory muscle performance with incremental threshold loading: a comparison of two techniques. Thorax. 1993;48:354–359.
Belman, M.J., Gaesser, G.A. Ventilatory muscle training in the elderly. J. Appl. Physiol. 1988;64:899–905.
Belman, M.J., Shadmehr, R. Targeted resistive ventilatory muscle training in chronic obstructive pulmonary disease. J. Appl. Physiol. 1988;65:2726–2735.
Belman, M.J., Shadmehr, R. A target feedback device for ventilatory muscle training. J. Clin. Monit. 1991;7:42–48.
Boutellier, U., Piwko, P. The respiratory system as an exercise limiting factor in normal sedentary subjects. Eur. J. Appl. Physiol. Occup. Physiol. 1992;64:145–152.
Boutellier, U., Buchel, R., Kundert, A., et al. The respiratory system as an exercise limiting factor in normal trained subjects. Eur. J. Appl. Physiol. Occup. Physiol. 1992;65:347–353.
Brown, P.I., Sharpe, G.R., Johnson, M.A. Inspiratory muscle training reduces blood lactate concentration during volitional hyperpnoea. Eur. J. Appl. Physiol. 2008;104:111–117.
Brown, P.I., Sharpe, G.R., Johnson, M.A. Loading of trained inspiratory muscles speeds lactate recovery kinetics. Med. Sci. Sports Exerc. 2010;42:1103–1112.
Brown, P.I., Sharpe, G.R., Johnson, M.A. Inspiratory muscle training abolishes the blood lactate increase associated with volitional hyperpnoea superimposed on exercise and accelerates lactate and oxygen uptake kinetics at the onset of exercise. Eur. J. Appl. Physiol. 2011;112(6):2117–2129.
Buchman, A.S., Boyle, P.A., Wilson, R.S., et al. Respiratory muscle strength predicts decline in mobility in older persons. Neuroepidemiology. 2008;31:174–180.
Caine, M.P., McConnell, A.K. Development and evaluation of a pressure threshold inspiratory muscle trainer for use in the context of sports performance. Journal of Sports Engineering. 2000;3:149–159.
Chatham, K., Baldwin, J., Oliver, W., et al. Fixed load incremental respiratory muscle training: A pilot study. Physiotherapy. 1996;82:422–426.
Chatham, K., Baldwin, J., Griffiths, H., et al. Inspiratory muscle training improves shuttle run performance in healthy subjects. Physiotherapy. 1999;85:676–683.
Chatham, K., Conway, J., Enright, S., et al. A new test of incremental respiratory endurance (TIRE). Am. J. Respir. Crit. Care Med. 1995;151:A416.
Chen, R.C., Que, C.L., Yan, S. Introduction to a new inspiratory threshold loading device. Eur. Respir. J. 1998;12:208–211.
Chiappa, G.R., Roseguini, B.T., Vieira, P.J., et al. Inspiratory muscle training improves blood flow to resting and exercising limbs in patients with chronic heart failure. J. Am. Coll. Cardiol. 2008;51:1663–1671.
Downey, A.E., Chenoweth, L.M., Townsend, D.K., et al. Effects of inspiratory muscle training on exercise responses in normoxia and hypoxia. Respir. Physiol. Neurobiol. 2007;156:137–146.
Edwards, A.M., Cooke, C.B. Oxygen uptake kinetics and maximal aerobic power are unaffected by inspiratory muscle training in healthy subjects where time to exhaustion is extended. Eur. J. Appl. Physiol. 2004;93:139–144.
Edwards, A.M., Wells, C., Butterly, R. Concurrent inspiratory muscle and cardiovascular training differentially improves both perceptions of effort and 5000 m running performance compared with cardiovascular training alone. Br. J. Sports Med. 2008;42:523–527.
Enright, S., Chatham, K., Ionescu, A.A., et al. Inspiratory muscle training improves lung function and exercise capacity in adults with cystic fibrosis. Chest. 2004;126:405–411.
Enright, S.J., Unnithan, V.B., Heward, C., et al. Effect of high-intensity inspiratory muscle training on lung volumes, diaphragm thickness, and exercise capacity in subjects who are healthy. Phys. Ther. 2006;86:345–354.
Gea, J., Hamid, Q., Czaika, G., et al. Expression of myosin heavy-chain isoforms in the respiratory muscles following inspiratory resistive breathing. Am. J. Respir. Crit. Care Med. 2000;161:1274–1278.
Geddes, E.L., O'Brien, K., Reid, W.D., et al. Inspiratory muscle training in adults with chronic obstructive pulmonary disease: An update of a systematic review. Respir. Med. 2008;102:1715–1729.
Gosselink, R., Wagenaar, R.C., Decramer, M. Reliability of a commercially available threshold loading device in healthy subjects and in patients with chronic obstructive pulmonary disease. Thorax. 1996;51:601–605.
Gosselink, R., De Vos, J., van den Heuvel, S.P., et al. Impact of inspiratory muscle training in patients with COPD: what is the evidence? Eur. Respir. J. 2011;37:416–425.
Griffiths, L.A., McConnell, A.K. The influence of inspiratory and expiratory muscle training upon rowing performance. Eur. J. Appl. Physiol. 2007;99:457–466.
Hakkinen, K., Komi, P.V., Alen, M., et al. EMG, muscle fibre and force production characteristics during a 1 year training period in elite weight-lifters. Eur. J. Appl. Physiol. Occup. Physiol. 1987;56:419–427.
Hanel, B., Secher, N.H. Maximal oxygen uptake and work capacity after inspiratory muscle training: a controlled study. J. Sports Sci. 1991;9:43–52.
Harver, A., Mahler, D.A., Daubenspeck, J.A. Targeted inspiratory muscle training improves respiratory muscle function and reduces dyspnea in patients with chronic obstructive pulmonary disease. Ann. Intern. Med. 1989;111:117–124.
Hill, K., Jenkins, S.C., Philippe, D.L., et al. High-intensity inspiratory muscle training in COPD. Eur. Respir. J. 2006;27:1119–1128.
How, S.C., McConnell, A.K., Taylor, B.J., et al. Acute and chronic responses of the upper airway to inspiratory loading in healthy awake humans: an MRI study. Respir. Physiol. Neurobiol. 2007;157:270–280.
Hsiao, S.F., Wu, Y.T., Wu, H.D., et al. Comparison of effectiveness of pressure threshold and targeted resistance devices for inspiratory muscle training in patients with chronic obstructive pulmonary disease. J. Formos. Med. Assoc. 2003;102:240–245.
Inbar, O., Weiner, P., Azgad, Y., et al. Specific inspiratory muscle training in well-trained endurance athletes. Med. Sci. Sports Exerc. 2000;32:1233–1237.
Johnson, B.D., Babcock, M.A., Suman, O.E., et al. Exercise-induced diaphragmatic fatigue in healthy humans. J. Physiol. 1993;460:385–405.
Johnson, M.A., Sharpe, G.R., Brown, P.I. Inspiratory muscle training improves cycling time-trial performance and anaerobic work capacity but not critical power. Eur. J. Appl. Physiol. 2007;101:761–770.
Jones, D.A., Rutherford, O.M., Parker, D.F. Physiological changes in skeletal muscle as a result of strength training. Q. J. Exp. Physiol. 1989;74:233–256.
Kilding, A.E., Brown, S., McConnell, A.K. Inspiratory muscle training improves 100 and 200 m swimming performance. Eur. J. Appl. Physiol. 2009;108:505–511.
Klusiewicz, A., Borkowski, L., Zdanowicz, R., et al. The inspiratory muscle training in elite rowers. J. Sports Med. Phys. Fitness. 2008;48:279–284.
Larson, J.L., Kim, M.J., Sharp, J.T., et al. Inspiratory muscle training with a pressure threshold breathing device in patients with chronic obstructive pulmonary disease. Am. Rev. Respir. Dis. 1988;138:689–696.
Leddy, J.J., Limprasertkul, A., Patel, S., et al. Isocapnic hyperpnea training improves performance in competitive male runners. Eur. J. Appl. Physiol. 2007;99:665–676.
Leith, D.E., Bradley, M. Ventilatory muscle strength and endurance training. J. Appl. Physiol. 1976;41:508–516.
Lisboa, C., Munoz, V., Beroiza, T., et al. Inspiratory muscle training in chronic airflow limitation: comparison of two different training loads with a threshold device. Eur. Respir. J. 1994;7:1266–1274.
Lisboa, C., Villafranca, C., Leiva, A., et al. Inspiratory muscle training in chronic airflow limitation: effect on exercise performance. Eur. Respir. J. 1997;10:537–542.
Lomax, M. Inspiratory muscle training, altitude, and arterial oxygen desaturation: a preliminary investigation. Aviat. Space Environ. Med. 2010;81:498–501.
Lomax, M., McConnell, A.K. Influence of prior activity (warm-up) and inspiratory muscle training upon between- and within-day reliability of maximal inspiratory pressure measurement. Respiration. 2009;78:197–202.
Lotters, F., van Tol, B., Kwakkel, G., et al. Effects of controlled inspiratory muscle training in patients with COPD: a meta-analysis. Eur. Respir. J. 2002;20:570–577.
Mador, M.J., Deniz, O., Aggarwal, A., et al. Effect of respiratory muscle endurance training in patients with COPD undergoing pulmonary rehabilitation. Chest. 2005;128:1216–1224.
Markov, G., Orler, R., Boutellier, U. Respiratory training, hypoxic ventilatory response and acute mountain sickness. Respir. Physiol. 1996;105:179–186.
McConnell, A.K., Lomax, M. The influence of inspiratory muscle work history and specific inspiratory muscle training upon human limb muscle fatigue. J. Physiol. 2006;577:445–457.
McConnell, A.K., Romer, L.M. Respiratory muscle training in healthy humans: resolving the controversy. Int. J. Sports Med. 2004;25:284–293.
McConnell, A.K., Sharpe, G.R. The effect of inspiratory muscle training upon maximum lactate steady-state and blood lactate concentration. Eur. J. Appl. Physiol. 2005;94(3):277–284.
Mickleborough, T.D., Stager, J.M., Chatham, K., et al. Pulmonary adaptations to swim and inspiratory muscle training. Eur. J. Appl. Physiol. 2008;103(6):635–646.
Mickleborough, T.D., Nichols, T., Lindley, M.R., et al. Inspiratory flow resistive loading improves respiratory muscle function and endurance capacity in recreational runners. Scand. J. Med. Sci. Sports. 2009;20(3):458–468.
Moritani, T., deVries, H.A. Neural factors versus hypertrophy in the time course of muscle strength gain. Am. J. Phys. Med. 1979;58:115–130.
Mota, S., Guell, R., Barreiro, E., et al. Clinical outcomes of expiratory muscle training in severe COPD patients. Respir. Med. 2007;101:516–524.
Mujika, I., Padilla, S. Detraining: loss of training-induced physiological and performance adaptations. Part I: short term insufficient training stimulus. Sports Med. 2000;30:79–87.
Mujika, I., Padilla, S. Detraining: loss of training-induced physiological and performance adaptations. Part II: long term insufficient training stimulus. Sports Med. 2000;30:145–154.
Nickerson, B.G., Keens, T.G. Measuring ventilatory muscle endurance in humans as sustainable inspiratory pressure. J. Appl. Physiol. 1982;52:768–772.
Nicks, C.R., Morgan, D.W., Fuller, D.K., et al. The influence of respiratory muscle training upon intermittent exercise performance. Int. J. Sports Med. 2009;30:16–21.
O'Kroy, J.A., Coast, J.R. Effects of flow and resistive training on respiratory muscle endurance and strength. Respiration. 1993;60:279–283.
Pardy, R.L., Leith, D.E. Ventilatory muscle training. In: Roussos C., Macklem P.T., eds. The thorax. New York: Marcel Dekker; 1995:1353–1371.
Pardy, R.L., Rochester, D.F. Respiratory muscle training. Seminars in Respiratory Medicine. 1992;13:53–62.
Preusser, B.A., Winningham, M.L., Clanton, T.L. High- vs low-intensity inspiratory muscle interval training in patients with COPD. Chest. 1994;106:110–117.
Ramirez-Sarmiento, A., Orozco-Levi, M., Guell, R., et al. Inspiratory muscle training in patients with chronic obstructive pulmonary disease: structural adaptation and physiologic outcomes. Am. J. Respir. Crit. Care Med. 2002;166:1491–1497.
Rassler, B., Hallebach, G., Kalischewski, P., et al. The effect of respiratory muscle endurance training in patients with myasthenia gravis. Neuromuscul Disord. 2007;17:385–391.
Romer, L.M., McConnell, A.K. Specificity and reversibility of inspiratory muscle training. Med. Sci. Sports Exerc. 2003;35:237–244.
Romer, L.M., McConnell, A.K., Jones, D.A. Effects of inspiratory muscle training on time-trial performance in trained cyclists. J. Sports Sci. 2002;20:547–562.
Romer, L.M., McConnell, A.K., Jones, D.A. Effects of inspiratory muscle training upon recovery time during high intensity, repetitive sprint activity. Int. J. Sports Med. 2002;23:353–360.
Romer, L.M., McConnell, A.K., Jones, D.A. Inspiratory muscle fatigue in trained cyclists: effects of inspiratory muscle training. Med. Sci. Sports Exerc. 2002;34:785–792.
Smart, N.A., Giallauria, F., Dieberg, G. Efficacy of inspiratory muscle training in chronic heart failure patients: A systematic review and meta-analysis. Int. J. Cardiol. 2012. [(in press)].
Smeltzer, S.C., Lavietes, M.H., Cook, S.D. Expiratory training in multiple sclerosis. Arch. Phys. Med. Rehabil. 1996;77:909–912.
Smith, K., Cook, D., Guyatt, G.H., et al. Respiratory muscle training in chronic airflow limitation: a meta-analysis. Am. Rev. Respir. Dis. 1992;145:533–539.
Spengler, C.M., Roos, M., Laube, S.M., et al. Decreased exercise blood lactate concentrations after respiratory endurance training in humans. Eur. J. Appl. Physiol. Occup. Physiol. 1999;79:299–305.
Stuessi, C., Spengler, C.M., Knopfli-Lenzin, C., et al. Respiratory muscle endurance training in humans increases cycling endurance without affecting blood gas concentrations. Eur. J. Appl. Physiol. 2001;84:582–586.
Sturdy, G., Hillman, D., Green, D., et al. Feasibility of high-intensity, interval-based respiratory muscle training in COPD. Chest. 2003;123:142–150.
Suzuki, S., Sato, M., Okubo, T. Expiratory muscle training and sensation of respiratory effort during exercise in normal subjects. Thorax. 1995;50:366–370.
Tobin, M.J., Laghi, F., Jubran, A. Narrative review: ventilator-induced respiratory muscle weakness. Ann. Intern. Med. 2010;153:240–245.
Tong, T.K., Fu, F.H., Chung, P.K., et al. The effect of inspiratory muscle training on high-intensity, intermittent running performance to exhaustion. Appl. Physiol. Nutr. Metab. 2008;33:671–681.
Topin, N., Matecki, S., Le Bris, S., et al. Dose-dependent effect of individualized respiratory muscle training in children with Duchenne muscular dystrophy. Neuromuscul. Disord. 2002;12:576–583.
Turner, L.A., Tecklenburg-Lund, S.L., Chapman, R.F., et al. Inspiratory muscle training lowers the oxygen cost of voluntary hyperpnea. J. Appl. Physiol. 2012;112:127–134.
Tzelepis, G.E., Vega, D.L., Cohen, M.E., et al. Pressure-flow specificity of inspiratory muscle training. J. Appl. Physiol. 1994;77:795–801.
Tzelepis, G.E., Vega, D.L., Cohen, M.E., et al. Lung volume specificity of inspiratory muscle training. J. Appl. Physiol. 1994;77:789–794.
Verges, S., Boutellier, U., Spengler, C.M. Effect of respiratory muscle endurance training on respiratory sensations, respiratory control and exercise performance: a 15-year experience. Respir. Physiol. Neurobiol. 2008;161:16–22.
Verges, S., Kruttli, U., Stahl, B., et al. Respiratory control, respiratory sensations and cycling endurance after respiratory muscle endurance training. Adv. Exp. Med. Biol. 2008;605:239–244.
Verges, S., Renggli, A.S., Notter, D.A., et al. Effects of different respiratory muscle training regimes on fatigue-related variables during volitional hyperpnoea. Respir. Physiol. Neurobiol. 2009;169:282–290.
Villafranca, C., Borzone, G., Leiva, A., et al. Effect of inspiratory muscle training with an intermediate load on inspiratory power output in COPD. Eur. Respir. J. 1998;11:28–33.
Volianitis, S., McConnell, A.K., Koutedakis, Y., et al. Inspiratory muscle training improves rowing performance. Med. Sci. Sports Exerc. 2001;33:803–809.
Watsford, M., Murphy, A. The effects of respiratory-muscle training on exercise in older women. J. Aging Phys. Act. 2008;16:245–260.
Weiner, P., Weiner, M. Inspiratory muscle training may increase peak inspiratory flow in chronic obstructive pulmonary disease. Respiration. 2006;73:151–156.
Weiner, P., Magadle, R., Beckerman, M., et al. Comparison of specific expiratory, inspiratory, and combined muscle training programs in COPD. Chest. 2003;124:1357–1364.
Weiner, P., Magadle, R., Beckerman, M., et al. Specific expiratory muscle training in COPD. Chest. 2003;124:468–473.
Weiner, P., Magadle, R., Beckerman, M., et al. Maintenance of inspiratory muscle training in COPD patients: one year follow-up. Eur. Respir. J. 2004;23:61–65.
Wells, G.D., Plyley, M., Thomas, S., et al. Effects of concurrent inspiratory and expiratory muscle training on respiratory and exercise performance in competitive swimmers. Eur. J. Appl. Physiol. 2005;94:527–540.
West, C.R., Taylor, B.J., Campbell, I.G., et al. Effects of inspiratory muscle training in Paralympic athletes with cervical spinal cord injury. Med. Sci. Sports Exerc. 2009;41:S31–S32.
Witt, J.D., Guenette, J.A., Rupert, J.L., et al. Inspiratory muscle training attenuates the human respiratory muscle metaboreflex. J. Physiol. 2007;584:1019–1028.
Wylegala, J.A., Pendergast, D.R., Gosselin, L.E., et al. Respiratory muscle training improves swimming endurance in divers. Eur. J. Appl. Physiol. 2007;99:393–404.