Chapter 38 Pregnancy and Parturition
PREGNANCY
The Development of an Embryo Involves Fusion of an Oocyte and Spermatozoon Within the Oviduct
The development of a new individual requires the transfer of male gametes to the female genital tract for fertilization of the female gamete(s). Spermatozoa, which have been concentrated and stored in the epididymis, gradually change from oxidative (aerobic) to glycolytic (anaerobic) metabolism as they progress through the epididymis. In this situation, spermatozoa are in a state of reduced metabolism. Mature sperm are only able to metabolize a special sugar, fructose, within the reproductive tract. Lactose, glucose, dextrose and fructose have all been used in commercially available semen extenders.
Sperm are ejaculated usually into the vagina, although some domestic species (dog, horse, and pig) ejaculate directly into the cervix and uterus. The movement of sperm through the cervix is aided by estrogen-induced changes in cervical mucus, which result in the formation of channels that facilitate movement of sperm. This has been particularly emphasized in primates, in which the thinning of mucus occurs just before ovulation, a factor that can be used to predict the time of ovulation.
The environment of the female genital system is generally inhospitable to the survival of sperm; for example, white blood cells are quickly attracted to the uterine lumen because sperm cells are foreign to the female genital tract. Special reservoirs have evolved in the female tract to aid in the survival of sperm during transport; these include the cervix and oviduct, the latter involving areas at the uterotubule junction and within the ampulla. The reservoirs are progressively filled (from caudal to cranial in the tract), requiring hours before the oviductal reservoirs are full. Finally, the reservoir within the ampulla is able to release a few sperm on a continuous basis, so that fertilization can occur shortly after the arrival of oocytes within the oviduct.
The first studies in sperm transport emphasized the rapidity of the process, with sperm reported passing from the vagina to the fimbriated end of the oviduct within minutes. It is now known that sperm undergoing so-called fast transport are not involved in fertilization; in fact, they are damaged by the rapid transport.
Sperm need to undergo changes within the female genital tract that are a prerequisite for fertilization; the process is called capacitation. One of the effects of capacitation is the removal of glycoproteins from the sperm cell surface.
The glycoproteins, perhaps added for protective purposes, interfere with fertilization. This change allows sperm to undergo the acrosome reaction when they come in contact with oocytes. The acrosome reaction involves the release of hydrolytic enzymes from the acrosomal cap; this may be important for penetration of the sperm through the granulosa and zona pellucida to the oocyte plasma membrane. Hyaluronidase causes breakdown of hyaluronic acid, an important component of the intercellular matrix of granulosa cells that surround the oocyte. Acrosin, a proteolytic enzyme, digests the acellular coating around the oocyte. Both enzymatic events allow the sperm to penetrate to the oocyte. The acrosome reaction also changes the surface of the sperm, which allows it to fuse with the oocyte. The acrosomal reaction results in tail movements that feature a flagellar beat that tends to drive sperm in a forward direction.
Because of the changes that spermatozoa must undergo within the female reproductive tract before fertilization, the deposition of sperm before ovulation is the preferred timing for producing maximal fertility. An exception to this takes place when sperm with reduced longevity are used, such as the case with chilled-extended semen or frozen semen. In these cases, deposition of semen into the female reproductive tract should occur close to the time of ova maturation associated with fertilization. Females are usually sexually receptive for at least 24 hours before ovulation and, in the natural setting (free interaction between genders), insemination usually occurs a number of hours before the occurrence of ovulation. Even with induced ovulators, such as cats, the interval from copulation to ovulation is usually 24 hours or more. In essence, the system has evolved to have ready-to-fertilize sperm at the fertilization site when oocytes arrive. This concurs with the finding that the life span of male gametes tends to be twice that of female gametes.
The presentation of male gametes before female gametes in the oviduct implies that oocytes are ready for fertilization on arrival in the ampulla; this is likely true for a majority of animals. A prerequisite for fertilization of the oocyte is that it must undergo the first meiotic division before fertilization. Although this occurs in a number of species before ovulation, in the horse and dog the first meiotic division does not occur until after ovulation (in the dog, not for at least 48 hours). In this situation, spermatozoa often wait for oocytes to mature in the oviduct before fertilization can occur. One means of adaptation to delayed completion of meiosis is that spermatozoa have a longer life span in the dog (6-11 days) and horse compared with other domestic species.
Once fertilization has occurred, the embryo usually develops to the morula, or early blastocyst stage, within the oviduct before moving into the uterus. This period, usually 4 to 5 days, affords the uterus time to finish its inflammatory response involving the removal of spermatozoa. This period also allows the endometrial glands time to secrete nutrients under the influence of progesterone from the developing corpus luteum (CL); the nutrients are essential for the development of embryos during their preimplantation stage.
An interesting finding in the mare is her ability to distinguish fertilized from unfertilized oocytes; unfertilized oocytes from previous cycles are retained within the oviduct, whereas recently fertilized oocytes (embryos) move through the oviduct to the uterus. It is likely that all animals recognize pregnancy by the presence of an embryo(s) at the early oviductal stage. However, this recognition does not necessarily result in prolongation of the CL and the continued production of progesterone, which is essential for the maintenance of pregnancy. In the bitch, despite ovulation and ova maturation spanning several hours, embryonic ages are synchronized by some mechanism inherent to the bitch’s reproductive tract.
Extension of the Life Span of the Corpus Luteum in Large Domestic Species and Cats Is Essential for Pregnancy Maintenance
For those domestic animals (cattle, goats, horses, pigs, sheep) whose luteal activity is controlled by the uterus, modification of uterine prostaglandin F2α (PGF2α) synthesis and release is critical for the establishment of pregnancy. The embryo apparently produces substances that modify uterine production of PGF2α. Estrogen synthesis by the embryo is one way the endometrium may be informed regarding the presence of an embryo. A specific protein of embryonic origin called trophoblastin, produced before day 14 of pregnancy (or postovulation) in both sheep and cattle, is of immunological interest for the establishment of pregnancy; it has a close structural relationship to the molecule interferon. Movement of the embryo(s) in the tract is also important for pregnancy recognition. In the mare the embryo moves throughout both horns before being fixed at day 16. In pigs a minimal number of embryos need to be present (about four), presumably to occupy a sufficiently large area of the endometrium. Litter-bearing animals also use transuterine migration to maximize the opportunity for fetal development, a procedure that aids the recognition of pregnancy process. The end result is either suppression of PGF2α synthesis, as seen in the cow (Figure 38-1), or modification of the secretion mode (continuous instead of pulsatile), as seen in sheep. The absence of pulsatile secretion of PGF2α seems to be critical for the extension of the life span of the CL and the establishment of pregnancy in large domestic species.
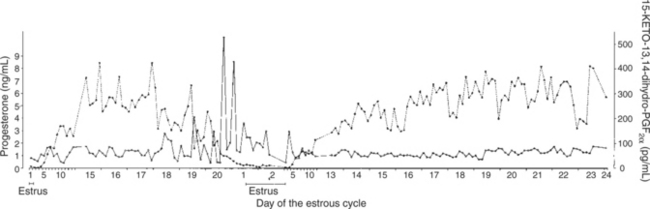
FIGURE 38-1 Relationship between prostaglandin release, as indicated by the measurement of 15-keto-13,14-dihydroprostaglandin F2α, and progesterone production by the corpus luteum during a nonfertile cycle and after a conception in the same cow.
(From Kindahl H, Edqvist LE, Bane A: Blood levels of progesterone and 15-keto-13,14-dihydroprostaglandin F-2α during the normal oestrous cycle and early pregnancy of heifers, Acta Endocrinol 82:134, 1976.)
In the cat the CL lasts for 35 to 40 days after ovulation regardless of the presence of pregnancy, and thus early modification of luteal activity is not essential for the establishment of pregnancy. Implantation occurs at about day 13, which allows the fetoplacental unit to influence and extend luteal activity that is compatible with pregnancy maintenance. The luteotropic hormone that is responsible for luteal maintenance in the cat is not known. One hormone that likely synergizes with progesterone for the support of pregnancy is relaxin, a placental hormone produced in the cat beginning at about day 20 of gestation (see later discussion).
The dog does not extend its luteal phase during pregnancy; the luteal phase in the nonpregnant animal is often slightly longer (70 days) than in pregnant animals. Nevertheless, enhancement of luteal activity occurs through a placental luteotropin, likely relaxin, with progesterone secretion enhanced beginning at about day 20 of gestation or a few days after implantation. Early in the luteal phase, luteal function in the bitch is likely autonomous. During the second half of the luteal phase, luteinizing hormone (LH) and prolactin are likely luteotrophs (Figure 38-2).
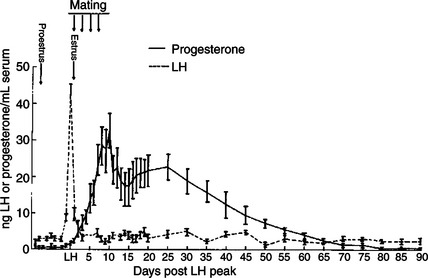
FIGURE 38-2 Luteinizing hormone (LH) and progesterone concentrations during pregnancy in nine dogs. Vertical bars represent the standard error of the mean.
(From Smith MS, McDonald LE: Serum levels of luteinizing hormone and progesterone during the estrous cycle, pseudopregnancy and pregnancy in the dog, Endocrinology 94:404, 1974. Copyright © by The Endocrine Society.)
The rescue of the CL at the onset of pregnancy in primates involves the production of a luteotropin called chorionic gonadotropin (CG; for humans, hCG), which is produced by trophoblastic cells (syncytiotrophoblasts) of the embryo (Figure 38-3). In order for trophoblast tissue to produce CG, it must have intimate contact with the interstitium of the endometrium. This contact occurs by a type of implantation called interstitial, in which the embryo penetrates the endometrium at about 8 to 9 days after fertilization in humans and nonhuman primates. Secretion of CG begins 24 to 48 hours after implantation, with immediate enhancement of luteal progesterone production. Rescue of the CL in human pregnancy occurs as late as 4 to 5 days before the end of the luteal phase.
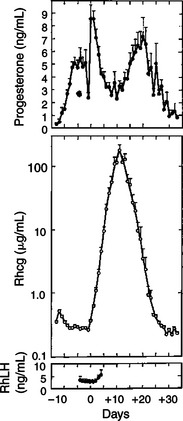
FIGURE 38-3 Summarization of 15 early pregnancies in normal rhesus monkeys normalized to the day of corpus luteum rescue (day 0). Points are means plus or minus standard error. Note the temporal relationship between luteal progesterone production (before day +10) and chorionic gonadotropin output. RhCG, Rhesus chorionic gonadotropin; RhLH, rhesus luteinizing hormone.
(From Knobil E: On the regulation of the primate corpus luteum, Biol Reprod 8:246, 1973.)
As indicated, interstitial implantation is essential to the development of pregnancy in primates. Implantation is less invasive in the dog and cat, with the type termed eccentric. In the large domestic species, “invasion” of the endometrium is minimal; implantation occurs within special endometrial protrusions called caruncles in ruminants and by relatively minor villus invasion of the endometrium in horses and pigs. Domestic animals depend more on uterine secretions for the support of pregnancy than do primates. For cattle and horses the first indications of implantation begin about days 25 to 30, and another week to 10 days likely passes before a significant amount of embryonic nutrition is obtained through the implantation site. Subclinical uterine infections, or an inadequate number of endometrial glands, can interfere with the establishment of pregnancy in the species in which a long interval exists from fertilization to implantation. The cervix forms an important barrier to contamination of the uterine lumen in both the nonpregnant and the pregnant animal; in the latter the cervix becomes sealed.
The Placenta Acts as an Endocrine Organ
Besides the essential role of providing nutrients and oxygen for embryonic metabolism, the placenta functions as an endocrine organ. One of the most important functions of the placenta is the production of progesterone. In primates this function is established early in gestation, and the placenta likely can maintain pregnancy within 2 to 3 weeks after implantation in primates. Placental production of sufficient progesterone to maintain pregnancy occurs later in domestic animals (sheep, day 50 of 150-day gestation; horse, day 70 of 340-day gestation; cat, day 45 of 65-day gestation); in some species the placenta never produces enough progesterone to support pregnancy (cattle, goats, pigs).
The production of estrogen, in contrast to that of progesterone, requires interaction between the fetus and the placenta. This interaction has been best described in primates, in particular by the experiments of the Hungarian immigrant to Sweden Dycfaluszy. He and his co-workers found that the primate placenta is unable to produce estrogen from progesterone even though the steroids are only separated by androgens in the steroid biochemical synthetic pathway (see Figure 38-5). The placenta simply does not possess the enzymes necessary for the conversion of progesterone to androgens. Therefore a system has evolved in which the placenta supplies pregnenolone, the immediate precursor of progesterone, to the fetus, and the fetal zone of the adrenal cortex transforms pregnenolone to a C-19 androgen, dehydroepiandrosterone. This is returned to the placenta, which is able to convert dehydroepiandrosterone to an estrogen. In humans the primary estrogen of pregnancy is estriol. Because the fetus is involved in the production of estriol, the well-being of the fetus can be judged by determining estriol concentrations in the plasma of the mother.
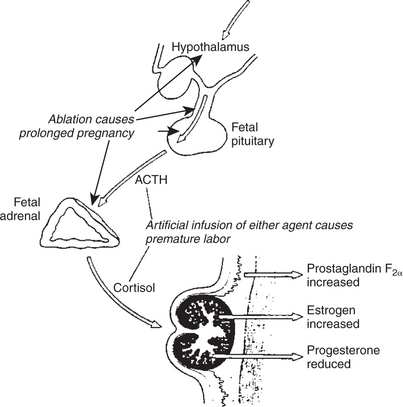
FIGURE 38-5 Diagrammatic summary explaining how the fetal lamb controls the onset of labor. Experimental procedures that lengthen or shorten pregnancy are shown. ACTH, Adrenocorticotropic hormone (corticotropin).
(Redrawn from Liggins CG: The foetal role in the initiation of parturition in the ewe. In Wolstenholme GEW, O’Connor M, editors: Foetal autonomy, London, 1969, Churchill Livingstone.)
The production of estrogen in the mare also involves an interaction between the placenta and fetus (Figure 38-4). From the work of Pashen and Allen, we know that the fetal gonads replace the fetal adrenals in primates as the key fetal endocrine organ involved in the cooperative synthesis of estrogen. The interstitial cells of the gonads appear to be the interactive cells, with fetal gonads enlarging to a size greater than the maternal gonads during the latter part of gestation. The production of estrogens during pregnancy in other domestic species, occurring relatively late in gestation, may involve the development of placental enzymes that allow progesterone to be metabolized to estrogens without the direct intervention of a fetal endocrine organ. (Fetal cortisol, however, is important for the induction of these placental enzymes, particularly in sheep; see next section.)
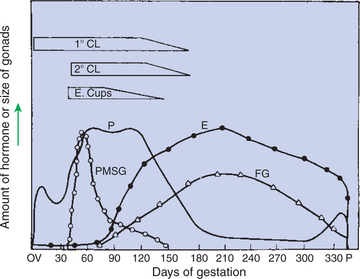
FIGURE 38-4 Summary of the temporal relationships among changes in hormonal concentrations and morphological changes throughout the gestational period of the mare. 1° CL, Primary corpus luteum; 2° CL, secondary corpora lutea; E, estrogens; E. Cups, endometrial cups; FG, fetal gonads; P, progesterone; PMSG, pregnant mares’ serum gonadotropin (equine chorionic gonadotropin).
(From Daels PF, Hughes JP, Stabenfeldt GH: Reproduction in horses. In Cupps PT, editor: Reproduction in domestic animals, ed 4, New York, 1991, Academic Press.)
The protein hormones that are produced during pregnancy tend to be of placental origin. For example, relaxin is a hormone produced by the placenta in the cat, dog, and horse beginning at about days 20, 20, and 70, respectively. Besides its importance for preparing the soft tissues of the pelvic canal for passage of the fetus at birth (see Parturition), relaxin may be important for the support of pregnancy through a synergistic action with progesterone. In exception to the general rule of protein hormone production by the placenta, relaxin is produced by the CL in the pig, cow, and primates during pregnancy, with prepartum release occurring in conjunction with luteolysis.
The only CG identified in domestic animals to date is equine CG (eCG, formerly called “pregnant mares’ serum gonadotropin” by its discoverer, Harold Cole) (see Figure 38-4). The eCG is produced by trophoblast cells that initially form as a band on the chorion (chorionic girdle), detach themselves around day 35 of pregnancy, penetrate the endometrium, and form associations of cells called endometrial cups. The eCG enhances progesterone production by the primary CL of pregnancy and aids in the formation of additional (secondary) CL through the luteinization, or ovulation, of preformed follicles. The essentiality of eCG for pregnancy maintenance is not known, because the primary CL is adequate for maintaining pregnancy.
Placental lactogen is another placental protein hormone. Its production increases in primates as CG secretion wanes during pregnancy. Placental lactogen has been reported in goats and sheep, with secretion increasing during the latter part of gestation. The hormone appears to have both somatotropic and lactogenic effects on the basis of growth hormone–like and prolactin-like properties. In dairy cattle, for example, placental lactogen may be important for mammary gland alveolar development, setting the stage for the next lactation. Another hormone whose production is increased during pregnancy, prolactin, also is important for alveolar development during the prepartum period. Prolactin is not a hormone of placental origin; prolactin increases during the latter part of gestation due to the effect of estrogen on its release from the adenohypophysis.
PARTURITION
Fetal Cortisol Initiates Delivery Through Increased Secretion of Estrogen and Thus Prostaglandin F2α
During pregnancy the uterus progressively enlarges and stretches because of the growing fetus. Progesterone plays an important role in maintaining the quiescence of the myometrium as well as promoting a tightly contracted cervix. During the latter part of gestation, estrogen begins to influence uterine muscle by stimulating the production of contractile protein and the formation of gap junctions; the former increases the contractile potential of the uterus, and the latter facilitates the contractile process through increased communication among smooth muscle cells. Thus, important changes that set the stage for parturition begin weeks before the actual process begins. In the end, the uterus is converted from a quiescent to a contractile organ, and importantly, the cervix relaxes and opens to allow the fetus to be delivered.
The most important question about parturition concerns what initiates the process. In domestic animals, maturation of the fetus eventually brings about changes that initiate the delivery process. The key organ system of the fetus responsible for initiating the process is the fetal adrenal cortex, with the hypothalamus and adenohypophysis playing important supporting roles. This concept came from the work at the University of California (UC)–Davis by Liggins and Kennedy, who showed that destruction of the anterior pituitary of the sheep fetus resulted in prolongation of gestation; Drost subsequently found the same results after fetal adrenalectomy. Critical changes in cortisol secretion by the fetus eventually result in the synthesis and release of PGF2α from the uterus, which produces muscle contraction and relaxation of the cervix. The following details of the initiation of parturition emphasize ruminants. It is unclear if elevated cortisol levels contribute to the initiation of parturition in the dog.
The maturation of the fetal adrenal cortex is of critical importance in the initiation of parturition. The adrenal cortex likely becomes progressively sensitive to fetal adrenocorticotropic hormone (ACTH, corticotropin) (Figure 38-5). The time of adrenal maturation is under fetal genetic control, as shown by studies conducted on fetal lambs of different breeds in the same uterus (produced by embryo transfer) in which the prepartum initiation of cortisol production occurred at times that were characteristic (and different) for the breed. Fetal cortisol induces placental enzymes (17-hydroxylase and C17-20 lyase) that direct steroid synthesis away from progesterone to estrogen. This process occurs at different times prepartum in domestic species, beginning at prepartum days 25 to 30 in cattle, 7 to 10 in pigs, and 2 to 3 in sheep. The end result of increased estrogen secretion is the secretion of prostaglandins, particularly PGF2α. PGF2α is the pivotal hormone for the initiation of parturition; once its secretion begins, the acute phase of delivery is activated. The role of oxytocin in the initiation of delivery is not certain; it likely complements PGF2α once the delivery process has started.
The synthesis of PGF2α is thought to come about through increased availability of the substrate arachidonic acid, which is the main rate-limiting step in the synthesis of PGF2α. Estrogens are proposed to influence the system by making available the enzyme phospholipase A, a membrane-bound lysosomal enzyme that initiates the subsequent hydrolysis of phospholipids and release of arachidonic acid. This likely results from an increasing estrogen/progesterone ratio, with progesterone initially stabilizing, then estrogens destabilizing, lysosomal membranes. The end result is increased availability of arachidonic acid for the synthesis of PGF2α. The onset of PGF2α synthesis results in the immediate release of the hormone because PGF2α is not synthesized and stored. The critical effect of PGF2α on the myometrium is to release intracellular calcium ion, which binds to actin and myosin to initiate the contractile process. Prostaglandins, both PGE and PGF2α, also have important effects on the cervix, which allow it to relax and dilate, permitting the passage of the fetus. The end result is a direct effect of PGF2α on the intracellular matrix of the cervix in which there is a loss of collagen with a concomitant increase in glycosaminoglycans, the latter affecting the aggregation of collagen fibers.
In some animals, such as the cow, goat, dog, and cat, PGF2α synthesis and release initiate regression of the CL beginning 24 to 36 hours before delivery, with complete withdrawal of progesterone occurring 12 to 24 hours before delivery. Although essential for delivery in these species, progesterone withdrawal per se does not initiate delivery; it is the release of PGF2α that both causes luteolysis and drives myometrial contractions.
In the mare, as in primates, delivery occurs even though progesterone concentrations remain elevated during the process. In this situation, PGF2α is able to overcome the suppressive effects of progesterone on myometrial activity. For animals dependent on placental production of progesterone for pregnancy maintenance, it is not possible to turn off one function (i.e., steroid synthesis) and still continue with other functions that are necessary for the support of the fetus through the time of delivery.
Oxytocin is also important to the delivery process (Figure 38-6). Estrogen induces oxytocin receptor formation in the myometrium. Recent information indicates that significant amounts of oxytocin are released only with the entry of the fetus into the birth canal. Oxytocin release occurs through the Ferguson reflex. The afferent arm of the reflex involves passage of impulses through sensory nerves in the spinal cord to the appropriate nuclei in the hypothalamus; the efferent arm involves transport of oxytocin from the neurohypophysis by the vascular system. Oxytocin is synergistic with PGF2α in promoting contraction of the uterus.
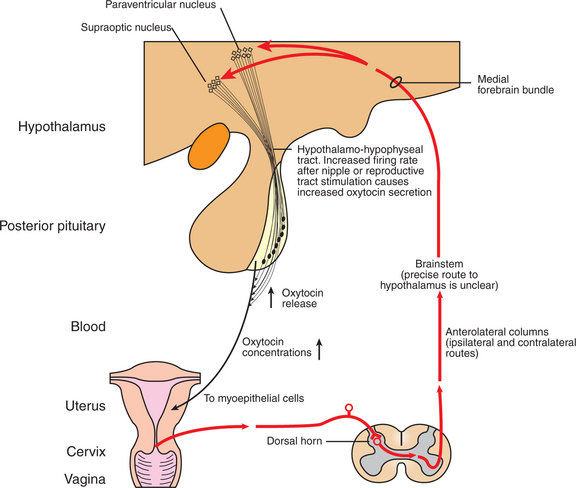
FIGURE 38-6 The neuroendocrine reflex (Ferguson reflex) underlying oxytocin synthesis and secretion.
(From Johnson M, Everitt B, editors: Essential reproduction, ed 3, London, 1988, Blackwell Scientific.)
As noted earlier, a hormone important for the preparation of parturition is relaxin. This hormone was first identified as responsible for the separation of the pubic symphysis through relaxation of the interpubic ligament. Relaxin causes the ligaments and associated muscles surrounding the pelvic canal to relax, which allows the fetus to expand the pelvic canal to its fullest potential. In the mare, a well-defined area of muscle softening can be discerned on the midline from the top of the croup through the ventral commissure of the vulva. In the cow, muscles posterior to the hip become relaxed to the point that they undulate as the animal walks in the final 24 hours before parturition. In the cow and pig, the CL is the source of relaxin. In both these species the prepartum release of PGF2α causes luteolysis, with a concomitant decline in progesterone production and the release of preformed relaxin. In other domestic species, such as cats, dogs, and horses, the source of relaxin is the placenta. In these species, significant relaxin production begins during the first part of gestation, with values sustained through parturition. A relaxin assay has been developed for the diagnosis of pregnancy in the dog, with good accuracy after 25 days of gestation. Relaxin may be important in these species for the maintenance of pregnancy in synergism with progesterone (see Figure 38-2).
The first stage of parturition involves presentation of the fetus at the internal os of the cervix. This likely results from increased myometrial activity caused by PGF2α release. Once the cervix opens and the fetus passes into the pelvic canal, myometrial contractions become less important for delivery of the fetus; abdominal press, accomplished by closure of the epiglottis and contraction of maternal abdominal muscles, becomes the main force involved in the delivery process. The actual delivery process is called the second stage of parturition.
The third stage of parturition involves the delivery of the fetal membranes. In litter-bearing animals, such as the cat, dog, and pig, the placental membranes are delivered often with, or immediately after, the appearance of each fetus. In single-bearing species, the placenta may be delivered immediately or within a few hours. From studies done on the mare at UC–Davis, we know that major, sustained surges of PGF2α occur in the immediate postpartum period and are important for expulsion of placental membranes and reduction of uterine size through myometrial contraction. PGF2α is likely the most important component of uterine size reduction in the immediate postpartum period for all domestic species. This can be inferred from the episodes of discomfort that parturient animals undergo during the hours immediately after delivery.
The neonate must make a major physiological adjustment to life on the outside. The major change involves the vascular system, in particular the respiratory system. During fetal life, blood bypasses the lungs (except for the perfusion of lung tissue in support of development) by two routes: through the ventricles by way of the foramen ovale, and from the pulmonary artery to the aorta by the ductus arteriosus. The foramen ovale is closed functionally at birth by a flap of tissue in the left ventricle through the development of higher pressures within the left versus the right ventricle. Although the ductus arteriosus immediately constricts at birth, it requires months before it is completely closed. This course of closure is also true for the ductus venosus, which serves as a hepatic shunt during fetal life. The rapid conversion from a fluid to a gaseous environment, as occurs at birth, is a truly remarkable adaptation.
CLINICAL CORRELATIONS
Prolonged Gestation
History.
You are called to examine a purebred Holstein cow that is 12 days overdue compared with the herd gestation average of 280 days. She was artificially inseminated, was diagnosed pregnant 35 days later, and has not been observed in estrus since insemination. You inquire about the presence of bulls on the dairy farm, but there are none.
Clinical Examination.
The cow has a greatly enlarged abdomen. On palpation of the uterus per rectum, you find the presence of a large calf. The cow certainly appears to be term as far as the size of the calf. You are puzzled, however, by the lack of colostrum in the udder.
Comment.
The history and physical examination findings are compatible with an animal that has a fetus that is defective in terms of the initiation of parturition. A normal fetal hypothalamic-pituitary-adrenocortical system is essential for the production of cortisol, which initiates the delivery process. In the cow, this can begin 3 to 4 weeks prepartum, with fetal cortisol directing the increased production of estrogen; this in turn eventually initiates PGF2α synthesis and release. The deficit could be caused by a malformed adrenal gland, pituitary, or hypothalamus. In one syndrome described for Holsteins, the critical defect was a lack of corticotropin-producing cells in the pituitary, which led to inadequate stimulation of the adrenal cortex and inadequate fetal cortisol production. The lack of lactogenesis reflects that the endocrine changes beginning 3 to 4 weeks prepartum as a prelude to delivery are also important for lactogenesis, and in their absence, colostral formation is delayed.
Treatment.
The animal can respond to glucocorticoids, with delivery usually occurring 2 to 3 days later. The placenta is normal in this situation, and the systemic administration of glucocorticoids substitutes for fetal cortisol in initiating the endocrine events that lead to parturition. Lactogenesis is usually initiated by glucocorticoid treatment, although the process is usually less advanced than that expected at normal delivery. Because the calf continues to grow in utero in this syndrome, it is often too large to be delivered per vaginam, and a cesarean section may have to be performed 2 to 3 days after treatment in concert with dilation of the cervix.
You need to tell the owner that the calf will likely not survive because of inadequate adrenal secretion. If the calf were an extremely valuable bull prospect, one could administer both glucocorticoids and mineralocorticoids for a number of months with the hope the animal would eventually be able to take over its own adrenal support (this actually occurred in one case at the University of California–Davis). It would be questionable, however, to initiate treatment of the calf on the basis that the disease is an autosomal recessive inherited condition.
Austin CR, Short RV, eds. Reproduction in mammals, vols 1-6. Cambridge, UK: Cambridge University Press, 1986.
Concannon PW, Morton DB, Weir BJ. Dog and cat reproduction, contraception and artificial insemination. J Reprod Fertil Suppl. 1989;1:39.
Cupps PT, ed. Reproduction in domestic animals, ed 4, New York: Academic Press, 1991.
Feldman EC, Nelson RW. Canine and feline endocrinology and reproduction. Philadelphia: Saunders, 2004.
Hafez ESE, Hafez B. Reproduction in farm animals, ed 7, Baltimore: Lippincott Williams & Wilkins, 2000.
Johnson MH, Everitt BJ. Essential reproduction, ed 5, London: Blackwell Scientific, 2000.
Lennoz-Roland M: Practical uses of aglepristone: review of a recent expert meeting. Presented at 5th Biannual Congress, European Veterinary Society for Small Animal Reproduction (EVSSAR), Budapest, Hungary, 2006.
Neill JD, ed. Knobil and Neill’s physiology of reproduction, ed 3, vols 1 and 2. Philadelphia: Elsevier, 2005.
Olson PN, Nett TM, Bowen RA, et al. Endocrine regulation of the corpus luteum of the bitch as a potential target for altering fertility. J Reprod Fertil Suppl. 1989;39:27.
Pineda MH, Dooley MP. McDonald’s veterinary endocrinology and reproduction, ed 5, Ames: Iowa State University Press, 2003.
Silva LDM, Verstegen JP. Comparisons between three different extenders for canine intrauterine insemination with frozen-thawed spermatozoa. Theriogenology. 1995;44:571.
Van der Weyden GC, Taverne MA, Dieleman SJ, et al. Physiological aspects of pregnancy and parturition in dogs. J Reprod Fertil Suppl. 1989;39:211.
PRACTICE QUESTIONS
1. Active rescue of luteal activity through suppression of pulsatile prostaglandin synthesis and release by the production of embryonic signals must occur in which of the following species in order for a developing pregnancy to have the early progestational support essential for pregnancy maintenance? (Select more than one letter.)
2. In primates it has been established that estrogen production during much of pregnancy is a cooperative venture between fetal adrenals and the placenta. The domestic species most extensively studied in this regard is the horse. In this species the main two interactive organs involved in the synthesis of estrogen during pregnancy are the placenta and the:
3. Which of the following hormones initiates the final process that eventually leads to parturition?
4. The hormone that initiates the myometrial contractile process that acutely initiates parturition is:
5. The hormone released by the passage of the fetus into the pelvic canal through the cervix is: