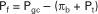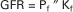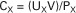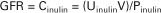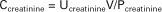Chapter 41 Glomerular Filtration
1. Introduction to the physiology of the kidney.
2. The glomerulus filters the blood.
3. The structure of the glomerulus allows efficient, selective filtration.
4. Glomerular filtration rate is determined by the mean net filtration pressure, permeability of the filtration barrier, and area available for filtration.
5. The filtration barrier is selectively permeable.
6. Glomerular filtration rate is regulated by systemic and intrinsic factors.
7. Glomerular filtration rate is measured by determining the plasma clearance rate of a substance.
Introduction to the Physiology of the Kidney
The kidney has diverse roles in maintaining homeostasis. In mammals the two kidneys receive approximately 25% of the cardiac output. The kidneys filter the blood and thereby excrete metabolic waste, while retrieving the filtered substances that are needed by the body, including low-molecular-weight proteins, water, and electrolytes. The kidneys respond to water, electrolyte, and acid-base disturbances by specifically altering the rate of reabsorption or secretion of these substances. The kidneys also produce hormones that regulate systemic blood pressure and red blood cell production.
These myriad functions are accomplished by an extensive variety of cell types, each with specific responses to direct and indirect signals, arranged in a particular pattern to form the functional unit of the kidney, the nephron. The nephron is composed of the glomerulus, where the blood is filtered, and its associated renal tubule segments, where filtered substances are absorbed from, and plasma components are secreted into, the tubule fluid. In the renal cortex the nephrons merge into the collecting duct system, which traverses the kidney and empties into the renal pelvis. Figure 41-1 provides an overview of the anatomical arrangement of nephrons within the kidney and the major functions of the nephron and collecting duct segments.
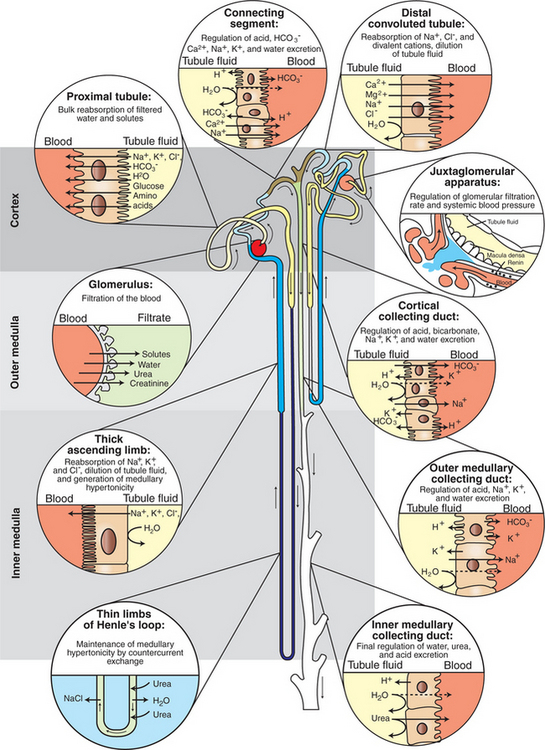
FIGURE 41-1 Schematic illustration of juxtamedullary and superficial nephrons, listing functions of the segments of nephron and collecting duct. The glomerulus of a juxtamedullary nephron is located deep in the cortex near the corticomedullary junction. The thin limb of Henle’s loop extends deep into the inner medulla. The glomerulus of a superficial nephron is located in the outer cortex, and Henle’s loop extends only into the outer medulla. Arrows indicate the direction of tubule fluid flow. Segments are numbered in sequential order of modification of the tubule fluid, beginning with the glomerulus.
(Modified from Madsen KM: Anatomy of the kidney. In Tisher CC, Wilcox CS, editors: Nephrology for the house officer, Baltimore, 1989, Williams & Wilkins.)
Most of our knowledge of renal physiology has been obtained from experimental evidence from the mouse, rat, and rabbit. Our understanding of renal physiology will continue to evolve as more information is gathered.
The Glomerulus Filters the Blood
The first step in renal function is filtration of the blood. Filtration takes place in the glomerulus, which is a network of capillaries that retains cellular components and medium- to high-molecular-weight proteins within the vasculature while extruding a fluid nearly identical to plasma in its electrolyte and water composition. This fluid is the glomerular filtrate; the process of its formation is glomerular filtration.
The rate of glomerular filtration is a renal function parameter that is frequently evaluated in clinical practice. The glomerular filtration rate (GFR) is expressed as milliliters of glomerular filtrate formed per minute per kilogram of body weight (mL/min/kg). To understand GFR, it may help to think of these numbers in more tangible terms. An average-size beagle of 10 kg body weight with a typical GFR of 3.7 mL/min/kg would produce approximately 37 mL of glomerular filtrate per minute, or 53.3 L (~14 gallons) of glomerular filtrate per day, almost 27 times the beagle’s extracellular fluid volume.
The Structure of the Glomerulus Allows Efficient, Selective Filtration
The glomerular tuft is composed of a network of capillaries (Figure 41-2). In mammals, blood from the renal artery is delivered to the afferent arteriole, which divides into numerous glomerular capillaries. The capillaries coalesce to form the efferent arteriole, which conducts the filtered blood away from the glomerulus (Figure 41-3). Birds have three pairs of renal arteries: anterior, middle, and posterior. Avian kidneys contain both mammalian-type and reptilian-type nephrons; in glomeruli of reptilian-type nephrons, the capillaries have few branches.
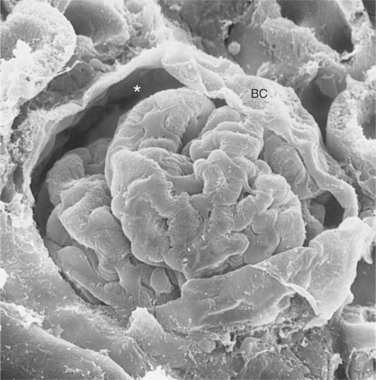
FIGURE 41-2 Scanning electron micrograph of rat glomerulus. The glomerular tuft is a complex network of capillaries that is encased in visceral epithelial cells and Bowman’s capsule (BC). Between the visceral epithelial cells and BC is Bowman’s space (asterisk), where the glomerular filtrate is collected and delivered to the proximal tubule. (Magnification ″600.)
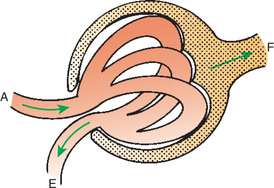
FIGURE 41-3 Schematic illustration of glomerulus. The afferent arteriole (A) carries blood to the glomerulus and subdivides into numerous glomerular capillaries. Water and solutes cross the glomerular capillary wall into Bowman’s space, forming the glomerular filtrate (F, stippled area), which flows into the proximal tubule. The glomerular capillaries coalesce, and the filtered blood leaves the glomerulus through the efferent arteriole (E).
The glomerular tuft is encased within Bowman’s capsule, which is lined with a single layer of epithelium. The area between the glomerular tuft and Bowman’s capsule is known as Bowman’s space and is the site of collection of the glomerular filtrate, which is funneled directly into the first segment of the proximal tubule.
The structure of the glomerular capillaries is important in determining the rate and selectivity of glomerular filtration. The wall of the capillary consists of three layers: the capillary endothelium, the basement membrane, and the visceral epithelium (Figure 41-4). The capillary endothelium is a single layer of cells with cytoplasmic extensions that are pierced by fenestrae (windows). The endothelial fenestrae provide channels for the passage of water and noncellular components from the blood to the second layer of the glomerular capillary wall, the glomerular basement membrane. This is an acellular structure composed of various glycoproteins, including type IV and type V collagens, proteoglycans, laminin, fibronectin, and entactin. The glomerular basement membrane is arranged in three layers, presumably created during development by the fusion of the basement membranes of the endothelial and epithelial cell layers. The three layers are named according to both their density with respect to an electron beam and their relative position. As shown in Figure 41-4, the lamina densa (dense layer) is relatively dark because it is relatively resistant to the passage of electrons when viewed with a transmission electron microscope. The lamina densa is composed of tightly packed glycoprotein fibrils. It is sandwiched between the lamina rara interna (inside thin layer) on the endothelial side of the glomerular basement membrane and the lamina rara externa (outside thin layer) on the epithelial side of the glomerular basement membrane. The laminae rarae are composed of a loose network of glycoprotein fibrils.
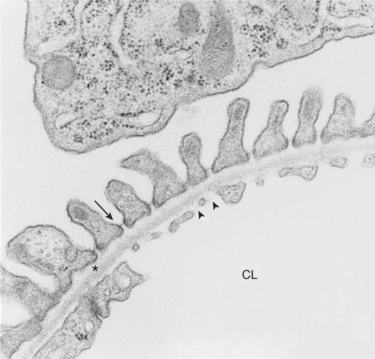
FIGURE 41-4 Transmission electron micrograph of rat glomerular capillary wall. The three main layers of the capillary wall are viewed in cross section. A single layer of glomerular capillary endothelial cells lines the capillary lumen (CL). Numerous fenestrae (arrowheads) pierce the endothelial cells. On the outside of the capillary is a single layer of visceral epithelial cells. At the top of the micrograph is a portion of the cell body of a visceral epithelial cell. The secondary foot processes are aligned along the capillary wall, and the spaces between them are spanned by the slit diaphragm (arrow). Between the endothelial and epithelial cell layers is the glomerular basement membrane, consisting of the electron-lucent lamina rara interna adjacent to the endothelial cells, the lamina densa (asterisk), and the lamina rara externa adjacent to the visceral epithelial cells. (Magnification ″19,000.)
The third compartment of the glomerular capillary wall is the visceral epithelium, which is a layer of intricate, interlocking cells called podocytes. Numerous long, narrow extensions, called primary and secondary foot processes, interdigitate with foot processes from other podocytes and wrap around the individual capillaries (Figure 41-5). Spanning the space between adjacent foot processes is the epithelial slit diaphragm (see Figure 41-4).

FIGURE 41-5 Scanning electron micrograph of the surface of rat glomerular capillaries viewed from Bowman’s space. The cell bodies (P) of visceral epithelial cells, or podocytes, nestle between the capillary loops. The primary foot processes (arrowheads) radiate outward and wrap around the capillaries. Secondary foot processes extend from the primary foot processes and interdigitate with secondary foot processes from other podocytes. (Magnification ″1500.)
Glomerular Filtration Rate Is Determined by the Mean Net Filtration Pressure, Permeability of the Filtration Barrier, and Area Available For Filtration
The glomerular capillary wall creates a barrier to the forces favoring and opposing filtration of the blood. The forces favoring filtration—that is, movement of water and solutes across the glomerular capillary wall—are the hydrostatic pressure of the blood within the capillary and the oncotic pressure of the fluid within Bowman’s space (the ultrafiltrate). Normally, the oncotic pressure of the ultrafiltrate is inconsequential because medium- to high-molecular-weight proteins are not filtered. Therefore the main driving force for filtration is the glomerular capillary hydrostatic pressure. Forces opposing filtration are the plasma oncotic pressure within the glomerular capillary and the hydrostatic pressure in Bowman’s space. Figure 41-6 illustrates the direction and magnitude of these forces under normal conditions.
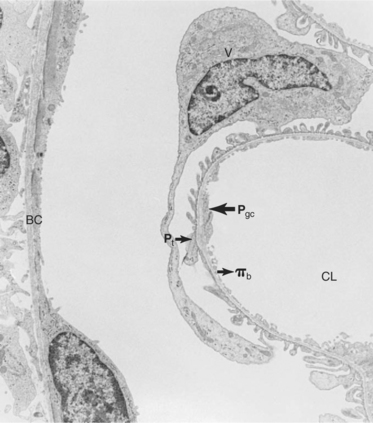
FIGURE 41-6 Transmission electron micrograph of rat glomerular capillary and Bowman’s capsule (BC) illustrating the forces favoring and opposing filtration. The main force favoring filtration is the hydrostatic pressure of the glomerular capillary (Pgc). The forces opposing filtration are the hydrostatic pressure of Bowman’s space (Pt) and the oncotic pressure of the blood (πb). CL, Capillary lumen; V, visceral epithelial cell. (Magnification ″2400.)
The net filtration pressure (Pf) at any point along the glomerular capillary is the difference between the capillary hydrostatic pressure (Pgc) favoring filtration and the capillary oncotic pressure (πb) plus hydrostatic pressure of the ultrafiltrate (Pt) opposing filtration. This relationship is expressed mathematically as follows:
As blood travels through the glomerular capillary, a large proportion of the fluid component of the plasma is forced across the capillary wall, whereas the plasma proteins are retained in the capillary lumen. Therefore the plasma oncotic pressure increases significantly along the capillary bed. At the same time, the loss of plasma volume along the capillary bed causes a decrease in the hydrostatic pressure in the capillary, although this change is small because of resistance in the efferent arteriole. The result is that the net filtration pressure tends to decrease along the capillary bed.
The GFR is the product of the mean net filtration pressure (Pf), the permeability of the filtration barrier, and the surface area available for filtration. The permeability of the filtration barrier is determined by the structural and chemical characteristics of the glomerular capillary wall. The product of the filtration barrier permeability and its surface area is the ultrafiltration coefficient (Kf). Thus the combined effects of the determinants of GFR are mathematically represented by the following equation:
The Filtration Barrier Is Selectively Permeable
In addition to determining the hydraulic permeability of the filtration barrier, the structural and chemical characteristics of the glomerular capillary wall establish the selective permeability (permselectivity) of the filtration barrier. The permselectivity of the filtration barrier is responsible for differences in the rate of filtration of blood components. Normally, all cellular components and plasma proteins the size of albumin molecules or larger are retained within the bloodstream, whereas water and solutes are freely filtered. In general, substances with a molecular radius of 4 nm or more are not filtered, whereas molecules with a radius of 2 nm or less are filtered without restriction.
However, characteristics other than size affect the ability of blood components to cross the filtration barrier. The net electrical charge of a molecule has a dramatic effect on its rate of filtration. The cationic (positively charged) form of many substances is more freely filtered than is the neutral form, which is more freely filtered than the anionic (negatively charged) form of the same molecule. For example, the cationic form of albumin is excreted at a rate approximately 300 times that of native albumin, which has a net negative charge. These differences are caused by a charge-selective barrier in the glomerular capillary wall that is created by negatively charged residues of glycoproteins incorporated in the glomerular basement membrane and coating the endothelial and epithelial cells. These fixed negative charges repel negatively charged plasma proteins and thus reduce their passage across the filtration barrier. The shape and deformability of the molecule also affect its ability to cross the filtration barrier. Neutral dextran, a long, flexible molecule, crosses the filtration barrier approximately seven times as easily as horseradish peroxidase, a globular protein with a similar molecular radius and net charge.
Glomerular Filtration Rate Is Regulated by Systemic and Intrinsic Factors
The kidney normally maintains the GFR at a relatively constant level despite changes in systemic blood pressure and renal blood flow. The GFR is maintained within the physiological range by renal modulation of systemic blood pressure and intravascular volume and by intrinsic control of renal blood flow, glomerular capillary pressure, and Kf. Renal effects on systemic blood pressure and volume are mediated primarily through humoral factors, particularly the renin-angiotensin-aldosterone system. Intrinsic control of glomerular capillary perfusion is mediated by two autoregulatory systems that control the resistance to flow in the afferent and efferent arterioles: the myogenic reflex and tubuloglomerular feedback.
The renin-angiotensin-aldosterone system is an important regulator of GFR and renal blood flow. Renin is a hormone produced by specialized cells of the wall of the afferent arteriole, the granular extraglomerular mesangial cells, which are specialized juxtaglomerular cells. Renin release is stimulated by a decrease in renal perfusion pressure, most often caused by systemic hypotension. Renin catalyzes the transformation of angiotensinogen produced by the liver to angiotensin I. Angiotensin I is converted to the more active angiotensin II by angiotensin-converting enzyme (ACE), which is located primarily in the vascular endothelium of the lung. ACE is also present in the vascular endothelium of the kidneys and other organs, and thus the conversion of angiotensin I to angiotensin II may occur in extrapulmonary sites as well.
Angiotensin II is a potent vasoconstrictor and thus directly increases systemic blood pressure and renal perfusion pressure. Angiotensin II directly activates sodium uptake in both the proximal tubule and the collecting duct, and it stimulates the release of aldosterone from the adrenal gland and vasopressin from the pituitary. Thus, angiotensin II directly and indirectly enhances salt and water retention, intravascular volume increase, and vascular resistance, all of which contribute to increased systemic blood pressure and renal perfusion pressure. Renin release is suppressed by both the improved renal perfusion and the elevated plasma angiotensin II, creating a negative-feedback system that maintains renal perfusion and GFR within the physiological range (Figure 41-7).
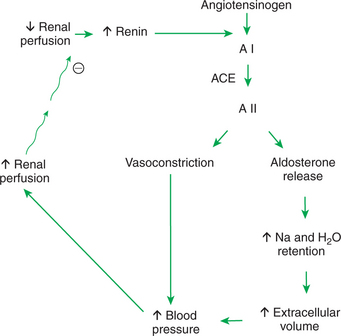
FIGURE 41-7 Schematic illustration of renin-angiotensin-aldosterone system. Circled minus sign represents inhibition. A I, Angiotensin I; ACE, angiotensin-converting enzyme; A II, angiotensin II.
Angiotensin II also stimulates production and release of at least two vasodilative renal prostaglandins: prostaglandin E2 (PGE2) and prostaglandin I2 (prostacyclin). This response is an important moderator of the renin-angiotensin-aldosterone system. The intrarenal production of these vasodilators counteracts the vasoconstrictive effect of angiotensin II on the intrarenal vasculature and helps to maintain renal vascular resistance at normal or near-normal levels. Without this protective effect, generalized vasoconstriction would result in reduced renal blood flow and GFR, despite an elevation of systemic blood pressure.
Within the kidney itself, there appears to be direct control of glomerular capillary perfusion by two systems previously mentioned: the myogenic reflex and tubuloglomerular feedback. A mechanism of autoregulation of renal blood flow and GFR was proposed after the observation that glomerular arterioles respond to changes in arteriolar wall tension. This response is called the myogenic reflex; the result is almost immediate afferent arteriolar constriction after an increase in arteriolar wall tension and arteriolar dilation after a decrease in arteriolar wall tension. Arcuate and interlobular arteries respond similarly. Thus, arteriolar dilation and constriction respectively decrease and increase the resistance to blood flow in the afferent arteriole. These changes in vascular resistance contribute to maintenance of GFR and renal blood flow at a constant level, despite marked alterations in the blood pressure in the renal artery. This reflex is independent of renal innervation but may be influenced by chemical mediators, such as nitric oxide.
The second intrinsic control mechanism is tubuloglomerular feedback. To understand this concept, it is important to review the anatomical arrangement of an individual nephron (see Figure 41-1). Specifically, recall that the distal tubule is closely associated with the glomerulus of the same nephron. An anatomically distinct cluster of epithelial cells, known as the macula densa, is located in the distal portion of the thick ascending limb of the loop of Henle. The macula densa is situated between the afferent and efferent arterioles and adjacent to the extraglomerular mesangial region. These four structures together are known as the juxtaglomerular apparatus, and they are closely related functionally as well as anatomically.
An increase in sodium chloride concentration in the tubule fluid at the macula densa causes a signaling cascade in the macula densa cells. This leads to generation of paracrine factors, such as nitric oxide, adenosine, and adenosine triphosphate (ATP), that suppress renin release from juxtaglomerular cells, increase resistance in the afferent arteriole, decrease glomerular capillary perfusion pressure, trigger mesangial cell contraction, and reduce Kf. These responses lead to reduced GFR in the individual nephron (single-nephron GFR), which prevents tubule fluid flow rates that exceed the tubule’s transport capacity and thus prevents excessive fluid and solute loss.
In addition, it is now evident that the endothelium itself contributes to local control of renal vascular tone by producing potent vasoconstrictors and vasodilators. Endothelium-derived constricting factors include the potent vasoconstrictor endothelin thromboxane A2 (a metabolite of arachidonic acid) and angiotensin II. Endothelium-derived relaxing factors include nitric oxide (NO), prostacyclin, and PGE2. Of these, NO was the first endothelium-derived vasodilating agent to be identified, and thus endothelium-derived relaxing factor is sometimes used as a synonym for nitric oxide. NO is now known to have important protective effects on the kidney. NO is produced in the kidney by oxidation of L-arginine, catalyzed by isoforms of NO synthase. NO prevents renal damage by quenching reactive oxygen species, thus inhibiting intrarenal vasoconstriction, glomerular hypertension, mesangial cell proliferation, and mesangial matrix production.
In addition to controls exerted by the kidney itself, systemic factors can contribute to changes in GFR. These include systemic control of blood volume and vessel tone. Many hormones regulate blood volume. Angiotensin II, aldosterone, and vasopressin (antidiuretic hormone) enhance water and solute reabsorption by the kidney and thus increase blood volume. Atrial natriuretic peptides, produced in the cardiac atria, cause both natriuresis (sodium wasting) and diuresis (water wasting) and thereby reduce blood volume.
Systemic factors that affect vessel tone also affect systemic blood pressure, renal perfusion, and ultrafiltration. Vasopressin and circulating catecholamines can cause systemic vasoconstriction and increase blood pressure. Beta-adrenergic stimulation can activate the renin-angiotensin system, and alpha-adrenergic stimulation can cause renal vasoconstriction, which can both reduce and redistribute renal blood flow. In addition to altering renal perfusion, vasoconstrictors can affect the other determinant of GFR, the ultrafiltration coefficient Kf. Vasoconstrictors may cause contraction of the mesangial cells within the glomerulus and thus reduce the area available for filtration. Because Kf is the product of the area available for filtration and the hydraulic permeability, mesangial cell contraction in vivo would reduce Kf and thus reduce GFR.
Insulin-like growth factor and high dietary protein levels can enhance GFR. Insulin-like growth factor increases GFR in kidneys of normal animals and after experimental renal ischemia. Chronically high dietary protein intake causes sustained increases in renal blood flow and GFR. Renal blood flow and GFR are transiently elevated after a single high-protein meal. These observations are clinically relevant in the management of chronic renal insufficiency and renal failure. Although it may seem desirable to increase GFR by any means in patients with chronic renal disease, in fact the increase in GFR resulting from some high-protein diets can cause more rapid progression of glomerular injury and renal failure in animals and in humans.
In birds the GFR is more variable than in mammals, but the regulatory mechanisms are not well understood. Birds, unlike mammals, exhibit intermittent filtration in reptilian-type glomeruli; this occurs during dehydration and decreases the GFR. This may result from the release of arginine vasotocin, the avian analogue of mammalian arginine vasopressin, which decreases GFR in birds by causing constriction of the afferent arteriole of reptilian-type nephrons. Although some authors report that a juxtaglomerular apparatus is present in avian kidneys, the macula densa is either absent or rudimentary, and tubuloglomerular feedback has not yet been demonstrated.
Glomerular Filtration Rate Is Measured by Determining Plasma Clearance Rate of a Substance
Both in experimental settings and in clinical practice, GFR is one of the most important parameters of renal function. Determination of GFR relies on the concept of clearance, that is, the rate the plasma is cleared of a substance. The rate of clearance is measured by the rate of elimination of a substance divided by its plasma concentration, mathematically expressed as follows:
where CX is the volume of plasma cleared of substance X per unit time, UX is the urine concentration of substance X, V is the volume of urine collected divided by the time period of the collection, and PX is the plasma concentration of substance X.
The net clearance rate of a substance is the sum of the rates of filtration and secretion minus the rate of reabsorption of the substance. To determine the filtration rate accurately, the rates of secretion and reabsorption must be determined or excluded from the equation. This is neatly done by using inulin as the substance for the measurement of clearance. Inulin is freely filtered by the glomerulus but is neither reabsorbed nor secreted by the renal tubule cells. Because of these properties, and because inulin is not produced by the body, the rate of its disappearance from the blood after intravascular injection is strictly related to the rate of glomerular filtration. Therefore, measurement of GFR can be expressed mathematically by the clearance equation, in which substance X is inulin:
where GFR is in milliliters per minute, Cinulin is the rate of clearance of inulin from the plasma in milliliters per minute, Uinulin is the inulin concentration in a urine sample collected over a time T in minutes, V is the volume of the urine collected over time T, and Pinulin is the mean plasma inulin concentration during time T.
Although the standard method of determination of GFR is by the rate of clearance of inulin from the blood, GFR can be measured in a variety of ways. In clinical situations the most widely used measure of glomerular filtration is endogenous creatinine clearance. Creatinine is a byproduct of muscle metabolism that is handled similar to inulin by the kidney. It is freely filtered, is not reabsorbed by the tubule, and, at least in the dog, is not secreted by the tubule. In some species, however, approximately 10% of the excreted creatinine is secreted by the tubule. Nevertheless, depending on the accuracy of the assay used for creatinine, the endogenous creatinine clearance test provides an estimate of GFR. In practice the measurement of endogenous creatinine clearance requires collecting the urine produced by a patient over a 24-hour period. The volume of urine produced over 24 hours is recorded, and the creatinine concentration is measured. The mean plasma concentration of creatinine is represented either by the plasma concentration measured at the midpoint of the collection period or by the mean of the plasma concentration measured at the beginning and end of the collection period. These values are used in the clearance equation as follows:
This results in an approximation of GFR in milliliters per minute. In veterinary medicine the GFR is better expressed on the basis of body weight or body surface area—that is, as milliliters per minute per kilogram or milliliters per minute per square meter—because of the large variation in size within individual species.
In birds, creatinine clearance cannot be used for determination of GFR because avian renal tubules can secrete creatinine when the plasma level is elevated and can reabsorb creatinine when the plasma level is normal.
CLINICAL CORRELATIONS
Chronic Renal Failure
History.
You examine a 15-year-old male Siamese cat. The owner reports that her cat is listless, inappetent, and thin. The cat has been drinking more water than usual lately, urinating large volumes, and vomiting frequently.
Clinical Examination.
The cat is very thin and moderately dehydrated. The mucous membranes are pale. Both kidneys are easily palpable and feel small, firm, and slightly irregular. The hematocrit is 22% (normal, 30%-42%), serum creatinine level is 8.7 mg/dL (normal, 0.5-1.2 mg/dL), and urine specific gravity is 1.012. The urine sediment is unremarkable.
Comment.
The cat has chronic renal failure, which is seen frequently in geriatric patients in small animal practice. The serum creatinine level is elevated because progressive loss of glomerular function has severely reduced the GFR, and creatinine is not cleared from the plasma normally. The urine is not concentrated in response to dehydration because tubular function is also compromised. The smallness of the kidneys is an indication of chronicity and is a result of gradual nephron loss and scarring. Anemia is common in chronic renal failure and results from many factors, including decreased production of erythropoietin by the kidney.
Treatment.
In veterinary medicine the treatment of chronic renal failure is usually supportive and symptomatic. This cat would probably benefit initially from rehydration with intravenous fluids and correction of electrolyte and acid-base disturbances as dictated by the serum chemistry profile. Chronic support may greatly improve the cat’s quality of life and slow the progression of disease. This should include a diet containing low total protein that is high in bioavailability, low sodium, and low phosphorus. Supplementing with the water-soluble vitamins may be beneficial. Anabolic steroids may help improve the anemia, although exogenous erythropoietin has become standard treatment in humans with anemia caused by chronic renal failure and is now being used in veterinary medicine as well.
Glomerulonephritis
History.
A client presents his 3-year-old, spayed female springer spaniel. He reports that the dog has not been eating well for several days and seems to tire easily.
Clinical Examination.
The dog seems bright and alert and is in good flesh. The only abnormality detected by physical examination is slight pitting edema in the distal extremities. The left kidney is palpable and feels smooth and of normal size. A urinalysis yields normal results except for 3+ protein (normal, negative to trace amounts) and the presence of a few red blood cell casts. A complete blood cell count is normal, and the only abnormality on a serum chemistry profile is a serum albumin level of 1.5 g/dL (normal, 2.3-4.3 g/dL).
Comment.
This dog has acute glomerulonephritis. Proteinuria is indicative of glomerular disease because normally the filtration barrier established by the glomerular capillary wall prevents the passage of proteins into the tubular fluid. When the glomerulus is damaged, it becomes leaky, and protein appears in the urine. The loss of albumin in this case appears to be marked because the serum albumin level has dropped below normal levels. The peripheral edema is probably caused by the hypoalbuminemia, and thus the lowered intracapillary oncotic pressure and leakage of fluid into the extravascular space.
In this case, acute glomerulonephritis is suspected because of the recent onset of clinical signs, the absence of renal failure, and the presence of red blood cell casts in the urine. Additional tests that are helpful in assessing the patient and guiding therapy include a 24-hour urine collection to measure the severity of the protein loss and an endogenous creatinine clearance test done at the same time to determine whether the GFR has been altered. A renal biopsy is necessary to verify the type and severity of glomerular injury. Potential causes of acute glomerulonephritis, such as recent or concurrent bacterial or viral infections or autoimmune diseases, should be explored. Renal ultrasound may provide additional information on the condition of the kidneys.
Treatment.
The treatment of glomerulonephritis varies. Occasionally, the initiating cause can be determined and removed. Some cases resolve spontaneously; at other times, various combinations of immunosuppressive and antiinflammatory agents are used to combat ongoing damage from immune complex deposition and glomerular inflammation. If either renal failure or pulmonary edema secondary to hypoalbuminemia is present, the hypoalbuminemia must be treated with plasma or other colloids to sustain the animal until the glomerular lesion is resolved or controlled. Frequent assessment of urine protein/creatinine ratios as well as serum creatinine is warranted to monitor disease progression.
Bell PD, Lapointe J, Peti-Peterdi J. Macula densa cell signaling. Annu Rev Physiol. 2003;65:481.
Brenner BM, ed. Brenner & Rector’s the kidney, ed 7 e-dition, Philadelphia: Saunders, 2004.
Deen WM, Lazzara MJ, Myers BD. Structural determinants of glomerular permeability. Am J Physiol Renal Physiol. 2001;281:F579.
Kone BC. Nitric oxide synthesis in the kidney: isoforms, biosynthesis, and functions in health. Semin Nephrol. 2004;24(4):299.
Schnermann J, Levine DZ. Paracrine factors in tubuloglomerular feedback: adenosine, ATP, and nitric oxide. Annu Rev Physiol. 2003;65:501.
Seldin SW, Giebish G. The kidney: physiology and pathophysiology, ed 3, Philadelphia: Lippincott Williams & Wilkins, 2000.
PRACTICE QUESTIONS
1. The major force favoring filtration across the glomerular capillary wall is the:
2. The glomerular filtration rate (GFR) is the:
3. In clinical practice the GFR is often estimated by determining the rate of creatinine clearance. The rate of creatinine clearance is the:
4. The two major characteristics that determine whether a blood component is filtered or retained in the capillary lumen are its: