Chapter 11 Disinfectants, Antiseptics, and Related Biocides
Disinfectants, antiseptics, and biocides are expected to play an even more important role in the future in controlling microbes in both the veterinary patient and hospital.1 When used properly, disinfectants, antiseptics, and biocides contribute to both the prevention and the treatment of disease. Despite the fact that there are as many as 300 biocidal products available, about 14 of these are in more than 90% of the registered products in the United States.2 The veterinary clinician need be familiar with relatively few biocidal products to make an informed decision regarding their selection and use.
Definitions
Definitions of appropriate terms, characteristics of disinfectants and antiseptics by chemical type, factors affecting disinfection and antisepsis, and disinfection and antiseptic practices germane to veterinary practice are reviewed in this chapter (Box 11-1). It is hoped that through greater understanding of the properties of disinfectants and antiseptics, veterinary clinicians will use them appropriately.
Box 11-1 Disinfection and Antisepsis Definitions
(From Block SS: Disinfection, sterilization, and preservation, ed 5, Philadelphia, 2001, Lippincott Williams & Wilkins; and Greene CE: Infectious diseases of the dog and cat, ed 3, St Louis, 2006, Saunders)
The distinction between disinfectants and antiseptics is not always clear (Table 11-1). Antiseptics are usually the weakest and least toxic of the surface antimicrobials.3 Antiseptics may be used on intact skin or mucous membranes before a surgical procedure or in the treatment of open wounds. Regardless of their use, antiseptics should exert a sustained effect against microorganisms without causing tissue damage.4 Although some specific biocides may be used as both disinfectants and antiseptics (e.g., alcohols and iodines), it is not generally recommended that an antiseptic be used for the purpose of disinfection, and vice versa.
Table 11-1 Categorization of Biocides: Disinfectants, Antiseptics, or Both
| Biocides Most Appropriately Used as Disinfectants | Biocides Most Appropriately Used as Antiseptics | Biocides Effective as Both Disinfectants and Antiseptics |
|---|---|---|
| Aldehyde compounds (formaldehyde and glutaraldehyde) | Chlorhexidine | Alcohols |
| Chlorine and chlorine compounds | Dilute sodium hypochlorite solution (Dakin’s solution) | Chloroxylenols |
| Ethylene oxide | EDTA | Iodines |
| Hydrogen peroxide | Iodophors | |
| Phenols | ||
| Quaternary ammonium compounds |
Characteristics of Disinfectants and Antiseptics by Chemical Type
The characteristics of the following 10 types of disinfectants, antiseptics, and biocides are presented: alcohols, aldehyde compounds (formaldehyde and glutaraldehyde), chlorhexidine, chlorine and chlorine compounds, ethylenediaminetetraacetate (EDTA), ethylene oxide, iodine and iodine compounds, peroxygen compounds (including hydrogen peroxide), phenols (including bisphenols and halophenols), and quaternary ammonium compounds. Mechanism of action (presumed or established), classification as to level of biocidal activity, commonly available preparations, efficacy, and uses are presented for each chemical type (Figure 11-1).
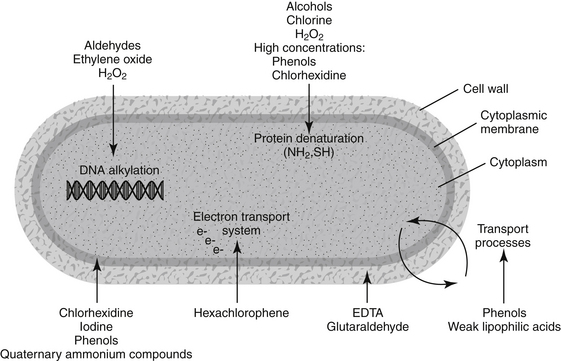
Figure 11-1 Diagram showing targets within the microbe for selected disinfectants, antiseptics, and biocides.
Alcohols
Alcohols possess the following features desirable for a disinfectant: bactericidal action against vegetative forms, relative inexpensivness, ease of availability, and relative nontoxicity when used topically.5 Alcohols are used alone or in combination with phenols, chlorhexidine, iodines, and quaternary ammonium compounds.6 Alcohols appear to exhibit their antimicrobial effect by denaturing proteins. Lysis of some microorganisms may occur, although the bacteriostatic action of alcohols is due to the inhibition of cell metabolites. Some water is required for alcohols to be most effective. Alcohols are considered to have intermediate-level biocidal activity. Two forms of alcohol are used most commonly: ethyl alcohol and isopropyl alcohol. Ethyl alcohol has enhanced virucidal properties and reduced toxicity compared with isopropyl alcohol, which has slightly greater bactericidal action.
When used alone, alcohols are more effective antiseptics than disinfectants. Alcohols are not good cleaning agents and are not recommended in the presence of physical dirt.7 Alcohols are widely used for both hard-surface disinfections and skin antisepsis.8 In appropriate concentrations, alcohols provide the most rapid and greatest reduction in microbial counts on intact skin. Alcohols should not be used in open wounds. Alcohols should be allowed to evaporate thoroughly from the skin to be fully effective and to decrease irritation.7 Because of their inability to destroy bacterial spores, alcohols are not recommended for disinfection of surgical instruments.
Aldehyde Compounds (Formaldehyde and Glutaraldehyde)
Formaldehyde (3% to 8% solutions) exhibits intermediate-level to high-level disinfection. It is sporicidal, with its mechanism of action being the ability to combine with protein, RNA, and DNA in the spore.9 Formaldehyde does not penetrate well, and its fumes are irritating.6 It is used infrequently as a disinfectant in veterinary hospitals.
Glutaraldehyde (1,5-pentanedial) has been used as a chemosterilizing agent for approximately 40 years.10,11 It displays potent bactericidal, fungicidal, virucidal, mycobactericidal, and sporicidal activity.11-13 Glutaraldehyde acts on proteins by denaturation and on nucleic acids by alkylation.14 It is classified as an intermediate-level to high-level biocide.
Factors that influence the activity of glutaraldehyde include time of contact, temperature, concentration, pH, and the presence of soiling material.12 Glutaraldehyde shows a very marked, temperature-dependent activity.12 The pH affects the stability and biocidal activity of glutaraldehyde. Glutaraldehyde is more stable at acid pH, but it is more active at alkaline pH (around 8 to 8.5). As pH increases, the number of reactive sites to which glutaraldehyde binds is increased, thus enhancing its lethal effect.12 At alkaline pH, glutaraldehyde penetrates more extensively into the spore, where it fixes the cortex.11 The negative effect of organic matter is more apparent when lower concentrations of glutaraldehyde are used. Alkaline glutaraldehyde (2%) takes longer to be effective in the presence of organic matter.6 Glutaraldehyde has a dual role against bacterial biofilms (a characteristic of some bacteria that makes them more resistant to disinfection): an ability to penetrate the biofilm and inhibit microbial cells protected by the film and an acceleration of the detachment rate of bacteria from the biofilm.12
Disinfectants containing 2% glutaraldehyde are considered high-level disinfectants, with recommended contact times of 10 to 30 minutes. Exposure times of 6 to 10 hours frequently result in sterilization. Glutaraldehyde is used widely as a disinfectant for heat-labile equipment (e.g., endoscopes). Glutaraldehyde disinfectants are not as noxious, irritating to the skin, or corrosive as formaldehyde; however, precautions should be taken with their use. Gloves, safety goggles, and proper ventilation are recommended to minimize risks to those disinfecting the equipment, and glutaraldehyde-disinfected equipment should be thoroughly rinsed with sterile water before use to reduce risk to the patient.11 Stabilized glutaraldehyde solutions are safe and effective preoperative skin antiseptics for elective clean-contaminated surgical procedures in dogs.15
Chlorhexidine
Chlorhexidine is a cationic bisbiguanide, not related to hexachlorophene, that was first synthesized in 1950.16 It is available as a solution and as a scrubbing agent. Chlorhexidine solution is used principally as a topical antiseptic on skin wounds and mucous membranes, but it is also used as a pharmaceutical preservative. Chlorhexidine scrub is used to preoperatively prepare the surgeon and patient. Chlorhexidine exhibits a broad spectrum of antibacterial activity, strong binding to the skin, ability to adsorb to negatively charged surfaces in the mouth (e.g., tooth and oral mucosa), persistence, low toxicity, and a minimal negative effect on activity by blood or other organic material.7 Chlorhexidine has its major antibacterial action by interference with the function of cellular membranes, with the primary site of action being the cytoplasmic membrane.8,13,14,16,17 Rupture of the cytoplasmic membrane of the microbe occurs without lysis of the cell wall. Chlorhexidine has low-level to intermediate-level biocidal activity.
Chlorhexidine has a very rapid bactericidal effect as well as persistence of action.14 It has limited fungicidal and virucidal properties.6 Chlorhexidine is more effective against gram-positive than gram-negative bacteria and exhibits a bacteriostatic effect against some bacteria.6,7 Acquired resistance to chlorhexidine has been observed, notably in staphylococci.8,18 The optimum range of pH for activity of chlorhexidine is 5.5 to 7. Chlorhexidine is incompatible with anionic detergents and inorganic anionic compounds; 6 thus standing soap lather should be removed by rinsing before chlorhexidine solution is applied to the skin. Chlorhexidine forms a precipitate when diluted with electrolyte solutions, but this precipitate does not affect antimicrobial activity.19
Chlorhexidine is available as an alcoholic solution (scrub) that is used in the preoperative skin preparation of the surgeon and patient and as an aqueous solution. Two preparations that are used commonly in veterinary hospitals are chlorhexidine gluconate (scrub) and chlorhexidine diacetate or gluconate (solution). There are few reports of adverse reactions with chlorhexidine. Chlorhexidine scrub should be used only on intact skin, never in wounds. Negligible absorption from the alimentary tract occurs, and the incidences of skin irritation and hypersensitivity are low. Chlorhexidine is ototoxic when placed in the middle ear cavity, and its use on the brain or meninges is contraindicated. In general, chlorhexidine solution (0.05%) is an effective and well-tolerated wound antiseptic in veterinary patients.19,20
Chlorine and Chlorine Compounds
Chlorine disinfectants are readily available, inexpensive, have a broad antimicrobial spectrum, and present minimal environmental hazards.3 Mechanism of action appears to be through oxidation of peptide links and denaturation of proteins.14 Intracellular accumulation results in inhibition of essential enzyme systems.13 Chlorine compounds are classified as intermediate-level disinfectants. Two factors that affect the biocidal activity of chlorine are pH and the presence of organic material. The greatest influence on the antimicrobial activity of chlorine in solution appears to be pH. With decreasing pH there is increasing biocidal activity. This increased activity at lower pH is due to a higher concentration of undissociated hypochlorous acid, which has a greater bactericidal action than the dissociated form. Organic matter consumes available chlorine and reduces its antibacterial efficacy.21 This negative effect is particularly evident in solutions with low levels of chlorine. Small additions of iodine or bromine to chlorine solutions greatly enhance their bactericidal activity. 21
Sodium hypochlorite appears to be the chlorine compound most frequently used as a disinfectant in veterinary hospitals. It is an effective virucidal agent.22 Sodium hypochlorite was first introduced as an antiseptic in 1915 as Dakin’s solution (0.4% available chlorine).23 Although still used as a wound antiseptic, sodium hypochlorite is used more frequently as a disinfectant in veterinary hospitals as a 0.16% solution (1:32 dilution of 5.25% stock solution) of liquid bleach.
EDTA
EDTA, especially in combination with Tris buffer ([hydroxymethyl]aminomethane), has been shown to have antibacterial properties, particularly against certain gram-negative bacteria. Tris-EDTA acts by increasing cell wall and membrane permeability through chelation of divalent cations and by slowing degradation of ribosomes.13,24,25 The clinical use of Tris-EDTA has been limited largely to four major pathogens: Pseudomonas aeruginosa, Proteus vulgaris, Escherichia coli, and Staphylococcus aureus.24 Tris-EDTA decreases the minimal inhibitory concentration for these bacteria when selected antimicrobials are added in vitro.26 It also potentiates the antimicrobials effects of chlorhexidine diacetate in lavage solutions.27
Tris-EDTA solution is inexpensive and readily available. It has been used as an irrigant in combination with antimicrobials in the treatment of otitis externa, bacterial rhinitis, and multiple fistulae in dogs.24 Formulations of other biocidal agents (e.g., chloroxylenol) may contain EDTA. Such preparations have enhanced efficacy against P. aeruginosa organisms. 28
Ethylene Oxide
Recognition of the biocidal activity of ethylene oxide did not take place until more than 60 years after its discovery in the 1850s. Ethylene oxide was patented in the United States in 1936 for use as a gas-phase biocide.10 It is a high-level disinfectant when used under proper conditions. Variables that are critical to the action of ethylene oxide include prehumidification, temperature, and concentration. Ethylene oxide sterilization is used for heat-labile equipment. Ethylene oxide is an alkylating agent that exhibits its bactericidal and sporicidal activities because of its reaction with nucleic acids.8,29 It may be combined with either carbon dioxide or fluorocarbons. Because of its toxicity, mutagenicity, carcinogenicity, and capacity to irritate eyes and mucous membranes, ethylene oxide sterilization for heat-labile equipment is being challenged by other, safer techniques, such as plasma sterilization (see later discussion of peroxygen compounds).
Iodine and Iodine Compounds
The first reference to the use of iodine for wounds was made in 1839.10,30 Iodine is an excellent, prompt, effective biocide with a broad range of action. Of the seven different forms of iodine that are present in pure aqueous iodine solutions, only two play a role in the disinfection processes: molecular iodine (I2) and hypoiodic acid (HOI). Molecular iodine has superior sporicidal and cysticidal properties compared with HOI. Iodine acts by decreasing the oxygen requirements of aerobic microorganisms.14 Iodine also interacts preferentially with thiol groups in proteins of the cytoplasmic membrane.14,30,31 Although bacterial resistance to iodine seems to be uncommon, bacterial resistance to povidone–iodine has been reported.18 Iodine has comparatively low reactivity with proteins, except blood, and pH has little effect on antimicrobial efficacy. Blood reduces the efficacy of iodine by converting it into nonbactericidal iodide.
Two main groups of iodine preparations are used clinically: preparations releasing free iodine and those containing complex-bound iodine. Preparations that release free iodine have intermediate-level biocidal activity. Preparations that release free iodine include iodine topical solution, an aqueous solution containing 2% iodine and 2.4% sodium iodide; strong iodine solution (Lugol’s solution), an aqueous solution containing 5% iodine and 10% potassium iodide; iodine tincture, containing 2% iodine and 2.4% sodium iodide in aqueous ethanol (1:1); and strong iodine tincture, containing 7% iodine and 5% potassium iodide in 95% ethanol. Iodine tincture preparations are both more efficacious and more toxic than aqueous solutions, including iodophors.
Preparations containing complex-bound iodine have low-level to intermediate-level activity. Iodophors are the most commonly used preparations that contain complex-bound iodine. The iodine in iodophors is bound to a carrier of high molecular weight (e.g., polyvinylpyrrolidone). Such carriers tend to increase the solubility of iodine; provide a sustained-release reservoir; reduce the equilibrium concentration of free molecular iodine; improve the wetting properties of the iodine; and aid in penetration of iodine in organic soil, including fat.30
The best known iodophor is povidone–iodine. Povidone–iodine compounds have a pH of about 5. The bactericidal properties of iodophors depend on the liberation of free iodine.32 The amount of free iodine in iodophor preparations depends on concentration, being greatest in a 0.07% solution of povidone–iodine.33 Dilutions of iodophors demonstrate more rapid bactericidal action than a full-strength povidone–iodine solution.34 Although in vitro studies have shown povidone–iodine to be highly effective against selective bacteria, including methicillin-resistant S. aureus,35 in vivo studies provide conflicting data regarding efficacy.23 LeVeen et al.32 conclude that on the basis of clinical and experimental evidence, free iodine is not liberated from povidone–iodine in therapeutic concentrations. Clinical studies indicate that povidone–iodine is suitable as an antiseptic on intact skin.32
Iodine-containing preparations, particularly iodophors, are used frequently as antiseptics. Iodophors are available in a variety of forms, including 10% solution, 2% cleansing solution (scrub), and 2% aerosol spray. Presurgical skin disinfection of surgeon and patient, disinfection of mucous membranes, and wound disinfection are the most common uses of iodophors. Povidone–iodine also has been reviewed favorably as an oral antiseptic.36 Cutaneous absorption of iodine, particularly in traumatized skin, can lead to increased serum iodine levels. Systemic iodine toxicity is also possible when iodophors are used to treat large, open wounds.23 Also, iodophors produce adverse effects when placed in the peritoneal cavity; hence their use in the peritoneal cavity is not recommended.32
Peroxygen Compounds
Hydrogen peroxide has historical and current application as a disinfectant and antiseptic. As a 3% solution, hydrogen peroxide has limited bactericidal effectiveness and is usually classified as an intermediate-level biocide.23 It is an oxidizing agent and acts by denaturing proteins and lipids of microorganisms.14,29 Hydrogen peroxide (3%) provides an effervescent cleansing action; however, because of its cytotoxicity, hydrogen peroxide is inappropriate as an antiseptic.23,37 As a 58% solution, however, and in the presence of an electromagnetic field, hydrogen peroxide becomes a gas plasma. As a gas plasma, hydrogen peroxide destroys microorganisms and is classified as a high-level disinfectant. Hydrogen peroxide (58% solution) is sporicidal by virtue of its effect on the outer spore layers and the spore core.38 Because the process temperature associated with plasma sterilization does not exceed 50° C, plasma sterilization is particularly well suited to heat-labile materials that cannot be steam sterilized.39 Because of its relative safety, plasma sterilization is emerging as an alternative to ethylene oxide gas in the sterilization of heat-labile articles. Other peroxygen compounds, such as peracetic acid and ozone, have limited use in veterinary medicine.
Phenols
Carbolic acid, a phenol, is the oldest example of an antiseptic compound.40 Phenols have a wide spectrum of activity against bacteria, viruses, and fungi, but they have minimal sporicidal activity.6 They act on the cytoplasmic membrane, producing leakage and disrupting membrane transport.13 Phenols are classified as low-level to intermediate-level biocides. While used sparingly in veterinary medicine, phenolic biocides are still widely used throughout the world.41 Two classes of phenol derivatives that have potential interest are the bisphenols (e.g., triclosan and hexachlorophene) and halophenols (chloroxylenol).8
Triclosan, a broad-spectrum antimicrobial agent, is used as a topical antiseptic and is frequently formulated with EDTA.8,42 Hexachlorophene, a chlorinated phenol derivative, was used as a presurgical antiseptic primarily by surgeons, but its toxicity has limited its use.43 Chloroxylenol has been used extensively as a preservative, disinfectant, and topical antiseptic.28
Quaternary Ammonium Compounds
Introduced in 1916, quaternary ammonium compounds are surface-active cations that exhibit low-level biocidal activity.10 They bind irreversibly to the phospholipids and proteins of the cytoplasmic membrane of microbes, impairing permeability.13,14 Quaternary ammonium compounds are much more effective in preventing the growth of bacteria than in killing them, and they are far more effective against gram-positive than gram-negative bacteria.6 Quaternary ammonium compounds possess a narrow margin of safety and can fail when exposed to resistant microorganisms.43
Quaternary ammonium compounds with a carbon chain length of 14 demonstrate the highest level of bactericidal activity.44 They were used initially as an adjunct to surgery, such as in presurgical patient preparation, but this use has been curtailed, in part because of the observation that skin bacteria survive beneath the layer of applied quaternary ammonium compound. Additionally, quaternary ammonium compounds tend to be inactivated by lipids in organic matter, and their activity is adversely affected by soap, hard water, and gauze.43 Quaternary ammonium compounds are currently used primarily for environmental disinfection of floors, walls, and equipment surfaces. Benzalkonium chloride is used as a topical antifungal agent for horses at a concentration of 0.15%.
Factors Affecting Disinfection and Antisepsis
A successful disinfection plan requires initial consideration of microbial susceptibility and environmental conditions.45 Factors of importance when selecting a chemical biocide include the degree of microbial killing required; the nature and composition of the surface item or device to be treated; amount of organic matter present; number and resistance of microorganisms present; presence of microbial biofilms; and cost, safety, and ease of use of the available agents.2 Critical items, which, if contaminated, impart a substantial risk of infection, must be sterilized. Noncritical items, which touch only the intact skin of the patient during routine use, can be disinfected. The greater the risk of infection associated with the use of a device, the more complete must be the degree of microbial killing on that device. The nature and composition of the device or surface to be treated affects the ease with which that device may be disinfected. Smooth, nonporous, and cleanable surfaces (e.g., table surfaces) are easiest to disinfect, whereas crevices, joints, and pores (e.g., surgical clamps) constitute barriers to the penetration of liquid biocides.2
The amount of organic material present has a major impact on the efficacy of most disinfectants. Physical cleaning before disinfection is often the most important step in the disinfection process.2 Endoscopes and accessories are particularly challenging to clean properly. Organic material has an especially profound effect on the biocidal efficacy of chlorine and iodine-based disinfectants and quaternary ammonium compounds.2 Negative effects of organic material are particularly profound with weak concentrations and with low-level disinfectants.
The number and resistance of microorganisms present can influence biocidal efficacy. In general, the higher the level of microbial contamination, the longer must be the exposure to the chemical biocide before the entire microbial population is killed.2 Microorganisms vary widely in their resistance to chemical biocides, with bacterial spores being most resistant, then protozoal cysts, coccidial oocysts, tubercle bacilli, small nonlipid viruses, fungi, vegetative bacteria, and medium-size lipid viruses.37 Differences in resistance exhibited by various vegetative bacteria are relatively minor, with staphylococci and enterococci being the more resistant gram-positive bacteria and Pseudomonas, Proteus, Klebsiella, Enterobacter, and Serratia spp. showing greater resistance than other gram-negative bacteria.2,8 The resistance of some bacteria, such as Pseudomonas and Serratia spp., may relate in part to the production of a glycocalyx-based biofilm.8,43,46
Some bacteria (e.g., Klebsiella pneumoniae, Serratia and Pseudomonas spp.) produce biofilms.43,47,48 Biofilms are collections of bacteria in a community that form in an exopolysaccharide extracellular matrix.48 They surround bacteria and make them more resistant to disinfection. Biofilms may be encountered in medical implants, such as catheters, sutures, and orthopedic prostheses, and seem to present a barrier to penetration by disinfectants and antiseptics.8,25,43,46,48 Biofilms serve as nidi of contamination as well as sources of bacterial products, such as toxins.48 The reduced accessibility of the bacteria included in the biofilm appears to be the major factor in resistance development.47 Resistance depends on the nature of the disinfectant, with greater resistance reported with selected quaternary ammonium compounds (e.g., benzalkonium chloride) and less resistance with oxidizing agents (e.g., sodium hypochlorite and hydrogen peroxide) and phenolic derivatives.47 Intrinsic and acquired resistances to disinfectants have been observed, notably Staphylococci, Pseudomonas, and Enterobacter spp.3,8,18,46 Potential for acquired bacterial resistance is rated very low, low, and moderate for alcohols, triclosan, and chlorhexidine, respectively.42 Although concerns about the use of chlorhexidine, triclosan (a bisphenol), and quaternary ammonium compounds and possible bacterial resistance to them and to antimicrobials have been raised,49 antimicrobial-resistant pathogens (e.g., methicillin-resistant S. aureus) did not demonstrate resistance to biocides at the currently used contact times and concentrations.34
Cost may be a factor in selection of disinfectants and antiseptics. One of the major impacts on cost of a disinfection procedure is the dilution of biocide used. Although overdilution of a biocide will reduce its net cost, overdilution may also significantly reduce its biocidal potency. The manufacturer’s recommendations should be followed when diluting a biocide. Selection of biocides should be based primarily on efficacy and safety, not cost. Ease of use of a biocide can affect disinfection practices. Those agents with wider safety margins are likely to be easier to use. Other causes of disinfection failure include poor disinfectant penetration or coverage, insufficient contact time, and inadequate temperature and humidity while the disinfectant is being applied.3,46
Disinfection and Antiseptic Practices
Veterinary Hospital Disinfection
Types of disinfection practices in a veterinary hospital include immersion disinfection; disinfection of surfaces, including cabinets, tables (examination, treatment, and surgery), kennels, lights, and chairs; and environmental disinfection.40 Agents that have been used for immersion disinfection include 2% alkaline glutaraldehyde, isopropyl alcohol (thermometers), chlorhexidine diacetate, and quaternary ammonium compounds. Of these agents, only 2% alkaline glutaraldehyde is reliable. Because the disinfection of surgical instruments by immersion lacks the safety of heat-pressure sterilization, immersion disinfection is not recommended for instruments to be used during aseptic surgery.
Surface disinfection in a veterinary hospital can be particularly important in minimizing spread of disease. Thorough cleaning of the surface before disinfection is a critical step. Other factors that improve the efficacy of surface disinfection include adequately covering the surface with the disinfectant and maintaining contact for a sufficient time. Agents that are used as surface disinfectants include sodium hypochlorite and quaternary ammonium compounds. Sodium hypochlorite is used as a 0.16% solution (1:32 dilution of 5.25% solution) of liquid bleach to disinfect kennels and tables. Such a solution has been shown to be effective in neutralizing parvovirus.50 Quaternary ammonium compounds are surfactants that have both cleansing and disinfecting properties. Despite their cleansing properties, quaternary ammonium compounds have reduced biocidal efficacy in the presence of organic material; hence cleaning of surfaces before their use is indicated. Quaternary ammonium compounds have been recommended for the routine disinfection of environmental surfaces.43 Effective environmental disinfection of large-animal holding facilities using a peroxygen compound (4% peroxymonosulfate) has been described.51
Surgical Antisepsis
Surgical antisepsis is the application of antimicrobial chemicals to skin, mucosa, and wounds to reduce the risk of infection.52 Surgical antisepsis most commonly involves the removal or reduction of normal flora by the topical application of antimicrobial substances to the intact skin before a surgical procedure. Distinguishing between antiseptic use on intact skin and that on mucous membranes or in wounds is important. Different formulations and concentrations of antiseptics are indicated depending on their use. Preparations containing alcohol or detergents (scrubs) are to be used only on intact skin. The concentration of antiseptic used in wounds is less, in general, than that of preparations applied to intact skin.
Surgical Preparation of the Skin
The purpose of a surgical hand scrub or alcohol-based hand rub is to remove transient flora and reduce resident flora for the duration of surgery in case of glove tears.7,53 Regardless of the biocidal agent used, the technique of hand washing is important. The primary problem with hand hygiene is not a paucity of good products but rather the laxity of practice.7 Nails should be short, and artificial nails and nail polish are discouraged.7 Debris should be removed from under the fingernails with a nail cleaner after the hands and forearms have been washed thoroughly. The subungual area has higher microbial counts, and contamination of the hands can increase when gloves create a warm, moist environment.7,40 Duration of washing is important both for mechanical action and to allow antimicrobial products sufficient contact time to achieve the desired effect.7 Although the American College of Surgeons suggests that a surgical scrub of 120 seconds, including brushing of the nail and fingertip areas, is adequate,7 longer scrubs may be performed by veterinary surgeons.
Selection of an appropriate biocidal agent for surgical hand scrubbing should be made in three stages: One should determine what characteristics are desired, review and evaluate the evidence of safety and efficacy in reducing microbial counts, and consider the personnel acceptance of the product and the costs.7 Antiseptic treatment of the skin should not be toxic, should not cause skin reactions, and should not interfere with the normal protective function of the skin.52 Antiseptic agents used to prepare the skin of the surgeon or patient include alcohols, chlorhexidine, iodophors, and chloroxylenols.
Alcohols, in appropriate concentrations (60% to 90%), provide the most rapid and greatest reduction in microbial counts on skin.7,52 Alcohols are not good cleansing agents, and they are not recommended in the presence of physical dirt. They should be allowed to evaporate thoroughly from the skin to be fully effective and to decrease irritation.7 Immersion of the surgeon’s hands and arms in alcohol has been shown to be an effective technique.7 Waterless, alcohol-based hand rinse products (rubs) effectively reduce microbial counts on skin.54 Hand rubs have been shown to be effective within application times of 90 to 180 seconds.53 Alcohol is also used on the intact skin of the veterinary patient before surgery as a defatting agent.55
Chlorhexidine gluconate has both rapid and persistent antibacterial activity when used as a presurgical scrub. Its persistence is probably the best of any agent currently available for hand washing.7 The activity of chlorhexidine is not significantly affected by blood or other organic material.7 The incidence of skin irritation to chlorhexidine scrub seems low. Chlorhexidine scrub may be the ideal agent for surgical preparation of the skin.33 Both 2% and 4% formulations in a detergent base are readily available.
Iodophors, particularly povidone–iodine, are used frequently in the presurgical preparation of surgeons and veterinary patients. The antimicrobial effects of iodophors are similar to those of iodine. Recommended levels of free iodine for antiseptics are 1 to 2 mg/L.7 Povidone–iodine scrub has been found to be equally effective as chlorhexidine gluconate scrub in reducing the number of bacteria on canine skin for up to 1 hour after application.56 Iodophors are rapidly neutralized in the presence of organic materials such as blood.7 They have a propensity toward skin irritation, and cutaneous absorption can cause thyroid dysfunction. Iodophors are available as a surgical scrub (2%) and as a solution (10% and 2%).
Chloroxylenols and bisphenols are synthetic phenol derivatives that have been used sparingly as a presurgical scrub of surgeons and veterinary patients. They are less effective than either chlorhexidine or iodophors in reducing skin flora, but chloroxylenols may have a lower incidence of skin irritation than iodophors.7 Their activity is only minimally affected by organic matter.7 Formulations used as a presurgical scrub include 3% chloroxylenol, 1.5% parachlorometaxylenol, and 1% to 2% triclosan.7,42
Surgical Preparation of Mucous Membranes
Antisepsis of mucous membranes, particularly the oral mucosa, presents particular problems. The bacterial colonization of the oral cavity is very high, and the efficacy of oral antiseptics is affected by dilution effects as well as inactivation due to salivary proteins.36 Also, an increase in antiseptic concentration is limited by local irritation and a high absorption rate with the risk of systemic intoxication.36 Only a few solutions are useful as oral antiseptics: povidone–iodine, chlorhexidine, and hexetidine.36 Povidone–iodine solution has been shown to reduce inflammation and the progression of periodontal disease as well as bacteremia after dental extractions.36
Chlorhexidine solution is an effective agent for the prevention and treatment of oral disease.16 Its effectiveness stems from its ability to adsorb to negatively charged surfaces in the mouth, such as the tooth and mucosa. Hexetidine is used as a 0.1% solution for local infections and oral hygiene. It has been shown to have similar antimicrobial efficacy against common buccal organisms as 0.2% chlorhexidine.57
Wound Antisepsis
When topically treating a contaminated wound with an antiseptic solution, the clinician should choose an appropriate type and concentration of antiseptic that has both antibacterial properties and minimal negative effects on wound healing. Antiseptics that appear to fulfill these criteria include chlorhexidine solution, povidone–iodine solution, and sodium hypochlorite solution (Dakin’s solution). Chlorhexidine diacetate solution (0.05%) has a wide spectrum of antimicrobial activity as well as minimal deleterious effects on wound healing.58 Its sustained residual activity seems to be an advantage in wound therapy. Dilution of the stock solution with sterile water, 0.9% sodium chloride, or lactated Ringer’s solution does not adversely affect its antibacterial activity.19
Povidone–iodine solution appears to be most effective and least tissue toxic in concentrations of 0.1% to 1%.33 Povidone–iodine concentrations greater than 0.5% are cytotoxic to the canine fibroblast in vitro.33 Povidone–iodine should be used judiciously on large wounds because of the potential for systemic absorption of iodine.
A dilute Dakin’s solution (0.005% sodium hypochlorite) has been shown to be both bactericidal and not damaging to fibroblasts.58 Dakin’s solution has been used as an effective irrigant for human wounds since World War I, and its use has persisted to the present. In vivo studies on the efficacy of Dakin’s solution in canine wounds are not available.33
1. King L.J. History and future perspectives of the use of disinfectants in animal health. Rev Sci Tech. 1995;14:41-46.
2. Favero M.S., Bond W.W. Chemical disinfection of medical and surgical materials. In: Block S.S., editor. Disinfection, sterilization, and preservation. ed 5. Philadelphia: Lippincott Williams & Wilkins; 2001:881.
3. Kahrs R.F. General disinfection guidelines. Rev Sci Tech. 1995;14:105-122.
4. Brown C.D., Zitelli J.A. A review of topical agents for wounds and methods of wounding—guidelines for wound management. J Dermatol Surg Oncol. 1993;19:732-737.
5. Ali Y., Dolan M.J., Fendler E.J., et al. Alcohols. In: Block S.S., editor. Disinfection, sterilization, and preservation. ed 5. Philadelphia: Lipipncott Williams & Wilkins; 2001:229.
6. Jeffrey D.J. Chemicals used as disinfectants: active ingredients and enhancing additives. Rev Sci Tech. 1995;14:57-74.
7. Larson E.L. APIC guideline for handwashing and hand antisepsis in health care settings. Am J Infect Control. 1995;23:251-269.
8. McDonnell G., Russell A.D. Antiseptics and disinfectants: Activity, action, and resistance. Clin Microbiol Rev. 1999;12:147-179.
9. Rossmoore H.W. Nitrogen compounds. In: Block S.S., editor. Disinfection, sterilization, and preservation. ed 5. Philadelphia: Lippincott Williams & Wilkins; 2001:383.
10. Hugo W.B. A brief history of heat, chemical and radiation preservation and disinfection. Int Biodeterioration Biodegrad. 1995;36:197-217.
11. Scott E.M., Gorman S.P. Glutaraldehyde. In: Block S.S., editor. Disinfection, sterilization, and preservation. ed 5. Philadelphia: Lippincott Williams & Wilkins; 2001:361.
12. Russell A.D. Glutaraldehyde: current status and uses. Infect Control Hosp Epidemiol. 1994;15:724-733.
13. Denyer S.P., Stewart G.S.A.B. Mechanisms of action of disinfectants. Int Biodeterioration Biodegrad. 1998;41:261-268.
14. Maris P. Modes of action of disinfectants. Rev Sci Tech. 1995;14:47-55.
15. Lambrechts N.E., Hurter K., Picard J.A., et al. A prospective comparison between stabilized glutaraldehyde and chlorhexidine gluconate for preoperative skin antisepsis in dogs. Vet Surg. 2004;33:636-643.
16. Denton G.W. Chlorhexidine. In: Block S.S., editor. Disinfection, sterilization, and preservation. ed 5. Philadelphia: Lippincott Williams & Wilkins; 2001:321.
17. Barrett-Bee K., Newboult L., Edwards S. The membrane destabilising action of the antibacterial agent chlorhexidine. FEMS Microbiol Lett. 1994;119:249-254.
18. Chapman J.S. Characterizing bacterial resistance to preservatives and disinfectants. Int Biodeterioration Biodegrad. 1998;41:241-245.
19. Lozier S., Pope E., Berg J. Effects of four preparations of 0.05% chlorhexidine diacetate on wound healing in dogs. Vet Surg. 1992;21:107-112.
20. Amber E.I., Henderson R.A., Swaim S.F., et al. A comparison of antimicrobial efficacy and tissue reaction of four antiseptics on canine wounds. Vet Surg. 1983;12:63-68.
21. Dychdala G.R. Chlorine and chlorine compounds. In: Block S.S., editor. Disinfection, sterilization, and preservation. ed 5. Philadelphia: Lippincott Williams & Wilkins; 2001:135.
22. Kennedy M.A., Mellon V.S., Caldwell G., et al. Virucidal efficacy of the newer quaternary ammonium compounds. J Am Anim Hosp Assoc. 1995;31:254-258.
23. Doughty D. A rational approach to the use of topical antiseptics. J Wound Ostomy Continence Nurs. 1994;21:224-231.
24. Ashworth C.D., Nelson D.R. Antimicrobial potentiation of irrigation solutions containing Tris-(hydroxymethyl) aminomethane-EDTA. J Am Vet Med Assoc. 1990;197:1513-1514.
25. Russell A.D. Biocide use and antibiotic resistance: the relevance of laboratory findings to clinical and environmental situations. Lancet Infect Dis. 2003;3:794-803.
26. Wooley R.E., Jones M.S. Action of EDTA-Tris and antimicrobial agent combinations on selected pathogenic bacteria. Vet Microbiol. 1983;8:271-280.
27. Klohnen A., Wilson D.G., Hendrickson D.A., et al. Effects of potentiated chlorhexidine on bacteria and tarsocrural joints in ponies. Am J Vet Res. 1996;57:756-761.
28. Stubbs W.P., Bellah J.R., Vermaas-Hekman D., et al. Chlorhexidine gluconate versus chloroxylenol for preoperative skin preparation in dogs. Vet Surg. 1996;25:487-494.
29. Joslyn L.J. Gaseous chemical sterilization. In: Block S.S., editor. Disinfection, sterilization, and preservation. ed 5. Philadelphia: Lippincott Williams & Wilkins; 2001:337.
30. Gottardi W. Iodine and iodine compounds. In: Block S.S., editor. Disinfection, sterilization, and preservation. ed 5. Philadelphia: Lippincott Williams & Wilkins; 2001:159.
31. Russell A.D. Similarities and differences in the responses of microorganisms to biocides. J Antimicrob Chemother. 2003;52:750-763.
32. LeVeen H.H., LeVeen R.F., LeVeen E.G. The mythology of povidone–iodine and the development of self-sterilizing plastics. Surg Gynecol Obstet. 1993;176:183-190.
33. Lemarié R.J., Hosgood G. Antiseptics and disinfectants in small animal practice. Compend Contin Educ Pract Vet. 1995;17:1339-1351.
34. Weber D.J., Rutala W.A., Sickbert-Bennett E.E. Outbreaks associated with contaminated antiseptics and disinfectants. Antimicrob Agents Chemother. 2007;51:4217-4224.
35. Goldenheim P.D. In vitro efficacy of povidone–iodine solution and cream against methicillin-resistant Staphylococcus aureus. Postgrad Med J. 1993;69:S62-S65.
36. Rahn R. Review presentation on povidone-iodine antisepsis in the oral cavity. Postgrad Med J. 1993;69:S4-S9.
37. Greene C.E. Environmental factors in infectious disease. In: Greene C.E., editor. Infectious diseases of the dog and cat. ed 3. St Louis: Saunders; 2006:991.
38. Block S.S. Peroxygen compounds. In: Block S.S., editor. Disinfection, sterilization, and preservation. ed 5. Philadelphia: Lippincott Williams & Wilkins; 2001:185.
39. Jacobs P.T., Lin S. Sterilization processes utilizing low-temperature plasma. In: Block S.S., editor. Disinfection, sterilization, and preservation. ed 5. Philadelphia: Lippincott Williams & Wilkins; 2001:747.
40. Heit M.C., Riviere J.E. Antiseptics and disinfectants. In: Adams H.R., editor. Veterinary pharmacology and therapeutics. ed 8. Ames, Iowa: Iowa State University Press; 2001:783.
41. Goddard P.A., McCue K.A. Phenolic compounds. In: Block S.S., editor. Disinfection, sterilization, and preservation. ed 5. Philadelphia: Lippincott Williams & Wilkins; 2001:255.
42. Kampf G., Kramer A. Epidemiologic background of hand hygiene and evaluation of the most important agents for scrubs and rubs. Clin Microbiol Rev. 2004;17:863-893.
43. Terleckyj B., Elsinger E.C., Axler D.A. Antiseptics and disinfectants—current issues. J Am Podiatr Med Assoc. 1995;85:439-445.
44. Merianos J.J. Surface-active agents. In: Block S.S., editor. Disinfection, sterilization, and preservation. ed 5. Philadelphia: Lippincott Williams & Wilkins; 2001:283.
45. Grow A.G. Writing guidelines to require disinfection. Rev Sci Tech. 1995;14:469-477.
46. Russell A.D. Mechanisms of bacterial resistance to biocides. Int Biodeterioration Biodegrad. 1995;36:247-265.
47. Ntsama-Essomba C., Bouttier S., Ramaldes M., et al. Resistance of Escherichia coli growing as biofilms to disinfectants. Vet Res. 1997;28:353-363.
48. Morck D.W., Olson M.E., Ceri H. Microbial biofilms: prevention, control, and removal. In: Block S.S., editor. Disinfection, sterilization, and preservation. ed 5. Philadelphia: Lippincott Williams & Wilkins; 2001:675.
49. Russell A.D. Bacterial adaptation and resistance to antiseptics, disinfectants and preservatives is not a new phenomenon. J Hosp Infect. 2004;57:97-104.
50. McGavin D. Inactivation of canine parvovirus by disinfectants and heat. J Small Anim Pract. 1987;28:523-535.
51. Patterson G., Morley P.S., Blehm K.D., et al. Efficacy of directed misting application of a peroxygen disinfectant for environmental decontamination of a veterinary hospital. J Am Vet Med Assoc. 2005;227:597-602.
52. Crabtree T.D., Pelletier S.J., Pruett T.L. Surgical antisepsis. In: Block S.S., editor. Disinfection, sterilization, and preservation. ed 5. Philadelphia: Lippincott Williams & Wilkins; 2001:919.
53. Suchomel M., Gnant G., Weinlich M., et al. Surgical hand disinfection using alcohol: the effects of alcohol type, mode and duration of application. J Hosp Infect. 2009;71:228-233.
54. Larson E.L., Aiello A.E., Heilman J.M., et al. Comparison of different regimens for surgical hand preparation. AORN J. 2001;73:412-420.
55. Shmon C. Assessment and preparation of the surgical patient and the operating team. In: Slatter D., editor. Textbook of small animal surgery. ed 3. Philadelphia: Saunders; 2003:162.
56. Osuna D.J., DeYoung D.J., Walker R.L. Comparison of three skin preparation techniques in the dog. Part I: Experimental trial. Vet Surg. 1990;19:14-19.
57. Ashley K.C. The antimicrobial properties of two commonly used antiseptic mouthwashes—corsodyl and oraldene. J Appl Bacteriol. 1984;56:221-225.
58. Swaim S.F., Lee A.H. Topical wound medications: a review. J Am Vet Med Assoc. 1987;190:1588-1593.