Chapter 13 Drugs for the Treatment of Helminth Infections
Anthelmintics
Practicing veterinarians commonly use drugs to treat and prevent helminth infections in small animals. The life cycle and biology of the most important parasites are well understood by graduate veterinarians and are not discussed here; however, current textbooks of parasitology may be consulted for review.1-4 Gastrointestinal parasites are among the most common infectious agents that veterinarians in small animal practice face. A landmark parasite prevalence study evaluated more than 6000 canine fecal specimens from all 50 states and the District of Columbia.5 The results indicate that parasites are common in American dogs. Nationwide, 36% of the samples tested were positive for roundworm (Toxocara canis), hookworm (Ancylostoma caninum), or whipworm (Trichuris vulpis). Even more surprising, 52% of the samples from the southeastern United States were positive for at least one nematode. In a recent study of the results of heartworm and fecal testing in the western United States, the importance of annual testing and routine use of preventives was highlighted.6 Clinics in 11 states were surveyed, and local dogs with no history of travel were diagnosed with heartworms in every state but Idaho and Wyoming. The prevalence of intestinal parasites in companion animals in Ontario and Quebec, Canada, during the winter was recently evaluated.7 The fact that 30% of feline and 39% of canine fecal samples were positive for gastrointestinal parasites prompted the authors to recommend that all veterinarians follow the Companion Animal Parasite Council (CAPC) guidelines8 regarding use of year-round broad-spectrum deworming protocols. Another reason for following CAPC guidelines, in this instance regarding routine heartworm testing and prophylaxis, is concern about animals moving from heartworm-endemic areas to those with limited heartworm exposure. These concerns were realized when Hurricane Katrina resulted in thousands of dogs and cats being shipped from Louisiana, where heartworm prevalence is quite high, to shelters across the United States.9
KEY POINT 13-2
Testing, treatment, and prevention of helminth infections in pets have profound zoonotic implications.
Although these parasites are important to the health of dogs, several are also important zoonotic pathogens. Ascarid larvae migrate through human tissues, causing a variety of signs correlated to the location of the migration. These are primarily Toxocara species ascarids, but the raccoon ascarid, Baylisascaris procyonis, is being increasingly implicated as a cause of human disease in the United States.10 The Centers for Disease Control and Prevention (CDC) have published Guidelines for Veterinarians: Prevention of Zoonotic Transmission of Ascarids and Hookworms of Dogs and Cats, which is an excellent resource and is available online as a PDF download.11
Worldwide, helminth infections are a major animal and human health concern,12 with hookworms infecting large numbers of people worldwide, especially those of low economic status.13 More than 30% of the human population, in vast areas of South America and Asia, is infected with hookworms. More than half of the population is infected with hookworms in many southern areas of the African continent. Experts estimate that a billion people, more than a fifth of the planet’s human inhabitants, harbor hookworms.14 One study showed that nearly all dogs in a remote community of northeastern India were infested with one or more zoonotic gastrointestinal parasites.15 This study demonstrated that dogs played a major zoonotic role both in transmitting parasites that use dogs as their definitive and paratenic host and in mechanically transmitting and spreading the dissemination range of an array of human-specific parasites. A recent feline study in metropolitan Rio de Janeiro revealed an 89.6% prevalence of overall gastrointestinal helminth parasites in cats.16
KEY POINT 13-4
The practicing veterinarian who judiciously uses anthelmintics is in a unique position to have a dramatic impact on the health of pets and people.
Through the prudent use of anthelmintics in companion animals, the practicing veterinarian is in a unique position to positively affect not only the patient’s health but also public health.
Anthelmintics
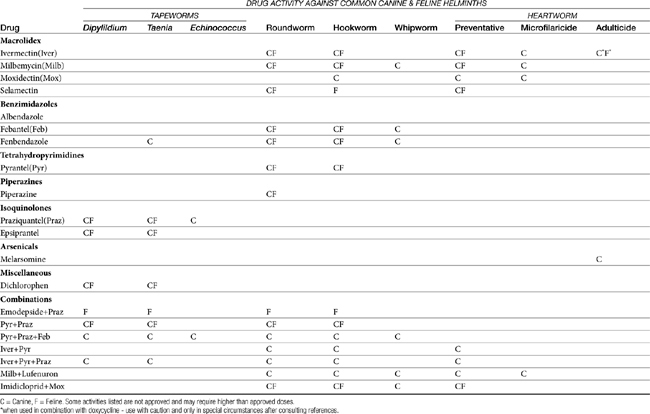
In this chapter anthelmintics that are approved by the U.S. Food and Drug Administration (FDA) and commercially available in the United States are grouped together by class according to their generic names. The literature on antiparasitic drugs is enormous. In the interest of both economy and readability, only a few references are listed for each drug. These will guide the veterinarian who needs more specific information about the subject. Table 13-1 provides a general overview of anthelmintic drug spectrum against the common canine and feline helminths.
Since the last edition of this text was published, there have been considerable changes, most notably the more widespread use of ivermectin and the emergence of other macrocyclic lactones. New information on avermectin toxicity is covered in the ivermectin section. Drug manufacturers have discontinued production of many tried-and-true anthelmintics such as dichlorvos (Task Capsules) and diethylcarbamazine citrate (Filaribits). In addition, some drugs, such as N-butyl chloride, have simply been superseded. For simplicity’s sake, discontinued products and drugs that are not widely available do not appear in the current edition; however, an earlier edition of this text can certainly be consulted for information about them. The latest information about diethylcarbamazine citrate can be obtained from the American Heartworm Society 2005 guidelines for the diagnosis, prevention, and management of heartworm infection in dogs.17 This organization produced similar guidelines for cats in 200718 and updated the guidelines for dogs in 2010.18a
New anthelmintic products are continuously researched and frequently launched. The Compendium of Veterinary Products provides a comprehensive list of commercially available products approved by the FDA.19 An exhaustive review of the pharmacology, mechanism of action, pharmacokinetics, and efficacy of anthelmintics is beyond the scope of this chapter. There are excellent texts available for those interested in more exhaustive information on anthelmintics.20-22 Pharmacologic activity against nonhelminths, such as flukes, fleas, and ticks, by some anthelmintics and anthelmintic drug combinations may be mentioned, but such activity is not the focus of this chapter.
Macrolides
Macrolides, which include both avermectins and milbemycins, have revolutionized the control of parasites in both humans and animals. Ivermectin is the best known agent in this class. The dual activity of some macrolides, such as ivermectin and selamectin, against endoparasites, such as helminths, and ectoparasites, such as fleas, gave rise to the term endectocide. These products are similar in that they are large complex macrocyclic structures produced by soil microorganisms in the Streptomyces genus. They are generally regarded as the most effective and least toxic parasiticides yet developed. Commercially, they have crushed the competition. Many conventional drugs that were direct competitors of this class were retired from common use and eventually discontinued by the manufacturer.
KEY POINT 13-5
Macrolides are large complex macrocyclic products of soil microorganisms. This class includes the most effective, least toxic parasiticides.
Macrolides are excreted in the feces as active drug. Drugs in this class, especially the avermectins, are toxic to dung-feeding insects, but not birds, plants, and earthworms. Elimination of coprophagous insects appears to delay processing of nutrients, but the overall environmental impact of this finding is unclear.23
Although originally believed to act by disturbing gamma-aminobutyric acid (GABA)–mediated neurotransmission, it now appears that they act with high affinity to a nematode-specific glutamate-gated chloride channel.24,25 Macrolides trigger chloride ion influx, which hyperpolarizes the parasite neuron and prevents initiation or propagation of normal action potentials. The selectivity of macrolides is due to different glutamate function in invertebrates compared with that of vertebrates. Glutamate acts as an inhibitory neurotransmitter in invertebrates and an excitatory neurotransmitter in mammals.2 The net effect is paralysis and death of the target parasite.
Despite their beneficial activities, macrocyclic lactones have several flaws. They are ineffective against cestodes and trematodes, and they are sometimes expensive. That said, the U.S. patent on ivermectin has now expired, allowing generic competitors to enter the market and reduce the cost of ivermectin treatment, but to date the cost savings have not been realized on products for dogs and cats to the same degree as those for horses and food animals. Although macrolides are generally regarded as the most effective and least toxic parasiticides yet developed, toxicity may occur with overdosage, especially in Collies, many of which are unusually sensitive to macrolide endectocides. An excellent summarized overview of the pharmacology, indications, dosing, precautions, and side effects of commercially available macrocyclic lactones is available online as a PDF download from the United States Pharmacopeial Convention, Inc.23
Ivermectin
Ivermectin was the first commercially available macrolide, released for use in animals in 1981.24 The avermectins were isolated from the fermentation broth of Streptomyces avermitilis. The discovery of its anthelmintic activity was made after administration of the actinomycetic broth to mice infected with the nematode Nematospiroides dubius. Ivermectin is effective against many nematodes and arthropods. In particular, ivermectin is very effective against immature Dirofilaria immitis.
KEY POINT 13-7
Ivermectin, the first macrolide used commercially, revolutionized the control of parasites in humans and other animals.
Administration of ivermectin to pregnant rats, mice, and rabbits produced teratism only at or near doses that were maternally toxic. There was no teratogenesis in cattle, sheep, and dogs when ivermectin was administered to pregnant animals at four times the recommended dose. Although toxicity for aquatic animals is high, the binding of ivermectin in soil reduces its concentration to levels that have minimal effect on the environment, as previously discussed. The acute oral LD50 in mice varied from 11.6 to 87.2 mg/kg; and the LD50 for rats was 42.8 to 52.8 mg/kg. In a 14-week study with rats, the “no-effect” level was 0.4 mg/kg.
Ivermectin is well absorbed (95%) after oral administration and well distributed to most tissues except the central nervous system. It is largely eliminated unchanged in the feces and is metabolized to a small degree in the liver by oxidation. In dogs the terminal half-life is approximately 48 hours. No teratism was observed in fetuses when pregnant bitches received repeated oral doses of ivermectin of 0.5 mg/kg. A single oral dose of 2 mg/kg and repeated oral doses of 0.5 mg/kg per day for 14 weeks were well tolerated by dogs.26
Avermectin Toxicity
It is important not to administer avermectins concurrently with drugs that could increase avermectin blood–brain barrier penetration, such as ketoconazole, itraconazole, cyclosporine, and calcium-channel blockers.27 Selamectin toxicity information is addressed in detail later, in the selamectin section. According to a popular veterinary pharmaceutical clinical text, signs of ivermectin toxicity in dogs, in order of frequency, are ataxia, blindness, mydriasis, tremors, and vomiting.28 Other signs include dehydration, depression, diarrhea, hyperthermia, bradycardia, sinus arrhythmia, coma, seizures, and death.26,29,30 A recent retrospective study of ivermectin toxicosis cases evaluated at a poison control center revealed clinical signs in the following order of frequency: ataxia, lethargy, tremors, mydriasis, and blindness.30a
The apparent LD50 for Beagles is 80 mg/kg.26 The primary clinicopathologic sign in dogs is decreased serum iron values.22 A common reference used by clinical veterinarians states that death could occur with doses above 40 mg/kg, tremors at 5 mg/kg, and mydriasis at 2.5 mg/kg and that signs of toxicity rarely occur at doses below 1 mg/kg.28 But a recent retrospective poison control center study revealed that clinical signs may develop between 0.2 to 2.5 mg/kg.30a A wide variety of signs were noted, including more severe signs like coma and seizure, at doses below 1 mg/kg. In fact, death was noted in dogs that received 1 to 2.5 mg/kg doses of ivermectin.
A common presenting history is that of a dog that was in close proximity to horses during deworming and later started showing signs of disease. Dogs that develop clinical signs within 4 to 6 hours of ivermectin ingestion typically develop severe clinical signs, whereas dogs with signs developing 10 to 12 hours after exposure tend to have much milder clinical signs.29 It is not uncommon for dogs with ivermectin toxicity to have seizures. When seizures are severe and uncontrolled for a considerable period of time, hemolytic anemia and muscle damage may occur. Some severe cases present with seizures and miosis, which warrants a poor prognosis. It is possible that severe seizures and miosis on presentation may be associated with severe brain damage.31
Clinical signs of ivermectin toxicity in cats, in order of frequency, are ataxia, mydriasis, tremors, hyperesthesia, and hypothermia.28 Dogs are about 10 times as likely as cats to have ivermectin toxicity.28
Some Collies are unusually sensitive to the toxic effects of ivermectin, although it is safe for all breeds at the approved dose of 0.006 mg/kg (6 mcg/kg). Early studies indicated that some genetic lines of Collies developed severe adverse reactions when ivermectin was given at a dose of 100 to 200 mcg/kg (16 to 32 times the label dose), producing mydriasis, ataxia, tremors, drooling, paresis, recumbency, excitability, stupor, and coma. At that time Australian Shepherds, Border Collies, Shetland Sheepdogs, and Old English Sheepdogs were also reported to be sensitive to ivermectin. The lethal dose for some Collies was reported to be 1/200th that of Beagles.32
After Collies were also found to be more sensitive to loperamide,33 the canine multidrug resistance (MDR1) gene was identified and found to be mutated in ivermectin-sensitive Collies.34 MDR1 codes for a glycoprotein that is an integral part of the blood–brain barrier. There are many excellent sources of information about the MDR1 gene mutation and mechanisms of ivermectin toxicity associated with increased GABA activity.29,30,35 The mutant MDR1 allele was found in 35% of the 40 Collies that were tested in one study, about the same percentage of Collies that are sensitive to ivermectin.36 A survey of DNA from 4000 purebred dogs revealed that the MDR1 mutation was present in seven breeds of Collie lineage and two sighthound breeds, although the mutation was not identified in all breeds known to have ivermectin sensitivity.37
It was found that the potential for ivermectin sensitivity could be estimated by genotypic or polymerase chain reaction (PCR)–based testing for the MDR1 mutation.38 In this study the mutant MDR1 allele was found in Australian Shepherds, Miniature Australian Shepherds, English Shepherds, German Shepherd Dogs (white), Longhaired Whippets, McNab Shepherds, Old English Sheepdogs, Shetland Sheepdogs, Silken Windhounds, and Longhaired Whippet.23,37 Sensitivity to ivermectin was also noted in Australian Cattle Dogs, Bearded Collies, and Border Collies, but the mutant MDR1 allele was not found in these breeds.37 More recently, the relationship with P-glycoprotein, the product of the ABCB1 (formerly MDR1) gene, was studied in depth by looking for the ABCB1-1Δ allele in a DNA study of 5368 dogs.39 The ABCB1-1Δ allele was found in Collies, Longhaired Whippets, Standard and Miniature Australian Shepherds, Shetland Sheepdogs, Old English Sheepdogs, Border Collies, Silken Windhounds, and German Shepherd Dogs.
The fact is, until a particular dog is tested, its susceptibility to ivermectin toxicity is unknown. Washington State University, College of Veterinary Medicine, Veterinary Clinical Pharmacology Lab, provides genetic testing to determine the presence of the MDR1 mutant gene. Table 13-2 was adapted from information available at the aforementioned institution’s website.40 The general practitioner can also use this table when presented with a dog that has had a known exposure to ivermectin to determine prognosis and the appropriate level of treatment. As noted previously, a common finding when taking history on ivermectin and moxidectin exposure cases is that the dog was present while the owner was deworming a horse. It is not unusual for horses to spit out a small amount of dewormer, which is ingested by the dog. If the amount ingested can be quantified, then this table can help the veterinarian estimate prognosis and determine how aggressive to get with treatment. Obviously, the risk of a severe toxicity is much greater with a Collie than with another breed.
Table 13-2 Breeds Affected by MDR-1 Mutation
| Breed | Approximate Frequency |
|---|---|
| Collie | 70% |
| Longhaired Whippet | 65% |
| Australian Shepherd | 50% |
| Australian Shepherd, Mini | 50% |
| Silken Windhound | 30% |
| McNab Shepherd | 30% |
| Shetland Sheepdog | 15% |
| English Shepherd | 15% |
| German Shepherd Dog | 10% |
| Herding Breed Cross | 10% |
| Mixed Breed | 5% |
| Old English Sheepdog | 5% |
| Border Collie | < 5% |
(Data from Washington State University, College of Veterinary Medicine, Veterinary Clinical Pharmacology Lab. (2010). Affected breeds Retrieved Jan 26, 2010, from http://www.vetmed.wsu.edu/depts-VCPL/breeds.aspx.)
Another presentation to consider is dogs with the habit of eating horse feces. Dogs that are not ivermectin sensitive are probably not at risk, but an ivermectin-sensitive dog that eats the feces of a horse that has been treated with ivermectin within the last few days may have a severe, even fatal reaction. Ivermectin reaches maximum fecal concentration 2 to 3 days after the horse is treated.41 By 4 days posttreatment, 90% of the drug has been excreted in the feces. Owners of ivermectin-sensitive coprophagic dogs should treat feces from ivermectin-treated horses as toxic waste and dispose of it in a manner that will prevent the dog from eating it.
Regarding treatment of ivermectin toxicity, although there is some evidence that intravenous administration of physostigmine may be of some benefit for dogs42 and neostigmine may help treat cats43 suffering from severe ivermectin intoxication, adverse events associated with these treatments typically outweigh benefit, thus the mainstay of care given by most veterinarians is supportive and symptomatic.26 Inducing emesis, giving activated charcoal, providing fluid therapy, supplying parenteral alimentation, and maintaining respiratory support and normal body temperature are essential. This supportive care may be needed for an extended period of time because the half-life of ivermectin is 2 days and the half-life of moxidectin is 19 days.30
There is no antidote for ivermectin and moxidectin toxicity, but veterinarians should consider lipid rescue, a promising therapy adapted from human medicine. Dr. Guy Weinberg initially described the use of an intravenous lipid emulsion (Intralipid) to treat local anesthetic toxicity (bupivacaine) in humans. He coined the term “lipid rescue.” One of the studies to support human use of lipid rescue was an experiment on dogs that were overdosed with bupivacaine and rescued from certain toxicity with intravenous lipid emulsion.44 Dr. Weinberg established a noncommercial website (www.lipidrescue.com) to disseminate information and foster discussion of cases. Since then, lipid rescue has been used to treat nonbupivacaine toxicities in other species. Although support is certainly anecdotal, in 2008 a veterinary online contributor to the lipid rescue website described an ivermectin-overdosed dog that had clinical signs of toxicity and recovered nicely after activated charcoal, supportive care, and an intravenous lipid emulsion were administered. More recently, a case report of a puppy with moxidectin toxicosis was published describing the use of an intravenous lipid emulsion given as a bolus of 2 mL/kg, followed by 4 mL/kg/hr for 4 hours beginning 10 hours after exposure and repeated at 0.5 mL/kg/min for 30 minutes beginning 25.5 hours after exposure.45 The 16-week-old dog presented with acute onset seizures, paralysis, and coma soon after exposure to moxidectin. Diazepam, glycopyrrolate, and intravenous fluids were given along with respiratory ventilation and other supportive care. The puppy improved dramatically within 30 minutes of the second dose of Intralipid. Although ideal dosages have not been established, the typical recommendation is for bolus administration of 1.5 mL/kg of intravenous lipid emulsion, followed by 0.25 mL/kg/min for 30 to 60 minutes.46 Other brands of intravenous lipid emulsion, such as Liposyn, can also be considered. It is best to have the product available ahead of time rather than try to acquire it in the midst of an emergency. Dr. Weinberg’s lipid rescue website (mentioned previously) describes preparation of a kit to have on hand.
Dogs
Ivermectin (Heartgard) tablets are administered orally at a dose level of 0.006 mg/kg (6 mcg/kg) at monthly intervals to prevent the establishment of the D. immitis. The initial dose should be given within 1 month of the first exposure to mosquitoes and throughout the period of the year when mosquitoes are active. The last treatment must be given to dogs within 1 month after the last exposure to mosquitoes. Ivermectin alone has minimal activity against the adult heartworm in the short term. It is active on the third- and fourth-stage larvae and the circulating microfilariae. A single oral dose of ivermectin administered within 2 months of infection prevents the establishment of adult worms in the heart. A single oral dose of 0.05 mg/kg is adequate to clear the circulating microfilariae when given to dogs 4 weeks after the administration of an adulticide, although ivermectin is not approved as a microfilaricide.47 Review of the original reference is suggested for more complete information. When ivermectin (0.006 mg/kg) is given to heartworm-positive dogs over several months, the circulating microfilariae are eliminated, resulting in an occult infection. Thus dogs receiving monthly ivermectin should be tested annually with an occult heartworm test.17,48,49
Knight provides an excellent review of heartworm testing and suggested chemoprophylaxis timing for various regions in the United States.50 The American Heartworm Society guidelines for diagnosis, prevention, and management of heartworm infection in dogs should also be consulted.17 Although there is no FDA-approved microfilaricide, macrocyclic lactones are the safest and most effective microfilaricidal drugs available for use in heartworm-positive dogs.17 Compared with ivermectin, milbemycin is a more potent microfilaricide and causes quicker clearance of microfilariae.17
Short-term use of ivermectin alone has minimal effect on adult heartworms, but when given continuously over a prolonged period, for 1 to 2 years, or when combined with doxycycline, it may have some utility for treating dogs with adult heartworm infection. The older the adult heartworms are when first exposed to ivermectin, the longer it takes them to die; because they continue to cause damage during this time, long-term ivermectin therapy generally is not a substitute for melarsomine (Immiticide) therapy.17 In addition, a mild hypersensitivity reaction has been observed in dogs with circulating microfilariae that are treated with ivermectin. Many products that contain ivermectin have precautions suggesting removal of adult heartworms and microfilariae before initiating ivermectin heartworm prophylaxis.
Regarding the combination of ivermectin and doxycycline as a heartworm adulticide; it has been found that Wolbachia spp. bacteria are filarial species endosymbionts—that is, their presence is necessary for filial worm survival—and that eliminating this bacteria from heartworm-positive dogs and cats will decrease host antigenic response.51,52 In fact, one study of heartworm-positive dogs comparing groups that were treated with three drugs (i.e., melarsomine, doxycycline, and ivermectin), two drugs (i.e., doxycycline and ivermectin), doxycycline alone, ivermectin alone, and melarsomine alone, led the authors to conclude that the combination of doxycycline and ivermectin was synergistic and could eliminate adult heartworms with less potential for severe thromboembolism than melarsomine alone.52 This is discussed in greater depth in the melarsomine section.
Ivermectin given as a single subcutaneous injection or orally administered at 0.2 mg/kg demonstrated high efficacy against the immature and adult T. canis, A. caninum, Ancylostoma braziliense, Uncinaria stenocephala, Strongyloides stercoralis, Capillaria spp., and Filaroides hirthi.53 At that dose its activity against Toxascaris leonina and T. vulpis is erratic.23 When treating respiratory nematode parasites a higher dose, 0.4 mg/kg by subcutaneous injection or orally every 2 weeks for 2 to 3 doses has been recommended recently for Oslerus (Filaroides) osleri, F. hirthi, Aelurostrongylus abstrusus, and Capillaria aerophila infections.54
Several combination products containing ivermectin are available. A combination product (Heartgard Plus) containing ivermectin and pyrantel pamoate is available. See the discussion of combination products for more information.
Cats
Ivermectin is FDA approved as a monthly heartworm preventive in cats (Heartgard Chewable for Cats). The approved monthly oral dose of 0.024 mg/kg is effective in preventing the development of D. immitis and hookworms (A. braziliense and Ancylostoma tubaeforme).55-57 Feline roundworm (Toxocara cati) infections have been controlled with 0.2 to 0.3 mg/kg of ivermectin and lungworm (A. abstrusus) infections with 0.4 mg/kg of ivermectin.58-60 Capillaria species are rarely implicated in feline cystitis, and infestations are thought to be self-limiting usually, but in one case a single dose of ivermectin 0.2 mg/kg, administered subcutaneously, was successfully used to treat the condition in a cat.61
Milbemycin Oxime
Milbemycin oxime was the second macrocyclic lactone approved by the FDA. It is a fermentation product of Streptomyces hygroscopicus aureolacrimosis. It has structural similarities to ivermectin and is believed to work by a similar mechanism of action and have similar pharmacokinetic properties with regard to absorption, metabolism, and excretion. Although an LD50 was never determined for dogs, single oral doses of 200 mg/kg were tolerated in laboratory Beagles. Collie dogs tolerated single oral doses of 10 mg/kg without toxicity.
Dogs
Milbemycin oxime tablets (Interceptor) are formulated to deliver a minimum dose of 0.5 mg/kg of body weight. When given every 30 days it is effective in preventing heartworms (D. immitis).62-64 It also kills A. caninum, T. canis, and T. vulpis.65-6823 One study indicates that milbemycin may help control raccoon roundworm (B. procyonis) infections in dogs and thus decrease the zoonotic potential of a parasite that can have devastating effects in humans, including death.69
Milbemycin oxime has been extensively tested with regard to safety. It is nontoxic to Collies at up to 20 times the recommended dose and is safe when given to pregnant and nursing animals.70,71 Milbemycin oxime, like ivermectin, is known to kill heartworm microfilariae and inhibit the release of new microfilariae. Thus all dogs receiving routine monthly heartworm prophylaxis with milbemycin should be tested with adult antigen tests.72-74,49,17
Moxidectin
Moxidectin was the third macrolide to enter the parasite market. It is a chemically altered product of Streptomyces aureolacrimosus noncyanogenus and has a similar range of activity and safety margin as ivermectin and milbemycin oxime. Moxidectin is currently approved in the United States for injectable use as a heartworm (D. immitis) preventive and hookworm (A. caninum and U. stenocephala) treatment in dogs (ProHeart 6) and for topical use in combination with imidacloprid to prevent heartworms, treat fleas, and control and treat ear mites and intestinal parasites in dogs and cats (Advantage Multi). The combination product is reviewed later in this chapter. The oral use of moxidectin in dogs is covered in the previous edition of this text. Moxidectin toxicity was discussed previously in the Avermectin Toxicity section.
KEY POINT 13-11
Moxidectin, the third macrolide used commercially, is also similar to ivermectin and milbemycin.
Dog
Moxidectin injection (ProHeart 6) was launched in the United States in 2001 with an indication to prevent heartworms and treat hookworms in dogs. The label for the sustained-release injectable product instructed that it was to be given no more often than once every 6 months. The FDA had concerns about safety as a result of adverse event reports that it received, and the manufacturer voluntarily recalled the product from the U.S market in 2004 to address those safety concerns.77 During that time the product remained on the market in Australia, Japan, and parts of Europe. In 2008 the product was reintroduced to the U.S. market with a new label under a postmarketing surveillance initiative based on human drug programs and known as a Risk Minimization Action Plan (RiskMAP), which includes veterinarian training and use of a pet-owner consent form. This is the first veterinary drug to be marketed under RiskMAP, a strengthened risk minimization and restricted distribution program.78 The new label advises not to administer the drug to sick, debilitated, or underweight dogs or those with a history of weight loss and states that the product should be used with caution in dogs with preexisting allergic disease, including food allergy, atopy, and flea allergy dermatitis. The label also warns not to administer moxidectin injection within 1 month of vaccinations.79
Injectable moxidectin is indicated for use in dogs 6 months of age and older for the prevention of heartworm disease. It should be given at 0.17 mg/kg by subcutaneous injection within 1 month of the dog’s first exposure to mosquitoes or within 1 month of the dog’s last dose of monthly heartworm preventive. The sustained-release injection provides a 6-month window of protection from heartworms.79 However, it does not clear microfilariae or remove adult heartworms.23 A challenge study comparing efficacy and adverse reactions among four groups of dogs given placebo or moxidectin at 0.06, 0.17, or 0.5mg/kg and inoculated with 50 D. immitis third-stage larvae 180 days later revealed 100% efficacy at the label dose (0.17 mg/kg).80 However, one of eight dogs given the lower dose (0.06 mg/kg) was infected. The authors speculated that the failure of protection was a result of individual pharmacokinetic variation because the moxidectin serum concentration in the unprotected dog was at the limit of quantitation (lowest detectable quantity) 8 days after treatment and undetectable thereafter compared with others in that group that had detectable concentrations until at least day 14 and for as long as 55 days for most of the other dogs.80 Both the frequency and size of injection-site granulomas correlated positively with the moxidectin dose.80
Moxidectin sustained-release injection is also indicated for the treatment of hookworms A. caninum and U. stenocephala; although it will eliminate larval and adult stages of those parasites, reinfection may occur in less than 6 months, making its use less than ideal when attempting to control recurrent hookworm disease.
Selamectin
Prepared by semisynthetic modification of doramectin,81 selamectin (Revolution) is the latest macrolide to enter the U.S. marketplace. This product is unique among other macrolides used in small animals in that it is formulated for convenient topical administration. It is simply “spotted” onto the skin of the pet. It was the first macrocyclic lactone approved for use in dogs and cats to provide activity against both internal and external parasites. However, the discussion here focuses on the activity of selamectin against internal parasites.
KEY POINT 13-12
Moxidectin is available as a sustained-release injectable heartworm preventive and hookworm treatment.
KEY POINT 13-13
Moxidectin combined with imidacloprid is available as a topical heartworm preventive and flea treatment that controls ear mites and internal parasites.
KEY POINT 13-15
Selamectin is unique among macrolides in its topical formulation and extreme broad spectrum against internal and external parasites.
It is produced as a fermentation product of S. avermitilis, which is then chemically modified.82 The pharmacokinetics after topical administration has been studied extensively. The topical bioavailability varies greatly among species. In dogs the bioavailability is only 4%, but it is much greater in cats (74%). The terminal half-life was much longer in both species after topical administration than after intravenous administration, suggesting sustained release from an extravascular depot. The half-life after topical administration was 11 days for dogs and 8 days for cats.23 The product is absorbed in sufficient quantities and persists for sufficient time to control the target parasites. The approved topical dose is a minimum of 6 mg/kg for dogs and cats.
Selamectin is approved for the prevention of heartworm (D. immitis) in both dogs and cats when applied topically every month.8 Extensive studies proved that the drug’s efficacy against heartworms remained84,85 even when bathing followed application. The bathing study demonstrated that after topical application efficacy is not likely to be decreased by inadvertent swimming or exposure to rainstorms. The drug is not effective in clearing microfilariae. In cats selamectin is also effective in removing hookworm (A. tubaeforme) and roundworm (T. cati). This effect is undoubtedly due to the greater topical bioavailability observed in cats.82 Selamectin is effective against roundworms in dogs23 and lungworms (A. abstrusus) in cats,86 but these are not label-approved indications. The drug has labeled indications in dogs for activity against ear mites, sarcoptic mange mites, and ticks and in cats for activity against ear mites, but these indications are not the focus of this chapter.
KEY POINT 13-16
Selamectin has much greater bioavailability after topical administration on cats compared with dogs.
The safety of selamectin has been established in both dogs and cats and even for use in puppies and kittens over 6 weeks of age. It is also safe to use in ivermectin-sensitive Collies and in breeding dogs and cats.84 Selamectin did not cause any abnormalities when applied topically to ivermectin-sensitive Collies at 40 mg/kg.22 However, avermectin-sensitive Collies given a topical overdose of 10 times the label dose had hypersalivation.28 Clinical signs of dogs reported to the ASPCA Animal Poison Control Center are, in order of frequency, hypersalivation, agitation, diarrhea, facial edema, and hyperactivity.28 In cats the signs in decreasing frequency are vomiting, anorexia, hyperesthesia, hyperthermia, and mydriasis.28 If cats are exposed orally to selamectin they invariably have hypersalivation and sometimes vomit, but topical overdoses at 10 times the label dose in cats did not cause any abnormality.28 Although the insert recommends that dogs should be tested for existing heartworm infections before selamectin administration, it also notes that hypersensitivity reactions were not observed when heartworm-infected dogs were treated with selamectin at 3 times the label dose.87 Clinical studies have confirmed the wide safety margin demonstrated in the laboratory studies.
Benzimidazoles
The benzimidazoles represent a large family of broad-spectrum agents that have been in widespread use for many years in a vast array of animal species. Several excellent review articles88-90 discuss the history, mode of action, and spectrum of activity of this useful class of anthelmintics.
Thiabendazole, the first benzimidazole discovered, represented a major step forward when it became available in the early 1960s.91 At the time of its introduction, thiabendazole was a true broad-spectrum product that was very safe to the host animal. Since that time, parasite resistance to the benzimidazoles has been discovered in several species.
KEY POINT 13-17
Introduced to the market in the early 1960s, thiabendazole was the first benzimidazole used commercially and was a major advancement in anthelmintics. It is not commercially available as an anthelmintic for small animals.
Considerable effort has been devoted to determining benzimidazole mechanism of action. Conventional wisdom holds that benzimidazoles bind to tubulin molecules, which inhibit the formation of microtubules and disrupt cell division.92,93 It has a much higher affinity for nematode tubulin versus mammalian tubulin, thus providing selective activity against parasites. Evidence also indicates that the benzimidazoles can inhibit fumarate reductase, which blocks mitochondrial function and kills the parasite by depriving it of energy.
The benzimidazoles are poorly soluble and thus are generally given by mouth. They are more effective in horses and ruminants because of their slow transit through the cecum and rumen. Proper use in small animals requires that the benzimidazoles be given for a minimum of 3 days in a row. The dose is usually more effective when divided into two doses per day, thus prolonging the contact time with the parasite.
Because both albendazole and oxfendazole were found to be teratogenic, their use is limited to nonpregnant animals. For simplicity, febantel, a nonbenzimidazole drug that is metabolized to a benzimidazole, thus sharing efficacy and mechanism of action, is included in this section with the other benzimidazoles.
Albendazole
Albendazole is the newest benzimidazole. It has potent broad-spectrum anthelmintic activity, but is not approved for use in dogs and cats. Albendazole has demonstrated a broad spectrum of anthelmintic activity against gastrointestinal nematodes; lung nematodes, including inhibited larval forms; cestodes; and lung and liver trematodes in farm animals, companion animals, and humans. Albendazole (Albenza or Zentel) is used overseas to treat humans with intestinal helminth infections, hydatid disease, or cysticercosis. It is commonly used for treatment of nematode and trematode infections in large animals (Valbazen).
KEY POINT 13-19
Albendazole is the latest benzimidazole to be used commercially, but it is not approved for use in dogs or cats.
Albendazole, like other benzimidazoles, is well absorbed (about 50% bioavailable) and converted in the liver to the active metabolites albendazole sulfoxide and albendazole sulfone. These active metabolites are thought to bind to tubulin and inhibit fumarate reductase. The parent drug and its metabolites are excreted primarily in the urine.
Because albendazole was shown to be teratogenic, its use is limited to nonpregnant animals. Dogs treated with 50 mg/kg twice daily may develop anorexia. Cats may exhibit lethargy, depression, and anorexia when treated.28 When cats were given 100 mg/kg/day for 14 to 21 days, they displayed weight loss, neutropenia, and mental dullness.28 When used clinically, albendazole may be associated with significant toxicity, including myelosuppression (leukopenia, anemia, and thrombocytopenia), abortion, teratism, anorexia, depression, ataxia, vomiting, and diarrhea.94,95 Recent evidence suggests that it may cause aplastic anemia in dogs and cats.28 Veterinarians are advised to use due caution with this product.
Albendazole is available as an oral suspension (Valbazen) containing 113.6 mg/mL. Dogs can be treated for lungworms (F. hirthi) at a dose of 25 to 50 mg/kg twice daily for 5 days, with the treatment repeated in 2 to 3 weeks, and for bladder worms (Capillaria plica) at a dose of 50 mg/kg twice daily for 10 to 14 days.28 Both dogs and cats can be treated for the lung fluke (Paragonimus kellicotti) at a dose of 25 mg/kg twice daily for 14 days or 50 mg/kg orally once daily for 21 days.28 Although albendazole is effective against these uncommon parasites, ivermectin and praziquantel are more convenient therapies and likely to be just as effective.
Febantel
Febantel is a prodrug that is metabolized to fenbendazole and oxfendazole, which are undoubtedly the active parasiticides.90 The oral acute toxic dose in mice, rats, and dogs is more than 10 g/kg (10,000 mg/kg). At oral doses above 150 mg/kg per day for 6 days, transient salivation, diarrhea, vomiting, and anorexia may be seen in dogs and cats. Febantel is not available in a single-entity formulation but only in combination with praziquantel and pyrantel, which are discussed in the section on combination products.
Fenbendazole
Fenbendazole (Panacur) is a commercially successful benzimidazole that is widely used in dogs. The oral LD50 for rats and mice is more than 10 g/kg (10,000 mg/kg).28 Fenbendazole does not have embryotoxic or teratogenic effects in rats, sheep, or cattle. In the rabbit fenbendazole was fetotoxic but not teratogenic, and no carcinogenesis was observed in lifetime studies of rats and mice. In a 6-month toxicity study in dogs, no effect was observed at 4 mg/kg or less.
KEY POINT 13-20
Febantel is metabolized to fenbendazole and oxfendazole, which are probably the active molecules.
KEY POINT 13-21
Fenbendazole is used in a wide variety of species, including dogs, cats, livestock, horses, and zoo animals.
Fenbendazole is a broad-spectrum anthelmintic with activity against a wide variety of nematodes and cestodes in dogs, cats, cattle, sheep, goats, horses, and many zoo animals. Absorbed fenbendazole is metabolized to at least two active metabolites, oxfendazole sulfoxide and oxfendazole sulfone. It undergoes enterohepatic cycling in ruminants, which prolongs effective blood levels.96 In the United States fenbendazole is approved for control of helminth parasites of horses, cattle, and dogs.
Dogs
Fenbendazole is approved only for use in dogs 6 weeks of age and older. Fenbendazole granules (Panacur) are mixed in the feed at a dose level of 50 mg/kg and given to dogs for 3 consecutive days for the removal of T. canis, T. leonina, A. caninum, U. stenocephala, T. vulpis, and Taenia pisiformis.97 It has shown excellent activity against Giardia spp. in dogs at the approved dose.95,98 At longer-than-approved duration of therapy (i.e., 50 mg/kg daily for 10 to 14 days), it has been used to treat the lung fluke, P. kellicotti, in dogs.28 Fenbendazole is relatively safe, and there are no known contraindications to its use in dogs.
Tetrahydropyrimidines
The tetrahydropyrimidines include the numerous salts of pyrantel, morantel, and the investigational compound oxantel, which is available outside the United States. They all act as nicotinic agonists, which disturb the neuromuscular system, causing contraction and subsequent tonic paralysis.99-102 In vitro experiments indicate that pyrantel is 100 times more powerful than acetylcholine. It seems that the nicotinic acetylcholine receptors of invertebrate parasites are essential for neurologic function but differ in physiology and distribution in mammals.103 In ruminants these products are rapidly metabolized to inactive metabolites; therefore higher doses are required to treat ruminants compared with monogastric animals.20
KEY POINT 13-23
Tetrahydropyrimidines are nicotinic agonists. They cause contraction and subsequent tonic paralysis of parasites.
Pyrantel
Pyrantel was introduced in the United States as a broad-spectrum anthelmintic for sheep in 196622 and is now available under a wide variety of trade names in the form of tablet, paste, oral suspension, and medicated feed.104 The tartrate salt of pyrantel is a white powder, soluble in water, that is used in horses and swine. Pyrantel tartrate is well absorbed after oral administration in the rat, dog, and pig. Plasma levels peak within 3 to 6 hours.22 Pyrantel tartrate is rapidly metabolized and in dogs is primarily eliminated by way of the urinary tract, but pyrantel pamoate is poorly absorbed from the gastrointestinal tract and is primarily eliminated through the feces with less than 15% excretion through the urinary tract.104
KEY POINT 13-25
Pyrantel pamoate is poorly absorbed, which contributes to its safety in young animals.
The pamoate salt of pyrantel is a yellow powder, insoluble in water. It is available as a ready-to-use suspension and as tablets for dogs and horses. The fact that pyrantel pamoate is poorly absorbed from the intestine adds to its safety in very young or weak animals. Pyrantel salts are stable in solid form but photodegrade when dissolved or suspended in water, resulting in reduction of potency.
Dogs
Pyrantel pamoate is available as a tablet, chewable tablet, and palatable suspension (Nemex and many other trade names) and is indicated for the removal of T. canis, T. leonina, A. caninum, and U. stenocephala from dogs and puppies.105-108 The drug has also been used to treat Physaloptera stomach worms in dogs, although such use is not approved.104,109 Pyrantel may have some effect on tapeworms as well, but other drugs are commonly used to treat tapeworm infections in small animals. The recommended dose of 5 mg/kg of pyrantel pamoate suspension is administered orally or mixed with a small amount of feed.104 For animals weighing 2.25 kg or less, the dose is increased to 10 mg/kg. Tablets may be administered directly or placed in a small portion of food. Pyrantel pamoate is safe for nursing and weanling pups, pregnant bitches, males used for breeding, and dogs infected with D. immitis. The oral LD50 is greater than 690 mg/kg in dogs.22 In chronic toxicity studies dogs had no adverse effects when given 20 mg/kg daily for 3 months but did have ill effects at 50 mg/kg or above daily.22 Pyrantel pamoate is compatible with organophosphates and other antiparasitic and antimicrobial agents.
Cats
Pyrantel pamoate products used in dogs are not labeled for cats but are considered very safe and effective against some common feline parasites, the ones that are similar to those afflicting dogs and are listed as indications on the canine label. The oral dose range of 5 to 20 mg/kg is reportedly efficacious against ascarids, hookworms, and Physaloptera spp., with the dose repeated in 2 to 3 weeks in some cases.22,27,28,104 Pyrantel pamoate is labeled for cats as a combination product with praziquantel (Drontal), which is described later, in the combination product section.
Cyclic Depsipeptides
The cyclodepsipeptide PF1022A, isolated from Mycelia sterilia, was found to have low toxicity and strong anthelmintic properties when tested against Ascaridia galli in chickens, making it one of the most promising deworming prospects to emerge since the discovery of avermectins and milbemycin.
Emodepside
Emodepside is the first cyclic depsipeptide to be approved for use against animal parasites in the United States. It is a semisynthetic derivative of PF1022A, mentioned previously. The product binds to a presynaptic latrophilin receptor in parasitic nematodes, which results in flaccid paralysis and death.110 It has low to moderate acute toxicity in mammalian species. The oral LD50 in rats is greater than 500 mg/kg and is more than 2000 mg/kg when applied to the skin. Studies in rats and rabbits suggest that emodepside may interfere with fetal development.111 Women who are pregnant or may become pregnant should avoid direct contact with emodepside and wear disposable gloves if product handling is necessary. Emodepside is only available commercially as a combination product, combined with praziquantel, and as such is discussed in detail later, in the section on broad-spectrum combinations.
Piperazines
Piperazine was used to treat human gout in the early 1900s because it acts as an excellent uric acid solvent. Its anthelmintic activity was discovered in the 1950s.22 Since then, a wide variety of piperazine salts have been derived. The various salts of piperazine (adipate, hydrochloride, sulfate, monohydrate, citrate, and dihydrochloride) are used as anthelmintics in swine, poultry, horses, dogs, and cats. Piperazine is quite safe to use in these species but has a narrow spectrum of action, limited primarily to roundworms.93,27 Anthelmintic activity depends on the salt freeing its piperazine base in the gastrointestinal tract. The amount of piperazine (base) in each salt varies widely. The citrate salt contains 35% piperazine; adipate salts, 37%; phosphate salts, 42%; and dihydrochloride salts, 50% piperazine base.96
Piperazine paralyzes worms by blocking the action of acetylcholine and GABA at the neuromuscular junctions, and the worms are eliminated by intestinal peristalsis.20,112 It acts by hyperpolarizing nerve membranes at the neuromuscular junction, leading to flaccid paralysis of the parasite.22 Piperazine is also one of the active ingredients in a number of combination anthelmintic products. Piperazine should not be used in combination with pyrantel pamoate because the modes of action are antagonistic.
KEY POINT 13-28
Piperazine paralyzes worms by blocking acetylcholine and GABA, and the worms are eliminated by intestinal peristalsis; thus it may not work well in the face of gastrointestinal hypomotility.
Piperazine is rapidly absorbed from the gastrointestinal tract and rapidly cleared by urinary excretion. Elimination is virtually complete within 24 hours.96 It may not be effective in animals with intestinal hypomotility because the paralyzed worms may recover from the drug effect before they are passed in the stool. Piperazine should be used with caution in animals with hepatic or renal dysfunction. Occasional adverse reactions observed in dogs include ataxia, diarrhea, and vomiting.
Piperazine is available as tablets, solution, and soluble powder under many proprietary names (Pipatabs, Puppy Paste, Happy Jack KittyKat Paste, Tasty Paste). It is practically nontoxic. The oral LD50 for rats is 4.9 g/kg and for mice is 11.4 g/kg. Treatment for intoxication is symptomatic and supportive. Piperazine can be administered to animals of all ages.
Isoquinolones
The cesticidal isoquinolones are represented by two closely related drugs: praziquantel and epsiprantel. This cesticidal class is the safest and most effective yet approved in the United States. They attack the parasite neuromuscular junction and the tegument. These drugs cause increased cell membrane permeability to calcium and resulting loss of intracellular calcium. This effect produces an instantaneous contraction and paralysis of the parasite.116 The second effect is a devastating vacuolization and destruction of the protective tegument.92,117 The combined effects of paralysis and tegmental destruction provide excellent activity against cestodes.
KEY POINT 13-29
Isoquinolones are the safest and most effective cesticidal drugs approved in the United States.
Praziquantel
Praziquantel was the first cesticidal isoquinolone approved in the United States. It has marked anthelmintic activity against a wide range of adult and larval cestodes and trematodes of the genus Schistosoma. Oral administration results in nearly complete absorption and rapid distribution throughout the body and across the blood–brain barrier. Although 80% of the drug is eliminated in the urine, the main site of inactivation is the liver, with only trace amounts of the unchanged drug excreted primarily in the urine.22,91 Praziquantel has high oral bioavailability, high protein binding, and a marked first-pass effect. The oral half-life in dogs is reported to be 30 to 90 minutes22 to 3 hours.28
KEY POINT 13-30
Praziquantel, the first approved cesticidal isoquinolone in the United States, has marked anthelmintic activity against cestodes and trematodes.
Praziquantel is a very safe anthelmintic. Rats tolerated daily administration of up to 1000 mg/kg for 4 weeks, and dogs tolerated up to 180 mg/kg per day for 13 weeks. Vomiting is typically observed at high dosage rates. Injected doses of 200 mg/kg were lethal in cats.28 Praziquantel did not induce embryotoxicity, teratogenesis, mutagenesis, or carcinogenesis, nor did it affect the reproductive performance of test animals. Occasional adverse experiences in clinical use include pain on injection, anorexia, diarrhea, salivation, vomiting, sleepiness, staggering, and weakness. Overdoses have been reported to cause diarrhea, depression, incoordination, tremors, salivation, and vomiting.
Dogs and cats
Praziquantel (AmTech, Droncit) is administered orally or injected subcutaneously at 2.5 to 7.5 mg/kg for the removal of Dipylidium caninum, Taenia taeniaeformis, T. pisiformis, T. hydatigena, T. ovis, Mesocestoides corti, Echinococcus granulosus, Echinococcus multilocularis, Spirometra spp., Diphyllobothrium latum, D. erinacei, and Joyeuxiella pasquali.118-12496 The product insert has extensive information about using this drug to help control E. multilocularis, including the life cycle of the parasite, difficulty of diagnosis, and other public health considerations.125 Praziquantel injection is not intended for use in puppies or kittens younger than 4 weeks of age. Several combination products contain praziquantel; see the section on combination products for more information.
Epsiprantel
Epsiprantel (Cestex) was the second cesticidal isoquinolone to be approved in the United States. Unlike its cousin praziquantel, epsiprantel is poorly absorbed after oral administration. Less than 0.1% is recovered from the urine; there are no known metabolites.28 It is eliminated in the feces unchanged.22 Because of the low bioavailability, systemic toxicity and teratogenic effects are very unlikely, but the safety of epsiprantel in pregnant dogs and cats has not been proved. In acute toxicity studies in mice and rats, the oral minimum lethal dose of epsiprantel was shown to be more than 5000 mg/kg. Doses as high as 36 times the label dose were well tolerated in dogs and caused vomiting in some kittens.28 Cats given the drug at 40 times the label dose for 4 days had minimal signs. Dogs given 90 times the label dose for 14 days had no significant adverse events.126
KEY POINT 13-32
Like praziquantel, epsiprantel, the second cesticidal isoquinolone approved in the United States, has a wide margin of safety, but unlike praziquantel, it is poorly absorbed orally.
Epsiprantel treatment as an oral film-coated tablet, at 2.75 mg/kg for cats or 5.5 mg/kg for dogs, effectively removes D. caninum, T. taeniaeformis, T. pisiformis, and T. hydatigena after a single dose.127,128 Evidence suggests that the drug is effective against E. granulosa and E.multilocularis, but the data are insufficient to recommend a dosage that will completely clear the infection from those treated.129 Epsiprantel was given concurrently with diethylcarbamazine (in dogs), antiinflammatory drugs, insecticides, and nematocides with no incompatibilities observed.126 It should not be used in puppies and kittens younger than 7 weeks of age.
Arsenicals
Heavy metals like arsenic and antimony are well represented in the history of anthelmintics. To date, safer and more effective drugs for the most common parasites have largely replaced arsenicals. Their use is now limited to removal of adult D. immitis. Thiacetarsemide (Caparsolate) is no longer available commercially in the United States130 and thus will not be covered in this chapter. The therapeutic effect of arsenicals depends on a reaction between the arsenic salt and sulfhydryl-containing enzymes.131 Inactivation of parasite enzyme systems causes death. Because arsenic is widely known as a toxin in man and animal, caution is required when using these products.
Melarsomine
Although contraindicated in cats, melarsomine dihydrochloride (Immiticide), the only arsenical anthelmintic commercially available in the U.S. veterinary market, has 92% to 98% efficacy against adult D. immitis in dogs.132-136 The arsenic content of the product is less than that of thiacetarsemide, making melarsomine less toxic to the patient. Melarsomine is labeled to be administered intramuscularly at a dose of 2.5 mg/kg for two injections given 24 hours apart to dogs at low risk of thromboembolic complications.17 Dogs that have moderate risk of thromboembolism may be treated with an alternative three-injection regimen of a single injection followed by a rest period of 1 to 2 months, after which two standard injections are given.17 This later three-injection regimen is reportedly less hazardous for the patient and more efficacious and therefore is the preferred regimen recommended by the American Heartworm Society, regardless of the stage of disease (unless melarsomine is otherwise contraindicated).17,18a Melarsomine is contraindicated in dogs with severe heartworm disease associated with caval syndrome.28 Injections should only be made deep into the lumbar epaxial muscles along L3 to L5. Peak blood level is achieved in about 11 minutes after injection, and the half-life is 3 hours.28
About one third of dogs treated will have injection site reactions, most of which resolve within a week, but firm nodules at the injection site can persist indefinitely.28 Additional adverse reactions include elevated hepatic enzymes, coughing, gagging, depression, lethargy, anorexia, fever, pulmonary congestion, and vomiting.27 This drug exemplifies the problem of parasite removal by poisoning the patient just enough to kill the parasite, hopefully without damaging the patient too much. It has a narrow therapeutic range. The toxic dose is only 2.5 to 3 times the recommended dose and can result in panting, pulmonary inflammation, salivation, vomiting, edema, and death. Safety has not been determined in breeding, pregnant, or lactating dogs. That said, clinical studies indicate that the treatment is well tolerated even in dogs that have clinical signs of heartworm disease.135,137,138
As previously mentioned in the ivermectin section of this chapter, the American Heartworm Society guidelines for diagnosis, prevention, and management of heartworm infection in dogs should be consulted before treating a heartworm-infected dog with melarsomine.18a Treatment with a macrocytic lactone before administration of melarsomine should be considered along with other methods to reduce the potential for melarsomine adverse reactions. For example, as previously mentioned in the ivermectin section, one study of heartworm-positive dogs comparing groups that were treated with three drugs (i.e., melarsomine, doxycycline and ivermectin), two drugs (i.e., doxycycline and ivermectin), doxycycline alone, ivermectin alone, or melarsomine alone led the authors to conclude that the combination of doxycycline and ivermectin was synergistic.52 All dogs treated with ivermectin plus doxycycline (with or without melarsomine) were free of microfilariae in 9 weeks. This may be related to the elimination of Wolbachia sp. bacteria, which are filarial endosymbionts. The authors found that the administration of doxycycline plus ivermectin for several months before (or without) melarsomine resulted in elimination of adult heartworms with less severe thromboembolism than did treatment with melarsomine alone.52
Miscellaneous
Dichlorophen
Dichlorophen (Happy Jack Tapeworm Tablets) is a chlorinated analog of diphenylmethane. It has low toxicity for mammals. The oral LD50 is 2690 mg/kg for rats, and the acute oral LD50 in dogs is 1000 mg/kg. Dichlorophen has bacteriostatic, fungicidal, and cesticidal properties. It causes electron transport–linked phosphorylation to uncouple in the parasite mitochondria and is relatively safe in the host because of low gastrointestinal absorption.91 Dichlorophen may be given orally as an “aid in the removal” of D. caninum and T. pisiformis tapeworms from dogs.139 The drug may be administered orally in tablet or capsule form at 220 mg/kg after an overnight fast. The tapeworms are killed, digested, and eliminated in an unrecognizable form. Animals occasionally vomit or develop diarrhea after treatment with dichlorophen.
Broad-Spectrum Combinations
The veterinary practitioner is always looking for anthelmintic products that cover an ever-increasing spectrum of parasites. Broad-spectrum products provide two important advantages. First, they obviate dosing with several different products at once when a patient has a mixed parasite infection, making administration easier. Second, they provide peace of mind that a treated animal will be cleared of possibly undiagnosed parasites. For instance, a puppy from the animal shelter will be better served by use of a product that is effective in removing both roundworms and hookworms than a product that is effective against only roundworms. There are two ways to increase the spectrum of anthelmintics: either by tackling the arduous task of discovering a single broad-spectrum chemical or by combining several compatible active ingredients to build the desired spectrum of activity.
KEY POINT 13-38
Broad-spectrum anthelmintic combinations make treating mixed parasitic infections easier and may treat common undiagnosed parasites; therefore they are associated with increased veterinary and owner confidence.
Combination anthelmintic products are briefly reviewed in this section, but not combinations that are formulated to treat or prevent fleas or other nonhelminth parasites. In many cases combination-product formulation and dosing regimens are different from those of the single-entity drug ingredients. The toxicity and mechanism of action of the individual ingredients are covered earlier in this chapter.
Emodepside Plus Praziquantel
This product is formulated for use in cats (Profender) as a topical spot-on that contains 1.98% emodepside and 7.94% praziquantel. The prefilled applicators deliver a minimum dose of 3 mg/kg emodepside and 12 mg/kg praziquantel when applied to the skin. The active ingredients are readily absorbed through the skin, enter systemic circulation, and act on target parasites in the gastrointestinal tract. It is labeled for use in cats and kittens that are at least 8 weeks of age and is considered safe to use in heartworm-positive cats.111 The product is safe and effective in removing roundworms, T. cati (adults and fourth-stage larvae); hookworms, A. tubaeforme (adults, immature adults, and fourth-stage larvae); and tapeworms, D. caninum and T. taeniaeformis.140-142 The topical emodepside–praziquantel product was very effective and safe when used in a large-scale clinical study comparing it with topical selamectin–oral epsiprantel.143
Pyrantel Plus Praziquantel
Two-way combination products containing pyrantel and praziquantel are approved for use in the United States in both dogs (Virbantel) and in cats (Drontal).
Dogs
The canine product is formulated to deliver 5 mg of praziquantel and 5 mg of pyrantel pamoate per kilogram. A single dose is given to dogs to remove tapeworms (D. caninum and T. pisiformis), hookworms (A. caninum, A. braziliense, and U. stenocephala), and roundworms (T. canis and T. leonina).
Cats
The feline product is formulated to deliver at least 5 mg of praziquantel and 20 mg of pyrantel pamoate per kilogram. A single dose is given to cats and kittens to remove tapeworms (D. caninum and T. taeniaeformis), hookworms (A. tubaeforme), and roundworms (T. cati). The product is 98% effective and well tolerated. Cats maintained in conditions of heavy or constant parasite exposure should be reevaluated in 2 to 4 weeks. This combination product should not be used in kittens weighing less than 1.5 pounds or those younger than 4 weeks of age.
Pyrantel Plus Praziquantel Plus Febantel
A three-way combination of pyrantel, praziquantel, and febantel (Drontal Plus) is available in the United States and many other parts of the world. This product is formulated to deliver at least 25 mg febantel, 5 mg praziquantel, and 5 mg pyrantel pamoate per kilogram. A single dose is given to dogs to remove tapeworms (D. caninum, T. pisiformis, E. granulosus, E. multilocularis), hookworms (A. caninum, U. stenocephala), roundworms (T. canis, T. leonina), and whipworms (Trichuris vulpis).144,145 It is interesting to note that a single dose of this combination is effective against nematodes, especially whipworms, but that febantel alone requires three daily doses to effectively remove nematodes. This combination of ingredients may be synergistic. This product should not be used in pregnant dogs, dogs weighing less than 2 pounds, or puppies younger than 3 weeks of age.
Ivermectin Plus Pyrantel
Ivermectin combined with pyrantel pamoate is available in flavored chunks or tablets (Heartgard-30 Plus, Iverhart Plus, Tri-Heart Plus) for dogs. Pyrantel pamoate is added to provide action against gastrointestinal parasites because the heartworm-preventive dose of ivermectin, which is safe for Collies, is not effective against these important parasites. The product is formulated to deliver a target dose of 0.006 mg (6 mcg) of ivermectin and 5 mg of pyrantel pamoate per kilogram of body weight. Given orally to dogs every 30 days, it treats and controls roundworms (T. canis, T. leonina) and hookworms (A. caninum, A. braziliense, and U. stenocephala) and prevents heartworms (D. immitis).146 At a minimum, the product should be given at monthly intervals during the heartworm season. Adult heartworms do not produce detectable levels of microfilariae when exposed to ivermectin, so an antigen test should be used to reveal the presence of adult heartworms.17 Safety tests have revealed that the ivermectin–pyrantel combination is well tolerated.147 This medication should not be given to dogs younger than 6 weeks of age. (See the ivermectin section of this chapter for a discussion of administration of ivermectin to dogs harboring adult heartworms, a procedure that carries some risk and is not a labeled indication for use.)
Ivermectin Plus Pyrantel Plus Praziquantel
Ivermectin combined with pyrantel pamoate and praziquantel is available in flavored tablets (Iverhart Max) for dogs. Adding praziquantel to the two-way combination product previously mentioned extends the parasite spectrum to include tapeworms. This product is formulated to deliver a target dose of 0.006 mg (6 mcg) of ivermectin, 5 mg of pyrantel pamoate, and 5 mg of praziquantel per kilogram. Given orally to dogs every 30 days, it treats and controls roundworms (T. canis, T. leonina), hookworms (A. caninum, A. braziliense, and U. stenocephala), and tapeworms (D. caninum, T. pisiformis) and prevents heartworms (D. immitis). At a minimum, the product should be given at monthly intervals during the heartworm season. Adult heartworms do not produce detectable levels of microfilariae when exposed to ivermectin, so an antigen test should be used to reveal the presence of adult heartworms.17 This medication should not be given to dogs younger than 8 weeks of age or those with existing heartworm infections. (See the ivermectin section of this chapter for a discussion of administration of ivermectin to dogs harboring adult heartworms, a procedure that carries some risk and is not a labeled indication for use.)
Milbemycin Oxime Plus Lufenuron
A two-way combination of milbemycin oxime and lufenuron (Sentinel) is approved for use in dogs. It is formulated to deliver a minimum dose of 0.5 mg of milbemycin oxime and 10 mg of lufenuron per kilogram of body weight. When given every 30 days, it is effective in preventing heartworms (D. immitis). The product also kills hookworms (A. caninum), removes and controls roundworms (T. canis and T. leonina) and whipworms (T. vulpis), and controls fleas. This medication should not be used in puppies younger than 4 weeks of age or those that weigh less than 2 pounds. This product is approved for concurrent administration with nitenpyram (Capstar) for quick knockdown of existing flea populations.
Imidacloprid Plus Moxidectin
A new combination product (Advantage Multi) contains imidacloprid for external parasites and moxidectin for internal parasites. The canine product provides a minimum of 10 mg/kg of imidacloprid and 2.5 mg/kg moxidectin, whereas the feline product provides the same dose of imidacloprid and 1 mg/kg moxidectin. It is important not to use the canine product on cats because cats are more sensitive to moxidectin than dogs.
Dogs
The canine product is approved for the prevention of heartworms (D. immitis), for the treatment and control of adult and larval hookworms (A. caninum, U. stenocephala), adult and larval roundworms (T. canis, T. leonina), and whipworms (T. vulpis)148 The canine product has not been tested in dogs that weigh less than 1.36 kg (3 lb) or are younger than 7 weeks of age. Nor has it been tested in breeding, pregnant, or lactating dogs. Dogs should be tested for the presence of heartworm before administration. The canine product is not effective against adult heartworm or for clearing microfilariae. It was well tolerated at 5 times the label dose. Oral ingestion of the product by dogs may cause serious reactions, including depression, salivation, dilated pupils, incoordination, panting, and generalized tremors. Thus it is important to prevent dogs from licking the product from the application site.
Cats
The feline product is approved for the prevention of heartworm, D. immitis; for the treatment and control of adult and larval hookworm, A. tubaeforme; and adult and larval roundworm, T. cati. It should not be used on cats that weigh less than 0.9 kg (2 lb) or on cats younger than 9 weeks of age. This product was well tolerated when given at 5 times the label dose in 9-week-old kittens. Cats dosed with a single dose at 10 times the label dose exhibited mild transient hypersalivation. Oral ingestion of the product may cause hypersalivation, tremors, vomiting, and decreased appetite.
This combination is also effective in treating fleas in dogs and cats and ear mites in cats.
1. Foreyt W.J. Veterinary parasitology reference manual, ed 5. Ames, Iowa: Blackwell; 2001.
2. Mehlhorn H., editor. Encyclopedic reference of parasitology: diseases, treatment, therapy, ed 2, New York: Springer-Verlag, 2001.
3. Taylor M.A., Coop R.L., Wall R.L. Veterinary parasitology, ed 3. Ames Iowa: Blackwell; 2007.
4. Bowman D.D. Georgi’s parasitology for veterinarians, ed 9. Philadelphia: Saunders; 2008.
5. Blagburn B.L., Lindsay D.S., Vaughan J.L., et al. Prevalence of canine parasites based on fecal floatation. Compend Cont Educ Pract Vet. 1996;18(5):483-523.
6. Bowman D.D. Spread of companion animal vector-borne parasitic disease in the US and Europe: Concerns relative to travel, national disasters, shelter-source animals and wildlife. 2nd Canine Vector-Borne Disease (CVBD) Symposium. Mazara del Vallo, Sicily, Italy. 2007.
7. Blagburn B.L., Schenker R., Gagne F., et al. Prevalence of intestinal parasites in companion animals in Ontario and Quebec, Canada, during the winter months. Vet Ther. 2008;9(3):169-175.
8. Companion Animal Parasite Council. Accessed February 19, 2010, at http://www.capcvet.org/.
9. Bowman D.D., Torre C.J., Mannella C. Survey of 11 western states for heartworm (Dirofilaria immitis) infection, heartworm diagnostic and prevention protocols, and fecal examination protocols for gastrointestinal parasites. Vet Ther. 2007;8(4):293-304.
10. Murray W.J., Kazacos K.R. Raccoon roundworm encephalitis. Clin Infect Dis. 2004;39(10):1484-1492.
11. Centers for Disease Control (CDC): Guidelines for veterinarians: prevention of zoonotic transmission of ascarids and hookworms of dogs and cats. Accessed February 19, 2010, at http://www.cdc.gov/ncidod/dpd/parasites/ascaris/prevention.pdf.
12. Urbani C., Albonico M. Anthelminthic drug safety and drug administration in the control of soil-transmitted helminthiasis in community campaigns. Acta Trop. 2003;86(2-3):215-221.
13. Hotez P.J., Bethony J., Bottazzi M.E., et al. Hookworm: “The Great Infection of Mankind,”. PLoS Med. 2005;2(3):e67.
14. Hotez P.J., Pritchard D.I. Hookworm infection. Sci Am. 1995;272(6):68-75.
15. Traub R.J., Robertson I.D., Irwin P., et al. The role of dogs in transmission of gastrointestinal parasites in a remote tea-growing community in northeastern India. Am J Trop Med Hyg. 2002;67(5):539-545.
16. Labarthe N., Serrao M.L., Ferreira A.M., et al. A survey of gastrointestinal helminths in cats of the metropolitan region of Rio de Janeiro, Brazil. Vet Parasitol. 2004;123(1-2):133-139.
17. American Heartworm Society 2005 Guidelines for the diagnosis, prevention and management of heartworm (Dirofilaria immitis) infection in dogs, American Heartworm Society. Accessed January 25, 2009, at http://www.heartwormsociety.org/article_48.html.
18. American Heartworm Society 2007 Guidelines for the diagnosis, prevention and management of heartworm (Dirofilaria immitis) infection in cats, American Heartworm Society. Accessed January 26, 2009, at http://www.heartwormsociety.org/article_47.html.
18a. American Heartworm Society 2010 Guidelines for the diagnosis, prevention and management of heartworm (Dirofilaria immitis) infection in dogs, American Heartworm Society. Accessed July 18, 2010, at http://www.heartwormsociety.org/veterinary-resources/canine-guidelines.html.
19. North American Compendiums. Compendium of veterinary products, ed 10. Port Huron, Mich: North American Compendiums; 2007.
20. Campbell W.C., Rew R.S., editors. Chemotherapy of parasitic diseases. New York: Plenum Press, 1985.
21. Vanden Bossche H., Thienpoint D., Janssens P.G., editors. Chemotherapy of gastrointestinal helminths. New York: Springer-Verlag, 1985.
22. Riviere J.E., Papich M.G., editors. Veterinary pharmacology and therapeutics, ed 9, Ames, Iowa: Wiley-Blackwell, 2009.
23. United States Pharmacopeia: Macrocyclic lactones (veterinary-systemic). The United States Pharmacopeial Convention, Inc. Accessed Jan 26, 2009, at http://www.usp.org/pdf/EN/veterinary/macrocyclicLactones.pdf.
24. Shoop W.L., Mrozik H., Fisher M.H. Structure and activity of avermectins and milbemycins in animal health. Vet Parasitol. 1995;59(2):139-156.
25. Wolstenholme A.J., Rogers A.T. Glutamate-gated chloride channels and the mode of action of the avermectin/milbemycin anthelmintics. Parasitology. 2005;131(Suppl):S85-S95.
26. Paul A., Tranquilli W. Ivermectin. In: Kirk R.W., editor. Current veterinary therapy X. ed 10. Philadelphia: Saunders; 1989:140-142.
27. Papich M.G. Saunders handbook of veterinary drugs, ed 2. Philadelphia: Saunders; 2007.
28. Plumb D.C. Plumb’s veterinary drug handbook. Ames, Iowa: Blackwell; 2008.
29. Dorman D.C. Neurotoxic drugs in dogs and cats. In: Bonagura J.D., editor. Kirk’s current veterinary therapy XII. Philadelphia: Saunders; 1995:1140-1145.
30. Rumbeiha W.K. Parasiticide toxicosis: avermectins. In: Bonagura J.D., editor. Kirk’s current veterinary therapy XIV. St Louis: Saunders; 2009:125-127.
30a. Merola V., Khan S., Gwaltney-Brant S. Ivermectin toxicosis in dogs: a retrospective study. J Am Anim Hosp Assoc. 2009;45(3):106-111.
31. Gwaltney-Brant S. ASPCA Animal Poison Control Center—veterinary toxicologist. Personal Communication. January 26, 2010.
32. Pulliam J.D., Seward R.L., Henry R.T., et al. Investigating ivermectin toxicity in Collies. Vet Med. 1985:33-40. (June)
33. Hugnet C., Cadore J.L., Buronfosse F., et al. Loperamide poisoning in the dog. Vet Hum Toxicol. 1996;38(1):31-33.
34. Mealey K.L., Bentjen S.A., Gay J.M., et al. Ivermectin sensitivity in collies is associated with a deletion mutation of the mdr1 gene. Pharmacogenetics. 2001;11(8):727-733.
35. Mealey K.L. Therapeutic implications of the MDR-1 gene. J Vet Pharmacol Ther. 2004;27(5):257-264.
36. Mealey K.L., Bentjen S.A., Waiting D.K. Frequency of the mutant MDR1 allele associated with ivermectin sensitivity in a sample population of collies from the northwestern United States. Am J Vet Res. 2002;63(4):479-481.
37. Neff M.W., Robertson K.R., Wong A.K., et al. Breed distribution and history of canine mdr1-1Delta, a pharmacogenetic mutation that marks the emergence of breeds from the collie lineage. Proc Natl Acad Sci U S A. 2004;101(32):11725-11730.
38. Geyer J., Doring B., Godoy J.R., et al. Development of a PCR-based diagnostic test detecting a nt230(del4) MDR1 mutation in dogs: verification in a moxidectin-sensitive Australian Shepherd. J Vet Pharmacol Ther. 2005;28(1):95-99.
39. Mealey K.L., Meurs K.M. Breed distribution of the ABCB1-1Delta (multidrug sensitivity) polymorphism among dogs undergoing ABCB1 genotyping. J Am Vet Med Assoc. 2008;233(6):921-924.
40. Washington State University, College of Veterinary Medicine, Veterinary Clinical Pharmacology Lab: (2010). Affected breeds. Accessed January 26, 2010, at http://www.vetmed.wsu.edu/depts-VCPL/breeds.aspx.
41. Perez R., Cabezas I., Sutra J.F., et al. Faecal excretion profile of moxidectin and ivermectin after oral administration in horses. Vet J. 2001;161(1):85-92.
42. Tranquilli W.J., Paul A.J., Seward R.L., et al. Response to the physostigmine administration in collie dogs exhibiting ivermectin toxicosis. J Vet Pharmacol Ther. 1987;10:96-100.
43. Muhammad G., Abdul J., Khan M.Z., et al. Use of neostigmine in massive ivermectin toxicity in cats. Vet Hum Toxicol. 2004;46(1):28-29.
44. Weinberg G., Ripper R., Feinstein D.L., et al. Lipid emulsion infusion rescues dogs from bupivacaine-induced cardiac toxicity. Reg Anesth Pain Med. 2003;28(3):198-202.
45. Crandell D.E., Weinberg G.L. Moxidectin toxicosis in a puppy successfully treated with intravenous lipids. J Vet Emerg Crit Care (San Antonio). 2009;19(2):181-186.
46. Weinberg G. Lipid rescue resuscitation from local anaesthetic cardiac toxicity. Toxicol Rev. 2006;25(3):139-145.
47. Hribernik T.N. Canine and feline heartworm disease. In: Kirk R.W., editor. Current veterinary therapy. Philadelphia: Saunders; 1989:263-270. X
48. Bowman D.D., Johnson R.B., Ulrich M.E., et al. Effects of long-term administration of ivermectin or milbemycin oxime on circulating microfilariae and parasite antigenemia in dogs with patent heartworm infections. Tex: American Heartworm Society; 1992. Proceedings of the Heartworm Symposium Austin
49. Lok J.B., Knight D.H. Macrolide effects on reproductive function in male and female heartworms. Auburn: Ala, American Heartworm Society; 1995. Proceedings of the Heartworm Symposium
50. Knight D.H. CVT update: heartworm testing and prevention in dogs. In: Bonagura J.D., editor. Kirk’s current veterinary therapy: XIII. Philadelphia: Saunders; 2000:777-782.
51. Bazzocchi C., Ceciliani F., McCall J.W., et al. Antigenic role of the endosymbionts of filarial nematodes: IgG response against the Wolbachia surface protein in cats infected with Dirofilaria immitis. Proc Biol Sci. 2000;267(1461):2511-2516.
52. McCall J.W., Genchi C., Kramer L., et al. Heartworm and Wolbachia: therapeutic implications. Vet Parasitol. 2008;158(3):204-214.
53. Bowman D.D. Georgi’s parasitology for veterinarians, ed 7. Philadelphia: Saunders; 1999.
54. Sherding RG: Respiratory parasites. In Bonagura JD, Twedt DC, editors: Kirk’s current veterinary therapy XIV, St Louis, 2009, pp 666-671.
55. Nolan T.J., Niamatali S., Bhopale V., et al. Efficacy of a chewable formulation of ivermectin against a mixed infection of Ancylostoma braziliense and Ancylostoma tubaeforme in cats. Am J Vet Res. 1992;53(8):1411-1413.
56. Knight D.H. Guidelines for diagnosis and management of heartworm (Dirofilaria immitus) infection. In: Bonagura J.D., editor. Kirk’s current veterinary therapy: XII. Philadelphia: Saunders; 1995:879-887.
57. Merial LTD: Heartgard® chewables for cats [package insert], 2006.
58. Blagburn B.L., Hendrix C.M., Lindsay D.S., et al. Anthelmintic efficacy of ivermectin in naturally parasitized cats. Am J Vet Res. 1987;48(4):670-672.
59. Kirkpatrick C.E., Megella C. Use of ivermectin in treatment of Aelurostrongylus abstrusus and Toxocara cati infections in a cat. J Am Vet Med Assoc. 1987;190(10):1309-1310.
60. Hawkins EC: Diseases of the lower respiratory system. In Ettinger SJ, Feldman EC, editors: Textbook of veterinary internal medicine, ed 4, Philadelphia, 1995, pp 767–811.
61. Bedard C., Desnoyers M., Lavallee M.C., et al. Capillaria in the bladder of an adult cat. Can Vet J. 2002;43(12):973-974.
62. Bater A.K. Efficacy of oral milbemycin against naturally acquired heartworm infection in dogs. Charleston, SC: American Heartworm Society; 1989. Proceedings of the Heartworm Symposium 1989
63. Bradley R.E. Dose titration and efficacy of milbemycin oxime for prophylaxis against Dirofilaria immitis infection in dogs. Charleston, SC: American Heartworm Society; 1989. Proceedings of the Heartworm Symposium 1989
64. Grieve R.B., Frank G.R., Stewart V.A., et al. Chemoprophylactic effects of milbemycin oxime against larvae of Dirofilaria immitis during prepatent development. Am J Vet Res. 1991;52(12):2040-2042.
65. Bowman D.D., Parsons J.J., Grieve R.B., et al. Effects of milbemycin on adult Toxocara canis in dogs with experimentally induced infection. Am J Vet Res. 1988;49(11):1986-1989.
66. Bowman D.D., Johnson R.C., Hepler D.I. Effects of milbemycin oxime on adult hookworms in dogs with naturally acquired infections. Am J Vet Res. 1990;51(3):487-490.
67. Bowman D.D., Lin D.S., Johnson R.C., et al. Effects of milbemycin oxime on adult Ancylostoma caninum and Uncinaria stenocephala in dogs with experimentally induced infections. Am J Vet Res. 1991;52(1):64-67.
68. Blagburn B.L., Hendrix C.M., Lindsay D.S., et al. Efficacy of milbemycin oxime against naturally acquired or experimentally induced Ancylostoma spp. and Trichuris vulpis infections in dogs. Am J Vet Res. 1992;53(4):513-516.
69. Bowman D.D., Ulrich M.A., Gregory D.E., et al. Treatment of Baylisascaris procyonis infections in dogs with milbemycin oxime. Vet Parasitol. 2005;129(3-4):285-290.
70. Blagburn B.L., Hendrix C.M., Lindsay D.S., et al. Milbemycin: Efficacy and toxicity in beagle and collie dogs. Charleston, SC: American Heartworm Society; 1989. Proceedings of the Heartworm Symposium 1989
71. Sasaki Y., Kitagawa H., Murase S., et al. Susceptibility of rough-coated collies to milbemycin oxime. Nippon Juigaku Zasshi. 1990;52(6):1269-1271.
72. Blagburn B.L., Hendrix C.M., Lindsay D.S., et al. Post-adulticide milbemycin oxime microfilaricidal activity in dogs naturally infected with Dirofilaria immitis. Austin, Tex: American Heartworm Society; 1992. Proceedings of the Heartworm Symposium 1992
73. Bowman D.D. Anthelmintics for dogs and cats effective against nematodes and cestodes. Compend Cont Educ Pract Vet. 1992;14(5):597-599.
74. Lok J.B., Knight D.H., LaPaugh D.A., et al. Kinetics of microfilaremia suppression in Dirofilaria immitis-infected dogs during and after a prophylactic regimen of milbemycin oxime. Austin, Tex: American Heartworm Society; 1992. Proceedings of the Heartworm Symposium 1992
75. Stewart V.A., Hepler D.I., Grieve R.B. Efficacy of milbemycin oxime in chemoprophylaxis of dirofilariasis in cats. Am J Vet Res. 1992;53(12):2274-2277.
76. Blagburn B.L., Hendrix C.M., Vaughan J.L., et al. Efficacy of milbemycin oxime against Ancylostoma tubaeforme in experimentally infected cats. Boston, Mass: 37th Annual meeting of the American Association of Veterinarian Parasitologists; 1992.
77. Glickman L.T., Glickman N.W., Moore G.E., et al. Safety profile of moxidectin (Proheart 6) and two oral heartworm preventives in dogs. Intern J Appl Res Vet Med. 2005;3(2):49-61.
78. Food and Drug Administration, Center for Veterinary Medicine: ProHeart® 6 (moxidectin) Questions and Answers, June 5, 2008. Accessed January 29, 2009, at http://www.fda.gov/cvm/Proheart6FAQ.htm.
79. Anonymous: ProHeart 6 client information and product label. Fort Dodge Animal Health, 2008. Accessed January 29, 2009, at http://www.proheart6.com/.
80. Lok J.B., Knight D.H., Wang G.T., et al. Activity of an injectable, sustained-release formulation of moxidectin administered prophylactically to mixed-breed dogs to prevent infection with Dirofilaria immitis. Am J Vet Res. 2001;62(11):1721-1726.
81. Bishop B.F., Bruce C.I., Evans N.A., et al. Selamectin: a novel broad-spectrum endectocide for dogs and cats. Vet Parasitol. 2000;91:163-176.
82. Thomas C.A. Revolution: a unique endectocide providing comprehensive conveient protection. Compend Cont Educ Pract Vet. 1999;21(Suppl 9):2-25.
83. McTier T.L., McCall J.W., Jernigan A.D., et al. UK-124,114, a novel avermectin for the prevention of heartworms in dogs and cats. Tampa, Fla: American Heartworm Symposium, American Heartworm Society, Batavia, Ill; 1998.
84. Thomas C.A. Revolution safety profile. Compend Cont Educ Pract Vet. 1999;21(Suppl 9):26-31.
85. Boy M.G., Six R.H., Thomas C.A., et al. Efficacy and safety of selamectin against fleas and heartworms in dogs and cats presented as veterinary patients in North America. Vet Parasitol. 2000;91(3-4):233-250.
86. Fisher M.A., Shanks D.J. A review of the off-label use of selamectin (Stronghold/Revolution) in dogs and cats. Acta Vet Scand. 2008;50:46.
87. Pfizer Animal Health. Revolution (selamectin) package insert. Accessed January 29, 2009, at http://www.revolution4dogs.com/PAHimages/compliance_pdfs/US_EN_RV_compliance.pdf.
88. Campbell W.C. Benzimidazoles; veterinary uses. Parasitology Today. 1990;6(4):130-133.
89. Lacey E. Mode of action of benzimadzoles. Parasitology Today. 1990;6(4):112-115.
90. McKellar Q.A., Scott E.W. The benzimidazole anthelmintics: a review. J Vet Pharmacol Ther. 1990;13:223-247.
91. Adams H.R., editor. Veterinary pharmacology and therapeutics, ed 7, Ames, Iowa: Iowa State University Press, 1995.
92. Frayha G.J., Smyth J.D., Gobert J.G., et al. The mechanisms of action of antiprotozoal and anthelmintic drugs in man. Gen Pharmacol. 1997;28(2):273-299.
93. Reinemeyer C.E., Courtney C.H. Antinematodal drugs. In: Adams H.R., editor. Veterinary pharmacology and therapeutics. ed 8. Ames, Iowa: Iowa State University Press; 2001:947-979.
94. Stokol T., Randolph J.F., Nachbar S., et al. Development of bone marrow toxicosis after albendazol administration in a dog and cat. J Am Vet Med Assoc. 1997;210:1753-1756.
95. Meyer E.K. Adverse events associated with albendazole and other products used for treatment of giardiasis. J Am Vet Med Assoc. 1998;213(1):44-46.
96. United States Pharmacopeia. USP Drug Information Update (September, pp 1289-1586). Rockville, MD: United States Pharmacopeial Convention; 1998.
97. Intervet/Schering-Plough Animal Health. Panacur C Canine Dewormer Product Insert. Accessed January 30, 2009, at http://www.compasnac.com/intervet.htm.
98. Barr S. Enteric protozoal infections. In: Greene C., editor. Infectious diseases of the dog and cat. St Louis: Saunders; 2006:736-750.
99. Aubry M.L., Cowell P., Davey M.J., et al. Aspects of the pharmacology of a new anthelmintic: pyrantel. Br J Pharmacol. 1970;38:332-344.
100. Eyre P. Some pharmacodynamic effects of the nematodes: methypyrdine, tetramisole and pyrantel. J Pharm Pharmacol. 1970;22:26-36.
101. Martin R.J. Neuromuscular transmission in nematode parasites and antinematodal drug action. Pharmacol Ther. 1993;58(1):13-50.
102. Martin R.J. Modes of action of anthelmintic drugs. Vet J. 1997;154(1):11-34.
103. Londershausen M. Approaches to new parasiticides. Pestic Sci. 1996;48(4):269-292.
104. United States Pharmacopeia (2005). Tetrahydropyrimidines (veterinary—oral-local). The United States Pharmacopeial Convention, Inc. Accessed January 26, 2009, at http://www.usp.org/pdf/EN/veterinary/tetrahydropyrimidines.pdf.
105. Linquist W.D. Drug evaluation of pyrantel pamoate against Ancylostoma, Toxocara, and Toxascaris in eleven dogs. Am J Vet Res. 1975;36(9):1387-1389 & 1695.
106. Klein J.B., Bradley R.E., Conway D.P. Anthelmintic efficacy of pyrantel pamoate against the roundworm, Toxocara canis, and the hookworm, Ancylostoma caninum, in dogs. Vet Med Small Anim Clin. 1978;73:1011-1013.
107. Jacobs D.E. Control of Toxocara canis in puppies: a comparison of screening techiques and evaluation of a dosing programme. J Vet Pharmacol Ther. 1987;10:23-29.
108. Clark J.A. Physaloptera stomach worms associated with chronic vomition in a dog in Western Canada. Can Vet J. 1990;31(12):840.
109. Clark J.N., Daurio C.P., Barth D.W., et al. Evaluation of a beef-based chewable formulation of pyrantel against induced and natural infections of hookworms and ascarids in dogs. Vet Parasitol. 1991;40:127-133.
110. Harder A., Holden-Dye L., Walker R., et al. Mechanisms of action of emodepside. Parasitol Res. 2005;97(Suppl 1):S1-S10.
111. Bayer Healthcare: Profender (emodepside/praziquantel) Topical Solution Product Insert, Bayer Healthcare, 2007. Accessed January 31, 2009, at http://www.bayerdvm.com/resources/docs/Profender-Label.pdf.
112. Roberson E.L. Chemotherapy of parasitic diseases. In: Booth N.E., McDonald L.E., editors. Veterinary pharmacology and therapeutics. ed 6. Ames, Iowa: Iowa State University Press; 1988:882-927.
113. English P.B., Sprent J.F.A. The large roundworms of dogs and cats: effectiveness of piperazine salts against immature Toxocara canis in prenatally infected puppies. Austr Vet J. 1965;41:50-53.
114. Sharp M.L., Sepesi J.P., Collins J.A. A comparative critical assay on canine anthelmintics. Vet Med Small Anim Clin. 1973;68:131-132.
115. Jacobs D.E. Anthelmintics for dogs and cats. Int J Parasitol. 1987;17(2):511-518.
116. Andrews P., Thomas H., Pohlke R., et al. Praziquantel. Med Res Rev. 1983;3(2):147-200.
117. Arundel J.H., Hvd Bossche, Thienpoint D., et al. Chemotherapy of gastrointestinal helminths. New York: Springer-Verlag; 1985.
118. Gemmell M.A., Johnstone P.D., Oudemans G. The effect of praziquantel on Echinococcus granulosus, Taenia hydatigena and Taenia ovis infections in dogs. Res Vet Sci. 1977;23:121-123.
119. Andersen F.L., Conder G.A., Marsland W.P. Efficacy of injectable and tablet formulations of praziquantel against mature Echinococcus granulosus. Am J Vet Res. 1978;39(11):1861-1862.
120. Thakur S.A., Prezioso U., Marchevsky N. Efficacy of droncit against Echinococcus granulosus infection in dogs. Am J Vet Res. 1978;39(5):859-860.
121. Thomas H., Gonnert R. The efficacy of praziquantel against cestodes in cats, dogs, and sheep. Res Vet Sci. 1978;24:20-25.
122. Andersen F.L., Conder G.A., Marsland W.P. Efficacy of injectable and tablet formulations of praziquantel against immature Echinococcus granulosus. Am J Vet Res. 1979;40(5):700-701.
123. Gemmell M.A., Johnstone P.D., Oudemans G. The effect of route of administration on the efficacy of praziquantel against Echinococcus granulosus infections in dogs. Res Vet Sci. 1980;29:131-132.
124. Kruckenberg S.M., Meyer A.D., Eastman W.R. Preliminary studies on the effect of praziquantel against tapeworms in dogs and cats. Vet Med Small Anim Clin. 1981;76:689-697.
125. Bayer Healthcare. Droncit (praziquantel) injectable cestocide for dogs and cats. Accessed January 30, 2009, at https://www.bayerdvm.com/Products/droncit/droncit-labels.cfm.
126. Pfizer Animal Health: Cestex (epsiprantel) veterinary tablets, 2000. Accessed January 30, 2009, from http://www.pfizerah.com/PAHimages/compliance_pdfs/US_EN_CE_compliance.pdf.
127. Corwin R.M., Green S.P., Keefe T.J. Dose titration and confirmation tests for determination of cestocidal efficacy of epsiprantel in dogs. Am J Vet Res. 1989;50(7):1076-1077.
128. Manger B.R., Brewer M.D. Epsiprantel, a new tapeworm remedy, preliminary efficacy studies in dogs and cats. Br Vet J. 1989;145:384-388.
129. United States Pharmacopeia: Epsiprantel veterinary—oral-local, 2008, The United States Pharmacopeial Convention, Inc., Accessed Jan 26, 2009, at http://www.usp.org/pdf/EN/veterinary/epsiprantel.pdf.
130. Plumb D.C. Veterinary drug handbook, ed 5. Stockholm, Wisconsin: PharmaVet; 2005.
131. Gilman A.G., Rall T.W., Nies A.S., et al, editors. Goodman and Gilman’s the pharmacological basis of therapeutics, ed 8, New York: Pergamon Press, 1990.
132. Dzimianski M.T., McCall J.W., McTier T.L., et al. Preliminary results of the efficacy of RM 340 administered seasonally to heartworm antigen and microfilaria positive dogs living outdoors in a heartworm endemic area. Austin, Tex: American Heartworm Society; 1992. Proceedings of the Heartworm Symposium 1992
133. Keister D.M., Dzimianski M.T., McTier T.L., et al. Dose selection and confirmation of RM 340, a new filaricide for the treatment of dogs with immature and mature Dirofilaria immitis. Austin, Tex: American Heartworm Society; 1992. Proceedings of the Heartworm Symposium 1992
134. Keister DM, Tanner PA, Meo NJ: Immiticide: review of discovery, development and utility. Proceedings of the Heartworm Symposium 1995, Auburn, Ala, 1995.
135. Miller MW, Keister DM, Tanner PA, et al: Clinical efficacy and safety trial of melarsomine dihydrochloride (RM340) and thiacetarsemide in dogs with moderate (Class 2) heartworm disease. Proceedings of the Heartworm Symposium 1995, Auburn, Ala, 1995.
136. Rawlings C.A., McCall J.W. Melarsomine: a new heartworm adulticide. Compend Cont Educ Pract Vet. 1996;18(4):373-379.
137. Vezzoni A., Genchi C., Raynaud J.P. Adulticide efficacy of RM 340 in dogs with mild and severe natural infections. Austin, Tex: American Heartworm Society; 1992. Proceedings of the Heartworm Symposium 1992
138. Case JL, Tanner PA, Keister DM, et al: A clinical field trial of melarsomine dihydrochloride (RM340) in dogs with severe (Class 3) heartworm disease. Proceedings of the Heartworm Symposium 1995, Auburn, Ala, 1995.
139. Reinemeyer C.R., Courtney C.H. Anticestodal and antitrematodal drugs. In: Adams H.R., editor. Veterinary pharmacology and therapeutics. ed 8. Ames, Iowa: Iowa State University Press; 2001:980-991.
140. Altreuther G., Borgsteede F.H., Buch J., et al. Efficacy of a topically administered combination of emodepside and praziquantel against mature and immature Ancylostoma tubaeforme in domestic cats. Parasitol Res. 2005;97(Suppl 1):S51-S57.
141. Charles S.D., Altreuther G., Reinemeyer C.R., et al. Evaluation of the efficacy of emodepside+praziquantel topical solution against cestode (Dipylidium caninum, Taenia taeniaeformis, and Echinococcus multilocularis) infections in cats. Parasitol Res. 2005;97(Suppl 1):S33-S40.
142. Reinemeyer C.R., Buch J., Charles S.D., et al. Evaluation of the efficacy of emodepside plus praziquantel topical solution against ascarid infections (Toxocara cati or Toxascaris leonina) in cats. Parasitol Res. 2005;97(Suppl 1):S41-S50.
143. Altreuther G., Buch J., Charles S.D., et al. Field evaluation of the efficacy and safety of emodepside/praziquantel spot-on solution against naturally acquired nematode and cestode infections in domestic cats. Parasitol Res. 2005;97(Suppl 1):S58-S64.
144. Bowman DD, Arthur RG: Laboratory evaluation of Drontal plus (febantel/praziquantel/pyrantel) tablets for dogs. Proceedings of the American Association of Veterinary Parasitology, Minneapolis, Minn, 1993.
145. Cruthers LR, Slone RL, Arthur RG: Efficacy of Drontal plus (praziquantel/pyrantel/febantel) tablets for removal of Ancylostoma caninum, Uncinaria stenocephala and Toxascaris leonina Proceedings of the American Association of Veterinary Parasitology, Minneapolis, Minn, 1993.
146. Clark J.N., Daurio C.P., Plue R.E., et al. Efficacy of ivermectin and pyrantel pamoate combined in a chewable formulation against heartworm, hookworm, and ascarid infections in dogs. Am J Vet Res. 1992;53(4):517-520.
147. Clark J.N., Pulliam J.D., Daurio C.P. Safety study of a beef-based chewable tablet formulation of ivermectin and pyrantel pamoate in growing dogs, pups and breeding adult dogs. Am J Vet Res. 1992;53(4):608-612.
148. Arther R.G., Atkins C., Ciszewski D.K., et al. Safety of imidacloprid plus moxidectin topical solution applied to cats heavily infected with adult heartworms (Dirofilaria immitis). Parasitol Res. 2005;97(Suppl 1):S70-S75.