Chapter 23 Rational Use of Reproductive Hormones
The use of reproductive hormones as pharmaceuticals should be based on knowledge of both the reproductive physiology of the species under treatment and the pathophysiology of the disorder being treated. Too often, the use of hormonal therapies in small animal reproduction is based on their documented action in other species. Hormonal therapies are also used when the underlying reproductive disorder has not been fully studied nor understood in small animals. Although there are specific indications for the rational (evidence-based) use of reproductive hormones in small animal theriogenology, they are commonly used inappropriately. This chapter includes a review of the basic physiology of hormones used therapeutically in small animal theriogenology, a discussion of their clinical availability, their appropriate applications, and common misuses.
Physiologic Principles of Reproductive Hormones
Hypothalamic Hormones
Gonadotropin-Releasing Hormone and Its Analogs
Gonadotropin-releasing hormone (GnRH) is a highly conserved hypothalamic decapeptide with the same amino acid sequence in all mammals. After puberty GnRH is released in a pulsatile manner from the hypothalamus, traverses the hypothalamic–hypophyseal portal system, and activates anterior pituitary gonadotroph receptors. The pituitary responds by releasing luteinizing hormone (LH) and follicle-stimulating hormone (FSH) in a pulsatile pattern.1 Stimulation of the pituitary by GnRH must be in pulsatile form for repeated release of LH and FSH; after receptor activation, GnRH is rapidly deactivated and cleared. The frequency and amplitude of GnRH pulses vary depending on the phase of the reproductive cycle. The frequency of GnRH pulsatile release in primates during folliculogenesis is every 70 to 90 minutes.2 It is this natural pulsatile secretion of GnRH and its short biological half-life that cause difficulties when attempting to use GnRH as a pharmaceutical. Investigations with specialized infusion devices for pulsatile delivery have successfully augmented fertility in a variety of species,3-5 but the clinical application of such a method lacks practicality for routine use in small animal patients.
GnRH analogs are synthetically prepared substances that differ from GnRH by various amino acid substitutions in the peptide sequence. Analogs with a few amino acid substitutions can act as GnRH agonists because of their increased binding affinity and decreased clearance compared with GnRH. Heavily substituted analogs can cause receptor blockade and have an antagonist function. The result is a suppressive effect on the pituitary–gonadal axis. This axis is easily downregulated such that frequent or high dosing of a GnRH agonist will suppress the release of LH and FSH also.6 GnRH agonists and antagonists thus may have the same ultimate physiologic effect.
Oxytocin
A nonapeptide hormone synthesized by neurons in the hypothalamus, oxytocin is transported axonally to the posterior pituitary, where it is stored. This peptide hormone is released from the posterior pituitary into the general circulation after appropriate neural stimulation. Its primary effects are on mammary tissue and the myometrium. The effects of oxytocin on the milk let-down reflex and parturition have been well described.7 The ability of oxytocin to induce myometrial contraction is enhanced by prior estrogen sensitization to “prime” the myometrium for maximal response.8 The half-life of oxytocin in the blood is short, approximately 1.5 minutes; thus secretion pulse frequency and amplitude are important for its physiologic effects. The release of neurotransmitters may alter the amplitude and frequency of oxytocin release; for example, dopamine enhances burst frequency and amplitude, whereas cholinergic antagonists may be inhibitory.9
Pituitary Gonadotropins
Luteinizing Hormone and Follicle-Stimulating Hormone
The pituitary gonadotropins LH and FSH are relatively large glycoproteins, each consisting of two covalently bound structural subunits (α and β). Within a species the amino acid sequence of the α-subunits are identical for all anterior pituitary glycoproteins, with high sequence homology existing across species. The β-subunits are specific for individual hormones (i.e., thyroid-stimulating hormone) and provide the functional specificity of each. The overall size of these glycoproteins precludes economic synthetic production of these hormones.8
The secretion patterns of these hormones differ depending on the species and the phase of the ovarian cycle. A unique pattern of LH secretion in the bitch has been documented10 (Figure 23-1). Serum concentration of LH is at basal levels during anestrus. Significant increases in both the amplitude and frequency of LH pulsatile release occur before proestrus. The frequency of LH pulsatile release during anestrus is 3 to 7 hours; before proestrus LH pulse frequency is 60 to 120 minutes.11 This increase in LH release before the onset of proestrus is likely involved with termination of the anestrus phase.1 The factors that lead to the LH increase at that time are unknown. Serum estrogen concentration at that time also decreases; estrogen production inhibits LH release. What causes the relative decrease in estrogen production at that time is also unknown.
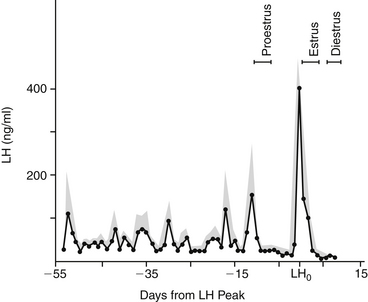
Figure 23-1 Concentrations of luteinizing (LH) hormone in canine serum throughout late anestrus, proestrus, estrus, and early diestrus. Stippled area is the standard error of the mean (bars indicate ranges of proestrus, estrus, and diestrus).
(From Olson PN, Bowen RA, Behrendt MD et al: Concentrations of reproductive hormones in canine serum throughout late anestrus, proestrus, and estrus, Biol Reprod 27:1196, 1982.)
Serum LH concentration returns to basal levels for most of proestrus (i.e., folliculogenesis). The increase in serum estrogen concentration during folliculogenesis contributes to an inhibition of LH release during that phase.1 When estrogen production decreases, the inhibition of LH release is discontinued. At that time a preovulatory surge in LH release (the preovulatory LH peak) occurs and is thought to trigger ovulation. The duration of the preovulatory LH peak is 1 to 3 days, after which LH secretion returns to a basal state in early diestrus. Additionally, LH is luteotrophic throughout most of the luteal phase in the bitch.
Similar to the pattern of LH secretion, FSH secretion also appears to be inhibited by relatively high concentrations of estrogen during proestrus. Inhibin, a modulary hormone released from the ovary during folliculogenesis, also inhibits FSH release during this phase. When estrogen secretion declines in late proestrus, FSH secretion surges to maximal levels before ovulation, in concert with the preovulatory LH peak1,10 (Figure 23-2). After this FSH surge, serum FSH concentration remains relatively high during diestrus and pregnancy. Interestingly, the bitch is also unique with regard to the pattern of FSH secretion during anestrus. During anestrus serum FSH concentration can be 50% to 100% of the concentration found at the preovulatory FSH peak and is 5 to 10 times higher than during proestrus.1 It is unclear why FSH produced during anestrus is unable to stimulate folliculogenesis. It has been postulated that perhaps the FSH measured during anestrus is in a biologically inactive form.1
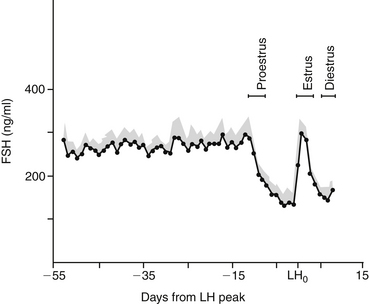
Figure 23-2 Concentrations of follicle-stimulating hormone (FSH) in canine serum throughout late anestrus, proestrus, and early diestrus. Stippled area is the standard error of the mean (bars indicate ranges of proestrus, estrus, and diestrus).
(From Olson PN, Bowen RA, Behrendt MD et al: Concentrations of reproductive hormones in canine serum throughout late anestrus, proestrus, and estrus, Biol Reprod 27:1196, 1982.)
The patterns of LH and FSH secretion in the queen have not been clearly determined. It has been documented that after adequate copulation LH release begins within minutes. Queens are induced ovulators; thus this response is expected. Apparent spontaneous ovulation, without copulation or other tactile stimulation, has been reported in the queen.12 The pattern of LH secretion during the apparent spontaneous ovulation has not been determined, however.
The release of LH and FSH is also pulsatile in response to GnRH in the male. Both glycoprotein hormones are necessary for spermatogenesis. Testicular interstitial cells bind LH and respond by increasing testosterone production. Activation of receptors on Sertoli cells by FSH promotes spermatogenesis and produces inhibin, a hormone that, as in the female, regulates FSH release by the pituitary. After neutering the loss of negative feedback inhibition causes serum FSH concentrations to increase dramatically.13 This has also been documented in cases of infertility resulting from primary testicular degeneration.14 Postcastration elevations in LH serum concentrations also occur, but overlap in measured values between intact and neutered dogs is possible.13
Some species also produce placental gonadotropins. A luteotrophic gonadotropin produced by the human placenta (human chorionic gonadotropin [hCG]) has a potent LH-like effect in many species. A gonadotropin produced by fetal trophoblastic cells in horses is referred to as equine chorionic gonadotropin (eCG) or pregnant mare serum gonadotropin (pmSG). In most species eCG has a combination of FSH and LH activity. Additionally, eCG products commonly contain components of equine serum that can be antigenic when used as a pharmaceutical in another species.
Prolactin
A relatively large polypeptide produced by the anterior pituitary, prolactin is luteotrophic in the bitch and queen.15,16 It is suspected that prolactin is also involved in the maintenance of the anestrus phase in the bitch.1 Prolactin is under negative control by dopamine such that the administration of a dopamine agonist will inhibit prolactin secretion. Inhibition of prolactin secretion can promote luteolysis during the second half of diestrus or pregnancy in the bitch15 and queen.16 Various dopamine agonists have been investigated for inducing abortion and shortening anestrus (i.e., estrus induction). Bromocriptine is a dopaminergic drug that has been used in various protocols. Its use has not gained acceptance, however, because it commonly causes vomiting and diarrhea. The highly potent dopamine agonists cabergoline and metergoline have fewer or no apparent side effects, respectively. The effect of metergoline to decrease prolactin secretion may primarily result from blockade of central serotonin receptors and may function as a dopamine agonist only at higher doses.17 Metoclopramide, more commonly used as a central serotonin antagonist, may be used to enhance prolactin release in the bitch and queen, and thus enhance milk let-down, through its central dopamine (D2) agonist effects.
Gonadal Steroids
Estrogen
Produced by the ovary during folliculogenesis, estrogens affect target tissues, causing vulvar edema, vaginal mucosal hyperplasia, sanguineous vulvar discharge in the bitch, and sexual attraction in both the bitch and queen. The bitch uniquely exhibits sexual receptivity when estrogen production decreases; serum concentration of estrogen peaks 1 to 2 days before the end of proestrus. A concurrent increase in progesterone is required for complete expression of sexual receptivity. Estrogen acts synergistically with progesterone to stimulate growth of endometrial and mammary glands, and estrogen priming may be important before the natural effect of oxytocin on the myometrium during parturition.8 Male production of estrogen occurs in both the Leydig and Sertoli cells, by way of de novo steroidogenesis in the Leydig cell and P450 aromatase conversion of testosterone alone in the Sertoli cell. Considerable interspecies variation occurs with respect to the relative ratios of estrogen produced by each cell. Excessive production of estrogens by Sertoli cell tumors and, less commonly, by other testicular or adrenal tumors results in male feminizing syndrome. Estrogens are involved in receptor sensitivity and feedback influence on the hypothalamic–pituitary–gonadal axis.
The adverse effects of estrogen (i.e., bone marrow aplasia, cystic endometrial hyperplasia/pyometra, infertility) have been documented in dogs after administration of any estrogen preparation.18,19 The unique sensitivity of dogs to estrogen toxicity may be due to the relatively weak binding affinity of sex-steroid binding proteins for estrogen in the dog. The result is a decrease in the inherent buffering mechanism that would otherwise regulate the amount of free hormone available to penetrate cell membranes. Estrogen toxicity can occur with exogenous or endogenous estrogens (e.g., Sertoli cell tumors, ovarian follicular cysts, and ovarian neoplasia).
Progesterone
As the main progestational hormone in both bitches and queens, progesterone is produced by corpora lutea just before and after ovulation. The bitch continues to produce progesterone during nonpregnant diestrus; serum concentrations of progesterone are indistinguishable in concentration or duration of production from that of pregnancy. The decline of progesterone and increase in prolactin production at the termination of diestrus cause the clinical manifestations of pseudopregnancy in the nonpregnant bitch. This is a demonstration of a normal physiologic occurrence that should not be confused with a pathologic state and in fact suggests normal ovarian function. There is some variation among bitches regarding the maximum amount of progesterone produced in midgestation (or mid-diestrus), with levels reaching 80 to 100 ng/mL in some individuals. The minimum serum progesterone concentration necessary to maintain pregnancy appears to be 2 ng/mL.20 The minimum amount of progesterone required to sustain pregnancy in the queen has not been determined; however, serum progesterone concentrations of less than 1 ng/mL for several days occurred before termination of pregnancy were observed in one study.16
Placental production of progesterone by the queen during the latter half of pregnancy had been suggested as a requirement to maintain pregnancy. It has been learned, however, that corpora lutea of the queen are necessary for progesterone production throughout pregnancy.21 During pseudopregnancy in queens (i.e., nonfertile ovulation), peak luteal activity appears to occur at days 10 to 15 and then declines to basal values by days 35 to 40.22
In the bitch progesterone is produced before ovulation by follicular luteinization, approximately concurrent with the preovulatory LH peak. Serial evaluations of serum progesterone concentrations during proestrus and estrus can be used to indirectly determine the preovulatory LH peak and thus determine the time of ovulation in the bitch. This methodology, known as ovulation timing, is used to improve breeding management. The bitch typically exhibits sexual receptivity (behavioral estrus) when the serum concentration of estrogen is decreasing and progesterone is increasing.
Synthetic progestational compounds (progestagens) are commercially available. Megestrol acetate has been marketed as a drug to suppress estrus or inhibit ovulation, depending on the time and dose of administration. The method by which progestagens inhibit folliculogenesis and ovulation is not precisely understood. It appears that although megestrol acetate will not decrease the serum concentration of LH that is already at low basal levels, it may be able to prevent the increases in LH that normally occur at the end of anestrus.1
Testosterone
Produced by the interstitial cells of the testes, testosterone is necessary for gonadal development, spermatogenesis, and libido in the male. The normal function of Sertoli cells to promote spermatogenesis depends on an intratesticular testosterone concentration that greatly exceeds circulatory levels.8 Pharmacologic administration of androgens, including testosterone, can induce infertility by negative inhibition of LH and FSH release, which are necessary for spermatogenesis. Testosterone is converted in the prostate to dihydrotestosterone (DHT), which promotes development of this gland and the eventually contributes to the anticipated formation of benign prostatic hyperplasia in mature dogs. DHT is an androgen with greater biological activity than testosterone.
Testosterone and the androgenic steroid mibolerone have been used to suppress ovarian activity and thereby prevent estrus cycles in the bitch. Mibolerone was specifically approved for this use, although the duration of time from discontinuing administration to the occurrence of the next estrous cycle was variable. Silent heats following mibolerone therapy were common. Persistent anestrus (i.e., lack of return to estrous cycles) has been a problem in some Greyhound bitches treated with injectable testosterone to prevent estrus cycles during racing.
Inhibitors of Gonadal Steroids
Tamoxifen is both an estrogen agonist and antagonist that has been used as adjunctive therapy for women with mammary carcinoma. The nature of its effect depends on tissue estrogen receptor type. Tamoxifen appears to have, at least in part, a direct estrogenic effect in the bitch. Some bitches treated with tamoxifen had observable vulvar edema; sanguineous vaginal discharge; and, in some cases, pyometra of the uterine stump.23 Tamoxifen and clomiphene, another antiestrogenic compound, have been used to promote superovulation in women, possibly by promoting an increase in endogenous FSH release. Tamoxifen has been used in the bitch as a mismate therapy but was associated with pyometra, endometritis, and cystic ovaries in a high percentage of bitches; was reliably effective only when given during the first 14 days of diestrus; and is not advised.24
Antigestagens are agents that inhibit the effect of progesterone by binding to and altering the progesterone receptor and have been investigated as abortion agents in humans (i.e., mifepristone, RU-486). Preliminary studies have documented the effectiveness of mifepristone to terminate pregnancy in bitches.25
Finasteride is another useful inhibitor of gonadal steroids. Testosterone is converted to DHT in the prostate by the action of the enzyme 5α-reductase. Trophic in its effect, DHT induces benign prostatic hyperplasia as a dog ages. Finasteride inhibits the action of 5α-reductase, thereby reducing DHT concentrations in the prostate. Finasteride may also alter prostatic angiogenesis and reduce hemospermia through a change in microvessel density. Developed for use in men with prostatic hyperplasia, finasteride has been investigated for successful similar use in dogs.26 Benign prostatic hyperplasia causing hemospermia can be detrimental to efforts to successfully freeze and thaw canine semen; otherwise, it is minimally problematic in the dog unless accompanied by infection or neoplasia.
Autacoids
Prostaglandin F2α
Prostaglandins, potent autacoids, have therapeutic indications in small animal theriogenology. Administration of prostaglandin F2α(PGF2α) induces a direct luteolytic effect in bitches and queens during pregnancy or diestrus. Induction of luteolysis depends on dose, frequency of drug administration, and stage of diestrus that the drug is administered. After day 30 of diestrus, PGF2α is reliably luteolytic in both the bitch27 and queen.22 Corpora lutea are relatively resistant to luteolysis by PGF2-alpha during the first 5 days of diestrus in the bitch.28 There is evidence that PGF2α will cause either a transient decrease in progesterone production or complete luteolysis when administered during early diestrus after day 6.28 In addition to a direct luteolytic effect, PGF2α also has a stimulatory action on the myometrium, promoting evacuation of uterine luminal contents. The drug is therefore effective as an abortifacient and for the treatment of open cervix pyometra in the bitch and queen.
Additional effects of PGF2-alpha administration include panting, nausea, vomiting, diarrhea, hypersalivation, and tachycardia in dogs and vocalization, mydriasis, and possibly vomition and diarrhea in cats. These adverse clinical signs usually stop within 20 to 30 minutes of drug administration. The drug is preferably administered after fasting in the dog and cat to decrease the incidence of vomiting. The adverse effects of PGF2α are potentially dose related, although there is individual sensitivity, and signs usually abate after repeated dosing. Although extremely rare, cardiovascular collapse after PGF2-alpha administration is possible.
Most protocols using PGF2α are based on the formulation of dinoprost tromethamine. This product is not approved for use in dogs or cats in the United States. Informed owner consent is advised, although the use of PGF2α is established in the veterinary literature. The more potent synthetic PGF2α analogs (cloprostenol and fluprostenol) are now used clinically in both the dog and the cat; studies regarding the use of these compounds indicate efficacy with fewer side effects, owing to the increased specificity of the compounds for uterine smooth muscle.
Commercial Availability of Reproductive Hormones
Gonadotropin-Releasing Hormone And Its Analogs
Lack of availability is a major hindrance to widespread use of GnRH analogs to control reproduction in humans and animals. Analogs are expensive to produce and are often available only as investigational products for research studies; availability also varies throughout the United States. One marketed GnRH analog (an antagonist) is leuprolide (Lupron, TAP Pharmaceuticals, Deerfield, Ill.). The native GnRH hormone (gonadorelin, Rhone Merieux) is commercially available (Cystorelin, or Factrel, Fort Dodge Laboratories, Fort Dodge, Iowa). Implant formulations of GnRH agonists such as deslorelin have been shown to be effective for reversible long-term suppression of reproductive function in the male and female dog and estrus suppression in the queen29,30 and have been manipulated for estrus induction in the bitch.31
Gonadotropins
Currently, FSH is not available in the United States; hCG is readily available (Chorulon, chorionic gonadotropin, Butler, Columbus, Ohio). In the United States eCG is not commercially available but may be available in Canada (Equinex, Ayerst Laboratories, Quebec) and in some European countries. Purified canine LH is not commercially available, but human LH can be purchased in a 1:1 ratio with human FSH in a product used to treat human infertility (Repronex, menotropin, Serono Laboratories, Norwell, Mass.). Additionally, purified human FSH is available (Metrodin, Bravelle, Serono Laboratories). The use of human menotropins has not been extensively studied in small animal medicine, and these products are likely to be too costly for routine use.
Dopamine Agonists
Bromocriptine (Parlodel, Sandoz Pharmaceuticals, East Hanover, NJ) is available, as is the newer dopamine agonist cabergoline (Dostinex, Pharmacia and Upjohn); metergoline (Virbac Laboratories, Carros, France) is available in Europe.
Gonadal Steroids
Other than antigestagens, which are not available in the United States, gonadal steroids and their inhibitors are widely available in many formulations. Some hormones are available in repositol formulation (i.e., medroxyprogesterone acetate, Depo-Provera, Upjohn, Kalamazoo, Mich.), and others are available in esters that exert a more potent effect (i.e., esters of estradiol). Some commonly available products include compounded diethylstilbestrol (DES), progesterone in oil (Eli Lilly), and testosterone propionate (Steris Laboratories, Phoenix, Ariz.).
Inhibitors of Gonadal Steroids
Tamoxifen (Nolvadex, Zeneca Pharmaceuticals, Wilmington, Del.) and finasteride (Proscar, Merck and Co., West Point, Pa.) are commonly available through pharmacies for humans. One product that is available (but not recommended on account of the side effects of progesterone administration in intact bitches) for contraception (heat prevention) in bitches is megestrol acetate (Ovaban, Schering-Plough).
Indications for the Rational (Evidence-Based) use of Reproductive Hormones
Some reproductive disorders are common and well described in the literature (e.g., pyometra, dystocia). Others are either incompletely understood or are less common (e.g., estrus induction, luteal insufficiency) such that information can be difficult for the practicing veterinarian to obtain. The discussion presented here is intended to provide an overview of several reproductive problems of dogs and cats that commonly require veterinary intervention (Table 23-1). Many of these disorders are topics of current research, which is needed to better understand and manage them.
Table 23-1 Uses of Reproductive Hormones for Dogs and Cats
| Indication | Hormone Used | Considerations |
|---|---|---|
| Estrus induction | See Table 23-3 | |
| Induction of ovulation | ||
| Ovulatory failure | hCG or GnRH to induce ovulation | Difficult to accurately determine follicular maturity to administer at correct time |
| Ovarian cysts | hCG or GnRH to induce luteinization | Often ineffective, may require ovariectomy need to differentiate neoplasia |
| Vaginal hyperplasia | hCG or GnRH to hasten ovulation | Not proven to shorten time for spontaneous recovery |
| Luteal insufficiency | Progesterone to maintain gestation | Potentially teratogenic, inhibits lactation development, luteal insufficiency is rare; need diagnosis before treatment attempted |
| Estrus prevention | ||
| Signs of pseudocyesis, galactostasis, mastitis | Cabergoline | Mild GI side effects |
| Pyometra/postpartum metritis | PGF2, cloprostenol | Transient side effects of PGF2, need careful evaluation and supportive clinical care |
| Pregnancy termination | PGF2, cloprostenol, dopamine agonists, dexamethasone. | Transient side effects of PGF2; unavailability of some dopamine agonists, steroid side effects (transient) |
| Dystocia | Can induce uterine tetany and fetal demise if overdosage occurs Use less concentrated solution | |
| Mammary gland carcinoma | Tamoxifen | Controversy as to efficacy, possibly exacerbates condition |
| Urinary incontinence | DES | Phenylpropanolamine can be synergistic if ineffective alone |
| Cryptorchidism | hCG or GnRH | Ethical concerns, not proven to be effective |
| Benign prostatic hyperplasia | Finasteride | Costly, requires compounding in small dogs |
| Hypogonadism | Gonadotropins | Rare condition, difficult to document |
hCG, Human chorionic gonadotropin; GnRH, gonadotropin-releasing hormone; PGF2, prostaglandin F2; DES, diethylstilbestrol.
KEY POINT 23-2
Medical management of reproductive conditions best treated surgically (i.e., neutering) should be reserved for individuals with good reproductive potential.
Estrus Induction
Induction of Estrus in the Bitch
A reliable, practical method to successfully induce fertile estrus in the bitch has long been sought. Protocols that are successful in other species usually do not produce the same effect in the bitch. This is primarily because of the unique reproductive physiology of the bitch and a lack of understanding of all the factors that initiate a new estrous cycle. Various protocols have been tested using all classes of reproductive hormones: GnRH, GnRH analogs, gonadotropins, dopamine agonists, and DES (Table 23-2). Some relatively successful protocols use compounds that are not commercially available at this time. It is important to thoroughly evaluate all potential causes of reproductive failure before estrus induction is attempted and realize that protocols are designed and tested using bitches that are reproductively normal. The outcome of estrus induction in bitches that have reproductive pathology is not known.
Table 23-2 Hormones Used to Induce Estrus in Bitches
| Hormone Classification | Considerations | Drawbacks |
|---|---|---|
| GnRH | Leaves normal feedback mechanisms intact; drug is available, not expensive | Requires expensive, cumbersome pump for drug delivery |
| GnRH analogs | Success in preliminary reports; subcutaneous administration or delivery by implanted infusion devices | Leuprolide, lutrelin, deslorelin, lupron |
| Gonadotropins | Many different protocols proposed | |
| Estrogen | Oral administration of DES, readily available compounded | Reliably induces signs of proestrus, ovulation less predictable. Information available based on one study at this time. Better protocols exist. |
| Dopamine agonists | Shortened interestrous interval | Cost of the newer dopamine agonist cabergoline, which has decreased adverse effects, but usually requires compounding; bromocriptine available but associated with vomiting and diarrhea. Must allow 8 week anestrus period. |
GnRH, Gonadotropin-releasing hormone; eCG, equine chorionic gonadotopin (also known as pregnant mare’s serum gonadotropin [PMSG]); hCG, human chorionic gonadotropin; FSH, follicle-stimulating hormone; DES, diethylstilbestrol.
Investigation into the use of GnRH as an agent to induce estrus in the bitch resulted in seven of eight Beagle bitches that ovulated and conceived (GnRH 140 μg/kg per pulse every 90 minutes intravenously for 11 to 13 days).5 This protocol used a programmable, portable pulsatile infusion device (Pulsamat, Ferring Laboratories, Ridgewood, N.J.) that delivered the GnRH from a reservoir within the pump to a jugular intravenous catheter. The pump, attached to a harness worn by the bitch, was well tolerated by laboratory Beagles. The pump and harness were not well tolerated by privately owned Labrador Retriever bitches, however. The fragility of the pump and its expense preclude its routine use in veterinary medicine.
GnRH analogs, many of which are not currently commercially available, have been investigated as agents to induce estrus in bitches. The advantage of GnRH analogs is their relatively longer bioavailability and increased potency. Concannon32 reported the use of [D-Trp6NmeLeu7Pro9NEt] GnRH administered by a constant-rate infusion device. Twenty-four bitches received 1.7 to 2.5 μg/kg/day subcutaneously for 14 days; nine bitches ovulated and whelped litters. In a preliminary report, the use of [D-Trp6Pro9NEt] GnRH was administered to bitches (1 μg/kg subcutaneously every 8 hours) by the following protocol: every 8 hours subcutaneous injections until the observation of behavioral estrus, after which treatment was continued for another 3 days at half the original dose (i.e., 0.5 μg/kg subcutaneously every 8 hours).33 Four of six bitches treated with this protocol whelped litters as a result of an induced estrus.
Deslorelin implants have also been used successfully as vulval implants for 10 days to induce estrus in the bitch.31 They must be removed before downregulation occurs.
The gonadotropins FSH and LH have been investigated with numerous protocols as agents to induce estrus in the bitch. Most protocols use either eCG or FSH to stimulate folliculogenesis and hCG to induce ovulation. Some protocols additionally use DES administered before gonadotropins; estrogen can increase the responsiveness to gonadotropins. Gonadotropin protocols are largely unreliable and can induce adverse effects (e.g., ovarian hyperstimulation with hyperestrogenism), and with some protocols an abnormal luteal phase was detected.34 One report indicated great success with the currently unavailable product PLH (Burns-Biotech),35 although it is unknown what the relative biopotency of FSH and LH was in the product marketed as PLH. That protocol was investigated using Greyhound bitches that had previously received testosterone to suppress estrus during racing. Those bitches had not received testosterone during the 12 months before inclusion in the study, and none of the bitches had an observed proestrus or estrus during that period of time.
The protocol was as follows: DES was administered (5 mg/bitch per day orally) until proestrus was evident and then for 2 days thereafter. If no signs of proestrus were observed by day 7 of DES treatment, the dose was doubled (10 mg/bitch per day orally) until a response was elicited (not to exceed 7 additional days of DES therapy). On day 5 of proestrus, PLH was administered (5 mg/bitch intramuscularly), and on days 9 and 11 of proestrus FSH was administered (10 mg/bitch intramuscularly).35 All seven bitches in this report became pregnant when bred during an induced estrus. This method used gonadotropin administration in a reverse sequence to that of other reported studies. Because the LH used in this study was no longer available, an attempt to modify the protocol using hCG in place of LH was attempted, but results were disappointing.36
Subsequent studies into the use of DES as a sole agent to induce estrus may indicate that the DES used in other protocols was the primary active agent, and perhaps the administration of additional hormone products decreases the response. Fertile estrus has been induced in bitches by the sole administration of oral DES. In a preliminary report DES (5 mg/bitch orally) was administered daily until proestrus was observed and then for 2 days thereafter.37 The DES therapy continued for 6 to 9 days and resulted in an induced proestrus lasting 0 to 2 days (range) and an estrus duration of 15 to 26 days (range). The preovulatory LH peak occurred 13.5 ± 3.7 days after the last day of DES treatment, although one treated bitch did not have a detectable preovulatory LH peak. All five treated bitches in this study became pregnant, four bitches whelped at term, and one bitch aborted. The lengths of proestrus and estrus and the sizes of the litters did not significantly differ between treated and control groups (n = 5 in each group). The bitches used in this study were treated during a defined period of anestrus (95 to 129 days; mean 107 ± 13 days), which resulted in a shorter period of anestrus than is normal for the colony (175 ± 87 days). Additional investigation into the use of DES as an agent to induce fertile estrus is needed before client-owned bitches are treated. The optimum dosage schedule and potential for toxicity must be assessed. It may be necessary to adjust the dose in smaller bitches to avoid toxicity.
Another method to shorten the naturally long interestrus interval in the bitch is by the administration of a dopamine agonist to decrease prolactin secretion. Prolactin secretion may alter ovarian responsiveness to gonadotropins; the continued presence of prolactin during anestrus may be responsible for the duration of this phase of the estrous cycle. Decrease in prolactin secretion can shorten the length of anestrus and can induce estrus if treatment occurs during late anestrus. Recent work indicates that the effect of bromocriptine causing shortening of anestrus is due to a yet-undefined action other than its lowering of plasma prolactin concentration and can shorten either diestrus or anestrus to have this effect.38 Bromocriptine (20 μg/kg twice daily administered 112 ± 4 days after the last onset of proestrus) was used in one study to decrease the interestrus interval in bitches.39 Bromocriptine causes vomiting, and investigation into its use has been largely replaced by the dopaminergic drugs cabergoline and metergoline. Termination of anestrus and induction of fertile estrus with minimal side effects has been reported by several authors in dogs using cabergoline.40,41 One protocol uses cabergoline at 5 μg/kg/day until the second cytologic day of proestrus. Treatment for up to 40 days has been reported as effective in inducing fertile estrus in 70% to 90% of normal bitches when started in late anestrus.42 Ovulation timing and appropriate breeding management result in normal pregnancies. Two protocols using metergoline were reported in one study.43 Bitches were treated with metergoline (12.5 mg/bitch intramuscularly every 3 days) until the onset of proestrus. Response to therapy was considered positive if proestrus was observed within 40 days after the first day of treatment; 18 of 20 bitches so responded. Ten of these bitches received no additional treatment and were bred, and nine produced litters. Eight other bitches responding to metergoline were additionally treated with hCG (500 IU/bitch intramuscularly) during late proestrus. Six of these bitches ovulated, and four achieved pregnancy. Overall, the metergoline protocol without hCG was more effective in producing pregnancy.
The administration of PGF2α can also shorten the interestrus interval if administered during diestrus. Diestrus normally has a duration of 65 to 70 days in the bitch, and luteolytic therapy with PGF2α abbreviates this phase of the estrous cycle. Bitches that have undergone luteolytic therapy will still enter a phase of anestrus for a variable period, but the return to proestrus or estrus can be sooner than expected, by 1 to 2 months.
Induction of Estrus in the Queen
The domestic cat has been used as a model for reproductive techniques that can apply to preservation of the nondomestic large felines.44 Queens are apparently sensitive to effects of gonadotropins administered to induce folliculogenesis, but production of anovulatory follicles or cysts can result. The administration of eCG as a single bolus (100 IU) to anestrus queens, followed in 5 to 7 days by a single injection of hCG (50 IU), resulted in a pregnancy rate that was comparable with that produced by natural matings.45 Daily administration of FSH-P (2 mg/queen per day intramuscularly) for 5 to 7 days results in 72% of queens that will mate and deliver normal offspring.46
Induction of Ovulation
Ovulatory Failure in the Bitch
Ovulation failure is rare in the bitch. Too often, bitches are suspected of ovulatory failure when they have seemingly long proestrous and estrous phases, produce small litters, or fail to conceive. Bitches can have signs of estrus, and a fully cornified vaginal smear, for up to 21 days before natural spontaneous ovulation. Also, bitches can have a split estrus: signs of proestrus or estrus without ovulation, a period of anestrus for several weeks, and then return to proestrus. Often the second estrus will be ovulatory and fertile. Split estrus is more common in pubertal bitches but can occur in mature bitches.
Ovulation can be reliably detected with ultrasonography but requires thrice-daily monitoring during the ovulatory period and technical expertise. Direct visualization by laparoscopy is not possible because of the ovarian bursa (it requires bursal resection and is done only experimentally); thus the determination of ovulation is usually attempted indirectly. Serum progesterone concentration can be measured in bitches that historically fail to conceive. The timing of the progesterone measurement is important. It is recommended to evaluate progesterone during the first few weeks of diestrus. Serum concentration of progesterone should be in excess of 5 ng/mL (and generally in excess of 20 ng/mL) at this time. A progesterone level of less than 2 mg/mL indicates either ovulation failure or luteal insufficiency. Values between 2 and 5 ng/mL are suspect and can indicate ovulatory failure, luteal insufficiency, or an uncommonly low progesterone production during diestrus.
Bitches that have normal proestrus and estrus but apparently do not ovulate can be given hormonal products in an attempt to induce ovulation. To mimic the preovulatory LH peak, either GnRH or hCG can be administered at the point of follicular maturation. The determination of follicular maturity is difficult. Incorrect administration (i.e., inappropriate timing) of either preparation can cause preovulatory luteinization of follicles without ovulation or the ovulation of immature, nonviable ova. It has been recommended to administer either GnRH (50 μg/bitch intramuscularly) or hCG (500 to 1000 IU/bitch intramuscularly) on either the day before or the day after the first breeding.47 Bitches can begin sexual receptivity several days before or after spontaneous ovulation; therefore this protocol is questionable and not documented. It may be advisable to measure serum LH concentration daily during proestrus and estrus and administer either GnRH or hCG on the day of the natural preovulatory LH peak. This presumes that the bitch produces enough LH to measure a peak but either does not produce enough LH to cause ovulation or has another factor inhibiting ovulation. Bitches that fail to ovulate may not produce a measurable LH peak, in which case this recommendation would also fail. Clearly, more investigation is needed in this area.
Ovulatory Failure in the Queen
Queens can be sexually receptive before follicular maturation; sexual receptivity may not correlate with follicular development because estrogen may not return to baseline. Limited mating before the third to fourth day of estrus can result in an attenuated LH secretion and ovulatory failure.48 Additionally, although an LH response after one effective mating will occur, a maximal LH response ensuring ovulation of all mature ova will more likely occur if numerous matings over a several-day period is allowed. In one report 10 of 48 queens ovulated after a single mating, whereas 30 of 36 ovulated after multiple matings.49 Optimal breeding management includes mating three times per day at 4-hour intervals throughout estrus. Matings starting on the third day of estrus are advised.
If it is determined that a queen fails to ovulate despite appropriate management, induction of ovulation can be attempted. Protocols with either hCG or GnRH have been reported as follows: hCG 500 IU/queen intramuscularly on day 1 of estrus50 and GnRH 25 μg/queen intramuscularly on day 2 of estrus.51
Treatment of Follicular and Luteal Ovarian Cysts in Bitches
Estrogen-producing follicular cysts can cause prolonged proestrus, nymphomania, estrogen toxicity, and resultant infertility. A luteinized follicular cyst producing progesterone can cause persistent diestrus and resultant infertility. Nonfunctional ovarian cysts can prevent normal cycling and result in infertility. Ovarian cysts are often detected during ovariohysterectomy of older bitches as incidental findings. The detection of a cystic ovarian structure by ultrasonography can be a significant finding. Normal ovarian follicles, luteal structures, and subepithelial cysts should be ruled out, often by sequential ultrasonographic examinations in concert with serum hormone (estradiol and progesterone) measurements. The possibility of a cystic ovarian neoplasm, which can produce clinical signs identical to those of benign cysts, must be considered and is more likely in bitches older than 5 years of age. The measurement of estrogen and progesterone concentrations from the fluid of percutaneously aspirated ovarian cysts can assist the diagnosis of a functional cyst; this will not, however, differentiate between a cyst and neoplasm.
Whether to attempt therapeutic pharmacologic intervention when an ovarian cyst is detected is controversial; ovarian cysts can spontaneously regress.52 Estrogen-producing follicular cysts can be luteinized with GnRH (50 μg/bitch intramuscularly) or hCG (500 to 1000 IU/bitch intramuscularly). Either treatment can be used as a single injection or repeated daily for three treatments. Response to therapy is a termination of estrous behavior or decrease of estrogen and increase of progesterone serum concentrations. Efficacy of these regimens has not been reported, and surgical removal of the cyst by unilateral ovariectomy is usually required if aggressive intervention is deemed necessary. Similarly, lysis of luteal ovarian cysts with PGF2-alpha can be attempted but is usually unrewarding in the authors’ experience. Ovariectomy permitting histopathologic evaluation of both follicular and luteal ovarian cystic disorders is advised. Ultrasound-guided aspiration of follicular cysts has been advocated as a successful method of terminating prolonged proestrus or estrus in the bitch and deserves further controlled evaluation.
Treatment of Vaginal Hyperplasia and Prolapse
Estrogen produced during folliculogenesis normally causes a hyperplastic response of the vaginal mucosal epithelium and cornification of the vaginal epithelial cells, in preparation for the copulatory lock. The estrogen response can induce a hyperplastic vaginal periurethral papillary mass in some bitches that can prolapse through the vulvar cleft. Follicular luteinization can be attempted to prematurely decrease estrogen production with GnRH (50 μg/bitch intramuscularly) or hCG (500 to 1000 IU/bitch intramuscularly). It is doubtful that medical intervention is of benefit given that most bitches resolve this condition after ovulation. Successful surgical methods of amputating the hyperplastic tissues have been reported; the condition resolves in normal bitches when estrogen levels fall.53 Prolonged vaginal hyperplasia or prolapse most commonly occurs with ovarian pathology (follicular ovarian cysts), requiring ovariectomy for resolution.
Diagnosis and Treatment of Premature Labor
Corpora lutea in both the queen and bitch produce progesterone during gestation. Evidence of spontaneous abortion or fetal reabsorption associated with serum progesterone concentrations below 1 to 2 ng/mL can suggest luteal insufficiency, which is usually associated with inappropriate myometrial contractility. By the time fetal death is observed clinically, progesterone secretion has likely decreased secondarily.54 Also, a serum progesterone concentration below 1 to 2 ng/mL after estrus can indicate lack of ovulation rather than luteal insufficiency. Primary luteal insufficiency has not yet been documented in the bitch and queen.
Bitches with a documented history of fetal reabsorption (i.e., ultrasonographic confirmation with no other associated pathology) should be evaluated after the next breeding with both uterine monitoring, ultrasonography, and serum progesterone concentrations.55 If inappropriate uterine contractility is determined with no contributory pathology evident, tocolytic therapy can be considered (terbutaline 0.03 mg/kg orally every 8 to 12 hours to effect). Serum progesterone concentration should be evaluated; if it is less than 3 to 5 ng/mL and viable fetal vesicles are detected, progesterone supplementation can be considered. Because excessive or inappropriate progesterone administration during pregnancy can cause masculinization of female fetuses and interfere with lactogenesis, it should be undertaken only when indicated (i.e., when tocolytic therapy alone is not effective). Progesterone administration will also prevent normal spontaneous parturition; therefore treatment must be discontinued 3 days before the calculated date of parturition. Therapeutic intervention in suspected hypoluteoidism can be accomplished with the administration of injectable natural progesterone or oral synthetic progestagens. Total serum levels of progesterone can be monitored only when supplemented with the natural product. Progesterone in oil is given intramuscularly at 2 mg/kg every 72 hours.56 Altrenogest (Regu-Mate, Hoechst-Roussel), a synthetic progestagen manufactured for use in the mare, is dosed orally at 0.088 mg/kg every 24 hours.57 Both forms of supplementation must be discontinued in a timely fashion so as not to interfere with normal parturition, within 24 hours of the due date with the oral synthetic product, and within 72 hours with the natural, injectable depot form. This requires accurate identification of gestational length using prior ovulation timing (parturition is expected to occur 64 to 66 days from the LH surge or initial rise in progesterone or 56 to 58 days from the first day of cytologic diestrus). Less accurate identification of gestational length can be made from breeding dates (58 to 72 days from the first breeding), radiography, or ultrasound.
Luteal insufficiency is also uncommon in the queen, although it is frequently suspected in queens that experience late-term abortion (i.e., day 50 to 55 of gestation). As in the bitch, luteal insufficiency is diagnosed by determination of viable pregnancy and inadequate progesterone secretion.
Evaluation of such queens early in gestation for inappropriate myometrial activity is advisable. Evaluation of protocols designed to cause medical abortion in queens has determined that a measured serum progesterone concentration of 1 ng/mL may be sufficient to support pregnancy, at least for a short time.16 The criteria to diagnose luteal insufficiency in the queen, therefore, remains undetermined. Premature labor in an otherwise benign uterus can be effectively treated with tocolytics (terbutaline 0.03 mg/kg orally as needed), the exact dose based on response to therapy, as evaluated by uterine monitoring.
Prevention or Termination of Estrus
Megestrol acetate is the only substance currently licensed in the United States to delay or suppress estrus in bitches. There are no products currently licensed for this purpose in the queen. It is advised not to use progestational products to delay or suppress estrus in bitches intended for future breeding because of the predictable side effects of progesterone in the intact bitch. A progestagen hormone, megestrol acetate, promotes the development of endometrial gland proliferation and suppresses the local uterine immune response. These effects can increase the incidence of pyometra and infertility. Progestagens can cause pathologic changes in mammary glands, the endocrine pancreas, and prolactin-producing and growth hormone–producing cells of the pituitary gland.58 The drug is contraindicated in cases of previous uterine or mammary gland disease or diabetes mellitus.
If megestrol acetate therapy is chosen despite potential adverse effects, the treatment protocol depends on the stage of the estrous cycle in which treatment is begun. To prevent estrus, megestrol acetate (0.55 mg/kg per day orally for 32 days) is given beginning at least 7 days before the onset of proestrus. After discontinuation of therapy, the bitch will likely begin an estrous cycle. Alternatively, megestrol acetate (2.2 mg/kg per day orally for 8 days) administration can be started during proestrus to abbreviate the signs of estrus and prevent ovulation. In addition to a dose-related effect, this high-dose protocol can be potentially more deleterious than the low-dose protocol because the uterine effects of megestrol acetate can be enhanced by endogenous estrogens increased during proestrus.
Although not yet commercially available in most of the United States, GnRH analogues in a repository form offer good options for preventing estrous cycles for approximately 6 months resulting from down regulation.
Prolongation of Interestrus Intervals in the Bitch
Synthetic androgen (previously available as mibolerone, the availability of which is currently variable) therapy was recommended as a therapeutic protocol to delay estrus in bitches that have interestrus intervals of less than 4 months and are infertile as a result of an abbreviated anestrus. Frequent ovulatory estrous cycles may not allow sufficient time for the endometrium to recover from the trophic influences of progesterone during a nonpregnant diestrus.59 Synthetic androgen suppression of ovarian activity for 6 to 9 months was previously recommended after ruling out other causes of infertility in bitches with short interestrus intervals.54 Fertility rates after the use of mibolerone for this purpose have not been reported; the benefit of this protocol have never been proved. As there is no documented effective therapy for abbreviated anestrus, breeding affected bitches at the earliest opportunity, before progesterone-mediated changes in the endometrium have occurred, is advised. The heritability of this tendency has not been established. Because of problems with human abuse of androgenic substances, the availability of such compounds is variable.
Treatment of Clinically Significant Pseudocyesis
The decrease in serum progesterone and increase in serum prolactin concentrations at the end of diestrus can cause overt signs of pseudocyesis (e.g., nesting, galactorrhea, reclusiveness, and possibly aggression) in some bitches. Because this is a normal phenomenon, clinical signs rarely warrant therapeutic intervention. Rarely, mastitis occurs during pseudocyesis. Cabergoline, a prolactin inhibitor, at 5 μg/kg orally every 24 hours for 3 to 5 days, alleviates the signs of pseudopregnancy if problematic.
Pyometra and Postpartum Metritis
The pathophysiology and diagnostic criteria of pyometra and postpartum metritis are well described.54,60 Case selection must be considered carefully because medical therapy should be reserved for animals that are medically stable and have future reproductive potential. Ovariohysterectomy, after appropriate medical stabilization, remains the treatment of choice for bitches or queens older than 6 to 7 years of age or if signs of systemic sepsis are present.
Many protocols have been proposed for the effective treatment of open-cervix pyometra and postpartum metritis in the bitch and queen using PGF2. These protocols use the natural hormone dinoprost tromethamine, marketed as Lutalyse or Prostin (Upjohn). Dosage recommendations range from 0.1 to 0.2 mg/kg body weight every 12 to 24 hours for 5 to 7 days. Alternatively, the synthetic prostaglandin cloprostenol has fewer side effects and appears to be equally effective, using doses of 1 to 3 μg/kg subcutaneously every 12 to 24 hours, to effect. Monitoring with ultrasonography aids in the documentation of disease resolution. Some individuals may require repeated or prolonged treatment if the initial regimen is unsuccessful. The administration of concurrent appropriate systemic antimicrobial therapy, either broad spectrum in anticipation of or based on culture and sensitivity results from a guarded sample of the cranial vaginal vault, is indicated.
Similar management of closed-cervix pyometra is problematic. Treatment can be attempted in the bitch with closed-cervix pyometra that is medically stable and carefully monitored during the treatment process. The use of a PGE1 analog (misoprostol) intravaginally in conjunction with PGF2α has been advocated as a method of establishing cervical patency.61 Failure to respond to therapy or worsening of clinical signs indicates the need for ovariohysterectomy.
Pregnancy Termination
There are now several options for the management of an unwanted breeding (i.e., misalliance or mismating) of a bitch that has future reproductive potential. The administration of estrogen is no longer recommended because of potential toxicity, induction of a pyometra, or future infertility (see additional information in the later discussion of misuses of reproductive hormones). Besides allowing the pregnancy to proceed to term or performing an ovariohysterectomy during early gestation, there are safe, reliable methods to induce medical abortion in the bitch and queen.
Early Diestrus Protocol
Administration of PGF2α (dinoprost tromethamine) to bitches in early diestrus can result in transient or permanent luteolysis and thus prevent continuation of pregnancy.28 Fetal contents are reabsorbed, and outward signs of abortion are not detected. In one study the following protocol was successful for all 25 bitches treated with PGF2α: 0.25 mg/kg subcutaneously twice daily for 4 days between days 5 and 19 of diestrus.28 Clinicians are advised to determine the onset of diestrus cytologically by evaluating sequential vaginal cytology after the mismating. The major drawback to this regimen is that treatment occurs before documentation of pregnancy can be performed. Because many mismatings (60%) do not result in pregnancy, bitches can be unnecessarily treated with this protocol.27 This regimen can be performed on an outpatient basis, and its relatively short treatment period (i.e., 4 days) is favorable.
Midgestation Protocols
After day 30 of gestation, PGF2α therapy induces luteolysis and myometrial contractions, resulting in fetal expulsion. Treatment is begun after documentation of pregnancy by ultrasonography. Ultrasonography is repeated during the treatment period to determine the end point of therapy because some bitches can partially abort their litter and carry the remaining pups to term. The following protocol has been reliably successful in bitches treated after day 30 of gestation: PGF2α 0.1 mg/kg subcutaneously every 8 hours for 2 days and then increasing the dose to 0.2 mg/kg subcutaneously every 8 hours until the abortion is complete.27 The range of therapy is from 3 to 9 days, with excellent subsequent reproductive capability in treated bitches. Queens have also been similarly treated with good results.54 Cloprostenol can be substituted for PGF2α with fewer undesirable physical side effects.
An adjunctive therapy to PGF2α has been proposed to hasten the treatment period (i.e., the duration of treatment needed to produce effect). Administration of misoprostol, a prostaglandin E compound, intravaginally daily (1 to 3 μg/kg) concurrently with the previously described PGF2α protocol was found to decrease the treatment period by 1 to 2 days.61 The proposed action of the misoprostol in this regimen is to soften and open the cervix, thus permitting evacuation of uterine contents.
Dexamethasone has been used successfully to induce abortion and resorption in the bitch.24 Dexamethasone administered at 0.2 mg/kg orally twice daily for 10 days initially is advised and effective. Pregnancies of less than 40 days generally are resorbed with minimal vaginal discharge. Successful pregnancy was reported in 18 of 20 bitches bred at the subsequent estrus. It is imperative that ultrasonographic evaluation of the pregnancy take place after the 10-day treatment period, insofar as fetal death occurs between 8 and 12 days, and additional days of treatment may be needed to complete termination. Progesterone levels, when measured, were below 1 ng/mL, suggesting luteolysis. Side effects include polydipsia, polyuria, polyphagia, and panting, which were tolerable for the treatment period and resolved when the drug was discontinued. Dexamethasone-induced abortion permits outpatient treatment of unwanted pregnancy with minimal expense.
Abortion can be induced in bitches and queens by the antiprolactin effect of dopamine agonists. Because bromocriptine is not well tolerated, studies investigating the use of cabergoline and metergoline to terminate pregnancy in the bitch and queen have been conducted.15,16 Pregnancy termination with a dopamine agonist results in fetal reabsorption more commonly than the uterine evacuation that occurs with the PGF2α regimen. The protocols appear to be effective and well tolerated; the availability and expense of the latter generation dopamine agonists are the major drawback.
Medical Management of Dystocia
Oxytocin is the most commonly used reproductive hormone in general veterinary practice. Its use in the medical management of uterine inertia has been well described elsewhere.54 It is important to accurately assess the indication for the use of oxytocin and ensure that fetal malposition or obstruction is not present. Additionally, overuse of oxytocin can result in a tetanic uterus and can impede fetal (placental) blood supply and cause fetal compromise.55 Judicious use of oxytocin to treat uterine inertia can be beneficial. Dose ranges are lower than previously reported, from 0.25 to 2 units subcutaneously or intramuscularly per dose, and can be repeated every 30 minutes to effect. Previously suggested doses of more than 1U/kg are superphysiologic and contraindicated because they may induce tetanic contractions, compromise fetal (placental) blood supply, and cause uterine rupture. It has been recommended to administer calcium gluconate subcutaneously 10 to 15 minutes before the oxytocin therapy despite the typical eucalcemic status of bitches experiencing dystocia, suggesting that the benefit of exogenous calcium occurs at the cellular level. Calcium improves strength of uterine contractions, whereas oxytocin increases their frequency.55 The use of uterine and fetal monitoring systems permitting insightful mediation (WhelpWise) is strongly advocated during the management of labor.
Adjunct Therapy for Mammary Gland Carcinoma
The use of the antiestrogen tamoxifen has been investigated as an adjunct in the treatment of mammary gland adenocarcinoma in the bitch.62 Although the bitch exhibits direct estrogenic effects to the reproductive tract (i.e., vulvar edema, stump pyometra) after the administration of tamoxifen, this drug may have an antiestrogenic effect on mammary tissue. In one report tamoxifen therapy (mean dose 0.42 mg/kg orally twice daily) was effective for five of seven bitches with nonresectable or metastatic mammary carcinoma.62 In another study tamoxifen was administered (0.7 mg/kg every 24 hours orally for 4 to 8 weeks) but had no observable effect in 10 bitches that had advanced mammary cancer.62 The use of tamoxifen as an adjunct in the treatment plan for mammary neoplasia requires more investigation before it can be advised routinely.
Urinary Incontinence in Spayed or Neutered Dogs
Supplementation with reproductive hormones can be considered when a diagnostic evaluation for chronic urinary incontinence in a neutered dog detects decreased urethral sphincter tone with no other pathology evident. Supplementation with DES or testosterone in ovariectomized bitches or castrated dogs, respectively, can enhance the sensitivity of α-adrenergic receptors to endogenous α-agonists. Oral treatment with DES can be started at 0.1 to 1 mg/bitch per day for 7 days, after which the frequency is diminished to the lowest effective dose. If signs cannot be controlled with infrequent therapy (i.e., 1 mg or less every 3 to 5 days in a 30-kg bitch), alternative or adjunctive drug therapy with phenylpropanolamine should be considered. Potential side effects of DES treatment include estrogen-induced bone marrow toxicity (not reported with oral diethylstilbesterol), attraction of male dogs, and dermatologic disorders. Supplementation of incontinent male dogs with testosterone (testosterone cypionate 2.2 mg/kg intramuscularly every 30 days) can be considered.63 Adverse effects include prostatic hyperplasia and behavioral changes such as inappropriate urination, aggression, and sexual excitability. When treating idiopathic urinary incontinence in either the neutered female or male dog, the clinician may prefer to consider a compounded α-agonist phenylpropanolamine. Essentially no side effects are expected with this type of therapy, but the owner may need to administer the drug multiple times per day for a consistent effect. The role of gonatotropins in idiopathic incontinence has been reported; a depot administration of GnRH has been used for the treatment of urinary sphincter incompetence in the bitch with good results, suggesting a future role for adjunct therapy of urethral sphincter incompetence in the bitch.64
Cryptorchidism
Cryptorchidism is an inherited congenital disorder for which bilateral castration is recommended. Medical treatment to cause descent of a retained testicle is unethical if performed for the purpose of enabling the dog to be shown or bred and likely ineffective. Alternatively, descent of a retained testicle before castration will allow a prescrotal surgical approach. Protocols using either GnRH or hCG have been recommended, although no controlled studies to document efficacy have been published. The precise method of action of these hormones to induce testicular descent is unknown. One protocol for GnRH is 50 to 100 μg/dog subcutaneously or intramuscularly, repeated after 4 to 6 days if no improvement is observed. An alternative protocol is to administer hCG 100 to 1000 IU/dog intramuscularly 4 times spaced over a 2-week period.55 This protocol has been unsuccessful when used in dogs older than 16 weeks of age, and no actual controls existed in the study describing its use.54
Cryptorchidism is uncommon in cats. To the authors’ knowledge, the medical treatment of this disorder in cats has not been investigated.
Benign Prostatic Hyperplasia
Chronic administration of a GnRH analog can cause downregulation, and serum testosterone concentrations can decline to castrate levels. This is the basis of therapy for the administration of leuprolide (a GnRH analog) to men with prostatic carcinoma. Prostatic carcinoma occurs with equal frequency in dogs castrated before puberty as in intact dogs, indicating that testicular androgens are not the sole inciting factor in the development of prostatic neoplasia in the dog.65 Adrenal androgens may play a role in the development of prostatic neoplasia in dogs and in men refractory to downregulation therapy.
It is possible that downregulation therapy could successfully treat other androgen-dependent conditions in the dog (e.g., perianal adenoma, perineal hernia formation, benign prostatic hyperplasia). Factors such as cost, lack of appropriate dosing information, and the routine acceptance of surgical castration in the dog make treatment with GnRH analogs for such cases unlikely. The advantage of medical treatment versus surgical castration is the potential reversibility of infertility with cessation of downregulation therapy, although the androgen-dependent disorder would likely recur.
Successful treatment of benign prostatic hyperplasia using finasteride has been reported.26 The advantage of finasteride administration is the maintenance of spermatogenesis, because finasteride decreases intraprostatic DHT concentrations and does not affect intratesticular or circulating testosterone concentrations. Recommendations have been to administer finasteride to dogs at the dosage currently used for men: 5 mg/dog orally once daily. Whether a lower or less frequent dosing regimen is possible remains to be investigated. The disadvantage of finasteride administration in men, as a potentially teratogen, is not of concern in dogs because of the minimal exposure associated with typical breedings. One problem resulting from the long-term use of finasteride is a reduction in the volume of the prostatic fraction of the ejaculate, perhaps necessitating the use of semen extenders. The impact of this lack of prostatic fluid on natural breedings (in which the volume of prostatic fluid forces semen into the uterus) is not known. The use of progestagens (i.e., medroxyprogesterone and megestrol acetate) has also been reported as a method to decrease prostatic hypertrophy and maintain sperm production. The potential adverse systemic effects of these compounds (i.e., effects on growth hormone production, liver function, insulin secretion, mammary gland disease) make this treatment less appealing.
Hypogonadism
Hypogonadotropic hypogonadism, a congenital condition in which the pituitary fails to produce gonadotopins, occurs in men. Affected men can respond to gonadotropin replacement therapy with resultant fertility. Hypothalamic or hypogonadotropic hypogonadism has not been documented to occur in a congenital form in dogs. Acquired pituitary dysfunction can occur in dogs with space-occupying neoplasms, but the diagnosis of hypogonadism is difficult. Lack of negative feedback inhibition causes dogs with primary testicular degeneration or atrophy to have high serum LH and FSH concentrations. If primary pituitary failure was the cause of azoospermia, the serum FSH and LH concentrations would be low. At present, FSH and LH assays are not readily available for the dog; a semiquantitative LH kit is available (Synbiotics). Because of the nature of LH secretion, challenge testing is recommended to evaluate pituitary function.
Treatment of this form of infertility depends on the etiology of the pituitary disease; fertility may be unimportant in view of the dog’s general health. Although the prognosis for return to fertility is guarded, one protocol using gonadotropin replacement therapy is to administer 500 IU hCG biweekly (subcutaneously or intramuscularly) and FSH at either 1 mg/kg intramuscularly every 48 hours or 25 mg/dog subcutaneously once weekly.54 Because spermatogenesis and spermatozoa maturation requires approximately 77 days in the dog, therapy must be continued for 3 months before the effectiveness of this protocol can be evaluated.
Management of Retrograde Ejaculation; Improvement of Ejaculate
Retrograde ejaculation causes semen to flow into the urinary bladder, reducing the effective ejaculate. Retrograde ejaculation is treated with pseudoephedrine hydrochloride (3 to 5 mg/kg given 3 hours and 1 hour before collection) or phenylpropanolamine (3 mg/kg every 12 hours).42
It is often desirable to increase the sperm quantity in an ejaculate to optimize the sample for freezing for shipping. In the dog it has been theorized that GnRH administration before ejaculation will induce an LH surge with a primary increase in testosterone and thus improve the total spermatozoa per ejaculate; however, a recent study evaluating eight mature mixed-breed dogs showed no objective improvement in semen parameters or libido. Interestingly, administration of PGF2-alpha (0.1 mg/kg 15 minutes before collection) significantly increased the total number of sperm per ejaculate compared with control dogs and dogs receiving oxytocin and GnRH before collection. It is thought that administration of PGF2-alpha before ejaculation facilitates the movement of spermatozoa from the epididymis to the ductus deferens, thus enhancing the ejaculatory output.66
Misuses of Reproductive Hormones
Some reproductive hormones have been misused for many years (Table 23-3). Assumptions based on incomplete information about canine reproductive physiology led to the development of these protocols in the past. Current knowledge and other alternatives (i.e., for mismating in bitches) have resulted in the discontinuation of these practices, which are now considered inappropriate.67
Treatment of Idiopathic Infertility
Infertility in the Bitch
Physiologic processes that control folliculogenesis in the bitch are complex and involve precise, minute amounts of hypothalamic and pituitary hormones that are sensitively controlled by the ovarian feedback loop. To interrupt the hypothalamic–pituitary–ovarian axis pharmacologically leads to dysfunction, rather than an augmentation, of the system. The rational administration of GnRH requires pulsatile administration, the use of GnRH analogs results in downregulation, and the use of gonadotropins is largely unsuccessful to induce fertile estrus in known fertile bitches. There is no evidence to support the use of these hormones in bitches with a history of infertility or decreased fecundity. Careful evaluation of the underlying causes of infertility and optimal breeding management and husbandry with ovulation timing (i.e., serial evaluation of measured parameters to determine the time of ovulation) is recommended.
Infertility in the Stud Dog
Decreased production of sperm is usually the result of primary testicular degeneration and atrophy. Gonadotropin secretion is increased as a result of the lack of negative feedback inhibition. The administration of GnRH or gonadotropins in such cases is therefore inappropriate. Careful investigation to determine the cause of infertility (e.g., testicular atrophy, bacterial prostatitis, infectious or immune-mediated orchitis, spermatic tubular obstruction) is recommended.
As is true of GnRH and gonadotropins, the use of gonadal steroids to enhance fertility is contraindicated. The most common misuse of gonadal steroids to potentiate fertility is the administration of testosterone to heighten libido. Pharmacologic administration of testosterone inhibits steroidogenesis by interruption of the sensitive feedback mechanisms of the hypothalamic–pituitary–gonadal axis. The concentration of testosterone within the seminiferous tubules normally exceeds that found in circulation. An increase of circulating testosterone will serve to decrease output of FSH and LH and thereby decrease spermatogenesis. Additionally, many dogs with low libido have normal concentrations of serum testosterone but have a decrease in libido for other reasons (e.g., prostatic disease, behavioral issues).
Treatment of Mismating and Pregnancy Termination
Estrogens, in the form of either estradiol cypionate (ECP) or DES, have been used historically to prevent pregnancy after mismating in the bitch. The administration of estrogen in any form is no longer recommended for this condition because estrogens are either ineffective or unsafe to use. All bitches treated with estrogens are at risk for the development of bone marrow aplasia, pyometra, or infertility. Although most cases of estrogen-induced bone marrow toxicity have been associated with ECP administered at high doses (i.e., >1 mg), aplastic anemia has been observed in bitches receiving a lower dose. One study determining the efficacy of estrogens to prevent pregnancy found that DES (75 μg/kg orally for 7 days) was ineffective when treatment began in proestrus, estrus, or day 2 of diestrus.19 Also, ECP (22 μg/kg intramuscularly) was ineffective when administered once during proestrus or estrus, preventing pregnancy in only 50% of treated bitches.19 The administration of estrogens during diestrus increases the risk of the development of pyometra because the progesterone effect on the uterus to promote glandular secretion and decrease local uterine immunity is enhanced in the presence of estrogen. To prevent pregnancy in mismated bitches, protocols using PGF2α, dexamethasone, or dopamine agonists should be considered.
Treatment of Benign Prostatic Hyperplasia
The use of estrogens to treat benign prostatic hyperplasia in dogs is not recommended. In addition to potential toxicity with the administration of estrogens, estrogen-induced squamous metaplasia of the prostate gland may increase the risk of bacterial prostatitis and cyst formation.
1. Concannon P.W. Biology of gonadotrophin secretion in adult and prepubertal female dogs. J Reprod Fertil. (Suppl 47):1993.
2. Hull M.E., Kenigsberg D.J. Gonadotropin-releasing hormone function and clinical use. Lab Manag. 1987;25:51.
3. Leyendecker G., Wildt L., Hansmann M. Pregnancies following chronic intermittent (pulsatile) administration of GnRH by means of a portable pump (Zyklomat): a new approach to the treatment of infertility in hypothalamic amenorrhea. J Clin Endocrinol Metab. 1980;51:1214.
4. Johnson A.L. Induction of ovulation in anestrous mares with pulsatile administration of gonadotropin-releasing hormone. Am J Vet Res. 1986;47:983.
5. Cain J.L., Cain G.R., Feldman E.C., et al. Use of pulsatile intravenous administration of gonadotropin-releasing hormone to induce fertile estrus in bitches. Am J Vet Res. 1993;49:1988.
6. McRae G.I., Roberts B.B., Worden A.C., et al. Long-term reversible suppression of oestrus in bitches with nafarelin acetate, a potent LHRH agonist. J Reprod Fertil Suppl. 1985;74:389.
7. Johnston S.D. Parturition and dystocia in the bitch. In: Morrow D.A., editor. Current therapy in theriogenology. ed 2. Philadelphia: Saunders; 1986:501.
8. Carruthers T.D. Principles of hormone therapy in theriogenology. In: Morrow D.A., editor. Current therapy in theriogenology. ed 2. Philadelphia: Saunders; 1986:3.
9. Delouis C., Richard P. Lactation C., Thibault M., R. Hunter. 1993. Reproduction in mammals and man. Paris. Edition Marketing, p. 503
10. Olson P.N., Bowen R.A., Behrendt M.D., et al. Concentrations of reproductive hormones in canine serum throughout late anestrus, proestrus, and estrus. Biol Reprod. 1982;27:1196.
11. Concannon P.W., Whaley S., Anderson S.P. Increased LH pulse frequency associated with termination of anestrus during the ovarian cycle of the dog. Biol Reprod. 1986;34:119.
12. Lawler D.F., Johnston S.D., Hegstad R.L., et al. Ovulation without cervical stimulation in domestic cats. J Reprod Fertil Suppl. 1993;47:57.
13. Olson P.N., Mulnix J.A., Nett T.M. Concentrations of luteinizing hormone and follicle-stimulating hormone in the serum of sexually intact and neutered dogs. Am J Vet Res. 1992;53:762.
14. Soderberg S.F. Infertility in the male dog. In: Morrow D.A., editor. Current therapy in theriogenology. ed 2. Philadelphia: Saunders; 1986:544.
15. Onclin K., Silva L.D.M., Donnay I., et al. Luteotrophic action of prolactin in dogs and the effects of a dopamine agonist, cabergoline. J Reprod Fertil Suppl. 1993;47:403.
16. Verstegen J.P., Onclin K., Silva L.D.M., et al. Abortion induction in the cat using prostaglandin F2alpha and a new anti-prolactinic agent, cabergoline. J Reprod Fertil Suppl. 1993;47:411.
17. Krulich L., McCann S.M., Mayfield M.A. On the mode of the prolactin release-inhibiting action of the serotonin receptor blockers metergoline, methysergide and cyproheptadine. Endocrinology. 1981;108:1115.
18. Teske E. Estrogen-induced bone marrow toxicity. In: Kirk R.W., editor. Current veterinary therapy IX: small animal practice. Philadelphia: Saunders; 1986:495.
19. Bowen R.A., Olson P.N., Behrendt M.D., et al. Efficacy and toxicity of estrogens commonly used to terminate canine pregnancy. J Am Vet Med Assoc. 1985;186:783.
20. Root M.V., Johnston S.D. Pregnancy termination in the bitch using prostaglandin F2alpha. In: Bonagura J.D., editor. Current veterinary therapy XII: small animal practice. Philadelphia: Saunders; 1995:1079.
21. Verstegen J.P., Onclin K., Silva L.D.M., et al. Regulation of progesterone during pregnancy in the cat: studies on the roles of corpora lutea, placenta and prolactin secretion. J Reprod Fertil. Suppl 47. 1993. 165.
22. Shille V.M., Stabenfeldt G.H. Luteal function in the domestic cat during pseudopregnancy and after treatment with prostaglandin F2-alpha. Biol Reprod. 1979;21:1217.
23. Kitchell B.E., Fidel J.L. Tamoxifen as a potential therapy for canine mammary carcinoma. Proc Vet Cancer Soc Annual Forum. 1992;12:91.
24. Eilts B.E. Pregnancy termination in the bitch and queen. In: Greco D.S., Davidson A.P., editors. Clinical techniques in small animal practice: Reproductive techniques in small animals. Philadelphia: Saunders; 2002:116-123.
25. Concannon P.W., Yeager A., Frank D., et al. Termination of pregnancy and induction of premature luteolysis by the antiprogestagen, mifepristone, in dogs. J Reprod Fertil. 1990;88:99.
26. Cohen S.M., Taber K.H., Malatesta P.F., et al. Magnetic resonance imaging of the efficacy of specific inhibition of 5 alpha reductase in canine spontaneous benign prostatic hyperplasia. Magn Reson Imaging Med. 1991;21:55.
27. Feldman E.C., Davidson A.P., Nelson R.W., et al. Prostaglandin induction of abortion in pregnant bitches after misalliance. J Am Vet Med Assoc. 1993;202:1855.
28. Romagnoli S.E., Camillo F., Cela M., et al. Clinical use of prostaglandin F2alpha to induce early abortion in bitches: serum progesterone, treatment outcome and interval to subsequent oestrus. J Reprod Fertil Suppl. 1993;47:425.
29. Trigg T.E., Wright P.J., Armour A.F., et al. Use of a GnRH analogue implant to produce reversible long-term suppression of reproductive function in male and female domestic dogs. J Reprod Fert Suppl. 2001;51:255.
30. Munson L., Bauman J.E., Asa C.S., et al. Efficacy of the GnRH analogue for suppression of oestrous cycles in cats. J Reprod Fertil Suppl. 2001;57:269.
31. Kutzler MA, Wheeler R, Volkmann DH: Serum deslorelin and progesterone concentrations in bitches after ovuplant administration. In Proceedings Ann Meet Soc Theriogenology, 2003.
32. Concannon P.W. Induction of fertile oestrus in anoestrus dogs by constant infusion of GnRH agonist. J Reprod Fertil Suppl. 1989;39:143.
33. Cain JL, Davidson AD, Cain GR, et al: Induction of ovulation in bitches using subcutaneous injections of gonadotropin-releasing hormone analog (abstr), Proc 8th Ann Vet Med Forum, ACVIM, 1990, p 1126.
34. Cain J.L. Induction of estrus and ovulation in the bitch. In: Kirk R.W., editor. Current veterinary therapy X: small animal practice. Philadelphia: Saunders; 1989:1288.
35. Moses D.L., Shille V.M. Induction of estrus in greyhound bitches with prolonged idiopathic anestrus or with suppression of estrus after testosterone administration. J Am Vet Med Assoc. 1988;192:1541.
36. Shille V.M., Thatcher M.J., Lloyd M.L., et al. Gonadotrophic control of follicular development and the use of exogenous gonadotrophins for induction of oestrus and ovulation in the bitch. J Reprod Fertil Suppl. 1989;39:103.
37. Bouchard G.F., Gross S., Ganjam V.K., et al. Oestrus induction in the bitch with the synthetic oestrogen diethylstilboestrol. J Reprod Fertil Suppl. 1993;47:515.
38. Beijerink N.J., Dieleman S.J., Kooistra H.S., et al. Low doses of bromocriptine shorten the interestrous interval in the bitch without lowering plasma prolactin concentration. In Proceedings Reproduction in Companion, Exotic and Laboratory Animals, 2nd Course: Pathology of Canine and Feline Reproduction. Physiology and Pathology of the Neonate. 1. 2003. 112.
39. Van Haaften B., Vieleman S.J., Okkens A.C., et al. Induction of a fertile oestrus in beagle bitches with PMSG and bromocriptine. Proc 11th Int Congr Anim Reprod AI Dublin. 1988;4:463.
40. Verstegen J.P., Onclin K. Early termination of anestrus and induction of fertile estrus in dogs by the dopamine superagonist cabergoline. Biol Reprod Suppl. 1989;50(Abstr 14):12.
41. Gobello C., Castex G., Corrada Y. Use of cabergoline to treat primary and secondary anestrus in dogs. J Am Vet Med Assoc. 2002;11:1653.
42. Romagnoli S.E. Aspermia/oligozoospermia caused by retrograde ejaculation in the dog. In: Bonagura J.D., Twedt D.C., editors. Kirk’s current veterinary therapy XIV: small animal practice. St Louis: Saunders, 2009.
43. Handaja Dusma P.S. Tainturier D: Comparison of induction of oestrus in dogs using metergoline, metergoline plus human chorionic gonadotrophin, or pregnant mares’ serum gonadotrophin. J Reprod Fertil Suppl. 1993;47:363.
44. Goodrowe K.L., Howard J.G., Schimdt P.M., et al. Reproductive biology of the domestic cat with special reference to endocrinology, sperm function and in-vitro fertilization. J Reprod Fertil Suppl. 1989;39:73.
45. Cline E.M., Jennings L.L., Sojka N.J. Breeding laboratory cats during artificially induced estrus. Lab Anim Sci. 1980;30:1003.
46. Wildt D.E., Kinney G.M., Seager S.W.J. Gonadotropin induced reproductive cyclicity in the domestic cat. Lab Anim Sci. 1978;28:301.
47. Burke T.J. Causes of infertility. In: Burke T.J., editor. Small animal reproduction and infertility: a clinical approach to diagnosis and treatment. Philadelphia: Lea & Febiger; 1986:227.
48. Banks D.H., Stabenfeldt G.H. Luteinizing hormone release in the cat in response to coitus on consecutive days of estrus. Biol Reprod. 1982;26:603.
49. Wildt D.E., Seager S.W.J., Chakraborty P.K. Effect of copulatory stimuli on incidence of ovulation and on serum luteinizing hormone in the cat. Endocrinology. 1980;107:1212.
50. Wildt D.E., Seager S.W.J. Ovarian response in the estrual cat receiving varying dosages of hCG. Horm Res. 1978;9:144.
51. Chakraborty P.K., Wildt D.E., Seager S.W.J. Serum luteinizing hormone and ovulatory response to luteinizing hormone releasing hormone in the estrous and anestrous cat. Lab Anim Sci. 1979;29:338.
52. Davidson A.P., Feldman E.C. Ovarian and estrous cycle abnormalities in the bitch. In Ettinger S.J., Feldman E.C., editors: Textbook of veterinary internal medicine, ed 6, St Louis: Saunders, 2005.
53. Schaefers-Okkens A. Vaginal oedema and vaginal fold prolapse in the bitch, including surgical management. In Proceedings Reproduction in Companion, Exotic and Laboratory Animals, 2nd Course: Pathology of Canine and Feline Reproduction. Physiology and Pathology of the Neonate. 1. 2003. 51.
54. Feldman E.C., Nelson R.W. Canine and feline endocrinology and reproduction, ed 3. St Louis: Saunders; 2004.
55. Davidson AP: Uterine and fetal monitoring in the bitch. In Davidson AP, editor: Vet Clin North Am Small Anim Pract: Clinical Theriogenology 31(2):305-313, 2001
56. Scott-Moncrieff J.C., Nelson R.W., Bill R.L., et al. Serum disposition of exogenous progesterone after intramuscular administration in bitches. Am J Vet Res. 1990;51:893.
57. Eilts B.E. Pregnancy maintenance in the bitch using Regumate. Proc Ann Meet Soc Theriogenology. 1992:144-147.
58. Concannon P.W., Meyers-Wallen V.N. Current and proposed methods for contraception and termination of pregnancy in dogs and cats. J Am Vet Med Assoc. 1991;198:1214.
59. Al-Bassam M.A., Thompson R.G., O’Donnell L. Normal post-partum involution of the uterus in the dog. Can J Comp Med. 1981;45:217.
60. Davidson A.P. Medical treatment of pyometra with prostaglandin F2-alpha in the dog and cat. In: Bonagura J.D., editor. Current veterinary therapy XII: small animal practice. Philadelphia: Saunders; 1995:1081-1083.
61. Davidson AP, Nelson RW, Feldman EC: Induction of abortion in 9 bitches with intravaginal misoprostol and parenteral PGF 2alpha (abstr), Proc 15th Ann Vet Med Forum, ACVIM, 1997, p 670.
62. Henry C.J. Mammary cancer. In: Bonagura J.D., Twedt D.C., editors. Kirk’s current veterinary therapy XIV. St Louis: Saunders, 2009.
63. Forrester S.D. Urinary incontinence. In Ettinger S.J., Feldman E.C., editors: Textbook of veterinary internal medicine, ed 6, St Louis: Saunders, 2005.
64. Reichler I.M., Hubler M., Jochle W., et al. The effect of GnRH analogs on urinary incontinence after ablation of the ovaries in dogs. Theriogenology. 2003;60(7):1207-1216.
65. Orbradovich J., Walshaw R., Goullaud E. The influence of castration on the development of prostatic carcinoma in the dog. J Vet Int Med. 1987;1:183.
66. Kustritz M.V., Hess M. Effects of administration of prostaglandin F2 alpha or presence of an estrous teaser bitch on characteristics of the canine ejaculate. Theriogenology. 2007;67(2):255-258.
67. Wiebe VJ, Howard JP: Pharmacologic advances in canine and feline reproduction. In Davidson AP, editor: Topics in Companion Animal Medicine, Reproduction 24(2):71, 2009.