24 Anesthetic Agents
Preanesthetic Medications
The use of preanesthetic medications before general anesthesia in dogs and cats has several advantages. The advantages include helping to decrease stress and anxiety, providing analgesia, decreasing the amount of subsequent anesthetic drugs used, and minimizing the cardiopulmonary depression associated with the commonly used anesthetic agents. Additionally, certain preanesthetic medications will facilitate recovery from anethesia.
The preanesthetic medications used routinely in dogs and cats include the anticholinergics, phenothiazine and benzodiazepine tranquilizers, opioids, and alpha2-adrenergic agonists. Certain drugs discussed in this chapter are not labeled for use in dogs or cats or for the dose and route of administration suggested. Decisions regarding extralabel use should be based on the judgment of the veterinarian and the current laws governing extralabel use of drugs.
KEY POINT 24-1
Selecting an appropriate anesthetic regimen for the small animal patient requires a thorough understanding of the commonly used anesthetic premedication, induction, and maintainence agents, including their indications, contraindications, and potential adverse effects.
Anticholinergics
General Pharmacology
Anticholinergics competitively antagonize acetylcholine at postganglionic terminations of cholinergic fibers in the autonomic nervous system. They are used as preanesthetic medications to decrease salivary secretions, decrease gastric fluid acidity, and inhibit the bradycardic effects of vagal stimulation. Other effects include mydriasis, decreased tear formation, decreased intestinal motility, and bronchodilation. Atropine and glycopyrrolate are the two anticholinergic drugs used in dogs and cats.
Atropine Sulfate
Atropine sulfate (0.02 to 0.04 mg/kg) can be administered intramuscularly, subcutaneously, or intravenously. The duration of action is 60 to 90 minutes. Atropine may stimulate vagal nuclei in the medulla and cause an initial bradycardia before the desired effect is seen, particularly when the drug is administered intravenously. Other central effects of atropine include depression, restlessness, and delirium. Atropine administration may cause cardiac arrhythmias and sinus tachycardia. Atropine does cross the placental barrier and may lead to central and peripheral anticholinergic effects in the fetus when administered to the dam. Arrhythmias are more common after intravenous administration and include second-degree atrioventricular block, unifocal ventricular premature contractions, and ventricular bigeminy.1 Atropine is contraindicated in animals with preexisting tachycardia.
Glycopyrrolate
Glycopyrrolate is a synthetic quaternary ammonium anticholinergic. Glycopyrrolate may be given intramuscularly, subcutaneously, or intravenously at a dose of 0.011 mg/kg. The duration of action of vagal inhibition is 2 to 4 hours, significantly longer than with atropine. The antisialagogue effect may persist for up to 7 hours. The cardiovascular effects of glycopyrrolate are similar to those of atropine. Because of its large structure, glycopyrrolate does not cross the blood–brain or placental barrier readily and therefore has minimal central or fetal effects.2
Tranquilizers
Acepromazine
Acepromazine (0.05 to 0.1 mg/kg intravenously, intramuscularly, or subcutaneously, not to exceed a total dose of 3 mg; oral dose is 1 to 2 mg/kg), a phenothiazine tranquilizer, is used commonly as a premedication before general anesthesia in dogs and cats to relieve anxiety. Through depression of the reticular activating system and antidopaminergic actions in the central nervous system (CNS), acepromazine produces mental calming, decreased motor activity, and increased threshold for responding to external stimuli. Acepromazine does not produce analgesia but may act synergistically when administered concurrently with other drugs with analgesic activity. Administration of acepromazine will decrease the dose of subsequent anesthetic agents. Other effects include antiemetic activity and antihistaminergic properties. Hypotension and hypothermia can result from depression of vasomotor reflexes. Acepromazine is metabolized by the liver and should not be used in patients with liver disease. Because of the potential for hypotension, acepromazine should be used cautiously in compromised patients, particularly those with significant cardiovascular disease. Acepromazine may inhibit platelet function and should be avoided in patients with coagulopathies.2
In many veterinary textbooks, authors cautioned against the use of acepromazine in animals at risk for seizures. Recently, this caution is being questioned on account of a lack of references. In two retrospective studies, no evidence was found that acepromazine lowered the seizure threshold in dogs with a history of seizure disorders.3,4 It was further concluded that a controlled prospective study is necessary for a more thorough evaluation of the use of acepromazine both in this patient population and in the general canine population.
Diazepam
Diazepam (0.1 to 0.2 mg/kg intravenously) is a benzodiazepine tranquilizer that possesses muscle relaxant and anticonvulsant properties. Benzodiazepines exert their effect by enhancing the CNS inhibitory neurotransmitters gamma-aminobutyric acid (GABA) and glycine and by combining with CNS benzodiazepine receptors.1 Diazepam may produce a mild calming effect in some patients, but agitation and excitement can also occur. Diazepam is solubilized by mixing with propylene glycol. Diazepam has minimal cardiovascular effects; bradycardia and hypotension may be seen after rapid intravenous administration. Propylene glycol is associated with pain on injection and incompatibility when mixed in the same syringe with other drugs. Clinical uses of diazepam in small animal anesthesia include providing muscle relaxation when given concurrently with dissociative anesthetics and as a co-induction agent with injectable anesthetics (thiopental, propofol, etomidate) to decrease their doses or side effects (or both). The effects of diazepam can be reversed with the benzodiazepine antagonist flumazenil.
Midazolam
Midazolam (0.1 to 0.2 mg/kg intravenously and intramuscularly) is a benzodiazepine tranquilizer with behavioral effects and clinical uses similar to those of diazepam. Midazolam is more potent and has a shorter duration of action than diazepam. Midazolam is water soluble at a pH of 3.5. At a pH above 4, the chemical structure changes to become lipid soluble.5 Unlike diazepam, midazolam can be mixed with other anesthetic agents and can be administered intramuscularly without causing irritation. Flumazenil can be used to antagonize the effects of midazolam.
Midazolam is often used as a component of a total intravenous anesthetic technique (TIVA). The advantage of TIVA is that general anesthesia can be maintained without the use of inhalant agents, which can cause significant cardiovascular depression in certain high-risk patients. Midazolam, 8 μg/kg/min, combined with fentanyl, 0.8 μg/kg/min, are delivered as a constant-rate infusion (CRI) after induction of anesthesia. The dose can be adjusted as needed to produce a desirable level of anesthesia. Typically, the dose is lowered periodically during the anesthetic period, based on the judgment of the level of anesthesia. This technique makes recovery less prolonged. In certain patients a low concentration of inhalant (e.g., sevoflurane) may be added if the TIVA is inadequate to maintain a desirable level of anesthesia. This is particularly true in animals that are alert and active preoperatively. These patients have significant cardiovascular disease precluding a primary inhalant regimen but are asymptomatic or well-compensated. Examples include young dogs with valvular stenosis or patent ductus arteriosis without evidence of heart failure.
Opioids
Opioids act by combining with one or more specific receptors in the brain and spinal cord to produce analgesia, sedation, euphoria, dysphoria, and excitement. The mu receptors are thought to mediate supraspinal analgesia, respiratory depression, and euphoria. Kappa receptors mediate spinal analgesia, miosis, dysphoria, and sedation; the sigma receptors mediate hallucinations, psychomimetic activity, and respiratory and vasomotor stimulation. Delta receptors are thought to primarily modify mu receptor activity.1 Opioids are classified as agonists, agonist–antagonists, or antagonists according to their receptor activity.
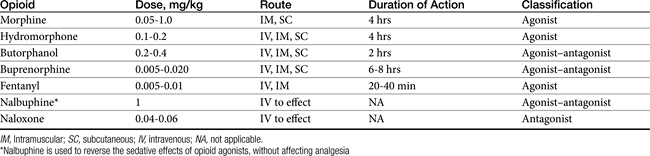
Opioids, agonists or agonist–antagonists, are used before, during, and after surgery in dogs and cats to provide analgesia. Certain opioids may produce sedation in some patients. The antagonists are used to reverse the effects of the agonists or agonist–antagonists. The opioid chosen is based on the degree and duration of expected pain and physical status of the patient. Understanding the differences between the commonly used opioids and their possible side effects are also important when choosing which drug to use. Dose, route, classification, and duration of action of the commonly used opioids in dogs and cats during the perioperative period are shown in Table 24-1.
The most common side effects of opioids preanesthesia include bradycardia and second-degree atrioventricular blockade. These effects may be prevented or treated with an anticholinergic agent. When given as a preanesthetic medication, certain opioids may cause vomiting. It is more commonly seen after administration of a mu agonist (morphine, hydromorphone). Respiratory depression can also occur, especially at high doses. The respiratory depressant effects may be additive to those caused by inhalant anesthetic agents. The user should be prepared to assist or control ventilation if necessary. This is especially crucial in patients with suspected space-occupying masses or lesions of the brain. Hypercapnia resulting from respiratory depression causes cerebral vasodilation and can lead to a life-threatening increase in intracranial pressure. The opioids can cause histamine release and should not be used before intradermal skin testing for allergies.
CRIs of many of the opioids can be used both intraoperatively and postoperatively to provide analgesia and decrease the amount of inhalant anesthetic agent required. Fentanyl (2 to 5 μg/kg/hr intravenously), morphine (0.1 to 0.2 mg/kg/hr intravenously), hydromorphone (0.02 to 0.05 mg/kg/hr), and butorphanol (0.1 to 0.2 mg/kg/hr intravenously) will provide acceptable analgesia for most patients. Remifentanil (18 to 36 μg/kg/hr intravenously) is unique in that it is undergoes rapid hydrolysis by nonspecific tissue and plasma esterases. Therefore it has a rapid onset and offset and is noncumulative, and clearance is unaffected by decreased hepatic function.
Alpha2-Adrenergic Agents
The alpha2-adrenergic agonists are used to produce sedation, muscle relaxation, and analgesia in dogs and cats by stimulating presynaptic alpha2-adrenoreceptors and causing a decrease in norepinephrine release both centrally and peripherally. This action leads to a decrease in both CNS sympathetic outflow and circulating catecholamines.2 Cardiopulmonary effects can be significant with these drugs and include respiratory depression, bradycardia, first- or second-degree atrioventricular blockade, decreased cardiac output, and increased peripheral vascular resistance. Because of these effects, careful patient monitoring should be employed after administering these drugs in dogs and cats. Alpha2-adrenergic agonists should not be used in compromised patients. Other effects seen with the use of alpha2-adrenergic agonists include vomiting, hyperglycemia, decreased gut motility, and dieresis. The commonly used drugs are xylazine, medetomidine, and dexmedetomidine.
Xylazine
Xylazine (0.2 to 1 mg/kg intravenously and intramuscularly) can be given as a premedication agent alone or in combination with opioids to facilitate intravenous catheter placement and decrease dose requirements of subsequent injectable and inhalant anesthetic agents. It is also used commonly as an adjuvant with dissociative anesthetic agents to improve muscle relaxation and provide visceral analgesia for short surgical procedures.
Medetomidine
Medetomidine is an alpha2-adrenergic agonist approved for use in dogs. Although the clinical effects are similar, medetomidine is more potent and possesses a higher alpha2 receptor selectivity profile than xylazine. The preanesthetic dose in dogs is 5 to 10 μg/kg, intravenously and intramuscularly and up to 20 μg/kg for sedation. Medetomidine is not approved for use in cats. However, it has been used successfully, either alone or in combination with an opioid or dissociative agent, for immobilization or surgical anesthesia. The preanesthetic dose in cats is 10 to 15 μg/kg, but a dose range up to 40 to 80 μg/kg intramuscularly is reported.6 This author recommends using the lowest dose needed to produce the desired effect. Combining medetomidine with an opioid may have a synergistic effect, allowing a lower dose of each drug to be used. The use of medetomidine (20 μg/kg intramuscularly) in feline hypertrophic cardiomyopathy patients with concurrent left ventricular outflow obstruction has been shown to improve the hemodynamics of these patients.7
When medetomidine is used as a preanesthetic medication, the dose of subsequent anesthetic agents will be markedly reduced. This includes both induction and inhalational agent requirements. The patients should be monitored carefully throughout the anesthetic period. Profound bradycardia is common after medetomidine administration but can be minimized by preemptive treatment with an anticholinergic agent. If severe bradycardia or hypotension occurs, the medetomidine can be reversed with atipamezole.
Failure to achieve adequate sedation, or no effect at all, can occur in stressed, agitated animals because of high levels of endogenous catecholamines. These animals should be allowed to rest before medetomidine is given. Repeat dosing is not recommended in dogs that do not respond satisfactorily to medetomidine. It is important to remember that sudden arousal and the potential for biting after minimal stimulation or handling can occur. To prevent harm, the user is warned against a false sense of security when handling animals sedated with medetomidine.8
Dexmedetomidine
Dexmedetomidine is the active, dextrorotary enantiomer of the racemic mixture medetomidine. The same indications for medetomidine apply to the clinical use of dexmedetomidine at equal pharmacologic doses. Dexmedetomidine has approximately twice the potency as medetomidine; therefore it is dosed at one half the amount of medetomidine to achieve the same level of sedation and analgesia. The pharmacologically inactive enantiomer levomedetomidine has no sedative, analgesic, or cardiorespiratory effects, but its absence may influence the pharmacokinetic and pharmacodynamic effects of dexmedetomidine. Differences would be expected in the drug metabolism of dexmedetomidine compared with medetomidine given that only half of the racemic mixture is administered and subsequently metabolized. One study in cats comparing medetomidine and dexmedetomidine before ketamine anesthesia reported that cats premedicated with dexmedetomidine recovered more quickly that the cats receiving medetomidine.9
Romifidine
Romifidine is the newest alpha2-adrenergic agonist to be evaluated for use in small animal veterinary patients. It is not approved for use in small animals in the United States. Compared with xylazine, the onset of action is slower and the duration of action is longer. At equipotent doses the cardiovascular effects are similar to those of xylazine. In healthy dogs 10 to 20 μg/kg administered intramuscularly produced mild to moderate sedation with limited effects on cardiovascular function.10 In healthy cats 40 μg/kg adminisered intramuscularly produced moderate sedation.11
Alpha2-Adrenergic Antagonists
Reversal of the clinical effects of xylazine and medetomidine can be accomplished with specific alpha2-adrenergic antagonists. Yohimbine (0.1 mg/kg intravenously), tolazoline (2 mg/kg IV), and atipamezole are most commonly used in dogs and cats. Atipamezole is used to reverse the effects of medetomidine. The dose of atipamezole is determined by the amount of the agonist given and the time elapsed since the agonist was administered. To prevent neurologic (excitement and muscle tremors), cardiovascular (hypotension and tachycardia), and gastrointestinal (salivation and diarrhea) side effects, atipamezole should be given intramuscularly and at the lowest dose needed to produce reversal of the CNS and cardiovascular effects of medetomidine.
Injectable Anesthetics
Injectable anesthetic agents are used to rapidly produce unconsciousness. They usually are given before maintenance of general anesthesia with an inhalant anesthetic agent but may also be administered by repeated injection or infusion, alone or in combination with other injectable agents, to maintain anesthesia. The major disadvantage of injectable agents once administered is that the effects are not immediately eliminated, including any unwanted cardiopulmonary changes. The injectable agents used in veterinary patients include the barbiturates, dissociative agents, propofol, and etomidate.
Barbiturates
The barbiturates cause depression of the CNS by interfering with passage of impulses to the cerebral cortex. Barbiturates are categorized according to their duration of action. The ultrashort-acting barbiturates thiopental and methohexital are the two most commonly used in dogs and cats to produce a rapid induction of anesthesia. The transition to inhalant anesthesia is smooth, and recovery is relatively rapid because of redistribution. Methohexital is cleared from the body at a faster rate and is preferred in sighthounds, which have a more prolonged recovery with the thiobarbiturates.
The barbiturates decrease cerebral blood flow, cerebral metabolic rate of oxygen, and electrical activity of the brain.2 Because of these CNS effects, anesthetic induction using a barbiturate is preferred in patients with certain neurologic diseases (seizure disorders, space-occupying lesion of the brain). Other organ system effects include cardiovascular and respiratory depression that is dependent on the dose and rate of administration. Cardiac arrhythmias may occur, with ventricular extrasystoles and bigeminy being the most common.2
Maximal effect from an intravenous injection of an ultrashort-acting thiobarbiturate is reached within 30 seconds. The duration of action depends on redistribution to lean body tissues. Barbiturates are primarily metabolized by the liver and eliminated by renal excretion. Care should be taken when administering barbiturates to patients with liver disease because the duration of action may be prolonged.
Thiopental
Thiopental can be used as a 2% to 5% solution in dogs and cats. More concentrated solutions may cause severe tissue damage if accidentally administered perivascularly. For induction of anesthesia, thiopental is administered in small increments, 2 to 6 mg/kg intravenously, until the desired effect is reached. A total dose of 10-12 mg/kg is usually sufficient for induction, before intubation and maintenance with an inhalant agent. Repeated injections of thiopental for maintenance of anesthesia have a cumulative effect and can cause a prolonged recovery.
Methohexital
Methohexital is similar in its effects to thiopental, except it is more rapidly metabolized and is not cumulative.2 Methohexital is reconstituted as a 2.5% solution, and a calculated dose of 6 to 10 mg/kg is drawn up in a syringe. One half is administered initially and the remainder given to the desired effect. In patients that have not been premedicated, involuntary excitement or emergence delirium can be seen during the recovery period. Treatment is accomplished with intravenous administration of diazepam, 0.2 mg/kg.
Dissociative Agents
Dissociative anesthesia is an anesthetic state caused from interruption of ascending transmission from the unconscious to conscious parts of the brain.12 This group includes ketamine and tiletamine. Tiletamine is a component of Telazol. Dissociative anesthesia is characterized by a catalepsy; somatic analgesia; and intact ocular, laryngeal, and pharyngeal reflexes. Because control of the airway may not be complete, intubation with a cuffed endotracheal tube is recommended. Visceral analgesia is poor. Muscle rigidity or reflexive skeletal muscle movements can also occur. Dissociative agents are commonly used for induction and maintenance of anesthesia in cats and dogs.
Ketamine
Both ketamine and tiletamine increase cerebral blood flow and intracranial pressure and therefore should be avoided in patients in which these effects could be detrimental.12 Seizure activity may be seen particularly in dogs. As a result of sympathetic stimulation, the cardiovascular effects include an increase in heart rate and arterial blood pressure. The myocardium becomes sensitized to catecholamine-induced arrhythmias. Although ventilation and arterial oxygenation generally remain adequate, an apneustic or irregular breathing pattern may be observed after administration of a dissociative agent. Transient apnea may be seen after rapid intravenous administration. Excessive salivation may occur and is controlled by administration of an anticholinergic. Hallucinatory behavior, emergence delirium, or CNS excitement may be observed during the recovery period. Prior administration of a tranquilizer will attenuate these effects.
Whereas ketamine is metabolized primarily by the liver in the dog, it is excreted intact by the kidneys in the cat. Ketamine should be used with caution in animals with hepatic or renal disease. In cats with urethral obstruction, ketamine can be used if renal disease is absent and the obstruction is relieved. Tiletamine is excreted predominantly by the kidneys. Telazol is contraindicated in patients with pancreatic disease or impairment of renal function.
Ketamine can be administered intravenously (1-2 mg/kg) or intramuscularly (2-20 mg/kg) for induction or maintenance of anesthesia in cats and dogs. Intravenous administration is used for induction before intubation and maintenance with an inhalant agent or for anesthesia for short procedures. Intramuscular administration, in combination with agents providing analgesia and muscle relaxation (e.g., dexmedetomidine), is used for maintenance of anesthesia for surgical procedures. Duration of action is dose dependent. Recovery from large intramuscular doses of ketamine may be associated with prolonged recoveries. Ketamine should be combined with a tranquilizer, muscle relaxant, or opioid to provide muscle relaxation and additional analgesia and to smooth the recovery period. Using adjuvants is important when administering ketamine to dogs because when it is used alone, extreme muscle tone, spontaneous movements, violent recovery, and convulsions can occur.
Ketamine may also be administered as an intravenous CRI both intraoperatively and postoperatively to provide analgesia. At subanesthetic doses, ketamine blocks the N-methyl-d-aspartate (NMDA) receptor responsible for the transmission of painful stimuli from the peripheral nervous system to the CNS. Low-dose ketamine administered as a CRI helps to prevent “wind-up,” an exaggerated and prolonged response to pain caused by NMDA receptor activation. In dogs undergoing forelimb amputation, an initial bolus of 0.5 mg/kg of ketamine administered intravenously, followed by a CRI dose of 10 μg/kg/min intraoperatively and 2 μg/kg/min for 18 hours after surgery, resulted in lower pain scores that dogs receiving saline infusions. Both groups also received a fentanyl CRI (1 to 5 μg/kg/hr) during the 18-hour postoperative period.13
Telazol
Telazol is a combination of tiletamine and zolazepam. Tiletamine has a longer duration of action and greater analgesic effect than ketamine. Zolazepam, a benzodiazepine tranquilizer, provides muscle relaxation and is an effective anticonvulsant. Telazol has been used intramuscularly (4 to 15 mg/kg), alone or in combination with xylazine or an opioid, for induction and maintenance of anesthesia for surgical procedures. Lower doses (2 to 4 mg/kg) can be given intravenously for induction before intubation and maintenance with an inhalant agent. Adverse responses to Telazol can occur during the recovery period, particularly in dogs. These responses include muscle rigidity, convulsions, and emergence delirium. Using the lowest dose of Telazol possible and treatment with a tranquilizer will minimize these effects.
Propofol
Propofol (2,6-diisopropylphenol) is classified as a nonbarbiturate sedative–hypnotic agent. It is an alkylphenol, poorly soluble in water, and is solubilized in a lecithin-containing emulsion (Intralipid). Because propofol emulsion is capable of supporting microbial growth, any unused propofol, in either an ampule or vial, should be discarded within 6 hours. The advantage of propofol over other injectable anesthetic agents is its rapid recovery profile.14
Propofol causes a decrease in both intracranial and cerebral perfusion pressures and therefore can be used in patients with neurologic disease. Propofol has cardiovascular effects similar to those of the thiobarbiturates, including a dose-dependent decrease of arterial blood pressure, cardiac output, and systemic vascular resistance. Heart rate may remain unchanged or increased. Ventricular arrhythmias may also be observed. Propofol should be used with caution in patients with severe cardiovascular disease. Propofol does cause a dose-dependent respiratory depression and may also cause transient apnea. Methods for ventilatory support should be available.
Propofol is noncumulative. Termination of the anesthetic effects from propofol is due to redistribution from vessel-rich tissues followed by rapid biotransformation by the liver. Propofol is rapidly cleared from the body by hepatic and extrahepatic metabolism.
Propofol can be administered as an intravenous bolus (4 to 6 mg/kg) for induction of anesthesia as well as maintenance of anesthesia by a CRI (0.4 mg/kg/min intravenously). Propofol does not provide analgesia; therefore painful procedures should not be performed when given alone. Premedication with an opioid or alpha2 agonist will provide analgesia, but dose requirements for propofol will be lowered. Propofol allows for a rapid recovery with little to no hangover effect compared with the thiobarbiturates. Side effects include excitement during induction or recovery in patients that have not been premedicated, pain on injection, and occasional muscle tremors or myoclonic activity.15 The main disadvantages of propofol are cost and limited shelf-life.
Etomidate
Etomidate is an imidazole derivative classified as a rapid-acting, nonbarbiturate anesthetic agent.16 Etomidate is not a good analgesic. Etomidate (1 to 3 mg/kg) is used as an intravenous induction agent before intubation and maintenance with an inhalant agent. The main advantage of etomidate over other injectable induction agents is its minimal cardiopulmonary depressant effects; therefore it is very useful in severely compromised patients.
Etomidate is a good induction agent for neurologic procedures insofar as it depresses cerebral blood flow and cerebral metabolic rate of oxygen. Administration of etomidate produces little change in heart rate, arterial blood pressure, and cardiac output. Transient apnea may be observed after induction. In patients that have not been premedicated, administration of etomidate may be associated with pain on injection, involuntary muscle movements, gagging, or retching.16 Premedication with a tranquilizer or opioid will attenuate these effects. Etomidate causes transient adrenocortical suppression.17 The effect may be seen for 2 to 3 hours after a single intravenous bolus. Although it is believed that this suppression is not clinically significant after a single dose, long-term infusion with etomidate is not recommended. Etomidate is noncumulative, is rapidly redistributed, and undergoes some ester hydrolysis.
Inhalant Anesthetics
Inhalant anesthetic agents are used to produce general anesthesia in dogs and cats. These drugs produce unconsciousness, muscle relaxation, and analgesia. Inhalant anesthetic agents are administered directly to the respiratory system, absorbed from the alveoli into the bloodstream, and passed to the brain. The advantage of using inhalant agents instead of injectable agents for maintenance of general anesthesia is the ability to adjust the depth of anesthesia by increasing or decreasing the amount of inhalant delivered to the patient. Also, the commonly used inhalant agents permit a rapid induction and recovery from anesthesia because of its elimination through the lungs. Delivering inhalant anesthetic agents requires the use of an anesthetic machine. A proper machine consists of a vaporizer for drug delivery, a source of oxygen, a patient breathing circuit, and methods for eliminating carbon dioxide and scavenging waste gases. Additionally, the patient is often intubated; therefore ventilation can be supported if necessary, and arterial oxygenation is improved because of the high levels of oxygen present in the breathing circuit.
Potency of inhalant anesthetic agents is expressed by its MAC value, which is the minimum alveolar concentration of anesthetic that produces no responses in 50% of patients exposed to a painful stimulus. MAC is measured as the end-tidal concentration of anesthetic. The lower the MAC value of the inhalant agent, the greater its potency. Surgical anesthesia is approximately 1.5 to 2 times the MAC. Several factors control the partial pressure of inhalant anesthetic in the brain. The brain tension mirrors the alveolar concentration of anesthetic agent, which depends on the amount delivered to the lungs and uptake from the lungs. Several factors determine uptake of anesthetic from the lungs. These include solubility (blood–gas partition coefficient), cardiac output, the alveolar–venous anesthetic tension difference, and the presence of shunts or any pathologic change in the alveoli that may cause a diffusion barrier. Elimination of inhalants is primarily by the lungs. Some anesthetic agents undergo varying degrees of biotransformation by the liver. Toxic metabolites are formed by several of the inhalant anesthetic agents. The most commonly used inhalant anesthetic agents in dogs and cats are nitrous oxide, isoflurane, and sevoflurane. Another inhalant agent, which is not used routinely in dogs and cats, is desflurane.
Nitrous Oxide
Nitrous oxide has a MAC value of greater than 100% in the dog and cat.18 It is used as a mild analgesic or to add to the effects of other inhalant anesthetic agents. As it crosses the alveolar membranes, it will speed the uptake of the primary inhalant agent (second gas effect). A period of denitrogenation with 100% oxygen should be performed before nitrous oxide is introduced into the breathing circuit. Up to 70% nitrous oxide is then administered to the patient. Typically, nitrous oxide is administered as 1:1 or 2:1 N2O:O2 ratio. Care must be taken to prevent hypoxia by delivering a minimum of 30% oxygen and, when nitrous oxide is discontinued, delivering 100% oxygen for a minimum of 5 minutes before allowing the patient to breathe room air. Nitrous oxide is eliminated through the lungs rapidly after its discontinuation.
Nitrous oxide has minimal effects on the cardiopulmonary system. Because nitrous oxide is 30 times more soluble in blood than nitrogen, it diffuses into air containing cavities faster than nitrogen diffuses out. Therefore the administration of nitrous oxide is contraindicated in patients with pneumothorax, obstructed bowel, or other closed-air cavities. Nitrous oxide should not be used with closed-circuit or low-flow anesthesia because of the significant risk that a hypoxic mixture will be delivered.
Isoflurane
The MAC value for isoflurane is 1.28% in the dog and 1.63% in the cat.19 Although isoflurane causes a dose-dependent increase in intracranial pressure, it can be used safely in patients with neurologic disease if less than 1 MAC is delivered and hypoventilation is avoided. Vasodilation caused by increased muscle and skin blood flow can be significant and result in hypotension. Isoflurane is a respiratory depressant; therefore it may be necessary to assist ventilation at higher anesthetic depths. Less than 1% of isoflurane undergoes biodegradation. The majority is eliminated unchanged by the lungs. The rapid recovery produced by isoflurane in patients that have not been premedicated may lead to emergence delirium. Treatment with a tranquilizer such as acepromazine may be required. Opioids are strongly recommended if the animal is thought to be in pain. Isoflurane is an excellent choice for either induction or maintenance of anesthesia in most small animal patients. As with any anesthetic technique, vigilant monitoring of the cardiopulmonary system is encouraged.
Sevoflurane
The MAC value of sevoflurane is 2.36% in the dog20 and 2.58% in the cat.21 The principle advantage of sevoflurane over isoflurane is its extremely rapid induction and recovery times. The cardiovascular and respiratory effects are similar to those produced by isoflurane. Sevoflurane does not sensitize the myocardium to catecholamine-induced arrhythmias. Sevoflurane undergoes minimal metabolism by the liver; the majority is eliminated unchanged by the lungs. Sevoflurane reacts with carbon dioxide absorbents and decomposes to compound A. Compound A is a vinyl halide that has been shown to be nephrotoxic in laboratory rats. There have been no case reports of compound A–associated renal injury in human or veterinary patients. Although compound A formation is an area of controversy among researchers, sevoflurane should not be used during low-flow or closed-circuit anesthesia to prevent the accumulation of the potentially nephrotoxic substance.
Because of its low lipid solubility, sevoflurane produces a rapid and smooth induction and a rapid recovery. As with isoflurane, patients that have not been premedicated may experience emergence delirium. Sevoflurane may be advantageous for outpatient procedures in which a rapid surgery-to-discharge time is desired. During the anesthetic management of critically ill patients, wherein it may be necessary to make rapid changes in the depth of anesthesia, sevoflurane may be preferred over isoflurane. Also, sevoflurane is preferred for maintenance of anesthesia for cesarean sections because the neonates, if ventilating adequately, will quickly eliminate residual inhalant agent. The primary disadvantages of using sevoflurane is the higher cost compared with isoflurane.
Desflurane
The MAC value of desflurane is approximately 7.2% in the dog22 and 9.79% in the cat.23 Desflurane has an extremely low blood–gas partition coefficient allowing for a very rapid induction and recovery from general anesthesia. A dose-dependent respiratory depression is seen. The cardiovascular depressant effects are similar to isoflurane. Desflurane is very pungent, which may make mask inductions difficult in some patients. Desflurane is eliminated from the lungs and has not been reported to cause hepatic or renal toxicity. Desflurane requires a special, electrically heated vaporizer for delivery because of its high vapor pressure. Desflurane is not used routinely in veterinary patients at this time.
1. Thurmon J.C., Tranquilli W.J., Benson G.J. Injectable anesthetics. In Thurman J.C., Tranquilli W.J., Benson G.J., editors: Lumb and Jones’ veterinary anesthesia, ed 3, Baltimore: Williams & Wilkins, 1996.
2. Muir W.W., Hubbell J.A.E. Handbook of veterinary anesthesia, ed 4. St Louis: Mosby; 2007.
3. Garner J.L., Kirby R., Rudloff E. The use of acepromazine in dogs with a history of seizures. J Vet Emerg Crit Care. 2004;14:S1.
4. Tobias K.M., Marioni-Henry, Wagner R. A retrospective study on the use of acepromazine maleate in dogs with seizures. J Am Anim Hosp Assoc. 2006;42:283.
5. Ilkiw J.E. Other potentially useful new injectable anesthetic agents. Vet Clin North Am Small Anim Pract. 1992;22(2):281.
6. Plumb D.C. Veterinary drug handbook, ed 5. Ames, Iowa: Blackwell; 2005.
7. Lamont L.A., Bulmer B.J., Sisson D.D., Grimm, et al. Doppler echocardiographic effects of medetomidine on dynamic left ventricular outflow tract obstruction in cats. J Am Vet Med Assoc. 2002;221(9):1276.
8. Tranquilli W.J. α2-agonists. In: Greene S.A., editor. Veterinary anesthesia and pain management secrets. Philadelphia: Hanley & Belfus, 2002.
9. McKusick B.C., Westerholm F.C., Väisänen M. Clinical evaluation of dexmedetomidine premedication prior to ketamine anesthesia in cats [abstract]. Proc Assoc Vet Anaesth Spring Meet. 66, 2005.
10. Lemke K.A. Sedative effects of intramuscular administration of a low dose of romifidine in dogs. Am J Vet Res. 1999;60:162.
11. Selmi A.L., Barbudo-Selmi G.R., Moreira C.F., et al. Evaluation of sedative and cardiorespiratory effects of romifidine and romifidine-butorphanol in cats. J Am Vet Med Assoc. 2002;221:506.
12. Lin H.C. Dissociative anesthetics. In Thurman J.C., Tranquilli W.J., Benson G.J., editors: Lumb and Jones’ veterinary anesthesia, ed 3, Baltimore: Williams & Wilkins, 1996.
13. Wagner A.E., Walton J.A., Hellyer P.W., et al. Use of low doses of ketamine administered by constant rate infusion as an adjunct for postoperative analgesia in dogs. J Am Vet Med Assoc. 2002;221:72.
14. Branson K.R., Gross M.E. Propofol in veterinary medicine. J Am Vet Med Assoc. 1888;204:1994.
15. Smith J.A., Gaynor J.S., Bednarski R.M., et al. Adverse effects of administration of propofol with various preanesthetic regimens in dogs. J Am Vet Med. 1993;202:1111.
16. Muir W.W., Mason D.E. Side effects of etomidate in dogs. J Am Vet Med. 1989;194:1430.
17. Kruse-Elliott K.T., Swanson C.R., Aucoin D.P. Effects on adrenocortical function in canine surgical patients. Am J Vet Res. 1987;48:1098.
18. Steffey E.P., Gillespie J.R., Berry J.D., et al. Anesthetic potency (MAC) of nitrous oxide in the dog, cat, and stumptail monkey. J Appl Physiol. 1974;36:530.
19. Steffey E.P., Howland D.Jr. Isoflurane potency in the dog and cat. Am J Vet Res. 1977;38:1833.
20. Kazama T., Ikeda K. Comparison of MAC and the rate of rise of alveolar concentration of sevoflurane with halothane and isoflurane in the dog. Anesthesiology. 1988;68:435.
21. Doi M., Yunoki H., Ikeda K. The minimum alveolar concentration of sevoflurane in cats. J Anesth. 1988;2:113.
22. Doorley M.B., Waters S.J., Terrell R.C., et al. MAC of I-653 in beagle dogs and New Zealand white rabbits. Anesthesiology. 1988;69:89.
23. McMurphy R.M., Hodgson D.S. The minimum alveolar concentration of desflurane in cats. Vet Surg. 1995;24:453.