Chapter 25 Muscle Relaxants
Muscle relaxants may be chosen for use in veterinary patients for several reasons. Because of their muscle-relaxing effects, they are used during certain surgical procedures. Orthopedic procedures such as dislocation and fracture reductions can be performed more easily because of abolished skeletal muscle tone.1 When a balanced anesthesia technique is used that combines opioids, nitrous oxide, and low-dose inhalant agents, muscle relaxation is greatly improved if a neuromuscular blocking agent is given. This technique is especially beneficial for critically ill patients in which high doses of inhalant agents may lead to unwanted cardiovascular depression.2 During intraocular procedures or for patients with penetrating eye injuries requiring surgery, muscle relaxants may be beneficial by producing a central pupil, motionless eye, and soft globe. When used during induction and intubation, they can help prevent an increase in intraocular pressure that can occur during coughing or vomiting.2
KEY POINT 25-1
The use of muscle relaxants in anesthetized small animal patients can help facilitate the procedure being performed, but it is important to remember that these drugs do not provide analgesia or analgesia.
When using a muscle relaxant, the clinician must remember that these agents do not provide either anesthesia or analgesia. The patient must be adequately anesthetized, which may be even more difficult to determine because some of the usual indicators of depth of anesthesia is abolished. These indicators include purposeful movement, palpebral response, and degree of jaw tone. Owing to paralysis of the muscles of respiration, ventilation must also be controlled until neuromuscular function is restored.
Neuromuscular Blocking Agents
Anatomy and Physiology of the Neuromuscular Junction
The components of the skeletal neuromuscular junction include the somatic motor nerve terminal, synaptic cleft, and the motor end plate of the muscle fiber. The cell bodies of the somatic motor neurons are located within the spinal cord. The axon divides into multiple branches, each of which innervates a single muscle fiber in mammalian species. At each neuromuscular junction, the terminal portion of the axon loses its myelin sheath and forms an arborization that lies in close proximity to the motor end plate of the muscle fiber (Figure 25-1). The site of action of neuromuscular blocking agents is at the nicotinic cholinergic receptors located at the motor end plate of the muscle fiber.
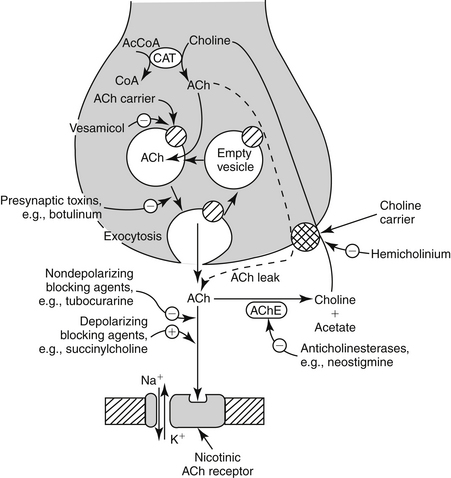
Figure 25-1 Neuromuscular junction with sites of drug action. AcCoA, Acetyl coenzyme A; ACh, acetylcholine; AChE, acetylcholinesterase; CAT, catecholamine; CoA, coenzyme A.
(From Rang HP, Dale MM, Ritter JM et al: Pharmacology, New York, 1995, Churchill Livingstone.)
The nicotinic receptors at the neuromuscular junction bind and respond to the endogenous neurotransmitter, acetylcholine (ACh). ACh is synthesized from the acetylation of choline by the enzyme choline acetyl transferase, using acetyl coenzyme A as the source of the acetyl groups. ACh is then packaged at high concentrations into synaptic vesicles by carrier-mediated transport. When a motor nerve is stimulated and action potential subsequently generated reaches the nerve terminal, ACh is released rapidly into the synaptic cleft by way of calcium-mediated exocytosis. One presynaptic nerve impulse releases 100 to 500 vesicles or approximately 3 million ACh molecules. ACh then diffuses across the synaptic cleft and, upon binding with a nicotinic cholinergic receptor, stimulates opening of an ion channel located on the muscle fiber membrane. This allows sodium ions to move and allow sufficient current through them that the resting membrane potential is shifted toward threshold, generating an action potential that triggers muscle contraction. Unbound ACh within the synaptic cleft is rapidly hydrolyzed by the enzyme acetylcholinesterase to choline and acetate. The choline is taken up by the nerve terminal and recycled for continued synthesis of ACh (this is the rate-limiting step in ACh synthesis).
Pharmacology
Neuromuscular blocking agents exert their effects by interfering with the postsynaptic action of ACh. They can be divided into two classes, depolarizing and nondepolarizing neuromuscular blocking agents. Nondepolarizing drugs produce muscle relaxation by preventing ACh from binding to its receptors on the motor end plate. As a result of this competitive antagonism, the ion channels will not open, no shift in the resting membrane potential will occur, the motor end plate will not depolarize, and the muscle becomes flaccid. Complete neuromuscular blockade will occur when approximately 90% to 95% of the receptors are occupied.3
Depolarizing drugs elicit their pharmacologic effect by binding to the ACh receptor in the same way ACh does, causing transient muscle fasciculations. Because these drugs are not immediately metabolized by acetylcholinesterase, however, they bind for a much longer period than ACh does, causing persistent depolarization of the muscle fiber endplate. In mammals the result of this maintained depolarization is a loss of electrical excitability by the postsynaptic muscle fiber, producing neuromuscular blockade.
Individual Agents
The development of newer muscle relaxants has given the practitioner many options when selecting which agent to use. These drugs differ in their onset and duration of action, recovery time, cardiovascular effects, and route of elimination. When making the decision on which muscle relaxant to use, it is important to consider the reason for neuromuscular blockade, the desired duration of action, and the physical status of the patient. Organ dysfunction and concurrent drug administration may alter the clinical effect of muscle relaxants. Doses for depolarizing and nondepolarizing neuromuscular blocking agents for dogs and cats are listed in Table 25-1.
Table 25-1 Doses of Neuromuscular Blocking Agents for Dogs and Cats
| Drug | Dose (mg/kg IV) |
|---|---|
| Succinylcholine∗ | |
| Atracurium∗ | 0.22 |
| Cisatracurium† | 0.1 |
| Vecuronium∗ | 0.1 |
| Pancuronium∗ | 0.044-0.11 |
| Mivacurium‡ | 0.01 – 0.05 |
| Rocuronium§ | 0.18 |
| Doxacurium¶ | 0.008 |
IV, Intravenous.
∗ Plumb DC: Veterinary drug handbook, Ames, Iowa, 2005, Blackwell.
† Adams WA, Robinson KJ, Senior JM: The use of the neuromuscular blocking drug cis-atracurium in dogs, Vet Anaesth Analg 28:156, 2001.
‡ Smith LJ, Moon PF, Lukasik VM et al: Duration of action and hemodynamic properties of mivacurium chloride in dogs anesthetized with halothane, Am J Vet Res 60:1047, 1999.
§ Cason B, Baker DG, Hickey RF et al: Cardiovascular and neuromuscular effect of three steroidal neuromuscular blocking drugs in dogs, Anesth Analg 70:382, 1990.
¶ Savarese JJ, Wastila WB, Basta SJ et al: Pharmacology of BW A938U, Anesth 59(3):A274, 1987.
Depolarizing Agents
Succinylcholine
Succinylcholine is the only depolarizing neuromuscular blocking agent in clinical use today. Succinylcholine has a rapid onset of action, and its short duration of action is primarily due to rapid hydrolysis by plasma cholinesterase.4 For these reasons succinylcholine is used routinely to facilitate endotracheal intubation in human patients. Its use in small animal patients has been limited because the larynx of the dog and cat is easily visualized and does not routinely exhibit excessive laryngospasm, making neuromuscular blockade unnecessary for intubation.5
Because of the short duration of action of succinylcholine, frequent redosing or a constant-rate infusion is required if long-term neuromuscular blockade is desired. This may lead to tachyphylaxis (increased dose requirement) and change the character of the initial block (phase I block) to one similar to that produced with nondepolarizing blocking agents (phase II block), which may increase recovery time and require the use of a reversal agent (anticholinesterase therapy).6
Use of succinylcholine may be associated with significant side effects. A transient increase in serum potassium concentration occurs because of the leakage of potassium from the interior of cells. Severe, life-threatening hyperkalemia can occur after succinylcholine administration in patients with severe burns, trauma, nerve damage, neuromuscular disease, closed head injury, intraabdominal infections, and renal failure.7 Succinylcholine should also be avoided in patients in which increases in intraocular, intracranial, and intragastric pressures are undesirable. Other side effects of succinylcholine administration include myalgia and cardiac arrhythmias (e.g., sinus bradycardia, catecholamine-induced ventricular arrhythmias).7
Any agent than inhibits plasma cholinesterase (organophosphates, procaine) will prolong the duration of action of succinylcholine. Certain disease states such as liver disease, malnutrition, and chronic anemia can decrease the plasma cholinesterase level. Succinylcholine must be used cautiously, if at all, in these patients.4
The current goal in the research and development of newer neuromuscular blocking agents is to find a drug that can be offered as an alternative to succinylcholine. This drug would have both a rapid onset and duration of action and possess minimal unwanted side effects. Although the ideal replacement has yet to be discovered, drugs such as rocuronium and mivacurium have proved useful in certain clinical situations. Both are discussed in detail later in this chapter.
Nondepolarizing Agents
Atracurium besylate
Atracurium is an intermediate-acting nondepolarizing agent with an onset of action of 3 to 5 min and a duration of action of 20 to 35 min.8 It is metabolized primarily through Hofmann elimination and ester hydrolysis.9 For this reason atracurium is the muscle relaxant of choice in patients with hepatic or renal disease. The rate of spontaneous degradation through Hofmann elimination is pH and temperature dependent. Both acidemia and hypothermia will prolong atracurium-induced neuromuscular blockade. Administration of atracurium can cause histamine release at higher doses, resulting in hypotension and tachycardia. Atracurium should be avoided in patients in which cardiovascular stability is desired. Administration of large doses slowly will attenuate these effects.10 Repeated doses or an infusion of atracurium produces a consistent degree of block and duration of action because of its noncumulative effects, making it an attractive choice for a constant-rate infusion for long-term paralysis.11,12 A metabolite of atracurium, laudanosine, can cause central nervous system (CNS) stimulation and cardiovascular depression, but this problem is rarely seen when clinical doses are used.12
Cisatracurium besylate
Cistracurium is the purified form of one of the 10 stereoisomers of atracurium. Because of this, the neuromuscular-blocking profile of cisatracurium is similar to that of atracurium. However, it is less likely to lead to histamine release. When administered to cats at up to 60 times the effective dose in 95% of cases (ED95), plasma histamine levels were unchanged.13 In human patients Hofmann elimination accounts for 77% and renal clearance accounts for 16% of total body clearance.14 A study in anesthetized dogs reported the onset of action as 3.8 minutes and duration of action as 27.2 minutes.15
Vecuronium bromide
Vecuronium is an intermediate-acting nondepolarizing agent with an onset of action of 2 minutes and duration of action of 25 minutes.8 The lack of cardiovascular or histamine-releasing effects, even at higher doses, is an advantage.12 Vecuronium is the muscle relaxant of choice for patients when hemodynamic stability is needed. Recovery from vecuronium-induced muscle relaxation depends on hepatic elimination. Animals with hepatic disease may exhibit a prolonged duration of action. Vecuronium is noncumulative and is well suited for repeated doses or constant-rate infusions.12
Pancuronium bromide
Pancuronium is a long-acting nondepolarizing agent with an onset of action of 2 to 3 minutes and duration of action of 30 to 45 minutes.8 It lacks histamine-releasing effects but does possess vagolytic and sympathomimetic effects, which can result in tachycardia, increased arterial blood pressure, and catecholamine-induced ventricular arrhythmias.16 Pancuronium is mainly eliminated by the kidney, with the remainder undergoing hepatic metabolism; therefore is should be used with caution in patients with renal or hepatic disease.17 Repeated doses or infusions of pancuronium are cumulative and can produce a delayed recovery.18
Mivacurium chloride
Mivacurium was recently developed as an alternative to succinylcholine for intubation in human patients. Its onset of action is 1 to 2 minute, with a duration of action of 15 to 20 minutes.19 Because its metabolism is by way of plasma cholinesterase, prolonged recoveries are possible in patients with hepatic disease, renal disease, or organophosphate toxicity.20 Mivacurium can cause histamine release, is noncumulative, and can be used for infusion administration.19 In human patients the onset and depth of blockade has a high interpatient variability. Preliminary work in dogs suggested that the dose should be reduced below doses used in human patients.21 In a later study, it was shown that one third of the human dose given to dogs resulted in a duration of action five times longer than that with human patients.22 The species differences may be explained, in part, by the fact that normal plasma cholinesterase levels in dogs vary from 19% to 76% of human patients.23 Also, the canine pseudocholinesterase enzyme may exhibit different affinity for the three primary isomers of mivacurium.23 Mivacurium has not been available in the United States since 2006. It is listed on the Food and Drug Administration’s Discontinued Drug Product List.
Rocuronium bromide
Like mivacurium, rocuronium was developed as an alternative to succinylcholine for intubation in human patients because of its rapid onset of action. In halothane-anesthetized dogs, rocuronium had an onset and duration of action of 1.1 ± 0.49 and 13.7 ± 0.49 minutes, respectively.24 Rocuronium lacks significant cardiovascular and histamine-releasing effects.25 It is metabolized primarily by the liver, with a small fraction eliminated by the kidney.26 In small animal anesthesia, rocuronium may be chosen if a rapid onset of action without significant hemodynamic effects is desired.
Doxacurium chloride
Doxacurium is the most potent nondepolarizing agent available for use at this time.19 It is a long-acting muscle relaxant with a slow onset of action and long duration of action in human patients. Doxacurium has minimal cardiovascular or histamine-releasing effects.27 Because metabolism is through renal elimination, a prolonged or more variable duration of action is seen in patients with renal disease.28 Doxacurium is not commonly used in veterinary medicine. Becaue of its long duration of action, doxacurium may not be suitable for routine clinical use in small animal patients, but it may be an attractive choice for researchers when long-term relaxation with minimal hemodynamic effects is desired.
Drug Interactions
Many medications that are given to veterinary patients during the perioperative period can alter the pharmacodynamics and pharmacokinetics of nondepolarizing agents, leading to an increased or decreased effect. Table 25-2 lists the medications and their effects on the muscle relaxant.
Table 25-2 Effect of Medication on Nondepolarizing Neuromuscular Blockade
| Drug | Effects on Depth/Duration | Comments |
|---|---|---|
| Increased | Calcium may reverse effect | |
| Anticholinesterases | Decreased | |
| Increased | After chronic therapy (>2 weeks) | |
| Dantrolene | Increased | |
| Increased | Dose-dependent potentiation | |
| Inhalational anesthetics | Increased | |
| Increased | ||
| Steroids | Increased or decreased | Most likely to decrease but may also increase or exert no effect |
| Succinylcholine | Increased | When given before nondepolarizing agents |
| Theophylline | Decreased |
From Silverman DG, Mirakhur RK: Effects of other agents on nondepolarizing relaxants. In Silverman DG, editor: Neuromuscular block in perioperative and intensive care, Philadelphia, 1994, JB Lippincott.
Monitoring Neuromuscular Blockade
Whenever a muscle relaxant is administered, the neuromuscular junction should be monitored to allow the proper dose of relaxant and antagonist to be determined accurately. Also, the degree of residual blockade during the recovery period, if any, can be detected and treated appropriately. Evoked responses are used to evaluate neuromuscular blockade. This involves stimulating a peripheral motor nerve in order to evaluate the resultant motor response. Several hand-held peripheral nerve stimulators are available (Figure 25-2).
Figure 25-2 A-C, Several hand-held peripheral nerve stimulators available to monitor neuromuscular blockade in small animals.
Sites of Stimulation
Sites for stimulation of peripheral motor nerves in dogs and cats include the peroneal and ulnar nerves; the more accessible is chosen (Figure 25-3).
Electrical Stimulus Characteristics
There are standard methods of stimulating peripheral motor nerves because the physical characteristics of electrical stimuli influence the motor response they evoke. The output from the peripheral nerve stimulator should be a square wave stimulus having duration of 0.2 to 0.3 milliseconds. Ideally, the output current is adjustable and should be sufficient to produce a supramaximal impulse.
After the two electrodes are placed over the nerve to be stimulated, the stimulus is adjusted to deliver a supramaximal current, slightly greater than that required to elicit a maximum motor response. This ensures that all neurons in the bundle are depolarized, which will cause the muscle fibers to contract in an all-or-none fashion. Any subsequent changes in the motor response are from effects at the neuromuscular junction.
Patterns of Stimulation
Peripheral nerve stimulators should provide single-twitch, tetanus of 50 Hz, and train-of-four stimulus patterns. Newer stimulators may also have the capability to deliver a double-burst pattern (Figure 25-4).
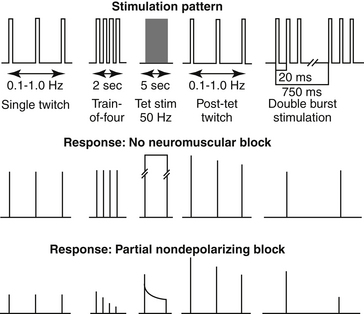
Figure 25-4 Diagram showing different nerve stimulation patterns for monitoring neuromuscular blockade. Stim, stimulation; tet, tetanic.
(Courtesy Cullen LK: Muscle relaxants and neuromuscular block. In Thurman JC, Tranquilli WJ, Benson GJ, editors: Lumb and Jones’ veterinary anesthesia, ed 3, Baltimore, 1996, Williams & Wilkins.)
Single-twitch method
The single-twitch method is used to evaluate the degree of relaxation by dividing the elicited response by the control response. The control response is taken before the administration of the relaxant. The frequency should not be greater than 0.15 Hz (1 twitch/7-10 seconds). This is due to the prejunctional effects of the relaxant, which greatly decreases the amount of ACh release.29 The resultant motor response will be less than the baseline values, making accurate determination of the degree of relaxant difficult. The disadvantages of using the single-twitch method are that a baseline response is necessary before administration of the muscle relaxant and that it is insensitive for the detection of residual blockade.30
Train-of-four method
The train-of-four method consists of four supramaximal impulses delivered at a frequency of 2 Hz (2 twitches/second). The degree of blockade is evaluated by comparing the ratio of the fourth twitch to the first twitch (T4/T1 ratio). The train-of-four serves as its own control; therefore no baseline values before muscle relaxant administration are necessary. The train-of-four stimulus can be delivered intermittently or at regular intervals 10 to 20 seconds apart. In the absence of muscle relaxants, the T4/T1 ratio is approximately 1. Following the administration of a nondepolarizing muscle relaxant, the fourth, third, second, and first twitches disappear (fade) in that order as the block becomes more profound. The degree of fade, strength of the remaining twitches, and length of time that the twitches are absent depends on the dose of relaxant given. A T4/T1 ratio of 0.7 or higher correlates with clinical signs of adequate recovery from neuromuscular blockade.31
During phase 1 block with succinylcholine, there is a flat T4 response (no fade). Repeated or prolonged infusions of succinylcholine changes the character of the block to phase 2, where fade during a train-of-four stimulus is seen.32
Tetanic stimulation
Serial supramaximal stimulation at high frequency, 50 Hz, for 5 seconds causes sustained muscle contraction.32 After administration of a nondepolarizing muscle relaxant, fade is seen as the muscle is unable to maintain the strength generated by the tetanic stimulus.18 Although it is a sensitive indicator of residual paralysis during the recovery period, this method is painful and will elicit a physiologic response (tachycardia, hypertension, movement) in the lightly anesthetized animal.12
Double-burst stimulation
Double-burst stimulation consists of the delivery of two minitetanic (50 Hz) bursts. Each burst consists of three impulses and is 750 milliseconds apart.33 The ratio of the second burst compared with the first burst (D2/D1) correlates highly to the T4/T1 ratio and is preferable to train-of-four monitoring to some individuals because fade is more readily seen.34,35 Another advantage is that D1 is still detected at slightly deeper levels of block than is T1.36
Quantifying Evoked Responses
During routine clinical use of muscle relaxants in veterinary patients, visual observation of the evoked response is used to detect the degree of block present. This method is unreliable, especially in detecting residual blockade.37 With experience in using muscle relaxants in small animals, visual means are adequate in detecting clinical recovery, but more accurate methods are needed in research settings. The two methods most commonly used to accurately assess the motor response after peripheral nerve stimulation are mechanomyography (MMG) and electromyography (EMG). A third method, accelerography, has also been used in veterinary research patients but to a much lesser extent.
Mechanomyography
MMG measures the evoked contractile response of the stimulated muscle by force translation. This method is the most commonly used and has been well described in the cat, dog, and horse.24,38,39 Simply put, the paw or hoof of the front or rear limb is immobilized, the stimulating electrodes (surface or needle) placed over the nerve supplying the muscle to be studied, and a force transducer attached to the paw or hoof perpendicular to the twitch angle. A resting tension of 100 to 300 grams is applied to provide maximum tension development. After supramaximal single-twitch, train-of-four, tetanic, or double-burst stimulation, the evoked response is recorded on a strip chart. Although this method is extremely accurate, use of MMG in the clinical setting is limited because the limb must be immobilized, and no movement can occur throughout the duration of recording period because it will affect resting tension and twitch angle.37
Electromyography
EMG involves the measurement of the compound action potential of the muscle fibers during a supramaximal stimulus of a peripheral motor nerve. Two stimulating electrodes are placed over the peripheral nerve. The active recording electrode is placed over the innervation zone of the muscle to be studied, usually midway between the origin and insertion. The reference electrode is placed over the insertion of the muscle. A ground electrode is positioned between the stimulating and recording electrodes to decrease stimulation artifact. Its advantages over MMG is that it requires no limb immobilization, no resting tension, and greater flexibility in the muscles monitored. Disadvantages are that proper skin preparation and electrode placement is crucial to obtain valid results.37 Although used on human patients, this method has not been described for veterinary patients. This author has used EMG (Relaxograph, Datex) successfully on dogs and horses. In the dog the ulnar nerve is stimulated with recording electrodes placed over the abductor digiti quinti. In the horse the superficial peroneal nerve is stimulated with recording electrodes placed over the lateral digital extensor. Skin preparation involves shaving the hair with a single-edged razor, rubbing the skin vigorously with isopropyl alcohol, and allowing the skin to dry completely before surface electrode placement.
Mechanomyography Versus electromyography
Although MMG remains the gold standard for quantifying evoked responses in veterinary patients in the research setting, EMG may become an attractive choice for use in the clinical patients once the methodology is validated. In human patients differences exist between the two methods. Compared with MMG, the EMG method tends to show a lesser degree of relaxation with nondepolarizing agents and overestimates the degree of relaxation with depolarizing agents.40,41 Future studies in veterinary patients will help in the development of different criteria for onset and reversal on neuromuscular blockade based on the type of monitoring used.
Accelerography
Accelerography is based on Newton’s second law: Force equals mass times acceleration. In other words, acceleration is directly proportional to the force if the mass is constant. During electrical stimulation of a peripheral nerve, a piezoelectric sensor assesses the acceleration during an isotonic contraction of the innervated muscle. A study in human patients comparing accelerography with EMG concluded that the wide variations in individual patients and the differences in clinical duration and recovery index do not allow the two monitoring techniques to be used interchangeably.42 Compared with MMG, published studies in human patients present inconsistent results. In one study accelerography showed good correlation and was considered a simple and reliable monitoring tool.43 In two other studies, the limits of agreement were unacceptably wide.44,45 A study in dogs comparing the neuromuscular effect of vecuronium during either propofol or sevoflurane anesthesia used accelerography to quantify neuromuscular function.46 The fibular nerve was stimulated with the acceleration transducer fixed over the cranial tibial muscle. Accelerography appeared to be an acceptable technique for quantifying neuromuscular blockade in anesthetized dogs. More studies are needed, both in dogs and other species, to determine if it can be used easily and reliably in clinical veterinary patients.
Reversal of Neuromuscular Blockade
Recovery from succinylcholine (phase 1 block) and mivacurium-induced neuromuscular blockade is usually rapid and spontaneous owing to rapid hydrolysis by plasma cholinesterases. Delayed recovery will be seen in patients with decreased plasma cholinesterase levels.
Residual neuromuscular blockade from nondepolarizing agents and phase 2 block from succinylcholine may be reversed at the conclusion of surgery to prevent potentially serious complications in the recovery period. Complications include muscle weakness and inadequate ventilation, which can lead to life-threatening hypoxia or respiratory acidosis.
Before reversal of blockade, it is desirable to have 3 to 4 twitches of the train-of-four visible. This is achieved by close monitoring of neuromuscular function throughout surgery and not redosing the patient close to the time of anticipated reversal.
The anticholinesterase drugs used clinically include neostigmine, pyridostigmine, and edrophonium. Doses are listed in Table 25-3. They reverse neuromuscular blockade by inhibiting the enzyme acetylcholinesterase, which is responsible for the hydrolysis of ACh. The effect is an accumulation of ACh at muscarinic and nicotinic receptor sites. Because nondepolarizing agents and ACh compete for the same receptor binding sites, anything that increases the concentration of ACh tips the balance of competition in favor of ACh and restores neuromuscular transmission. Reversal of neuromuscular blockade requires only the nicotinic cholinergic effects of anticholinesterase drugs; therefore the muscarinic effects are attenuated or prevented by concurrent administration of an anticholinergic such as atropine or glycopyrrolate.
Table 25-3 Doses of Neuromuscular Reversal Agents for Dogs and Cats
| Drug | Dose (mg/kg IV) |
|---|---|
| Neostigmine∗ | 0.04 |
| Edrophonium† | 0.5 |
| Pyridostigmine† | 0.2 |
∗ Muir WW, Hubbell JAE,: Handbook of veterinary anesthesia, ed 4, St Louis, 2007, Mosby.
† Dose used at Veterinary Teaching Hospital, Texas A&M University.
Sugammadex is the first selective relaxant binding agent (SRBA). It is a novel agent used to reverse neuromuscular blockade produced by rocuronium and, to a lesser extent, other steroidal muscle relaxants (rocuronium > vecuronium > pancuronium). Sugammadex is a modified gamma cyclodextrin that binds and encapsulates the rocuronium molecule. Once bound within the lipophilic core of sugammadex, it is rendered unavailable to bind to the ACh receptor.47 A significant advantage of sugammadex is the ability to reverse neuromuscular blockade without the use of anticholinesterase drugs. In addition, because there is no need to co-administer antimuscarinic drugs with sugammadex, its use is associated with greater cardiovascular and autonomic stability. Sugammadex is approved in the European Union but is not currently approved for use in the United States.
Monitoring neuromuscular function and support of ventilation must be continued until complete reversal has been accomplished. An adequate plane of anesthesia must be maintained while monitoring the degree of block with a peripheral nerve stimulator because this can cause the patient some discomfort, especially if tetanic stimulation is used. A T4/T1 ratio of 0.7 or greater correlates well to clinical recovery. Once reversal is complete and the use of the nerve stimulator is discontinued, the animal is allowed to recover from anesthesia. Although recurarization is uncommon after reversal, spontaneous respiratory efforts, sufficient to maintain adequate ventilation, should be present and monitored closely during the recovery period.
Skeletal Muscle Relaxants (Spasmolytics)
Although neuromuscular blocking agents are effective for skeletal muscle spasticity, these agents are too nonselective to be an appropriate choice for this purpose. Several other drugs are available and commonly used to reduce muscle tone without abolishing voluntary skeletal muscle contraction. Some of these drugs may also possess sedative effects, which may be beneficial to animals that are experiencing both anxiety and pain.
Physiology
Skeletal muscle spasticity may be due to an increase in tonic stretch reflexes originating from the CNS, with involvement of descending pathways, and results in hyperexcitability of motor neurons in the spinal cord. Several diseases involving increased muscle tone caused by defective neuronal control of muscle activity have been described in Scottish Terriers (Scotty cramp), Dalmatians, and Norwich Terriers.48,49 These dogs are normal at rest and on initiation of exercise, but episodic muscle rigidity or cramping are seen during heavy exercise or excitement. Familial reflex myoclonus has been described in Labrador Retrievers. Symptoms appear in young puppies and are characterized by paroxysmal muscle spasms and progressive muscle stiffness. Either a defect in the major inhibitory neurotransmitter glycine or altered genetic regulation of the spinal cord glycine receptor is thought to be the basis for this condition.50 Intervertebral disk disease in dogs may cause painful spasms of the neck and shoulder (cervical disk) or back (thoracolumber disk) muscles. In certain clinical cases, skeletal muscle relaxants are used as part of a conservative management protocol for animals with intervertebral disk disease.
Pharmacology
Skeletal muscle relaxants act in the CNS or directly on skeletal muscle to reduce muscle tone and relieve spasticity without abolishing voluntary motor control. Centrally acting muscle relaxants block interneuronal pathways in the spinal cord and in the midbrain reticular activating system. These drugs have little effect on the diaphragm and respiratory muscles at therapeutic doses. Dantrolene, a hydantoin derivative, is a peripherally acting muscle relaxants that acts by decreasing the amount of calcium released from the sarcoplasmic reticulum.
Individual Agents
Centrally acting skeletal muscle relaxants used in small animal veterinary medicine include guaifenesin, methocarbamol, and the benzodiazepines. The most common side effect seen with these drugs is CNS depression, which is manifested as sedation and lethargy. Ataxia and muscle weakness are also possible. Additive depression can occur when these drugs are given concurrently with other CNS depressant agents. Anticholinesterase agents given with either guaifenesin or methocarbamol may result in severe muscle weakness. Dantrolene is the only clinically used peripherally acting skeletal muscle relaxant.
Guaifenesin
Although more commonly used in large animals, guaifenesin is used in small animal veterinary patients to induce muscle relaxation as an adjunct to general anesthesia. The dose in dogs is 33 to 88 mg/kg adminstered intravenously. Guaifenesin has also been used in the treatment of strychnine intoxication in dogs.
Methocarbamol
Methocarbamol is labeled for adjunctive therapy of acute inflammatory and traumatic conditions of skeletal muscle and the reduction of muscle spasms in dogs and cats. The dose is 44 mg/kg intravenously or 61 to 132 mg/kg orally, initially divided every 8 to 12 hours. For muscle relaxation as a part of conservative management of intervertebral disk disease in dogs, methocarbamol is given at a dose of 15 to 20 mg/kg orally three times a day. To control the severe effects of strychnine and tetanus, the dose in dogs and cats is 55 to 220 mg/kg intravenously (not to exceed 330 mg/kg daily). Half of the dose is given rapidly, whereupon the clinician waits until the patient begins to relax and then continues administering the drug to effect.
Diazepam
Diazepam is the primary benzodiazepine used for the purpose of muscle relaxation. Although its clinical indications include seizure control, appetite stimulation, and sedation, only its use as a muscle relaxant will be discussed here. Diazepam may be effective in reducing skeletal muscle spasticity in dogs with episodic muscle cramping such as Scotty cramp or in certain myopathic syndromes. The dose range is 0.5 to 2 mg/kg intravenously or orally three times daily.
Diazepam is also used to treat intraurethral obstruction secondary to acquired lower urinary tract disease in male cats. It is believed that muscle spasms of the urethra, along with inflammation of the urethral tissue, make removal of the obstructing plug more difficult. Additionally, these factors may create a “functional” obstruction of the urinary tract even after the obstructing plug has been removed. Diazepam has been recommended for treatment of external urethral sphincter hypertonus at a dose of 2 to 10 mg/kg (total) orally every 8 hours. Because the urethral musculature contains a predominance of smooth muscle, it may be necessary to combine skeletal muscle relaxants (which affect only the external urethral sphincter) with smooth muscle relaxants, such as prazosin.
Dantrolene
The clinical indications for dantrolene include functional urethral obstruction resulting from increased urethral tone and treatment of malignant hyperthermia. For treatment of functional urethral obstruction, dantrolene is administered at a dose of 1 to 5 mg/kg orally every 8 hours (dogs) or 0.5 to 2 mg/kg every 8 hours (cats). Dantrolene has been shown to decrease urethral pressure in the postprostatic/penile urethral segment but has no effect on intraurethral pressures in the prostatic or preprostatic urethral segment.51 Concurrent use of smooth muscle relaxants may be of benefit in relieving urethral obstruction. For treatment of malignant hyperthermia, the dose of dantrolene is reported in dogs at 0.29 and 0.69 mg/kg intravenously.52,53 Dantrolene has moderate to poor oral bioavailability and is highly bound to albumin. Dantrolene undergoes hepatic metabolism with metabolites excreted in the urine. Adverse effects include hepatotoxicity, sedation, muscle weakness, and gastrointestinal effects.
1. Hall L.W., Clarke K.W. Relaxation of skeletal muscles during anaesthesia. In Hall L.W., Clarke K.W., editors: Veterinary anesthesia, ed 8, London: Baillière Tindall, 1983.
2. Ilkiw J.E. Advantages of and guidelines for using neuromuscular blocking agents. Vet Clin North Am Small Anim Pract. 1992;22(2):347.
3. Hunter J.M. New neuromuscular blocking drugs. N Engl J Med. 1995;332:1691.
4. Benson G.J., Thurmon J.C. Clinical pharmacology of succinylcholine. J Am Vet Med Assoc. 1980;176(7):646.
5. Hubbell J.A.E. Disadvantages of neuromuscular blocking agents. Vet Clin North Am Small Anim Pract. 1992;22(2):351.
6. Silverman D.G., Donati F. Neuromuscular effects of depolarizing relaxants. In: Silverman D.G., editor. Neuromuscular block in perioperative and intensive care. Philadelphia: JB Lippincott, 1994.
7. Miller R.D., Savarese J.J. Pharmacology of muscle relaxants and their antagonists. In Miller R.D., editor: Anesthesia, ed 2, New York: Churchill Livingston, 1986.
8. Plumb D.C. Veterinary drug handbook, ed 5. Ames, Iowa: Blackwell; 2005.
9. Fisher D.M., Canfell P.C., Fahey M.R., et al. Elimination of atracurium in humans: contribution of Hofmann elimination and ester hydrolysis versus organ-based elimination. Anesthesiology. 1986;65:6.
10. Scott R.P.F., Savarese J.J., Basta S.J., et al. Atracurium: clinical strategies for preventing histamine release and attenuating the hemodynamic response. Br J Anaesth. 1985;57:550.
11. Hildebrand S.V. Neuromuscular blocking agents. Vet Clin North Am Small Anim Pract. 1992;22(2):341.
12. Miller R.D., Rupp S.M., Fisher D.M., et al. Clinical pharmacology of vecuronium and atracurium. Anesthesiology. 1984;61:444.
13. Wastila W.B., Maehr R.B., Turner G.L., et al. Comparative pharmacology of cistracurium (51W89), atracurium, and 5 isomers in cats. Anesthesiology. 1996;85:169.
14. Kisor D.F., Schmith V.D., Wargin W.A., et al. Importance of the organ-independent elimination of cisatracurium. Anesth Analg. 1996;83:1065.
15. Adams W.A., Robinson K.J., Senior J.M. The use of the neuromuscular blocking drug cis-atracurium in dogs. Vet Anaesth Analg. 2001;28:156.
16. Stoelting R.K. Pharmacology and physiology in anesthetic practice, ed 3. Philadelphia: Lippincott-Raven; 1999.
17. Silverman D.G., Mirakhur R.K. Nondepolarizing relaxants of long duration. In: Silverman D.G., editor. Neuromuscular block in perioperative and intensive care. Philadelphia: JB Lippincott, 1994.
18. Hildebrand S.V. Neuromuscular blocking agents in equine anesthesia. Vet Clin North Am Equine Pract. 1990;6(3):587.
19. Mirakhur R.K. Newer neuromuscular blocking drugs: an overview of their clinical pharmacology and therapeutic use. Drugs. 1992;44(2):182.
20. Basta S.J. Clinical pharmacology of mivacurium chloride: a review. J Clin Anesth. 1992;4(2):153.
21. Lukasik V.M. Neuromuscular blocking drugs and the critical care patient. J Vet Emerg Crit Care. 1996;5(2):99.
22. Smith L.J., Moon P.F., Lukasik V.M., et al. Duration of action and hemodynamic properties of mivacurium chloride in dogs anesthetized with halothane. Am J Vet Res. 1999;60:1047.
23. Smith L.J., Schwark W.S., Cook D.R., et al. Pharmacokinetic variables of mivacurium chloride after intravenous administration in dogs. Am J Vet Res. 1999;60:1051.
24. Cason B., Baker D.G., Hickey R.F., et al. Cardiovascular and neuromuscular effect of three steroidal neuromuscular blocking drugs in dogs. Anesth Analg. 1991;70:382.
25. Hudson M.E., Rothfield K.P., Tullock W.C., et al. Haemodynamic effects of rocuronium bromide in adult cardiac surgical patients. Can J Anaesth. 1998;45:139.
26. Morris R.B., Cahalan M.K., Miller R.D., et al. The cardiovadcular effects of vecuronium (ORG NC45) and pancuronium in patients undergoing coronary bypass grafting. Anesthesiology. 1983;58:438.
27. Faulds D., Clissold S.P. Doxacurium: a review of its pharmacology and clinical potential in anaesthesia. Drugs. 1991;42(4):673.
28. Cook R.D., Freeman J.A., Lai A.A., et al. Pharmacokinetics and pharmacodynamics of doxacurium in normal patients and in those with hepatic or renal failure. Anesth Analg. 1991;72:145.
29. Ali H.H., Savarese J.J. Stimulus frequency and dose-response curve to d-tubocurarine in man. Anesthesiology. 1980;52:39.
30. Ali H.H., Miller R.D. Monitoring of neuromuscular function. In Miller R.D., editor: Anesthesia, ed 2, New York: Churchill Livingstone, 1986.
31. Brand JB, Cullen DJ, Wilson NF, et al: Spontaneous recovery from nondepolarizing neuromuscular blockade: correlation between clinical and evoked response, Anesth Analg 56:55, 177.
32. Klein L.V. Neuromuscular blocking agents. In: Short C.E., editor. Principles and practice of veterinary anesthesia. Baltimore: Williams & Wilkins, 1987.
33. Silverman D.G., Brull S.J. Patterns of stimulation. In: Silverman D.G., editor. Neuromuscular block in perioperative and intensive care. Philadelphia: JB Lippincott, 1994.
34. Drenck N.E., Ueda N., Olsen N.V., et al. Manual evaluation of residual curarization using double-burst stimulation: comparison with train-of-four. Anesthesiology. 1989;70:578.
35. Saddler J.M., Bevan J.C., Donati F., et al. Comparison of double-burst and train-of-four stimulation to assess neuromuscular blockade in children. Anesthesiology. 1990;73:401.
36. Braude N., Vyvyan H.A.L., Jordan M.J. Intraoperative assessment of atracurium-induced neuromuscular block using double-burst stimulation. Br J Anaesth. 1991;67:574.
37. Law S.C., Cook D.R. Monitoring the neuromuscular junction. In: Lake C.L., editor. Clinical monitoring. Philadelphia: Saunders, 1990.
38. Forsyth S.F., Ilkiw J.E., Hildebrand S.V. Effect of gentamicin administration on the neuromuscular blockade induced by atracurium in cats. Am J Vet Res. 1990;57(10):1675.
39. Hildebrand S.V., Hill T. Interaction of gentamycin and atracurium in anaesthetized horses. Equine Vet J. 1994;26:209.
40. Kopman K.F. The relationship of evoked electromyographic and mechanical responses following atracurium in humans. Anesthesiology. 1985;63:208.
41. Weber S., Muravchick S. Monitoring technique affects measurement of recovery from succinylcholine. J Clin Monit. 1987;3:1.
42. Dahaba A.A., Rehak P.H., List W.F. Assessment of accelerography with the TOF-GUARD: a comparison with electromyography. Eur J Anaesthesiol. 1997;14:623.
43. Viby-Mogensen J., Jensen E., Werner M.U., et al. Measurements of acceleration: a new method of monitoring neuron-muscular function. Acta Anaesthesiol Scand. 1988;32:45.
44. Harper N.J.N., Martlew R., Strang T., et al. Monitoring neuromuscular block by accelerography: comparison of the Mini-Accelerograph with the Myograph 200. Br J Anaesth. 1994;72:411.
45. Loan P.B., Paxton L.D., Mirakhur R.K., et al. The TOF-Guard neuromuscular transmission monitor: a comparison with the Myograph 200. Anaesthesia. 1995;50:699.
46. Kastrup M.R., Marsico F.F., Ascoli F.O. Neuromuscular blocking properties of atracurium during sevoflurane or propofol anaesthesia in dogs. Vet Anaesth Analg. 2005;32:222.
47. Naguib M. Sugammadex: Another milestone in clinical neuromuscular pharmacology. Anesth Analg. 2007;104:575.
48. Meyers K.M., Clemmons R.M. Scotty cramp. In: Kirk R.W., editor. Current veterinary therapy VIII. Philadelphia: Saunders, 1983.
49. Woods C.B. Hyperkinetic episodes in two Dalmatian dogs. J Am Anim Hosp Assoc. 1977;13:225.
50. Furber R.M. Cramp in Norwich terriers. Vet Rec. 1984;115:46.
51. Straeter-Knowlen I.M., Marks S.L., Rishniw M., et al. Urethral pressure response to smooth and skeletal muscle relaxants in anesthetized, adult male cats with naturally occurring urethral obstruction. Am J Vet Res. 1995;56:919.
52. Kirmayer A.H., Klide A.M., Purvance J.E. Malignant hyperthermia in a dog: case report and review of the syndrome. J Am Vet Med Assoc. 1984;185:978.
53. Bagshaw R.J., Cox R.H., Rosenberg H. Dantrolene treatment of malignant hyperthermia. J Am Vet Med Assoc. 1981;178:1029.