CHAPTER 54 Disorders of Metabolism
POLYPHAGIA WITH WEIGHT LOSS
In most dogs and cats polyphagia is usually accompanied by weight gain, and weight loss is accompanied by partial or complete anorexia. In some, however, polyphagia with concurrent weight loss is the presenting complaint. The most common cause of polyphagia with concurrent weight loss is inadequate caloric intake (Table 54-1). Daily caloric needs may not be met if inadequate quantities of food are being fed or if the diet is not complete and balanced or is of poor quality. Alternatively, the client may not recognize changes in nutritional needs (e.g., during pregnancy and lactation and at times of strenuous exercise, such as during hunting season) and may continue to feed the animal at previously adequate caloric levels.
 TABLE 54-1 Differential Diagnosis for Polyphagia and Weight Loss
TABLE 54-1 Differential Diagnosis for Polyphagia and Weight Loss
| ETIOLOGY | DEFINITIVE DIAGNOSTIC TESTS |
|---|---|
Endocrinopathies and gastrointestinal tract disorders also cause polyphagia and weight loss in some dogs and cats (see Table 54-1) as a result of an increase in basal metabolism (hyperthyroidism), inadequate assimilation of dietary nutrients (gastrointestinal tract disorders), or inappropriate use of nutrients (diabetes mellitus). Gastrointestinal tract disorders include parasitism, pancreatic exocrine insufficiency, infiltrative bowel disorders, lymphangiectasia, and neoplasia (most notably lymphoma). In most of these disorders the history and physical findings usually provide valuable clues to the diagnosis. For example, polyuria and polydipsia are common signs in diabetes mellitus. A thyroid nodule is usually palpable in dogs and cats with hyperthyroidism. Bulky, voluminous stools are noted in animals with pancreatic exocrine insufficiency. Diarrhea and vomiting may occur in animals with gastrointestinal tract disorders, and palpation of the abdomen may reveal abnormal loops of intestine and mesenteric lymphadenopathy. The last condition may be discernible in animals with any of the infiltrative diseases but is especially noticeable in those with gastrointestinal tract lymphoma, eosinophilic enteritis, or histoplasmosis.
In addition to routine questions posed to the client, the clinician should assess the type of foods offered, daily caloric intake, feeding routines, and competition for food from other dogs or cats. Daily caloric requirements in cats and dogs are quite variable and depend on numerous factors, such as signalment and the amount of daily physical activity. The average daily caloric intake can be calculated using the equation for the resting energy requirement (RER): 70× body weight in kilograms raised to the ¾ power. This can be calculated on a simple calculator with a square root button. The body weight in kilograms is multiplied by itself three times, and the square root of the result is taken twice before multiplying by 70. This value for RER has a unit of kcal per day and is multiplied by a factor to derive the maintenance energy requirement (MER). The factor for a neutered cat is 1.2, an intact cat’s factor is 1.4, a neutered dog’s factor is 1.6, and an intact dog’s is 1.8. The daily caloric requirements in any individual dog or cat may vary by as much as 50% more or less than this calculation. Although this represents a large range for normal caloric intakes, the clinician may have a greater suspicion that an inadequate amount of calories is being fed if the amount based on the diet history is closer to 50% of MER. At the same time, consumption of calories closer to 150% of MER may increase the suspicion that adequate calories are being fed but that an endocrinopathy and/or gastrointestinal tract disorder may be leading to polyphagia with concurrent weight loss. If the results of comparing the caloric intake to the calculated MER prove equivocal or cannot be attained, simply feeding more food or calories and reassessing the patient’s weight may be illuminative.
A complete blood count, serum biochemistry panel, measurement of baseline thyroxine concentration, urinalysis, and fecal examination for parasites should be done if the history and physical findings are unremarkable. Results of these tests usually help identify additional specific diagnostic tests that may be required to establish a definitive diagnosis (see Table 54-1). Inadequate nutrition should be suspected if the initial blood test results are unremarkable. Changes in the type of foods provided, daily caloric intake, and feeding routine should be made to ensure that the animal has an adequate caloric intake of a palatable and nutritionally complete and balanced food. The animal’s body weight should be determined 2 and 4 weeks after the start of an appropriate diet. The resolution of signs and weight gain confirm the diagnosis. Failure to gain weight indicates problems with client compliance or the presence of occult disease, most likely disease involving the gastrointestinal tract.
OBESITY
Obesity is a clinical syndrome that involves the excess accumulation of body fat. Obesity is considered the most common form of malnutrition in small animal practice. Indeed, surveys suggest that 25% to 40% of cats and dogs presented to veterinary clinics are overweight or obese. The significance of obesity pertains to its role in the pathogenesis of a variety of diseases and its ability to exacerbate preexisting disease and decrease lifespan. Obesity has been associated with an increased incidence of arthritis, diabetes mellitus, hepatic lipidosis, feline lower urinary tract disease (FLUTD), urine incontinence in spayed bitches, constipation, dermatitis, cardiovascular problems, respiratory problems, and increased anesthetic and surgical risk (Box 54-1). In addition, Scarlett et al. (1998) found a threefold increase in risk of death in obese middle-aged cats compared with the risk in lean middle-aged cats. In dogs Kealy et al. (2002) found that dogs that were kept lean throughout their life lived almost 2 years longer than control-group littermates that were overweight. The lean dogs also did not need treatment for co-morbidities such as osteoarthritis until later in life.
Etiology
Obesity develops when energy intake consistently exceeds daily energy expenditure. Numerous environmental and social factors contribute to the development of obesity (Box 54-2). These include decreased daily exercise as a result of confinement to the house and overfeeding of the pet. Clients may overfeed their pet because a good appetite is perceived as a sign of good health, they may use food as a palliative agent when they leave the pet on its own, they may replace exercise with food, and they often indulge begging behavior because they find it endearing. Clients also tend to feed the same volume of food each day despite changes in energy requirements and the energy density of foods provided. Daily energy requirements vary according to the environmental temperature, the life stage of the pet (i.e., growth, pregnancy, lactation, adult maintenance, old age), the neuter status, and the activity level of the pet. Therefore it is necessary to adjust the amount of food according to these factors. Feeding errors also arise when a client purchases a different type of food with a higher energy density but does not reduce the amount accordingly. It is worth noting that dry extruded foods can now range from 200 kcal per 8-ounce cup to over 600 kcal per cup. Overfeeding may also arise if the feeding guidelines provided by pet food manufacturers are incorrect. In some situations clients are simply not aware that they are overfeeding their pet. Ad libitum feeding may also predispose to overeating, particularly if the pet is bored and inactive. Likewise, highly palatable foods encourage overconsumption. Snacks and treats are a significant silent contributor to excess daily caloric intake as well. It takes only about 11 extra calories a day for a pet to gain 1 pound over the course of a year; many common treats provide between 50 and 100 extra calories apiece.
Obese clients may be more likely to have obese pets. The client’s sedentary lifestyle may contribute to a lack of exercise by the pet, and the consumption of high-fat foods by the client may increase the likelihood that these energy-dense scraps are fed to the pet. In addition, it is possible that obese clients do not believe (or recognize) that obesity is a major problem for their pet.
Because of genetic differences, some animals have significantly lower energy requirements and therefore require fewer calories per day to maintain their ideal body weight. These genetic differences may be reflected by the increased propensity of certain dog breeds to gain weight. Breeds commonly recognized as at risk for obesity include the Labrador Retriever, Golden Retriever, Cocker Spaniel, Collie, Dachshund, Cairn Terrier, Shetland Sheepdog, Beagle, Cavalier King Charles Spaniel, and Basset Hound. Neutering has been associated with an increased risk of obesity. It has been suggested that hormonal alterations secondary to neutering may alter energy expenditure and the regulation of food intake. Obesity has been reported to be more common in female neutered dogs and male neutered cats.
Obesity is less likely to result from a disease process or drug. Indeed, it has been suggested that less than 5% of obesity is due to a disease or drug. Endocrine abnormalities associated with obesity include hypothyroidism, hyperadrenocorticism, hyperinsulinism, and acromegaly. Drugs such as progestagens and corticosteroids have been associated with the development of obesity.
Diagnosis
Obesity is defined as a “pathological condition characterized by an accumulation of fat much in excess of that required for optimal body function” (Mayer, 1973). However, what is an excess amount of body fat, and what is an acceptable amount? To answer these questions, the clinician must accurately determine the amount of body fat. Body fat can be assessed by techniques such as morphometric measurements, dilutional methods, bioelectrical impedance analysis, dual energy X-ray absorptiometry, densitometry, computed tomography, magnetic resonance imaging, determination of total body electrical conductivity, determination of total body potassium, and neutron activation analysis. Although numerous methods exist to determine body fat, measurement of body weight, calculation of a body condition score (BCS), and morphometric measurements remain the most clinically useful techniques in small animal practice.
Measurement of body weight is the simplest technique available and should be included in the examination of every animal. Body weight provides a rough measure of total body energy stores, and changes in weight reflect energy and protein balance.
Body condition scoring provides a quick and simple subjective assessment of the animal’s body condition. The two most commonly used scoring systems in small animal practice are a 5-point system in which a BCS of 3 is considered ideal and a 9-point system in which a BCS of 5 is considered ideal. Larger numbers are used for patients with greater adiposity. Each point above and below 5 on the 9-point system has been validated to correspond with an increase or decrease in adiposity or weight of 10% to 15%. Thus a patient that has a BCS of 7 out of 9 is 20% to 30% overweight as a result of the accumulation of adipose tissue. Likewise, pets can be classified as being thin, lean, of optimal weight, overweight, or obese (Box 54-3). The BCS technique depends on operator interpretation and does not provide any precise quantitative information concerning alteration in fat-free or lean body mass relative to fat mass.
 BOX 54-3 Body Condition Scoring (BCS) System for Cats and Dogs Using a 5-Point System
BOX 54-3 Body Condition Scoring (BCS) System for Cats and Dogs Using a 5-Point System
| Thin (BCS 1/5) | Underweight; no obvious body fat |
| Lean (BCS 2/5) | Skeletal structure visible; little body fat |
| Optimal (BSC 3/5) | Rib cage easily palpable but not showing; moderate amount of body fat |
| Overweight (BCS 4/5) | Rib cage barely palpable; body weight more than normal |
| Obese (BCS 5/5) | Rib cage not palpable; large amount of body fat; physical impairment resulting from excess body fat |
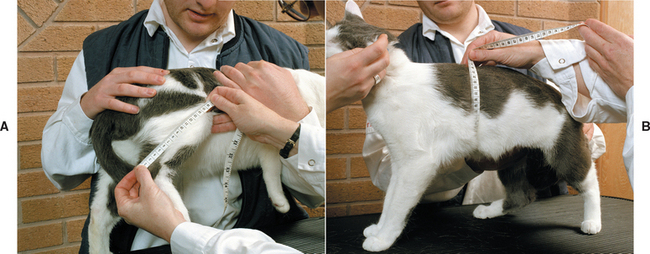
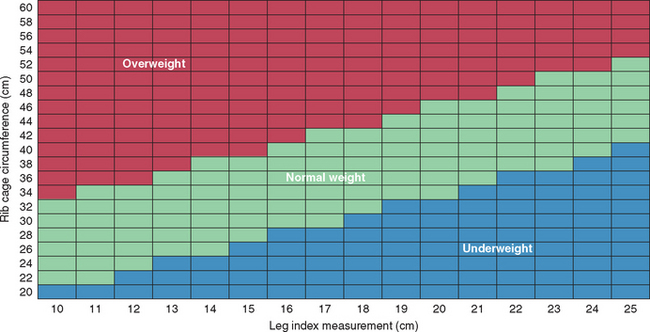
Height and circumferential measurements of the abdomen, hip, thigh, and upper arm are commonly used to estimate the percentage of body fat in humans. Circumferential measurements have also been developed to estimate the percentage of body fat in cats. The Feline Body Mass Index (FBMI) is determined by measuring the rib cage circumference at the level of the ninth cranial rib and deter mining the leg index measurement (LIM), which is the distance from the patella to the calcanealtuber (Fig. 54-1, A and B). The percentage of body fat can be calculated as 1.5 to 9 (rib cage measurement minus LIM) or determined by consulting a reference chart (Fig. 54-2). Cats with more than 30% body fat are candidates for a weight loss program. The FBMI is a very simple yet objective tool for determining the body fat content of the cat. In addition, it is particularly valuable in persuading clients that their cat is indeed overweight and in need of weight loss. Pelvic circumference in relation to the distance from hock to stifle has been shown to predict body fat in dogs. Whether morphometric measurements or BCS is used, providing a quantitative assessment of a patient’s degree of adiposity can be helpful in diagnosing obesity, which is typically defined as being approximately 25% over one’s ideal body weight.
Treatment
After determining that a patient is overweight or obese, the clinician should obtain a thorough dietary history to calculate the patient’s daily caloric intake. The clinician should gather the following information:
The patient’s current body weight should be recorded and a BCS assigned. The BCS can be used to determine the percentage of body weight that must be lost. Remembering that each point above a 5 on a 9-point scale represents an additional 10% to 15% of weight over ideal, the clinician can calculate the percentage of weight that should be lost. For example, a patient that has a BCS of 8 out of 9 is 30% to 45% overweight. For reasons that will be discussed later, patients should not lose more than 2% of their body weight per week. Therefore it should be expected that most overweight and obese patients will take at least several months to lose enough adipose tissue to attain their ideal body weight. Given the necessary length of time, it is imperative to break down the ultimate goal of an ideal body weight into smaller goals that can be achieved in shorter periods of time. Therefore the clinician may recommend that the patient lose 2% to 4% of body weight every 2 weeks; later, monthly goals of 4% to 8% may be set. These shorter-term goals are typically more manageable and provide more opportunities for adjustment of a weight loss plan if needed and for praise if the plan is proving effective.
A rate of weight loss of 1% to 2% of current body weight per week is typically recommended for several reasons. First, greater rates of weight loss will require that the patient receive a very small allowance of food, which is most likely to encourage begging behavior and garbage scavenging. These undesirable behaviors, along with the small volume of food to be provided, can jeopardize client compliance. Second, weight loss greater than 2% of body weight per week is considered unhealthy and has been associated with a greater loss of lean body mass compared with fat mass. Third, rapid weight loss is most likely to result in a rebound weight gain effect after cessation of the program.
Given the large variation in energy requirement that can be seen in cats and dogs, the best method to determine the amount of calories to feed a patient to induce weight loss is the use of an accurate diet history. Typically, the weight of overweight and obese patients is relatively stable at presentation; therefore feeding 80% of the patient’s current caloric intake based on an accurate diet history results in weight loss of 0.5% to 2% of body weight loss per week. In patients in which an accurate diet history cannot be determined or that are not roughly weight stable, the client may feed 80% of RER for cats and RER for dogs. Regardless of the method used to determine the number of calories to feed to initiate weight loss, clients should be told to expect to adjust the amount of food on the basis of frequent weigh-ins. Initially, it can be expected that some patients will gain weight on the new weight loss plan, some may stay weight stable, some may lose the desired amount, and some may even lose weight too quickly.
After determining the daily amount of calories to feed the patient, the clinician should consider the most suitable type of food. There are essentially two main dietary options: either feed a reduced amount of the regular maintenance food or feed a food that has been specifically formulated for weight reduction. It is not advisable to feed less of the regular food because this most likely was the food that resulted in the problem in the first place. More important, feeding a maintenance food decreases compliance and increases the risk of nutrient deficiency and unhealthy weight loss. Most foods designed for weight reduction are one-half to two-thirds less energy dense than typical maintenance foods. Therefore clients will not visually perceive as much of a decrease in “bowl fill” when feeding a food designed for weight reduction. Decreased energy density is achieved by decreasing the fat content of the food, air-puffing kibble, increasing the moisture content of canned or pouched foods, and/or by adding fiber. There does appear to be some satiety effect by increasing “bowel fill”. More significantly, canine and feline maintenance foods are formulated according to energy intake. This means that if a dog or cat eats its daily energy requirement, it will automatically consume the required amounts of additional essential nutrients, such as amino acids, essential fatty acids, minerals, and vitamins. By feeding less of the maintenance food, the client is reducing not only the amount of energy but also the amount of amino acids, fatty acids, minerals, and vitamins, thereby risking malnutrition, especially given the length of time that is often needed to achieve an ideal body condition. Conversely, foods that have been specifically formulated for weight reduction contain more essential nutrients relative to the energy content of the food. This means that the patient will receive the required amounts of essential nutrients even though it is ingesting fewer calories.
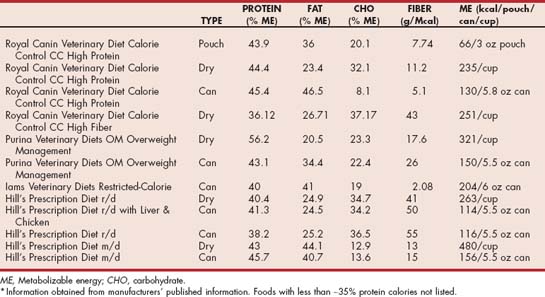
Foods formulated specifically for weight reduction typically vary according to energy density, fiber content, and caloric distribution (Tables 54-2 and 54-3). Most foods designed for weight reduction are less energy dense than maintenance foods. This enables a greater filling of both the bowl and the bowel, which should lead to increased compliance and satiety. Traditionally, higher-fiber foods are initially suggested for weight loss. Fiber is used as a bulking agent to decrease energy density and provide a satiating effect. However, there is conflicting research as to whether fiber increases satiety. Because some patients may not respond well to higher-fiber foods, some manufacturers do not use this nutritional strategy. Caloric distribution refers to the percentage of calories provided from protein, fat, and carbohydrate. Higher-protein foods have been reported to increase the proportion of fat loss while preserving or, indeed, increasing the lean body mass. The lean body mass is the most metabolically active portion of the body and includes skeletal muscle tissues. Preservation of lean body mass in humans has been shown to facilitate successful long-term maintenance of the ideal body weight once weight loss has been achieved. Lowering the percentage of calories from fat in foods helps reduce the energy density of the food because fat provides almost 2.5 times the amount of calories per gram as protein or carbohydrate does. Lower-carbohydrate foods specifically designed for weight reduction have become available. According to initial reports, these foods result in greater fat mass loss with the same amount of caloric restriction compared with higher-carbohydrate foods. The proposed mechanism for this difference relates to shifting metabolism from a lypogenic state to a lypolytic state, especially in the cat. One drawback of some lower-carbohydrate foods designed for weight reduction is their potential to be more energy dense and thus have a decreased bowl- and bowel-filling effect.
 TABLE 54-2 Level of Key Nutrients in Selected Therapeutic Commercial Foods Suitable for Weight Loss in Dogs*
TABLE 54-2 Level of Key Nutrients in Selected Therapeutic Commercial Foods Suitable for Weight Loss in Dogs*
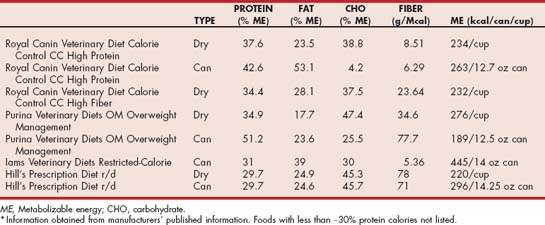
 TABLE 54-3 Level of Key Nutrients in Selected Therapeutic Commercial Foods Suitable for Weight Loss in Cats*
TABLE 54-3 Level of Key Nutrients in Selected Therapeutic Commercial Foods Suitable for Weight Loss in Cats*
Carnitine is an amino acid derivative that is vital for energy metabolism. Carnitine facilitates the movement of long-chain fatty acids across the mitochondrial membrane, where they are used for energy production. Carnitine supplementation is believed to facilitate weight loss by increasing the efficiency of “burning” fat as an energy source. However, a study evaluating the effect of carnitine supplementation on body weight loss failed to demonstrate any benefits (Center et al., 2000). Cats that received carnitine supplementation lost the same percentage of body weight in the same period of time as cats that did not receive carnitine supplementation. In addition, neither group of cats developed hepatic lipidosis.
As this chapter was being completed, a new drug (dirlotapide) has become available that helps reduce the appetites of dogs in need of weight loss. According to the manufacturer’s literature, dirlotapide is a selective microsomal triglyceride transfer protein inhibitor that blocks the assembly and release of lipoproteins into the bloodstream. The mechanism of action for producing weight loss is not completely understood, but it seems to result from reduced fat absorption and a satiety signal from lipid-filled enterocytes. Dirlotapide mainly acts locally in the gut to reduce appetite, increase fecal fat, and produce weight loss in the management of obesity in dogs. It appears that changes in long-term client feeding practices are important for prevention of weight regain after the use of this potentially promising new drug.
Once the daily caloric intake has been determined and the appropriate weight reduction food(s) chosen, the method of feeding should be determined. Ideally, the patient should receive meals rather than be fed ad libitum. The number of feedings per day can be selected to suit the client’s schedule, but two to four meals per day is adequate. One member of the household should be selected to feed the patient. This will reduce inadvertent overfeeding by additional family members. If treats are typically fed or are desired, the client should be instructed to limit the number of treats to less than 10% of the daily caloric intake. Ideally, low-calorie treats should be selected. Commercial treats are available, but fruits (excluding grapes or raisins) and/or vegetables (no garlic or onions and not in patients with calcium oxalate urolithiasis) can be good alternatives for dogs and even some cats. Baby carrots are an especially good vegetable treat for dogs and contain only 4 kcal each. A small amount of lean meat, such as skinless chicken breast, can be a good alternative treat for cats. It is also important to modify the behavior of the client such that the patient should not be allowed in the kitchen or dining room during meal preparation or eating if this is typically a tempting time to respond to begging. In addition, the client should inform and enlist the support of family members and neighbors so that they do not unknowingly give the patient additional calories. In some cases it may be useful for the client to use a food diary to record the amount of food and snacks fed each day. For other clients this technique is often met with resistance and should not be considered.
Multicat households in which one cat is obese and the remainder are of normal body weight or are lean can present some management problems. Ideally, cats should be fed in separate rooms, but this is not always possible. If it is possible, most cats can consume their caloric needs if given at least 4 hours of access to their food daily. Thus the time that cats are separated can be minimized. Moreover, fat cats usually cannot jump very high. Therefore it may be useful to place the food for the lean, healthy cats on an elevated bench or counter that the healthy cats can reach but the obese cat cannot. Alternatively, a hole can be cut into a cardboard box that is large enough to allow the lean cats to enter but small enough to restrict the entry of the overweight or obese cat. The lean cats are then fed in the box.
In addition to reducing the daily caloric intake, every effort should be made to increase the pet’s daily energy expenditure by encouraging exercise. Toys that the cat or dog can chase and play with should be encouraged. Laser pointers are particularly useful for encouraging cats to play. Ideally, dogs should receive two 20-minute walks per day. Swimming is an equally effective exercise, particularly for dogs with osteoarthritis. Providing the client with written instructions for weight loss will typically improve both compliance and success. Photographing the patient before institution of the weight reduction program will help clients see the effect of the weight loss on their pet. Institution of reward boards or incentive programs will also increase compliance with the weight reduction program.
Patients on weight reduction programs should be reevaluated every 2 weeks initially. The body weight, BCS, and/or FBMI should be recorded. The dietary history should be reviewed. Ideally, cats should achieve no more than a 2% body weight loss per week. More rapid weight loss in cats increases the risk of hepatic lipidosis. Dogs should achieve a 1% to 2% body weight loss per week. If the rate of weight loss exceeds a 2% body weight loss per week, then the amount of calories fed to the patient should be increased by 10% to 20%. If the patient has not lost any weight, the dietary history should be reevaluated for a source of additional calories and compliance with the weight loss plan confirmed. If no such reasons are found, the daily caloric intake should be further reduced by 10% to 20%.
Once the ideal body condition of the patient has been achieved, the daily caloric intake can be adjusted to maintain an ideal body condition. The patient’s regular food may be changed to one formulated for weight maintenance or a light food. The patient should be reevaluated every 2 to 3 months after weight loss to ensure that weight stability is maintained and that the patient is not gaining weight on its new diet regimen.
Prevention
Ideally, clinicians should focus more on obesity prevention than on treatment because treatment can be very challenging. Energy requirements significantly decrease when the animal has a gonadectomy. Therefore prevention should begin at the time that the pet is neutered. Clients should be counseled about the risk factors of obesity (e.g., male neutered cats, female neutered dogs, inactive and indoor lifestyle, inappropriate feeding practices, energy-dense foods) and the consequences of obesity (e.g., increased incidence of lower urinary tract disease, diabetes mellitus, arthritis, decreased life span). It is important that clients be instructed in both how to feed their pet and how to regularly determine the pet’s body condition such that they can maintain the ideal body condition of their pet. Weight education should be reinforced at least annually during the health examination.
HYPERLIPIDEMIA
Hyperlipidemia is defined as an increased concentration of triglycerides (hypertriglyceridemia), cholesterol (hypercholesterolemia), or both in the blood. In the fasted state (>10 hours without food), hyperlipidemia is an abnormal finding that represents either accelerated production or delayed degradation of lipoproteins. The lipoproteins function as a carrier system to transport water-insoluble triglycerides and cholesterol through the aqueous environment of blood. Lipoproteins consist of a triglyceride and cholesterol ester core surrounded by a surface layer of cholesterol, phospholipid, and apolipoproteins. The apolipoproteins (A, B, C, and E) are responsible for the structure of the lipoprotein particle, the binding of the particle to cell surface receptors, and the activation of enzymes. There are four major classes of lipoproteins. Each class differs in its lipid and apoprotein content and physicochemical characteristics, including size, density, and electrophoretic mobility. Lipoproteins are categorized according to their buoyant density on ultracentrifugation as chylomicrons, very-low-density lipoproteins (VLDLs), low-density lipoproteins (LDLs), or high-density lipoproteins (HDLs). The buoyant density is inversely proportional to the triglyceride content such that the chylomicrons are composed largely of triglyceride, whereas HDLs have virtually no triglyceride content. The classification system is somewhat arbitrary, and it should be understood that there is significant structural and functional heterogeneity within the classes. In addition, the system is a dynamic one, with one class producing another during its metabolism. Chylomicrons and VLDLs are primarily involved in triglyceride metabolism, whereas HDLs and LDLs are primarily involved in cholesterol metabolism. Dogs and cats are more resistant to the development of atherosclerosis than humans because HDLs predominate in dogs and cats, as opposed to the LDLs that predominate in humans.
Pathophysiology
After digestion and absorption occur, dietary cholesterol and triglyceride are packaged by the enterocyte into chylomicron particles. The chylomicron particles are secreted into the mesenteric lymph, through which they ultimately reach the systemic circulation via the thoracic duct. As the chylomicrons pass through the adipose and muscle tissue, they are exposed to lipoprotein lipase, an enzyme that is present on the surface of the capillary endothelial cells. After activation by apoprotein C-II, lipoprotein lipase hydrolyzes the triglyceride from the core of the lipoprotein to free fatty acids and glycerol. The free fatty acids diffuse into the adjacent tissue and are either resynthesized into triglycerides and stored (adipocytes) or used for energy by the cell (myocytes and other cells). The activity of lipoprotein lipase is influenced by several factors, including heparin, insulin, glucagon, and thyroid hormone. Depletion of the triglyceride component of the chylomicron alters the surface such that the chylomicron is converted into a chylomicron remnant. The remnant particle is rapidly recognized by specific hepatic receptors and removed from the circulation. Within the hepatocyte the contents of the chylomicron remnant are degraded and utilized. Chylomicrons are present in plasma 30 minutes to 2 hours after consumption of a fat-containing meal, and hydrolysis is normally complete within 6 to 10 hours.
The liver transforms excess free fatty acids that are not directly oxidized for energy into triglycerides. The free fatty acids may originate from residual dietary triglyceride present in chylomicron remnant particles, from endogenous production secondary to surplus dietary carbohydrate, and from excessive endogenous mobilization of free fatty acids. Free fatty acids can be mobilized from adipose tissue by the activation of the intracellular enzyme hormone-sensitive lipase (HSL). HSL hydrolyzes stored triglycerides into free fatty acids and glycerol. Stimulators of HSL include epinephrine, norepinephrine, adrenocorticotropic hormone (ACTH), corticosteroids, growth hormone, and thyroid hormone. In addition, HSL is activated by insulin deficiency. Activation of HSL is a normal physiologic response to provide the body with energy during periods of fasting. In addition, HSL can be inappropriately activated in several pathologic conditions associated with an altered metabolic state.
The triglycerides produced by the hepatocyte are packaged into VLDL particles and subsequently secreted into the bloodstream. VLDL particles are produced continuously by the liver and, in the fasting state, are the main carriers of triglycerides. In addition, VLDL particles are used to export cholesterol from the liver and therefore contain a significant proportion of cholesterol. Analogous to chylomicron metabolism, endothelial lipoprotein lipase hydrolyzes the triglyceride portion of the VLDL particle into free fatty acids and glycerol. The free fatty acids can either be oxidized for energy or reconstituted into triglycerides and stored. Removal of the triglyceride core converts the VLDL particle into a remnant particle, which may be removed and catabolized by the liver. Alternatively, a second endothelial lipase, hepatic lipase, can further remove any residual triglyceride and convert the VLDL remnant particle into an LDL particle.
The LDL particle is a cholesterol and phospholipid–rich entity that functions to transport cholesterol to tissues, where it may be used for membrane synthesis or steroid hormone production. Ultimately, the LDL particle can bind to LDL receptors and is removed by the liver. In addition to VLDL particles, the liver also secretes nascent HDL particles into the circulation. HDL particles act to scavenge excess unesterified cholesterol from the cells and other lipoproteins and return it to the liver for excretion into bile. This process is often referred to as reverse cholesterol transport.
Hypertriglyceridemia can develop secondary to increased chylomicron production (excessive dietary intake of lipid), ineffective clearance of the chylomicron particle, increased VLDL production (excessive dietary intake of lipid and/or carbohydrate, excessive endogenous production or mobilization of lipids), and ineffective clearance of the VLDL particle. Hypercholesterolemia can arise from increased production of the LDL precursor particle (VLDL) or as a result of reduced clearance of the LDL or HDL particle.
Classification
Postprandial hyperlipidemia is the most common cause of hyperlipidemia in dogs and cats. It is a normal physiologic manifestation that is due to the production of triglyceride-rich chylomicrons and usually resolves within 2 to 10 hours. Pathologic abnormalities in plasma lipids and lipoproteins may be of genetic or familial origin (primary) or arise as a consequence of disease (Box 54-4).
Primary hypertriglyceridemias include the idiopathic hyperlipidemia of Miniature Schnauzers and hyperchylomicronemia of cats. Idiopathic hyperlipidemia of Miniature Schnauzers is characterized by severe hypertriglyceridemia resulting from excessive VLDL particles with or without concurrent hyperchylomicronemia and by mild hypercholesterolemia. The exact mechanism and genetics have not been fully elucidated. Feline familial hyperlipidemia is characterized as a fasting hyperchylomicronemia with a slight increase in VLDL particles. The defect is due to the production of an inactive form of lipoprotein lipase. Idiopathic hyperchylomicronemia has also been observed in dogs. Similar to the situation with the cat, the disease in the dog is characterized by hypertriglyceridemia, hyperchylomicronemia, and normal serum cholesterol concentrations. Idiopathic hypercholesterolemia is rare but has been reported in Doberman Pinschers and Rottweilers. Lipid derangements consist of hypercholesterolemia caused by an increased serum LDL concentration. The etiology of this disorder is unknown.
Diseases associated with secondary hyperlipidemia include endocrine disorders (hypothyroidism, diabetes mellitus, hyperadrenocorticism), nephrotic syndrome, and pancreatitis. Hypothyroidism is the most common cause of hypercholesterolemia in the dog. Hyperlipidemia secondary to hypothyroidism can be attributed to both a decrease in lipid synthesis and degradation (lipid degradation is more severely affected). Decreased lipoprotein lipase activity contributes to the impaired removal of triglyceride-rich lipoproteins. In addition, thyroid hormone deficiency reduces the biliary excretion of cholesterol. The resultant increase in intrahepatic cholesterol concentration downregulates the hepatic LDL receptor, which increases the concentration of the circulating LDL and HDL cholesterol–rich particles.
Insulin deficiency (diabetes mellitus) reduces the production of lipoprotein lipase, which contributes to decreased clearance of triglyceride-rich lipoproteins. Furthermore, insulin deficiency activates HSL, causing the release of large quantities of free fatty acids into the blood. These free fatty acids are ultimately converted by the liver into triglycerides, packaged into VLDL particles, and secreted back into the circulation. Therefore the hypertriglyceridemia seen with diabetes mellitus is attributed to both a reduction of lipoprotein lipase and increased production and decreased clearance of VLDL particles. Insulin deficiency increases the synthesis of cholesterol in the liver. The increased intrahepatic cholesterol concentration downregulates the hepatocyte LDL receptor, consequently reducing the clearance of circulating LDL and HDL particles, which in turn causes hypercholesterolemia.
The mechanism of hypertriglyceridemia associated with hyperadrenocorticism is probably due to stimulation of HSL with release of free fatty acids into the circulation. Similar to the situation with diabetes mellitus, excess free fatty acids are converted into VLDL particles. In addition, glucocorticoids inhibit lipoprotein lipase activity, thereby reducing the clearance of triglyceride-rich lipoproteins.
Clinical Features
Waxing-and-waning vomiting, diarrhea, and abdominal discomfort are the most common clinical presentations associated with hypertriglyceridemia (Table 54-4). Severe hypertriglyceridemia (levels exceeding 1000 mg/dl) has been associated with pancreatitis, lipemia retinalis, seizures, cutaneous xanthomas, peripheral nerve paralysis, and behavioral changes. Cutaneous xanthomas, which represent lipid-laden macrophages and foam cells, are the most common manifestation of hypertriglyceridemia in the cat. Severe hypercholesterolemia has been associated with arcus lipoides corneae, lipemia retinalis, and atherosclerosis.
 TABLE 54-4 Clinical Signs and Potential Consequences of Hypertriglyceridemia and Hypercholesterolemia
TABLE 54-4 Clinical Signs and Potential Consequences of Hypertriglyceridemia and Hypercholesterolemia
| CLINICAL SIGNS | CONSEQUENCES |
|---|---|
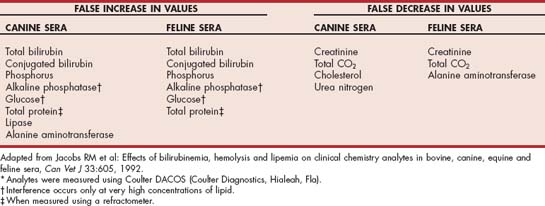
In addition to the clinical manifestations, hypertriglyceridemia may also interfere with the results of several routine biochemical tests (Table 54-5). The degree of interference depends on the specific assay used by the laboratory, the species (canine versus feline), and the severity of the hypertriglyceridemia. In addition, hyperlipidemia may also cause hemolysis, which in turn can interfere with the results of some biochemical assays. Alternatively, hyperbilirubinemia may cause the cholesterol concentration to be falsely lower. These potential alterations in biochemical data must be considered when interpreting results in animals with hyperlipidemia. Fortunately, many laboratories will attempt to clear the hypertriglyceridemia by ultracentrifugation before performing the biochemical assays.
Diagnosis
The presence of lipemic serum suggests that the animal is hypertriglyceridemic. Lactescence refers to the opaque and milklike appearance of serum samples that occurs when the elevation of the triglyceride level is sufficient. Animals with lactescent serum typically have triglyceride concentrations that exceed 1000 mg/dl. Conversely, animals that are purely hypercholesterolemic do not exhibit lipemic or lactescent serum because the cholesterol-rich LDL and HDL particles are too small to refract light. Blood samples to confirm hyperlipidemia should be obtained after a fast that lasts at least 12 hours. A serum sample rather than whole blood or plasma should be submitted for assessment. The sample can be refrigerated or frozen for several days without affecting the assays. When assessing the sample for hypertriglyceridemia, the technician should not clear the sample before determination of the triglyceride concentration. Clearing lipemic samples by centrifugation removes chylomicrons, which will artificially lower the triglyceride result. Reference intervals for serum triglyceride concentration are typically 50 to 150 mg/dl for the adult dog and 20 to 110 mg/dl for the adult cat. Reference intervals for serum cholesterol concentration are typically 125 to 300 mg/dl for the adult dog and 95 to 130 mg/dl for the adult cat.
The chylomicron test can be helpful to delineate whether the lipemia is predominantly a chylomicron or a VLDL defect. The test is performed by refrigerating a serum sample for 12 hours. Chylomicrons are less dense than the other particles and hence will float to the top of the sample to form an opaque cream layer over a clear infranatant of serum. If the hypertriglyceridemia is due to excess VLDL particles, the serum sample will remain turbid. Formation of a cream layer over a cloudy serum layer suggests both excess chylomicrons and VLDL particles.
Lipoprotein electrophoresis can be used to distinguish the lipoproteins, and ultracentrifugation can provide a quantitative measurement of each of the lipoprotein classes. However, both of these procedures are time consuming and are not routinely available for clinical application. The activity of lipoprotein lipase can be assessed by the heparin release test. Serum samples for the determination of triglyceride concentrations (and, if possible, lipoprotein concentrations) are obtained before and 15 minutes after the intravenous administration of heparin (90 IU/kg body weight in dogs; 40 IU/kg body weight in cats). Heparin causes the release of lipoprotein lipase from the endothelium and stimulates the hydrolysis of triglycerides. A defect in lipoprotein lipase is suspected if there is no difference between the serum triglyceride concentrations before and after the administration of heparin.
Treatment
Before therapy is recommended, every attempt should be made to determine whether the hyperlipidemia is primary or secondary to an underlying disease process. Hyperlipidemia secondary to an underlying disorder will typically resolve or improve with correction of the metabolic disturbance. Therefore each animal requires a full history, physical examination, complete blood count, serum biochemistry panel and thyroxine concentrations, and urinalysis. The results of the initial diagnostic evaluation may indicate the need for additional diagnostic tests such as abdominal ultrasound, pancreatic lipase immunoreactivity assay, and evaluation of an ACTH stimulation test. A recommendation to treat hyperlipidemia involves a lifelong commitment by the client and therefore must not be undertaken lightly. In general, severe hypertriglyceridemia (levels exceeding 1000 mg/dl) mandates treatment. In this circumstance catabolic mechanisms can be assumed to be overwhelmed, and the triglyceride level is very sensitive to a small increase from the intestine or liver. The triglyceride levels must be decreased to prevent possible complications, including pancreatitis. In other situations the recommendations will be influenced by additional variables, including the underlying disease process. A realistic goal of therapy is to reduce the triglyceride concentration to less than 400 mg/dl, even though such a level will still be above the reference interval.
Chylomicrons are produced from dietary fat. Therefore restriction of dietary fat is the cornerstone of therapy for hypertriglyceridemia. The dietary history should be reviewed, and the diet altered to one that contains less than 20% fat on an metabolizable energy (ME) basis for dogs (Table 54-6) or lower if the patient is already on a lower-fat diet. Nutritional management of hypertriglyceridemia in cats is more difficult because of the limited availability of lower-fat commercial therapeutic foods that have less than 24% fat on an ME basis (Table 54-7). Care should be taken when using over-the-counter foods that appear to be lower in fat. Because the proximate analysis that is reported on pet food labels requires only a minimum crude fat percentage to be reported, there is no guarantee that the fat content is not significantly higher. In contrast, therapeutic foods typically provide the average fat content in product guides, which should more accurately reflect the actual fat content of the food. Treats should be restricted to no more than 10% of the daily caloric intake and changed to low-fat commercial varieties. Fruit or brown rice crackers without seasoning are useful alternatives for dogs. In addition to the provision of a lower-fat diet, the absolute caloric intake should be evaluated. If the animal is overweight, caloric restriction is indicated and beneficial because it decreases the production of VLDL particles from excess dietary energy. The plasma triglyceride concentration should be reevaluated after 8 weeks of a lower-fat diet. If the reduction in triglyceride concentration is less than ideal, the dietary history should be reevaluated to ensure that there are no extra fat calories from treats, no access to other pet foods, and no additional family members or neighbors who are inadvertently providing the animal with dietary fat. In addition, the medical record should be reviewed to ensure the exclusion of underlying disorders that would contribute to hypertriglyceridemia. If the lower-fat commercial foods are not sufficient to control the hypertriglyceridemia, then a complete and balanced fat-restricted (10% to 14% ME for dogs, 15% to 19% ME for cats) home-prepared recipe can be formulated specifically for the animal using online software (such as at balanceit.com) or through a veterinary nutritionist (see acvn.org). Diets rich in omega-3 fatty acids have been suggested to improve hypertriglyceridemia in humans by decreasing the production of VLDL particles. In addition, fish oils are poor substrates for triglyceridesynthesizing enzymes, and their use leads to the formation of triglyceride-poor VLDL particles. Some clinicians have recommended fish oil rich in long chain omega-3 fatty acids (i.e., EPA and DHA) in the amount of 200 to 220 mg/kg body weight/day to assist in the management of hypertriglyceridemia, especially in dogs refractory or incompletely responsive to dietary fat restriction.
 TABLE 54-6 Level of Key Nutrients in Selected Therapeutic Commercial Foods Used for the Management of Canine Hypertriglyceridemia*
TABLE 54-6 Level of Key Nutrients in Selected Therapeutic Commercial Foods Used for the Management of Canine Hypertriglyceridemia*
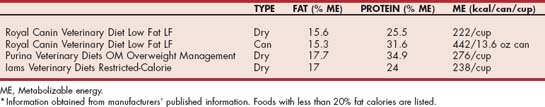
 TABLE 54-7 Level of Key Nutrients in Selected Therapeutic Commercial Foods Used for the Management of Feline Hypertriglyceridemia*
TABLE 54-7 Level of Key Nutrients in Selected Therapeutic Commercial Foods Used for the Management of Feline Hypertriglyceridemia*
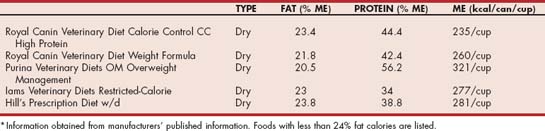
Treatment with drugs, all of which have the potential for toxicity, should be undertaken with particular care. In general, drugs should not be used in animals whose serum triglyceride concentration is less than 500 mg/dl. Several classes of drugs are used to treat hypertriglyceridemia in humans; however, there are few reports of their use in cats and dogs. Until there are further studies evaluating the dose, effect, and toxicity, drug therapy is indicated only in animals that have clinical signs associated with severe elevations in triglyceride concentrations that cannot be ameliorated by dietary therapy, which is very uncommon in one of the author’s (SJD) clinical experience.
Niacin (100 mg/day in dogs) reduces serum triglyceride concentrations by decreasing fatty acid release from adipocytes and reducing the production of VLDL particles. Adverse effects are frequent, mainly because of the associated release of the prostaglandin prostacyclin, and include vomiting, diarrhea, erythema, pruritus, and abnormalities in liver function tests. Fibric acid derivatives (clofibrate, bezafibrate, gemfibrozil, ciprofibrate, fenofibrate) lower plasma triglyceride concentrations by stimulating lipoprotein lipase activity, in addition to reducing the free fatty acid concentration, which decreases the substrate for VLDL synthesis. In humans the fibrates generally lower plasma triglyceride concentrations by 20% to 40%. Gemfibrozil has been used in the dog (200 mg/day) and cat (10 mg/kg q12h). Reported adverse effects include abdominal pain, vomiting, diarrhea, and abnormal liver function tests. The statins (lovastatin, simvastatin, pravastatin, fluvastatin, cerivastatin, atorvastatin) are hydroxymethyl-glutaryl coenzyme A (HMG-CoA) reductase inhibitors and therefore primarily suppress cholesterol metabolism. As a consequence of lower intracellular cholesterol concentrations, the hepatic LDL receptor is upregulated, thereby increasing the removal and clearance of LDL (VLDL remnant particles) from the circulation. In addition, the statins decrease hepatic production of VLDL. In humans the statins can lower triglyceride concentrations by 10% to 15%. Adverse effects include lethargy, diarrhea, muscle pain, and hepatotoxicity.
Hypercholesterolemia is most likely associated with the presence of an underlying disease and generally resolves with control of the altered metabolic state. Unlike the situation with humans, hypercholesterolemia rarely poses a health risk to the dog or cat. Specific therapy is indicated only for those animals with a prolonged marked increase in the serum cholesterol concentration (i.e., more than 800 mg/dl) that may be associated with the development of atherosclerosis. Nutritional therapy with a lower-fat diet is the initial treatment of choice for severe hypercholesterolemia. The addition of soluble fiber to the diet may also help to reduce plasma cholesterol concentrations by as much as 10%. Soluble fiber interferes with the enteric reabsorption of bile acids. Consequently, the liver uses cholesterol to increase the synthesis of bile acids.
Pharmacologic agents that can be considered for the management of severe hypercholesterolemia include bile acid sequestrates, HMG-CoA reductase inhibitors, and probucol. Bile acid sequestrates are ion exchange resins that interrupt the enterohepatic circulation of bile acids. Decreased reabsorption of bile acids stimulates the liver to synthesize bile acids, utilizing intrahepatic cholesterol. Depletion of intrahepatic cholesterol stores stimulates the hepatic LDL receptor to increase the removal of LDL and HDL particles from the circulation. Cholestyramine (1 to 2 g, administered orally q12h) is effective for lowering cholesterol concentrations; however, its use has been associated with constipation, it interferes with the absorption of several oral medications, and it may increase hepatic VLDL synthesis, resulting in an increase in plasma triglyceride concentrations. It may also increase the dietary requirement for sulfur amino acids because they serve as precursors for taurine synthesis in the dog, which conjugates bile acids exclusively with taurine. In cats the requirement for dietary taurine may be similarly increased. HMG-CoA reductase is the rate-limiting enzyme for cholesterol synthesis. The HMG-CoA reductase inhibitors (lovastatin, simvastatin, pravastatin, fluvastatin, cerivastatin, and atorvastatin) are the most potent cholesterol-lowering agents and in humans may reduce cholesterol concentrations by 20% to 40%. Lovastatin (10 to 20 mg, administered orally q24h) may be tried in dogs with persistent, severe idiopathic hypercholesterolemia that does not respond to diet alone. Potential adverse effects include lethargy, diarrhea, muscle pain, and hepatotoxicity. Lovastatin should not be administered to dogs with hepatic disease. Probucol is a cholesterol-lowering agent whose mechanism of action is not completely clear. Probucol is not widely recommended for the management of hypercholesterolemia because its effect on lowering cholesterol concentrations is variable and it has been associated with the development of arrhythmias.
Burkholder WJ. Body composition of dogs determined by carcass composition analysis, deuterium oxide dilution, subjective and objective morphometry and bioelectrical impedance. Blacksburg, Va: Virginia Polytechnic Institute and State University, 1994.
Burkholder WJ, et al. Foods and techniques for managing obesity in companion animals. J Am Vet Med Assoc. 1998;212:658.
Butterwick R, et al. A study of obese cats on a calorie-controlled weight reduction programme. Vet Rec. 1994;134:372.
Butterwick R, et al. Changes in the body composition of cats during weight reduction by controlled dietary energy restriction. Vet Rec. 1996;138:354.
Butterwick R, et al. Effect of amount and type of dietary fiber on food intake in energy-restricted dogs. Am J Vet Res. 1997;58:272.
Center SA, et al. The clinical and metabolic effects of rapid weight loss in obese pet cats and the influence of supplemental oral l-carnitine. J Vet Intern Med. 2000;14:598.
Edney AT, et al. Study of obesity in dogs visiting veterinary practices in the United Kingdom. Vet Rec. 1986;188:391.
Hawthorne AJ, et al. Predicting the body composition of cats: development of a zoometric measurement for estimation of percentage body fat in cats. J Vet Intern Med. 2000;14:365.
Kealy RD, et al. Effects of diet restriction on life span and age-related changes in dogs. J Am Vet Med Assoc. 2002;220:1315.
Mason E. Obesity in pet dogs. Vet Rec. 1970;86:612.
Mayer J. Obesity. In: Goodhart R, et al, editors. Modern nutrition in health and disease. Philadelphia: Lea & Febiger, 1973.
Scarlett JM, et al. Overweight cats—prevalence and risk factors. Int J Obes. 1994;18(1):S22.
Scarlett JM, et al. Associations between body condition and disease in cats. J Am Vet Med Assoc. 1998;212:1725.
Sloth C. Practical management of obesity in dogs and cats. J Small Anim Pract. 1992;33:178.
Barrie J, et al. Quantitative analysis of canine plasma lipoproteins. J Small Anim Pract. 1993;34:226.
Bauer JE. Evaluation and dietary considerations in idiopathic hyperlipidemia in dogs. J Am Vet Med Assoc. 1995;206:1684.
Bhatnagar D. Lipid-lowering drugs in the management of hyperlipidaemia. Pharmacol Ther. 1998;79:205.
Jacobs RM, et al. Effects of bilirubinemia, hemolysis, and lipemia on clinical chemistry analytes in bovine, canine, equine, and feline sera. Can Vet J. 1992;33:605.
Jones BR. Inherited hyperchylomicronaemia in the cat. J Small Anim Pract. 1993;34:493.
Jones BR, et al. Peripheral neuropathy in cats with inherited primary hyperchylomicronaemia. Vet Rec. 1986;119:268.
Schenck P. Canine hyperlipidemia: causes and nutritional management. In: Pibot P, et al, editors. Encyclopedia of canine clinical nutrition. Aimargines, France: Aniwa SAS on behalf of Royal Canin, 2006.
Watson TDG, et al. Lipoprotein metabolism and hyperlipidaemia in the dog and cat: a review. J Small Anim Pract. 1993;34:479.
Whitney MS, et al. Ultracentrifugal and electrophoretic characteristics of the plasma lipoproteins of miniature schnauzer dogs with idiopathic hyperlipoproteinemia. J Vet Intern Med. 1996;7:253.
 BOX 54-2
BOX 54-2