CHAPTER 75 Cytology
GENERAL CONSIDERATIONS
Evaluation of a cytologic specimen obtained by fine-needle aspiration (FNA) in small animals with suspected neoplastic lesions often yields information that can be used to make a definitive diagnosis, thereby circumventing the immediate need to perform a surgical biopsy. At our hospital, almost every mass or enlarged organ is evaluated cytologically before a surgical biopsy is performed because the risks and costs associated with FNA are considerably lower than those associated with surgical biopsy. Quite frequently, a definitive cytologic diagnosis allows for the clinician to institute a specific treatment (i.e., multicentric lymphoma treated with chemotherapy).
In a recent study of 269 cytologic specimens from dogs, cats, horses, and other animal species, the cytologic diagnosis completely agreed with the histopathologic diagnosis in approximately 40% of cases and partially agreed in 18% of the cases; complete agreement ranged from 33% to 66%, depending on the lesion and location, and was highest for skin/subcutaneous lesions and for neoplastic lesions (Cohen et al). Clinically applicable diagnostic cytologic techniques are summarized in this chapter, with emphasis on sample collection and the cursory interpretation of the specimens. Although some clinicians are able to obtain sufficient diagnostic information, a board-certified veterinary clinical pathologist should always evaluate a cytologic specimen before any prognostic or therapeutic decisions are made.
FINE-NEEDLE ASPIRATION
In FNA a single cell suspension is obtained using a small-gauge needle (i.e., 23 to 25 gauge) of the appropriate length for the desired target organ or mass; this needle can be coupled to a 12- or 20-ml sterile, dry plastic syringe, but frequently this is not necessary. Tissues easily accessible using this technique include the skin and subcutis, deep and superficial lymph nodes, spleen, liver, kidneys, lungs, thyroid, prostate, and intracavitary masses of unknown origin (e.g., mediastinal mass).
If the clinician is aspirating superficial masses, sterile preparation of the site is not necessary. However, clipping and sterile surgical preparation should always be done when aspirating organs or masses within body cavities. Once the mass or organ has been identified by palpation or radiography, it should be manually isolated; manual isolation is not necessary when performing ultrasound-, computed tomography (CT)–, or fluoroscopy-guided FNAs. A needle, either by itself or coupled to a syringe, is then introduced into the mass or organ; if the “needle alone” technique is used, the needle is reinserted into the tissue/mass several times. If the needle-syringe technique is used, suction is applied to the syringe three or four times. If the size of the mass or lesion allows it, the needle is then redirected two or three times and the procedure is repeated. Before withdrawing the needle and syringe, the clinician should release the suction so as not to aspirate blood that would contaminate the sample or air that would make the sample irretrievable from the barrel of the syringe. The needle is then detached, air is aspirated into the syringe, the needle is recoupled, and the sample is expelled onto a glass slide. In most cases no material is seen in the syringe, and the amount of cells present within the hub of the needle is usually adequate to obtain four to eight good-quality smears. When the clinician is using the “needle alone” technique, the mass or lesion is isolated as described, and the needle is inserted into the lesion four to six times. This allows the clinician to core out small samples, which will be completely contained within the hub of the needle. Once a sample has been obtained, a clean disposable syringe is loaded with air and coupled to the needle, and the specimen is then gently expelled onto slides.
An aspiration gun (or handle) facilitates the acquisition of specimens by FNA, particularly in hard-to-reach areas such as a solitary small mass in the abdominal cavity. A 12- or 20-ml AspirGun (The Everest Co., Linden, N.J.), which easily fits onto a Monoject syringe, can be used.
Superficial ulcerated masses can easily be sampled by scraping their surface with a sterile scalpel blade, wooden tongue depressor, or gauze. Smears are then made either by touching a glass slide onto the ulcerated lesion (see the following section on impression smears) or by further scraping the surface with a tongue depressor and transferring the material thus obtained onto the slide. “Pull” smears made using two glass slides are preferable over “push” smears. Once the smears have been made, they are air-dried and stained using any of the techniques described in the next section.
IMPRESSION SMEARS
Impression smears of surgical specimens or open lesions are commonly used in practice. At our clinic, we evaluate numerous intraoperative impression smears to determine the therapeutic course to follow in a given patient.
When making impression smears from surgical specimens, the clinician first gently blots the tissue onto a gauze pad or paper towel to remove any blood or debris, then gently grasps it with forceps from one end. Touch imprints are made on a glass slide by gently touching the slide with the tissue specimen. I usually make two or three rows of impressions along the slide and then stain it. It is advisable to submit a different tissue specimen for histopathologic evaluation.
STAINING OF CYTOLOGIC SPECIMENS
Several staining techniques are practical for in-office use, including rapid Romanowsky’s (e.g., Diff-Quik; various manufacturers) and new methylene blue (NMB) stains. Most commercial laboratories use Romanowsky’s stains, such as Wright’s or Giemsa.
There are some differences between these staining techniques. Romanowsky’s stains are slightly more time consuming, but they produce better cellular detail and offer worse contrast between nucleus and cytoplasm; moreover, the smears can be permanently archived. NMB, on the other hand, is a quick stain (it takes literally seconds to stain a smear), but it is not permanent, which means that slides cannot be saved for consultation; moreover, cellular details are not as sharp as they are on Romanowsky-stained smears. In addition, because nuclear DNA and RNA stain extremely well with this technique, most cells appear to be malignant. I routinely use Diff-Quik to get both a quick appreciation of the quality of the sample and, possibly, to arrive at a tentative diagnosis. This frequently allows a tentative diagnosis to be made while the client is still in the office. The main difference between rapid hematologic stains (e.g., Diff-Quik) and Giemsa or Wright-Giemsa stains is that, in a variable proportion of canine and feline mast cell tumors, the former do not stain the granules. In addition, rapid hematologic stains do not stain granules in some large granular lymphocytes (LGLs) or in eosinophils from Greyhounds (and some Golden Retrievers).
INTERPRETATION OF CYTOLOGIC SPECIMENS
Although the clinician should strive to evaluate cytologic specimens proficiently, the ultimate cytologic diagnosis should be made by a board-certified veterinary clinical pathologist. The following are guidelines for cytologic interpretation. As a general rule, cytologic specimens are classified into one of the following six categories: normal tissue, hyperplasia/dysplasia (difficult to diagnose), inflammation, neoplasia, cystic lesions (contains fluid of various types), or mixed cellular infiltrate. The latter is usually either a malignant tumor with ongoing inflammation (e.g., squamous cell carcinoma with neutrophilic inflammation) or a hyperplastic tissue secondary to chronic inflammation (e.g., chronic cystitis with epithelial hyperplasia/dysplasia). Cytology of cystic lesions will not be discussed in this chapter.
NORMAL TISSUES
Epithelial Tissues
Most epithelial cells, particularly those of the glandular or secretory epithelium, tend to cling together (i.e., they have desmosomes), forming clusters or sheets. Individual cells are easily identifiable and are round or polygonal; nuclei and cytoplasms are well differentiated. Most cells in Romanowsky-stained smears have blue cytoplasm and round nuclei.
Mesenchymal Tissues
Cells from mesenchymal tissues (e.g., fibroblasts, fibrocytes, chondroblasts) are difficult to obtain in routine FNA material or tissue scrapings because they are usually surrounded by intercellular matrix. Mesenchymal cells are typically spindle shaped, polygonal, or oval and have irregular nuclei; cytoplasmic boundaries are usually indistinct, and cell clumps are seen rarely.
Hematopoietic Tissues
A detailed morphologic description of circulating blood cells is beyond the scope of this chapter. Briefly, however, most cells from hemolymphatic organs are round, individual cells (with no tendency to clump); they have a blue cytoplasm on Romanowsky-stained smears and a variable nuclear size; most nuclei are round or kidney shaped. Tissue such as bone marrow has cells in different stages of development (i.e., from blasts to well-differentiated circulating cells).
HYPERPLASTIC PROCESSES
Hyperplasia of different tissues commonly results in enlargement of glandular organs and lymphoid structures. The cytologic features of epithelial and lymphoid hyperplasia differ; lymphoid hyperplasia is discussed later in this chapter. Cytologically, hyperplastic changes may be difficult to recognize because they may mimic either normal or neoplastic tissues. Care should be taken when evaluating specimens from organs such as enlarged prostates or thickened urinary bladders because the high degree of hyperplasia and dysplasia frequently suggests malignancy.
INFLAMMATORY PROCESSES
Most inflammatory reactions are characterized cytologically by the presence of inflammatory cells and debris in the smear. The type of cell present depends on the etiologic agent (e.g., neutrophils in pyogenic infections, eosinophils in parasitic or allergic reactions) and the duration of the inflammatory process (i.e., acute processes are usually characterized by a predominance of granulocytes, whereas macrophages and lymphocytes predominate in chronic processes). The following pathogens are frequently identified in cytologic specimens: Histoplasma, Blastomyces, Sporothrix, Cryptococcus, Coccidioides, Aspergillus/Penicillium, Toxoplasma, Leishmania, other rickettsial agents (e.g., salmon poisoning), bacteria, and Demodex (Fig. 75-1).
MALIGNANT CELLS
The cells that make up most normal organs and tissues (with the exception of bone marrow precursors) are well differentiated in that most of them are similar in size and shape, they have a normal nuclear : cytoplasmic (N : C) ratio, the nuclei usually have condensed chromatin and no nucleoli, and the cytoplasm may exhibit features of differentiation (e.g., keratin formation in squamous epithelium).
Malignant cells have one or more of the following features (Box 75-1): a high N : C ratio (i.e., larger nucleus and smaller cytoplasm); a delicate chromatin pattern; nucleoli (usually multiple); anisokaryosis (i.e., cells have nuclei of different sizes); nuclear molding (i.e., a nucleus in a multinucleated cell is compressed by a neighboring one); morphologic homogeneity (i.e., all cells look alike); pleomorphism (i.e., cells in different stages of development); vacuolization (primarily in malignant epithelial tumors); anisocytosis (i.e., cells are of different sizes); multinucleated giant cells; and, occasionally, phagocytic activity. Another feature of malignancy is heterotopia (i.e., the presence of a given cell type where it is not found anatomically); for example, epithelial cells can appear in a lymph node only as a consequence of metastasis from a carcinoma. In addition, malignant cells tend to be morphologically different from the progenitor cell population (see Box 75-1). On the basis of the predominant cytologic features, malignancies can be classified as carcinomas (epithelial), sarcomas (mesenchymal), or round (or discrete) cell tumors (Fig. 75-2).
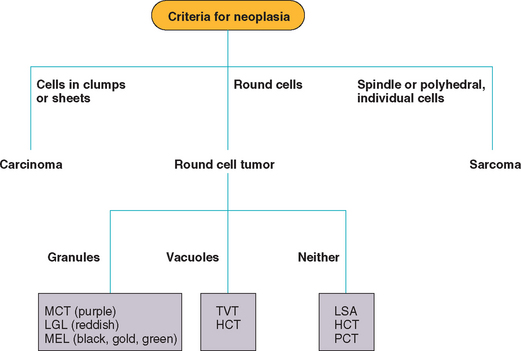
FIG 75-2 Flow chart for the cytologic diagnosis of tumors in dogs and cats. MCT, Mast cell tumor; LGL, large granular lymphoma; MEL, melanoma; TVT, transmissible venereal tumor; HCT, histiocytoma; LSA, lymphoma; PCT, plasma cell tumor.
Carcinomas
Most carcinomas are composed of round or polygonal cells that tend to cling together, forming clusters or large sheets. Their cytoplasms are usually deep blue, and in most adenocarcinomas vacuolization is evident. Cytoplasmic boundaries are difficult to recognize, and the cells resemble a mass of protoplasm rather than a sheet of individual cells. In squamous cell carcinomas cells are usually individualized, can be irregular or polygonal, have a deep blue cytoplasm (with an occasional eosinophilic fringe), and have large vacuoles; neoplastic cells in squamous cell carcinomas frequently exhibit leukophagia. Nuclei in both adenocarcinomas and squamous cell carcinomas are large, with a fine chromatin pattern and evident nucleoli (Fig. 75-3).
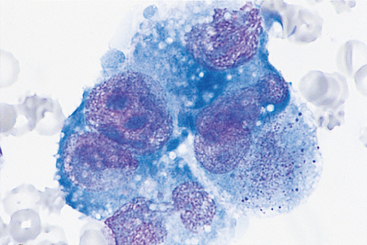
FIG 75-3 Photomicrograph of pleural fluid from an older female Irish Setter showing a cluster of deeply basophilic cells, with vacuolated cytoplasm, anisocytosis, anisokaryosis, and prominent nucleoli. The cytologic diagnosis was carcinomatosis (i.e., metastatic adenocarcinoma of unknown origin) (×1000).
Sarcomas
The cytologic features of sarcomas vary according to the histologic type. However, most mesenchymal tumors have spindle shaped, polygonal, polyhedral, or oval cells, with a reddish blue to dark blue cytoplasm and irregularly shaped nuclei. Most cells are individualized, although clumping may occur (particularly in impression smears). The cells in most sarcomas have a tendency to form “tails,” and the nuclei protrude from the cytoplasm (Fig. 75-4). The presence of spindle-shaped or polygonal cells with a vacuolated blue-gray cytoplasm is highly suggestive of hemangiosarcoma. Intercellular matrix (e.g., osteoid, chrondroid) is found occasionally; in these two tumor types the cells are usually round or ovoid. Multinucleated giant cells are common in some sarcomas in cats (Fig. 75-5).
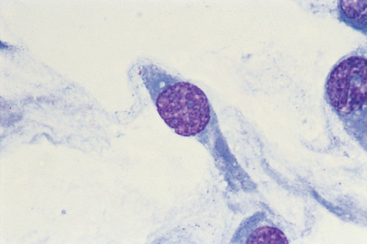
FIG 75-4 Photomicrograph of a fine-needle aspirate of a firm, lobulated, subcutaneous mass in an older dog. The cells are spindle shaped, have “tails,” and do not associate with other cells. The nuclei appear to be protruding from the cytoplasm (×1000). The cytologic diagnosis is spindle cell sarcoma. Histopathologic findings were diagnostic for fibrosarcoma.
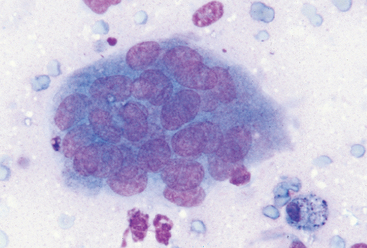
FIG 75-5 Photomicrograph of a multinucleated giant cell from a soft tissue sarcoma in a 13-year-old cat with tumor-associated hypercalcemia that resolved after surgical excision of the primary mass (×400).
As a general rule, because sarcoma cells do not exfoliate easily, aspirates of these masses may yield false-negative results. Therefore, if a mass is clinically suspected to be a sarcoma and FNA findings are negative, a core biopsy specimen of the mass should be obtained.
Round (Discrete) Cell Tumors
Tumors composed of a homogeneous population of round (or discrete) cells are referred to as round (or discrete) cell tumors (RCTs). These tumors are common in dogs and cats and include lymphoma, histiocytoma, mast cell tumor, transmissible venereal tumor, plasma cell tumor, and malignant melanoma; as discussed above, osteosarcomas and chondrosarcomas can be composed of round cells. RCTs are easily diagnosed on the basis of cytology; the presence or absence of cytoplasmic granules or vacuoles and the location of the nucleus aid in the classification of RCTs (see Fig. 75-2).
The cells that make up mast cell tumors (Fig. 75-6), LGL lymphomas (Fig. 75-7), and melanomas (Fig. 75-8) usually have cytoplasmic granules; cells in neuroendocrine tumors can also have granules. When hematologic stains are used, the granules are purple in mast cell tumors; red in LGL lymphomas; and black, green, brown, or yellow in melanomas. Lymphomas (Fig. 75-9), histiocytomas (Fig. 75-10), plasma cell tumors, and transmissible venereal tumors do not have cytoplasmic granules. Cells in osteosarcomas occasionally have small to large pink cytoplasmic granules (osteoid). Cytoplasmic vacuoles are common in transmissible venereal tumors and in histiocytomas.
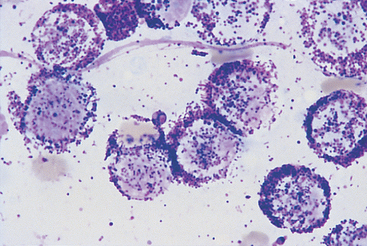
FIG 75-6 Photomicrograph of a fine-needle aspirate from a subcutaneous mass in an older Boxer with multiple dermoepidermal and subcutaneous masses and marked multifocal lymphadenopathy. Note the monomorphic population of round cells containing purple granules. The cytologic diagnosis was mast cell tumor (×1000).
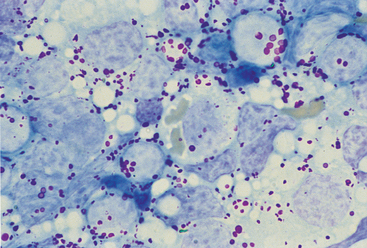
FIG 75-7 Photomicrograph of an impression smear from a mesenteric lymph node in an old cat evaluated because of vomiting and diarrhea. Note the large round cells with red, large cytoplasmic granules. The diagnosis was lymphoma of large granular lymphocytes (×1000).
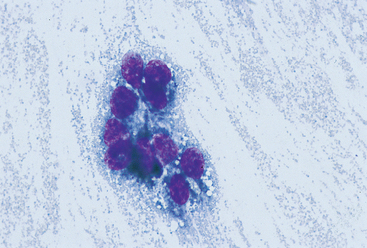
FIG 75-8 Photomicrograph of a fine-needle aspirate from a mass in the oral cavity of a 10-year-old Schnauzer. Note the dark, fine granules in the cytoplasm. The diagnosis was melanoma (×400).
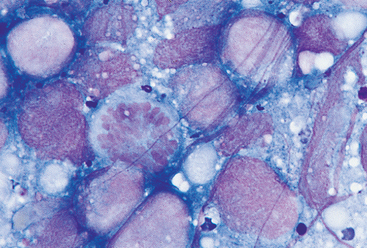
FIG 75-9 Photomicrograph of a fine-needle aspirate from the kidney of a middle-aged Boxer with bilateral renomegaly. Note the monomorphic population of round cells, with large nuclei, prominent nucleoli, and no cytoplasmic granules or vacuoles. A mitotic figure is seen in the center. The cytologic diagnosis was lymphoma (×1000).
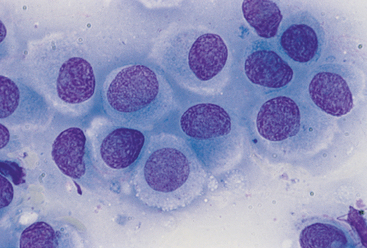
FIG 75-10 Photomicrograph of a fine-needle aspirate from a small, round, dermoepidermal mass in the head of a 1-year-old dog. Note the large round cells with abundant clear cytoplasm and fine chromatin pattern. The diagnosis was histiocytoma (×1000).
Briefly, large cell lymphomas are characterized by a monomorphic population of individual undifferentiated round cells with large nuclei, a coarse chromatin pattern, and one or two nucleoli; occasional cells may be vacuolated (see Fig. 75-9). Small and intermediate cell lymphomas may be difficult to recognize cytologically because the neoplastic population may resemble normal lymphocytes. Cells in histiocytomas are similar to those in lymphomas except that the chromatin pattern is fine rather than coarse, they have more abundant cytoplasm, and they are frequently vacuolated (see Fig. 75-10). Because inflammation is an important component of histiocytomas, inflammatory cells (i.e., neutrophils, lymphocytes) are commonly found in these tumors. Mast cell tumors are distinctive in that the cytoplasm of the cells contains purple (metachromatic) granules, which can be so numerous as to obscure the nuclear features; eosinophils are also a common feature in these tumors. Mast cell granules may be absent in poorly differentiated tumors or in tumors stained with Diff-Quik.
LYMPH NODES
Cytologic evaluation of lymph node aspirates is commonly done in practice. At our clinic, a cytologically based diagnosis is obtained in approximately 90% of dogs and 60% to 75% of cats with lymphadenopathy. If the cytologic findings of an enlarged lymph node are inconclusive, the node should be surgically excised and submitted for histopathologic evaluation.
When evaluating cytologic specimens prepared from lymph node aspirates or impression smears, the clinician should keep in mind that these organs react to a variety of stimuli following a distinct pattern. In general, four cytologic patterns are recognized: normal lymph node, reactive or hyperplastic lymphadenopathy, lymphadenitis, and neoplasia.
Normal Lymph Node
Cytologic specimens from normal nodes are composed predominantly (75% to 90%) of small lymphocytes. These cells are approximately 7 to 10 μm in diameter (1 to 1.5 times the diameter of a red blood cell) and have a dense chromatin pattern and no nucleoli. The remaining cells are macrophages, lymphoblasts, plasma cells, and other immune cells.
Reactive or Hyperplastic Lymphadenopathy
Lymphoid tissues reacting to different antigenic stimuli (e.g., bacterial, immunologic, neoplastic, fungal) are cytologically similar in that the cell population is composed of a mixture of small, intermediate, and large lymphocytes; lymphoblasts; plasma cells; and macrophages (Fig. 75-11). In addition, other cell types may be present, depending on the specific agent (e.g., eosinophils in parasitic or allergic reactions). The first impression when evaluating a reactive or hyperplastic node cytologically is that of a heterogeneous population of cells. The presence of cells in different stages of development indicates that the lymphoid tissue is undergoing polyclonal expansion (i.e., response to multiple antigens). Reactive lymph nodes in cats frequently lack plasma cells but contain large numbers of lymphoblasts, so they may be difficult to distinguish from lymphoma.
Lymphadenitis
Inflammatory processes affecting the lymph nodes produce cytologic changes similar to the ones seen in reactive lymphadenopathy, although there is a profusion of inflammatory cells (e.g., neutrophils in suppurative infections) and degenerative changes (e.g., pyknosis, karyorrhexis) in most cell lines. The etiologic agents may be visualized.
Neoplasia
Neoplastic cells can appear in a lymph node either as a result of lymphatic or vascular dissemination (i.e., metastasis from a primary tumor distal to the node) or as a primary process affecting these structures (i.e., lymphomas). Cytologic features of metastatic lymph node lesions consist of a reactive pattern and the presence of neoplastic cells; in advanced metastatic lesions it is frequently difficult to identify normal lymphoid cells. The morphology of the metastatic cells depends on the primary tumor type. As discussed in the preceding section, lymphomas are characterized by a monomorphous population of large, immature lymphoid cells; these cells are usually large and have an abnormally low N:C ratio, coarse chromatin, and evident nucleoli (see Fig. 75-9). As discussed previously, small cell lymphomas are difficult to diagnose cytologically.
Baker R, et al. Color atlas of cytology of the dog and cat. St Louis: Mosby, 2000.
Ballegeer EA, et al. Correlation of ultrasonographic appearance of lesions and cytologic and histologic diagnoses in splenic aspirates from dogs and cats: 32 cases (2002–2005). J Am Vet Med Assoc. 2007;230:690.
Barton CL. Cytologic diagnosis of cutaneous neoplasia: an algorithmic approach. Compend Contin Educ. 1987;9:20.
Bertazzolo W, et al. Canine angiosarcoma: cytologic, histologic, and immunohistochemical correlations. Vet Clin Pathol. 2005;34:28.
Bonfanti U, et al. Diagnostic value of cytologic examination of gastrointestinal tract tumors in dogs and cats: 83 cases (2001–2004). J Am Vet Med Assoc. 2006;229:1130.
Cohen M, et al. Evaluation of sensitivity and specificity of cytologic examination: 269 cases (1999-2000). J Am Vet Med Assoc. 2003;222:964.
Cowell RL, et al. Diagnostic cytology and hematology of the dog and cat, ed 3. St Louis: Elsevier, 2007.
Ghisleni G, et al. Correlation between fine-needle aspiration cytology and histopathology in the evaluation of cutaneous and subcutaneous masses from dogs and cats. Vet Clin Pathol. 2006;35:24.
Mills JN. Lymph node cytology. Vet Clin North Am. 1989;19:697.
Morrison WB, et al. Advantages and disadvantages of cytology and histopathology for the diagnosis of cancer. Semin Vet Med Surg. 1993;8:222.
Powe JR, et al. Evaluation of the cytologic diagnosis of canine prostatic disorders. Vet Clin Pathol. 2004;33:150.
Radin MJ, et al. Interpretation of canine and feline cytology. Wilmington, Del: Gloyd Group, 2001.
Raskin RE, et al. Atlas of canine and feline cytology. Philadelphia: WB Saunders, 2001.
Sharkey LC, et al. Maximizing the diagnostic value of cytology in small animal practice. Vet Clin N Am Small Anim Pract. 2007;37:351.
Stockhaus C, et al. A multistep approach in the cytologic evaluation of liver biopsy samples of dogs with hepatic diseases. Vet Pathol. 2004;41:461.
Vignoli M, et al. Computed tomography-guided fine-needle aspiration and tissue-core biopsy of bone lesions in small animals. Vet Radiol Ultrasound. 2004;45:125.
Wang KY, et al. Accuracy of ultrasound-guided fine-needle aspiration of the liver and cytologic findings in dogs and cats: 97 cases (1990-2000). J Am Vet Med Assoc. 2004;224:75.
Wellman ML. The cytologic diagnosis of neoplasia. Vet Clin N Am. 1990;20:919.
 BOX 75-1
BOX 75-1