CHAPTER 98 Polysystemic Mycotic Infections
BLASTOMYCOSIS
Etiology and Epidemiology
Blastomyces dermatitidis is a saprophytic yeast found primarily in the Mississippi, Missouri, and Ohio River valleys; the mid-Atlantic states; and southern Canada. Two cases in human beings have been reported in Colorado. An extracellular yeast form (5 to 20 μm in diameter) with broad-based budding develops in the vertebrate host (Table 98-1). The infectious mycelial phase occurs in the soil and in culture.
 TABLE 98-1 Morphologic Appearance of Systemic Canine and Feline Fungal Agents
TABLE 98-1 Morphologic Appearance of Systemic Canine and Feline Fungal Agents
| AGENT | CYTOLOGIC APPEARANCE |
|---|---|
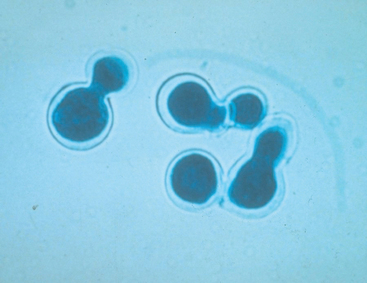
| Blastomyces dermatitidis | Extracellular yeast, 5 to 20 μm in diameter; thick, refractile, double-contoured wall; broad-based bud; routine stains are adequate |
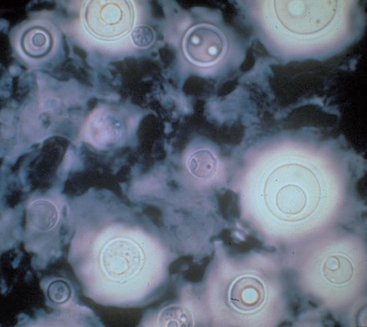
| Cryptococcus neoformans | Extracellular yeast, 3.5 to 7.0 μm in diameter; thick, unstained capsule; thin-based bud; violet color with light-red capsule with Gram stain; unstained capsule with India ink |
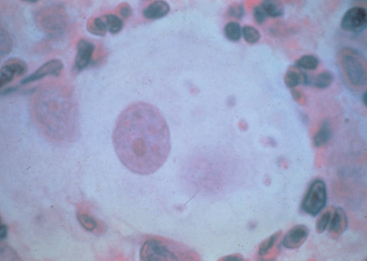
| Coccidiodes immitis | Extracellular spherules (20 to 200 μm in diameter) containing endospores; deep red to purple double outer wall with bright red endospores with PAS stain |
| Histoplasma capsulatum | Intracellular yeast in mononuclear phagocytes, 2 to 4 μm in diameter, basophilic center with lighter body with Wright’s stain |
| Sporothrix schenckii | Intracellular yeast in mononuclear phagocytes, 2 to 3 μm × 3 to 6 μm in diameter; round, oval, or cigar shaped |
Blastomycosis develops most frequently in areas exposed to high humidity, fog, excavation sites, and sandy, acidic soils near bodies of water. Potential for disease may vary with the virulence of the field strain, the inoculum dose, and the immune status of the host. Most clinical cases occur from point source exposure; multiple cases are diagnosed in an area, and clusters of infection in people and dogs have been reported (MacDonald et al., 2006).
Transmission is from inhalation or contamination of open wounds with spores from the environment. The organism probably replicates in the lungs initially and then spreads hematogenously to other tissues, including the skin and subcutaneous tissues, eyes, bones, lymph nodes, external nares, brain, testes, nasal passages, prostate, liver, mammary glands, vulva, and heart. The organism can be swallowed and passed in feces. Incomplete clearance of the organism by individuals with poor cell-mediated immune responses results in pyogranulomatous inflammation in affected organs, which can cause clinical signs of disease. Subclinical infection is believed to be uncommon in dogs and cats.
Clinical Features
Large-breed, young, male, sporting dogs are infected most commonly by B. dermatitidis most likely because of an increased chance for exposure to the organism. Anorexia, cough, dyspnea, exercise intolerance, weight loss, ocular disease, skin disease, depression, lameness, and syncope are the most common presenting complaints.
Fever occurs in approximately 40% of affected dogs. Interstitial lung disease and hilar lymphadenopathy result in cough, dry and harsh lung sounds, and dyspnea; hypertrophic osteopathy occurs in some dogs. Dyspnea from chylothorax caused by cranial vena cava syndrome has been described. Valvular endocarditis occurs as well, and conduction disturbances from myocarditis are detected in some dogs with cardiac blastomycosis (Schmiedt et al., 2006). Lymphadenopathy and cutaneous or subcutaneous nodules, abscesses, plaques, or ulcers occur in 20% to 40% of infected dogs. Splenomegaly is common. Lameness from fungal osteomyelitis of the spine or appendicular skeleton occurs in approximately 30% of dogs with blastomycosis. Infection of the testes, prostate, urinary bladder, and kidneys occurs rarely.
Ocular manifestations are recognized in approximately 30% of dogs with blastomycosis; anterior uveitis, endophthalmitis, posterior segment disease, and optic neuritis occur. Cataracts can result from chronic inflammation or rupture of the lens capsule (Hendrix et al., 2004). Depression and seizures from diffuse or multifocal central nervous system (CNS) involvement occur in some dogs.
Blastomycosis can occur in any cat but is most common in young males. Cats housed indoors and cats allowed outdoors have both been infected (Blondin et al., 2007). Infected cats develop respiratory tract disease, CNS disease, regional lymphadenopathy, dermatologic disease, ocular disease, gastrointestinal tract disease, and urinary tract disease. Pleural or peritoneal effusion resulting in dyspnea or abdominal distension occurs in some cats. Ocular disease usually involves the posterior segment.
Diagnosis
Hematologic abnormalities commonly identified in dogs or cats with blastomycosis are normocytic normochromic nonregenerative anemia, lymphopenia, and neutrophilic leukocytosis with or without a left shift and monocytosis. Hypoalbuminemia and hyperglobulinemia (i.e., polyclonal gammopathy) caused by chronic inflammation are common serum biochemical abnormalities; hypercalcemia occurs rarely in dogs. Most infected dogs and cats with respiratory disease have diffuse, miliary, or nodular interstitial lung patterns and intrathoracic lymphadenopathy on thoracic radiographs (Fig. 98-1); single masses and pleural effusion from chylothorax sometimes occur. Alveolar lung disease occurs in some cats (Gilor et al., 2006). Bone lesions induced by blastomycosis are lytic with a secondary periosteal reaction and soft tissue swelling.
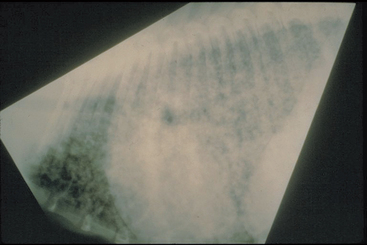
FIG 98-1 Miliary interstitial lung pattern consistent with blastomycosis in a dog.
(Courtesy Dr. Lynelle Johnson, College of Veterinary Medicine, University of California, Davis.)
Serum antibodies develop in some infected animals. Many cats with blastomycosis are negative for serum antibodies by agar gel immunodiffusion (AGID). False-negative results can occur in animals with peracute infection, immunosuppression, or advanced infection that overwhelms the immune system. Antibody titers do not always revert to negative after successful treatment. Because blastomycosis rarely causes subclinical infection, positive serologic results combined with appropriate clinical signs and radiographic abnormalities allow presumptive diagnosis if the organism cannot be demonstrated. Blastomyces antigens and serum antibodies were detected simultaneously in 36 dogs with confirmed infection (Shurley et al., 2005). However, the assay was not specific for Blastomyces. Definitive diagnosis of blastomycosis is based on cytologic, histopathologic, or culture demonstration of the organism (Fig. 98-2). Impression smears from skin lesions and aspirates from enlarged lymph nodes and focal lung lesions usually reveal pyogranulomatous inflammation and organisms that can usually be seen at low power. Recovery of organisms from urine is less consistent. Bronchoalveolar lavage is more sensitive than transtracheal wash for organism demonstration; organisms can also be found in samples obtained by percutaneous lung aspirates. Growth in culture requires 10 to 14 days and is of lower yield than cytology or biopsy.
Treatment
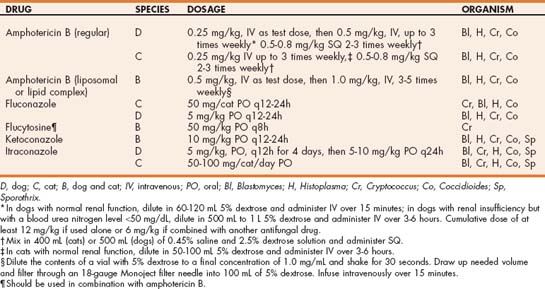
Amphotericin B, ketoconazole, both amphotericin B and ketoconazole, and itraconazole alone are used most frequently for the treatment of blastomycosis in dogs (Table 98-2). Amphotericin B is generally used in animals with life-threatening disease; the lipid or liposomal encapsulated product is less likely to cause toxicity. If regular amphotericin B is used, the animal should be well hydrated with 0.9% sodium chloride before treatment, and treatment should be discontinued if the blood urea nitrogen level exceeds 50 mg/dL. Because itraconazole is as effective as amphotericin B and ketoconazole alone or in combination and has fewer adverse effects, it is the drug of choice for the treatment of blastomycosis (see Table 98-2). Dogs should be treated with 5 mg/kg/day twice daily for the first 5 days and then 5 mg/kg. Treatment should be continued for 60 to 90 days or for 4 weeks beyond resolution of measurable disease (i.e., thoracic radiographic abnormalities or skin lesions). Fluconazole can also be used and may be effective for CNS, ocular, and urinary system blastomycosis.
 TABLE 98-2 Antifungal Drugs Used in the Management of the Systemic Canine and Feline Fungal Diseases
TABLE 98-2 Antifungal Drugs Used in the Management of the Systemic Canine and Feline Fungal Diseases
Relapses occur in 20% to 25% of treated dogs. When they occur a complete course of therapy should be reinstituted. Posterior segment ocular disease responds well to itraconazole, but anterior uveitis and endophthalmitis often require enucleation of the affected eye. In dogs with ocular blastomycosis resulting in euthanasia or enucleation of the affected eye, difference in the presence of the organism was not noted between treated and untreated dogs (Hendrix et al., 2004). In one study of 23 cats with blastomycosis, successful results were reported for two cats treated with amphotericin B and ketoconazole, one cat treated with amputation, and one cat treated with potassium iodide (Miller et al., 1990). In a more recent study of eight cats, two cats treated with itraconazole and one cat treated with fluconazole had clinical resolution of their disease (Gilor et al., 2006).
Zoonotic Aspects and Prevention
Direct zoonotic transmission from infected animals is unlikely because the yeast phase is not as infectious as the mycelial phase. One veterinarian was infected after material from a pulmonary aspirate from an infected dog was injected intramuscularly, and another developed disease after being bitten by an infected dog. The mycelial phase develops at temperatures lower than body temperature; positive cultures and contaminated bandages are infectious. Multiple reports have been made of canine and human blastomycosis that developed from the same environment exposure. Decreasing potential for exposure by avoiding lakes and creeks in endemic areas is the only way to prevent the disease.
COCCIDIOIDOMYCOSIS
Etiology and Epidemiology
Coccidioides immitis is a dimorphic fungus found deep in sandy alkaline soils in regions with low elevation, low rainfall, and high environmental temperatures, including the southwestern United States, California, Mexico, Central America, and South America. In the United States coccidioidomycosis is diagnosed most frequently in California, Arizona, New Mexico, Utah, Nevada, and southwest Texas. The environmental mycelial phase produces arthrospores (2 to 4 μm wide, 3 to 10 μm long) that enter the vertebrate host by inhalation or wound contamination. Large numbers of arthrospores return to the surface after periods of rainfall and are dispersed by the wind; the prevalence of coccidioidomycosis increases in the years after a high rainfall. Most cases (67%) of feline coccidioidomycosis are diagnosed between December and May. In one study of dogs residing in an endemic area (Arizona), the cumulative probability of infection (evidenced by seroconversion) by 2 years of age was 28%, and the cumulative probability of clinical infection by 2 years of age was 6% (Shubitz et al., 2005).
Inhaled arthrospores induce neutrophilic inflammation followed by infiltrates of histiocytes, lymphocytes, and plasma cells. Infection is cleared if cell-mediated immune responses are normal; most people, dogs, and cats exposed to the organism are subclinically affected. The organism disseminates to mediastinal and tracheobronchial lymph nodes, bones and joints, visceral organs (liver, spleen, kidneys), heart and pericardium, testicles, eyes, brain, and spinal cord of some individuals. Spherules (20 to 200 μm in diameter) containing endospores (see Table 98-1) form in tissues of infected hosts. Endospores are released by cleavage and produce new spherules. Respiratory signs and signs of disseminated disease occur 1 to 3 weeks and 4 months after exposure, respectively.
Clinical Features
Clinical disease in dogs is most common in young, male, large-breed dogs. Dogs that are allowed to roam or walk in the desert in endemic areas are most likely to be exposed (Butkiewicz et al., 2005). Approximately 90% of clinically affected dogs have lameness with swollen, painful bones or joints. Cough, dyspnea, anorexia, weakness, weight loss, lymphadenopathy, lameness, clinical signs of ocular inflammation, and diarrhea are other presenting complaints. Crackles, wheezes, or muffled lung sounds from pleural effusion are common. Restrictive pericarditis presenting with evidence of right heart failure, such as hepatomegaly, pleural effusion, and ascites, can occur (Heinritz et al., 2005). If subcutaneous abscesses, nodules, ulcers, and draining tracts occur, they are usually associated with infected bones. Myocarditis, icterus, renomegaly, splenomegaly, hepatomegaly, orchitis, epididymitis, keratitis, iritis, granulomatous uveitis, and glaucoma are detected in some dogs. Depression, seizures, ataxia, central vestibular disease, cranial nerve deficits, and behavioral changes are the most common signs of CNS infection.
The median age of cats with coccidioidomycosis is 5 years; no obvious sex or breed predilection exists. The most common clinical manifestations include skin disease (56%), respiratory disease (25%), musculoskeletal disease (19%), and either ophthalmic or neurologic disease (19%) (Greene et al., 1995).
Diagnosis
Normocytic, normochromic nonregenerative anemia; leukocytosis; leukopenia; and monocytosis are the most common hematologic abnormalities. Hyperglobulinemia (i.e., polyclonal gammopathy), hypoalbuminemia, renal azotemia, and proteinuria occur in some infected animals (Johnson et al., 2003).
Diffuse interstitial lung patterns are more common than bronchial, miliary interstitial, nodular interstitial, or alveolar patterns radiographically in dogs and cats with respiratory coccidioidomycosis. Pleural effusion from pleuritis, right-sided heart failure, or constrictive pericarditis can occur. Hilar lymphadenopathy is common in dogs and cats; however, sternal lymphadenopathy or calcification of lymph nodes is not. Bone lesions usually involve the distal diaphysis, epiphysis, and metaphysis of one or more long bones, and they are more proliferative than lytic.
Serum antibodies are detected by complement fixation (CF), AGID, and tube precipitin (TP) tests; TP detects immunoglobulin (Ig) M antibodies; CF and AGID detect IgG antibodies. False-negative results can occur in dogs and cats with early infections (less than 2 weeks), chronic infection, rapidly progressive acute infection, and primary cutaneous coccidioidomycosis. False-positive results in the CF test can occur as a result of anticomplementary serum, which may be caused by bacterial contaminants or immune complexes. The assays can cross-react with antibodies against H. capsulatum and B. dermatitidis. Serum antibodies develop in dogs with and without clinical signs of disease, and titer magnitude failed to correlate with the presence of illness in one study (Shubitz et al., 2005). Thus results of antibody test results alone should not be used to make a definitive diagnosis. The combination of positive serologic test results and radiographic signs of interstitial lung disease, dermatologic disease, or osteomyelitis in animals from endemic areas can be used to make a presumptive diagnosis if the organism cannot be demonstrated. Titers may persist for months to years after resolution of clinical disease.
Definitive diagnosis requires demonstration of the organism by cytology, biopsy, or culture. The organism is often difficult to demonstrate by cytology; transtracheal aspiration or bronchoalveolar lavage is commonly negative. Extracellular spherules (Fig. 98-3) are most commonly found in lymph node aspirates, draining masses, and pericardial fluid; wet mount examination of unstained smears or periodic acid–Schiff-stained smears are more suitable than are dry mounts.
Treatment
Ketoconazole is the drug of choice for treatment of coccidioidomycosis in dogs (see Table 98-1), but it commonly leads to inappetence, vomiting, diarrhea, weight loss, and increases in liver enzyme activities in some dogs and cats. In dogs, long-term use of ketoconazole can suppress testosterone and cortisol production and has been associated with cataracts. Amphotericin B should be used if life-threatening disease is present or if response to ketoconazole is poor. Itraconazole can be used in animals with toxicity from ketoconazole.
Fluconazole should be used for animals with meningoencephalitis. Cats and dogs should be treated for 60 to 90 days or until clinical illness has been resolved for at least 1 month. Bone infections are often incurable; therefore repeated treatments are often required. When treated with ketoconazole, itraconazole, or fluconazole, 32 of 44 cats with coccidioidomycosis were asymptomatic during or after treatment (Greene et al., 1995). Relapse occurred in 11 cats during or after treatment. Daily administration of lufenuron, a chitin synthesis inhibitor, has been evaluated in a limited number of dogs with coccidioidomycosis but should not be used in lieu of azoles.
Zoonotic Aspects and Prevention
People exposed to C. immitis develop asymptomatic infection or mild, transient respiratory signs. The organism is not transmitted from infected animals to people. However, the mycelial phase occurs outside the vertebrate host, so fomites, such as bandage material and cultures, should be handled carefully. Avoiding endemic areas is the only way to prevent the disease.
CRYPTOCOCCOSIS
Etiology and Epidemiology
Cryptococcus neoformans is a 3.5- to 7.0-μm yeastlike organism with worldwide distribution. It has a thick polysaccharide capsule and reproduces by narrow-based budding (see Table 98-1). Cryptococcus neoformans var grubii (serotype A) and C. neoformans var gattii (serotype B) are most commonly associated with disease. Clinical findings with either infection are similar. Many cases have been described in southern California and the eastern coast of Australia. An outbreak of Cryptococcus spp. infections recently occurred in people, dogs, cats, ferrets, and a bird in British Columbia (Lester et al., 2004; MacDougall et al., 2007). Most cases were on Vancouver Island and were caused by C. gattii. The organisms are acquired from the environment; risk factors significantly associated with infection in animals in the British Columbia outbreak included living near a site of soil disturbance such as logging sites, having an above-average level of activity, hunting, and having owners that hiked or visited a botanic garden (Duncan et al., 2006).
The route of transmission for C. neoformans is believed to be inhalation. Nasal and pulmonary disease manifestations are common; however, based on culture and serologic studies of healthy animals, an inapparent carrier state also occurs (Malik et al., 1997; Duncan et al., 2005a, 2005b). The organism probably spreads to extrapulmonary sites hematogenously; the CNS may also be infected by direct extension across the cribriform plate from the nasal cavity. Immunity is cell mediated; individuals with incomplete responses do not completely remove the organism, thus resulting in granulomatous lesions. The polysaccharide capsule of the organism inhibits plasma cell function, phagocytosis, leukocyte migration, and opsonization, potentiating infection.
Cryptococcus spp. can be primary pathogens. However, preexisting immunosuppressive conditions are documented in approximately 50% of people with cryptococcosis. Serologic evidence of coinfection with feline immunodeficiency virus or feline leukemia virus occurs in some cats with cryptococcosis. Potentially immunosuppressive conditions such as administration of corticosteroids, ehrlichiosis, heartworm disease, and neoplasia are identified in a small percentage of dogs with cryptococcosis.
Clinical Features
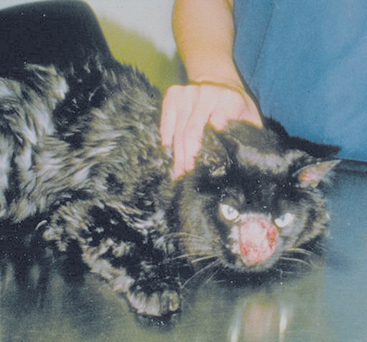
Cryptococcosis is the most common systemic fungal infection of cats and should be considered a differential diagnosis for cats with clinical evidence of upper or lower respiratory tract disease, subcutaneous nodules, lymphadenopathy, intraocular inflammation, fever, or CNS disease. All ages of cats have been infected, but young cats are generally overrepresented. In one study in Australia, Siamese, Himalayan, and Ragdoll breeds were overrepresented (O’Brien et al., 2004). Infection of the nasal cavity, resulting in sneezing and nasal discharge (Fig. 98-4), is reported most frequently. The nasal discharge can be unilateral or bilateral, range from serous to mucopurulent, and often contains blood. Granulomatous lesions extruding from the external nares, facial deformity over the bridge of the nose, and ulcerative lesions on the nasal planum are common. Mandibular lymphadenopathy is detected in most cats with rhinitis. The nasopharynx is the primary site of involvement in some infected cats and dogs, resulting in snoring and stertor as the predominant clinical signs. C. gattii has also been detected in pleural effusion (Barrs et al., 2005).
Single or multiple, small (less than 1 cm), cutaneous or subcutaneous masses also have been reported commonly in cats infected with C. neoformans. The masses can be either firm or fluctuant and have a serous discharge if ulcerated. Anterior uveitis, chorioretinitis, or optic neuritis occur in association with ocular infection; lens luxations and glaucoma are common sequelae. Chorioretinitis lesions can be punctate or large; suppurative retinal detachment occurs in some infected cats.
CNS signs of disease result from diffuse or focal meningoencephalitis or focal granuloma formation. Manifestations include depression, behavioral changes, seizures, blindness, circling, ataxia, loss of sense of smell, and paresis depending on the location of the lesion; peripheral vestibular disease can also occur (Beatty et al., 2000). Nonspecific signs of anorexia, weight loss, and fever occur in some infected cats.
Clinical findings in dogs with cryptococcosis depend on the organ systems involved and are similar to those that occur in the cat. Cryptococcosis is diagnosed most commonly in young purebred dogs; Doberman Pinschers, Great Danes, and German Shepherd dogs are commonly affected (Malik et al., 1995; O’Brien et al., 2004). Clinical manifestations include signs of upper or lower respiratory tract infection, disseminated disease including intraabdominal masses, CNS disease, disease of the orbit or eye, skin lesions, nasal cavity disease, and lymph node involvement. Seizures, ataxia, central vestibular syndrome, cranial nerve deficits, and clinical signs of cerebellar disease are the most common CNS manifestations in dogs. Dogs with Cryptococcus spp.–associated pyelonephritis (Newman et al., 2003) and gastrointestinal disease (Graves et al., 2005) have been reported.
Diagnosis
Nonregenerative anemia and monocytosis are the most common hematologic abnormalities; neutrophil counts and biochemical panels are generally normal. In dogs with CNS involvement, cerebrospinal fluid (CSF) protein concentrations vary from normal to 500 mg/dL, and cell counts vary from normal to 4500/μL; neutrophils and mononuclear cells predominate, but eosinophils are present in some cases. Radiographic changes consistent with cryptococcosis include increased soft tissue density in the nasal cavity caused by fungal granuloma formation as well as nasal bone deformity and lysis. Hilar lymphadenopathy and diffuse to miliary pulmonary interstitial patterns are common thoracic radiographic abnormalities.
Because circulating C. neoformans antibodies can be detected in both healthy and diseased animals, their presence does not document clinical disease. In addition, in one study all infected cats were seronegative (Flatland et al., 1996). Cryptococcal antigen can be detected in serum, aqueous humor, or CSF by latex agglutination (LA); serum antigen tests are positive in most cats and dogs with cryptococcosis. Animals with acute disease, chronic low-grade infections, drug-induced remission, or localized disease can be LA negative. The LA performed on CSF is positive in almost all animals with CNS cryptococcosis. Cryptococcal antigen can also be detected in subclinical carriers (Duncan et al., 2005a, 2005b).
A definitive diagnosis of cryptococcosis is based on positive antigen testing, or cytologic, histopathologic, or culture demonstration of the organism (Fig. 98-5) combined with appropriate clinical manifestations of disease. The organism is found during cytologic evaluation of nasal lesions, cutaneous lesions, lymph node aspirates, CSF, and bronchoalveolar lavage fluid in most affected animals; it can also be cultured. The organism can be cultured from the nasal cavity of some asymptomatic animals, so positive culture results do not always correlate to disease. One study evaluating subclinical carriage of C. gattii showed some animals eliminated the infection, some remained persistently colonized, and some progressed to clinical illness (Duncan et al., 2005a).
Treatment
Dogs and cats with cryptococcosis have been treated with amphotericin B, ketoconazole, itraconazole, fluconazole, and 5-flucytosine alone and in various combinations (see Table 98-2). Amphotericin B is usually not indicated unless life-threatening disseminated disease requiring rapid response to therapy is required. If amphotericin B is deemed necessary, lipid or liposomal encapsulated amphotericin is likely optimal because fewer adverse effects are associated with these formulations compared with regular amphotericin B. However, for owners who cannot afford this therapy, a less-expensive subcutaneous protocol for administration of regular amphotericin B has been used successfully for the treatment of cryptococcosis in dogs and cats and may be effective for other systemic fungi that are susceptible to the drug (Malik et al., 1996a; see Table 98-2).
Ketoconazole, itraconazole, or fluconazole are used as single agents in dogs or cats without life-threatening disease. Ketoconazole commonly leads to inappetence, vomiting, diarrhea, weight loss, and increases in liver enzyme activities in some dogs and cats. In dogs, long-term use of ketoconazole can suppresses testosterone and cortisol production and has been associated with cataracts. Because of these problems, ketoconazole is used less frequently than itraconazole and fluconazole. Fluconazole should be considered for dogs or cats with ocular or CNS infection. If clinical signs of toxicity develop (inappetence; drug eruptions) or increased activity of alanine aminotransferase is detected, drug therapy should be stopped and then reinstituted at 50% of the original dose after signs of toxicity abate.
Flucytosine crosses the blood-brain barrier better than ketoconazole or amphotericin B, so it has been used primarily for the treatment of CNS cryptococcosis. It must be used in combination with other antifungal drugs and has many adverse effects, including vomiting, diarrhea, hepatotoxicity, cutaneous reactions, and bone marrow suppression.
Clinical signs of nasal and cutaneous cryptococcosis generally resolve with treatment, but dogs or cats with CNS or ocular disease are less likely to respond. Treatment should continue for at least 1 to 2 months past resolution of clinical disease. Serum and CSF LA antigen titers can diminish with therapy and have been used to monitor response. Antigen titers fail to decrease in some animals without clinical evidence of disease, suggesting persistence of the organism in tissues.
HISTOPLASMOSIS
Etiology and Epidemiology
Histoplasma capsulatum is a saprophytic dimorphic fungus found in the soil in all regions with tropical and subtropical climates; histoplasmosis is diagnosed most frequently in the Mississippi, Missouri, and Ohio River valleys and in the mid-Atlantic states. The organism has also been associated with disease in a dog in Australia, a dog in Japan, and two indoor cats in California (Johnson et al., 2004). The microconidia (2 to 4 μm) and macroconidia (5 to 18 μm) of the mycelial phase are found in the environment. In the vertebrate host, the 2- to 4-μm yeast phase is found in the cytoplasm of mononuclear phagocytes (see Fig. 98-6 and Table 98-1).
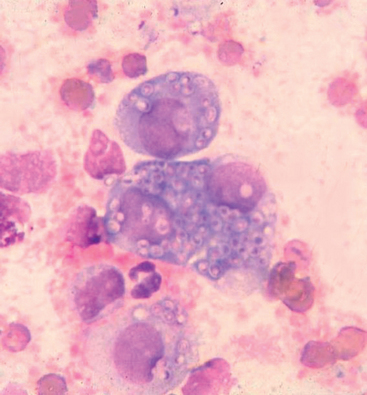
FIG 98-6 Histoplasma capsulatum (2 to 4 μm in diameter) in mononuclear cells.
(Courtesy Dr. Dennis Macy, College of Veterinary Medicine and Biomedical Sciences, Colorado State University.)
Histoplasma capsulatum is concentrated most heavily in soil contaminated with bird or bat excrement. Point sources for infection are found in endemic areas; two dogs and 20 people developed pulmonary histoplasmosis after removing a tree that had served as a bird roost (Ward et al., 1979). Subclinical infections are common in dogs. Dogs in endemic areas are commonly exposed but the incidence of disease is low. Immunosuppression may predispose to clinical infection in dogs and cats.
Infection is by ingestion or inhalation of microconidia from the environment. The organism is engulfed by mononuclear phagocytes, transformed to the yeast phase, and transported throughout the body in the blood and lymph. Granulomatous inflammation results in persistently infected organs and clinical signs of disease. Disseminated disease is common in cats.
Clinical Features
Most dogs with histoplasmosis are outdoor sporting breeds younger than 7 years. Subclinical infection, pulmonary infection, and disseminated infection are recognized most frequently. Most affected dogs are presented for evaluation of anorexia, fever, depression, weight loss, cough, dyspnea, or diarrhea. Large-bowel diarrhea is most common, but small-bowel diarrhea, mixed-bowel diarrhea, and protein-losing enteropathy occur in some.
Physical examination abnormalities often include depression, increased bronchovesicular sounds, respiratory wheezes, fever, evidence of diarrhea, pale mucous membranes, hepatomegaly, splenomegaly, icterus, ascites, and intraabdominal lymph node enlargement. Airway obstruction from massive hilar lymphadenopathy occurs in some dogs (Schulman et al., 1999). Lameness from bone infection or polyarthritis, peripheral lymphadenopathy, chorioretinitis, CNS disease, and skin disease occur occasionally. Subcutaneous nodules rarely drain or ulcerate and are less common than in dogs with cryptococcosis or blastomycosis.
Infected cats are either normal or develop disseminated disease. Most clinically affected cats are younger than 4 years, and some are coinfected with feline leukemia virus. Depression, weight loss, anorexia, lameness, and dyspnea are common presenting complaints. Weight loss can be severe and develop in as little as 2 weeks. Fever (103.5° to 105° F), pale mucous membranes, abnormal lung sounds, oral erosions or ulcers, peripheral or visceral lymphadenopathy, icterus, soft tissue swelling around osseous lesions, hepatomegaly, skin nodules and, rarely, splenomegaly are physical examination abnormalities potentially consistent with histoplasmosis. Disseminated disease has a grave prognosis in cats. Osseous histoplasmosis is most common in bones of the appendicular skeleton distal to the stifle or elbow joints, and one or more limbs can be involved. Feline ocular histoplasmosis manifests with conjunctivitis, chorioretinitis, retinal detachment, or optic neuritis and may induce glaucoma and blindness. Other than depression, CNS signs are uncommon.
Diagnosis
A variety of nonspecific clinicopathologic and radiographic abnormalities are associated with histoplasmosis. Normocytic, normochromic, nonregenerative anemia is the most common hematologic abnormality in both dogs and cats. Neutrophil counts can be normal, increased, or decreased. Unlike the other systemic fungi, H. capsulatum is occasionally seen in circulating cells, particularly on examination of a buffy coat smear; mononuclear cell infection is most common, followed by eosinophils. Thrombocytopenia from disseminated intravascular coagulation or microangiopathic destruction occurs in approximately 50% of dogs and some cats. Some affected cats develop pancytopenia from bone marrow infection. Hypoproteinemia and increased activities of alkaline phosphatase and alanine aminotransferase occur in some infected animals.
Lysis predominates in animals with bone infection; periosteal and endosteal new bone production occurs in some cases. In dogs with pulmonary infection, radiographic abnormalities include diffuse interstitial, miliary-to-nodular interstitial disease; hilar lymphadenopathy; pleural effusion; and calcified pulmonary parenchyma caused by chronic disease. In some dogs massive hilar lymphadenopathy is the only radiographic finding. Alveolar lung disease, tracheobronchial lymphadenopathy, and calcified lymph nodes are uncommon in cats. Colonoscopic findings in dogs with gastrointestinal infection include increased mucosal granularity, friability, ulceration, and thickness.
Several tests have been evaluated for the detection of circulating antibodies against H. capsulatum in the serum of dogs and cats, but the sensitivity and specificity are poor for all. Serologic diagnosis is unreliable and should be used only to establish a presumptive diagnosis when the organism cannot be demonstrated by cytology, histopathology, or culture and the clinical signs are suggestive of the disease.
Definitive diagnosis requires demonstration of the organism by cytology, biopsy, or culture (Fig. 98-6). The organism is found most frequently in rectal scrapings or biopsies from dogs with large-bowel diarrhea, in bone marrow or buffy coat cells from cats with disseminated disease, and in other locations (e.g., lymph nodes, lung, spleen, liver, skin nodules). The organism has also been identified in pleural and peritoneal effusions and in CSF.
Treatment
Because of its effectiveness and minimal toxicity, itraconazole is the initial drug of choice for dogs and cats with histoplasmosis (see Table 98-2). Animals should be treated for 60 to 90 days or until clinical evidence of disease has been resolved for at least 1 month. Amphotericin B can be used in animals with life-threatening disease or in those unable to absorb oral medications because of intestinal disease. Ketoconazole and fluconazole are also effective in some animals. However, ketoconazole has more adverse effects than itraconazole, and some cases that do not respond to fluconazole respond to intraconazole. The overall success rate for the treatment of histoplasmosis in cats was 33% in one study (Clinkenbeard et al., 1989c). In another study, all eight cats treated with itraconazole (5 mg/kg PO q12h) were eventually cured (Hodges et al., 1994). Pulmonary disease in dogs has a fair to good prognosis, whereas disseminated disease has a poor prognosis.
Administration of glucocorticoids with or without antifungal drugs lessened clinical signs associated with chronic hilar lymphadenopathy much more quickly than did administration of antifungal drugs alone and did not result in disseminated histoplasmosis (Schulman et al., 1999). However, if the infection is active, administration of glucocorticoids may exacerbate clinical disease.
Zoonotic Aspects and Prevention
Like blastomycosis, direct zoonotic transmission from infected animals is unlikely because the yeast phase is not as infectious as the mycelial phase. Care should be taken when culturing the organism. Prevention includes the avoidance of potentially contaminated soil. Organism numbers in contaminated areas can be decreased by application of 3% formalin.
Arceneaux KA, et al. Blastomycosis in dogs: 115 cases (1980–1995). J Am Vet Med Assoc. 1998;213:658.
Baumgardner DJ, et al. An outbreak of human and canine blastomycosis. Rev Infect Dis. 1991;13:898.
Baumgardner DJ, et al. Blastomycosis in dogs: a fifteen-year survey in a very highly endemic area near Eagle River, Wisconsin, USA. Wilderness Environ Med. 1996;7:1.
Baumgardner DJ, et al. Identification of Blastomyces dermatitidis in the stool of a dog with acute pulmonary blastomycosis. J Med Vet Mycol. 1997;35:419.
Blastomycosis acquired occupationally during prairie dog relocation—Colorado, 1998. Morb Mortal Wkly Rep. 1999;48:98.
Blondin N, et al. Blastomycosis in indoor cats: suburban Chicago, Illinois, USA. Mycopathologia. 2007;163:59.
Bloom JD, et al. Ocular blastomycosis in dogs: 73 cases, 108 eyes (1985–1993). J Am Vet Med Assoc. 1996;209:1271.
Breider MA, et al. Blastomycosis in cats: five cases (1979–1986). J Am Vet Med Assoc. 1988;193:570.
Bromel C, Sykes JE. Epidemiology, diagnosis, and treatment of blastomycosis in dogs and cats. Clin Tech Small Anim Pract. 2005;20:233.
Brooks DE, et al. The treatment of canine ocular blastomycosis with systemically administered itraconazole. Prog Vet Comp Ophthalmol. 1991;1:263.
Chester EM, et al. Blastomyces dermatitidis lysate antigens: antibody detection in serial serum specimens from dogs with blastomycosis. Mycopathologia. 2003;154:289.
Côté E, et al. Possible transmission of Blastomycosis dermatitidis via culture specimen. J Am Vet Med Assoc. 1997;210:479.
Dow SW, et al. Hypercalcemia associated with blastomycosis in dogs. J Am Vet Med Assoc. 1986;188:606.
Gilor C, et al. Clinical aspects of natural infection with Blastomyces dermatitidis in cats: 8 cases (1991-2005). J Am Vet Med Assoc. 2006;229:96.
Gnann JW, et al. Human blastomycosis after a dog bite. Ann Intern Med. 1983;98:48.
Hawkins EC, et al. Cytologic analysis of tracheal wash specimens and bronchoalveolar lavage fluid in the diagnosis of mycotic infections in dogs. J Am Vet Med Assoc. 1990;197:79.
Hendrix DV, et al. Comparison of histologic lesions of endophthalmitis induced by Blastomyces dermatitidis in untreated and treated dogs: 36 cases (1986-2001). J Am Vet Med Assoc. 2004;224:1317.
Howard J, et al. Blastomycosis granuloma involving the cranial vena cava associated with chylothorax and cranial vena caval syndrome in a dog. J Am Anim Hosp Assoc. 2000;36:159.
Krawieck DR, et al. Use of an amphotericin B lipid complex for treatment of blastomycosis in dogs. J Am Vet Med Assoc. 1996;209:2073.
Legendre AM, et al. Treatment of blastomycosis with itraconazole in 112 dogs. J Vet Intern Med. 1996;10:365.
MacDonald PD, et al. Human and canine pulmonary blastomycosis, North Carolina, 2001-2002. Emerg Infect Dis. 2006;12:1242.
McCune MB. A blastomycosis field investigation: canine outbreak suggests risk to human health. J Environ Health. 1988;51:22.
Miller PE, et al. Feline blastomycosis: a report of three cases and literature review (1961 to 1988). J Am Anim Hosp Assoc. 1990;26:417.
Ramsey DT. Blastomycosis in a veterinarian. J Am Vet Med Assoc. 1994;205:968.
Rudmann DG, et al. Evaluation of risks factors for blastomycosis in dogs: 857 cases (1980–1990). J Am Vet Med Assoc. 1992;201:1754.
Schmiedt C, et al. Cardiovascular involvement in 8 dogs with Blastomyces dermatitidis infection. J Vet Intern Med. 2006;20:1351.
Shurley JF, et al. Blastomyces dermatitidis antigen detection in urine specimens from dogs with blastomycosis using a competitive binding inhibition ELISA. Mycopathologia. 2005;160:137.
Wood EF, et al. Ultrasound-guided fine-needle aspiration of focal parenchymal lesions of the lung in dogs and cats. J Vet Intern Med. 1998;12:338.
Angell JA, et al. Ocular lesions associated with coccidioidomycosis in dogs: 35 cases (1980–1985). J Am Vet Med Assoc. 1987;190:1319.
Armstrong PJ, et al. Canine coccidioidomycosis: a literature review and report of 8 cases. J Am Anim Hosp Assoc. 1983;19:937.
Barsanti JA, et al. Coccidioidomycosis. In: Greene CE, editor. Infectious diseases of the dog and cat. ed 2. Philadelphia: WB Saunders; 1990:696.
Burtch M. Granulomatous meningitis caused by a dog. J Am Vet Med Assoc. 1998;212:827.
Butkiewicz CD, et al. Risk factors associated with Coccidioides infection in dogs. J Am Vet Med Assoc. 2005;226:1851.
Coccidioidomycosis—United States, 1991-1992. Morb Mortal Wkly Rep. 1993;42:21.
Greene RT, et al. Coccidioidomycosis in 48 cats: a retrospective study (1984–1993). J Vet Intern Med. 1995;9:86.
Heinritz CK, et al. Subtotal pericardectomy and epicardial excision for treatment of coccidioidomycosis-induced effusiveconstrictive pericarditis in dogs: 17 cases (1999-2003). J Am Vet Med Assoc. 2005;227:435.
Hinsch BG. Ketoconazole treatment of disseminated coccidioidomycosis in a dog. Mod Vet Pract. 1988;69:161.
Jackson JA, et al. Treatment of canine coccidioidomycosis with ketoconazole: serological aspects of a case study. J Am Anim Hosp Assoc. 1985;21:572.
Johnson LR, et al. Clinical, clinicopathologic, and radiographic findings in dogs with coccidioidomycosis: 24 cases (1995-2000). J Am Vet Med Assoc. 2003;222:461.
Millman TM, et al. Coccidioidomycosis in the dog; its radiographic diagnosis. J Am Vet Rad Soc. 1979;20:50.
Pappagianis D. Evaluation of the protective efficacy of the killed Coccidioides immitis spherule vaccine in humans. Am Rev Respir Dis. 1993;148:656.
Shubitz LF, et al. Constrictive pericarditis secondary to Coccidiodes infection in a dog. J Am Vet Med Assoc. 2001;218:537.
Shubitz LE, et al. Incidence of Coccidioides infection among dogs residing in a region in which the organism is endemic. J Am Vet Med Assoc. 2005;226:1846.
Barrs, et al. Feline pyothorax: a retrospective study of 27 cases in Australia. J Fel Med Surg. 2005;7:211.
Beatty JA, et al. Peripheral vestibular disease associated with cryptococcosis in three cats. J Feline Med Surg. 2000;2:29.
Berthelin CF, et al. Cryptococcosis of the nervous system in dogs. I. Epidemiologic, clinical, and neuropathological features. Prog Vet Neurol. 1994;5:88.
Berthelin CF, et al. Cryptococcosis of the nervous system in dogs. II. Diagnosis, treatment, monitoring, and prognosis. Prog Vet Neurol. 1994;5:136.
Como JA, et al. Oral azole drugs as systemic antifungal therapy. N Engl J Med. 1994;330:263.
Duncan C, et al. Clinical characteristics and predictors of mortality for Cryptococcus gattii infection in dogs and cats of southwestern British Columbia. Can Vet J. 2006;47:993.
Duncan CG, et al. Evaluation of risk factors for Cryptococcus gattii infection in dogs and cats. J Am Vet Med Assoc. 2006;228:377.
Duncan C, et al. Follow-up study of dogs and cats with asymptomatic Cryptococcus gattii infection or nasal colonization. Med Mycol. 2005;43:663.
Duncan C, et al. Sub-clinical infection and asymptomatic carriage of Cryptococcus gattii in dogs and cats during an outbreak of cryptococcosis. Med Mycol. 2005;43:511.
Flatland B, et al. Clinical and serologic evaluation of cats with cryptococcosis. J Am Vet Med Assoc. 1996;209:1110.
Foster SF, et al. Lower respiratory tract infections in cats: 21 cases (1995-2000). J Fel Med Surg. 2004;6:167.
Graves TK, et al. Diagnosis of systemic cryptococcosis by fecal cytology in a dog. Vet Clin Pathol. 2005;34:409.
Jacobs GJ, et al. Cryptococcal infection in cats: factors influencing treatment outcome and results of sequential serum antigen titers in 35 cats. J Vet Intern Med. 1997;11:1.
Lester SJ, et al. Clinicopathologic features of an unusual outbreak of cryptococcosis in dogs, cats, ferrets, and a bird: 38 cases (January to July 2003). J Am Vet Med Assoc. 2004;225:1716.
MacDougall L, et al. Spread of Cryptococcus gattii in British Columbia, Canada, and detection in the Pacific Northwest, USA. Emerg Infect Dis. 2007;13:42.
Malik R, et al. Cryptococcosis in cats: clinical and mycological assessment of 29 cases and evaluation of treatment using orally administered fluconazole. J Med Vet Mycol. 1992;30:133.
Malik R, et al. Cryptococcosis in dogs: a retrospective study of 20 consecutive cases. J Med Vet Mycol. 1995;33:291.
Malik R, et al. Combination chemotherapy of canine and feline cryptococcosis using subcutaneously administered amphotericin B. Aust Vet J. 1996;73:124.
Malik R, et al. A latex cryptococcal antigen agglutination test for diagnosis and monitoring of therapy for cryptococcosis. Aust Vet J. 1996;74:358.
Malik R, et al. Suspected drug eruption in seven dogs during administration of flucytosine. Aust Vet J. 1996;74:285.
Malk R, et al. Asymptomatic carriage of Cryptococcus neoformans in the nasal cavity of dogs and cats. J Med Vet Mycol. 1997;35:27.
Malik R, et al. Intra-abdominal cryptococcosis in two dogs. J Small Anim Pract. 1999;40:387.
Malik R, et al. Serum antibody response to Cryptococcus neoformans in cats, dogs and koalas with and without active infection. Med Mycol. 1999;37:43.
Newman SJ, et al. Cryptococcal pyelonephritis in a dog. J Am Vet Med Assoc. 2003;222:180.
O’Brien CR, et al. Retrospective study of feline and canine cryptococcosis in Australia from 1981 to 2001: 195 cases. Med Mycol. 2004;42:449.
O’Toole TE, et al. Cryptococcosis of the central nervous system in a dog. J Am Vet Med Assoc. 2003;222:1722.
Bromel C, Sykes JE. Histoplasmosis in dogs and cats. Clin Tech Small Animal Pract. 2005;20:227.
Clinkenbeard KD, et al. Identification of Histoplasma organisms in circulating eosinophils of a dog. J Am Vet Med Assoc. 1988;192:217.
Clinkenbeard KD, et al. Thrombocytopenia associated with disseminated histoplasmosis in dogs. Comp Cont Ed Pract Vet. 1989;11:301.
Clinkenbeard KD, et al. Canine disseminated histoplasmosis. Comp Cont Ed Pract Vet. 1989;11:1347.
Clinkenbeard KD, et al. Feline disseminated histoplasmosis. Comp Cont Ed Pract Vet. 1989;11:1223.
Davies C, et al. Deep mycotic infections in cats. J Am Anim Hosp Assoc. 1996;32:380.
Davies SF, et al. Concurrent human and canine histoplasmosis from cutting decayed wood. Ann Intern Med. 1990;113:252.
Hodges RD, et al. Itraconazole for the treatment of histoplasmosis in cats. J Vet Intern Med. 1994;8:409.
Johnson LR, et al. Histoplasmosis infection in two cats from California. J Am Anim Hosp Assoc. 2004;40:165.
Kagawa Y, et al. Histoplasmosis in the skin and gingiva in a dog. J Vet Med Sci. 1998;60:863.
Mackie JT, et al. Confirmed histoplasmosis in an Australian dog. Aust Vet J. 1997;75:362.
Pearce J, et al. Management of bilateral uveitis in a Toxoplasma gondii-seropositive cat with histopathologic evidence of fungal panuveitis. Vet Ophthalmol. 2007;10:216.
Schulman RL, et al. Use of corticosteroids for treating dogs with airway obstruction secondary to hilar lymphadenopathy caused by chronic histoplasmosis: 16 cases (1979–1997). J Am Vet Med Assoc. 1999;214:1345.
Vinayak A, et al. Treatment of thoracolumbar spinal cord compression associated with Histoplasma capsulatum infection in a cat. J Am Vet Med Assoc. 2007;230:1018.
Ward JI, et al. Acute histoplasmosis: clinical, epidemiologic and serologic finding of an outbreak associated with exposure to a fallen tree. Am J Med. 1979;66:587.
Wolf AM. Histoplasma capsulatum in the cat. J Vet Intern Med. 1987;1:158.
Wolf AM. Successful treatment of disseminated histoplasmosis with osseous involvement in two cats. J Am Anim Hosp Assoc. 1988;24:511.
Wolf AM. Histoplasmosis. In: Greene CE, editor. Infectious diseases of the dog and cat. ed 2. Philadelphia: WB Saunders Co; 1990:679.
Wolf AM, et al. The radiographic appearance of pulmonary histoplasmosis in the cat. Vet Radiol. 1987;28:34.