CHAPTER 101 Pathogenesis of Immune-Mediated Disorders
GENERAL CONSIDERATIONS AND DEFINITION
Immune-mediated disorders occur when the protective immune response is activated inappropriately, resulting in organ injury. Pathologic immune responses may occur in response to infectious pathogens and contribute to the clinical disease presentation for that pathogen (e.g., the hemolytic anemia associated with Mycoplasma hemofelis infection) or be stimulated by otherwise innocuous foreign substances (e.g., the allergic reactions that occur to house dust) or by self-antigens (primary autoimmunity). Autoimmunity is defined as a condition characterized by a specific humoral or cell-mediated immune response against constituents of the body’s own tissues (self-antigens or autoantigens). The term primary autoimmune disease is reserved for disorders in which no underlying cause can be identified and the cause of the autoimmunity is believed to be an underlying immune system dysfunction or imbalance. The term secondary autoimmunity (also termed secondary immune-mediated disease) is used to describe immune-mediated disorders in which an underlying reason for the autoimmune response can be identified. Examples of secondary causes of autoimmunity include infection, exposure to certain drugs or toxins, neoplasia, and vaccine administration.
IMMUNOPATHOLOGIC MECHANISMS
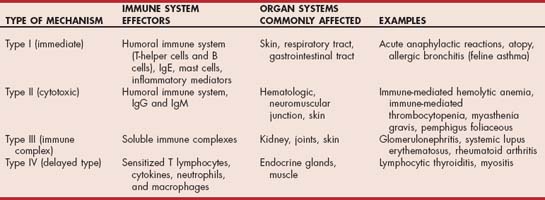
Immunopathologic injury occurs by four major mechanisms (Table 101-1). Each mechanism may either be part of an appropriate response to a foreign antigen or an inappropriate response that can lead to allergic or immune-mediated disease. More than one mechanism may be involved in a particular immune-mediated disorder.
Type I hypersensitivity involves the humoral immune system, immunoglobulin E (IgE), and mast cells. Exposure of the immune system to antigens by way of the skin, respiratory tract, or gastrointestinal tract leads to activation of antigen-specific subsets of T-helper lymphocytes and initiation of B-cell differentiation to plasma cells. Plasma cells secrete IgE, which attaches to receptors on mast cells. On future exposure to the same antigen, cross-linking of the IgE molecules on the mast cells occurs, which leads to mast cell degranulation. The potent inflammatory mediators that are released lead to vasodilation, edema, eosinophil chemotaxis, pruritus, and bronchoconstriction. Examples of diseases that are mediated primarily by a type I response include allergic bronchitis (feline asthma) and acute anaphylactic reactions.
Type II (cytotoxic) hypersensitivity involves the binding of antibody (IgG or IgM) to specific molecules on the surface of a cell. This binding typically results in destruction of the cell or receptors on the cell. In unusual situations antibodies may induce a biologic effect, such as stimulation of the thyroid-stimulating hormone receptor and induction of hyperthyroidism in humans with Graves disease. The target of antibody binding may be normal self-antigens, infectious agents bound to the cell surface, or nonbiologic antigens such as drugs bound to the cell surface. Antibodies to self-antigens may come from cell damage, resulting in exposure of previously hidden antigens, similarity between self-antigens and foreign antigens such as infectious agents and drugs, and primary immune system dysfunction or imbalance. Classic examples of diseases mediated by type II mechanisms include autoimmune hemolytic anemia, immune-mediated thrombocytopenia, pemphigus foliaceous, and myasthenia gravis. Antibodies involved in type II responses are usually tissue specific, and the consequence of antibody binding varies from tissue to tissue. For example, in autoimmune hemolytic anemia antibody binding results in either intravascular or extravascular red blood cell hemolysis, whereas in pemphigus foliaceous antibody binding results in disruption of keratinocyte adhesion and vesicle formation. In myasthenia gravis, antibodies directed against acetylcholine receptors cross-link and internalize the receptors, which results in failure of neuromuscular transmission.
Type III (immune complex) hypersensitivity involves the formation and deposition of soluble immune complexes (predominantly IgG) within tissues. Deposition of immune complexes in tissues results in complement fixation and a localized inflammatory response characterized by mast cell degranulation, platelet activation, and neutrophil chemotaxis. Phagocytosis of immune complexes by macrophages causes release of more inflammatory cytokines. In the presence of antibody excess the inflammatory reaction typically remains localized at the site of the initiating antibody; in the presence of antigen excess, however, soluble immune complexes enter the circulation and become deposited in vascular beds in the kidney, joints, eye, and skin. The location and extent of antibody deposition depend on a number of variables, including complex size, charge, degree of glycosylation, and Ig subclass. Classic examples of diseases mediated by type III mechanisms include infections (e.g., feline infectious peritonitis), glomerulonephritis, systemic lupus erythematosus, and rheumatoid arthritis.
Type IV (delayed–type) hypersensitivity involves the cell-mediated immune system. Exposure to either soluble or cell-associated antigen results in sensitization of specific subsets of T cells. Reexposure to the same antigen results in activation of sensitized lymphocytes, subsequent release of cytokines, and recruitment of neutrophils and macrophages. Cytotoxic destruction of target cells may also occur by this mechanism. Activation of sensitized lymphocytes requires 24 to 72 hours to occur, which is why this type of response is termed “delayed.” Persistence of the antigen can result in formation of multinucleate giant cells and tissue granulomas. Examples of diseases mediated by type IV immune responses include the protective immune response to intracellular microbes (e.g., leishmaniasis), contact hypersensitivity, polymyositis, and immune-mediated thyroiditis.
PATHOGENESIS OF AUTOIMMUNE DISORDERS
In normal animals the adaptive immune system should be tolerant of self. This is achieved by a number of mechanisms that prevent B and T lymphocytes from becoming selfreactive. Most autoreactive B and T cells are deleted during maturation in the thymus, and those that escape to the periphery may undergo peripheral deletion by apoptosis, remain hidden intracellularly, or be rendered anergic in the peripheral circulation. When autoimmunity occurs, these mechanisms responsible for tolerance break down. Factors that may play a role in loss of tolerance include genetics, environmental factors, age, hormonal influences, and other diseases that lead to perturbations of the immune system.
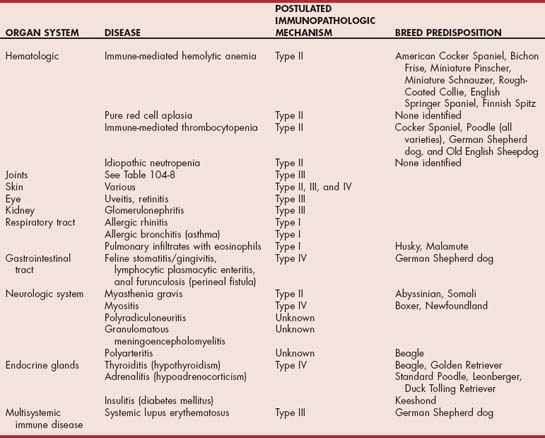
Genetics clearly has an important role in the development of autoimmune disease. In some autoimmune diseases certain breeds of dog are at increased risk (Table 101-2). Autoimmunity is also reported more commonly in some families than others. The inbreeding that occurs in many dog breeds exacerbates the effects of such familial traits. Familial autoimmunity is not as well documented in the cat, although the Abyssinian and Somali breeds are at increased risk of myasthenia gravis. The underlying genetic changes that result in such predispositions are not yet characterized in the dog and cat.
Environmental factors are believed to be important in the development of autoimmunity, with exposure to infectious agents either during natural infection or as a result of vaccination being the most common factor identified. Other possible environmental factors include environmental toxins and drug exposure. Some drugs have been clearly linked to induction of autoimmunity, and many other drugs can likely cause idiosyncratic autoimmune reactions. Examples include the risk of systemic immune disease (polyarthritis, glomerulonephritis, cutaneous lesions, retinitis, polymyositis, anemia, thrombocytopenia) in Doberman Pinschers treated with trimethoprim-sulfadiazine and development of immune-mediated hemolytic anemia in some cats treated with thioureylene drugs such as propylthiouracil and methimazole. Myasthenia gravis has also been reported in cats treated with methimazole.
Mechanisms by which infectious agents may induce autoimmunity include molecular mimicry, exposure of cryptic antigens after cellular damage, nonspecific polyclonal activation by superantigens, production of interferon-γ that induces major histocompatibility complex class II expression on cells that do not usually express them (e.g., thyroid follicular cells), and the innocent bystander effect in which the immune response is directed against a microbial antigen or other antigens on the surface of the cell. A complicating factor is that some infections (e.g., ehrlichiosis, borreliosis, and many other vector-borne diseases) may either mimic an autoimmune disease or cause true autoimmunity, and clinically differentiating which of the two is happening can be difficult. This is clinically relevant because the clinician is faced with a decision whether to include immunosuppressive drugs in the treatment protocol.
The role of vaccination in the precipitation of autoimmunity is unclear. Currently most of the evidence is based on anecdotal observation of a temporal association of immune-mediated disease with vaccination. A cause-and- effect relation has been difficult to establish definitively because of the high prevalence of vaccination and the low incidence of reported adverse effects. Specific evidence for association of individual disease syndromes with vaccination is discussed in the sections on individual diseases. Altered immunoregulation and evidence of immune-mediated disease may also occur in other underlying diseases such as lymphoid neoplasia, IgA deficiency, and after chemotherapy administration.
PRIMARY VERSUS SECONDARY IMMUNE-MEDIATED DISORDERS
Infection, drug therapy, neoplasia, and possibly vaccination may cause secondary autoimmunity. Investigation for the presence of these factors in dogs and cats with immune-mediated disease is important because the presence of underlying disease may influence the treatment and the prognosis. Clearly the presence of a serious underlying disorder such as neoplasia influences the prognosis negatively. Theoretically the presence of a treatable underlying disorder should make controlling the autoimmune process easier. Unfortunately, documentation of a better prognosis with immune-mediated disorders that have an identifiable and treatable underlying disease is lacking in the dog and cat. The presence of concurrent disease may also influence choice of treatment. In particular more potent immunosuppressive drugs may be initially withheld in the presence of an underlying infectious etiology.
ORGAN SYSTEMS INVOLVED IN AUTOIMMUNE DISORDERS
Any organ system in the body may be damaged by immune-mediated disease processes (see Table 101-2). The most common systems involved in the dog and cat are the joints, skin, kidney, and hematologic system, although in general immune-mediated diseases are less common in the cat than the dog. Other organs commonly involved in immune-mediated diseases are the eye, neurologic system, gastrointestinal tract, respiratory tract, and endocrine glands (see Table 101-2). Some immune-mediated diseases such as systemic lupus erythematosus involve multiple organ systems, although not all organ systems may be involved in every animal. Dogs with systemic immune-mediated disorders not uncommonly present with one manifestation of the disorder (e.g., immune-mediated hemolytic anemia) and later relapse with another (e.g., idiopathic thrombocytopenia purpura, polyarthritis). In some of these cases the underlying disorder may be systemic lupus erythematosus, but this is not always the case. A large number of autoimmune diseases affect the dog and cat. The autoimmune disorders discussed in detail in the sections that follow focus on the more-common autoimmune diseases, especially those in which the treatment of choice is immunosuppression. Other disorders in which the pathogenesis is immune mediated, but in which immunosuppression is not part of the treatment (e.g., hypothyroidism from thyroiditis), are discussed in the sections on diseases of the appropriate organ system.
Chabanne L, et al. Canine systemic lupus erythematosus. Part I. Clinical and biologic aspects. Compendium. 1999;21:135. (small animal/exotics)
Day MJ. Clinical immunology of the dog and cat. Ames, IA: Iowa State University Press, 1999;47.
Duval D, et al. Vaccine associated immune-mediated hemolytic anemia in the dog. J Vet Intern Med. 1996;10:290.
Miller SA, et al. Case control study of blood type, breed, sex, and bacteremia in dogs with immune-mediated hemolytic anemia. J Am Vet Med Assoc. 2004;224:232.
Tizard I. Veterinary immunology—an introduction, ed 5. Philadelphia: Saunders, 2004.
 TABLE 101-1
TABLE 101-1