Viscera of the Thorax
The Thorax –Partly Intricate Organs
The thoracic cage (Cavea thoracis) contains the heart (Cor) and the lungs (Pulmones). In ancient times, it was believed that life spirits along with the inhaled air reached the lungs, mixed with blood in the heart, which was at that time thought to be the seat of the soul, and distributed throughout the whole body by the blood vessels. Even today, the heart is still considered to be the engine of life and in colloquial terms it is also referred to as the centre of emotions. Scientifically, the heart is defined as a hollow muscle which pumps blood through the lesser circulation of the lungs (pulmonary circulation) and the greater circulation of the body (systemic circulation): The left side of the heart pumps oxygenated blood into the systemic circulation which transports the blood to the organs via arteries (leaving the heart). Blood vessels of the microcirculation branch out to allow the nutrient and gas exchange at the capillary level. The veins return deoxygenated blood to the right side of the heart from where the blood is forwarded to the pulmonary circulation. Pulmonary arteries transport deoxygenated blood to the lungs. In a network of pulmonary capillaries the deoxygenated blood finally reaches the alveoli, is enriched with oxygen and transferred via pulmonary veins to the left atrium. This completes the blood circulation.
The function of the heart as a pump is especially fascinating: On average the heart rate is 70 beats per minute and with every systolic contraction the heart forces 70 ml of blood into the circulation. Even without further stimulation of the heart in “excitement”, it beats more than 100,000 times per day and 36 million times per year. The volume of blood (206,000 m3), which is pumped by the heart in the course of 80 years, would be sufficient to fill 80 Olympic swimming pools. Conversely, no function of the body would be possible without the heart: in most cases cardiac arrest is an immediate cause of death.
In the dissection course, the opening of the thoracic cavity is perceived with mixed feelings of awe, excitement and interest by teaching professionals and students. The exposure of heart and lungs as well as the entitlement to touch and observe these vital organs is perceived as a great privilege during these training sessions.
The Mediastinum
A sagittal massive separation crosses the Thorax from the rear aspect of the Sternum to the ventral aspect of the thoracic vertebrae. It is called the Mediastinum (from Latin “in medio stans” = “standing in the middle”). Cranially the Mediastinum is continuous without sharp boundaries with the viscera of the neck through the superior thoracic aperture. Caudally it rests on the diaphragm and is sharply defined. The lungs are located within individual pleural cavities (Cavitates pleurales) to both sides of the Mediastinum.
In the Mediastinum, several organs are intertwined. The Thymus is located in the Mediastinum superius just behind the Sternum. It is an organ of the immune system but soon after puberty regresses to become an adipose body. The V. cava superior is displaced to the right from the median plane. Its tributaries – both Vv. brachiocephalicae – cover the large arterial trunks to the neck and the arms that emerge from the aortic arch. The cane-like curved main artery (Aorta) dominates on the left side of the Mediastinum. Hidden beneath the veins and the arch of the Aorta, the Trachea descends in the Mediastinum superius and branches into right and left main bronchi, Bronchi principales. The Oesophagus descends dorsal of the Trachea and in front of the vertebrae. Between the Oesophagus and the vertebrae there is the delicate thoracic duct, the Ductus thoracicus, which carries milky lymph (containing absorbed fats from meals) from the lower body.
The heart dominates in the Mediastinum inferius which is directed towards the diaphragm. It is located in a separate, thin-walled serous cavity, the Cavitas pericardiaca, and extends the Mediastinum towards the left side. The heart is only exposed after incision or removal of the cavity wall, the pericardium. A large area of the heart rests on the diaphragm with its apex (Apex cordis) pointing to the lower left side towards the left fifth intercostal space. Holding the heart by the apex, it can be freely moved in the cavity. Its only attachments are the large vessels that emerge at the upper pole (Aorta, A. pulmonalis) and enter at the its rear surface (Vv. pulmonales, Vv. cavae superior et inferior). The base of the heart (Basis cordis) with the origin of the blood vessels is opposite to the apex.
Immediately behind the Pericardium – more exactly: behind the left atrium of the heart – the Oesophagus descends to the oesophageal hiatus (Hiatus oesophageus) in the diaphragm. Slightly left side to the Oesophagus, also behind the Pericardium, the Aorta and the Ductus thoracicus descend and pass through the Hiatus aorticus in the diaphragm. The V. cava inferior traverses the diaphragm through a separate orifice (Foramen venae cavae), located slightly to the right and dorsal side of the centre of the diaphragm, and enters the pericardium and the Basis cordis from inferior. Additionally, numerous other structures, such as the Aa. thoracicae internae, Nn. phrenici, Nn. vagi, Vv. azygotes, and ganglia and nerves of the sympathetic trunk (part of the autonomic nervous system) descend in the mediastinum.
The Lungs and their Cavities
The larger trilobular right lung and the smaller bilobular left lung are located in separate serous cavities (Cavitates pleurales, pleural cavities) to the right and left side of the Mediastinum, respectively. Both lungs are covered by a thin, transparent, serous membrane (Pleura visceralis), through which a black, net-like pigment pattern is visible. This anthracotic pigment consists mainly of soot, the carbon which emanates from exhaust fumes and cigarette smoke. Numerous lymph nodes near the hilum of the lungs (see below) show an abundance of this pigment.
The lungs are supposed to move freely in their pleural cavities. They are attached only at the hilum where the bronchi, the Aa. pulmonales, and the Vv. pulmonales enter the lungs from the Mediastinum. Often, as a result of inflammation, the pleura covering the lungs (Pleura visceralis) adheres to the serous pleura of the ribs (Pleura costalis), the Mediastinum (Pleura mediastinalis), or the diaphragm (Pleura diaphragmatica), all of which comprise the Pleura parietalis. In exhaled condition, the parietal pleura is more substantial than the visceral pleura and reaches beyond the margins of the lungs. The virtual spaces in which the lungs may expand during deep inspiration are called the pleural recesses of the Pleura. During respiration, the lungs adapt to the shape of the thoracic wall and diaphragm. The lungs expand and retract as they slide in and out of the recesses. Therefore, adhesions of the Pleura parietalis to the Pleura visceralis restrain lung function.
→ Dissection Link
The Vasa thoracica interna, which are descending parallel to the Sternum, are presented by fenestration of the intercostal spaces to avoid damage during opening of the thoracic cavity. After removal of the Sternum with the anterior portions of the ribs, the lungs are separated at the hilum and removed. Now, the mediastinum is dissected: First the pericardium and the adjacent N. phrenicus are exposed. The pericardium is opened ventrally. The heart can be dissected in situ or after separation from the great vessels. The removal of epicardial adipose tissue serves the purpose of tracing the branches of the coronary arteries. Using scissors, the ventricles are opened from the direction of the aorta and the pulmonary trunk, respectively, and the right atrium is opened from the direction of both Vv. cavae. After removal of the pericardium, the Oesophagus and the course of the Aorta thoracica, the Vv. azygos and hemiazygos, the N. vagus, and the Ductus thoracicus are presented in the posterior mediastinum. The parietal pleura is removed to facilitate the dissection of the sympathetic trunk with the corresponding Nn. splanchnici as well as intercostal neurovascular structures. Finally, the preparation of the superior mediastinum exposes the residual Thymus and the passageways to the neck are traced.
Heart
Projection of the heart
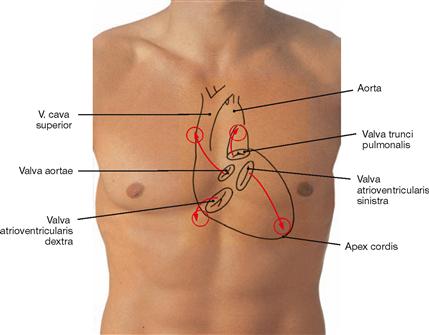
Fig. 5.1 Contours of the heart, cardiac valves and auscultation areas projected onto the ventral thoracic wall.
The right heart contour projects from the third to the sixth costal cartilage onto a line 2 cm lateral of the right sternal border.
The contour of the left heart projects onto a connecting line between the lower border of rib III (2–3 cm parasternal) and the left midclavicular line.
On each side, the heart contains an atrioventricular valve between the atrium and the ventricle and a semilunar valve between the ventricle and the respective artery.
The projection of the four cardiac valves forms a cross which is slightly deviating to the left side from the median axis.
The projection of the cardiac valves is of minor importance in clinical practice since the heart sounds and potential murmurs travel with the blood flow and are auscultated at the points of maximal intensity (circles).
| Surface Projection of Cardiac Valves | Auscultation Sites of Cardiac Valves | |
| Pulmonary valve | left (!) sternal border, 3rd costal cartilage | parasternal left 2nd ICS |
| Aortic valve | left sternal border, 3rd ICS | parasternal right 2nd ICS |
| Mitral valve | left 4th to 5th costal cartilages | in the midclavicular line 5th ICS |
| Tricuspid valve | retrosternal 5th costal cartilage | parasternal right 5th ICS |
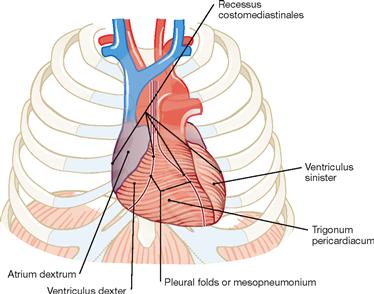
Fig. 5.2 Projection of the heart onto the thorax; ventral view (according to [2])
We distinguish four surfaces of the heart: The ventrally oriented Facies sternocostalis predominantly represents the right ventricle. The Facies diaphragmatica points inferiorly and consists of parts of both ventricles. The Facies pulmonalis is formed by the right atrium on the right side and by the left ventricle on the left side. Thus, the right ventricle does not contribute to any of the cardiac borders.
The major part of the Facies sternocostalis is covered by the Pleura. These areas represent the Recessus costomediastinales of the pleural cavity. The pleural borders separate from each other inferior to rib IV and form the boundary of the Trigonum pericardiacum where the Pericardium is directly adjacent to the ventral wall of the Thorax.
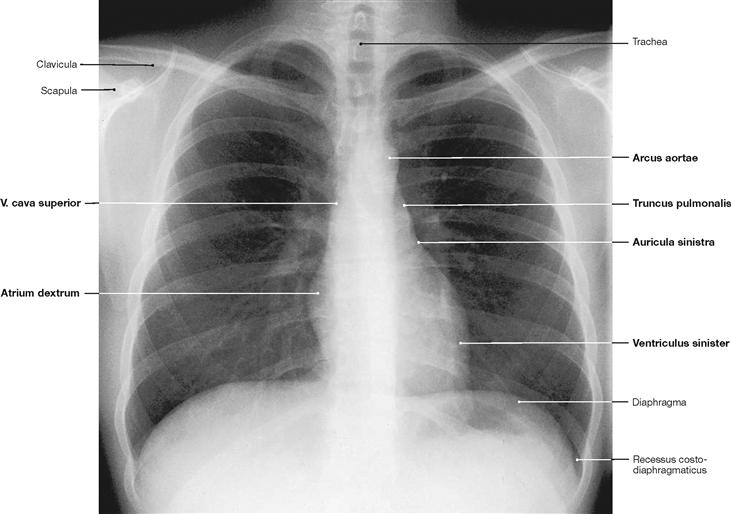
Fig. 5.3 Thoracic cage, Cavea thoracis, with thoracic viscera; radiograph in postero-anterior (PA) beam projection.
The radiograph can be used to assess the size of the heart. In addition to the absolute size, knowledge of the structures contributing to the heart contours is of importance.
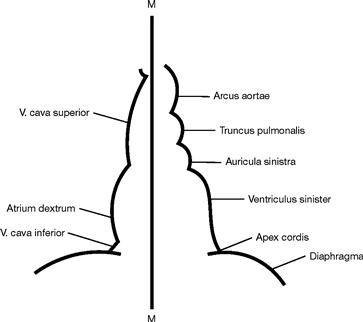
Fig. 5.4 Schematic drawing of the heart contours in the radiograph.
From cranial to caudal, the right border of the heart is formed by the following structures:
From cranial to caudal, the left border of the heart is formed by the following structures:
Thus, the right ventricle does not contribute to any of the cardiac borders!
M = Median axis of the body
Development
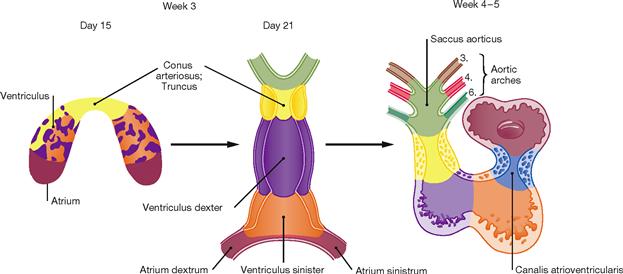
Fig. 5.5 Stages of cardiac development during weeks 3 to 5. (according to [2])
At week 3, the initially horseshoe-shaped endocardial tube develops from a vascular plexus in the cardiogenic mesoderm. Several gaps around the endocardial tube merge to establish the pericardial cavity which connects with the general body cavity. The inner layer of the pericardial cavity condenses to form the Myocardium. The Epicardium develops from cells which migrate from the Septum transversum and the liver primordium. The lateral crus of the endocardial tube fuse to build the cardiac tube which contracts rhythmically from the end of week 3 onwards. The cardiac tube initially comprises a paired atrium with the Sinus venosus collecting incoming blood, one ventricle, and the Conus arteriosus as the outflow segment. Caused by differential longitudinal growth and reorganisation of the respective segments, during weeks 4 – 5 the cardiac tube develops into the S-shaped heart loop. The transition between atrium and ventricle is constricted to form the unpaired atrioventricular canal. The latter originally opens into the left part of the ventricle, but is later shifted to the midline and partitioned into a right and left atrioventricular opening through endocardial cushions. These endocardial cushions later form the atrioventricular valves.
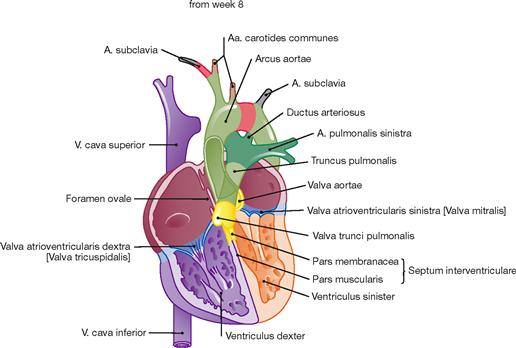
Fig. 5.6 Stages of cardiac development during weeks 5 to 7. (according to [2])
During weeks 5–7, the interventricular septum develops (Pars muscularis), which incompletely separates the two ventricles. The latter communicate until the end of week 7 when the formation of the Pars membranacea of the septum completes the ventricular separation. The Conus arteriosus of the outflow tract is separated spirally and, together with the adjacent Saccus aorticus, forms the Truncus pulmonalis and the Aorta.
The primitive aortic arches (arteries of the pharyngeal arches) derive from the Saccus aorticus. From the original six aortic aches, only the third, fourth and sixth contribute to the development of the great vessels. The A. carotis communis derives from the third aortic arch. Parts of the A. subclavia and the aortic arch develop from the fourth aortic arches on the right and left side, respectively. The proximal parts of the right and left pulmonary artery and the Ductus arteriosus develop from the right and left sixth aortic arches, respectively.
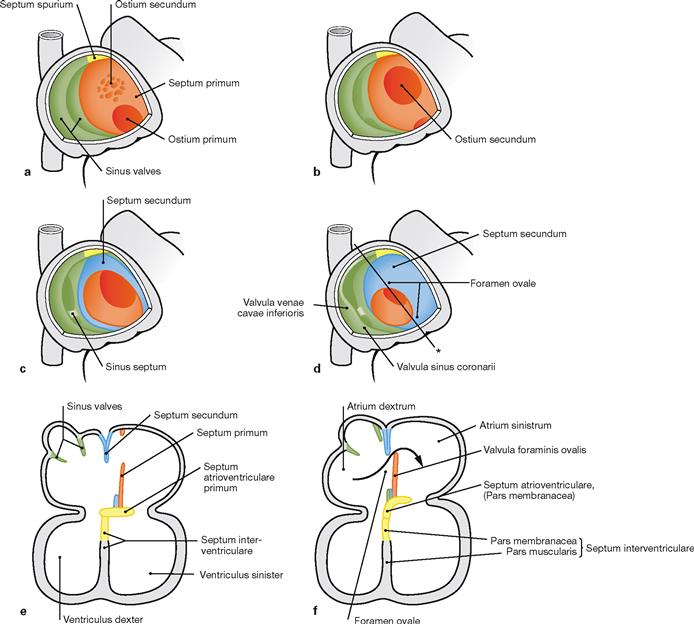
Figs. 5.7a to f Developmental steps in septum formation during weeks 5 (a, b), 6 (c, e), 7 and 8 (d, f); view from the opened right atrium (a–d) and in the four-chamber plane (e and f). (according to [2])
a. Septum formation in the atria occurs during weeks 5–7 and begins with the growth of the Septum primum from dorsal and cranial until the Ostium primum is formed.
b. Within the upper part of the Septum primum, the Ostium secundum is created through programmed cell death (apoptosis).
c, e. On the right side of the Septum primum, the Septum secundum develops. Both septa lie adjacent to each other and outline the Foramen ovale.
d, f. The Septum primum forms the Valvula foraminis ovalis which facilitates the directional blood flow from the right into the left atrium (→ Fig. 5.8). After birth, the Valvula foraminis ovalis closes the Foramen ovale due to the increased blood pressure in the left atrium (→ Fig. 5.10).
Prenatal circulation
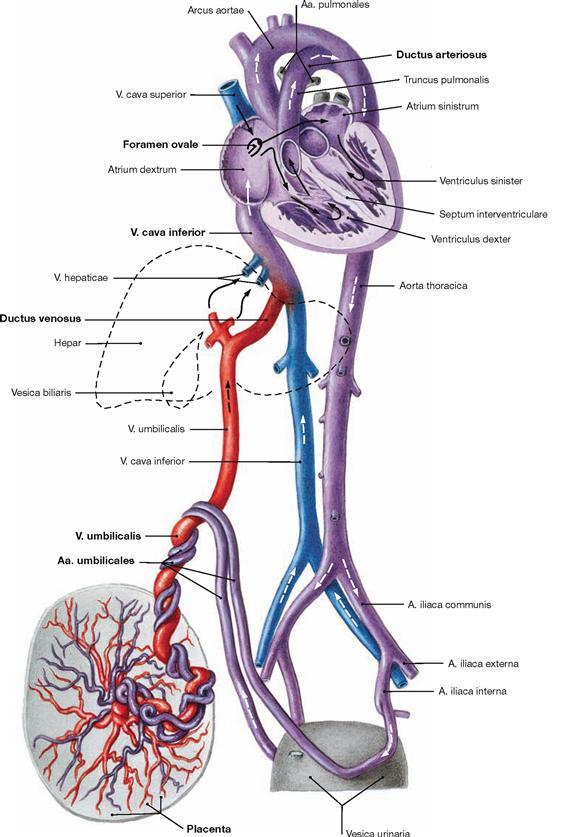
Fig. 5.8 Prenatal circulation (foetal circulation); schematic illustration.
This illustration distinguishes the different oxygen contents of the blood by colour codings: oxygenated blood (red), deoxygenated blood (blue), mixed blood (purple). The arrows mark the direction of blood flow.
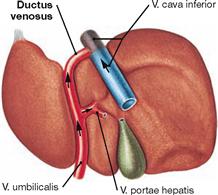
The following aspects distinguish the foetal circulation from the postnatal circulation: umbilical blood vessels, Ductus venosus, Ductus arteriosus, Foramen ovale (→ Fig. 5.10).
Deoxygenated blood from the foetus is conveyed to the placenta by the Aa. umbilicales which derive from the Aa. iliacae internae. After oxygenation the blood from the placenta reaches the foetus via the V. umbilicalis and bypasses the liver through the Ductus venosus due to the high flow resistance of the foetal liver. A valve at the opening of the inferior vena cava (Valvula venae cavae inferioris) directs the incoming blood predominantly through the Foramen ovale to the left atrium. This way, the oxygenated blood takes the shortest way to reach the foetal organs. Blood from the superior vena cava enters the right atrium and right ventricle. From the right ventricle it reaches the Truncus pulmonalis and is shunted through the Ductus arteriosus directly into the Aorta, thus bypassing the non-functional lung circulation.
Postnatal circulation
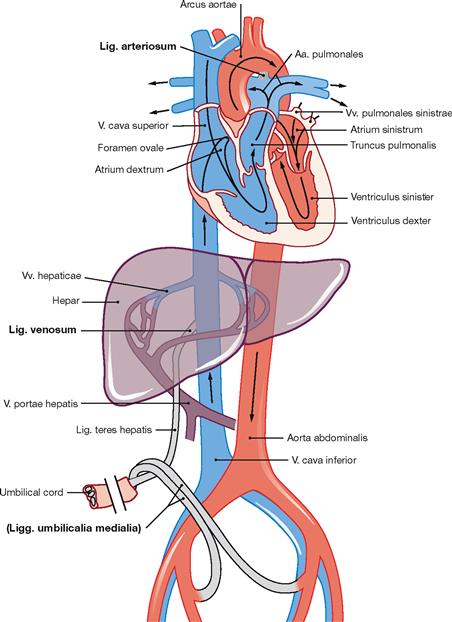
Fig. 5.10 Schematic illustration of the postnatal circulation.
After birth the placental circulation is interrupted. Inflation of the lungs due to breathing opens the pulmonary circulation and causes an increase in blood pressure in the left atrium. The switch from prenatal to postnatal circulation includes the following changes: The valve-like opening of the Foramen ovale between the right and left atrium is closed passively due to the increased blood pressure in the left atrium. Later, the Valvula foraminis ovalis fuses with the Septum secundum leaving the persistent Fossa ovalis in the right atrium.
The Ductus arteriosus functionally closes within a few days and later obliterates to the Lig. arteriosum (→ Fig. 5.13).
The Ductus venosus obliterates postnatally and remains as Lig. venosum at the hilum of the liver.
The umbilical vein obliterates and remains as Lig. teres hepatis between liver and ventral abdominal wall.
The distal parts of the umbilical arteries form the Lig. umbilicale mediale on the right and left side, which contribute to the formation of the respective Plica umbilicalis medialis at the internal relief of the ventral abdominal wall.
The heart in-situ
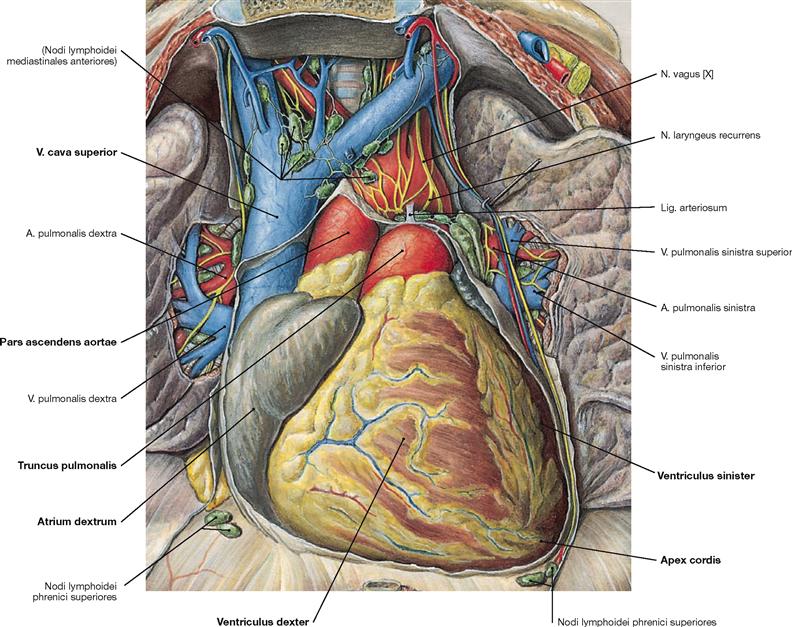
Fig. 5.11 Position of the heart, Cor, within the thorax, Situs cordis; ventral view; after opening of the Pericardium.
The heart is positioned within the pericardial cavity (Cavitas pericardiaca) in the inferior middle mediastinum. The broad base of the heart is oriented in an oblique direction towards the superior right side and corresponds to the valvular plane at the base of the great vessels. The apex of the heart (Apex cordis) points to the inferior left side and ventrally. Base and apex are connected by the longitudinal axis (12 cm) which shows an oblique course in the Thorax directed from the dorsal right side to the ventral left side. Thus, the longitudinal axis of the heart forms an angle of 45º with all three anatominal planes. The heart has four surfaces (→ Fig. 5.2). The anterior surface (Facies sternocostalis) is predominantly formed by the right ventricles. The inferior surface is adjacent to the diaphragm and consists of parts of the right and left ventricles. The inferior surface clinically represents the “posterior wall” in the diagnostic electrocardiogram (ECG) when referred to as posterior myocardial infarction. The Facies pulmonalis is determined by the right atrium on the right side and by the left ventricle on the left side.
Pericardium
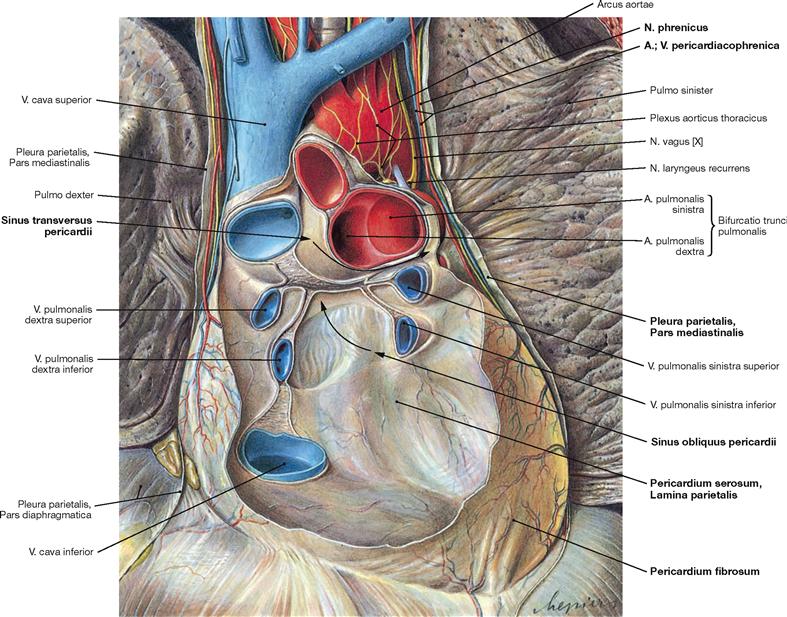
Fig. 5.12 Pericardium, Pericardium; ventral view; after removal of the anterior part of the Pericardium and the heart.
The Pericardium surrounds the heart, stabilises its position and enables the heart to contract without friction. The outer layer of dense connective tissue is the Pericardium fibrosum. Adjacent to the Pericardium fibrosum on the inner side is the Tunica serosa or Pericardium serosum which comprises the parietal layer (Lamina parietalis) of the Pericardium serosum. This Lamina parietalis is a continuation of the Lamina visceralis of the Pericardium (= Epicardium) folding back at the ventral side of the roots of the great cardiac vessels. At the posterior side of the atria, the reflection between the Epicardium and the parietal Pericardium forms a vertical fold between the V. cava inferior and superior and a horizontal fold between the upper pulmonary veins of the right and left side. These folds of the Pericardium create two sinuses of the pericardial cavity at the posterior side (Sinus pericardii, arrows):
• Sinus transversus pericardii: above the horizontal fold between the V. cava superior (posterior) and the Aorta and Truncus pulmonalis (anterior)
• Sinus obliquus pericardii: below the horizontal fold between the pulmonary veins on both sides
The Pericardium fibrosum is connected to:
• the Centrum tendineum of the diaphragm
• the posterior aspect of the Sternum (Ligg. sternopericardiaca)
At the outer side, the fibrous Pericardium is covered by the Pleura parietalis, Pars mediastinalis. The N. phrenicus and the Vasa pericardiacophrenica course between these two layers.
The Epicardium is the visceral layer of the Pericardium serosum.
Heart
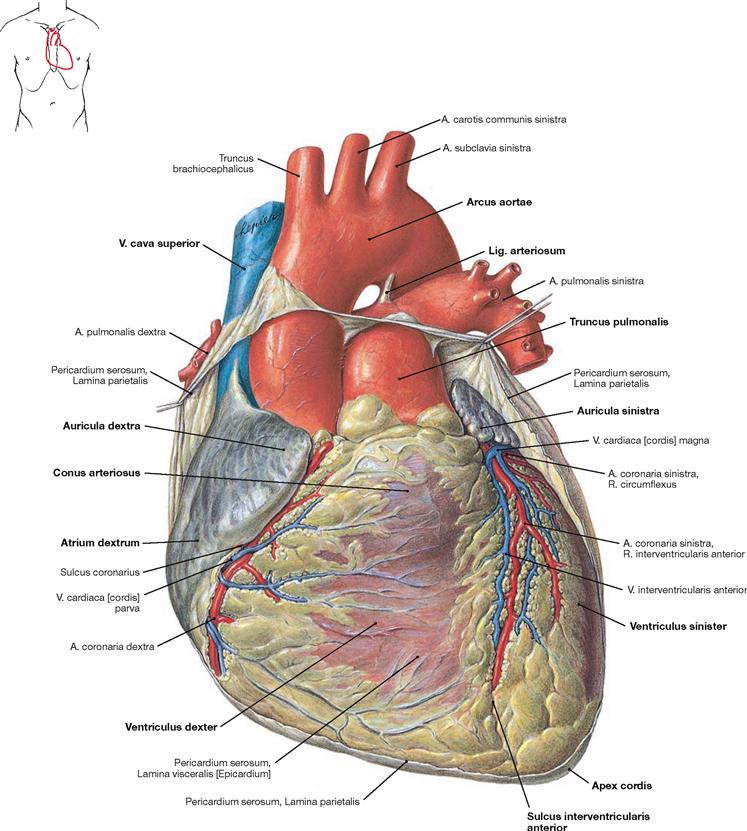
Fig. 5.13 Heart, Cor; ventral view.
The heart weighs 250–300 g and has approximately the size of the fist of the respective person. The apex of the heart (Apex cordis) is directed to the inferior left side. The base of the heart represents the position of the Sulcus coronarius which harbours, among other structures, the A. coronaria dextra. The heart consists of a ventricular chamber (ventricle) and an atrial chamber (atrium) on the right and left side, respectively. At the anterior surface (Facies sternocostalis), the Sulcus interventricularis anterior is visible. It depicts the position of the interventricular septum (Septum interventriculare) and contains the R. interventricularis anterior of the A. coronaria sinistra. At the inferior surface (Facies diaphragmatica), the border between the two ventricles is marked by the Sulcus interventricularis posterior (→ Fig. 5.14). Prior to the transition into the Truncus pulmonalis, the right ventricle is dilated as Conus arteriosus. The origin of the Aorta from the left ventricle is not visible from the outer surface due to the spiral course of the Aorta behind the Truncus pulmonalis. Therefore, the Aorta appears at the right side of the Truncus pulmonalis. The aortic arch is connected with the pulmonary trunk through the Lig. arteriosum, a developmental relict of the Ductus arteriosus of the foetal circulation (→ Fig. 5.8). Both atria have an anterior pouch which is referred to as auricle (Auriculae dextra and sinistra). The V. cava superior and inferior enter the right atrium, the four pulmonary veins enter the left atrium.
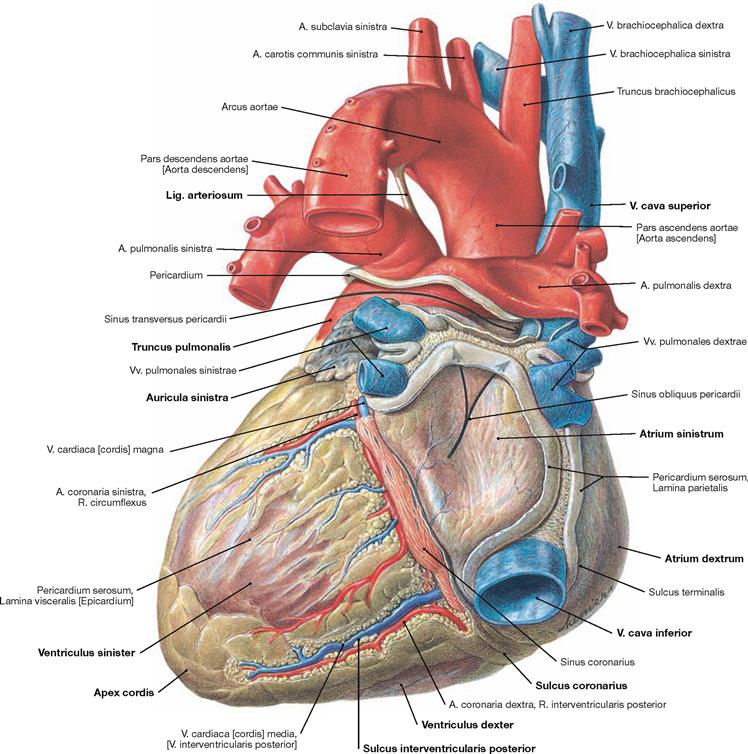
Fig. 5.14 Heart, Cor; dorsal view (explanation → Fig. 5.13).
Cardiac wall
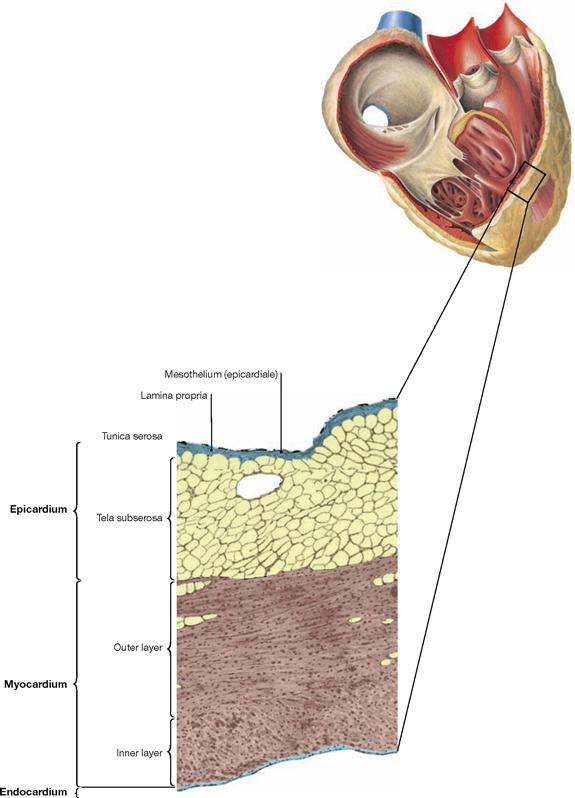
Fig. 5.15 Structure of the cardiac wall; microscopic detail from the right atrium. (according to [2])
The wall of the heart is composed of three layers:
• Endocardium: inner surface consisting of endothelium and connective tissue
• Myocardium: cardiac muscle with cardiomyocytes
• Epicardium: Tunica serosa and Tela subserosa at the outer surface of the heart, representing the visceral layer of the Pericardium serosum. In the human, the Tela subserosa contains plenty of white adipose tissue in which the coronary blood vessels and nerves are embedded.
Cardiac muscle
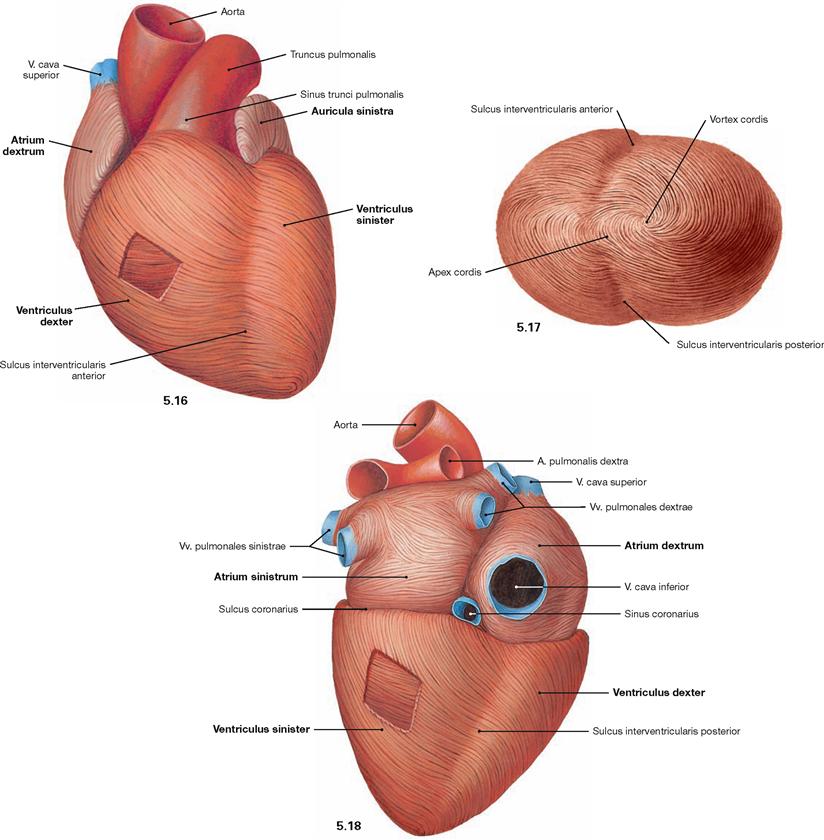
Fig. 5.16 to Fig. 5.18 Cardiac muscle, Myocardium; ventral view (→ Fig. 5.16), view from the apex (→ Fig. 5.17), and dorsocaudal view (→ Fig. 5.18).
The cardiac muscle fibres consist of cardiomyocytes and have a spiral arrangement within the cardiac wall. In the wall of the atria and the right ventricle they form two layers, in the wall of the left ventricle they even form three layers. Thus, the Myocardium and the cardiac wall are much thicker in the region of the left ventricle. In comparison to the right ventricle, this arrangement reflects the much higher pressure required in the left ventricle to pump the blood into the systemic circulation. The right ventricular wall is 3–5 cm thick, the left ventricular wall is 8–12 cm thick.
Heart valves and skeleton of the heart
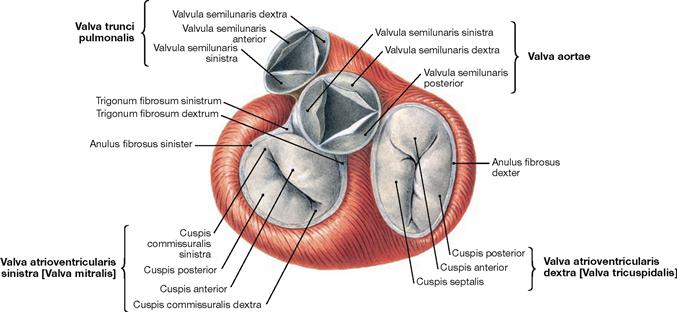
Fig. 5.19 Heart valves, Valvae cordis; cranial view; after removal of the atria, Aorta and pulmonary trunk.
The heart has two atrioventricular valves (Valvae cuspidales) between the atria and the ventricles of each side. The right atrioventricular valve (Valva atrioventricularis dextra) consists of three cusps (tricuspid valve). The left atrioventricular valve (Valva atrioventricularis sinistra) has two cusps (bicuspid valve, mitral valve). The cusps are anchored to the papillary muscles by tendinous cords (Chordae tendineae) to prevent a prolapse of the valves during ventricular contraction. In addition, between the ventricles and the great arteries lie the aortic valve (Valva aortae) on the left side and the pulmonary valve (Valva pulmonalis) on the right side, both of which consist of three semilunar cusps (Valvulae semilunares). When blood is ejected from the ventricles into the great arteries during the systole the semilunar valves are open and the atrioventricular valves are closed. When the ventricles are filled with blood from the atria during the diastole the atrioventricular valves are open and the semilunar valves are closed.
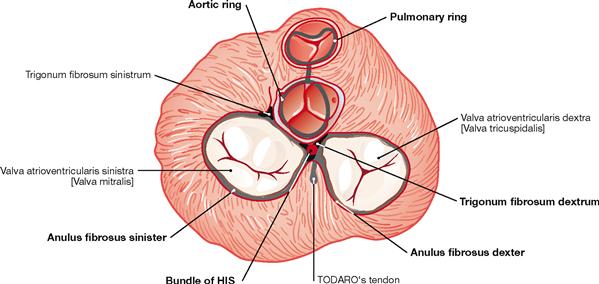
Fig. 5.20 Fibrous skeleton of the heart; cranial view, schematic illustration. (according to [2])
The valves are anchored to the cardiac skeleton. The latter consists of connective tissue forming a ring (Anuli fibrosi dexter and sinister) around the atrioventricular valves (Valvulae atrioventriculares) and a fibrous ring around the semilunar valves (Valvulae semilunares). Between the Anuli fibrosi lies the Trigonum fibrosum dextrum. Here, the bundle of HIS belonging to the conducting system of the heart passes over from the right atrium to the interventricular septum. In addition to the stabilisation of the valves, the fibrous skeleton of the heart serves as an electrical insulator between the atria and the ventricles because all cardiomyocytes are attached to the cardiac skeleton. Since there is no connection between atria and ventricles via cardiomyocytes, the electrical impulse reaches the ventricles exclusively through the bundle of HIS.
Chambers of the heart
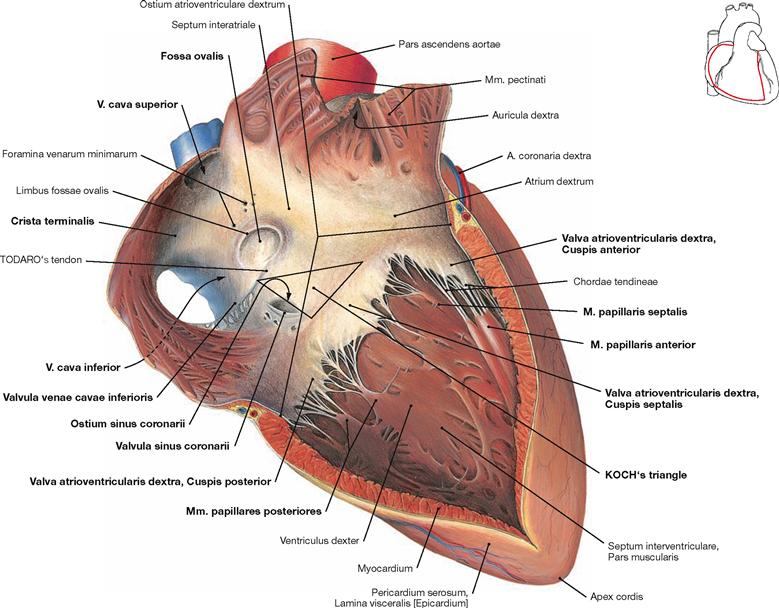
Fig. 5.21 Right atrium, Atrium dextrum, and right ventricle, Ventriculus dexter; ventral view.
The right atrium consists of a part with a smooth inner surface, the sinus of venae cavae (Sinus venarum cavarum), and of a muscular part with a rough inner surface consisting of the pectinate muscles (Mm. pectinati). Both parts are separated by the Crista teminalis, which serves as important landmark for the localisation of the sinu-atrial node (SA node) of the cardiac conducting system (→ pp. 20–22). The SA node is positioned at the outside (subepicardial) of this demarcation line between the entry of the V. cava superior and the right auricle (Auricula dextra). The interatrial septum (Septum interatriale) shows a remnant of the former Foramen ovale, the Fossa ovalis with its rim, the Limbus fossae ovalis. The opening of the Sinus coronarius (Ostium sinus coronarii), which represents the largest cardiac vein, has a valve (Valvula sinus coronarii) and the opening of the V. cava inferior is also demarcated by a valve (Valvula venae cavae inferioris). Both valves, however, are not able to close the respective lumen. Smaller cardiac veins enter the right atrium directly (Foramina venarum minimarum). An extension of the Valvula venae cavae inferioris is the TODARO’s tendon (Tendo valvulae venae cavae inferioris). It serves as a landmark and, together with the opening of the Sinus coronarius and the tricuspid valve (Valva atrioventricularis dextra), it forms the KOCH’s triangle which harbours the AV node (→ Figs. 5.25 to 5.27). In the right ventricle, the three cusps are attached via Chordae tendineae to the three papillary muscles (Mm. papillares anterior, posterior and septalis). Of the interventricular septum (Septum interventriculare) only the muscular part is visible in this illustration. Starting from the interventricular septum, specific fibres of the cardiac conducting system (moderator band described by LEONARDO DA VINCI, not visible here) course to the anterior papillary muscle (M. papillaris anterior). This connection is referred to as the Trabecula septomarginalis (→ Fig. 5.27).
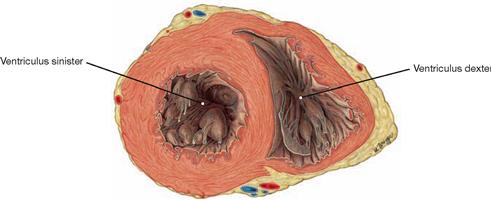
Fig. 5.22 Left and right ventricles, Ventriculus sinister and Ventriculus dexter; cross-section, cranial view.
Because of the substantially stronger muscle layer, the wall of the left ventricle is thicker than the wall of the right ventricle.
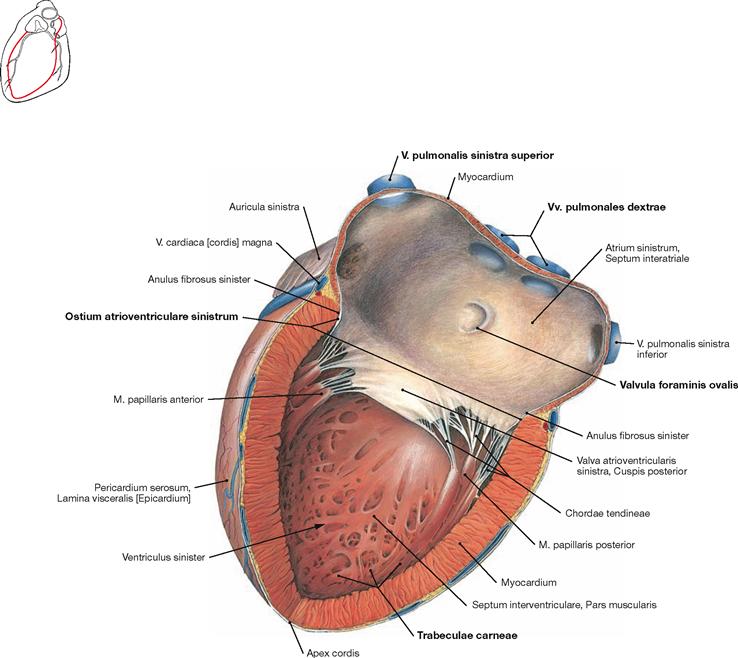
Fig. 5.23 Left atrium, Atrium sinister, and left ventricle, Ventriculus sinister; lateral view.
The auricle (Auricula sinistra) represents the muscular part of the left atrium. The four pulmonary veins (Vv. pulmonales) enter the smooth-walled part of the left atrium. The septal wall shows the crescent-shaped Valvula foraminis ovalis, a remnant of the Septum primum during the development of the heart (→ Fig. 5.7). The Ostium atrioventriculare sinistrum is the junction to the left ventricle and contains the Valva mitralis. The wall of the left ventricle is not smooth but structured by trabeculae of the ventricular Myocardium (Trabeculae carneae).
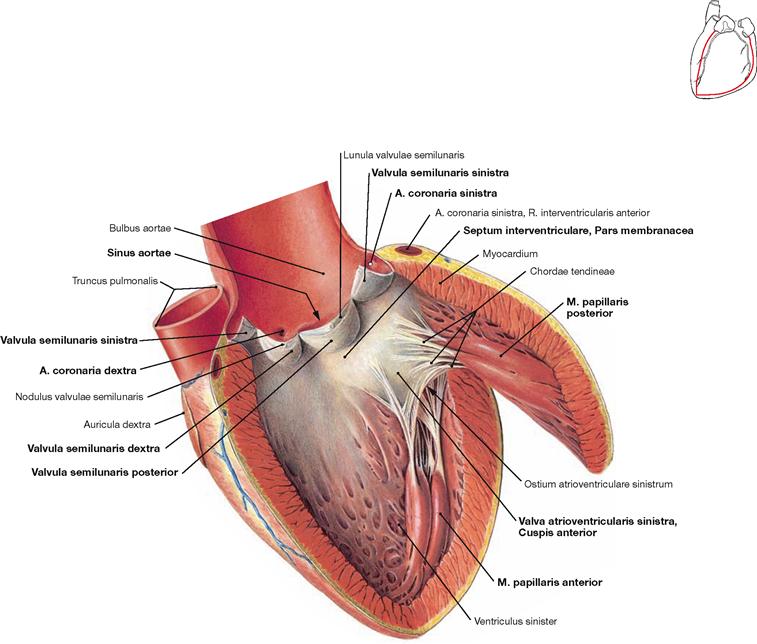
Fig. 5.24 Left ventricle, Ventriculus sinister; lateral view.
The mitral valve (Valva atrioventricularis sinistra) only consists of two cusps. Thus, only two papillary muscles are required (Mm. papillares anterior and posterior). Beneath the mitral valve, the approximately 1 cm2 large area of the Pars membranacea of the interventricular septum is located. However, the major part of the interventricular septum consists of cardiac muscle fibres (Pars muscularis). Blood from the left ventricle is pumped through the aortic valve (Valva aortae) into the dilated part of the Aorta (Bulbus aortae). The aortic valve consists of three semilunar valves (Valvulae semilunares) which cover the Sinus aortae from which the right and left coronary arteries originate (Aa. coronariae dextra and sinistra).
Electrical stimulation and conducting system of the heart
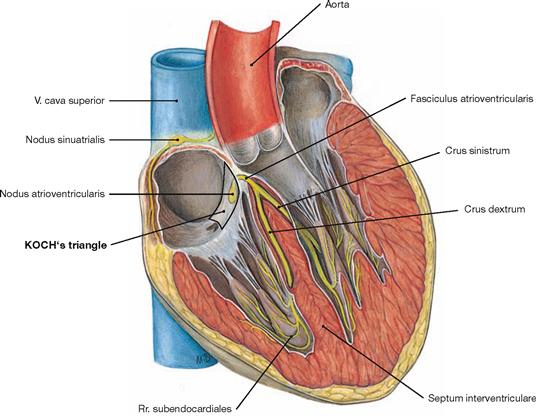
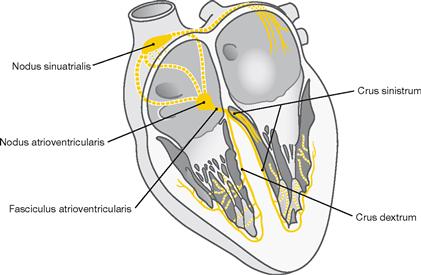
Fig. 5.25 Electrical stimulation and conducting system [Complexus stimulans et conducente cordis] along the axis of the sectioned heart.
The heart harbours an electrical stimulation and conducting system which consists of modified cardiomyocytes instead of nerve fibres. This system is divided into the following parts:
• sinu-atrial node (Nodus sinuatrialis, SA-node; node of KEITH-FLACK)
• atrioventricular node (Nodus atrioventricularis; AV-node, node of TAWARA)
• atrioventricular bundle (Fasciculus atrioventricularis, bundle of HIS)
• right and left bundle branch (Crus dextrum and sinistrum node of TAWARA)
The electrical stimulation is initiated independently within the sinuatrial node by spontaneous depolarisation in the specialised myocardial cells and has a frequency of approximately 70/min. The SA-node has a size of approximately 3 × 10 mm and is located within the wall of the right atrium in a groove (Sulcus terminalis cordis) between the entry of the V. cava superior and the right auricle. This groove corresponds to the Crista terminalis at the inner surface of the right atrium. The SA node is occasionally covered by an area of subepicardial adipose tissue making it visible from outside. The SA node is supplied by the sinu-atrial nodal branch (R. nodi sinuatrialis) which derives from the A. coronaria dextra in most cases. The electrical signal spreads from the SA node through the Myocardium of both atria (myogenic conduction) and reaches the AV node. The latter slows down the frequency of the electrical signal to allow a sufficient filling of the ventricles.
The AV node, approximately 5 × 3 mm in size, is embedded within the Myocardium of the atrioventricular septum at KOCH’s triangle. The KOCH’s triangle is confined by the TODARO’s tendon, the entry of the Sinus coronarius, and the septal cusp of the tricuspid valve (→ Fig. 5.21). The AV node is also supplied by a separate branch (R. nodi atrioventricularis) which usually derives from the dominant coronary artery (in most cases the A. coronaria dextra) near the branching of the R. interventricularis posterior.
From the AV node the electrical signal is conveyed by the bundle of HIS (approx. 4 × 20 mm) through the Trigonum fibrosum dextrum to the interventricular septum.
In the Pars membranacea of the interventricular septum the bundle of HIS divides into the right and left bundle branch. The left bundle branchsplits into the anterior, septal and posterior subendocardial fasciculi to the respective parts of the Myocardium including the papillary muscles and the apex of the heart. The right bundle branch descends subendocardially in the septum to the apex of the heart and reaches the anterior papillary muscle via the Trabecula septomarginalis (→ Fig. 5.27).
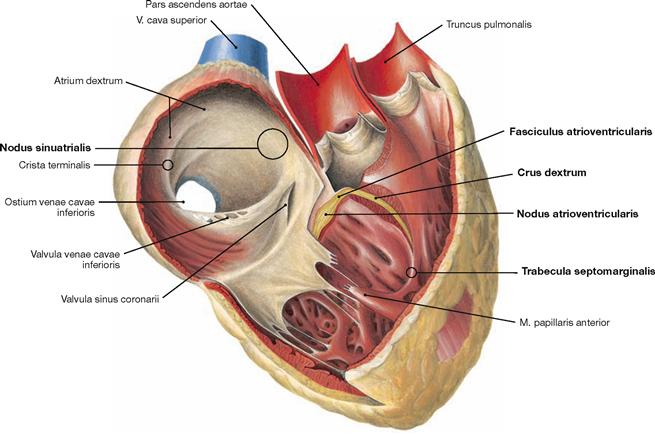
Fig. 5.27 Electrical stimulation and conducting system of the heart.
The electrical stimulation and conducting system is organised in four parts (→ Fig. 5.25).
The illustration demonstrates how a part of the right bundle branch (Crus dextrum) reaches the right anterior papillary muscle via the Trabecula septomarginalis.
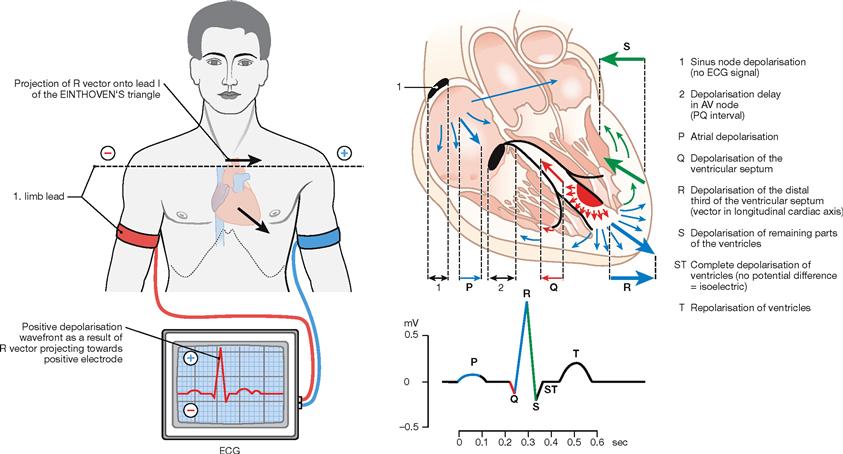
Fig. 5.28 Anatomical principles of the electrocardiogram (ECG). (according to [2])
The electrical signal spreads from the sinu-atrial node to the AV node which causes a delay in electrical conduction before reaching the interventricular septum via the bundle of HIS. The right and left bundle branches then divide and stimulate the ventricular Myocardium. This conduction of electrical impulses within the heart can be detected by electrodes on the surface of the body. If the electrical signal travels towards the electrode at the surface of the body, it results in a positive upward amplitude of the baseline voltage. Because of the small volume of the sinu-atrial node, the SA excitation is not detectable in the ECG. The depolarisation of the atria is represented by the P wave. The depolarisation delay by the AV node occurs during the PQ segment. The latter depicts the lack of polarisation changes during the depolarisation of the entire atrial Myocardium. The rapid retrograde direction of the depolarisation of the interventricular septum is illustrated by the Q wave. Depolarisation of the ventricular myocardium towards the apex of the heart is represented by the ascending limb of the R wave, whereas the propagation of the depolarisation away from the apex results in the descending limb of the R wave and in the short S wave. During the ST segment the entire ventricular Myocardium is depolarized. Since the repolarisation of the ventricular myocardium occurs in the same direction as the depolarisation, the T wave also shows a positive (upward) amplitude. Usually, three limb leads are recorded to determine the electrical axis of the heart according to the largest amplitude of the R wave. However, this electrical axis is influenced by the thickness of the Myocardium in both ventricles and by the excitability of the tissue and is therefore not identical with the anatomical axis of the heart.
Innervation of the heart
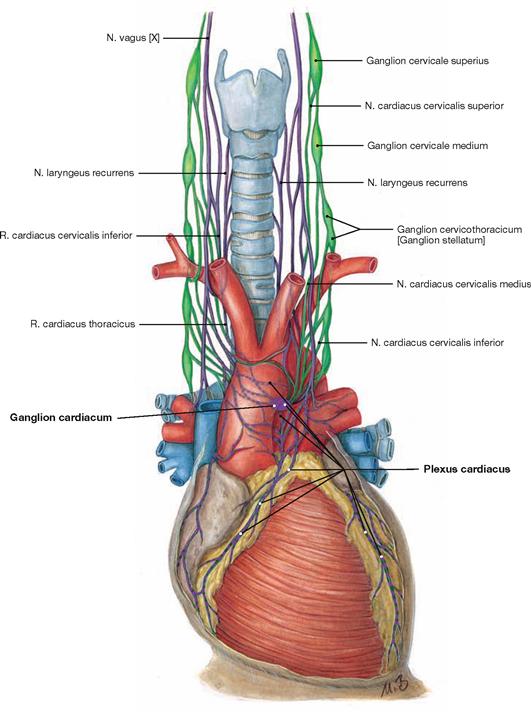
Fig. 5.29 Innervation of the heart: Plexus cardiacus with sympathetic (green) and parasympathetic (purple) nerve fibres; schematic illustration.
The function of the electrical conducting system and the Myocardium can be modified by autonomic innervation to adjust to the needs of the whole body. This is the purpose of the Plexus cardiacus as part of the autonomic nervous system. The Plexus cardiacus consists of sympathetic and parasympathetic nerve fibres. The cell bodies (Perikarya) of the postganglionic sympathetic nerve fibres reside within the cervical ganglia of the sympathetic trunk (Truncus sympathicus) and reach the Plexus cardiacus via three nerves (Nn. cardiaci cervicales superior, medius and inferior). Sympathetic stimulation increases the heart rate (positive chronotropic effect), the speed of conduction (positive dromotropic effect), and the excitability (positive bathmotropic effect) of the cardiomyocytes. In addition, sympathetic stimulation enhances the contractile force (positive inotropic effect) due to accelerated relaxation (positive lusitropic effect). Parasympathetic stimulation elicits negative chronotropic, dromotropic, and bathmotropic effects and, additionally, has negative inotropic effects on the atrial Myocardium. The parasympathetic nerve fibres derive as preganglionic nerve fibres from the N. vagus [X] and reach the Plexus cardiacus as Rr. cardiaci cervicales superior and inferior and as Rr. cardiaci thoracici. In the Plexus cardiacus, they are synapsed within numerous (up to 500) tiny ganglia (Ganglia cardiaca) onto postganglionic neurons.
Coronary arteries
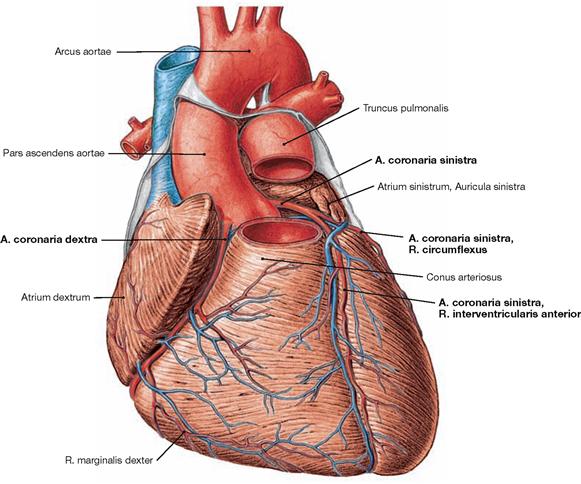
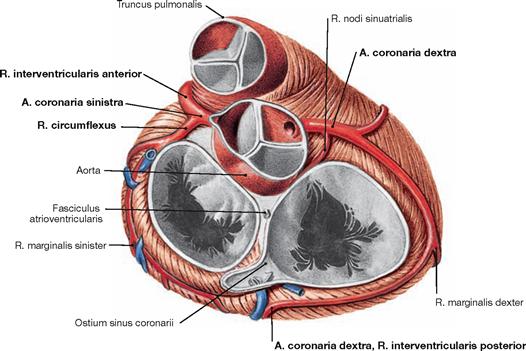
Fig. 5.30 Coronary arteries, Aa. coronariae; ventral view.
The right coronary artery (A. coronaria dextra) has its origin at the right aortic sinus and courses in the Sulcus coronarius to the inferior margin (Margo dexter). It continues to the Facies diaphragmatica where in most cases the R. interventricularis posterior branches off as a terminal branch.
The left coronary artery (A. coronaria sinistra) originates at the left aortic sinus and divides after 1 cm to form the R. interventricularis anterior, which courses to the apex of the heart, and the R. circumflexus. The latter courses in the Sulcus coronarius around the left cardiac margin to reach the posterior aspect of the heart.
Conventionally, the coronary dominance is determined by the artery that supplies the R. interventricularis posterior. In most cases the right coronary artery is dominant (in the “co-dominant” and the “right-dominant” perfusion type, together in 75% of all cases → pages 26 and 27).
Veins of the heart
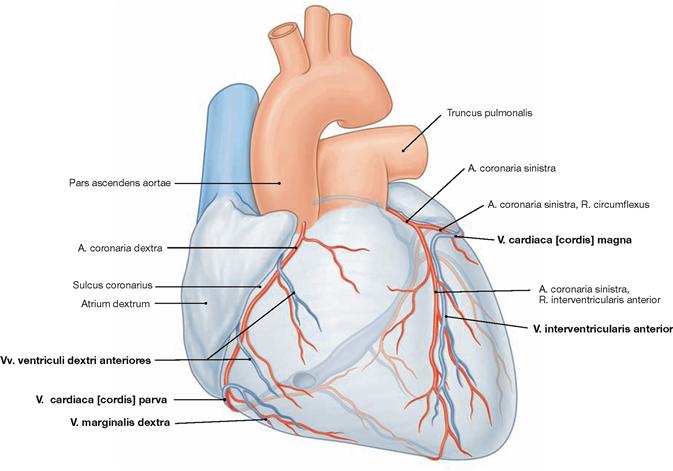
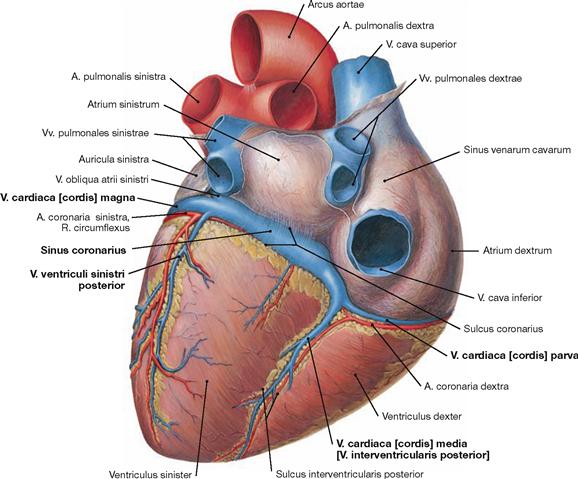
Fig. 5.32 Cardiac veins, Vv. cordis; ventral view. [8]
The venous blood from the heart is collected in three major systems. 75% of the venous blood are collected in the Sinus coronarius and drain into the right atrium. The remaining 25% of the venous blood drain into the atria and ventricles directly via the transmural and the endomural system (→ pages 17–19).
Coronary artery dominance
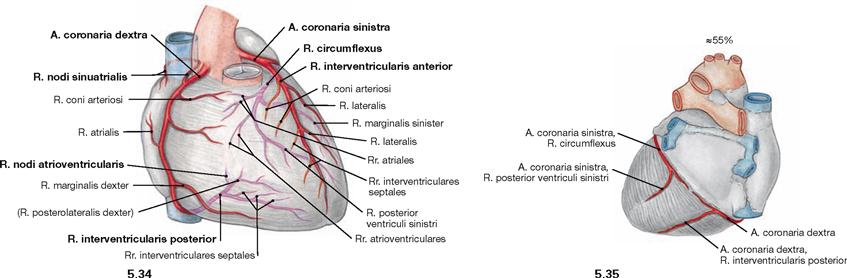
Fig. 5.34 and Fig. 5.35 “Balanced” or co-dominant coronary circulation between the coronary arteries, Aa. coronariae; ventral (→ Fig. 5.34) and dorsal (→ Fig. 5.35) views.
In 55% of all cases, the R. interventricularis posterior originates from the A. coronaria dextra but does not supply the posterior aspect of the left ventricle. This is referred to as a “balanced” or “co-dominant” perfusion type.
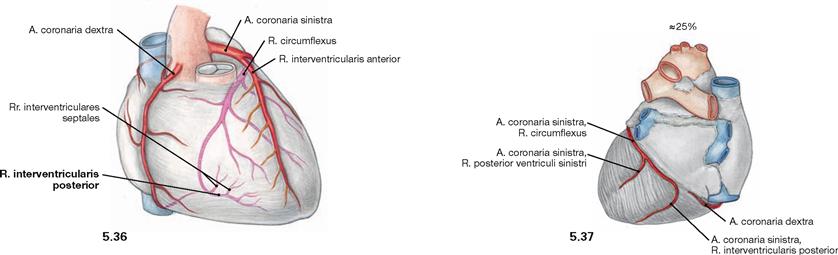
Fig. 5.36 and Fig. 5.37 “Left dominant” coronary circulation between the coronary arteries, Aa. coronariae; ventral (→ Fig. 5.36) and dorsal (→ Fig. 5.37) views.
In 25% of all cases, the R. interventricularis posterior originates from the A. coronaria sinistra.
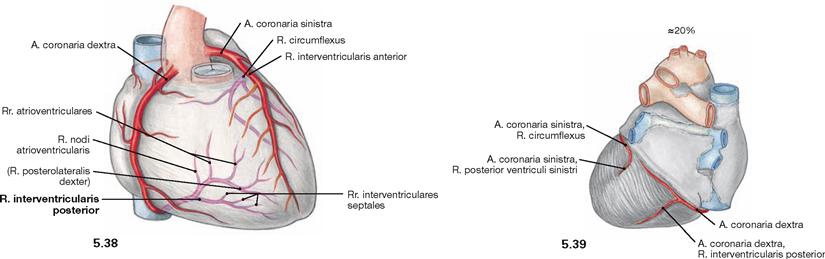
Fig. 5.38 and Fig. 5.39 “Right-dominant” coronary circulation between the coronary arteries, Aa. coronariae; ventral (→ Fig. 5.38) and dorsal (→ Fig. 5.39) views.
In 20% of all cases, the A. coronaria dextra not only branches off the R. interventricularis posterior but also supplies parts of the posterior aspect of the left ventricle.
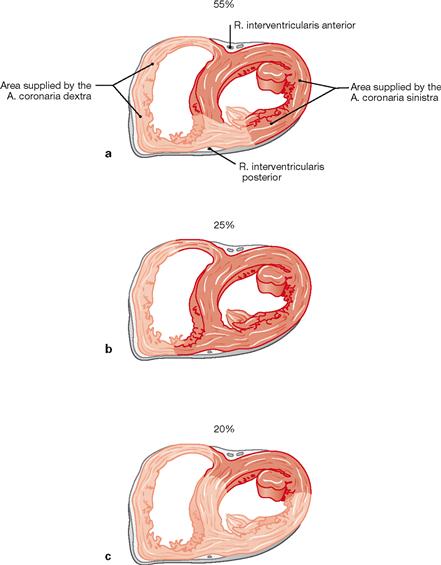
Figs. 5.40a to c Areas supplied by the A. coronaria dextra (light red) and sinistra (dark red) in the cross-section; caudal view. (according to [2]
a. Balanced or co-dominant perfusion type: The left coronary artery supplies the anterior two-thirds of the septum via the Rr. interventriculares septales of the R. interventricularis anterior (left anterior descending [LAD] branch). Corresponding branches derived from the R. interventricularis posterior of the right coronary artery supply the posterior third of the septum.
b. Left-dominant perfusion type: The left coronary artery supplies the entire septum and the AV node.
c. Right-dominant perfusion type: Two thirds of the septum and large areas of the posterior aspect of the left ventricle are supplied by the A. coronaria dextra.
The perfusion type has effects on the severity of a myocardial infarction due to an occlusion of one of the coronary arteries.
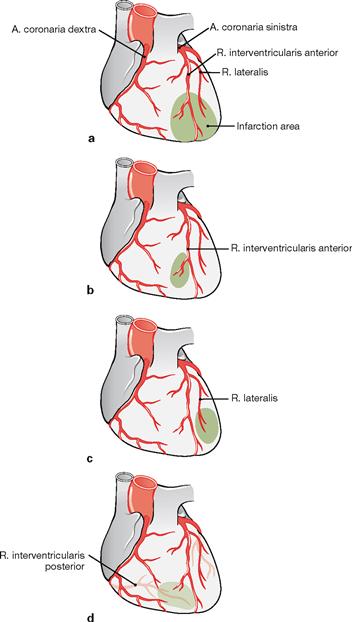
Figs. 5.41a to d Infarction pattern owing to the occlusion of the coronary arteries.
a. Isolated occlusion of the R. interventricularis anterior (left anterior descending [LAD] branch) results in an anterior myocardial infarction.
b. Distal occlusion of the R. interventricularis anterior results in myocardial infarction of the apex of the heart, often referred to as apical infarction.
c. If only the R. lateralis is occluded the myocardial infarction is restricted to the lateral wall of the ventricle.
d. Occlusion of the R. interventricularis posterior results in a posterior myocardial infarction (PMI) of the Facies diaphragmatica.
Projection of trachea and bronchi
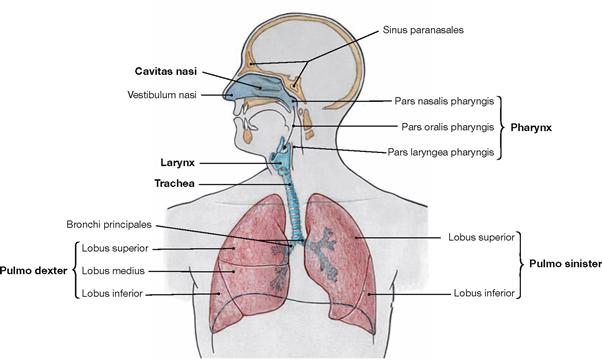
Fig. 5.42 Upper and lower respiratory tract; schematic illustration.
The respiratory system is devided in upper and lower parts.
The upper respiratory tract comprises:
The lower respiratory tract comprises:
The right lung (Pulmo dexter) has three lobes, the left lung (Pulmo sinister) has two lobes.
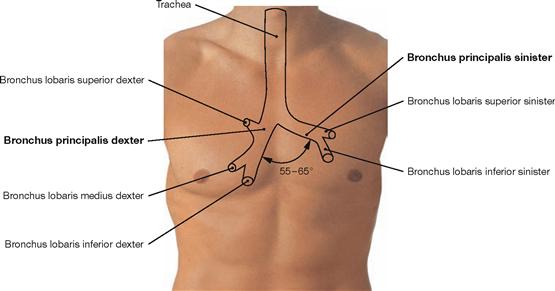
Fig. 5.43 Projection of the Trachea and main bronchi onto the anterior chest wall.
The Trachea is 10–13 cm long and elongates up to 5 cm during deep inspiration. The origin of the Trachea at the cricoid cartilage projects onto the 7th cervical vertebra; the bifurcation of the Trachea into the two main bronchi projects onto the 4th and 5th thoracic vertebrae (rib II to III). The angle between the main bronchi is 55º to 65º. The right main bronchus (Bronchus principalis dexter) is larger, 1–2.5 cm in length, and is positioned nearly vertically. The left main bronchus (Bronchus principalis sinister) is almost twice as long and located more horizontally.
Projection of the lungs
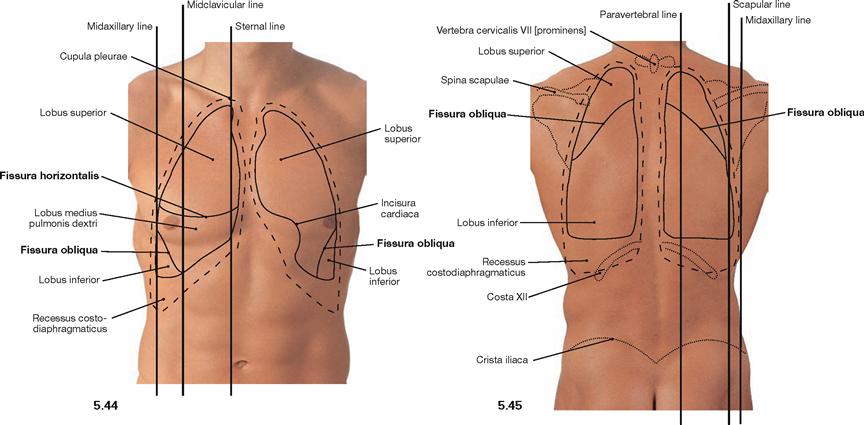
Fig. 5.44 and Fig. 5.45 Projection of the borders of the lungs and Pleura onto the anterior (→ Fig. 5.44) and posterior (→ Fig. 5.45) thoracic walls.
The right lung has three lobes which are separated by the Fissura obliqua and the Fissura horizontalis. On the dorsal side, the Fissura obliqua follows rib IV and, thus, separates the superior and the inferior lobes. From the midaxillary line onwards, the Fissura obliqua descends more steeply to reach rib VI at the midclavicular line. Anteriorly, the Fissura obliqua separates the middle and inferior lobes (→ Figs. 5.53 and 5.54). The Fissura horizontalis projects along rib IV on the anterior chest wall and separates the superior and the middle lobes.
The left lung only has two lobes which are separated by the Fissura obliqua. Because the heart enlarges the Mediastinum to the left side (Incisura cardiaca), the volume of the left lung is smaller and the position of the left lung differs in the sternal and midclavicular lines (see table).
Each pleural cavity (Cavitas pleuralis) is lined by the parietal pleura (Pleura parietalis). The Pleura parietalis is divided into Pars mediastinalis, Pars costalis, and Pars diaphragmatica (→ Fig. 5.65). The pleural cavities have four pleural recesses (Recessus pleurales). The largest recessus is the Recessus costodiaphragmaticus which expands laterally up to 5 cm in the midaxillary line.
| Borders of the Right Lung | Borders of the Left Lung | |
| Sternal line | crosses rib VI | crosses rib IV |
| Midclavicular line | parallel to rib VI | crosses rib VI |
| Midaxillary line | crosses rib VIII | as right side |
| Scapular line | crosses rib X | as right side |
| Paravertebal line | crosses rib XI | as right side |
pleural borders: one rib lower each
Development
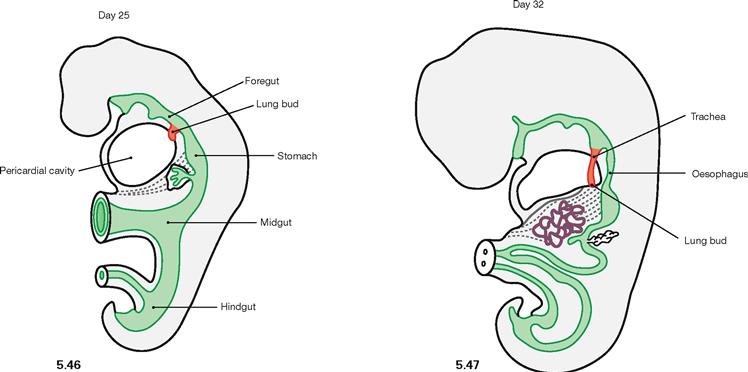
Fig. 5.46 and Fig. 5.47 Development of the lower respiratory tract on day 25 (→ Fig 5.46) and day 32 (→ Fig 5.47). (according to [3])
In week 4, the epithelial tissues of Larynx, Trachea, and lungs begin to develop from the endoderm of the foregut. Connective tissue, smooth muscles, and blood vessels derive from the surrounding mesoderm.
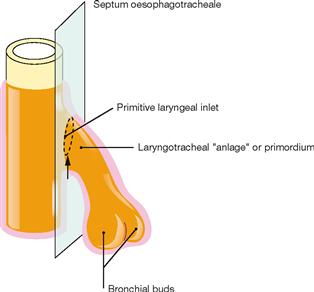
Fig. 5.48 Development of the Septum oesophagotracheale. [20]
During week 4 and 5, mesenchymal folds develop on both sides which fuse to the Septum oesophagotracheale and separate the primordium of the lower respiratory tract from the Oesophagus.
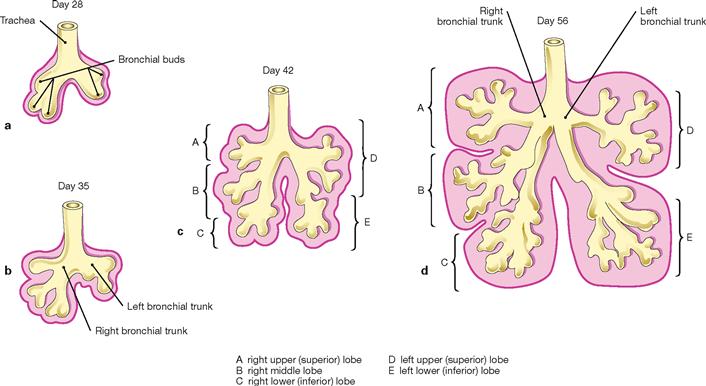
Figs. 5.49a to d Stages of the lung development. [20]
Three stages of the lung development are recognised which partly overlap:
Trachea and bronchi
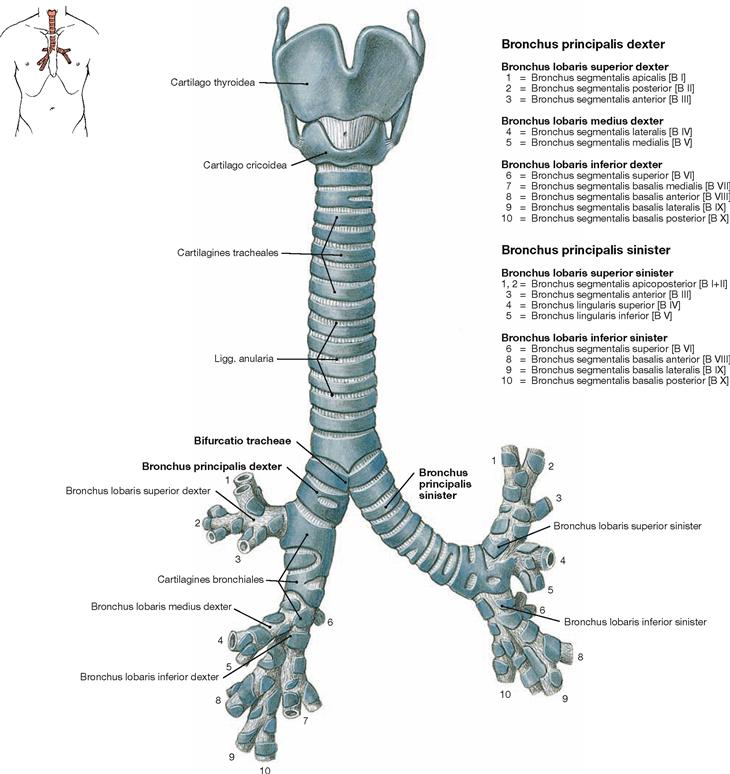
Fig. 5.50 Lower respiratory tract with larynx, Larynx, trachea, Trachea and bronchi, Bronchi; ventral view.
The Trachea is 10–13 cm long and extends from the cricoid cartilage of the Larynx to its division (Bifurcatio tracheae) into the two main (primary) bronchi (Bronchi principales). The Trachea is organised in a cervical part (Pars cervicalis) and a thoracic part (Pars thoracica). Projection and topography are described in → Fig 5.43. The main bronchi further divide in three and two lobar bronchi (Bronchi lobares) on the right and left sides, respectively. The lobar bronchi give rise to the segmental bronchi (Bronchi segmentales). The right lung has 10 segments and, thus, 10 segmental bronchi. In the left lung, however, segment 7 and the respective Bronchus are missing.
The more detailed systematic description of the bronchial tree is not illustrated here. The bronchi further divide six- to twelvetimes before continuing as bronchioles. Bronchioles have a diameter smaller than 1 mm and lack cartilage and glands within their walls. Each bronchiole is associated with a pulmonary lobule (Lobulus pulmonis) and further divides three- to fourtimes before continuing as terminal bronchioles (Bronchioli terminales). These represent the last segment of the air conducting part of the respiratory system which has a volume of 150–170 ml. Each Bronchiolus terminalis opens into a pulmonary acinus (Acinus pulmonis) which generates 10 additional generations of Bronchioli respiratorii with Ductus and Sacculi alveolares. All parts of the acinus contain alveoli and, thus, the acinus belongs to the gas-exchanging part of the respiratory system.
Structure of trachea and bronchi
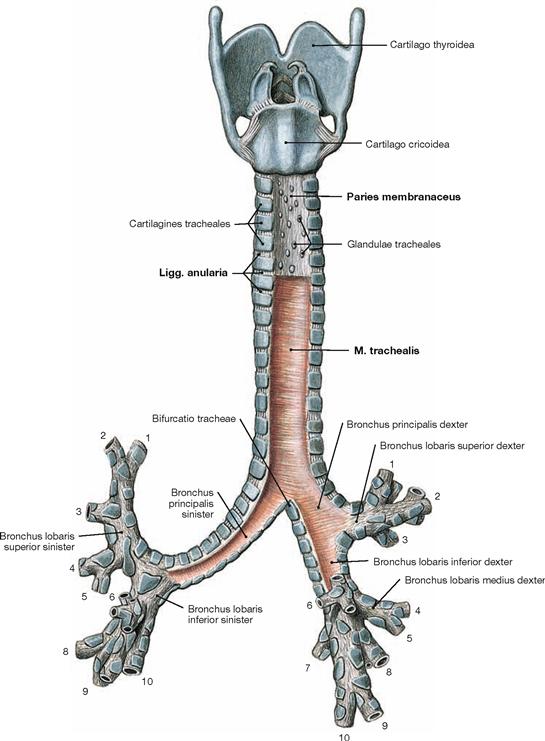
Fig. 5.51 Lower respiratory tract with larynx, Larynx, trachea, Trachea, and bronchi, Bronchi; dorsal view.
The systematic composition of the bronchial tree is described in → Figure 5.50. The dorsal view clearly shows that the dorsal walls of the Trachea and the main bronchi do not consist of cartilage (Paries membranaceus) but predominantly of smooth muscles (M. trachealis). The incomplete tracheal cartilages are connected by Ligg. anularia. These comprise elastic connective tissue and enable the elongation of the trachea for up to 5 cm during deep inspiration.
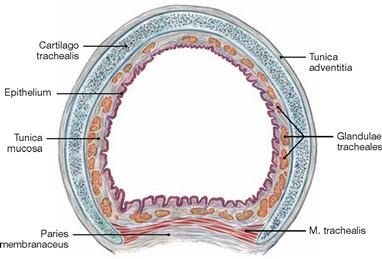
Fig. 5.52 Trachea, Trachea; cross-section, microscopic view.
The walls of the trachea and the main bronchi comprise a mucous membrane (Tunica mucosa) on the luminal side followed by the Tunica fibromusculocartilaginea and the Tunica adventitia. The Tunica fibromusculocartilaginea consists of 16 to 20 horseshoe-shaped incomplete tracheal cartilages of hyaline cartilage, which are bridged posteriorly by a smooth muscle (M. trachealis).
Lungs
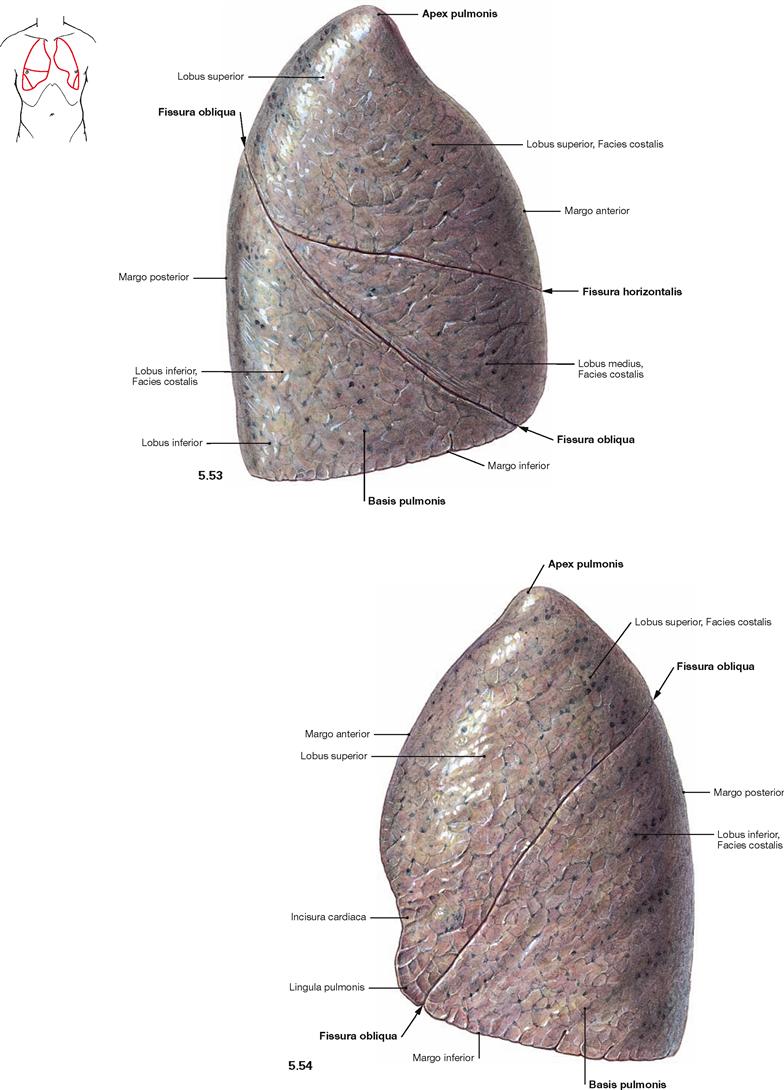
Fig. 5.53 and Fig. 5.54 Right lung, Pulmo dexter (→ Fig. 5.53), and left lung, Pulmo sinister (→ Fig. 5.54); lateral view.
The right lung has three lobes (Lobi superior, medius and inferior) which are separated by the Fissura obliqua and the Fissura horizontalis. The left lung has only two lobes (Lobi superior and inferior) separated by the Fissura obliqua. The Lingula pulmonis of the superior lobe is equivalent to the middle lobe of the right lung and forms a tongue-like extension inferior to the Incisura cardiaca.
The volume of the right lung encompasses 2–3 l, during maximal inspiration even 5–8 l. This volume is equivalent to a gas exchange area of 70–140 m2. Due to the left-shifted position of the heart the volume of the left lung is smaller by 10–20%.
The apex of the lung (Apex pulmonis) is cranial part, the broad base of the lung (Basis pulmonis) is the caudal part of the lung. The surface of the lung is covered by the Pleura visceralis and has three surface alignments. The Facies costalis is located laterally and continues at the Margo inferior as the Facies diaphragmatica (→ Figs. 5.55 and 5.56). At the Margo anterior and the blunt Margo posterior it continues as the Facies mediastinalis towards the Mediastinum.
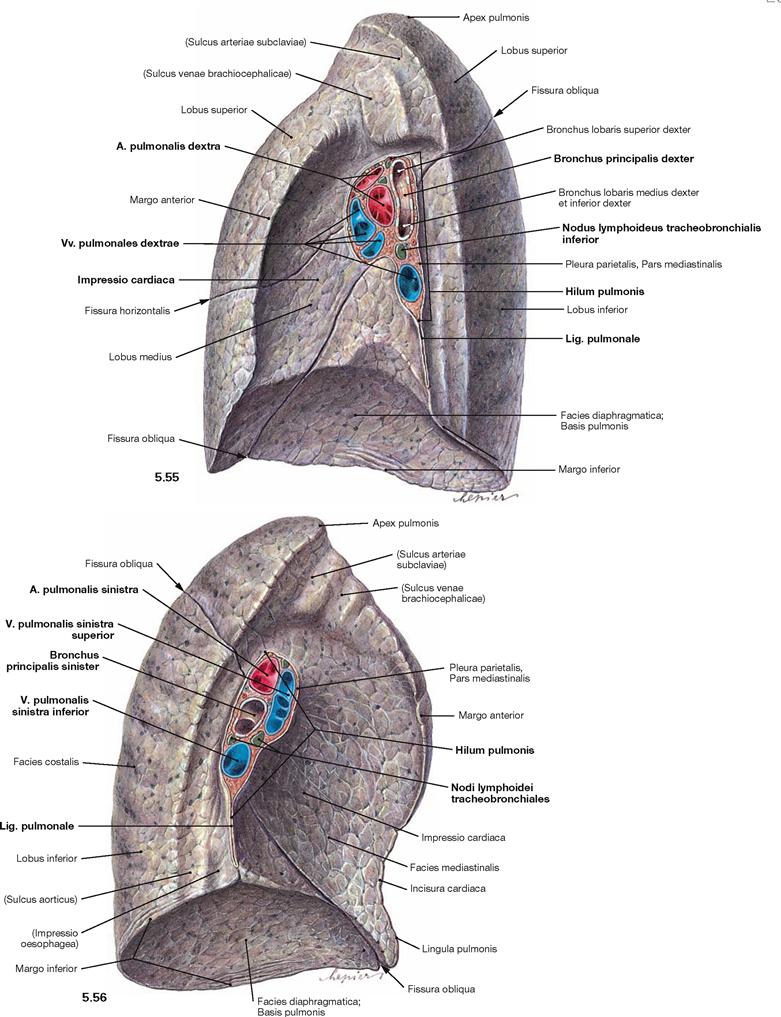
Fig. 5.55 and Fig. 5.56 Right lung, Pulmo dexter (→ Fig. 5.55), and left lung, Pulmo sinister (→ Fig. 5.56); medial view.
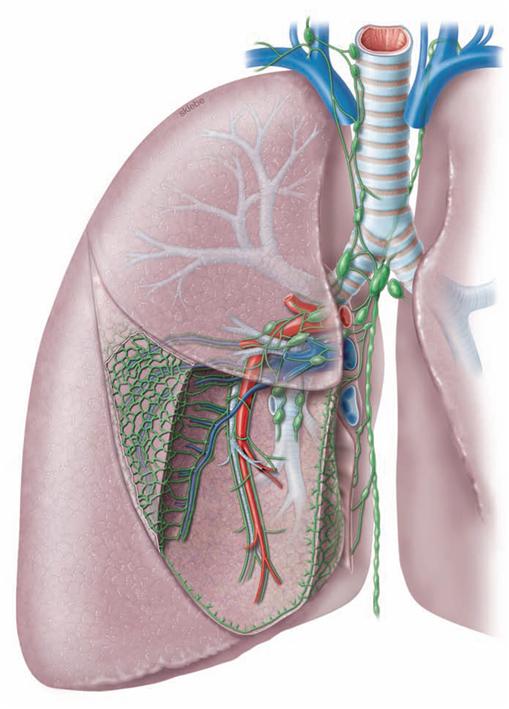
The Hilum pulmonis is the medially positioned entry for the main bronchi and the neurovascular structures to the lungs, which together are referred to as the root of the lung (Radix pulmonis). At the hilum, the Pleura visceralis is blends into the Pleura parietalis and both parts line the pleural cavity. This pleural fold extends inferiorly into the Lig. pulmonale.
The topographical orientation of the main bronchi in relation to the great blood vessels at the hilum of the lung is different for both lungs. At the right lung, the Bronchus principalis is the most superior structure and the Vv. pulmonales are positioned anteriorly. In contrast, the main bronchus of the left lung is positioned below the A. pulmonalis. When dissecting the root of the lung, the hilum frequently shows several lymph nodes (Nodi lymphoidei tracheobronchiales), which are normally black due to deposits of carbon dust. The Facies mediastinalis is concave-shaped (more pronounced at the left side) by the heart (Impressio cardiaca). Both lungs show impressions which are caused by adjacent blood vessels or, on the left side, the oesophagus. These impressions nicely demonstrate the topographical relations of the lungs to neighbouring organs but they are, similar to the margins of the lungs, only apparent in the fixed lungs (fixation artefacts).
Bronchopulmonary segments
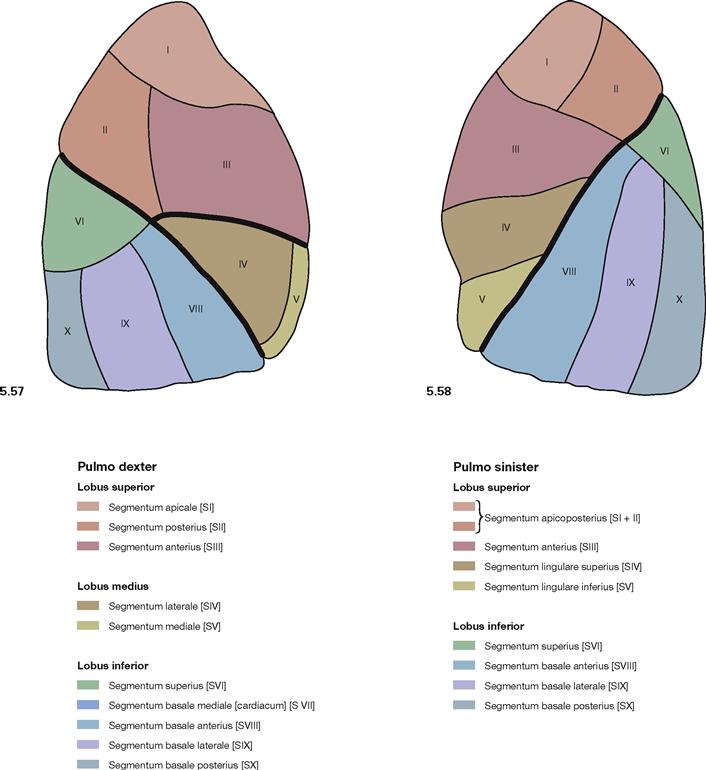
Fig. 5.57 and Fig. 5.58 Bronchopulmonary segments, Segmenta bronchopulmonalia, of the right lung (→ Fig. 5.57) and the left (→ Fig. 5.58) lung; lateral view.
The lobes of the lung are organised in cone-shaped lung (bronchopulmonary) segments which are incompletely divided by septations of connective tissue. The segmental borders are not visible on the surface of the lung. The lung segments are associated with segmental bronchi and segmental branches of the pulmonary artery. The right lung has ten segments, three in the superior, two in the middle, and five in the inferior lobe. The left lung only has nine segments since segment VII (Segmentum basale mediale → Fig. 5.59) on the left side is missing or drastically reduced and fused with segment VIII due to the larger extension of the Mediastinum. The organisation of the other lung segments is similar on both sides since the segments of the middle lobe of the right lung are equivalent to the two segments of the Lingula pulmonis in the left lung.
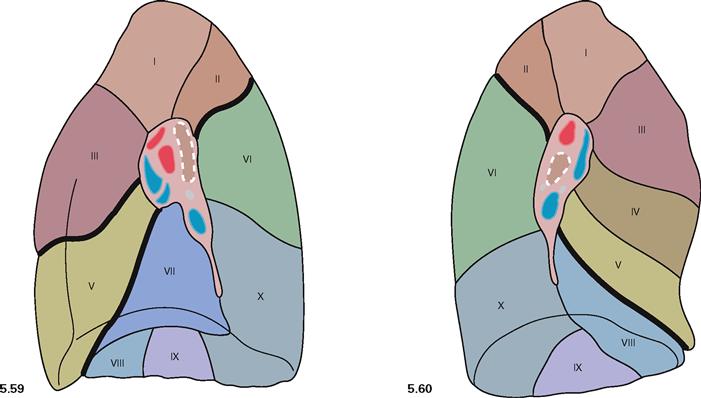
Fig. 5.59 and Fig. 5.60 Bronchopulmonary segments, Segmenta bronchopulmonalia, of the right lung (→ Fig. 5.59) and the left (→ Fig. 5.60) lung; medial view.
The right lung has ten segments. The left lung only has nine segments; segment VII (Segmentum basale mediale) is missing.
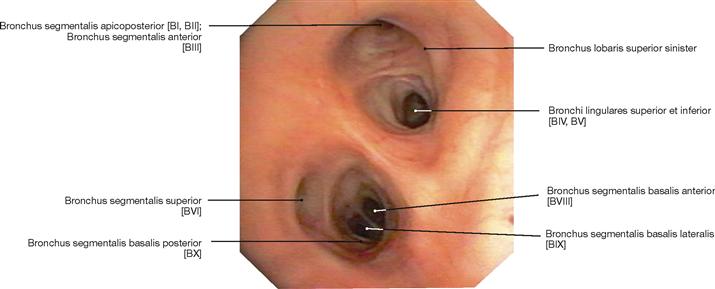
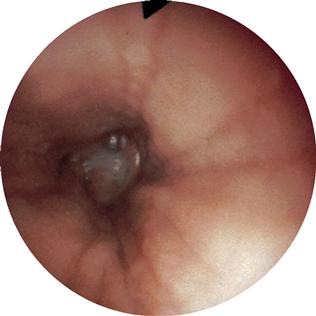
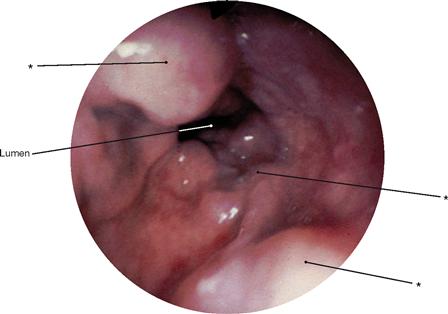
Fig. 5.61 Bronchi, Bronchi; bronchoscopy showing the segmental bronchi of the left side. It is apparent that the segmental bronchus VII is missing on the left side (→Fig. 5.60).
Blood vessels of the Lungs
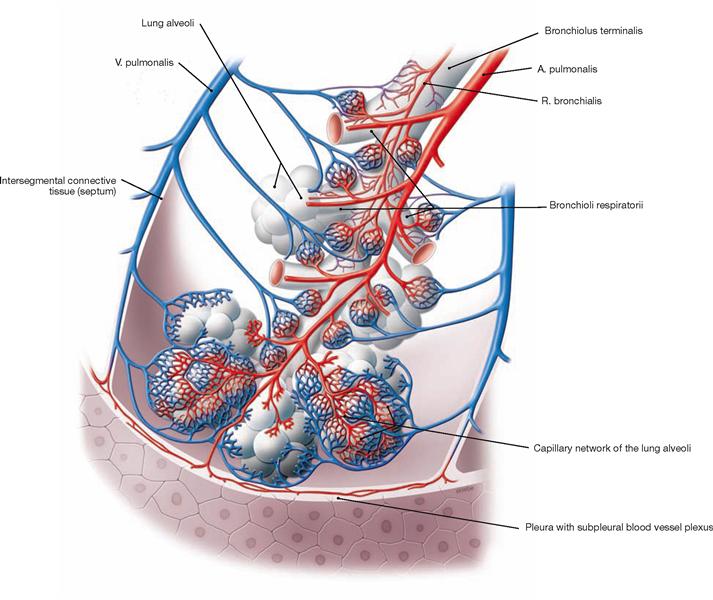
Fig. 5.62 Acinus of the lung, Acinus pulmonis, with blood vessels.
The lung has two blood vessel systems which communicate through their terminal branches in the wall of the alveoli (alveolar septa). The Aa. pulmonales and Vv. pulmonales of the pulmonary circulation constitute the Vasa publica which serve for the gas exchange of the blood. Branches of the Aa. pulmonales course in the peribronchial and subpleural connective tissue and transport the deoxygenated blood from the right heart to the alveoli. The Vv. pulmonales are located in the intersegmental connective tissue and transport the oxygenated blood to the left atrium.
The Vasa privata of the lung supply the lung tissue itself. The arterial Rr. bronchiales and the Vv. bronchiales course together with the bronchi.
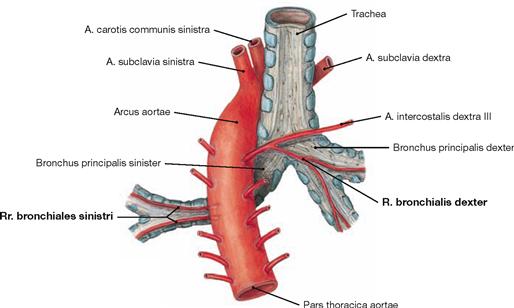
Fig. 5.63 Vasa privata of the lung; dorsal view.
The arterial Rr. bronchiales derive directly from the Aorta thoracica on the left side, but usually branch off the third intercostal artery (A. intercostalis dextra III) on the right side. The Vv. bronchiales drain into the azygos system (not shown here).
Lymph vessels and lymph nodes of the lung
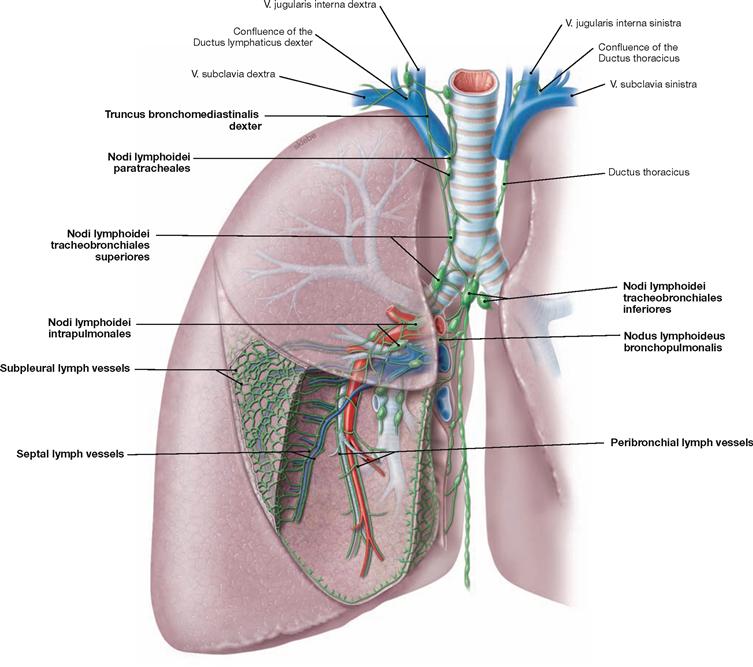
Fig. 5.64 Lymph vessels, Vasa lymphatica, and lymph nodes, Nodi lymphoidei, of the lung; ventral view; schematic illustration.
The lung has two lymph vessel systems which converge at the hilum. The peribronchial system follows the bronchi and feeds into several lymph node stations. The first station are the Nodi lymphoidei intrapulmonales at the transition from lobar to segmental bronchi. The second station comprises the Nodi lymphoidei bronchopulmonales at the hilum of the lung. The subsequent Nodi lymphoidei tracheobronchiales are located already at the root of the lung. Nodi lymphoidei tracheobronchiales superiores and inferiores are distinguished according to their location above and below the tracheal bifurcation. From here the lymph passes on to the Nodi lymphoidei paratracheales or to the Trunci bronchomediastinales on both sides. Thus, there is no strict separation of the lymph drainage from the different sides.
The subpleural and the septal lymph system drain into the Nodi lymphoidei tracheobronchiales as the first station. Their delicate lymph vessels form a polygonal network at the surface of the lung. This network represents the boundaries of distinct pulmonary lobules. Due to carbon dust deposits (exhaust fumes and cigarette smoke) these lymph vessels and the boundaries of the pulmonary lobules are clearly visible.
Lungs and pleural cavities
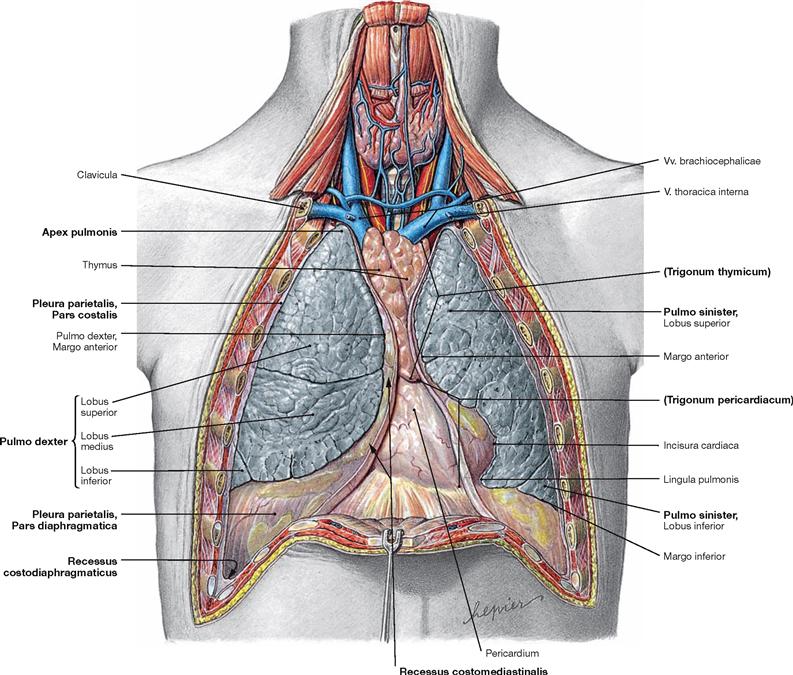
Fig. 5.65 Lungs, Pulmones, and pleural cavities, Cavitates pleurales, of an adolescent; ventral view; after removal of the anterior thoracic wall.
The pleural cavity (Cavitas pleuralis) is covered by the parietal Pleura (Pleura parietalis). The parietal Pleura is divided into Pars mediastinalis, Pars costalis, and Pars diaphragmatica. The visceral Pleura (Pleura visceralis) covers the outer surface of the lungs. The capillary space between both pleural layers contains 5 ml of a serous fluid which lubricates the pleural surfaces and reduces friction during breathing. The pleural cupula (Cupula pleurae) extends up to 5 cm above the superior thoracic aperture. The superior and inferior medial borders of the Pleura form the boundaries of the Trigonum thymicum and the Trigonum pericardiacum, respectively. The pleural cavities possesses four pleural recesses (Recessus pleurales) into which the lungs can expand during deep inspiration:
• Recessus costodiaphragmaticus:
lateral, in the midaxillary line up to 5 cm deep
• Recessus costomediastinalis:
ventral, to both sides of the Mediastinum and chest wall
• Recessus phrenicomediastinalis:
caudal, between diaphragm and Mediastinum
• Recessus vertebromediastinalis:
dorsal, adjacent to the vertebral column (→ Fig. 5.104)
Thoracic viscera, radiography
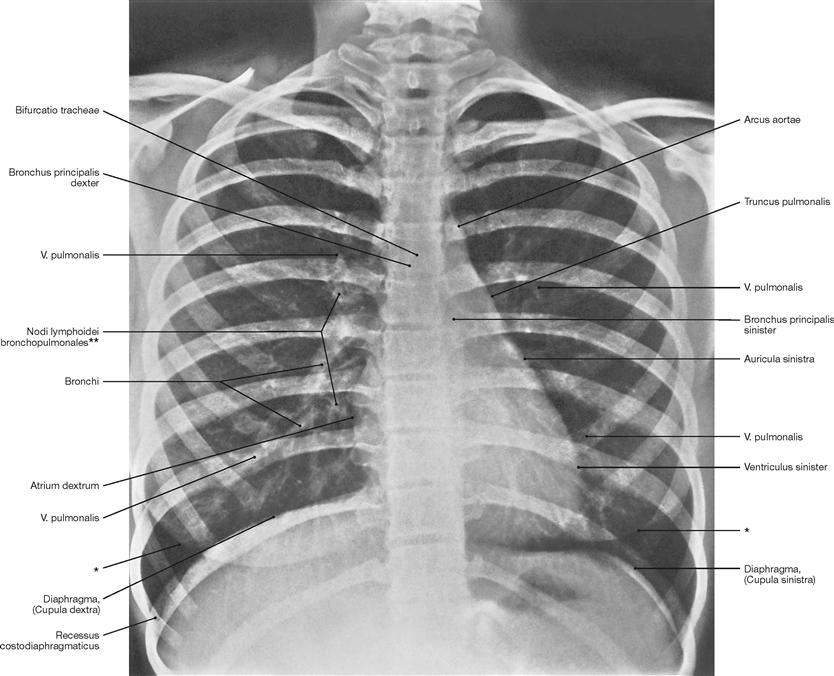
Fig. 5.66 Thoracic cage, Cavea thoracis, with thoracic viscera; radiograph in postero-anterior (PA) beam projection. [27]
The course of the bronchi is partly visible. On the right side, clusters of lymph nodes in the area of the hilum of the lung are visible.
* contour of the breast (mamma)
** clinical term: hilar lymph nodes
Projection of the oesophagus
Fig. 5.67 Overview of the digestive tract.
The Oesophagus is a muscular tube connecting the Pharynx with the stomach (Gaster). It transports the ingested food.
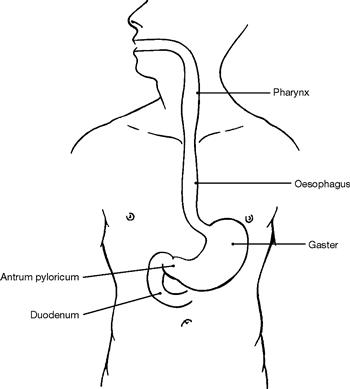
Fig. 5.68 Projection of the Oesophagus onto the ventral thoracic wall.
The Oesophagus is 25 cm long and originates at the cricoid cartilage which projects onto the 6th cervical vertebra. It ends at the Cardia of the stomach at the level of the 10th thoracic vertebra (beneath the Proc. xiphoideus of the Sternum).
Oesophagus
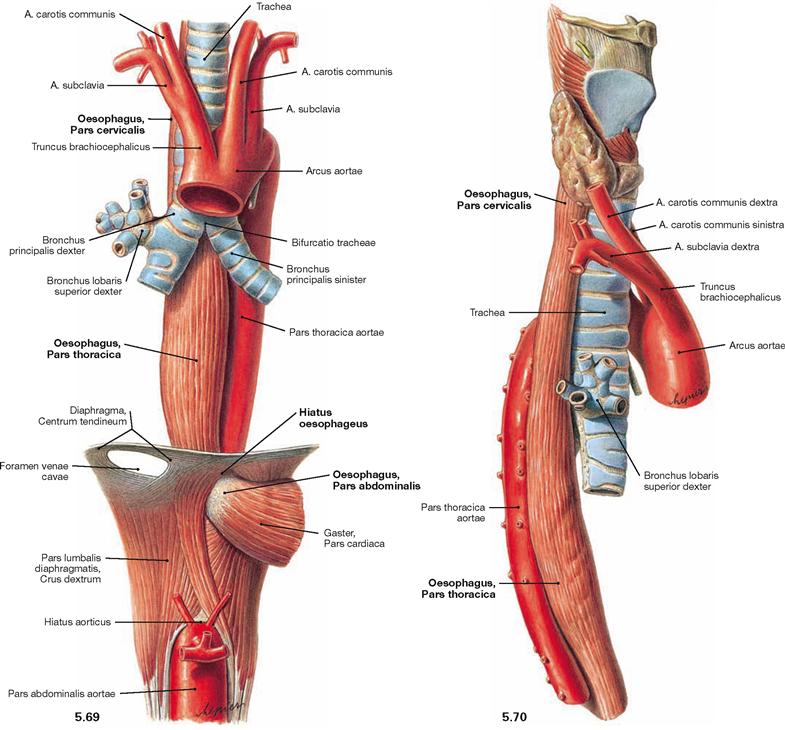
Fig. 5.69 and Fig. 5.70 Oesophagus, Oesophagus, trachea, Trachea, and thoracic aorta, Pars thoracica aortae; ventral view (→ Fig. 5.69) and view from the right side (→ Fig. 5.70).
The Oesophagus is 25 cm long and is organised in three parts:
The Pars cervicalis is adjacent to the vertebral column. The Pars thoracica crosses the aortic arch which is adjacent on the dorsal left side. This part runs along the left main bronchus and descends ventrally with increasing distance to the vertebral column. The dorsal view shows the close proximity of the Pars thoracica to the Pericardium and to the left atrium (→ Fig. 5.71). After traversing the Hiatus oesophageus of the diaphragm, the short intraperitoneally located Pars abdominalis begins.
Structure of the oesophagus
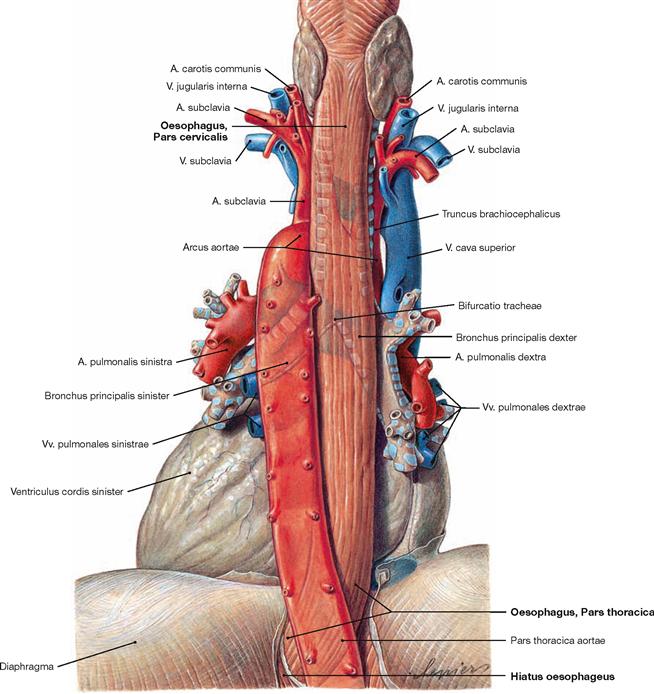
Fig. 5.71 Oesophagus, Oesophagus, pericardium, Pericardium, and thoracic aorta, Pars thoracica aortae; dorsal view.
The caudal part of the Pars thoracica of the Oesophagus is separated from the left atrium only by the pericardium.
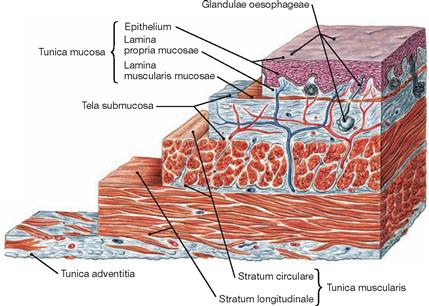
Fig. 5.72 Structure of the wall of the oesophagus, Oesophagus; microscopic view.
Similar to the entire gut, the wall of the Oesophagus consists of a luminal mucous membrane (Tunica mucosa) which is separated from the muscular layer (Tunica muscularis) by a loose connective tissue layer (Tela submucosa). The Partes cervicalis and thoracica are covered by the Tunica adventitia. The outer surface of the intraperitoneal Pars abdominalis is covered by visceral peritoneum (Peritoneum viscerale) which constitutes the Tunica serosa.
Constrictions and diverticula of the oesophagus
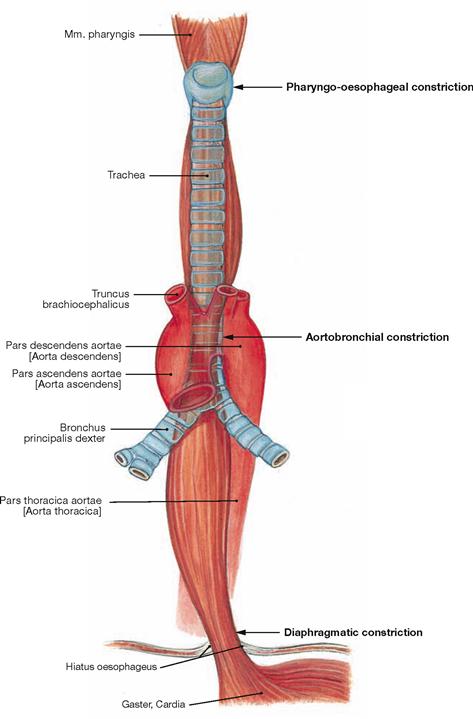
Fig. 5.73 Constrictions, Angustiae, of the Oesophagus; ventral view.
The Oesophagus has three constrictions:
• cervical constriction at the cricoid cartilage (Angustia cricoidea; pharyngo-oesophageal constriction)
• thoracic constriction at the Aorta (Angustia aortica; aortobronchial constriction)
The cervical constriction has the smallest lumen and is located at the level of the upper oesophageal sphincter and the 6th cervical vertebra. The thoracic constriction is created by the direct proximity of the aortic arch from the left and dorsal side (level of the 4th thoracic vertebra). The diaphragmatic constriction lies in the Hiatus oesophageus (level of the 10th thoracic vertebra). There is no true sphincter muscle but an angiomuscular mechanism that acts like a valve under extension (lower oesophageal sphincter, LES). Elastic connective tissue (Lig. phrenicooesophageale) attaches the outside of the Oesophagus to the Hiatus oesophageus.
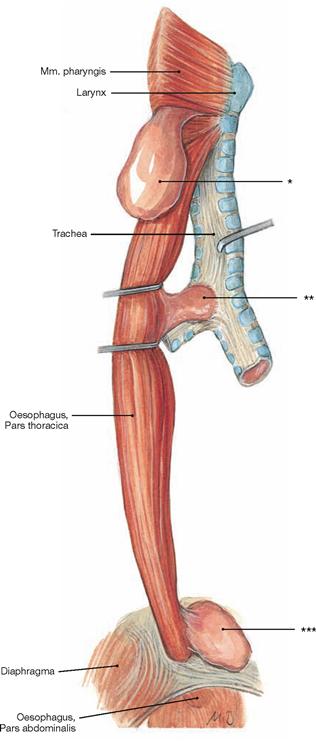
Fig. 5.74 Diverticula of the Oesophagus; view from the right dorsal side.
* clinical term: ZENKER’s diverticulum
** clinical term: traction diverticulum
*** clinical term: epiphrenic diverticulum
Blood vessels of the oesophagus
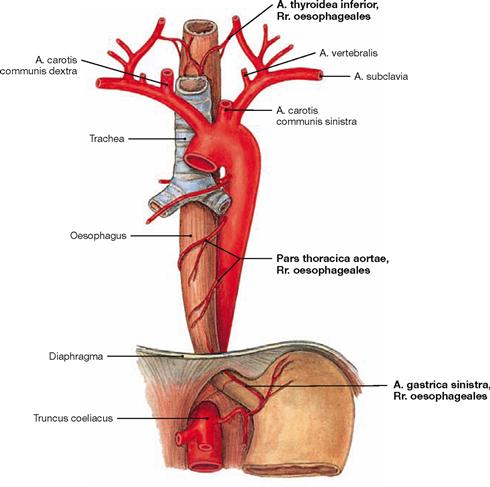
Fig. 5.75 Arteries of the Oesophagus; ventral view.
The different parts of the Oesophagus are supplied by surrounding arteries:
• Pars cervicalis: A. thyroidea inferior
• Pars thoracica: Rr. oesophageales of the Aorta
• Pars abdominalis: A. gastrica sinistra and A. phrenica inferior
The arterial and venous supply of the Trachea is equivalent to the blood vessels of the cervical and thoracic parts of the Oesophagus.
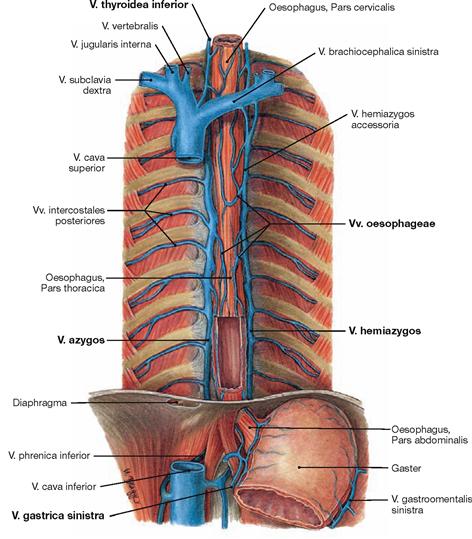
Fig. 5.76 Veins of the oesophagus, Vv. oesophageae; ventral view.
The complex venous network of the Tunica adventitia drains into different veins:
• Pars cervicalis: V. thyroidea inferior
• Pars thoracica and Pars abdominalis: via V. azygos and V. hemiazygos into the V. cava superior
The inferior parts gain access to the portal venous system via the gastric veins (V. gastrica sinistra). These veins may be utilised as portocaval anastomoses with increased pressure in the portal vein (portal hypertension) (→ Fig. 5.77).
Veins of the oesophagus
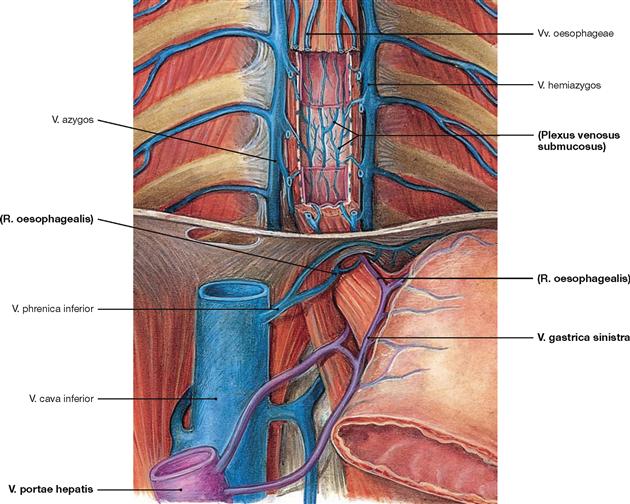
Fig. 5.77 Veins of the oesophagus, Vv. oesophageae, with illustration of the portocaval anastomoses between portal vein, V. portae hepatis, and V. cava superior; ventral view.
The extensive venous network in the Tunica adventitia is connected to the submucosal veins (Plexus venosus submucosus). The blood drains via V. azygos (right side) and V. hemiazygos (left side) upwards to the V. cava superior. The lower parts of the Oesophagus also connect to the V. portae hepatis via the veins at the lesser curvature of the stomach (V. gastrica sinistra).
Lymph vessels of the oesophagus
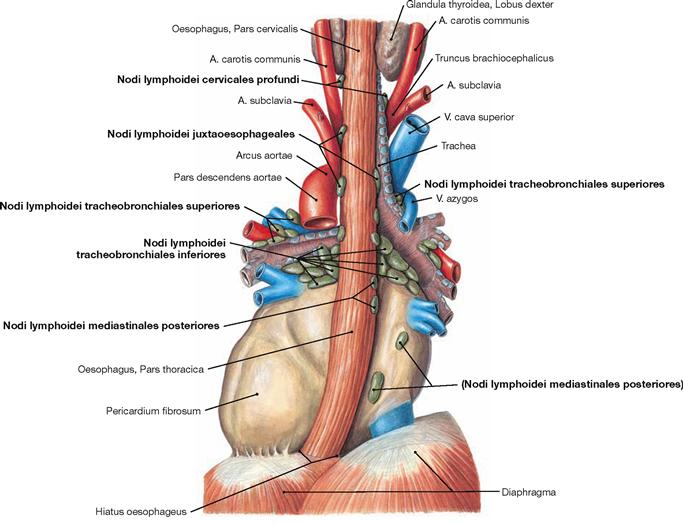
Fig. 5.78 Lymph nodes, Nodi lymphoidei, of the posterior mediastinum; dorsal view.
The lymph of the Oesophagus drains into the lymph nodes directly adjacent to the Oesophagus (Nodi lymphoidei juxtaoesophageales):
• Pars cervicalis: Nodi lymphoidei cervicales profundi
• Pars thoracica and Pars abdominalis: lymph nodes of the Mediastinum (Nodi lymphoidei mediastinales posteriores, Nodi lymphoidei tracheobronchiales and paratracheales) and of the peritoneal cavity (Nodi lymphoidei phrenici inferiores on the abdominal side of the diaphragm and Nodi lymphoidei gastrici on the lesser curvature of the stomach)
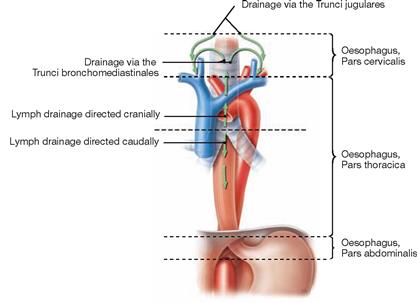
Fig. 5.79 Lymph drainage of the Oesophagus; ventral view.
The lymph of the Pars cervicalis reaches the Truncus jugularis via the deep cervical lymph nodes. The Pars thoracica drains in two directions: the upper part above the tracheal bifurcation drains via the mediastinal lymph nodes into the Truncus bronchiomediastinalis; the lower part beneath the tracheal bifurcation connects to the abdominal lymph nodes which are the regional lymph nodes for the Pars abdominalis. From here the lymph passes the Nodi lymphoidei coeliaci to reach the Truncus intestinalis.
Thymus
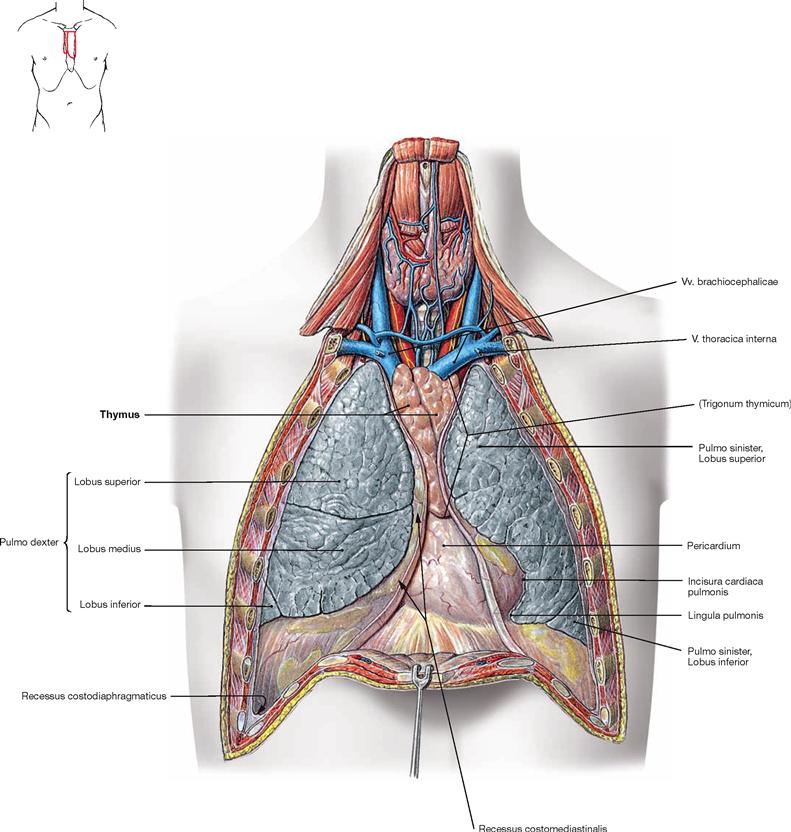
Fig. 5.82 Thymus, Thymus, mediastinum and pleural cavities, Cavitates pleurales, of an adolescent; ventral view; after removal of the anterior thoracic wall.
The Thymus is located in the Trigonum thymicum between the mediastinal borders of the pleural cavities. The Thymus is relatively large in a young adult. In an older individual it is almost completely replaced with adipose tissue. Thus, in the dissection of anatomical specimens only residual thymic tissue is found which is identified only due to smaller arterial branches derived from the A. thoracica interna or venous connections to the Vv. brachiocephalicae.
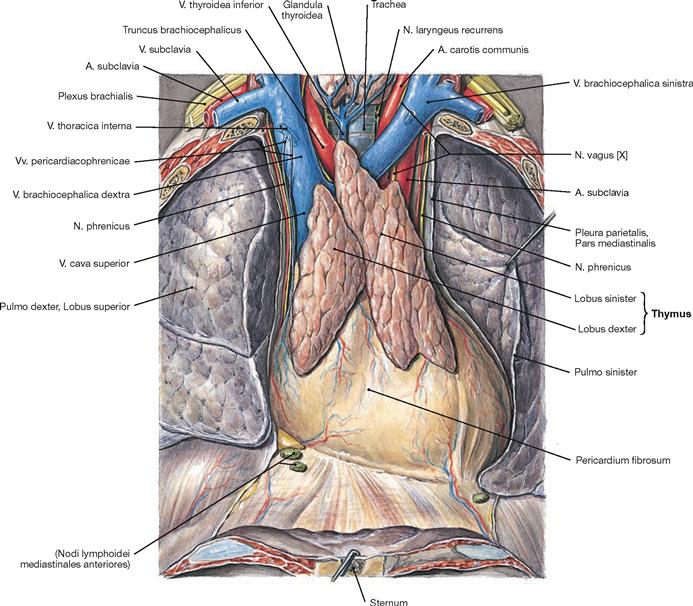
Fig. 5.83 Thymus, Thymus, of an adolescent; ventral view.
The Thymus is a primary lymphatic organ. It serves for the proliferation and selection of T-lymphocytes which then leave the Thymus to settle in secondary lymphatic organs to function in the adaptive immune responses.
The Thymus develops from the endoderm of the third pharyngeal pouch and the ectoderm of the third pharyngeal cleft. It consists of two lobes (Lobi dexter and sinister) which cover the great vessels of the superior Mediastinum. Microscopically, these lobes are subdivided into smaller lobules.
The composition of the thymic tissue changes continuously during life. Since its volume remains almost the same, its relative size is larger in the newborn than in the adult (→ Fig. 5.84). After puberty, the specific thymic parenchyma is gradually substituted by adipose tissue and the residual thymus is hardly visible in elder persons. However, functional thymic tissue remains present at all times to warrant adequate immune reactions.
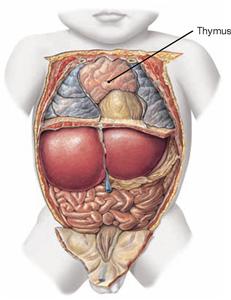
Fig. 5.84 Position of the thymus, Thymus, in a newborn; ventral view; after removal of the ventral thoracic wall.
Mediastinum
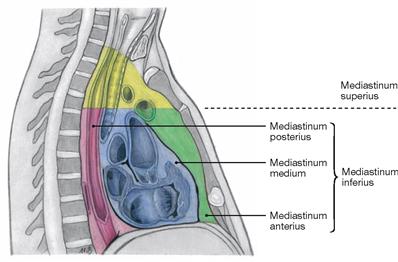
Fig. 5.85 Organisation of the Mediastinum.
The mediastinum is divided into a Mediastinum inferius which contains the heart, and a Mediastinum superius. The Mediastinum inferius is further divided into the Mediastinum anterius in front of the heart, the Mediastinum medium, containing the Pericardium, and the Mediastinum posterius behind the Pericardium.
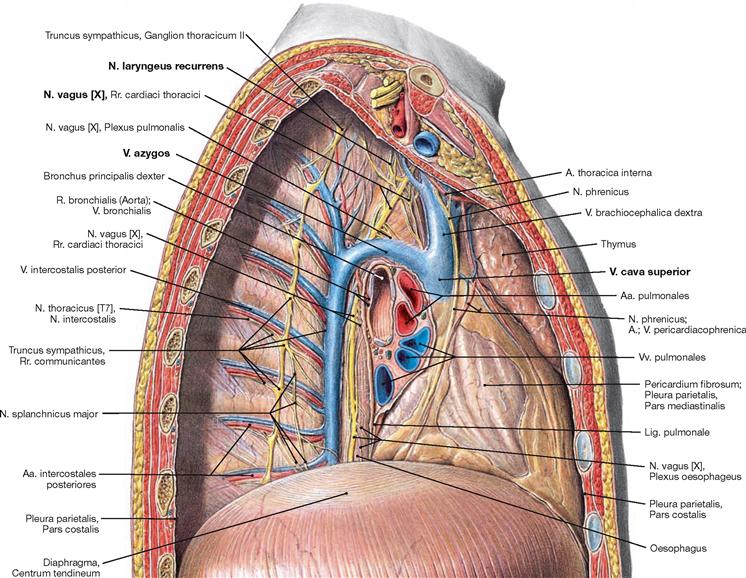
Fig. 5.86 Mediastinum and pleural cavity, Cavitas pleuralis, of an adolescent; view from the right side; after removal of the lateral thoracic wall and the right lung.
The view from the right side demonstrates clearly the V. azygos which ascends next to the vertebral column in the Mediastinum posterius. The V. azygos crosses the root of the right lung superiorly and enters the V. cava superior from dorsal at the level of the 4th / 5th thoracic vertebrae. After branching off the N. vagus [X], the N. laryngeus recurrens winds around the A. subclavia on the right side.
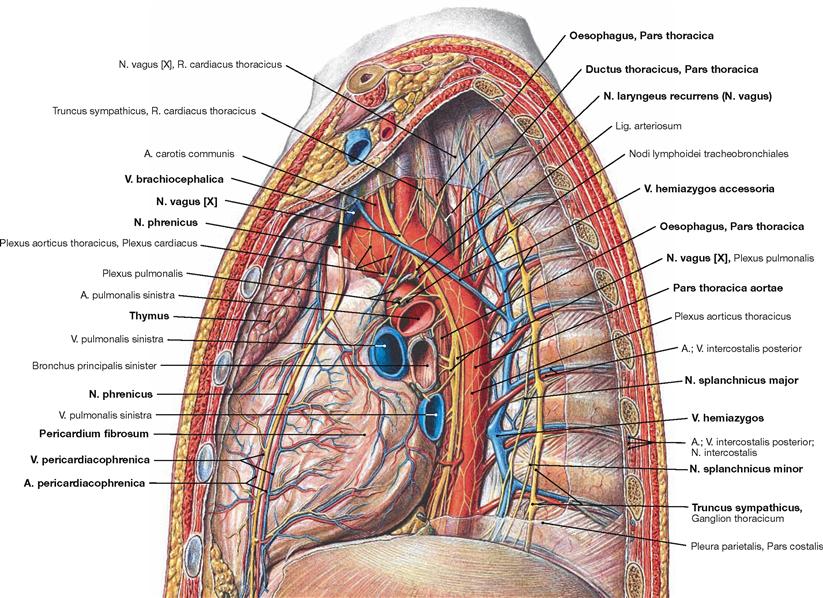
Fig. 5.87 Mediastinum and pleural cavity, Cavitas pleuralis, of an adolescent; view from the left side; after removal of the lateral thoracic wall and the left lung.
The view from the left side demonstrates clearly the Aorta thoracica which descends on the left side of the vertebral column in the Mediastinum posterius. The V. hemiazygos ascends on the lateral aspect of the vertebral bodies and drains ino the V. azygos at the level of the thoracic vertebrae 10th to 7th. Frequently, the V. hemiazygos communicates with the V. hemiazygos accessoria which collects the blood from the superior intercostal veins.
Further lateral, next to the heads of the ribs, the ganglia of the Truncus sympathicus are positioned which branch off the N. splanchnicus major and the N. splanchnicus minor. The N. vagus [X] descends behind the root of the lung next to the Oesophagus after releasing the N. laryngeus recurrens. On the left side, the N. laryngeus recurrens winds around the aortic arch. The Mediastinum medium harbours the Pericardium and the adjacent N. phrenicus accompanied by the Vasa pericardiacophrenica. In the Mediastinum superius the Thymus covers the great vessels ventrally.
N. phrenicus
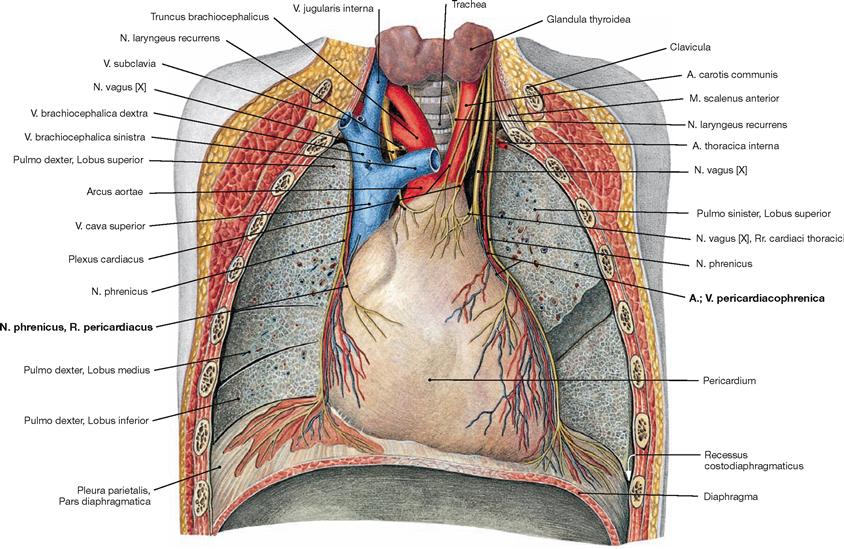
Fig. 5.88 Middle mediastinum; ventral view; after removal of the ventral thoracic wall, the lungs were dissected in the frontal plane.
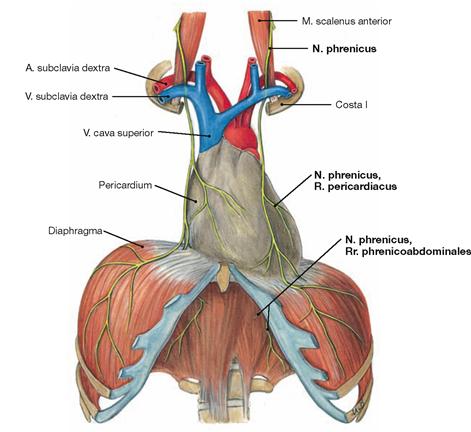
Fig. 5.89 Course of the N. phrenicus.
The N. phrenicus originates from the spinal cord segments C3 to C5 (predominantly C4) of the Plexus cervicalis and descends on the neck anterior to the M. scalenus anterior (guiding muscle!). Next the phrenic nerve courses anterior to the root of the lung and descends together with the Vasa pericardiacophrenica between the Pericardium and the Pleura mediastinalis to the diaphragm. The N. phrenicus provides motor innervation to the diaphragm and sensory innervation to the Pericardium (R. pericardiacus), the Pleura diaphragmatica, and the Peritoneum parietale at the abdominal side of the diaphragm (Rr. phrenicoabdominales). The Rr. phrenicoabdominales also convey sensory fibres to the Peritoneum viscerale on liver and gallbladder.
Aortic arch
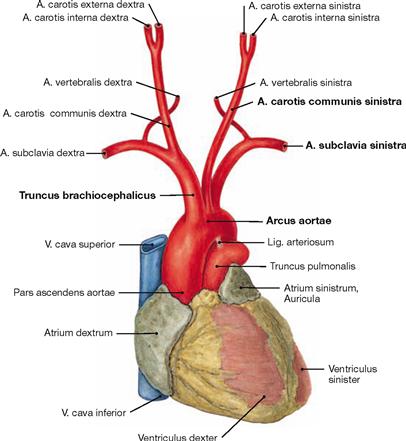
Fig. 5.90 Heart, Cor, and aortic arch, Arcus aortae, with branching of the great vessels; ventral view.
The Pars ascendens aortae continues as the aortic arch which is connected to the Truncus pulmonalis via the Lig. arteriosum. The aortic arch continues with the descending part (Pars descendens) of the Aorta thoracica (→ Fig. 5.92). The aortic arch has the following branches:
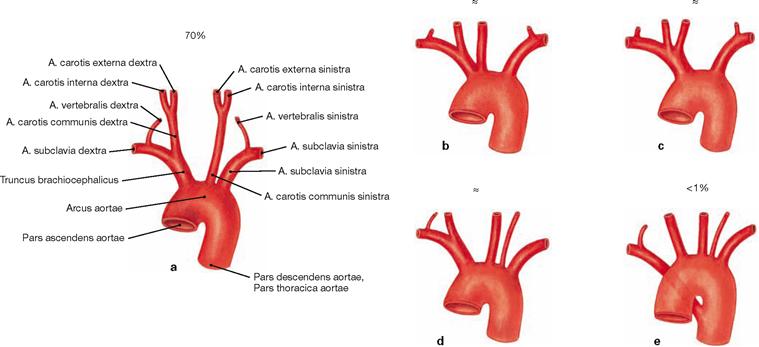
Figs. 5.91a to e Branching variations of the great vessels from the aortic arch.
b. common origin of Truncus brachiocephalicus and A. carotis communis sinistra
c. common stem for Truncus brachiocephalicus and A. carotis communis sinistra
d. independent branching of the A. vertebralis sinistra off the Arcus aortae
e. branching of the A. subclavia dextra as the last branch of the Arcus aortae. This unusual artery mostly courses behind the Oesophagus to the right side and may cause problems with swallowing (dysphagia lusoria).
The existence of an independent A. thyroidea ima coursing to the thyroid gland is uncommon. When existent, it either derives from the Truncus brachiocephalicus or as a second branch from the aortic arch.
Arteries of the posterior mediastinum
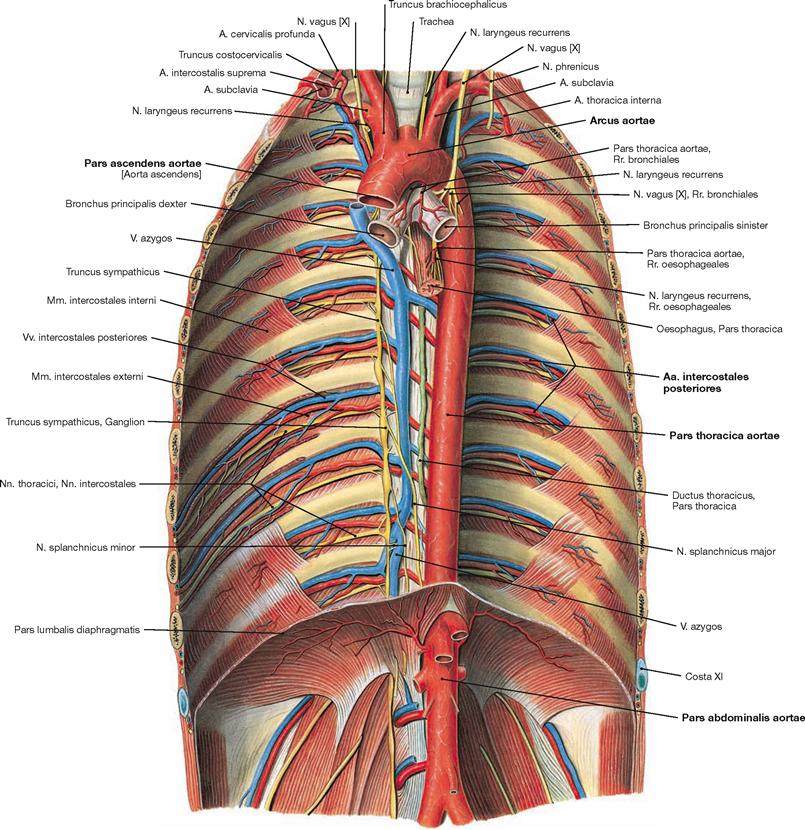
Fig. 5.92 Aorta and its branches; ventral view onto the posterior wall of the trunk.
The Pars descendens of the Aorta descends in the Mediastinum posterius (Pars thoracica) and traverses the diaphragm (Pars abdominalis).

Veins of the posterior mediastinum
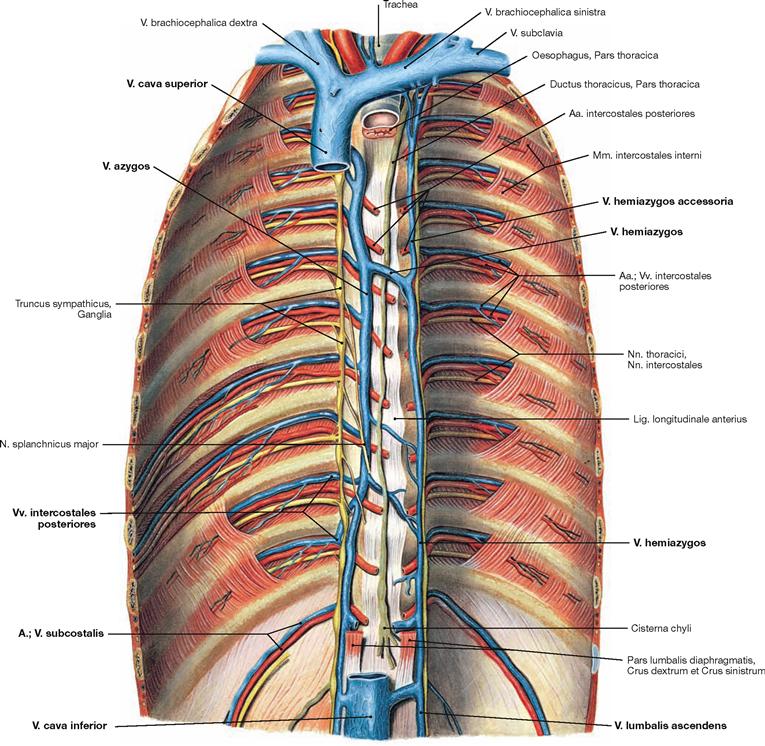
Fig. 5.93 Veins of the azygos system; ventral view onto the posterior wall of the trunk; after removal of the diaphragm.
The azygos system connects the Vv. cavae superior and inferior and its tributaries are equivalent to the branches of the Aorta. The V. azygos ascends on the right side of the vertebral column and drains into the V. cava superior from dorsal on the level of the 4th/5th thoracic vertebrae. The equivalent blood vessel on the left side is the V. hemiazygos which merges with the V. azygos at the level of the thoracic vertebrae 7th to 10th. Blood from the upper intercostal veins drains into the V. hemiazygos accessoria. Beneath the diaphragm, the V. lumbalis ascendens on each side continues the course of the azygos vein and connects to the V. cava inferior.
Tributaries:
Nerves of the posterior mediastinum
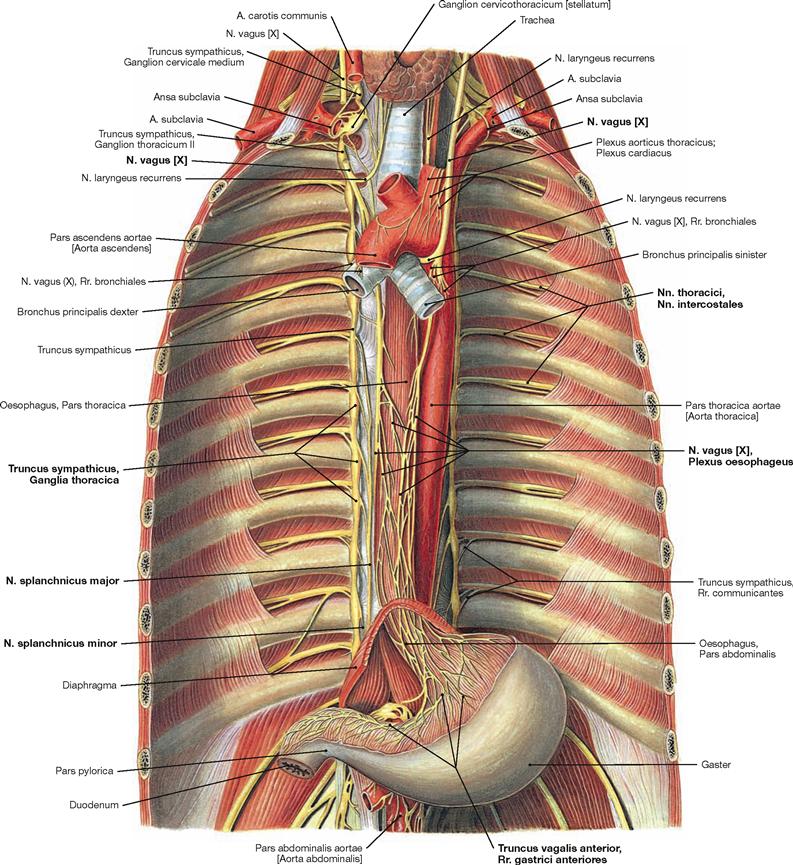
Fig. 5.94 Nerves of the posterior mediastinum; ventral view onto the posterior wall of the trunk; after removal of the diaphragm.
The posterior mediastinum contains the intercostal nerves (Nn. intercostales) of the somatic nervous system and parts of the sympathetic (Truncus sympathicus) and parasympathetic systems (Nn. vagi) as components of the autonomic nervous system. The sympathetic trunk (Truncus sympathicus) forms a paravertebral chain of twelve thoracic ganglia which are connected via Rr. interganglionares. The preganglionic sympathetic neurons are located in the lateral horns (C8 – L3) of the spinal cord and exit the vertebral canal with the spinal nerves. The Rr. communicantes albi guide the preganglionic fibres to the ganglia of the Truncus sympathicus where they are synapsed to postganglionic neurons. Axons of the postganglionic neurons join the spinal nerves and their branches again via the Rr. communicantes grisei. Some pregan-glionic fibres are not synapsed in the ganglia of the sympathetic trunk but continue as Nn. splanchnici major and minor to the nerve plexus around the Aorta abdominalis where they eventually synapse. The preganglionic fibres of the Nn. vagi course behind the root of the lung adjacent to the Oesophagus and form the Plexus oesophageus. The latter is the origin for the two vagal trunks (Trunci vagales anterior and posterior) which traverse the diaphragm together with the Oesophagus to reach the autonomic nerve plexus of the Aorta abdominalis. However, synapses to the postganglionic parasympathetic neurons mostly occur in closer proximity to the respective target organs.
Lymph vessels and lymph nodes of the mediastinum
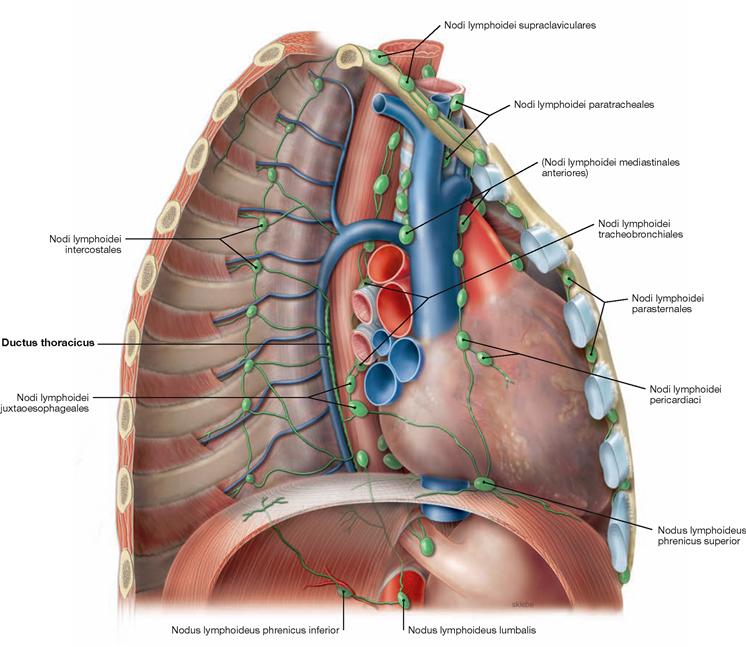
Fig. 5.95 Lymph vessels and lymph nodes of the mediastinum; view from the right ventrolateral side after removal of the lateral chest wall. (according to [2])
The Mediastinum harbours several different groups of lymph nodes which are categorised into parietal lymph nodes (drainage of the wall of the trunk) and visceral lymph nodes (drainage of the thoracic viscera). These drain into the large lymphatic trunks.
Parietal lymph nodes:
• Nodi lymphoidei parasternales: on both sides of the Sternum. They drain lymph from the anterior chest wall, the mammary glands and the diaphragm into the Truncus subclavius.
• Nodi lymphoidei intercostales: between the heads of the ribs. They drain lymph from the posterior chest wall. Their efferent lymph vessels drain directly into the Ductus thoracicus.
Visceral lymph nodes with connection to the Trunci bronchomediastinales:
• Nodi lymphoidei mediastinales anteriores: on both sides of the great vessels, tributaries from lungs and Pleura, diaphragm (Nodi lymphoidei phrenici superiores), heart and Pericardium (Nodi lymphoidei pericardiaci), and Thymus.
• Nodi lymphoidei mediastinales posteriores: at bronchi and Trachea (Nodi lymphoidei tracheobronchiales and paratracheales) and Oesophagus (Nodi lymphoidei juxtaoesophageales)
Lymphatic trunks:
The Ductus thoracicus traverses the diaphragm anterior to the vertebral column (→ Fig. 5.93) and ascends in the Mediastinum posterius, first behind the Aorta then behind the Oesophagus, to reach the 7th cervical vertebra. Next, the ductus crosses the left pleural cupula and opens into the left jugular-subclavian junction of veins from dorsal (between V. subclavia and V. jugularis interna). Shortly before draining into the jugular-subclavian junction, it collects the lymph of the Truncus bronchioMediastinalis sinister, which courses independently in the Mediastinum, the Truncus subclavius sinister (from the arm), and the Truncus jugularis sinister (from the neck). On the right side, a short (1 cm) Ductus lymphaticus dexter connects the respective lymphatic trunks and enters the right jugular-subclavian junction of veins.
Topography
Superior thoracic aperture
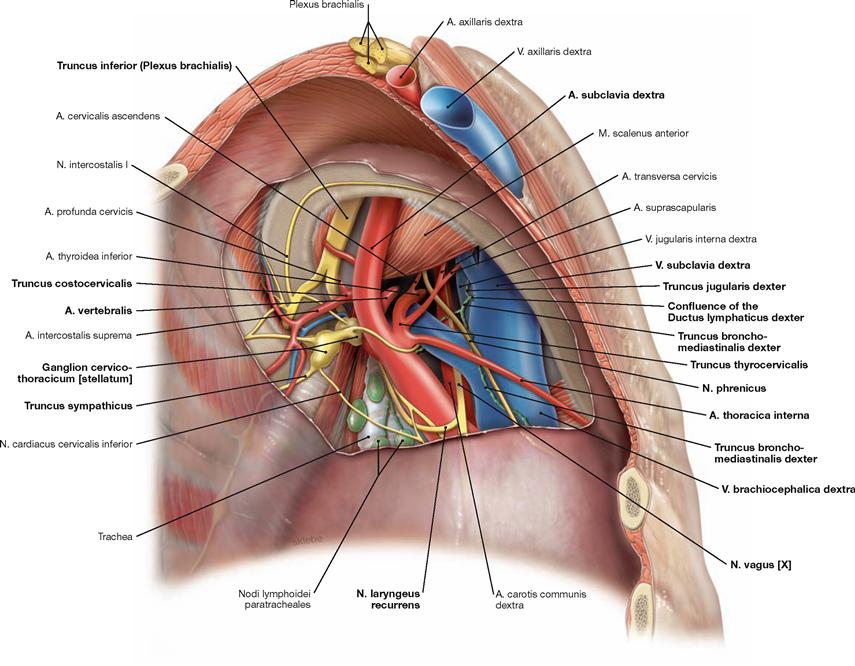
Fig. 5.96 Neurovascular structures of the superior thoracic aperture, right side; caudal view; after removal of the cervical pleural cupula.
The V.subclavia crosses the pleural cupula anterior to the M. scalenus, whereas the A. subclavia and the Plexus brachialis course posterior to the M. scalenus (scalene gap). Branches of the A. subclavia are the A. thoracica interna descending to the lateral aspect of the Sternum, the A. vertebralis, and the Truncus thyreocervicalis with its branches. The Truncus costocervicalis branches off dorsal of the M. scalenus anterior and divides into the A. profunda cervicis and the A. intercostalis suprema. The N. phrenicus is located ventral to the V. brachiocephalica. The N. vagus courses dorsal to the V. brachiocephalica and releases the N. laryngeus recurrens which winds around the A. subclavia to ascend to the neck. Posterior to the A. subclavia, the Truncus sympathicus with its Ganglion cervicothoracicum (stellatum) is found. Most difficult to identify is the short Ductus lymphaticus dexter which drains into the right venous angle (between V. subclavia and V. jugularis interna) after merging the Truncus bronchomediastinalis and the Truncus subclavius.
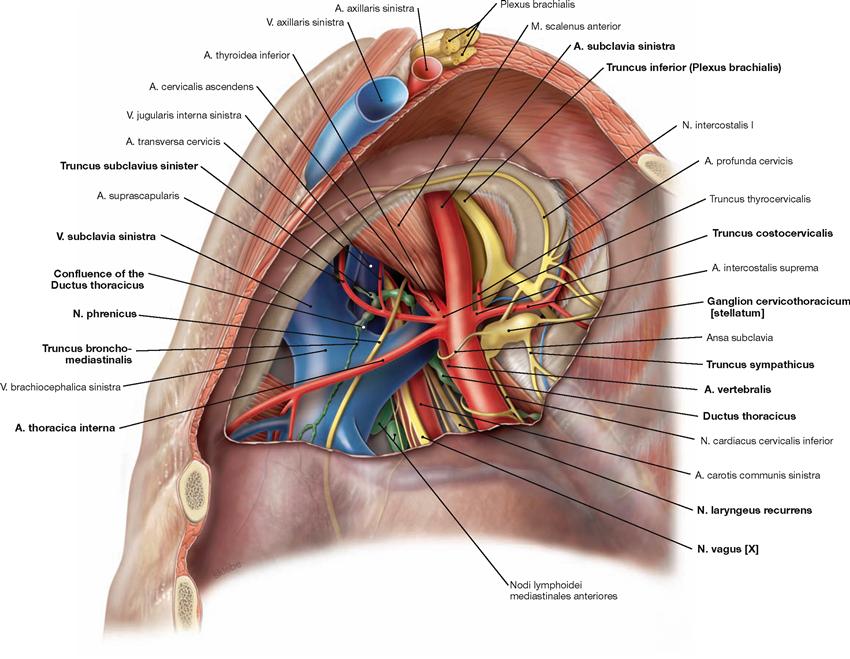
Fig. 5.97 Neurovascular structures of the superior thoracic aperture, left side; caudal view; after removal of the pleural cupula.
Here, only structures are described which differ in their course from the neurovascular structures of the right side (→ Fig. 5.96).
On the left side, the N. vagus [X] descends further before releasing the N. laryngeus recurrens which then winds around the aortic arch (not visible here) and ascends to the neck. Particular attention must be
paid to the Ductus thoracicus which is often injured during dissection in this region. The Ductus thoracicus ascends in the Mediastinum posterius and crosses the left pleural cupula before entering the left jugular-subclavian junction of veins (junction between V. subclavia and V. jugularis interna) from dorsal. Just before reaching the jugular-subclavian junction, it joins with the Truncus bronchomediastinalis, the Truncus subclavius, and the Truncus jugularis (not visible here).
Thoracic cavity, midsagittal section
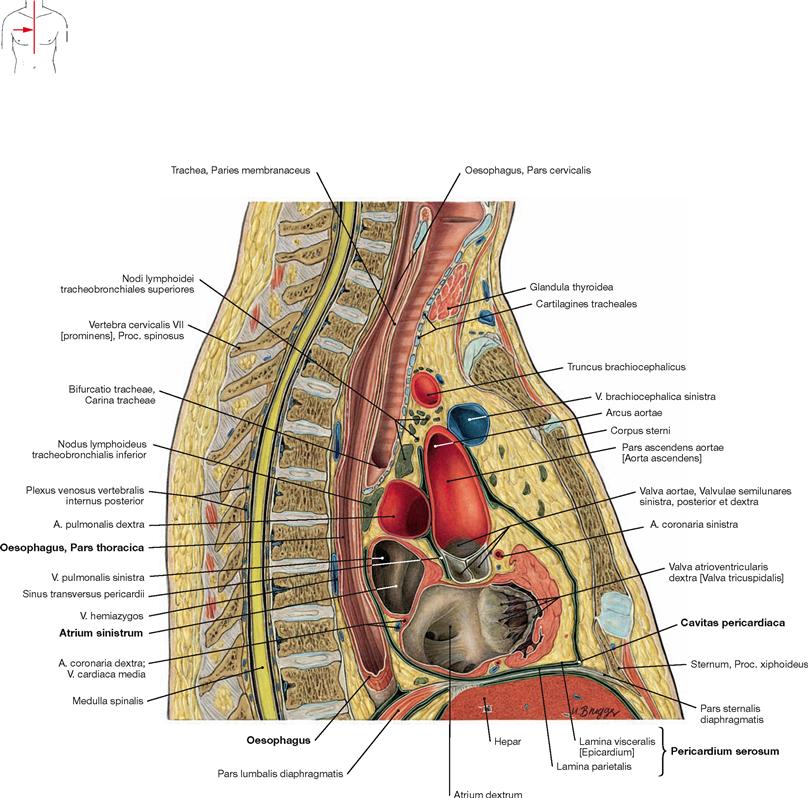
Fig. 5.98 Thoracic cavity, Cavitas thoracis; midsagittal section; lateral view from the right side.
In this section, the close proximity of the Oesophagus to the left atrium
of the heart (Atrium sinistrum) in the Mediastinum posterius is obvious. Both structures are only separated by the pericardial cavity (Cavitas pericardiaca).
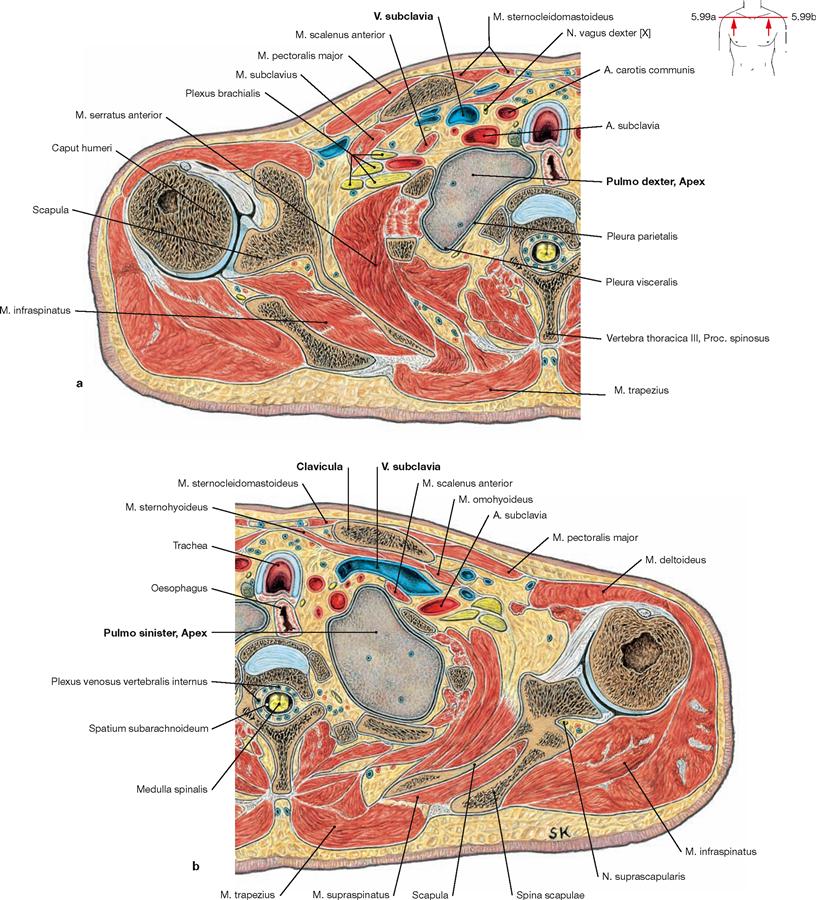
Figs. 5.99a and b Pleural cupula, Cupula pleurae; transverse sections; at the level of the shoulder joint; caudal view.
These sections demonstrate that the pleural cupula extends behind the neurovascular bundle of the arm above the superior thoracic aperture. Thus, the apex of the lung is positioned immediately posterior to the V. and A. subclavia.
Sections
Thoracic cavity, transverse sections
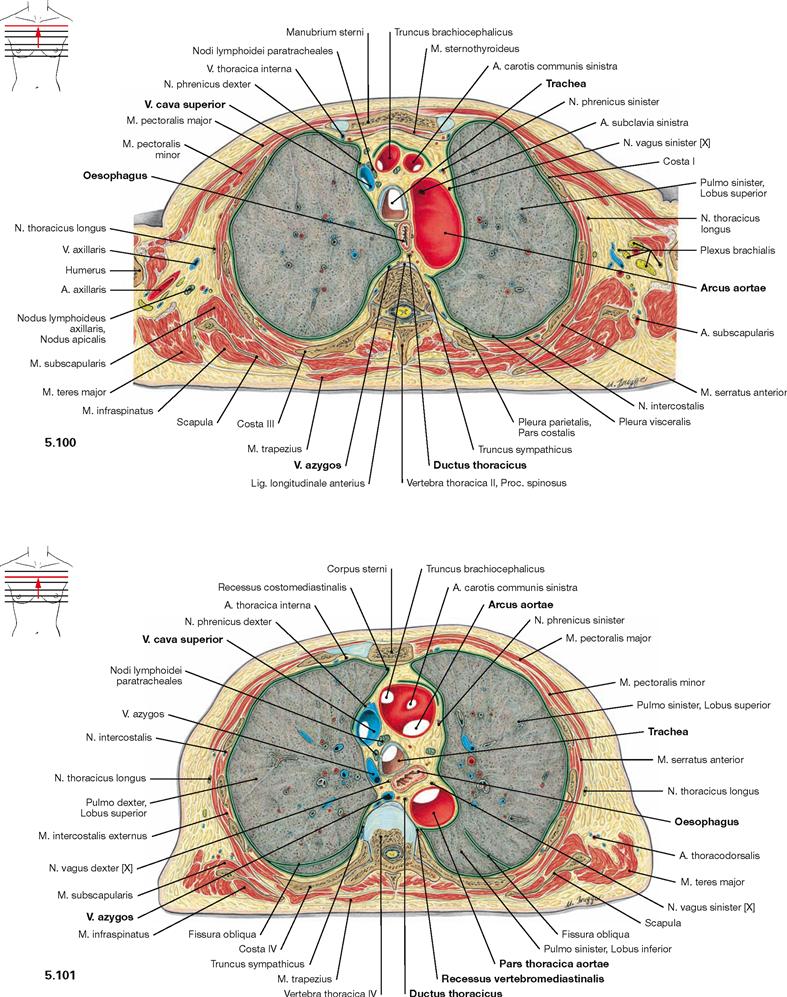
Fig. 5.100 and Fig. 5.101 Thoracic cavity, Cavitas thoracis; transverse sections at the level of the aortic arch; caudal view.
In the Mediastinum superius, the aortic arch is located ventrally and the V. cava superior is located at the right side of the aortic arch. Positioned dorsal to these blood vessels are the Trachea and, to the left side, the Oesophagus and the thoracic aorta. Posteriorly, the Aorta borders at the Recessus vertebromediastinalis of the pleural cavity. Positioned directly on the vertebral column are the V. azygos on the right side and the Ductus thoracicus on the left side.
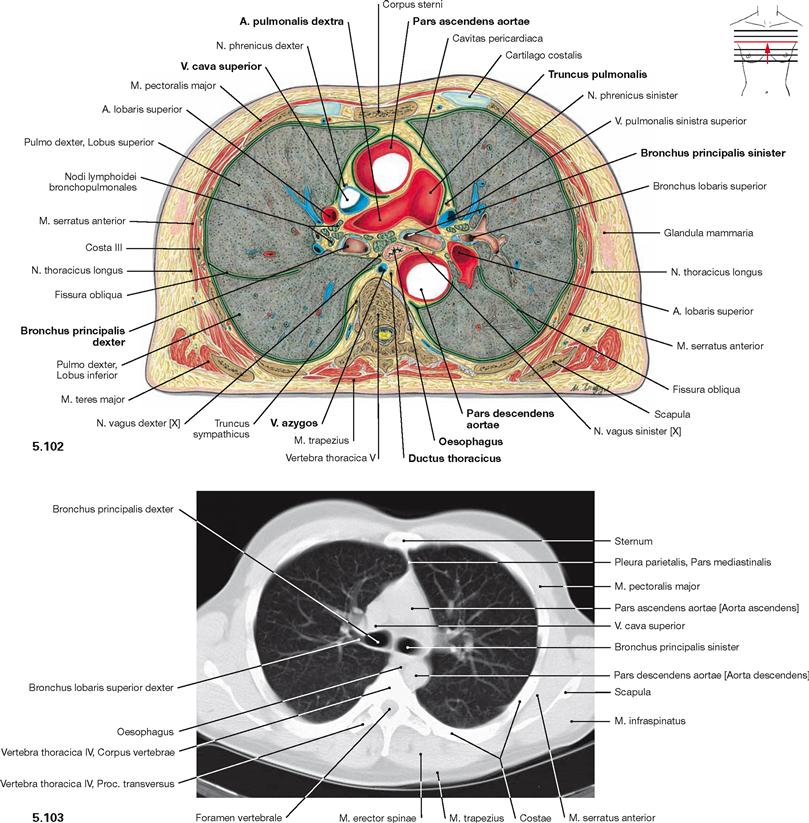
Fig. 5.102 and Fig. 5.103 Thoracic cavity, Cavitas thoracis; transverse section at the level of the Aorta descendens (→ Fig. 5.102) and computed tomographic cross-section (CT; → Fig. 5.103); caudal view.
In the Mediastinum superius, the Aorta ascendens is positioned most ventrally followed posteriorly and to the left side by the Truncus pulmonalis which branches into the pulmonary arteries. The V. cava superior is located at the right side of the Aorta. Behind the pulmonary arteries (Aa. pulmonales) are the main bronchi (Bronchi principales) and the Oesophagus. The Aorta descendens is visible on the left side of the vertebral column, the V. azygos on the right side.
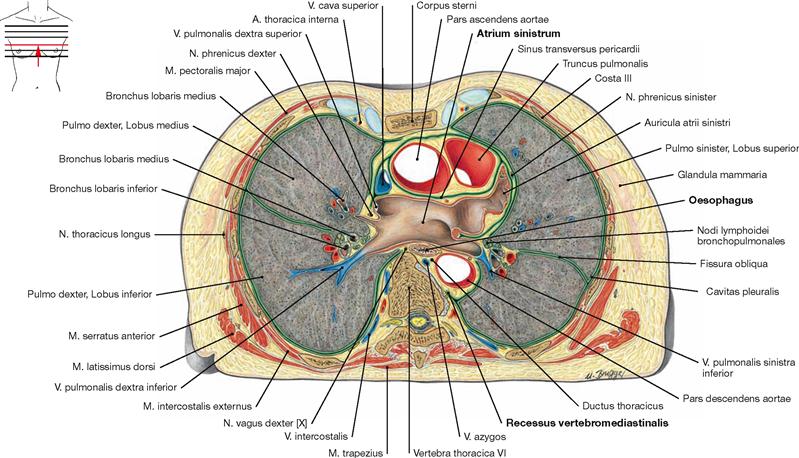
Fig. 5.104 Thoracic cavity, Cavitas thoracis; transverse section at the level of the left atrium; caudal view.
The left atrium of the heart (Atrium sinistrum) reaches further cranial than the right atrium and is positioned behind the great vessels. The Oesophagus is directly adjacent to the dorsal aspect of the left atrium.
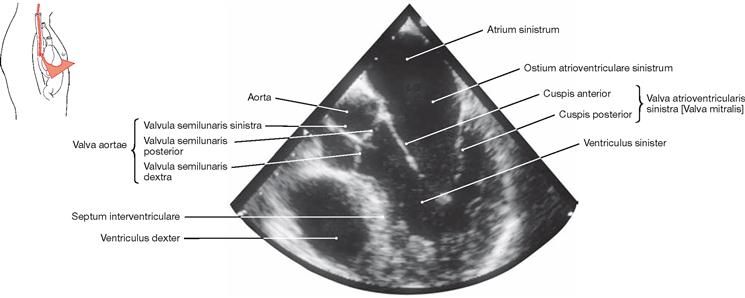
Fig. 5.105 Heart, Cor; ultrasound image taken from within the Oesophagus (transoesophageal echocardiography).
Thoracic cavity, transverse section
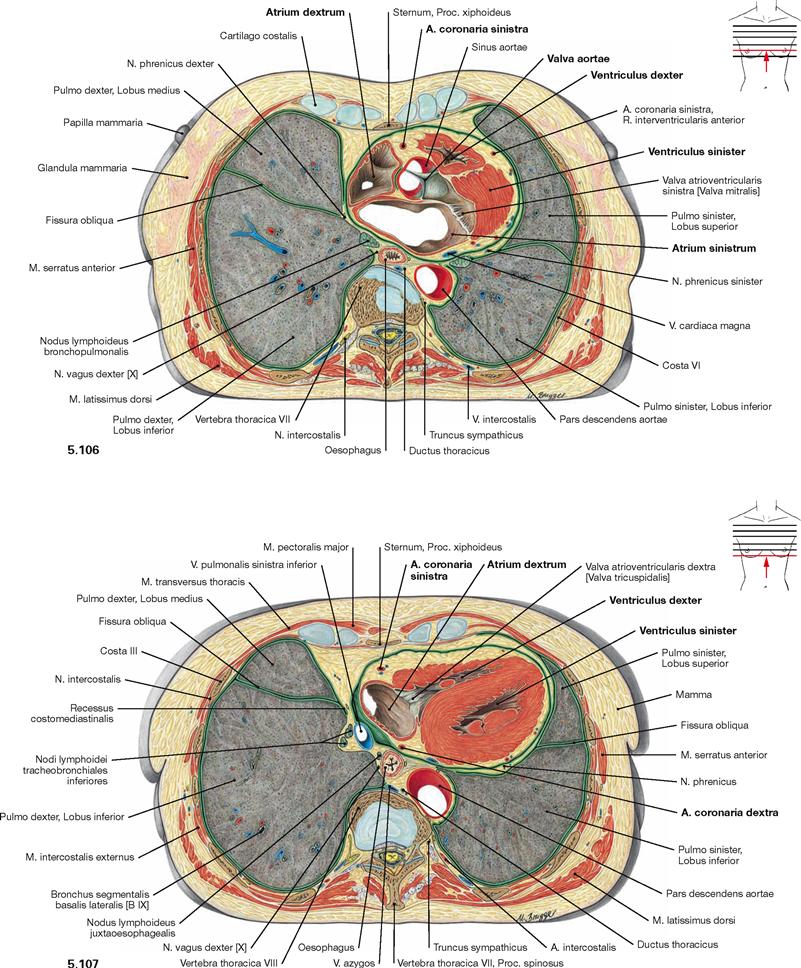
Fig. 5.106 and Fig. 5.107 Thoracic cavity, Cavitas thoracis; transverse sections at the level of the aortic valve (→ Fig. 5.106) and beneath the aortic valve (→ Fig. 5.107); caudal view.
These sections show that the Mediastinum medium, which contains the heart and the pericardium, extends further to the left side than to the right side. This results in a smaller volume of the left lung.
In the Pericardium, a thick layer of subepicardial adipose tissue is evident in which the coronary arteries are embedded. The lateral aspect of the heart (Facies pulmonalis of the heart) at this sectional level is confined by the right atrium on the right side and the left ventricle on the left side. The right ventricle does not participate in the borders of the heart but, instead, forms the anterior aspect of the heart (Facies sternocostalis).
Thoracic cavity, frontal sections
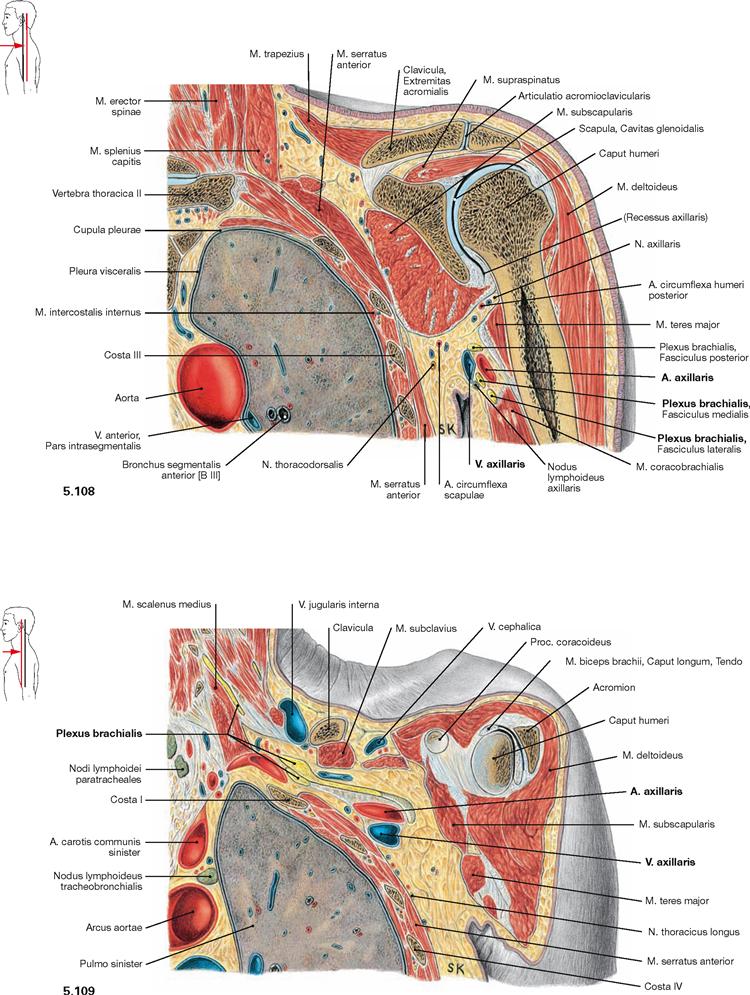
Fig. 5.108 and Fig. 5.109 Thoracic cavity, Cavitas thoracis, axillary fossa, Axilla, and shoulder joint, Articulatio humeri; frontal sections at the level of the shoulder joint (→ Fig. 5.108) and anterior to the shoulder joint (→ Fig. 5.109); ventral view.
These illustrations show that the neurovascular structures supplying the arm, A. and V. axillaris and the Plexus brachialis, course ventral to the shoulder joint in close topographical relation to the apex of the lung.