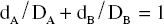Chapter 7 Synergy and other interactions in phytomedicines
The term ‘synergy’ (or synergism, from the Greek syn-ergo, meaning working together) refers to the phenomenon where two or more agents act together to produce an effect greater than would have been predicted from a consideration of individual contributions. Synergy is generally assumed to play a part in the effects of phytomedicines, and the use of combinations of herbs is fundamental to the philosophy of Western medical herbalism, traditional Chinese medicine (TCM) and Ayurveda. This attitude to their formulation and use differentiates herbal products from conventional medicines, even those originally obtained from plants. Modern phytomedicines are usually found as whole or semipurified extracts and should, ideally, be standardized for their active constituents, where known, to ensure clinical reproducibility. The likelihood of synergistic interactions is also recognized in reports from the European Pharmacopoeia Commission, where the most common type of extract, exemplified by Hypericum perforatum, is described as having ‘constituents with known therapeutic or pharmacological activity which are not solely responsible for the overall clinical efficacy of the extract’. To complicate matters further, herbalists use preparations and mixtures that are not necessarily intended to target a particular organ, cell tissue or biochemical system. This kind of application has been described as the ‘herbal shotgun’ approach, as opposed to the ‘silver bullet’ method of conventional medicine, to distinguish the multitargeted approach of herbals from the single-target approach of synthetic drugs.
WHAT IS SYNERGY?
The term ‘synergy’ is now used very widely, and mainly inaccurately, to describe any kind of positive interaction between drugs. In pharmacology the term has a specific definition, but is often misapplied in practice. Whether an effect can truly be described as synergy, or is merely addition, is rarely established and evidence to prove it conclusively in herbal medicines is sparse. The opposite, antagonism, meaning ‘working against’, is a reduction in the overall expected effect. Put simply, both antagonism and synergism can be defined in relation to an additivity expectation, which can be calculated from the potency of individual mixture components. Synergism is an effect larger than additive, whereas antagonism is smaller than additive. There are two ways of calculating additivity expectations: dose addition and also independent action.
Interactions can also involve a potentiation of effects. The terms ‘synergism’, ‘additivity’ and ‘antagonism’ are applied to combinations where all components induce the effect of interest, whereas the term ‘potentiation’ should be applied where one or several ‘inactive’ compounds enhance or exacerbate the effect of other actives.
Synergy and other interactions can take place between the constituents of a single extract as well as in a mixture of herbs. Medical herbalists have always insisted that better results are obtained with whole plant extracts and combinations of these rather than with isolated compounds. TCM, in particular, uses complicated recipes and it has sometimes been thought that the inclusion of some herbs was unnecessary, but the rationale for such combinations is gaining increasing acceptance. A TCM herbal treatment for eczema was the subject of a clinical trial of 37 young patients (M. P. Sheehan and J. D. Atherton, Br. J. Dermatol., 1992, 126: 179–184), and investigations were carried out to identify the ‘active constituent(s)’ of the mixture. However, a programme of pharmacological tests failed to find a single active herb or compound: it was the herbal mixture that was so effective (J. D. Phillipson, reported in European Phytotelegram, 1994, 6: 33–40).
MEASURING SYNERGY
Although the idea of synergy is easy to understand, the measurement of it is more problematic. It is fairly straightforward to identify synergy when one of the agents is inactive and a combination of this with an active agent produces an effect greater than that observed for the active alone (although this is more correctly termed potentiation), but difficulties in measurement arise when more than one (and there might easily be several) are active. Various methods for calculation have been devised over the years, but the following are now thought to be the most useful:
PREDICTION OF EFFECTS
Synergy is deemed present if the total effect of a combination is greater than would be predicted on the basis of expected additive effects of the mixture. Such additivity expectations can be derived from dose addition or independent action. Often, anticipated additivity is calculated by simply adding up the effects of individual mixture components, but this method can produce paradoxical and erroneous results and is therefore deemed unreliable (A. Kortenkamp and R. Altenburger, 1998; see legend for Fig. 7.1). The opposite applies for antagonism, which is observed less than would have been predicted.
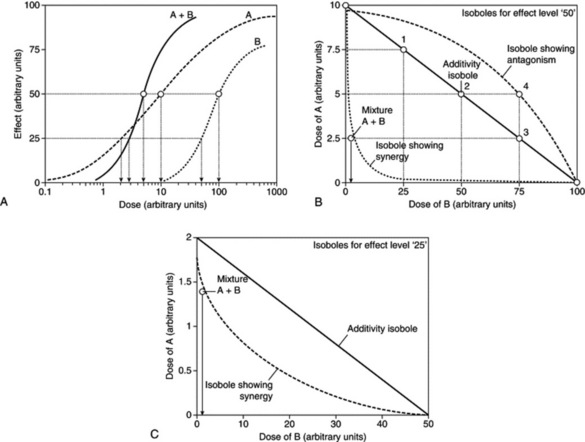
Fig. 7.1 An analysis of combination effects using the isobole method. A, Hypothetical dose–response curves for compounds A and B, and an equimolar mixture of A+B. An effect of 50 is produced by 10 (arbitrary) dose units of A or 100 dose units of B. The combination A+B yields this effect at 5 dose units (2.5 dose units A, 2.5 dose units B). Note that the curves for the individual compounds are dissimilar. B, Diagram showing isoboles for effect level ‘50’ derived from Fig. 7.1A. The solid line (additivity isobole) joining 10 dose units on the A axis and 100 dose units on the B axis describes combinations of A and B that are expected to yield an effect level of ‘50’, if the interaction between A and B is additive. For example, this should be the case with 7.5 dose units A plus 25 dose units B (point 1 on the additivity line), 5 dose units A plus 50 dose units B (point 2) or 2.5 dose units A plus 75 dose units B (point 3). However, the dose–response curves in Fig. 7.1A show that a combination of 2.5 dose units A and 2.5 dose units B is sufficient to produce this effect. Therefore a point below the additivity line is seen, yielding a concave-up isobole. It can be concluded that A and B interact with each other in a way that exacerbates their toxicity (synergism). Conversely, A and B antagonized each other if, e.g., 5 dose units of A plus 75 dose units of B were necessary to produce an effect level of 50 (open square 4). In this case, a point above the additivity line would appear, producing a concave-down isobole. C, Diagram showing isoboles for effect level ‘25’ derived from Fig. 7.1A
(From A. Kortenkamp and R. Altenburger 1998 Synergisms with mixtures of xenoestrogens: a re-evaluation using the methods of isoboles. Science of the Total Environment 221(1): 59–73, with permission.)
THE ISOBOLE METHOD
The isobole method is an application of dose addition. It is unequivocal proof of synergy because it is independent of any knowledge of mechanisms and applies under most conditions. It makes no assumptions about the behaviour of each agent and is applicable to multiple components of up to three constituents, so can be applied to the analysis of effects in herbal mixtures. The isobole method uses graphs constructed to show curves (isoboles) describing combinations of two compounds, A and B, which produce the same specified action – which can be any measurable effect (Fig. 7.1A,B). The axes of the graph (Fig. 7.1B) represent doses of the two compounds on a linear scale. A line joining the iso-effective doses A and B of the single agents predicts the combinations of A and B that will yield the same effect, provided the interaction between A and B is only additive. This is the ‘additivity line’, in which case, there is no interaction between the two agents and they could be considered to be behaving like dilutions of each other.
For additivity (zero interaction), the relationship can be expressed algebraically by the equation of Berenbaum (Pharmacol. Rev., 1989 41, 93–141; see Further reading):
However, if synergy occurs, then smaller amounts are needed to produce the effect (i.e. the effect of the combination exceeds expectation) and the equation becomes dA / DA + dB / DB < 1, and the isobole is said to be ‘concave-up’. The opposite applies for antagonism, and the equation becomes dA / DA + dB/DB > 1, producing a ‘concave-down’ isobole. It is actually possible to have synergy at a particular dose combination with antagonism at a different combination, and this would be reflected in the isobole. The position of isoboles varies depending on the effect level chosen for analysis (see Fig. 7.1B,C).
The isobole method can also be applied to mixtures in which only one of the two agents is active; in effect ‘potentiation’. In this case, the iso-effective dose of the agent lacking activity can be regarded as being infinitely large, so the additivity isobole runs parallel to the respective dose axis. Synergism will again yield a concave-up isobole and antagonism a concave-down isobole, as shown in Fig. 7.2.
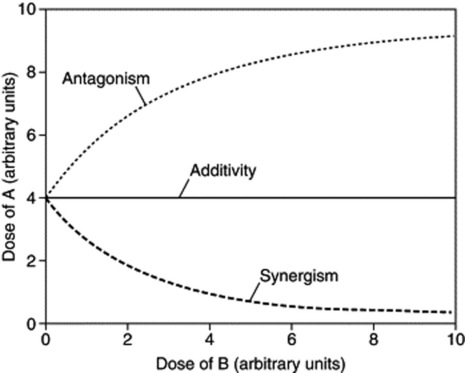
Fig. 7.2 The three types of combination effect for a mixture of an effective agent A and an ineffective compound B. When there is no interaction between A and B, the isobole is a straight line parallel to the dose axis of B (additivity). If there is a synergistic interaction, an isobole deviating towards the B axis is seen: in the presence of B smaller doses of A are sufficient to produce a predetermined effect. When there is antagonism, the isobole deviates away from the B axis. In this case, the presence of B requires higher doses of A to yield the same effect as in the absence of B.
(From: A. Kortenkamp and R. Altenburger 1998 Synergisms with mixtures of xenoestrogens: a re-evaluation using the methods of isoboles. Science of the Total Environment 221(1): 59–73, with permission.)
DEMONSTRATING SYNERGY AND POLYVALENT ACTION IN PHYTOMEDICINES
Proving the existence of true synergistic interactions, even within a single herbal extract, is remarkably difficult, and explains why this crucial aspect of herbal medicines is not well documented. To do so requires the extract to be fractionated, tested, recombined and retested in various permutations to see how each is interacting with the others. To complicate matters further, herbalists normally use mixtures of extracts, many of which are traditional combinations that are not necessarily intended to target a single biochemical system or enzyme (S. Y. Mills and K. Bone, Principles and Practice of Phytotherapy, Churchill Livingstone, 2000), making evaluation of additive or synergistic effects even more difficult. Simple examples of this practice would be the inclusion of laxative herbs in products used for haemorrhoids, or choleretic herbs in digestive preparations. This is not synergy but a way of approaching treatment from several angles concurrently, and could be described as ‘polyvalent action’. This term is used to cover the various effects of multiple active constituents acting in combination, in harmony and possibly in synergy. It therefore overcomes some of the problems of defining the overall effect as synergistic even when it includes antagonism, if that applies to a reduction of undesirable effects. As a preliminary step in looking for synergistic interactions, it is possible to test the effect of individual extracts singly and in combination, which will give an indication of synergy or antagonism although no real evidence as to which compounds are interacting.
In conventional medicine it is now common practice to use several drugs to treat a single complaint, such as in hypertension, psychoses and especially cancer, and this approach applies even more to plant extracts, because combinations are already present within the plant. There might be other sound reasons for not isolating individual components in some herbs such as ginkgo, St John’s wort and ginseng, because these are traditionally used as standardized extracts for which there is positive clinical data. In some cases, the active ingredients may not even be fully known, and if synergy is involved, then bioassay-led fractionation (the usual method for identifying actives) would not even be possible (see P. Houghton, Phytother. Res., 2000, 14(6): 419–423). The identities of the main actives of many important herbs are still under discussion, and it would be unwise to exclude, by overpurification, any constituents that might contribute to efficacy. Even if the phytochemistry of a plant is well documented, the actual contribution of individual components to the overall effect might not have been ascertained. Examples include hawthorn (Crataegus oxycantha) as a cardiac tonic, hops (Humulus lupulus) as a sedative, black cohosh (Cimifuga racemosa) and chasteberry (Vitex agnus-castus) as hormone-balancing agents in women, saw palmetto (Serenoa repens) as an antiandrogen for prostatic hyperplasia, and devil’s claw (Harpagophytum procumbens) as an anti-inflammatory agent. In other cases, the actives are unstable, and attempts to remove them from the ‘protection’ of the herb or whole extract could render them inactive. Here the obvious examples are garlic (Allium sativum) and valerian (Valeriana spp.). Garlic is often formulated as a product containing the precursor alliin, and the enzyme alliinase, which in solution (i.e. in the stomach) liberates the active allicin and other unstable, but still active, decomposition products. This is not synergy but, effectively, a drug-delivery system.
Interactions in vivo might also occur between combinations that enhance or hinder therapeutic activity by affecting absorption, metabolism or excretion. Some of these can be seen in vitro, such as the complexing of plant polyphenols and tannins with many drugs, which could theoretically reduce their effectiveness. This does not seem to be a real problem, otherwise tea drinkers would find many of their prescribed medicines inactive. Other interactions will only be seen clinically, such as the effects of cytochrome P450 enzyme induction, which are only seen after a period of treatment.
ENHANCEMENT OR REDUCTION OF ABSORPTION OR BIOAVAILABILITY
TCM and Ayurvedic formulae often have herbs such as liquorice or pepper included specifically to reduce or increase bioavailability of other ingredients, and this can be considered a form of synergy. Liquorice (Glycyrrhiza spp.) features as a synergist in a great many multiherbal preparations in TCM and the reason for its inclusion has not always been apparent. Glycyrrhiza extracts are used for their antiulcer, anti-inflammatory and antihepatotoxic properties and the saponin glycyrrhizin (formerly considered to be the ‘active’ constituent) exhibits activity in all of these areas. However, so also do the flavones, isoflavones and chalcones, and glycyrrhizin is certainly responsible for the more serious side effects related to its corticosteroid-like activity (such as Cushing’s syndrome) associated with ingestion of large amounts of liquorice. A study on the bioavailability of glycyrrhizin, when given on its own or as part of a liquorice-root extract, showed that absorption of glycyrrhizin is lower when taken in the form of an extract (G. Cantelli-Forti et al., Environ. Health Perspect., 1994, 102 Suppl. 9: 65–68). Although the sample size was small, when a similar experiment was carried out in rats the results were in agreement. The authors suggest that differences are due to an unspecified interaction taking place during intestinal absorption.
Ayurveda also uses fixed combinations of herbs, and an important ingredient of many recipes, some of which date back to 6000 BC, is ‘Trikatu’ (Sanskrit, meaning ‘three acrids’). Trikatu is a mixture of black pepper, Piper nigrum; long pepper, Piper longum; and ginger, Zingiber officinale and a theory for its use has been proposed that involves enhancement of bioavailability, not only by Trikatu, but especially by the alkaloid piperine, which is found in many Piper species (R. K. Johri and U. Zutshi, J. Ethnopharmacol., 1992, 37: 85–91). The effects on bioavailability probably result from the fact that piperine is a potent inhibitor of drug metabolism. Piperine also inhibits glucuronidation of epigallocatechin gallate in the small intestine, as well as slowing gastrointestinal transit, which would increase its availability and residence time in the intestine, allowing for greater absorption (J. D. Lambert et al., J. Nutr., 2004, 134(8): 1948–1952). Another example is the co-administration of piperine and curcumin to humans and rats, which enhanced the bioavailability of curcumin by 2000% and 154%, respectively, due to inhibition of the glucuronidation of curcumin (G. Shoba et al., Planta Med., 1998, 64(4): 353–356.)
EXAMPLES OF SYNERGY, POLYVALENT ACTION OR ANTAGONISM IN HERBAL MEDICINES
SYNERGISTIC EFFECTS DEMONSTRATED IN SINGLE PLANT EXTRACTS
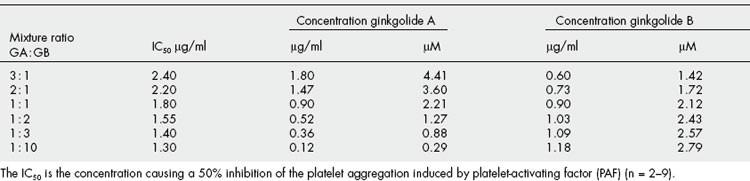
Synergy between ginkgolides present in Ginkgo biloba in a platelet aggregation test
Most ginkgo preparations are standardized for their terpene lactone (ginkgolide) and flavonoid content, and although these groups of compounds have discrete modes of action, it is likely that they work together. Recently, synergy has been demonstrated between ginkgolides A and B using a platelet aggregation assay. This is shown in the graph (Fig. 7.3) of the results obtained from investigating synergy between ginkgolides A and B as antithrombotic agents (Table 7.1). This is clinically significant because it means that if the most effective ratio ofginkgolides is selected, a lower total dose of extract is needed. Ginkgo is used mainly for vascular insufficiency, especially in the brain, where it can result in impairment of cognition, and numerous studies have confirmed its efficacy. The effect can be partly related to the different constituents. The ginkgolides are diterpenes and are known to be platelet-activating factor (PAF) antagonists. Numerous studies have shown that the ginkgolides antagonize many of the effects of PAF, including inflammation, bronchoconstriction, bronchial hyperresponsiveness, platelet aggregation and allergic responses. The ginkgolides might thus contribute to the efficacy of ginkgo in cerebral insufficiency but may also produce some benefit in inflammatory disorders, including asthma. Ginkgo flavones are also anti-inflammatory, the combination being considered additive and possibly synergistic in effect, as well as increasing blood circulation to the brain, and a total ginkgo extract acts as an antioxidant activity in brain preparations. Clinical studies have shown ginkgo to be effective in improving cognitive function as well as the early stages of dementia; the preparation used is a total extract not just the flavonoids. This suggests polyvalent as well as synergistic activity.
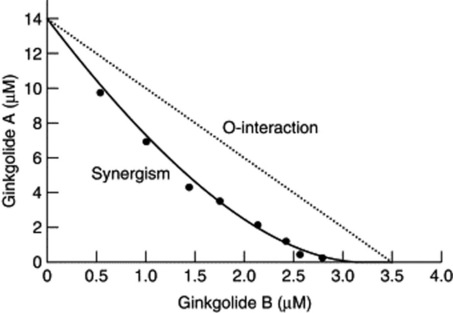
Fig 7.3 Synergy between ginkgolides A and B: the isobole drawn from values in Table 7.1
(From H. Wagner 2006 Multitarget therapy – the future of treatment for more than just functional dyspepsia. Phytomedicine 13: 122–129 with permission.)
Potentiation of the effect of berberine by 5′-methoxyhydnocarpin in Berberis extract in prevention of bacterial resistance
Although this has been cited as a clear example of synergy between components of a single plant extract, it is more correctly termed potentiation. The phenomenon is demonstrated by a compound isolated from Berberis fremontii on the antimicrobial effects of berberine, another constituent, as shown in Fig. 7.4. Multidrug-resistance pumps (MDRs) protect bacteria from antimicrobials, and berberine is readily extruded by such MDRs. Several Berberis species were found also to synthesize an inhibitor of the norA (a membrane-associated efflux protein) MDR pump of a human pathogen Staphylococcus aureus. The inhibitor is 5′-methoxyhydnocarpin (5′-MHC), originally found as a minor component of chaulmoogra oil. 5′-MHC had no antimicrobial activity alone but strongly potentiated the action of berberine and other norA substrates against S. aureus. MDR-dependent efflux of berberine and ethidium bromide (EtdBr; used for comparison because of its known mechanism of action and similarity in some properties to berberine) from S. aureus cells was completely inhibited by 5′-MHC. The level of accumulation of berberine in the cells was greatly increased in the presence of 5′-MHC, indicating that this compound effectively disabled the bacterial resistance mechanism. 5′-MHC has also been found to be present in B. aquifolia and B. repens suggesting that whole herbal extracts of these plants may have a superior antimicrobial effect to berberine alone where MDRs are involved (F. R. Stermitz et al., PNAS, 2000, 97(4): 1433–1437).
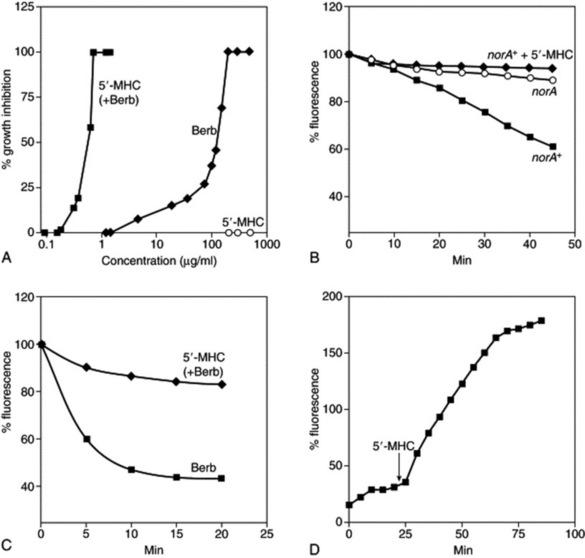
Fig. 7.4 Synergistic action of berberine and 5′-methoxyhydnocarpin (5′-MHC). A, Growth inhibition of Staphylococcus aureus. Berberine (Berb) was present at a concentration of 30 μg/ml when combined with 5′-MHC. Measurements were performed in triplicate, and the average values are shown. B, Inhibition of norA transport activity by 5′-MHC. S. aureus cells were loaded with ethidium bromide (EtdBr) and washed; the efflux was measured in the presence of 100 mM formate, a respiratory substrate. 5′-MHC was added at a final concentration of 10 μg/ml. C, Cells were loaded with berberine and efflux was measured in the presence of formate. D, Uptake of berberine added at time 0 by cells in the presence of formate. A small increase of fluorescence produced by 5′-MHC alone was subtracted from the plot.
(From: F. R. Stermitz, P. Lorenz, J. N. Tawara, L. A. Zenewicz, K. Lewis 2000 Synergy in a medicinal plant: antimicrobial action of berberine potentiated by 5′-methoxyhydnocarpin, a multidrug pump inhibitor. Proceedings of the National Academy of Sciences of the USA 97(4): 1433–1437 (with permission). Copyright (2000) National Academy of Sciences, USA.)
Enhancement of activity of Δ9–tetrahydrocannabinol in cannabis extract by other constituents
An example of the action of a known active compound being enhanced by the presence of other (inactive) compounds is shown by an experiment in which the antispastic effects of cannabis extract and isolated Δ9-tetrahydrocannabinol (Δ9-THC) were compared in an immunogenic model of multiple sclerosis. It can be seen from Fig. 7.5A that a cannabis extract (SCE) has a more rapid effect on relieving muscle spasticity than isolated Δ9-THC at matched concentrations. Fig. 7.5B shows that the extract from which the Δ9-THC has been removed (Δ9-THC-free SCE) has no effect on spasticity, confirming that THC alone is responsible for the effect. The extract had been passed through a high-performance liquid chromatography preparative column, so to ensure that this had no effect on the effect of the extract, it was recombined to give extract TSCE, which had similar properties to SCE in the biological model (J. D. Wilkinson et al., J. Pharm. Pharmacol., 2003, 55(12): 1687–1694).
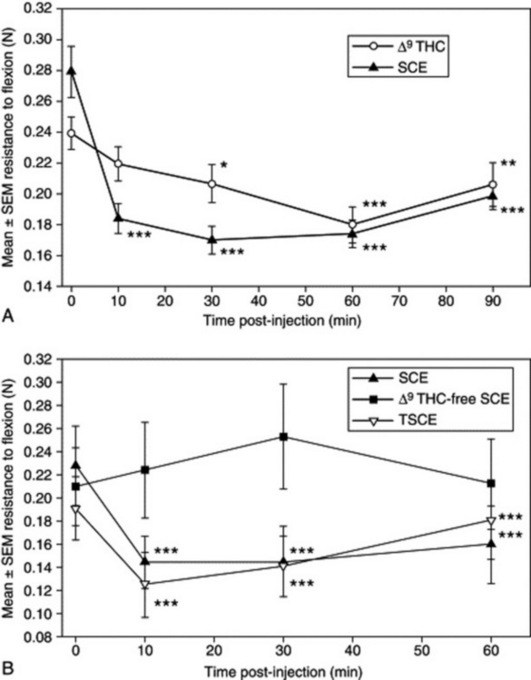
Fig 7.5 Effect of various cannabis extracts compared with isolated Δ9-tetrahydrocannabinol (Δ9-THC) on spasticity in an in-vivo model of multiple sclerosis. Following the induction of chronic relapsing experimental allergic encephalomyelitis, spasticity of the hind limbs developed. This was measured by the resistance to full flexion of the hind limbs against a strain gauge before and following intravenous administration of: A, 1 mg/kg Δ9-THC and, 1 week later, in the same group of animals (n = 8 mice) with 5 mg/kg SCE (a cannabis extract) containing 20% Δ9-THC in vehicle; or B, 5 mg/kg SCE, Δ9-THC-free SCE and TSCE in the same group of animals (n = 6 mice), separated by at least 48 h. The data points represent mean ± SEM of resistance force (Newtons, N) of 12 individual spastic hind limbs in each experiment. *P < 0.05, **P < 0.01, ***P < 0.001 are significantly different means compared with baseline control of individual experiments.
(From J. D. Wilkinson, B. J. Whalley, D. Baker, G. Pryce, S. Gibbons, A. Constanti, E. M. Williamson 2003 Medicinal cannabis: is Δ9THC responsible for all its effects? Journal of Pharmacy and Pharmacology 55(12): 1687–1694, with permission.)
ANTAGONISTIC OR OPPOSING EFFECTS DEMONSTRATED IN SINGLE PLANT EXTRACTS
Opposing effects on blood glucose levels of flavonoids in Pterospartum tridentatum
The isoflavonoid isoquercitrin and the flavonol sissotrin, both isolated from Pterospartum tridentatum, have been shown to have opposing actions on oral glucose tolerance in rats. The overall effect of the aqueous extract of P. tridentatum on blood glucose levels of normal rats given an oral glucose challenge was complex, in that it produced an antihyperglycaemic effect during the first 30 minutes, but subsequently blood glucose levels rose above those of control group (Fig. 7.6). This suggested the presence of compounds with different actions on glucose tolerance. An oral glucose tolerance test performed using isolated isoquercitrin and sissotrin, found these compounds to have opposing effects. Isoquercitrin showed a time-dependent antihyperglycaemic activity, by delaying the post-oral glucose load glycaemic peak, in a similar manner to the sodium-dependent glucose transporter inhibitor phloridzin (a flavonoid glucoside found in apples). By contrast, sissotrin produced an opposite effect by impairing glucose tolerance. These results show that the effect of the extract on blood glucose may be either antihyperglycaemic or hyperglycaemic, and that it depends on the relative concentrations of isoquercitrin and sissotrin in the extract (A. Paulo et al., Phytother. Res., 2008, 22(4), 539–543). This type of antagonism shows the importance of chemically characterizing an extract, because the relative flavonoid composition might vary among plant samples of the same species.
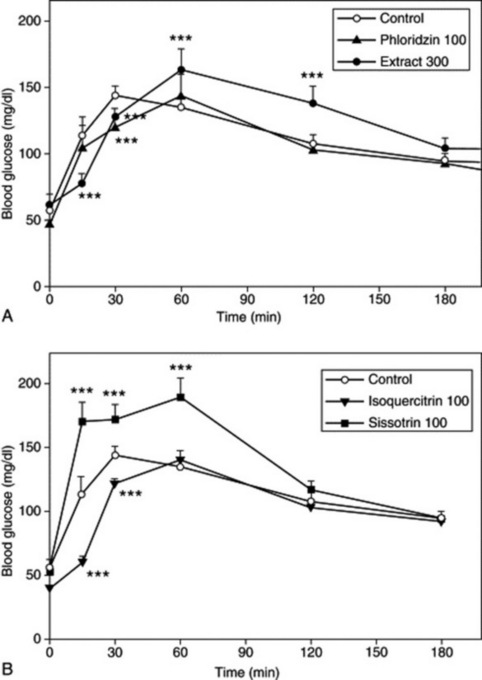
Fig 7.6 Opposing effects on blood glucose levels of flavonoids present in Pterospartum tridentatum. Effect of (A) aqueous extract of P. tridentatum (300 mg/kg; n = 5) and phloridzin (100 mg/kg; n = 6); (B) isoquercitrin (100 mg/kg; n = 5) and sissotrin (100 mg/kg; n = 6) on oral glucose tolerance in normal Wistar rats compared with a control group (n = 6). * P < 0.05, ** P < 0.01, *** P = 0.001.
(From: A. Paulo, S. Martins, P. Branco, T. Dias, C. Borges, A. Rodrigues et al 2008 The opposing effects of the flavonoids isoquercitrin and sissotrin, isolated from Pterospartum tridentatum, on oral glucose tolerance in rats. Phytotherapy Research, 22(4), 539–543. © John Wiley & Sons Ltd. Reproduced with permission.)
MULTIPLE PHARMACOLOGICAL EFFECTS DEMONSTRATED IN A SINGLE PLANT
Synergy and antagonism between compounds and fractions in Psyllium (ispaghula) husk
Psyllium husk, also known as ispaghula, is equally acceptable in traditional and modern medicine; it is also considered to be effective for use in both constipation and diarrhoea, which are two opposite disease states of the gut. The general perception is that its laxative effect is achieved mainly through its fibre content, which may be true, but what it makes more effective in chronic constipation than other fibre-containing remedies is not clear. However, evidence is now accumulating to suggest that it also contains constituents with gut-stimulatory properties, mediated partly through cholinergic activation, which is likely to supplement the laxative effect (A. H. Gilani et al., Phytother. Res., 1998, 12(S1): S63–S65). Interestingly, it also contains gut-inhibitory constituents, which not only are likely to offset the side effects associated with cholinergic components, but also provide a scientific explanation for the traditional use of ispaghula in diarrhoea (A. H. Gilani et al., Naunyn-Schmied. Arch. Pharmacol., 1998, 358(S1): 40–73). In addition to gut-stimulatory and gut-inhibitory constituents, ispaghula also contains antiamoebic constituents, explaining its traditional use in amoebic dysentery (V. Zaman et al., Phytother. Res., 2002, 16: 78–79), and thus demonstrating multiple effects, some supporting and some opposing a particular activity, in one medicinal plant.
SYNERGISTIC EFFECTS SHOWN BETWEEN TWO DIFFERENT PLANT EXTRACTS
Effect of soya and tea extracts on prostate tumour growth and angiogenesis in mice
A combination of a soya phytochemical concentrate (SPC) with tea extracts synergistically inhibited tumour growth and reduced serum concentrations of testosterone and dihydrotestosterone in a mouse model of androgen-sensitive human prostate cancer (Fig. 7.7). The inhibition of tumour progression was also associated with reduced tumour-cell proliferation and angiogenesis. SPC and black tea alone significantly reduced final tumour weights and, although green tea did not reduce final tumour weight, it tended to elevate serum dihydrotestosterone concentrations. This study is significant because it supports the idea that the chemopreventive properties of the Asian diet might result from interactions between several components. In Asia, where the intake of soy products and tea consumption are very high, aggressive prostate cancer is significantly less prevalent than in other parts of the world.
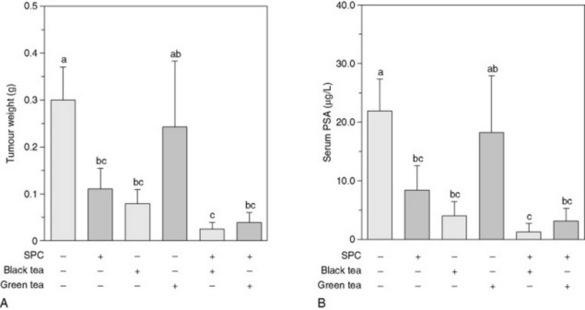
Fig. 7.7 Combined effects of soy phytochemicals and tea on final tumour weight and serum prostate-specific antigen (PSA) levels. Effects of soy phytochemicals and tea combinations on final tumour weight (A) and serum prostate-specific antigen levels (B) in severe combined immune deficient (SCID) mice bearing LNCaP human prostate cancer cells. Compared with the control (Fig. 7.7A), all treatments other than green tea alone significantly reduced the final tumour weight. The combined effects of the soya phytochemical concentrate (SPC)/black tea combination (93%) and the SPC/green tea combination (88%) on final tumour weight reduction were greater than the expected additive effects (91% and 70%, respectively), suggesting that the combination of SPC with either black tea or green tea synergistically inhibited final tumour weight. In parallel, serum levels of PSA, a marker that is secreted by LNCaP cells and reflects tumour size, in mice in the experimental groups other than the green tea group were reduced (Fig. 7.7B), compared with the control. Comparisons of expected and observed values suggest that SPC combined with black tea or green tea synergistically reduced serum PSA concentration. Values are means ± SEM, n = 14–16. Means without a common letter differ, P < 0.05.
(From: J.-R. Zhou, L. Yu, Y. Zhong, G. L. Blackburn 2003 Soy phytochemicals and tea bioactive components synergistically inhibit androgen-sensitive human prostate tumors in mice. Journal of Nutrition 133(2): 516–521, with permission.)
NEW TECHNOLOGIES FOR LOOKING AT SYNERGY AND OTHER INTERACTIONS
One outcome of the recent development of informatics tools is the advancement of systems biology, which has the potential to revolutionize natural product research and scientific-based herbal medicine. The integration of data into systems biology can enable the understanding of living systems from a holistic perspective and facilitate the study of multitarget approaches. Evidence obtained from the new ‘-transcriptomic’ technologies (genomics, proteomics and metabolomics) can hopefully support the identification of synergy and polyvalent pharmacological activities, as complex gene expression analysis by microarray can detect differences in cellular responses to drug combinations versus single agents (M. H. Cheok et al., Nat. Genet., 2003, 34: 85–90). It could further demonstrate whether drug combinations can lead to the activation of entirely different genes to those activated by individual agents. Thus, the mode of action of a combination can be, based on the gene expression, entirely different from the mode of action of the single agents contained in it. Although it is questionable whether the discriminating genes for treatment are the same as those responsible for the main action of the single agent, the method is still suitable for the discrimination of different treatments (see Further reading for reviews on the use of metabolomics and systems biology in research in phytomedicines).
CONCLUSION
There can be no doubt that most herbs rely for their effects on a variety of constituents, and the idea of synergy within and between them is also gaining acceptance. Whether they are acting in a truly synergistic way or by additive effects is not well documented, but it is important for both developing methods of standardization as well as furthering our knowledge of mechanisms of drug action. Clinical evaluation is also more difficult without knowing the extent to which synergy occurs within the herbal preparation, and it should be further investigated for all these reasons. In the meantime, evidence is accumulating to show that synergism does occur in extracts and mixtures, and that there is benefit in using whole extracts. However, it is still vital to ensure that extracts are standardized for the active principles known at the time and that any known synergistic interactions are taken into account. If done properly, this should lead to improved products with increased efficacy at lower doses and correspondingly reduced toxicity. Synergistic principles apply to all forms of drug treatment, not only those that are plant based, and although this is a well-known concept in phytotherapy, it is relatively new to other forms of conventional medicine.
Acknowledgement
The author wishes to thank Prof. Andreas Kortenkamp, University of London School of Pharmacy, for his expert advice on the different methods for the measurement of synergy.
Berenbaum MC. What is synergy? Pharmacological Reviews. 1989;41:93-141.
Duke JA, Bogenschutz-Godwin MJ. The synergy principle in plants, pathogens, insects, herbivores and humans. In: Kaufmann PB, et al, editors. Natural products and plants. New York: CRC Press; 1999:183-205.
Gilani AH, Atta-ur-Rahman. Trends in ethnopharmacology. Journal of Ethnopharmacology. 2005;100:43-49.
Kortenkamp A, Altenburger R. Synergisms with mixtures of xenoestrogens – a re-evaluation using the method of isoboles. Science of the Total Environment. 1998;221:59-73.
Wagner H. Trends and challenges in phytomedicine: research in the new millennium. In: Yaniv Z, Bachrach U, editors. Handbook of medicinal plants. Binghamtown, NY: Haworth Medical Press; 2001:3-28.
Williamson EM. Synergy and other interactions in phytomedicines. Phytomedicine. 2001;8(5):401-409.
Metabolomics and systems biology
Ulrich-Merzenich G, Zeitler H, Jobs D, Panek D, Vetter H, Wagner H. Application of the ‘-Omic-’ technologies in phytomedicine. Phytomedicine. 2007;14:70-82.
Verpoorte R, Choi YH, Kim HK. Ethnopharmacology and systems biology: a perfect holistic match. Journal of Ethnopharmacology. 2005;100:53-56.
Wang M, Lamers R-JAN, Korthout HAAJ, Nesselrooij JH, Witkamp RF, van der Heijden R, et al. Metabolomics in the context of systems biology: bridging traditional Chinese medicine and molecular pharmacology. Phytotherapy Research. 2005;19(3):173-182.