Chapter 12 Plant growth regulators
The growth and development of plants is regulated by a number of chemical substances which together exert a complex interaction to meet the needs of the plant. Five groups of plant hormones are well established; they are the auxins, gibberellins, cytokinins, abscisic acid and its derivatives, and ethylene. These substances are of wide distribution and may, in fact, occur in all higher plants. They are specific in their action, are active in very low concentrations, and regulate cell enlargement, cell division, cell differentiation, organogenesis, senescence and dormancy. Their action is probably sequential. Other hormones concerned with flower formation and reproduction, but as yet uncharacterized, have also been envisaged. The essential role of these substances is illustrated by cell and tissue cultures; without the addition of suitable hormones no development or cell division occurs.
The effects of these very active substances on the production of secondary metabolites, particularly with a view to producing plants containing an enhanced proportion of active constituent, are of interest to pharmacognosists. In such studies the manner in which the results are recorded is all-important, particularly as the treatment may also influence the size of the test plant compared with the controls. For commercial purposes yield per hectare is an obvious criterion, whereas for biosynthetic studies yield per plant or per cent fresh weight may be of more significance. For final drug evaluation per cent dry weight is the most likely requirement.
In spite of the early enthusiasm for research on drug enhancement by the use of hormones applied to medicinal field crops, very little in the way of useful practical application emerged; the results were, however, of interest and selected examples of this older work continue to be retained in this chapter.
AUXINS
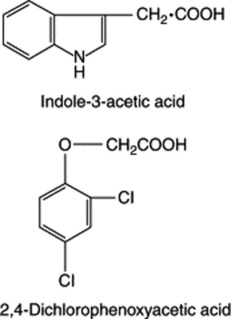
These growth-promoting substances were first studied in 1931 by Dutch workers who isolated two growth-regulating acids (auxin-a and auxin-b, obtained from human urine and cereal products, respectively). They subsequently noted that these had similar properties to indole-3-acetic acid (IAA), the compound now considered to be the major auxin of plants, and found particularly in actively growing tissues. Several similar acids, potential precursors of indoleacetic acid, have also been reported as natural products; they include indoleacetaldehyde, indoleacetonitrile and indolepyruvic acid. These compounds and IAA are all derived, in the plants, from tryptophan.
Typical effects of auxins are cell elongation giving an increase in stem length, inhibition of root growth, adventitious root production and fruit-setting in the absence of pollination. A number of widely used synthetic auxins include indole-3-butyric acid, naphthalene-1-acetic acid (NAA) and 2,4-dichlorophenoxyacetic acid (2,4-D).
In the plant, oxidative degradation of IAA to give a number of products is controlled by IAA oxidase. Some substances such as the orthodiphenols (e.g. caffeic and chlorogenic acids and quercetin) inhibit the action of the enzyme and, hence, stimulate growth themselves. Conversely, monophenols such as p-coumaric acid promote the action of IAA oxidase and so inhibit growth. IAA may also be conjugated in the plant with aspartic acid, glutamic acid, glycine, sugars and cyclitols; such bound forms may represent a detoxication mechanism or are inactive storage forms of the hormone.
The main practical uses of auxins are: (1) in low concentrations to accelerate the rooting of woody and herbaceous cuttings; and (2) in higher concentrations to act as selective herbicides or weed-killers. Placed for 24 h in a 1:500 000 solution of NAA, cuttings will subsequently develop roots. This includes cuttings from trees such as holly, which were formerly very difficult to propagate in this way and had to be raised from seed or by grafting. Similarly, indole-3-butyric acid was successful with Cinchona cuttings, saving some 2 or 3 years compared with growth from seed. Similar results have been obtained with cuttings of Carica, Coffea, Pinus and other species. In biogenetic studies, use has been made of auxins to induce root formation on isolated leaves such as those of Nicotiana and Datura species. Auxins used in suitable concentration (usually stronger than when used for rooting cuttings) selectively destroy some species of plant and leave others more or less unaffected. They have, therefore, a very important role as selective weed-killers in horticulture and agriculture. Thus 2,4-D is particularly toxic to dicotyledonous plants while, in suitable concentration, having little effect on monocotyledons. It can, therefore, be used to destroy such dicotyledonous weeds as dandelion and plantain from grass lawns. (N.B. Certain carbamate and urea derivatives have an opposite effect and can be used to destroy grass without serious injury to dicotyledonous crops.)
There have been several reports on the effects of auxins on the formation of secondary metabolites on medicinal plants.
Seedlings and young plants of Mentha piperita, when treated with derivatives of NAA, gave in the mature plants an increased yield (30–50%) of oil which itself contained 4.5–9.0% more menthol than the controls. The study of the effects of auxins on alkaloid formation has concentrated principally on the tropane alkaloids of Datura species. Morphological changes in the plants were observed (2,4-D, for example, produced abnormal and bizarre forms of D. stramonium; an increase in trichome production, particularly in branched non-glandular forms; smooth fruits as distinct from those with spines; and a proliferation of vascular tissue). Generally, workers found no marked effect on alkaloid production or on the type of alkaloids produced, although a Russian paper records that with thornapple and scopolia tissue cultures a stimulating effect on alkaloid production was obtained with NAA and an inhibiting effect with 2,4-D; similar results were reported for Rauwolfia serpentina tissue cultures with these two hormones. An increased alkaloid production has been reported for submerged cultures of certain ergot strains when treated with various auxins (IAA; NAA; 2,4-D; indole propionic acid; indole butyric acid), whereas unpredictable irregular quantitative and qualitative effects on ergoline alkaloid production were observed with the same hormones in Ipomoea, Rivea and Argyreia (Convolvulaceae) suspension cultures. Experiments carried out in Hungary involving the injection of IAA into poppy capsules 1 and 2 days after flowering produced a relatively elongated capsule form and, in general, a reduced alkaloid content. In studies on anthraquinone production by cell suspension cultures of Morinda citrifolia, Zenk and co-workers have shown that cells grown in the presence of NAA have a substantial anthraquinone production but those with 2,4-D as sole auxin do not. IAA appears to have no beneficial effect on the production of sennosides in Cassia angustifolia.
GIBBERELLINS
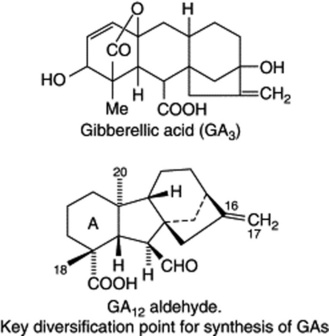
This group of plant growth regulators was discovered by Japanese workers in connection with the ‘bakanae’ (foolish seedlings) disease of rice. In this, the affected plants become excessively tall and are unable to support themselves; through a combination of the resulting weakness and parasite damage they eventually die. The causative organism of the disease is Gibberella fugikuroi, and in 1926 Kurosawa found that extracts of the fungus could initiate the disease symptoms when applied to healthy rice plants. Some 10 years later, Yabuta and Hayashi isolated a crystalline sample of the active material which they called ‘gibberellin’. Preoccupation with the auxins by western plant physiologists, the existence of language barriers and the advent of World War II meant that a further 10 years elapsed before the significance of these findings was appreciated outside Japan. In the 1950s groups in Britain, the USA and Japan further investigated these compounds, which were shown to have amazing effects when applied to plants. It soon became apparent that a range of gibberellins was involved, and they are now distinguished as GA1, GA2, GA3, etc. GA3, commonly referred to as gibberellic acid and produced commercially by fungal cultivation, is probably the best-known of the series; its structure was finally determined in 1959. The first good indications that gibberellins actually existed in higher plants came with West and Phinney’s observation in 1956 that the liquid endosperm of the wild cucumber (Echinocytis macrocarpa) was particularly rich in substances possessing gibberellin- like activity and Radley’s report of a substance from pea-shoots behaving like gibberellic acid on paper chromatograms. Finally, in 1958 MacMillan and Suter isolated crystalline GA1 from Phaseolus multiflorus. By 1980, 58 gibberellins were known of which about half were derived from the Gibberella fungus and half from higher plants. GA117 was characterized from fern gametophytes in 1998 (G. Wynne et al., Phytochemistry, 1998, 49, 1837). Of these many GAs most are either dead-end metabolites or are intermediates in the formation of active compounds; only a limited number have hormonal activity per se. It is now considered possible that these substances are present in most, if not all, plants.
Gibberellins are synthesized in leaves and they accumulate in relatively large quantities in the immature seeds and fruits of some plants. The most dramatic effect of gibberellins can be seen by their application to short-node plants—for example, those plants producing rosettes of leaves (Digitalis, Hyoscyamus)—when bolting and flowering is induced; also, dwarf varieties of many plants, when treated with the hormone, grow to the same height as taller varieties. Other important actions of the gibberellins are the initiation of the synthesis of various hydrolytic and proteolytic enzymes upon which seed germination and seedling establishment depend.
The growth effect of gibberellins arises by cell elongation in the subapical meristem region where young internodes are developing. The effects of gibberellins and auxins appear complementary, the full stimulation of elongation by either hormone necessitating an adequate presence of the other.
As with auxins, gibberellins appear also to occur in plants in deactivated forms; thus, β-D-glucopyranosyl esters of GA1, GA4, GA8, GA37 and GA38 are known. As such they may serve a depot function. The glucosyl ester of GA3 has been prepared in several laboratories.
The biogenetic pathways of the gibberellins appear to be similar in both higher plants and Gibberella. They arise at the C20 geranylgeranyl pyrophosphate level of the isoprenoid mevalonic acid pathway (q.v.) with cyclizations giving the C20 tetracyclic diterpernoid entkaurenoic acid, which by a multistep ring contraction furnishes the gibbane ring system as exemplified by the key intermediate GA12-aldehyde. Several pathways diverge from GA12-aldehyde to give the known 90 or so gibberellins. All GAs have either the ent-gibberellane (C20 GAs) or the ent-20-norgibberellane (C19 GAs) (loss of C-20) carbon skeleton. Both types are modified by the position and number of OH groups, oxidation state of C-18 and C-20, lactone formation, presence and position of double bonds on ring A, epoxide formation, the presence of a carboxyl group and hydration of the C16–C17 double bond.
The gibberellins have been used to treat many plants which contain useful secondary metabolites. A summary of some of the findings, for different groups, is given below.
Volatile oils and terpenoids
Early work involving GA treatment of volatile oil-containing plants was concerned with resultant changes in morphological characters in genera such as Citrus, Eucalyptus and Foeniculum. GA spraying of the flowers of Humulus lupulus advanced the maturity of the hops by 10 days and gave a more evenly developed crop. Although the cones of treated flowers were more subject to wind burn than normal, their yield was increased by about 40%. The α-resin content of the hops, on which their commercial value depends, however, was 1.8% compared with 10.2% for the controls; the volatile oil composition also differed. Several studies have been made of the effects of GA treatment on Mentha piperita. The detailed results vary, but the general result is a lowering of the oil content (possibly by reduction of the number of glandular hairs) with little change in the oil composition. In contrast to the above, Kaul and Kapoor have reported favourably on the GA treatment of Chenopodium ambrosioides and Anethum spp. with respect to volatile oil content. The former afforded a 33% increase in volatile oil with no appreciable change in ascaridole content. With Anethum graveolens specific doses of GA increased the oil content by up to 50% and with A. sowa (Indian dill) by up to 30%. At the lower doses of GA treatment there was no significant change in the carvone content of the oil, but at higher concentrations there appeared to be a slight increase over the (then) official limit (53%, BP 1958). With Foeniculum vulgare and Coriandrum sativum Gjerstad found that foliar sprays of 100 parts/106 GA, applied bi-weekly, gave a difference in cauline length of 200–300% but no differences were detected in yield of fruits and quantity and quality of volatile oil.
Alkaloids
The seeds of the tropane alkaloid-producing species of Atropa, Hyoscyamus and Datura often exhibit protracted dormancy or erratic germination; GA treatment of the seeds can be used to assist in obtaining uniform germination and total emergence. Considerable work has been published on the effects of the hormone on the morphology and alkaloid content of treated plants. Hyoscyamus niger is a perennial and the hormone effects included stem elongation giving a two- to threefold increase in height; a spindly and vine-like growth; slightly chlorotic and narrow leaves; a more rapid onset of flowering; increases in the stem dry weights but decreases in the dry weights of leaves, tops and roots. The overall yield of alkaloids in the treated plants was reduced by about a half and the concentration of alkaloids in various morphological parts ranged from 43 to 84% of that of the controls, with the stems showing the greatest reduction. Subsequent experiments showed that with belladonna, increased alkaloid yields could be obtained by adjusting the dose of GA treatment to favour overall growth in the older plant.
Similar results have been reported with Datura spp., plants showing the predictable morphological effects and giving a reduced alkaloid yield. The effects were not transmitted to the second-generation plants. With Nicotiana tabacum and Duboisia hybrids, treatment again gave a generally reduced alkaloid content accompanied by characteristic morphological effects.
Other alkaloid-containing plants which have been subjected to GA treatment include Catharanthus roseus (generally a lowering of alkaloid content and some change in the relative proportion of vinblastine to other alkaloids); Rauwolfia serpentina (lowering of alkaloid concentration in the roots, the effect increasing with dose); and Thea sinensis (slight difference in caffeine content of leaves). It would appear, therefore, that with the alkaloid-containing plants so far tested, the substantial internodal growth produced by GA is offset by a lower overall accumulation of alkaloid.
Glycosides
In 1959 Sayed and Beal reported the effects of the daily GA treatment of first-year rosette Digitalis purpurea plants. Flowering occurred in the first year with treated plants and the leaves became longer and more linear, with an increase in dry weight. An increase in cardioactive glycosides was obtained but no increase in the digitoxose content was observed. Similar experiments by Burton and Sciuchetti in 1961 involving weekly treatments of D. lanata produced similar results, with ‘bolting’ after the twelfth week. The total glycoside per shoot of the treated plants was considerably increased in the first 8 weeks, and at harvest time showed about 30% increase with the lower dose of hormone (10 μg week−1), and a 50% decrease with the higher (50 μg week−1). The authors concluded that the effect of the treatment of glycoside production correlated more closely with the growth response than with the effect on carbohydrate formation. With both leaf and root cultures Lui and ? (1981) found GA to have a positive effect on the production of digoxin.
Application of GA to Cassia angustifolia (Bhatia et al., Planta Med., 1978, 34, 437) appeared to reduce the sennoside content of the leaves at all concentrations used, but slightly increased the dry weight of the shoot.
CELL DIVISION HORMONES: CYTOKININS
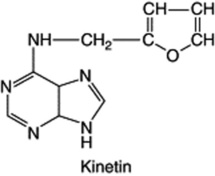
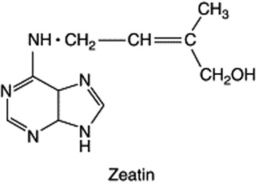
Auxins and gibberellins are concerned largely with cell enlargement; and although they influence cell-multiplication processes, there are other substances which have a more specific effect on cell division (cytokinesis). The activity of the latter is not only confined to cell division in a tissue per se; they also regulate the pattern and frequency of organ production as well as position and shape. They have an inhibitory effect on senescence. The presence of such compounds has been suspected for many years, the German botanist Haberlandt in 1913 having noted that phloem tissues contained water-soluble substances capable of promoting cell division in parenchymatous cells of wounded potato tubers. It was not until many years later (1954) that Miller, working at Wisconsin on tissue cultures, discovered that aged or autoclaved DNA from herring sperm stimulated cell division. This active degradation product was called kinetin and in 1955 was identified as 6-furfurylaminopurine (6-furfuryladenine). Kinetin itself has not been isolated from plants, but in 1964, after the indication of cytokinins in liquid endosperm of the coconut and in extracts of maize embryos at the milky stage, an active substance named zeatin was isolated from the latter source. Like most other cytokinins, it is a 6-substituted adenine derivative, 6-(4-hydroxy-3-methylbut-2-eny1)-aminopurine. It has since been shown to be associated in maize with zeatin riboside (1 β-D-ribofuranose) and with a phosphate ester of this compound. The hormone complex has been detected in the cambial region of various woody plants. Isopentenyladenine and dihydrozeatin are examples of cytokinin isolated from other sources; many more have been detected but not identified. The sidechain of cytokinins is of isoprenoid origin.
Cytokinins have been much employed in tissue culture work, in which they are used to promote the formation of adventitious buds and shoots from undifferentiated cells. In cell cultures, they have been shown to promote the biosynthesis of berberine (Thalictrum minus), condensed tannins (Onobrychis viccifolia) and rhodoxanthin (Ricinus).
A limited study only has been made of the effects of cytokinins on secondary metabolism in intact plants. Concerning plants producing tropane alkaloids, Ambrose and Sciuchetti have compared the action of kinetin and GA on Datura meteloides. Plants received weekly doses of 25 μg kinetin; in relation to the controls the following differences were noted:
| Kinetin treatment | Gibberellic acid treatment |
|---|---|
| Shorter and bushier plants | Taller and spindly plants |
| Decreased growth | Increased growth |
| No change in alkaloid content in plant organs | Decreased alkaloid production |
| Delayed response | Rapid response |
Luanratana and Griffin (J. Nat. Prod., 1980, 43, 546, 552; 1982, 45, 270) observed the beneficial effects of a commercial seaweed extract containing cytokinin activity on Duboisia hybrids grown both hydroponically and in a commercial plantation. In the latter there was an 18% increase in leaf yield and a 16% increase in hyoscine content compared with the controls. A further pointer to the usefulness of the treatment was that in field plants it led to a delay in the usual seasonal fall in alkaloid content (February–April in Australia), thus permitting an economically useful, extended collection period. Shah et al. (1990) reported favourable increases in growth and alkaloid yield with Hyoscyamus muticus treated with kinetin (50 p.p.m.).
Verzár-Petri injected benzyladenine and kinetin separately into developing poppy capsules; the effects were similar to those observed with auxins. Leaves of the coffee plant after kinetin treatment developed a transient increase of up to 10% in their caffeine content. The effect was transitory and passed after 6–12 days. With Cassia angustifolia plants low concentrations of hormone were found to increase slightly the sennoside content and to favour an increase in the dry weight of shoots.
GROWTH INHIBITORS
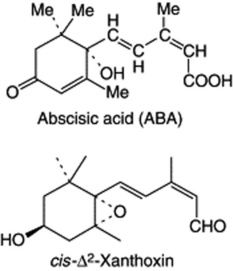
Natural growth inhibitors are present in plants and affect bud opening, seed germination and development of dormancy. One such substance, abscisic acid [3-methyl-5-(1-hydroxy-4-oxo-2,6,6-trimethyl-2-cyclohexane-1-y1)-cis,trans-2,4-pentadienoic acid] was isolated and characterized in 1965; it has also been isolated from the fungus Cenospora rosicola.
The structural similarity of abscisic acid (ABA) to the carotenoids prompted research on the relationship of these two groups of compounds and it has now been demonstrated that some xanthophylls, particularly violaxanthin, produce a germination inhibitor on exposure to light. Evidence has now accumulated in support of an indirect ‘apo-carotenoid’ pathway for ABA biosynthesis, the most likely precleavage precursors being 9′-cis-neoxanthin and 9-cis-violaxanthin which fracture across the 11,12 (11′,12′) double bond to produce xanthoxin which in plant tissues is readily transformed into ABA (see A. D. Parry and R. Horgan, Phytochem., 1991, 30, 815). However, it is also possible that ABA arises from farnesol at the C15 sesquiterpenoid level of the MVA pathway (q.v.). The theoretical postulation involves a number of steps in which, as with the apo-carotenoid pathway, cis-Δ2′-xanthoxin could also be involved. In accord with isoprenoid biosynthesis, mevalonic acid (MVA) has been demonstrated to be stereochemically incorporated into ABA in higher plants, and likewise labelled acetate in Cenospora rosicola. Other substances related to abscisic acid havebeen isolated from plants and include vomifoliol (several sources), which lacks the 2,4-pentadiene side-chain; it has the same activity as abscisic acid in stomatal closure tests. Little or no work appears to have been reported on the effects of abscisic acid on the production of pharmacognostically interesting substances.
A number of synthetic growth inhibitors have been studied; the first to be described was maleic hydrazide in 1949. N-Dimethyl-aminosuccinamic acid can be considered as a hydrazine derivative and acts as a shoot-elongation inhibitor by suppressing the oxidation of tryptamine to IAA. Sciuchetti and colleagues showed that this compound sprayed on to Datura stramonium and D. innoxia plants reduced the eventual height of the plants and lowered, overall, their total alkaloid content; however, significant increases were noted in the concentrations of stem alkaloid (56% increase in the second week and 90% in the fourth week compared with the controls). The inhibitor, tributyl 2,4-dichlorobenzylphosphonium chloride (phosphon) produced similar results with D. ferox. Trigonelline, an alkaloid of fenugreek seeds, promotes cell arrest in G2 (a specific period preceding mitotic division of the nucleus) in various legumes.
ETHYLENE
It has been known for many years that ethylene induces growth responses in plants, and in 1932 it was demonstrated that the ethylene evolved by stored apples inhibited the growth of potato shoots enclosed with them; it has a role in fruit ripening. Current thought maintains that this simple compound should be included among the natural plant hormones. Ethylene is synthesized in the plant from S-adenosylmethionine via the intermediate 1-aminocyclopropane-1-carboxylic acid (ACC). The gene for ACC synthase has been cloned from tomato squash (H. Klee and M. Estelle in Annu. Rev. Plant Physiol., 1991, 42, 529). One biochemical action of ethylene is the stimulation of the de novo synthesis and secretion of cell-wall dissolving enzymes such as cellulase during leaf abscission and fruit ripening. A compound that gives rise to a typical ethylene response in plants is (2-chloroethyl)phosphonic acid (ethephon) applied in aqueous solution in concentrations of the order of 100–5000 p.p.m. In the cell sap, at pH values above 4.0 it is broken down to ethylene and phosphate. It is marketed as Ethrel.
At low concentration ethylene has been shown to increase the sennoside concentration in Cassia angustifolia, and applied to tobacco leaves it stimulates production of the stress compounds phytuberin and phytuberol (these are compounds produced in response to tobacco mosaic virus); with Digitalis lanata tissue cultures, cardenolide accumulation is decreased. Ethephon is now increasingly used as standard practice for enhancing the flow of rubber latex. Sprayed on to the scraped bark (tapping groove) of the rubber tree it increases latex yields by from 36 to 130%.
Other growth regulators
In addition to the well-known plant growth substances discussed above, a very large number of other compounds have been isolated from natural sources which in some way influence plant growth. Some are widely distributed and others are of restricted occurrence. Generally they have a less specific action than the regulators already mentioned above. They have no common chemical structure and only a few recurrent functional groups (e.g. phenolic hydroxy groups and α-methylene-γ-butyrolactone moieties). This implies that these substances may be acting at many different sites along the growth regulatory process. Substances involved include aliphatic and aromatic carboxylic acids, phenolic and neutral compounds, salicylate, polyamines, S- and N-heterocyclic compounds, including alkaloids and terpenes. Acorus calamus produces a number of sesquiterpenes having the skeletal structures of cadinane, acorane and eudesmane which inhibit the germination of lettuce seeds (K. Nawamaki and M. Kuroyanagi, Phytochemistry, 1996, 43, 1175). A new class of plant growth regulators known as brassinosteroids is found in the seeds, pollens, galls, leaves, flower-buds and shoots of a considerable range of plants. Some 40 of these compounds are known; they stimulate cell enlargement and cell division and influence gene expression and nucleic acid metabolism at the molecular level, see V. A. Khripach et al. (1999), Brassinosteroids, a New Class of Plant Hormone, San Diego: Academic Press.