Chapter 15 Deterioration of stored drugs
The factors which must be considered in relation to drug deterioration are moisture content, temperature, light and the presence of oxygen; when these conditions are suitable, living organisms (bacteria, moulds, mites and insects) will rapidly multiply, using the drug as a source of nutrient. Drugs affected in this way are excluded by national pharmacopoeias.
PRIMARY FACTORS
As indicated in Chapter 11, air-dry drugs contain about 10–12% of moisture, and in some instances (e.g. digitalis) this may be sufficient to activate enzymes present in the leaves and bring about decomposition of the glycosides. Other drugs, such as powdered squill, which contain mucilage quickly absorb moisture and become a sticky mass. The containerized shipment of drugs which is now common practice can lead to spoilage due to excessive condensation of moisture on the inner metal walls. It is a particular problem with cargoes in transit from humid moist climates to temperate regions. An increase in temperature, in combination with moisture, may accelerate enzyme activity; a large temperature rise will obviously lead to a loss of volatile constituents (e.g. essential oils from dried plant material) and in the case of absorbent cotton-wool cause a reorientation of the small amount of fatty material present leading to non-absorbency or lower absorbency. Direct sunlight can cause decomposition of certain constituents (e.g. vitamins in cod-liver oil) as well as producing a bleaching of leaves and flowers. Oxygen assists in the resinification of volatile oils and in the rancidification of fixed oils.
MOULD AND BACTERIAL ATTACK
The moulds found in deteriorating drugs are usually the same as those associated with poorly stored food products. Species of Rhizopus, Mucor, Penicillium and Eurotium are common. Their presence is indicated by a mass of hyphae which bind the particles of drug and by a characteristic smell. Deterioration of drugs is only one aspect of the importance of moulds in pharmacognosy—see Chapter 30. Bacterial attack of crude drugs is less obvious unless chromogenic species are involved or effects produced such as dustiness in cotton-wool by attack on the fibres. Although not a cause of deterioration, certain pathogenic bacteria such as salmonellae and Escherichia coli are tested for in some crude drugs taken internally (digitalis, sterculia, tragacanth, gelatin). Also, as plant materials which have been dried under normal conditions contain viable bacteria and mould spores in variable amounts, the pharmacopoeias set limits for the total viable aerobic count per gram of drug (see Quality Control, Chapter 16).
COLEOPTERA OR BEETLES
Beetles are insects and constitute the largest order of the animal kingdom, comprising some 250 000 species, of which about 600 have been found associated with stored food products or drugs. Not all of these utilize the stored product itself but may be found in the wood of packing-cases or living predaciously. Beetles have a body which is divided into head, thorax and abdomen. To the lower side of the thorax are attached three pairs of legs, while the upper surface usually bears two membranous hind-wings which are folded beneath horny elytra (forewings). They show complete metamorphosis of egg, larval, pupal and adult stages, and those which constitute pests in stored products cause damage both as adults and as larvae. Among the characters which distinguish the larvae of most species which attack foodstuffs and vegetable drugs are the well-developed biting mouth-parts and a head which is darker in colour than the rest of the body. Table 15.1 lists beetles commonly found in stored drugs; for illustrations see Fig. 15.1.
Table 15.1 Beetles commonly found in stored drugs.
| Description | |
|---|---|
| Family Nitidulidae (sap-feeding beetles) | |
| Carpophilus spp., e.g. C. hemipterus (dried fruit beetles) | Obovate or oblong 2–4.5 mm long; 11-segmented antennae with a compact club. Elytra somewhat shortened exposing two–three apical abdominal segments. In this country about three species are found in granaries, food-stores and warehouses |
| Family Silvanidae | |
| Oryzaephilus mercator (merchant grain beetle) | Dark brown, narrow, distinctly flattened beetles, about 3 mm long. Clubbed antennae. Attack nuts and dried fruits |
| O. surinamensis (saw-toothed grain beetle) | |
| Family Curculionidae (weevils) | |
| Calandra granaria (granary weevil) | Dark brown to black insects, about 3–4 mm long. Hind-wings absent, characteristic snout and antennae. Bore into seeds and fruits and lay an egg in the cavity by means of the ovipositor. Larvae develop and pupate within the seeds |
| Calandra oryzae (rice weevil) | Similar, hindwings present. 2.3–4.5 mm long |
| Family Anobiidae (‘furniture beetles’) | |
| Stegobium paniceum (Sitodrepa panicea) Anobium paniceum (drugroom beetle) | Pale reddish-brown in colour, greyish hairs, 2–3 mm long. Antennae 11-segmented, with three terminal segments forming a loose club. Common in many stored vegetable drugs, formerly frequent in ships’ biscuits |
| Anobium punctatum (common furniture beetle) | Similar to Stegobium paniceum, 3–5 mm long. Viewed laterally, the prothorax exhibits a distinct hump. Does not attack drugs but may occur in wood of packing-cases, floors, etc. |
| Lasioderma serricorne (tobacco, cigar or cigarette beetle) | Reddish colour, 2–2.5 mm long. Found in many stored products, including ginger and liquorice |
| Family Ptinidae (‘spider beetles’) | |
| Ptinus fur (white-marked spider beetle)P. tectus (Australian spider beetle)P. hirtellus (brown spider beetle)Trigonogenius globulus Niptus hololeucus (goldenspider beetle, cloth bug)Gibbium psylloides | All rather similar, somewhat resembling spiders, with long legs and antennae, stout bodies and hairy covering, 2–4 mm long. Some species (e.g. Niptus hololeucus) are densely covered with hairs; others (e.g. Gibbium spp.) are glabrous with a shining cuticle. Of wide occurrence in stored products—food, spices, cocoa, cereals, almonds, capsicum, ginger, nutmegs, etc. |
| Family Tenebrionidae | |
| Tribolium confusum (confused flour beetle)T. castaneum (rust red flour beetle) | Reddish-brown beetles, 2–4 mm long. Found in many foodstuffs including flour and nuts. Infested flour has lingering pungent odour. Reported as becoming more common in crude drugs e.g. rhubarb |
LEPIDOPTERA
The Lepidoptera include the moths and butterflies, and although the number of species is large, only a relatively small number of moths cause injury to drugs. As in the case of the clothes moth, the damage is caused by the larva and not by the mature insect; but since moths are very mobile and lay eggs, infestation tends to spread rapidly. Moths reported in stored drugs are listed in Table 15.2.
Table 15.2 Moths of stored drugs.
| Moth | Products attacked |
|---|---|
| Ephestia kuehniella | Almond, capsicum, cocoa, cotton seed, ground-nut |
| E. cautella | Tonco bean, cocoa |
| E. elutella | Cocoa, tobacco, rose petals, pomegranate root bark |
| Plodia interpunctella | Cinnamon bark and yeast cake |
| Tinea pellionella | Aconite root, almonds, capsicums, mustard seed, ginger, linseed, orris, saffron and tobacco |
Ephestia kuehniella, the Mediterranean flour moth; E. cautella, the fig moth; E. elutella, the cocoa moth, and Plodia interpunctella, the Indian meal moth, all belong to the same family, the Phycitidae.Various members of the family Tineidae, which cause damage to clothes, carpets, etc., are also found in drugs.
Ephestia kuehniella is about 25 mm long. It has dark brownish-grey scaly forewings and dirty-white hindwings. The larvae are whitish except for the brownish anterior and the dark hairs on each segment; when fully grown, they remain in the food material, where they overwinter and form pupae. In contrast, the grubs of E. elutella migrate away from the food and in so doing often leave the food containers completely ‘webbed’ with their silky threads; they enter cracks in walls, etc., where they spin cocoons and remain until they pupate in the spring to finally emerge as adults in May.
ARACHNIDA
The arachnids or ‘mites’ differ from the true insects in that the mature forms have eight legs but possess no antennae. The members of the Tyroglyphidae (e.g. Tyroglyphus dimidiatus, the cheese-mite) are much smaller than the insects, and individuals can only be seen with a lens. If the suspected ‘dust’ is scraped into a pile, it will be seen to move and will gradually become flat if mites are present in considerable numbers.
Tyroglyphus farinae (Acarus siro) (Fig. 15.2A), the flour or meal mite, is 0.4–0.7 mm long. The body is oval in outline, with a truncated posterior, and is clearly divided into two regions—the anterior propodosoma and the posterior hysterosoma. It is common in cereal products, oil-seed cakes and many other commodities.
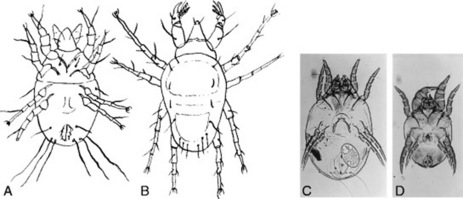
Fig. 15.2 Arachnids. A, Tyroglyphus farinae, female, ventral view, × 40. B, Cheyletus eruditus, female, dorsal view, × 40. C, Dermatophagoides culinae, female, ventral view. D, D. culinae, male, ventral view.
The Tyroglyphidae may themselves be attacked by other mites such as Cheyletus eruditus (Fig. 15.2B).
A number of different mites commonly attack cantharides, causing considerable damage. Ergot, quince and linseed are other drugs which seem very liable to attack.
Mites commonly exist in ordinary house dust as found under carpets, in mattresses, etc.; and these include Dermatophagoides pteronyssinus, D. culinae, Glycyphagus domesticus (the house mite) and Tyroglyphus farinae (the flour mite). It has been shown, comparatively recently, that these mites are the allergens responsible for house dust sensitivity, an allergy from which many people suffer. It is now possible to diagnose this condition by prick tests which utilize an extract prepared from Dermatophagoides culinae. This mite (Fig. 15.2C) does not show the marked division of the body into two parts exhibited by Tyroglyphus spp.
CONTROL OF INFESTATION
The detection, prevention and eradication of mite and insect infestation is an important hygienic and economic consideration for all who have occasion to store and use crude drugs. Effective preventive measures involve good hygiene in the warehouse (removal of spillages, old debris and packaging materials; elimination of sources of infections such as floor cracks and crevices), effective stock control (regular inspection, rotation of stock, early recognition of infestation), optimum storage conditions (maintenance of cool, dry environment) and good packaging (woven sacks and bags, multi-ply paper sacks stitched at the seams, paper, polythene film, flimsy cardboard are all penetrable by insects and mites)
If material becomes infested it may be wiser to sacrifice a small consignment rather than to risk contamination of other materials. After a contaminated drug has been removed, pallets, shelves, walls and floors should be thoroughly cleaned and sprayed with a contact insecticide such as chlorpyriphos-methyl or pirimiphos-methyl. Weekly air-spraying with pyrethrins or synthetic pyrethroids, and the use of slow-release dichlorvos strips, control air-borne insects. Various dust formulations may be used in cracks.
Fumigation is the only practical means of killing insects and mites in bulk consignments. Methyl bromide and ethylene oxide have been commonly used and the treatment needs to be applied under gas-proof sheets or in other suitable gas-tight enclosures or chambers. However in Europe the use of ethylene oxide has now been prohibited.
Owing to the possible hazards involved, the work should be performed by professional operators in compliance with the Health and Safety at Work Acts. Before drugs are subsequently used the fumigants must be completely removed as, if they are consumed, they may constitute a health hazard.
Low-temperature storage appears to offer great possibilities. It not only reduces insect attack, but also will, if a sufficiently low-temperature be employed, gradually destroy insects, larvae and eggs. The eggs of Ephestia elutella and E. kuehniella are rapidly destroyed at −15°C and more slowly at rather higher temperatures.
The eggs of the flour mite can withstand exposure to −10°C for up to 12 days or 0°C for several months; development is possible within the range 2.5–30°C, depending on humidity.
Studies on the effects of ionizing radiations (e.g. from a 60Co source) on cereal pests such as Tyroglyphus mites and various beetles show that small doses inhibit reproductive ability and larger ones destroy both mites and their eggs.
The quantitative determination of insect infestation in powdered vegetable drugs is of some significance. Melville (J. Pharm. Pharmacol., 1949, 1, 649; 1951, 3, 926) developed a method based on the acetolysis of the weighed sample for the quantitative isolation of the insect fragments present. These fragments are suspended in a suitable medium with a weighed quantity of lycopodium spores. Using the fact that the number of strial punctures per elytron is characteristic for a particular beetle, one can, by counting the strial punctures evident on the fragments of elytra present under the microscope, arrive at a figure for the number of beetles present. The use of lycopodium spores (94 000 spores mg−1) eliminates the necessity for counting all the strial punctures present in a particular volume (or weight) of the suspension; the use of lycopodium powder in this respect is described under ‘Quantitative Microscopy’ (Chapter 43).
SPOILAGE BY RODENTS
Rodent faeces usually contain the animal’s hairs, so that drugs which on microscopical examination show the presence of these hairs should be rejected.
Dales MJ. A review of plant materials used for controlling insect pests of stored products. Chatham, UK: Natural Resources Institute, 1996. Bulletin 65
Pedigo LP. Entomology and pest management, 4th edn. Upper Saddle River, NJ: Prentice Hall, 2002.
Rees D. Insects of stored products. Victoria and Manson, London: CSIRO, Collingwood, 2004.
Sabramanyam B, editor. Integrated management of insects in stored products. New York: Marcel Dekker, 1995.
