Jalap
Jalap consists of the dried tubercles or tuberous roots of Ipomoea purga, a large, twining plant indigenous to Mexico. Most of the drug is imported from eastern Mexico under the name of ‘Mexican’ or ‘Vera Cruz’ jalap. Convolvulaceous tubers with purgative properties were brought to Spain about 1565.
The traditional system of production in Central Vera Cruz has been described by A. Linajes et al. (Econ. Bot., 1994, 48, 84). Scarification of seeds prior to sowing is the secret of obtaining a 95% germination rate in 8 days. The productive period extends from July to February and the harvested tubers are smoke-dried in small wooden sheds using unseasoned Liquidamber macrophylla wood for fuel. During this process there is a weight loss of 50–75%. This method gives a product more resistant to fungal and insect attack than does simple drying. The yield is 2.4–3.0 tons of fresh root/hectare which can be increased, as in India, to 4.8 tons/hectare by the use of cow manure.
Jalap tubercles are fusiform, napiform or irregularly oblong in shape, and 3–5 cm long. They are extremely hard and heavy. The surface is covered with a dark brown, wrinkled cork, which is marked with lighter-coloured, transverse lenticels. The larger pieces may bear gashes, which have been made to facilitate drying. The tubercles may be softened for cutting by prolonged soaking in water. Cut transversely they show a greyish interior, a complete cambium ring fairly close to the outside and within it numerous irregular dark lines. The drug has a slight, smoky odour; the taste is at first sweetish, afterwards acrid. A description of the microscopy of jalap was given in the 11th edition.
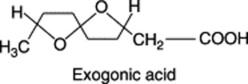
Jalap contains 9–18% of resin contained in secretion cells and giving a yellow stain with iodine water. It may be extracted from the powdered drug with boiling alcohol (90%). On pouring a concentrated tincture into water, the resin is precipitated and may be collected, washed and dried. The complexity of these convolvulaceous resins has prevented, until recently, their isolation in a pure form and they have been studied by investigating the products of their hydrolysis (short-chain volatile fatty acids, hydroxy fatty acids and sugars). The main constituent of jalap resin is convolvulin, a substance with some 18 hydroxyl groups esterified with valeric, tiglic and exogonic acids. Exogonic acid is 3,6-6,9-dioxidodecanoic acid. (For its stereochemical structure see E. N. Lawson et al., J. Org. Chem., 1992, 57, 353).
Jalap is a powerful hydragogue cathartic and was formerly extensively used either as standardized powder, as Jalap Resin or as Jalapin. The latter is the decolorized ether-insoluble portion of Jalap Resin.
Recently, using modern techniques, Japanese researchers have carried forward the investigation of those convolvulaceous species which are of relevance to the oriental market. Examples are given below.
Brazilian Jalap Rhizome
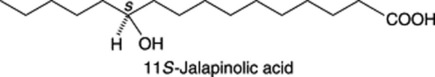
This is derived from Ipomoea operculata and constitutes a substitute for Mexican jalap. In a series of papers Ono and colleagues (see Chem. Pharm. Bull., 1992, 40, 1400 and references cited therein) characterized 18 operculins (ether-soluble resin glycosides). These resemble the other known jalapins in that they are monomers with similar intramolecular macrocyclic ester structures in the glycosidic acid moieties. However, their component acids (n-decanoic and n-dodecanoic acids) are characteristically different from those of previously known resin glycosides (isobutyric, 2-methylbutyric and tiglic acids), see ‘Jalap’ above. On alkaline hydrolysis a particular operculin will give a characteristic operculinic acid along with n-decanoic and n-dodecanoic acids. Operculinic acid E, for example, is 11S-jalapinolic acid 11-O-α-L-rhamnopyranosyl- (1→2)-β-D-glucopyranoside. The formula for 11S-jalapinolic acid (common to all operculinic acids) is given below:
Ipomoea batatus. This species, the sweet potato, is widely cultivated as a food but it has also traditional (Brazilian) medicinal uses and a number of pharmacological claims have been made for it. In 1979 Kawasaki et al. reported the roots to contain a mixture of hexa-, hepta- and octa-decylferulates; Noda et al. (Chem. Pharm. Bull., 1992, 40, 3163) isolated five new ether-soluble resin glycosides called simonins I–V. The arrangement of the acids in relation to the carbohydrate moieties of the molecule is illustrated by simonin I.
Similar compounds (stoloniferins) have been recorded in Ipomoea stolonifera (N. Noda et al., Phytochemistry, 1998, 48, 837).
The roots of Convolvulus scammonia (vide supra) contain ether-soluble resin glycosides called scammonins; they possess a glycosidic acid, e.g. scammonic acid A, and have an intramolecular macrocyclic ester structure involving various sugars (see H. Kogetsu et al., Phytochemistry, 1991, 30, 957).
VOLATILE OILS IN AROMATHERAPY
Aromatherapy is based primarily on the use of volatile oils, either singly or in admixture. They are administered in baths (drops of oil are added to the water and vigorously mixed), in compresses, in massage and as inhalations. For compress and massage usage the volatile oils are mixed with a suitable carrier (e.g. the fixed oils of apricot kernel, evening primrose, starflower, sweet almond to cite but a few) and for inhalation vaporizers and burners are available in addition to the traditional steam inhalation or use of the handkerchief or tissue.
A number of the oils used in aromatherapy have already been mentioned and include those from benzoin, black pepper, German chamomile, cinnamon leaf, clove, eucalyptus, fennel, frankincense, ginger, juniper berry, lavender, lemon, myrrh, neroli, orange, peppermint, pine, rose, sandalwood, spearmint, tea-tree and thyme. Others are listed below, citing: Botanical source; Geographical origin (but not necessarily the sole); Parts of the plant from which the oil is extracted; Extraction process; and Principal constituents. It must be remembered that volatile oils can contain over 100 constituents, most in very small amounts, and the principal components might not be those giving the oil its unique characteristics.