Chapter 38 Plants in African traditional medicine—some perspectives
In 1991, the World Health Organization (WHO) redefined traditional medicine (TM) as comprising ‘therapeutic practices that have been in existence, often for hundreds of years, before the development and spread of modern scientific medicine and are still in use today. These practices vary widely, in keeping with the social and cultural heritage of different countries’. The practice of TM in Africa, even today, contains considerable mysticism and secrecy. Therefore, the WHO’s original definition of TM, coined in the African region in 1976, and which took cognisance of the importance of ‘the concept of nature which includes the material world, the sociological environment whether living or dead and the metaphysical forces of the universe’ is still valid in Africa.
TRADITIONAL MEDICINE PRACTITIONERS AND THEIR TECHNIQUES
The practitioners of TM in Africa include herbalists, herb sellers, traditional birth attendants, bone setters, diviners, faith healers, traditional surgeons, spiritualists and others. The training of these practitioners is still by an apprenticeship of about 7 years minimum. The content of such training is not standardized. The techniques used in African TM derive from the basic understanding of the aetiology of disease, as conceived by traditional medical practitioners (TMPs), who believe that diseases arise not only from physical ailments and psychological causes (as in Western medicine) but also from astral influences, spiritual causes (due to evil thoughts and machination by enemies), esoteric causes (i.e. originating from the soul or caused by deeds of an individual before reincarnation). Because TMPs in Africa place so much emphasis on supernatural forces, they are consulted not only for sickness but also when misfortunes occur in the family or to an individual, as many such evil omens are ascribed in Africa to supernatural forces. TMPs observe their patients for symptoms and signs but do not perform any pathological examination because they lack training in such techniques. Diagnosis of the disease is made through anamnesis—observation of the patient for signs and symptoms, including visual examination, clinical examination, biological examinations (such as tasting of urine for the presence of sugar in the case of diabetics, or allowing the patient to urinate on the ground and watching for infestation by ants, smelling of sores for putrefaction etc.), divination, which can be by throwing of seeds (Sofowora, 2008) or bones, use of mind-changing plant drugs, use of astronomical signs and analysis of dreams. Although many of these methods can be utilized by TMPs, specializations do occur. The practitioners also refer patients to one another in appropriate cases.
Treatment types in African traditional medicine
African TM provides holistic treatment. The type of treatment varies and is sometimes indicative of the specialization of the practitioner.
Medicaments intended for internal and external application involve the use of vegetable organs (leaves, barks, roots, etc.), latex, resin, etc. Whole or parts of animals (snail, bone, etc.) and mineral substances (alum, kaolin, etc.) are also used. Although the medicine prescribed may contain only a single active item, it is often a multi-component mixture, some of the components of which act as preservatives, flavours or colouring agents. The multi-component preparation also contains ingredients for all the ailments (or symptoms) that need to be removed to restore the patient’s balance. In this way, African TM differs from Western medicine, where a patient can receive a prescription of various tablets, capsules, mixtures along with other dosage forms to eradicate a reported case of illness. The medicaments used in African TM can be administered in the form of a liquid (decoctions, oily mixtures, etc.), solid (powders, ointments), semi-solid (balsams, etc.) or gas (steam inhalation, incense, etc.). The only route of drug administration that is absent in TM in Africa is the intravenous (i.v.) route. The other routes are employed though in rather crude forms.
Other types of treatment used in African TM include therapeutic fasting and dieting, hydrotherapy, treatment of burns, dry heat therapy, blood letting (cupping or venesection), bone setting, spinal manipulation, massage, psychotherapy, faith healing (spiritual healing), therapeutic occultism and also obstetric and gynaecological practice.
Surgical operations carried out in African TM include male and female circumcision, tribal marks, whitlow operation, cutting of the umbilical cord, piercing ear-lobes, uvulectomy, tooth abstraction, trephination (or trepanation) and abdominal surgery. Common complications from the various surgical operations include tetanus, meningitis and septicaemia. No anaesthesia or X-ray diagnosis is used for these operative procedures. After each of the operations, the patient is treated with herbs to heal the wound.
Preventive medicine in African TM takes the form of simple hygiene in some cases, or the performance of regular sacrifices against the wrath of those gods, which, it is believed, leads to periodic epidemic diseases like smallpox and plague. However, health education is helping to modify these beliefs. Armlets, medicated rings, waist leather bands or special necklaces or charms are often worn as a preventive measure or talisman. Some charms are also used to prevent car crashes or to ward off evil spells from witchcraft; the efficacy of such preventive care has yet to be proven. Again, education by road safety corps personnel helps to dispel beliefs in charms for preventing road accidents.
Although there are minor differences all over Africa in TM practice, there is considerable similarity because of the closeness of the cultures of the African peoples, especially between neighbouring countries as the geographical barriers are artificial. For example, the sale of herbs is usually in the markets where food items (vegetables, etc.) can be purchased. This is so all over Africa, although a section of a big market may be set aside for herb sellers’ stalls.
In divination, bone throwing is done in southern Africa but seed throwing is done in Western and Central Africa. Seven seeds are used in Central Africa, whereas seed throwing of sixteen or eight seeds is used in Western Africa. The divination process involves, in all cases, the interpretation of the arrangement of the elements (seeds or bones) after being thrown on the ground by the TMP in order to predict or divine on a particular complaint or situation for the patient. These practices continue despite education, and diviners are consulted both by the educated elite as well as by the illiterate.
Ethnopharmacological themes, as illustrated by sub-Saharan art objects and utensils, have been discussed in an illustrated article by De Smet (1998).
Scientific evidence supporting some practices and remedies in African traditional medicine
Attempts have been made by scientists to justify or rationalize, on a scientific basis, many aspects of the practice of the African TMP. Some of these practices are inexplicable, whereas others, like the use of many of the herbs, can be rationalized.
Plants of Ageratum conyzoides L. collected at night are used to treat children who cry too often for no known cause, especially at night. Night collection of this herb is particularly indicated when the frequent crying is suspected to be due to the influence of witchcraft, or to persistent disturbance from the spirits of the child’s playmates (dead or alive), thus requiring the use of the ‘occult’ power of the herb. The following procedure is followed: A suitable location of A. conyzoides is found during the day. Very late at night, the collector approaches the plant and chews nine or seven seeds (for male or female, respectively) of melegueta pepper (Aframomum melegueta K. Schum.). The chewed grains are spat on the plant while the appropriate incantations are recited. The plant is then plucked and warmed over a fire at home before the juice is expressed. Palm oil (expressed from the mesocarp of Elais guiniensis family, Palmaceae) is added to the pressed juice and the mixture used to rub the whole body of the patient. Ageratum conyzoides is commonly used in TM for dressing wounds and ulcers, for scabies and as an eyewash. It is used as a styptic in East Africa (Kokwaro, 1993). These common uses result from its antimicrobial properties, which have been demonstrated scientifically but the special effect (occult power) it is claimed to possess when collected at night cannot easily be rationalized on a scientific basis, especially when there is no precise diagnosis of the disease. There are, however, other practices in African TM that are justifiable scientifically. Some examples are given below.
In many African homes, teeth are cleaned in the morning by chewing the root or slim stem of certain plants until they acquire brush-like ends. The fibrous end is then used to brush the teeth thoroughly. These chewing sticks impart varying taste sensations: a tingling, peppery taste and numbness is provided by Zanthoxylum zanthoxyloides Waterman (Fagara zanthoxyloides Lam.) root, a strong bitter taste and frothing by Masularia acuminata (G. Don.) Bullock ex Hoyle stem, and an initial bitterness becoming sweet later by Vernonia amygdalina Del. root. The root of Terminalia glaucescens Planch. produces a discoloration of the mouth. The most popular chewing sticks are those with a good flavour and texture, and a recognized effect on the teeth and supporting tissues. Freshly cut specimens are always desirable because they are more easily chewed into a brush. Some of them, however, possess such tough fibres that they penetrate the gums during use, thus causing some discomfort (Sofowora, 2008).
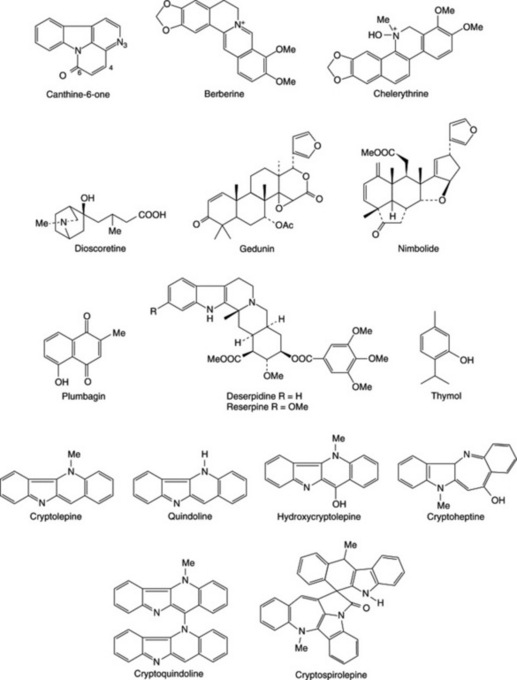
Buffered extracts of the common chewing sticks show antimicrobial activity against oral microbial flora but to varying degrees (Sote and Wilson, 1995; Taiwo et al., 1999; Almas, 2002; Ndukwe et al., 2005). Some African chewing sticks are also reported to contain fluoride ions, silicon, tannic acid, sodium bicarbonate and other natural plaque-inhibiting substances that can reduce bacterial colonization and plaque formation. The antimicrobial activity of the most effective (Z. zanthoxyloides) was shown to be due to berberine, chelerythrine and canthine-6-one (Fig. 38.1), which are most active at pH 7.5 (or during tooth decay) and simple benzoic acid derivatives, which are most active around pH 5 (or after an acid drink like lime juice). These data indicate that the chewing sticks, in addition to providing mechanical stimulation of the gums and removing food particles from the teeth crevices, also destroy oral microbes. Some African chewing sticks have been reported to contain fluoride ions, although their fluoride content was considered insufficient to produce a significant increase in the fluoride content of the dental enamel. Plant parts used as chewing sticks also have been shown to contain not only fluoride but also silicon, tannic acid, sodium bicarbonate and other natural plaque-inhibiting substances that could reduce bacterial colonization and plaque formation (Ogunmodede, 1991; Sote and Wilson, 1995; Taiwo et al., 1999; Almas, 2002; Ndukwe et al., 2005).
Other practices used in African TM, such as collecting certain plants only at certain seasons, using cold extraction instead of hot for some herbs, using young instead of old leaves of certain plants, using fallen dead leaves of certain plants rather than fresh ones, etc. have been rationalized as being due to seasonal, diurnal or age variations in active constituents of plants or the thermolability of the active ingredients of certain plants.
The following are the summarized results from a few examples of the investigations carried out to prove the efficacy claimed for medicinal plants used in African TM.
Dioscorea dumetorum (Kunth) Pax tubers are used in African TM, in carefully regulated doses, for the management of diabetes mellitus (Iwu, 1993). Crude extracts of the tuber were shown to possess a hypoglycaemic effect in normal rats and rabbits and were checked for hypoglycaemia produced by alloxan. From the active aqueous fraction, dioscoretine (Fig. 38.1) was characterized as the hypoglycaemic agent by using bioassay-guided fractionation of the extract. Of the solvent fractions tested for toxicity, the aqueous fraction used in TM was the least toxic LD50 = 1400 mg kg−1. Further work has been recommended by the researchers before dioscoretine or the extract of the tuber can be exploited commercially as careful control of the dosage was found necessary even by TMPs.
Polygala nyikensis is used by the highlanders of Malawi and bordering countries to treat various skin problems of fungal origin. The root of the plant was recently shown to exert its antifungal activity owing to the presence of xanthones (Marston et al., 1993).
Azadirachta indica leaves and stem bark are used in treating malaria and have been shown to be effective in vitro and in vivo. Rochanankij et al. (1985) associated the antimalarial activity with nimbolide while Khalid and Duddeck (1989), using a bioassay-directed purification procedure, named another limonoid, gedunin (Fig. 38.1), as the active principle. The possibility that the extract of neem acts by causing a redox perturbation by imposing substantial oxidant stress during malarial infection has been postulated. The antiplasmodial and larvicidal activity of neem has been confirmed by others (Dhar et al., 1998; Isah et al., 2003; Nathan et al., 2005; Udeinya et al., 2006; Okumu et al., 2007; Soh and Benoit-Vical, 2007).
The published scientific proof for the efficacy of other African plants was reviewed by Sofowora (1993) while the efficacy of others can be readily deduced from their active constituents. For example, the use of Rauwolfia vomitoria roots (containing reserpine) in treating some mentally ill patients; Plumbago zeylanica root (containing the naphthoquinone, plumbagin) for treating various fungal skin diseases; Ocimum gratissimum leaves (containing essential oils rich in thymol) for treating diarrhoea are all clearly justifiable (Sofowora, 2008). Other plants whose active constituents have not been characterized have also been demonstrated experimentally in the laboratory to be efficacious. Examples of these include remedies used in treating skin diseases and the use of Combretum mucronatum and Mitragyna stipulosa as anthelminthics (Sofowora, 2008).
Theories on the origin of herbal medicine in Africa
Although it is not known exactly when the humans first practised herbalism in Africa, a number of theories have been advanced by scholars and TMPs alike to explain the acquisition of this knowledge by early Africans. One such theory states that early man in Africa deliberately selected specific plant materials for the treatment of his ailments as man had the ability to rationalize rather than to rely on instinct as do lower animals. The choice was certainly not based on the knowledge of the plant constituents. Some anthropologists state that early man lived in fear, and that, to allay this, he indulged in mystical and religious rituals. Thus, it could well be that the initial selection of plant materials for medicinal purposes was influenced by religious thoughts and collections were accompanied by magical rituals. Some plants are still used in the rituals of traditional religion in many parts of Africa today.
It has been proposed that the knowledge of medicinal plants in Africa was gained by accident, although this theory has been refuted by a number of African TMPs, who claim that information on such plants was communicated to their ancestors in various ways. However, early Africans could have gained some specific knowledge by watching the effects produced by various plants when eaten by domestic animals. Even today, some herbalists try out remedies, in the presence of their patients, on domestic animals, especially when testing for toxicity, and on themselves or their relations. Such tests prove to the patient that the preparation is harmless and sometimes also confirm that the dosage prescribed is also justifiable. Such information on African medicinal and toxic plants has been passed on orally from generation to generation and even today there are many herbal cures in Africa that have not been written down (Sofowora, 2008).
According to some TMPs another possibility is that knowledge of traditional cures came from wizards and witches. It is believed that some witches, whether living or dead, attend village markets in strange forms—as goats, sheep or birds. If their presence in this disguise is detected by someone very shrewd or gifted, such as a TMP, the practitioner is promised some useful herbal cures in return for not exposing the witch in disguise. The same reward would be offered if a real-life witch was caught in the process of performing an evil act.
Hunters, especially in African countries, have been reported as the original custodians of some effective traditional herbal recipes. Such knowledge could have been acquired when, for example, a hunter shot an elephant. If the elephant ran away, chewed leaves from a specific plant and did not die, it is believed the hunter noted the plant as a possible antidote for wounds or for relieving pain. Similar observations were made in villages where, for example, a domestic animal chewed the leaf of a specific plant when that animal was ill and recovered later or when another animal accidentally chewed a leaf and died (Sofowora, 2008). Similar observations by scientists have confirmed that chimpanzees use medicinal plants in Africa for self-medication (Huffman and Wrangham, 1993).
TMPs also claim that, when in a trance, it is possible to be taught the properties of herbs by the spirit of an ancestor who practised herbalism. Spirits are said sometimes to assume various forms, e.g. an alligator, or a human being with one leg and one arm using a walking stick. If one encounters such a creature very late at night it can be a useful source of original information of herbal cures. In whatever manner the early Africans gained their knowledge of the curative powers of herbs, one must assume that they were able, thereafter, to recognize the plant, as the detailed flora available today describing medicinal plants were then non-existent.
RESEARCH INTO AFRICAN MEDICINAL PLANTS
Information on the use of medicinal plants has been obtained from herbalists, herb sellers and indigenous people in Africa over many years (Baba et al., 1992). Under the umbrella of Agence de Coopération Culturelle et Technique (ACCT) in Paris, ethnobotanical surveys with international teams had been carried out by 1988 in the following African countries: Central African Republic, Rwanda, Mali, Niger, Federal Islamic Republic of Comoros, Mauritius, Seychelles, Gabon, Dominica, Tunisia, Madagascar, Togo, Congo and Benin Republic. The African Union’s Scientific Technical and Research Commission (AU/STRC) carried out similar surveys in Western Nigeria, Uganda, Cameroon, Ghana, Swaziland and Mozambique as at 2004 (Adjanohoun et al., 1989, 1993, 1996; Mshana et al., 2000; Adeniji et al., 2001, 2004). All these ethnobotanical surveys have been published. Other ethnobotanical surveys on the region have also been published (Samuelsson et al., 1991).
As early as 1968, it was decided at a conference organized in Dakar by the AU/STRC that the efficacy of herbs used by TMPs should be tested, particularly in the following areas: anticancer, antimalarial, antihelminthic, antimicrobial, antihypertensive, cardiac activity, antisickling and antiviral. The following is a summary of the research to date, as indicated by publications on African medicinal plants collated by NAPRALERT database for natural products. Only 36% of all publications dealt with bioassay-guided isolation of plant constituents along with their pharmacological and toxicological testing. The remainder dealt with purely phytochemical research (including quantitative analysis). Biological screening work resulted in publications on antimicrobial activity (16%), molluscicidal (11%), antimalarial (7%), toxicity testing (7%) and antitumour related (4%), while other minor biological testing amounted to 55% of the total publications relating to bioassay-guided research on African medicinal plants to 1993 (Sofowora, 1993, 2008). In molluscicidal testing, three plants came out as having potential commercial exploitation, namely Phytolacca dodecandra, Swartzia madagascariensis and Tetrapleura tetraptera. The field trials on these and toxicity studies against non-target organisms have been carried out successfully in ponds where the intermediate host snail of schistosomiasis is prevalent (Hostettmann, 1991).
Cryptolepis sanguinolenta, which is used for treating urinary infections in TM, has been shown to be strongly antimicrobial. Cryptolepine was identified as the active alkaloid. The extract of this root has been formulated for therapeutic use by the Centre for Research into Plant Medicine in Ghana. A new alkaloid, named cryptospirolepine (Fig. 38.1), was characterized from this root by Tackie et al. (1993) whereas hydroxycryptolepine, cryptoheptine and cryptoquindoline (three new alkaloids) were reported from a specimen of the same root collected in Guinea Bisau by Houghton et al. (1993). Paulo et al. (1993) have examined the alkaloids characterized by Houghton et al. (1993) from this root for antibiotic activity. All the alkaloids showed activity but to varying degrees against the test organisms used. According to Cimanga et al. (1996, 1997), this plant showed potent antibacterial, anticomplementary and moderate antiviral activities, but no antifungal effect. The results obtained by Paulo et al. (1994a, 1994b) after testing the root extracts and its alkaloids against diarrhoeal and other bacteria suggested that the roots could be a therapeutic alternative for bacterial etiologic diarrhoea in West Africa. See Sofowora (2008) for more research on Cryptolepis sanguinolenta.
Garcinia kola seeds are chewed for protection against liver disease and were shown to contain biflavonoids (Iwu, 1993; Tarashima et al., 2002). The biflavonoids and the crude extracts of the seed have been shown to be effective in protecting against liver damage (Farombi et al., 2004, 2005; Odunola et al., 2005; Adaramoye and Adeyemi, 2006a) and they ameliorate di-n-butylphthalate-induced testicular damage in rats (Farombi et al., 2007). The mechanisms involved in the hepatoprotection were explained by Farombi in 2000. ‘Kolaviron’ has been patented for commercial exploitation and the methods for its isolation and quantification have been enunciated. Other activities reported for ‘Kolaviron’ and the extract of Garcinia kola include: Attenuation of indomethacin- and HCl/ethanol-induced oxidative gastric mucosa damage in rats, and hypoglycaemic and hypolipidaemic effects (Adaramoye and Adeyemi, 2006b), whereas toxicological investigations include on erythrocytes (Esomonu et al., 2005), alteration of oestrous cycle in rats (Akpantah et al., 2005) as well as the brine shrimp lethality and mutagenicity tests (Sowemimo et al., 2007). The amino acid composition of the seeds has been reported by Adeyeye et al. (2007).
Thaumatococcus danielli produces a red fruit, the aril of the seed of which contains the polypeptide thaumatin. Thaumatin is almost 5000 times as sweet as sucrose on molar basis. It is a low-calorie, high-intensity sweetener suitable for sweetening pharmaceuticals for diabetics. The plant grows readily in the moist areas of Africa and the early researches on its development were carried out jointly by researchers in Ife (Nigeria) and Tate and Lyle Ltd in UK. It is used in soft drinks in Japan (Sofowora, 2008). Thaumatin I and Thaumatin II have been cloned and synthesized through recombinant DNA. The cloning experiments showed that the N- and C-terminal regions of both of the thaumatin molecules do not play any important role in eliciting the sweet taste of thaumatin (Masuda et al., 2004; Zemanek and Wassermann, 2005; Ide et al., 2007).
Cassia podocarpa, which is used as a laxative in TM, has been shown to contain anthraquinone derivatives similar to those found in official senna of the British Pharmacopoeia. The leaves and pods were also compared for their biological efficacy with official senna and shown to be just as effective on a weight basis. C. podocarpa was also shown to be less toxic than official senna. This leaf has been formulated into tablets and recommended as a substitute for official senna in Africa through the work of African researchers (Abo and Adeyemi, 2002; Akomolafe et al., 2004). Danafco (Ghana) Ltd. produces standardized tea bags of this leaf on a commercial scale. Similar work on C. italica has led to the development of laxatives based on this plant, now commercially available in Mali and other African countries.
Euphorbia hirta is used traditionally in treating diarrhoea and dysentery in African TM. Although it contains phorbol derivatives this plant has been shown to be effective in vitro and in vivo against Entamoeba, which causes amoebic dysentry. The plant has been formulated into mixtures and a preparation of the whole plant is also available commercially in Mali for use against amoebic dysentery (Keita, 1994; see also Sofowora, 2008).
Zanthoxylum zanthoxyloides (Lam.) Waterm. The ‘antisickling’ property of the root of Z. zanthoxyloides was discovered when it was observed that the aqueous extract preserved the red colour of blood in blood-agar plates during a screen for its antimicrobial activity. The extract was later shown to revert sickled HbAS, HbSS and crenated HbAA red blood cells to normal in vitro. The activity was also demonstrated in the root of other Zanthoxylum species, and Z. gilletti was found to be just as active as Z. zanthoxyloides. This, and previous observations, led to postulation of a membrane-based activity earlier reported for the extracts. Activity-directed fractionation of the aqueous extract located the ether fraction as the active fraction. GC-MS analysis of the ether fraction indicated the presence of phenolic and fatty acids. These acids are 2-hydroxymethylbenzoic acid, p-hydroxybenzoic acid, vanillic acid, m-hydroxybenzoic acid, 2-hydroxy-3-phenylpropionic acid, traces of stearic acid, linoleic and palmitic acids. Further analysis of the fraction confirmed the presence of these acids and identified additional ones: p-coumaric, caffeic and ferulic acids. Xanthoxylol [2-dimethylallyl-4-(3-hydroxypropyl)phenol] was also isolated from the root. p-Hydroxybenzoic acid, 2-hydroxymethylbenzoic acid, vanillic acid, 2,2-dimethyl-2H-1-benzopyran-6-butyric acid (DBA; which is a chemical modification of xanthoxylol) and two uncharacterized non-acidic isomers of butyric acid isolated from the root have all been shown to possess antisickling activity. DBA also causes a slight increase in the pO2 of the HbSS. Although the extract from the root (Z. zanthoxyloides) and DBA have been reported as generally non-toxic to (whole) animals and intracellular enzymes of the red blood cell, such as glucose-6-phosphate and 6-phosphogluconate dehydrogenases, the extract was observed to revert sickled cells to round rather than discoid shapes in some experiments. DBA, however, has been shown to increase the activity of Ca2+-activated Mg2+-dependent ATPase in both normal HbAA and sickle HbSS cell membranes, suggesting an antisickling activity based on Ca2+ mobilization in the HbSS red cell membrane for the root extractives. Other synthetic benzoic acid derivatives known to possess antisickling activities are p-methoxybenzoic acid, 3,4-dihydroxybenzoic acid, 3,4-dimethylbenzoic acid and p-fluorobenzoic acid. Relating the observed antisickling activity to physicochemical parameters of substituted benzoic acids showed that increased lipophilicity enhances sickle-cell reversal activity and that electron-donating substituents play an important role in antisickling activity. Although the attempted preliminary clinical trial on sickle cell anaemia (SCA) patients was plagued with a high default rate, the results obtained appear to indicate significant diminution of painful episodes in treated individuals (Adesanya and Sofowora, 1994). A product developed from the extract of ‘Fagara’ is being marketed under the name DREPANOSTAT® in Togo and Benin Republics. In Burkina Faso and surrounding countries the herbal product FACA® which is a mixture of ‘Fagara’ and Calotropis procera is marketed for SCA (Sofowora 2008). The use of a leaf extract of Terminalia catappa as having antisickling potential has been supported by recent research involving human blood samples (Mgbemene and Ohiri, 1999). Research on other plants used in the management of SCA have been discussed by Adesanya and Sofowora (2008).
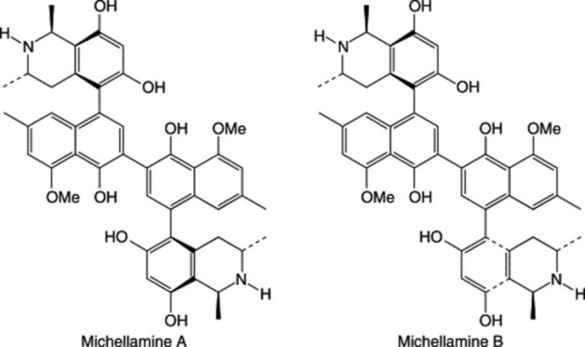
The development of bioassay techniques (Hostettmann, 1991) for antiviral activity in plants and the importance of finding a cure for HIV/AIDS has brought some African medicinal plants into prominence. About 120 plants have been reported to show antiviral activity (many of these grow in Africa, e.g. Diospyros, Spondias, Terminalia spp.), whereas others are reported to have immunomodulating properties, such as Aloe and Zingiber spp. etc. A new plant species, Ancistrocladus korupensis (Ancistrocladaceae), was discovered in Cameroon and found to contain new alkaloids: michellamines A and B, which have a wide spectrum of antiviral activity, including anti-HIV cytopathic activity. Efforts were made to develop michellamine B for use in HIV/AIDS treatment. It was characterized by collaborative effort of some Cameroon scientists and the National Cancer Institute in the USA. The plant is rare. Efforts are in progress to germinate the seeds in its natural habitat at Korup National Park, in glass houses and through tissue culture in collaboration with J. B. Johnson Biotech Laboratories (Manfredi et al., 1991; Jato et al., 1993). Readers should consult the review by Elujoba (2008) on plants used for the management of HIV/AIDS in Africa.
Nwosu (1999) has reported on 30 plants from 21 families which are used traditionally for the treatment of mental disorders in southern Nigeria.
In a review of over 240 higher plants that are used in Africa as arrow poisons, Neuwinger (1996) cites many as having medicinal properties.
TRADE IN MEDICINAL PLANTS IN AFRICA
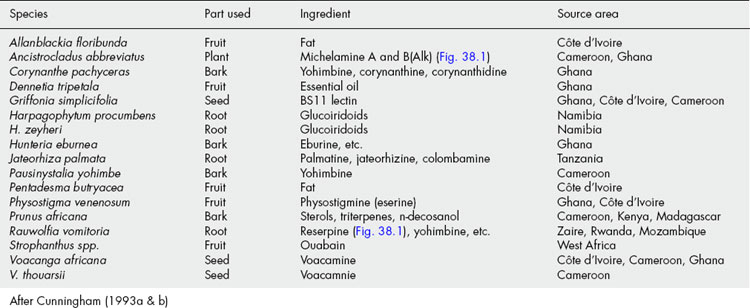
The amount of trade in the area of medicinal plants in some African countries is well documented (see Table 38.1). It is known, for example, that the government of Cameroon is the major source for the world market of Prunus africana bark, where it has been harvested since 1972. Over a 6-year period (1986–1991), 11 537 metric tons of the bark (reaching an average of 700 tons) were processed by Plantecam Medicam, a French-owned company based in south-west Cameroon. P. africana bark represents 86% of the medicinal plants exported by this company between 1985 and 1991 (Cunningham and Mbenkum, 1993). The bark is used in treating prostate gland hypertrophy and benign prostate hyperplasia (Shenouda et al., 2007; Dedhia et al., 2008). Another major plant material exported by Cameroon is the seed of Voacanga africana (Apocynaceae), which is used for the production of the alkaloid tabersonine, used as a CNS depressant in geriatric patients. Cameroon exported US$40 million worth of V. africana in 1993 alone. Cameroon also exports Tabernanthe iboga and Myrianthus arboreus, but in small quantities (Cunningham and Mbenkum, 1993).
Capsules containing the extract of P. africana bark are marketed in Europe, where the market value of this trade is estimated at US$150 million per year. In addition to Cameroon, Kenya (1923 tons per year), Uganda (193 tons per year), Zaire (300 tons per year) and Madagascar (78–800 tons per year) export this bark to various pharmaceutical companies in Europe, mainly to Madaus in Germany and Spain, Laboratoires Debat in France, Prosynthèse in France, Inverni Della Beffa and Indena Spa in Italy (Cunningham and Mbenkum, 1993).
Three plants out of the 24 000 indigenous species of the Republic of South Africa have been developed as export products. These are Rooibos tea (Asplathusa linearis), Marula (Sclerocarya birrea) and Aloe ferox. About 500 million South African Rands per annum are spent on traditional remedies in the Republic of South Africa.
Namibia exports 200 tons of Harpagophytum procumbens and H. zeyheri tubers annually to Germany (80.4%), France (12.8%), Italy (1.9%), USA (1.5%), Belgium (1%) and South Africa (1.2%) (Cunningham et al., 1992).
In Madagascar, the export sale of Catharanthus roseus and other plants represents a major export earner.
The roots of Swartzia madagascariensis and Entada africana are traded 500–800 kilometres from Burkina Faso and Mali to Abidjan in Côte d’Ivoire. Similarly, most of the common chewing sticks are sold across the borders of neighbouring countries in West Africa; 75–80 tons of Griffonia simplicifolia seeds are exported each year to Germany from Ghana; commercial gatherers in Côte d’Ivoire chop down Griffonia simplicifolia vines and Voacanga africana and Voacanga thouarsii trees in order to obtain the fruits for export (Cunningham, 1993a, 1993b). Large quantities of various medicinal plants are also exported to France by SETEXFARM in Senegal. These plants are collected from the wild and there is currently no evidence of any replanting. The harvesting of such large quantities of medicinal plants from the wild will eventually result in serious social or environmental consequences. To ensure the sustainable use of the medicinal plant resources of Africa, uncontrolled exportation of plants collected from the wild should give way to large-scale cultivation of the desired plants. Table 38.2 shows African medicinal plants whose demand exceeds supply.
Table 38.2 African medicinal plants whose demand exceeds supply.
| Species | Families |
|---|---|
| Alepidea amatymbica | Apiaceae |
| Asclepias cucullata | Asclepiadaceae |
| Begonia homonymma | Begoniaceae |
| Bowiea volubilis | Liliaceae |
| Cassia abbreviata | Fabaceae |
| Cassia sp. | (unidentified species known as muwawani) |
| Dianthus zeyheri | Illecebraceae |
| Garcinia afzellii | Clusiaceae* |
| Garcinia mannii | Clusiaceae* |
| Howorthia limifolia | Liliaceae |
| Monanthotaxis capea | Annonaceae |
| Pimpinella caffra | Apiaceae |
| Plectranthus grallatus | Lamiaceae |
| Siphonochilus aethiopicus | Zingiberaceae |
| Warburgia salutaris | Canellaceae* |
* Trees/shrubs with agroforestry potential
Compiled from Cunningham (1993a, b)
Office National de Développement des Forêts (ONADEF) in Cameroon has applied its experience of indigenous (e.g. Terminalia superba) and exotic timber to species with medicinal values. Three species cultivated for bark production (Prunus africana and two exotic Cinchona species) and Voacanga africana cultivated for its seed have been propagated on a large scale. The foresight of ONADEF in implementing medicinal tree cultivation in plantations and through enrichment planting is exceptional in Africa and is encouraging (Cunningham and Mbenkum, 1993).
Conservation of medicinal plants in Africa
More than 200 000 out of about 300 000 plants species identified in the whole of our planet are in the tropical countries of Africa and elsewhere. Among the potential uses of these African plants, those involving traditional medicines and pharmacopoeial drugs are foremost; 80% of the population of Africa living in rural areas relies on TM.
Approximately 1.8 million km2 of the world’s tropical rain forest (totalling roughly 9 million km2) are in Africa, the rest being in America, Asia and few patches in the Indian Ocean and Pacific Islands. One-fifth of the total 120 000 (including 30 000 undescribed species) seed plants present in the tropical moist forest has been estimated to be present in Africa (Farnsworth and Soejarto, 1991). All over the world, and especially in Africa, factors that cause forest depletion include direct human pressure as well as indirect factors: commercial logging in the forest, fuel wood consumption, cattle ranching (where either excessive grazing causes depletion or selective grazing by cattle results in prolific growth of poisonous species), forest farming and forest fires. Environmental factors such as desert encroachment, pollution, acid rain, the greenhouse effect and erosion are other factors causing loss of forests.
The collection of medicinal plants by herbalists and herb sellers (herb traders) for local use and export also has had a noticeable depletion effect on this important forest resource in Africa, where collectors now have to travel farther afield to obtain the herbs to be used in their practice, as few of these are cultivated. According to Cunningham (1993a, 1993b), indigenous forests cover only 0.3% of South Africa but are a source of over 130 commercially exploited traditional plants; over 400 indigenous species and 70 exotic species are commercially sold to Zulu people as herbal medicines. These indigenous species are causing concern because of the depletion of wild stocks when demand exceeds supply. Scarce, slow-growing forest species are particularly vulnerable to this over-exploitation.
Mauritius and Rodriguez have two of the most threatened flora in the world. Over 150 species of plants on these African islands are threatened with extinction, out of which at least 30 species are known from less than 10 collections (Owadally et al., 1991). According to Kokwaro (1991), high- and medium-potential land in Kenya constitutes about 17% of the country and supports 90% of the population, which is mostly rural. The plant communities in such areas are usually the most threatened by over-utilization. The depletion rates of the forest resources, which include medicinal plants, are very high. For example, Kakamega, North and South Mandi forests, which occur in high-potential areas, are being cleared at the rate of 245, 295 and 490 ha per year, respectively (Kokwaro, 1991).
The sustainable management of the forest resources and the medicinal plants in them is important, so that while the benefits to present generations are satisfied the potential to meet the needs and aspirations of future generations is not jeopardized. Conservation activities involving medicinal plant gardens maintained by herbalists, herbaria and various arboreta are scattered all over Africa. Some countries have also started special programmes to conserve the genetic resources of their medicinal plants. Although there is a need to utilize all the conventional methods of conservation (in situ and ex situ conservation, gene banks, biotechnology, etc.), the education of rural dwellers, particularly the herbalists and the herb sellers, in conservation awareness is important for an effective approach to the sustainable utilization of the medicinal plant resources in Africa. One group of TMPs in South Africa collaborates with the conservation of Traditional Healing Practices and Plants Project (CTHPPP) at Bulwer, in South Africa, for the conservation of medicinal plants. The project successfully cultivated more than 30 indigenous plant species. This young ethnobotanical reserve is currently being used to train TMPs in the Bulwer area in the identification of medicinal plants. This kind of approach, rather than merely relying on legislation, is to be encouraged. Attempts to stop the exploitation of Prunus africana in Cameroon by banning merely led to a rise in its exportation through illegal channels from 700 tons per year to over 1000 tons per year (Mbenkum and Thomas, 1993). Since 1995, P. africana has been included in CITES Appendix II as an endangered species. In 2000, Plantecam, the largest bark exporter in Africa, closed its extraction factory in Cameroon, due to complex ecological, social and economic factors. Wild collection is no longer sustainable (and probably never was) where harvest seriously affects morbidity and mortality rates of harvested populations (Stewart, 2003). Alternatives to wild collection to meet future market demand—including conservation practices, enrichment plantings, small- and large-scale production and protection of genetic resources—have been proposed by Stewart (2003). P. africana is at the beginning of a transition from an exclusively wild-collected species to that of a cultivated medicinal tree.
THE AFRICAN PHARMACOPOEIA
Reports of the uncontrolled dosage of herbal remedies used by TMPs necessitated a research programme to carry out quantitative pharmacognostical analysis on some common African medicinal plants. Data accumulated from such research were used to compile the first African Pharmacopoeia (AP) published by AU/STRC (1985, 1986). The AP specifies quality control standards to be met by the plants when used in commerce and in manufacturing pharmaceutical preparations. Volume 1 of the AP contains monographs on 100 medicinal plants, whereas volume 2 describes the methods to be used in their quality control. Some of the old reliable methods of plant analysis are still retained (along with the most modern techniques) as alternatives in this volume as many African countries cannot afford the sophisticated spectroscopic instruments used in quality control today. The AP is available in English, French and Arabic. Some African countries have produced their own national herbal pharmacopoeiae, e.g. Ghana and Nigeria.
CONCLUSION
With the development of simpler, inexpensive bench-top bioassays (Hostettmann, 1991), it is expected that in future many hitherto untested natural products isolated from African plants will be put through a variety of biological tests.
The current awareness of HIV/AIDS in Africa and the development of screening programmes for anti-HIV activity in plants will herald the screening of more African plants claimed by TMPs to be used in treating HIV/AIDS-related symptoms. This is of increasing importance because, in addition to being a sexually transmitted disease, HIV/AIDS is also contracted through blood transfusion when adequate care is not taken to use only HIV-free blood. Some patients with diseases like sickle-cell anaemia who require blood transfusion because of lack of an available cure (this disease kills 120 000 or more children annually in Africa) need to be considered especially in HIV/AIDS and primary health care programmes. More attention should also be given to the possibility of cures, from plant sources, for HIV/AIDS. However, it will be necessary to determine what indications in the treatment or diagnostic mode of the TMP should be looked for in identifying candidate plants for HIV/AIDS cure (see Elujoba, 2008).
Tissue and suspension cultures of some African medicinal plants have also been developed in various laboratories, but mainly outside Africa, for the future biotechnological production of the secondary plant metabolites of these plants for drugs needed world-wide. Examples include Catharanthus roseus, Ammi majus and A. visnaga, and Tribulus terrestris (see also Thaumatococcus danielli, above). This trend will probably be intensified to prepare for the future demand for drugs in relation to conservation efforts.
While national materia medica of herbs are being compiled, it is expected that efforts will continue to eliminate toxic plants from the recipes of the TMPs, and to encourage the use of the harmless ones, which will be made available on a large scale in standardized dosage forms not only for home use but also for export.
Many countries in Africa (for example, Rwanda, Egypt, Mali) now cultivate medicinal plants on a large scale for local processing into galenicals, teas, various dosage forms and other standardized preparations for use in health care. It is expected that activity in this direction will increase in Africa with assistance from UNIDO as more can be derived economically by making simple extracts of medicinal plants rather than exporting them as raw materials. Drug production from medicinal plants in Africa should be further intensified in African countries through public/private/partnership (PPP) arrangements, so that Africa can contribute more to the global trade in medicinal plant products.
The formation of networks of laboratories to bring African countries together for collaborative research and development work on natural products generally and medicinal plants in particular is expected to increase. The creation of the Natural Products of East and Central Africa (NAPRECA) network by UNESCO has given a boost to inter-African collaborative research efforts on medicinal plants in that subregion of Africa. This led to the creation of the West African network, also with UNESCO support. The International Organization for Chemistry in Development (IOCD) has encouraged the creation of a Network of Analytical and Bioassay Services in Africa (NABSA), with headquarters in Addis Ababa University, Ethiopia. This network was created to encourage the bioassay of natural products from phytochemical research by pooling existing analytical facilities in African laboratories. All these efforts are expected to boost output in research and development work in this field. With institutional strengthening of African laboratories taking place, it is hoped that more and more of the collaborative research with developed countries can take place in Africa where labour is relatively cheap.
The old methods and practices of traditional medicine in Africa are being transformed with the awareness of the TMPs for the need for more precise dosage in the use of their herbal remedies. Retraining programmes are going on in several countries, with a view to improving the competence of the TMPs and the quality of health care that they deliver on a continent where 80% of the people have only TM available to them. Research has provided evidence for the rationalization of some of the practices of the TMPs and the efficacy of some of the herbs they use while new natural products with potential for drug development for management of diseases rampant world-wide are emerging from African plants. It is hoped that increased inter-African and international collaborative research and sustainable use of biodiversity resources in Africa will help to develop new drugs from the rich untapped forests of Africa for the betterment of mankind.
Abo KA, Adeyemi AA. Seasonal accumulation of anthraquinones in leaves of cultivated Cassia podocarpa Guill et Perr. African Journal of Medicine and Medical Science. 2002;31(2):171-173.
Adaramoye OA, Adeyemi EO. Hepatoprotection of D-galactosamine-induced toxicity in mice by purified fractions from Garcinia kola seeds. Basic Clinical Pharmacology and Toxicology. 2006;98(2):135-141.
Adaramoye OA, Adeyemi EO. Hypoglycaemic and hypolipidaemic effects of fractions from kolaviron, a biflavonoid complex from Garcinia Kola in streptozotocin-induced diabetes mellitus rats. Journal of Pharmacy and Pharmacology. 2006;58(1):121-128.
Adesanya SA, Sofowora A. Phytochemical investigation of plants for the management of sickle cell anaemia. In: Hostettmann K, editor. Phytochemistry of plants used in traditional medicine. UK: Oxford University Press Oxford, 1994.
Adesanya SA, Sofowora A. Medicinal plants and management of sickle cell anaemia. In: Sofowora A, editor. Medicinal plants and traditional medicine in Africa. Ibadan, Nigeria: Spectrum Books, 2008.
Adeyeye EI, Asaolu SS, Aluko AO. Amino acid composition of two masticatory nuts (Cola acuminata and Garcinia kola) and a snack nut (Anacardium occidentale). International Journal of Food Science and Nutrition. 2007;58(4):241-249.
Adjanohoun EJ, Adjakidje V, Ahyi MRA, et al. Contribution aux études ethnobotaniques et floristiques en République du Benin. France: ACCT, Paris, 1989.
Adjanohoun E, Ahyi MRA, Ake-Assi L, et al. Contribution to ethnobotanic and floristic studies in Western Nigeria. Lagos, Nigeria: AU/STRC, 1991.
Adjanohoun E, Ahyi MRA, Ake-Assi L, et al. Contribution to ethnobotanic and floristic studies in Uganda. Lagos, Nigeria: OAU/STRC, 1993.
Adjanohoun JE, Aboubakar N, Dramane K, et al. Contribution to ethnobotanic and floristic studies in Cameroon. Lagos, Nigeria: AU/STRC, 1996.
Adeniji KO, Amusan OOG, Dlamini PS, et al. Contribution to the ethnobotanic and floristic studies in Swaziland. Lagos, Nigeria: AU/STRC, 2001.
Adeniji K, Agostinho AB, Amusan OOG, et al. Contribution to the ethnobotanic and floristic studies in Mozambique. Lagos, Nigeria: AU/STRC, 2004.
Akomolafe RO, Adeoshun IO, Ayoka AO, et al. An in vitro study of the effects of Cassia podocarpa fruit on the intestinal motility of rats. Phytomedicine. 2004;11(2–3):249-254.
Akpantah AO, Oremosu AA, Noronha CC, et al. Effects of garcinia kola seed extract on ovulation, oestrous cycle and foetal development in cyclic female sprague-dawley rats. Nigerian Journal of Physiological Science. 2005;20(1–2):58-62.
Almas K. The effect of Salvadora persica extract (miswak) and chlorhexidine gluconate on human dentin: a SEM study. Journal of Contemporary Dental Practice. 2002;3(3):27-35.
AU/STRC. African Pharmacopoeia. Vol 1. OAU/STRC, Lagos, Nigeria, 1985.
AU/STRC. African Pharmacopoeia. Vol 2. OAU/STRC, Lagos, Nigeria, 1986.
Baba S, Akerele O, Kawaguchi Y, editors. Natural resources and human health. Tokyo, Japan: Elsevier, 1992.
Cimanga K, De Bruyne T, Lasure A, et al. In vitro biological activities of alkaloids from Cryptolepis sanguinolenta.. Planta Medica. 1996;62(1):22-27.
Cimanga K, De Bruyne T, Pieters L, et al. In vitro and in vivo antiplasmodial activity of cryptolepine and related alkaloids from Cryptolepis sanguinolenta. Journal of Natural Products. 1997;60(7):688-691.
Cunningham AB. Ethics, ethnobiological research and biodiversity. Gland: WWF, 1993.
Cunningham AB. African medicinal plants. Setting priorities at the interface between conservation and primary healthcare. People and Plants Working Paper I. Paris, France: UNESCO, 1993.
Cunningham AB, Mbenkum FT. Sustainability of harvesting Prunus africana bark in Cameroon. People and Plants Working Paper 2. Paris, France: UNESCO, 1993.
Cunningham AB, Jasper PJ, Hansen LCB. The indigenous plant use programme. Paris, France: Foundation for Research Development, 1992.
De Smet PAGM. Journal of Ethnopharmacology. 1998;63:1-179.
Dedhia RC, Calhoun E, McVary KT. Impact of phytotherapy on utility scores for five benign prostatic hyperplasia/lower urinary tract symptoms health states. Journal of Urology. 2008;179(1):220-225.
Dhar R, Zhang K, Talwar GP, et al. Inhibition of the growth and development of asexual and sexual stages of drug-sensitive and resistant strains of the human malaria parasite Plasmodium falciparum by neem (Azadirachta indica) fractions. Journal of Ethnopharmacology. 1998;61(1):31-39.
Elujoba AA. Traditional medicine and HIV/AIDS in Africa. In: Sofowora A, editor. Medicinal plants and traditional medicine in Africa. Ibadan, Nigeria: Spectrum Books, 2008.
Esomonu UG, El-Taalu AB, Anuka JA, et al. Effect of ingestion of ethanol extract of Garcinia Kola seed on erythrocytes in Wistar rats. Nigerian Journal of Physiological Science. 2005;20(1–2):30-32.
Farnsworth NR, Soejarto DD. Global importance of medicinal plants. In: Akerele O, Heywood N, Synge H, editors. Conservation of medicinal plants. Cambridge, UK: Cambridge University Press, 1991.
Farombi EO. Mechanisms for the hepatoprotective action of kolaviron: studies on hepatic enzymes, microsomal lipids and lipid peroxidation in carbontetrachloride-treated rats. Pharmacological Research. 2000;42(1):75-80.
Farombi EO, Møller P, Dragsted LO. Ex-vivo and in vitro protective effects of kolaviron against oxygen-derived radical-induced DNA damage and oxidative stress in human lymphocytes and rat liver cells. Cell Biology and Toxicology. 2004;20(2):71-82.
Farombi EO, Adepoju BF, Ola-Davies OE, Emerole GO. Chemoprevention of aflatoxin B1-induced genotoxicity and hepatic oxidative damage in rats by kolaviron, a natural bioflavonoid of Garcinia kola seeds. European Journal of Cancer Prevention. 2005;14(3):207-214.
Farombi EO, Abarikwu SO, Adedara IA, Oyeyemi MO. Curcumin and kolaviron ameliorate di-n-butylphthalate-induced testicular damage in rats. Basic Clinical Pharmacology and Toxicology. 2007;100(1):43-48.
Hostettmann K. Assays for bioactivity. Methods in plant biochemistry. Vol 6. Academic Press, London, 1991.
Houghton PJ, Paulo MA, Gomez ET, New alkaloids of Cryptolepis sanguinolenta. Phytochemistry of plants used in traditional medicine—an international symposium of the Phytochemical Society of Europe, Lausanne September/October 1993. Book of Abstracts, Number P73, 1993.
Huffman MA, Wrangham RW. Diversity of medicinal plant use by wild chimpanzees. In: Heltne PG, Marquardt LA, editors. Chimpanzee behavioural diversity. Cambridge, MA: Harvard University Press; 1993:1-14.
Ide N, Kaneko R, Wada R, et al. Cloning of the thaumatin I cDNA and characterization of recombinant thaumatin I secreted by Pichia pastoris. Biotechnology Progress. 2007;23(5):1023-1030.
Isah AB, Ibrahim YK, Iwalewa EO. Evaluation of the antimalarial properties and standardization of tablets of Azadirachta indica (Meliaceae) in mice. Phytotherapy Research. 2003;17(7):807-810.
Iwu MM. A handbook of African medicinal plants. Boca Raton, FL: CRC Press, 1993.
Jato J, Symonds P, Thomas D, et al. Conserving a rare medicinal plant: the case of Ancistroclaudus korupensis (Ancistrocladaceae). Proceedings of the 5th OAU/STRC Symposium on African Traditional Medicine and Medicinal Plants. Lagos, Nigeria: OAU/STRC, 1993.
Keita A. Activities of the traditional medicine department in Mali. Mbarara, Uganda,: International workshop by the GIFTS of Health, 1994. December 6–9, 1994
Khalid SA, Duddeck H. Isolation and characterization of an antimalarial agent of the neem tree Azadirachta indica. Journal of Natural Products. 1989;52(5):922-927.
Kokwaro JO. Conservation of medicinal plants in Kenya. In: Akerele O, Heywood V, Synge H, editors. Conservation of medicinal plants. Cambridge: Cambridge University Press; 1991:315-319.
Kokwaro JO. Medicinal plants of East Africa, 2nd edn. Nairobi, Kenya: Kenya Literary Bureau, 1993.
Manfredi KP, Blunt JW, Cardelina JHII, et al. Journal of Medicinal Chemistry. 1991;34:3402-3405.
Marston A, Maillard M, Hostettmann K. Search for antifungal, molluscicidal and larvicidal compounds from African medicinal plants. Journal of Ethnopharmacology. 1993;38:215-223.
Masuda T, Tamaki S, Kaneko R, et al. Cloning, expression and characterization of recombinant sweet-protein thaumatin II using the methylotrophic yeast Pichia pastoris. Biotechnology and Bioengineering. 2004;85(7):761-769.
Mbenkum FT, Thomas DN. Sustainable use of secondary products from Cameroon’s forests: a survey of medicinal, insecticidal and molluscicidal plants. In: Proceedings of the 5th OAU/STRC Symposium on African Traditional Medicine and Medicinal Plants. Lagos, Nigeria: OAU/STRC; 1993.
Mgbemene CN, Ohiri FC. Pharmaceutical Biology. 1999;37:152.
Mshana NR, Abbiw DK, Addae-Mensah I, et al. Contribution to the ethnobotanic and floristic studies in Ghana. Lagos, Nigeria: AU/STRC, 2000.
Nathan SS, Kalaivani K, Murugan K. Effects of neem limonoids on the malaria vector Anopheles stephensi Liston (Diptera: Culicidae). Acta Tropica. 2005;96(1):47-55.
Ndukwe KC, Okeke IN, Lamikanra A, et al. Antibacterial activity of aqueous extracts of selected chewing sticks. Journal of Contemporary Dental Practice. 2005;6(3):86-94.
Neuwinger JD, (translated from the German by Aileen Porter). African ethnobotany—poisons and drugs. Weinheim Nwosu MO: Chapman and Hall, 1996. 1999 Fitoterapia 70: 58
Odunola OA, Adetutu A, Olorunnisola OS, Ola-Davis O. Protection against 2-acetyl aminofluorene-induced toxicity in mice by garlic (Allium sativum), bitter kola (Garcina kola seed) and honey. African Journal of Medicine and Medical Science. 2005;34(2):167-172.
Odusanya SA, Songonuga OO, Folayan JO. Fluoride ion distribution in some African chewing sticks. IRCS Medical Science. 1979;7:580.
Ogunmodede E. Dental care: the role of traditional healers. World Health Forum. 1991;12(4):443-444.
Okumu FO, Knols BG, Fillinger U. Larvicidal effects of a neem (Azadirachta indica) oil formulation on the malaria vector Anopheles gambiae. Malaria Journal. 2007;6:63.
Owadally AW, Dulloo ME, Straham W. Measures that are required to help conserve the flora of Mauritius and Rodriquez in ex situ collections. In: Heywood VH, Wyse-Jackson PS, editors. Tropical botanic gardens. Their role in conservation and development. London: Head Press, 1991.
Paulo A, Duarte A, Gomes ET. Antibiotic activity of some alkaloids isolated from Cryptolepis sanginolenta. International Symposium Phytochemistry of Plants used in Traditional Medicine, 1993. Book of Abstracts, Poster No. 108. Lausanne, September, 1993
Paulo A, Pimentel M, Viegas S, et al. Cryptolepis sanguinolenta activity against diarrhoeal bacteria. Journal of Ethnopharmacology. 1994;44(2):73-77.
Paulo A, Duarte A, Gomes ET. In vitro antibacterial screening of in vitro Cryptolepis sanguinolenta alkaloids. Journal of Ethnopharmacology. 1994;44(2):127-130.
Rochanankij S, Tebttearanonth Y, Yenjai Ch, Yuthavong Y. Journal of Tropical Medicine and S.E. Asian Public Health. 1985;16:66.
Samuelsson G, Farah MH, Claeson P, et al. Inventory of plants used in traditional medicine in Somalia. I. Plants of the families Acanthaceae—Chenopodiaceae. Journal of Ethnopharmacology. 1991;35:25-63.
Shenouda NS, Sakla MS, Newton LG, et al. Phytosterol Pygeum africanum regulates prostate cancer in vitro and in vivo. Endocrine. 2007;31(1):72-81.
Sofowora A. Recent trends in research into African medicinal plants. Journal of Ethnopharmacology. 1993;38:209-214.
Sofowora A. Medicinal plants and traditional medicine in Africa, 3rd edn. Ibadan, Nigeria: Spectrum Books Ltd, 2008.
Soh PN, Benoit-Vical F. Are West African plants a source of future antimalarial drugs? Journal of Ethnopharmacology. 2007;114(2):130-140.
Sote EO, Wilson M. In-vitro antibacterial effects of extracts of Nigerian tooth-cleaning sticks on periodontopathic bacteria. African Dental Journal. 1995;9:15-19.
Sowemimo AA, Fakoya FA, Awopetu I, et al. Toxicity and mutagenic activity of some selected Nigerian plants. Journal of Ethnopharmacology. 2007;113(3):427-432.
Stewart KM. The African cherry (Prunus africana): can lessons be learned from an over-exploited medicinal tree? Journal of Ethnopharmacology. 2003;89(1):3-13.
Tackie AN, Boye GI, Sharaff MHM, et al. Journal of Natural Products. 1993;56:653-670.
Taiwo O, Xu HX, Lee SF. Antibacterial activities of extracts from Nigerian chewing sticks. Phytotherapy Research. 1999;13(8):675-679.
Terashima K, Takaya Y, Niwa M. Powerful antioxidative agents based on garcinoic acid from Garcinia kola.. Bioorganism and Medicinal Chemistry. 2002;10(5):1619-1625.
Udeinya IJ, Brown N, Shu EN, et al. Fractions of an antimalarial neem-leaf extract have activities superior to chloroquine, and are gametocytocidal. Annals of Tropical Medicine and Parasitology. 2006;100(1):17-22.
WHO. African traditional medicine. Report of the African Regional Expert Committee, 1976. WHO Afro’s Technical Report Series, No 1
WHO. Traditional medicine and modern health care: Progress Report by the Director General. Geneva, Switzerland: World Health Organization, 1991. Document No A44/10 22 March 1991
Zemanek EC, Wasserman BP. Issues and advances in the use of transgenic organisms for the production of thaumatin, the intensely sweet protein from Thaumatococcus danielli. Critical Reviews of Food Science and Nutrition. 2005;35(5):455-466.