Management of the Patient Undergoing Radiotherapy or Chemotherapy
DENTAL MANAGEMENT OF PATIENTS UNDERGOING RADIOTHERAPY TO THE HEAD AND NECK
Radiation Effects on Oral Mucosa
Radiation Effects on Mandibular Mobility
Radiation Effects on Salivary Glands
Evaluation of Dentition Before Radiotherapy
Preparation of Dentition for Radiotherapy and Maintenance After Irradiation
Method of Performing Preirradiation Extractions
Interval Between Preirradiation Extractions and Beginning of Radiotherapy
Impacted Third Molar Removal Before Radiotherapy
Methods of Managing Carious Teeth After Radiotherapy
Tooth Extraction After Radiotherapy
Denture Wear in Postirradiation Edentulous Patients
DENTAL MANAGEMENT OF PATIENTS RECEIVING SYSTEMIC CHEMOTHERAPY FOR MALIGNANT DISEASE
DENTAL MANAGEMENT OF PATIENTS WITH BISPHOSPHONATE-ASSOCIATED OSTEONECROSIS OF THE JAW (BOJ)
DENTAL MANAGEMENT OF PATIENTS UNDERGOING RADIOTHERAPY TO THE HEAD AND NECK
Radiotherapy (i.e., radiation therapy and x-ray treatment) is a common therapeutic modality for malignancies of the head and neck. Approximately 30,000 cases of head and neck cancer occur each year. Many of these are managed by therapeutic irradiation. The use of therapeutic irradiation is ideally predicated on the ability of the radiation to destroy neoplastic cells while sparing normal cells. In practice, however, this is never actually achieved, and normal tissues experience some undesirable effect. Any neoplasm can be destroyed by radiation if the dose delivered to the neoplastic cells is sufficient. The limiting factor is the amount of radiation that the surrounding tissues can tolerate.
Radiotherapy destroys neoplastic (and normal) cells by interfering with nuclear material necessary for reproduction, cell maintenance, or both. The faster the cellular turnover, the more susceptible the tissue is to the damaging effects of radiation. Thus neoplastic cells, which are usually reproducing at higher rates than normal tissue, are selectively destroyed (relatively). In practice, normal tissues with rapid turnover rates are also affected to some degree. Therefore, hematopoietic cells, epithelial cells, and endothelial cells are affected soon after radiotherapy begins.
Early in the course of radiotherapy, the oral mucosa shows the effects of treatment. Most notable to dentistry are the changes in and around the oral cavity as the result of destruction of the fine vasculature. Salivary glands and bone are relatively radioresistant, but because of the intense vascular compromise resulting from radiotherapy, these tissues bear a considerable hardship in the long run.
Radiation Effects on Oral Mucosa
The initial effect of radiotherapy on the oral mucosa, which is seen in the first 1 or 2 weeks, is an erythema that may progress to a severe mucositis with or without ulceration. Pain and dysphagia may be severe and make adequate nutritional intake difficult. These mucosal reactions begin to subside after completion of the course of radiotherapy. The taste buds, also composed of epithelial cells, show similar reactions. Loss of taste is a prominent complaint early in treatment and gradually returns, depending on the quantity and quality of saliva that remains after treatment.
Relief from mucositis is not predictable. Antibiotic lozenges containing amphotericin, tobramycin, and neomycin may be of some benefit.1 When symptoms are severe, viscous lidocaine can be useful.
The long-term effects of radiotherapy to the oral mucosa are characterized by a predisposition to breakdown and delayed healing, even after minor insult. The epithelium is thin and less keratinized, and the submucosa is less vascular, which gives a pale appearance to the tissue. Radiotherapy induces submucosal fibrosis, which makes the mucosal lining of the oral cavity less pliable and less resilient. Minor trauma may create ulcerations that take weeks or months to heal. These ulcerations are often difficult to differentiate from recurrent malignant disease.
Radiation Effects on Mandibular Mobility
When irradiated, the pterygomasseteric sling and periarticular connective tissues become inflamed. Irradiated muscle becomes fibrotic and tends to contract, and the articular surfaces degenerate.2 These factors herald the onset of trismus. The decrease in ability to open the mouth may be insidious in onset, usually occurring over the first year after radiation therapy, and is painless. When the interincisal opening decreases to 20 mm, feeding becomes difficult. Additionally, inability to open the mouth wide makes it difficult to perform dental work and to provide a general anesthetic.
Radiation Effects on Salivary Glands
Salivary gland epithelium has a slow turnover rate; therefore the salivary glands might be expected to be radioresistant. However, because of the destruction of the fine vasculature by the radiation, the salivary glands show considerable damage, with resultant atrophy, fibrosis, and degeneration. This damage manifests clinically as xerostomia (the decreased production of saliva) and gives the patient a “dry mouth.” The severity of xerostomia depends on which salivary glands were within the field of radiation. A dry mouth may be the patient’s most significant complaint.
Loss of salivary function leads to a plethora of adverse sequelae, including difficulty with tasting, chewing, and swallowing; difficulty sleeping; esophageal dysfunction, including chronic esophagitis; nutritional compromises; higher frequency of intolerance to medications; increased incidence of glossitis, candidiasis, angular cheilitis, halitosis, and bacterial sialadenitis; decreased resistance to loss of tooth structure from attrition, abrasion, and erosion; loss of buffering capacity; increased susceptibility to mucosal injury; inability to wear dental prostheses; and rampant caries.
The effects of xerostomia on the oral cavity are devastating. Because saliva is the principal protector of the oral tissues, absence results in serious complications. Salivary proteins such as peroxidase, lysozyme, and lactoferrin are antibacterial and limit the growth of cariogenic bacteria. The film of salivary mucins on the teeth and mucosal surfaces is believed to protect these oral structures from wear. Histatins, a family of salivary proteins, have potent antifungal properties that limit the growth of oral yeast. These salivary components, in conjunction with the mucosal tissues, form part of the innate immune system that continually protects the human body from infection. The oral cavity also is protected by secretory immunoglobulins A and M, which are produced locally by B cells within the salivary glands. These antibodies include those with specificity against oral cariogenic bacteria. When salivary volume is reduced significantly, patients are at risk for serious oral complications.
The xerostomia makes it difficult for patients to eat a normal diet because of dysphagia. Patients therefore may adopt a more cariogenic diet. Rampant “radiation caries” can swiftly destroy the remaining dentition and predispose the patient to severe infections of the jaws (Fig. 18-1). Teeth thus affected exhibit decay around the entire circumference of the cervical portion (Fig. 18-2). Periodontitis is also accelerated in the absence of saliva. Dysgeusia, dysphonia, and dysphagia are also caused by xerostomia. Another sequela of low salivary flow is an increase in oral infections such as candidiasis.

FIGURE 18-1 Radiographs illustrating the rapidity with which dental caries can occur in an irradiated patient. A, Periapical radiographs taken just before radiation therapy. B, Periapical radiographs taken 16 months after radiation therapy. Note the prevalence and severity of dental caries that have occurred throughout the dentition (arrows).
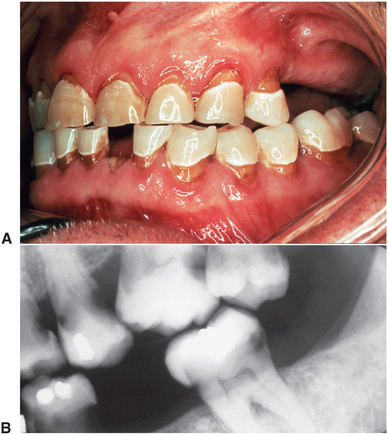
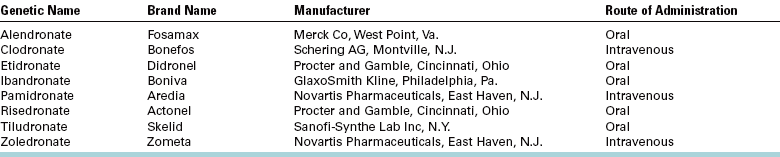
FIGURE 18-2 A, Typical clinical appearance of radiation caries. B, Typical radiographic appearance of radiation caries. Note the erosion around the cervical portion of the teeth.
Treatment of Xerostomia
After radiotherapy, patients often complain of chronic dry mouth. At present, no general agreement exists concerning how to prevent these changes. Unfortunately, in many cases, xerostomia never improves substantially, and exogenous replacement of saliva is necessary. For the simplest form of replacement, water can be sipped throughout the day. Sipping water during meals aids in chewing, swallowing, and taste perception. In addition, several saliva substitutes can be obtained without a prescription at the pharmacy. These substitutes contain several of the ions in saliva and other ingredients (e.g., glycerin) to mimic the lubricating action of saliva. Patients should be advised not to use products containing alcohol or strong flavors, which may irritate the mucosa. Patients should avoid sugar-containing products because of their increased susceptibility to dental caries. Many of the salivary substitutes available in the United States contain carboxymethylcellulose, whereas animal-derived mucin-based products are available in other countries. Studies have shown that the use of these products reduces the severity of symptoms associated with xerostomia.3,4
Unfortunately, artificial salivas on the market do not possess the protective proteins that are present in the salivary secretions. The patients are therefore still prone to the problems induced by xerostomia. For comfort, however, many patients seem to be just as satisfied with plain water as artificial salivas and keep small quantities available at all times to sip.
Efforts to stimulate the patient’s residual saliva have met with some success. Sugar-free chewing gum stimulates saliva production as long as there is some saliva being produced.5 The Food and Drug Administration has now approved the use of two medications to stimulate the flow of saliva: (1) pilocarpine hydrochloride and (2) cevimeline hydrochloride, which have been shown to relieve symptoms of xerostomia for patients with xerostomia.6 Both drugs are parasympathomimetic agents that function primarily as muscarinic agonists, causing stimulation of exocrine gland secretion. This stimulation can increase the production of saliva, even in patients whose salivary glands have been exposed to radiation. An oral dose of 5 mg of pilocarpine 4 times each day or 30 mg of cevimeline 3 times a day has been shown to improve many symptoms of xerostomia without significant drug-related side effects.7–12 The administration of these medications may prove to be beneficial for some patients with postradiation xerostomia.
Radiation Effects on Bone
One of the most severe and complicating sequelae of radiotherapy for patients with head and neck cancer is osteoradionecrosis (Fig. 18-3). Basically, osteoradionecrosis is devitalization of the bone by cancericidal doses of radiation. The bone within the radiation beam becomes virtually nonvital from an endarteritis that results in elimination of the fine vasculature within the bone. The turnover rate of any remaining viable bone is slowed to the point of being ineffective in self-repair. The continual process of remodeling normally found in bone does not occur, and sharp areas on the alveolar ridge will not smooth themselves, even with considerable time (Fig. 18-4). The bone of the mandible is denser and has a poorer blood supply than that of the maxilla. Thus the mandible is the jaw most commonly affected with nonhealing ulcerations and osteoradionecrosis.

FIGURE 18-3 Two cases of osteoradionecrosis of the mandible. A, Bone exposure occurred 3 weeks after tooth extraction. B, Severe osteoradionecrosis of the mandible with dehiscence of the facial soft tissues, exposing the necrotic bone externally.
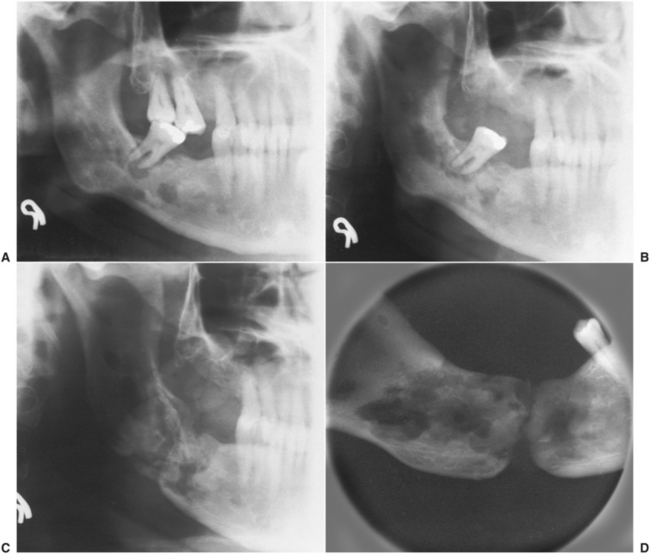
FIGURE 18-4 Progressive course of osteoradionecrosis. A, Radiograph showing radiolucencies in right mandible and around apex of molar tooth. B, Six months later, during which time antibiotics and local irrigations were used, radiolucent process is spreading into ramus. Molar was removed at this time. C, Five months after tooth removal, extraction site did not heal and destructive process spread, resulting in pathologic fracture of mandible. D, Radiograph after removal of devitalized bone, showing extent of process. (Courtesy Dr. Richard Scott, Ann Arbor, Mich.)
Other Effects of Radiation
Patients undergoing radiotherapy may have an alteration in the normal oral flora, with overgrowth of anaerobic species and fungi. Most researchers feel that oral flora colonizing the mucous membranes play an important role in the severity of mucositis and subsequent healing process.13,14 Candida albicans commonly thrives in the oral cavities of patients who have been irradiated. Whether the alteration in the flora is caused by the radiation itself or the resultant xerostomia is not known. Patients frequently require the application of topical antifungal agents, such as nystatin, to help control the number of Candida organisms present. Another oral rinse frequently prescribed is 0.1% chlorhexidine (Peridex). This agent has been shown to have potent in vitro antibacterial and antifungal effects. When used throughout the course of radiation treatment, it has been shown in at least one study to reduce greatly the prevalence and symptoms associated with radiation-induced mucositis.15 The use of chlorhexidine in other studies has been equivocal.13,16
Evaluation of Dentition Before Radiotherapy
The most feared side effect of radiotherapy is osteoradionecrosis. Most patients who have this complication have residual teeth throughout the course of radiotherapy. Thus the clinician may wonder what to do with the teeth before irradiation. Should teeth be extracted? This question has no categoric answer; however, several factors must be considered.17–20
Condition of Residual Dentition
All teeth with a questionable or poor prognosis should be extracted before radiotherapy. The more advanced the periodontal condition, the more likely the patient is to have caries and continued periodontitis. Although this may not be in keeping with usual dental principles, if in doubt, extract. Extraction in these cases may spare the patient months or years of suffering from osteoradionecrosis.
Patient’s Dental Awareness
The present state of the dentition and periodontium is a good clue to the past care they have received. In patients with excellent oral hygiene and oral health, the clinician should retain as many of the teeth as possible. Conversely, in patients who have neglected oral health for years, the chances are that they will continue to do so, especially in the face of severe xerostomia and oral pain, which will make oral hygiene even more difficult. Preradiotherapy patient preparation is similar to preorthodontic patient preparation. If an individual cannot or will not care for his or her mouth before the application of the braces, it will be impossible for him or her to do so when faced with future obstacles.
Immediacy of Radiotherapy
If the radiotherapist feels that therapy must be instituted urgently, there may not be time to perform the necessary extractions and allow for initial healing of the extraction sites. In this instance, the dentist may elect to maintain the dentition but must work closely with the patient throughout the course of radiotherapy and thereafter in an attempt to maintain oral health as optimally as possible.
Radiation Location
The more salivary glands and bone involved in the field of radiation, the more severe the resultant xerostomia and vascular compromise of the jaws. Thus the dentist should discuss with the radiotherapist the locations of the radiation beams and should estimate the severity of the probable xerostomia and bone changes. Xerostomia by itself may not result in severe problems if the dentition can be maintained because the bone is still healthy. The combination of xerostomia and irradiated bone usually causes the problem. In individuals who will have radiation to the major salivary glands and a portion of the mandible, preirradiation extractions should be considered. Frequently, the radiotherapist agrees to delay the institution of irradiation for 1 to 2 weeks if the dentist feels that time is necessary to allow the extraction sites to begin to heal.
Radiation Dose
The higher the radiation dose, the more severe the normal tissue damage. The radiotherapist should discuss with the dentist the amount of radiation planned for the individual. Frequently, the dose is not maximal, and tissue damage may be minimized. This allows the dentist to be more conservative in preirradiation extraction considerations.
Squamous cell carcinomas of the oral cavity make up approximately 90% of malignant tumors for which radiation therapy is used. Unfortunately, this cancer requires a large dose of radiation (greater than 6000 rad [60 Gy]) to effect a result. Other malignancies, such as lymphoma, require much less radiation for a response, and the oral cavity therefore is less affected. When the total dose falls below 5000 rad (50 Gy), long-term side effects, such as xerostomia and osteoradionecrosis, are dramatically decreased.
Preparation of Dentition for Radiotherapy and Maintenance After Irradiation
Every tooth to be maintained must be carefully inspected for pathologic conditions and restored to the best state of health obtainable. A thorough prophylaxis and topical fluoride application should be performed before radiotherapy. Oral hygiene measures and instructions should be demonstrated and reinforced. Any sharp cusps should be rounded to prevent mechanical irritation. Impressions for dental casts should be obtained for fabrication of custom fluoride trays to be used during and after treatment. Because tobacco use and alcohol consumption irritate the mucosa, the patient should be encouraged to stop these before commencement of radiation therapy.
During radiation treatment the patient should rinse the mouth at least 10 times a day with saline rinses. The patient should be placed on chlorhexidine mouth rinses twice a day to help minimize the bacterial and fungal levels within the mouth. The dentist should see the patient each week during the radiotherapy for observation and oral hygiene evaluations. If an overgrowth of C. albicans occurs, nystatin or clotrimazole topical applications will bring this under control relatively rapidly. The ability of the patient to open the mouth should be carefully monitored throughout the course of radiation treatment. Radiation causes a progressive fibrosis within the muscles of mastication that makes it difficult for the patient to open the mouth adequately. Patients should be instructed in physiotherapy exercises to maintain the preirradiation treatment interincisal dimension. All patients must be weighed weekly to determine whether they are maintaining an adequate nutritional status. The combination of mucositis and xerostomia makes oral intake extremely uncomfortable. However, malnutrition causes further difficulties by delaying healing of the oral tissues and giving the patient an overall feeling of generalized illness. In severe cases, it may be necessary to feed the patient via nasogastric tube to maintain a reasonable nutritional status.
After radiation treatment the dentist should see the patient every 3 to 4 months. A prophylaxis is performed during these postirradiation visits, and topical fluoride applications are made. The patient should be fitted with custom trays to deliver topical fluoride applications. The patient should be instructed in the use of the trays and in daily self-administration of topical fluoride applications. The use of a 1% fluoride rinse for 5 minutes each day has been found to decrease the incidence of radiation caries.21 Over-the-counter fluoride rinses currently available can be used without a customized delivery splint with good success and seem to have better patient acceptance.
All patients should also be monitored for the possible onset of trismus. It is easier to prevent trismus than to treat it. The patient should perform mouth-opening exercises when there is any decrease in the maximum interincisal dimension. For more established cases, the patient can use jaw exercising (i.e., Therabyte).
Method of Performing Preirradiation Extractions
If the decision has been made to extract some or all teeth before radiotherapy, the question becomes “How should the teeth be extracted?” In general, the principles of atraumatic exodontia apply. However, the concepts of bone preservation are disregarded, and an attempt is made to remove a good portion of the alveolar process along with the teeth and achieve a primary soft tissue closure. With the onset of radiotherapy, the normal remodeling process is inhibited; if any sharp areas of bone exist, ulceration occurs with bone exposure. Thus the teeth are usually removed in a surgical manner, with flap reflection and generous bone removal.
Atraumatic handling of the mucoperiosteal flaps is necessary to ensure a rapid soft tissue healing. Burs or files should be used to smooth the bony edges under copious irrigation because the remodeling capability of the tissues is greatly decreased after radiotherapy. Prophylactic antibiotics are indicated under these circumstances. Note: The dentist is in a race against time. If the wound fails to heal, the radiotherapy will be delayed. If the radiation is delivered before the wound heals, healing will take months or even years.
Interval Between Preirradiation Extractions and Beginning of Radiotherapy
No categoric answer exists to the question of how much time should be allowed after extractions before beginning radiotherapy. Obviously, the sooner radiotherapy is begun, the more beneficial it may be for treating the malignancy. Thus when the soft tissues have healed sufficiently, radiotherapy may begin. Traditionally, 7 to 14 days between tooth extraction and radiotherapy have been suggested.17,22,23 Most authors base their recommendations on the clinical impression that reepithelialization has occurred in this period. However, radiotherapy should be delayed for 3 weeks after extraction, if possible. This helps to ensure that sufficient soft tissue healing has occurred. The radiotherapy should be delayed further, if possible, if a local wound dehiscence has occurred. In this instance, daily local wound care with irrigations and postoperatively administered antibiotics are mandatory until the soft tissues have healed.
Impacted Third Molar Removal Before Radiotherapy
If the patient has a partially erupted mandibular third molar, removal may be prudent to prevent pericoronal infection. In general, however, allowing a tooth that is totally impacted within the bone of the mandible to remain in place is more expeditious than removing it and waiting for it to heal.
Methods of Managing Carious Teeth After Radiotherapy
Teeth that develop postradiotherapy caries must be immediately cared for in an attempt to prevent further spread of infection. Composites and amalgam are the materials of choice to repair the defects caused by caries. Full crowns are probably not warranted because recurrent caries is more difficult to detect under such restorations. Oral hygiene measures, including fluoride application, must be reinforced in any patient who has postirradiation caries.
If a tooth has a necrotic pulp, endodontic intervention with systemic antibiotics can be carefully performed and the tooth can be ground out of occlusion and maintained. Frequently, root canal treatment is difficult because of a progressive sclerosis of the pulp chamber that occurs in irradiated teeth. In such instances, the tooth can simply be amputated above the gingiva and left in place.
Tooth Extraction After Radiotherapy
Can teeth be extracted after radiotherapy, and if so, how? These are probably the most difficult questions to answer. Each dentist has a view on this subject, and the literature is contradictory. Postirradiation extractions are also the most undesirable extractions the dentist will ever perform because the outcome is always uncertain.
The answer to the question of whether extractions can be done after radiotherapy is certainly yes. The more important question is, How? If the tooth is to be extracted, the dentist can perform a routine extraction without primary soft tissue closure or a surgical extraction with alveoloplasty and primary closure. Either of these techniques yields similar results, with a certain concomitant incidence of osteoradionecrosis. The use of systemic antibiotics is recommended.
Another technique that has been shown to be effective and that is gaining in popularity is the use of hyperbaric oxygen (HBO) before and after tooth extraction. HBO therapy is the administration of oxygen under pressure to the patient. HBO has been shown to increase the local tissue oxygenation and vascular ingrowth into the hypoxic tissues.24,25 The usual protocol for such treatments is to have between 20 and 30 HBO dives before extraction and 10 more dives immediately after extractions. HBO chambers are not available in all communities and, when present, are usually in select hospitals. A physician that is experienced in hyperbaric medicine manages patients referred to these facilities. The patient usually undergoes one HBO session each day. Therefore, it takes 4 to 6 weeks to get the 20 to 30 treatments before surgery, and 2 weeks of treatment after surgery. In a prospective clinical trial comparing this regimen with the use of prophylactically administered antibiotics before dental extraction without hyperbaric oxygenation, Marx et al.26 found a significant decrease in the incidence of osteoradionecrosis (5.4% compared with 30%).
Because considerable controversy exists over how to manage an extraction surgically in a patient who has undergone irradiation, because few hyperbaric oxygenation chambers are available for use, and because the incidence of severe complications is relatively high, it is recommended that an oral and maxillofacial surgeon manage the patient who has received irradiation and requires extractions.
Denture Wear in Postirradiation Edentulous Patients
Patients who were edentulous before radiotherapy manage nicely with well-constructed dentures. However, patients rendered edentulous just before or after radiotherapy exhibit more problems with mucosal ulcerations and subsequent osteoradionecrosis. The normal remodeling process of the alveolar bone cannot smooth even the most minor irregularities left by extraction. With denture wear, these minor irregularities cause ulceration of the mucosa.
Soft denture liners might seem an ideal solution for patients who have received irradiation. However, the silicone soft liners proved to be not particularly useful for several reasons. At present, patients are probably best served with ordinary dentures.
Denture fabrication for patients who were previously edentulous can proceed once the acute effects of irradiation have subsided. For patients who underwent extractions just before or after radiotherapy, it is prudent to see them frequently after delivery of their dentures to make adjustments for sore spots that develop before they cause mucosal breakdown and bone exposure.
When dentures are constructed, the dentist must be certain that the denture base and occlusal table are designed so that forces are distributed evenly throughout the alveolar ridge and that lateral forces on the denture are eliminated.
Use of Dental Implants in Irradiated Patients
The dental rehabilitation of the edentulous patient who has received radiation therapy is one of the greatest challenges facing the reconstructive dentist. Many patients who have had ablative surgery for malignancy do not have the normal anatomy that makes denture wear possible. There may be no vestibules to accommodate a denture flange.
Often, portions of the tongue have been removed. The patient may have hard and soft tissue defects and deficits. When reconstructed, the bone may have poor form for support of a tissue-borne prosthesis. Frequently, such patients have thick, nonpliable soft tissue flaps that have been grafted from distant areas and are not adherent to the underlying bone. All of these combine to make conventional denture fabrication challenging. In such instances, the use of implant-borne prostheses are preferred from a functional standpoint.
For years, however, a history of irradiation has been a relative contraindication to the placement of dental implants.27 The effects of radiation on bone and soft tissue present a formidable challenge to the use of implanted metallic devices. It has been demonstrated that there is a 19% reduction of bone-to-implant contact of a cylindric titanium plasma–sprayed implants in rabbit tibiae after 4050 cGy irradiation during the initial healing time.28 Not surprising, numerous clinical studies evaluating the success rates of intraoral endosseous implants placed in previously irradiated bone beds with and without adjunct HBO treatment have demonstrated success rates slightly to substantially less than in nonirradiated patients.29–37
However, the benefits that can accrue from providing this group of patients with a functional and esthetic dental reconstruction are great. Such patients have been through a great deal of hardship. They have lost portions of their anatomy, are frequently deformed, and feel the uncomfortable effects of the radiation therapy, such as xerostomia, dysphagia, and dysgeusia. They relish the thought of being able to chew solid food with a functional dentition. Implant-borne prostheses can help achieve this goal in these difficult situations. However, the unpredictable reaction of soft and hard tissue in an irradiated patient and the surgical trauma of treatment have combined to promote caution in such cases.
Many variables must be evaluated when considering placement of dental implants into irradiated bone, including the radiation type, dose, sites, elapsed time since the treatment, protection provided to the bone during treatment, and the patient’s own physiologic responses (which themselves are affected by age, sex, genetics, smoking, and other systemic considerations). Other critical factors are whether the implants will be placed into irradiated host mandibular bone, irradiated bone grafts, or bone that has been transplanted after the radiation therapy. In the latter instance, if the mandible was reconstructed using a microvascular graft in which the blood supply to the bone is brought in from a distant source and has not been altered by the previous radiation therapy, no adverse tissue reaction should be expected after placement of dental implants.
When dental implants are to be placed into irradiated host or grafted bone, the dentist must proceed with caution. Consultation with the radiotherapist is recommended to determine the amount of radiation that has been delivered to the area of the jaws where the proposed implants will be placed. Studies have provided insight into the use of implants in irradiated bone. In general, they have shown the following:
1. The more radiation delivered, the higher the failure rate for endosseous implants.30,36
2. The longer the duration between radiation treatment and implantation, the higher the failure rate.36
3. When implants in irradiated patients fail, they usually fail early, before prosthetic reconstruction, indicating a failure of osseointegration.36
4. The combination of radiation and chemotherapy has a particularly negative effect on the outcome for osseointegration.36
5. Implant survival in irradiated patients tends to be higher in the maxilla than in the mandible.35,36,38,39
6. Shorter implants have the worst prognosis.36
7. HBO treatment reduces implant failure rates.36
It has been demonstrated that the success of implant retention is directly and positively correlated with the amount of radiation to which the bone was exposed.30,36 If the amount of radiation is less than approximately 4500 rad (45 Gy), implants may be placed with care. When the amount of radiation exceeds this amount, preoperative (20 to 30) and postoperative (10) HBO treatments should be considered. HBO treatments have been shown to be beneficial in such patients.36,40
The time required for osseointegration will be prolonged in irradiated patients because of the lower metabolic activity in the bone, so the implants should not be loaded for at least 6 months after placement. The dentist must pay particular attention to oral hygiene in such patients because their tissues will not be as able to resist bacterial invasion as tissues in patients who have not been irradiated. The prosthetic design should therefore be made as cleanable as possible, with frequent use of overdentures. However, prostheses that do not allow contact of denture flanges with the oral soft tissues help prevent ulceration. No matter what type of prosthesis is fabricated, these patients require more careful follow-up and hygiene measures.
In spite of the fear that implants placed into irradiated bone will lead to osteoradionecrosis, the condition is uncommonly reported in the literature (Fig. 18-5).41,42 However, there has been an insufficient duration of experience to predict the long-term outcome of implant prosthetics in the patient who has undergone radiation.

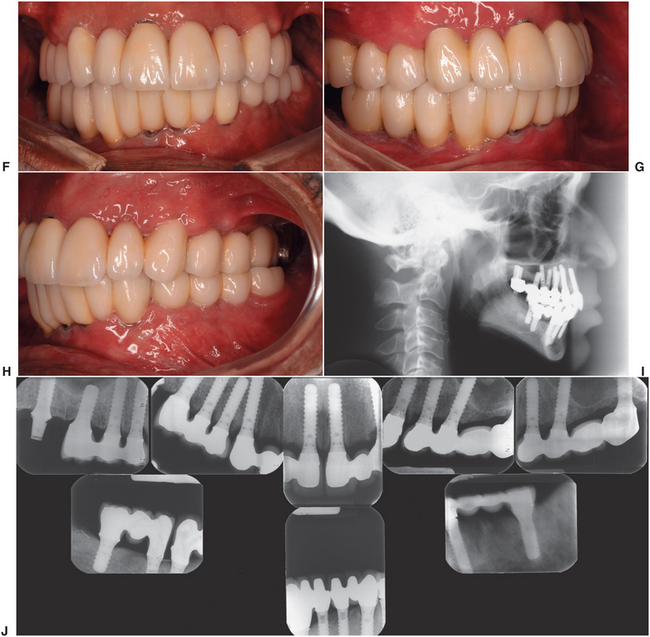
FIGURE 18-5 Photographs of a case of dental implant reconstruction in a patient who had full-course radiation treatment for squamous cell carcinoma. His existing dentition developed rampant dental caries (A to C) within a year after radiation therapy. After hyperbaric oxygen treatment, his teeth were extracted and implanted (D and E). After a waiting period of 6 months, full-fixed prosthetic restorations (crowns and bridges) were fabricated. His prosthesis (F, G, and H) and lateral cephalometric radiograph (I) 1 year after placement of his prostheses. J, The bone levels have been maintained around all of the implants.
Management of Patients Who Have Osteoradionecrosis
Most mucosal breakdown and subsequent osteoradionecrosis occur in the mandible (Fig. 18-4). These conditions occur most often in mandibles that have received radiation in excess of 6500 rad (65 Gy) and do not usually occur in mandibles that have received radiation doses less than 4800 rad (48 Gy).43–45 Severe pain may follow. The patient should discontinue wearing any prosthesis and try to maintain a good state of oral health. Irrigations should be instituted to remove necrotic debris. Only occasionally are systemic antibiotics necessary because osteoradionecrosis is not an infection of the bone but rather a nonhealing hypoxic wound.24 Because of the decreased vascularity of the tissues, systemic antibiotics do not gain ready access to the area to perform the function for which they are intended. However, in acute secondary infections, antibiotics may be useful to help prevent spread of the infection. Any loose sequestra are removed, but no attempt is made initially to close the soft tissues over the exposed bone. Most wounds smaller than 1 cm eventually heal, although it may take weeks to months.
For nonhealing wounds or extensive areas of osteoradionecrosis, surgical intervention may be indicated. In this instance, resection of the exposed bone and a margin of unexposed bone and primary soft tissue closure can be attempted (Fig. 18-6). This treatment is successful in many cases. Greatly improved results have recently been obtained by the use of HBO therapy in conjunction with surgical intervention.24
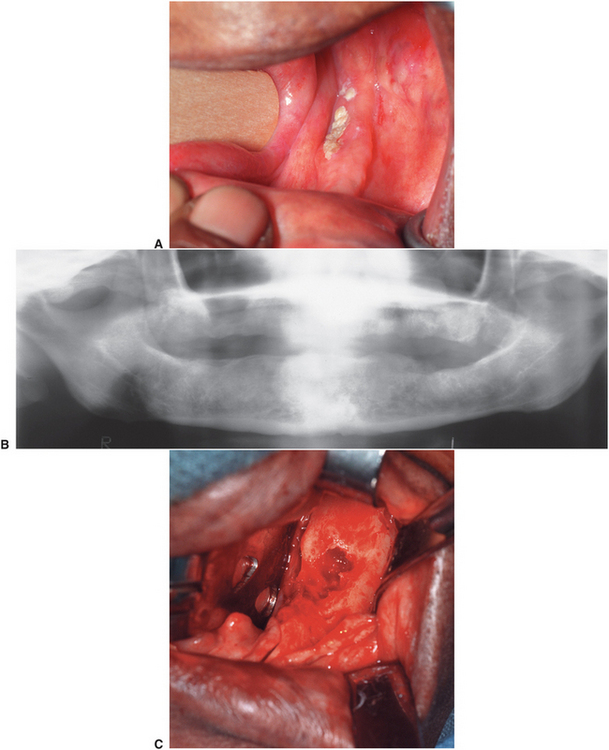
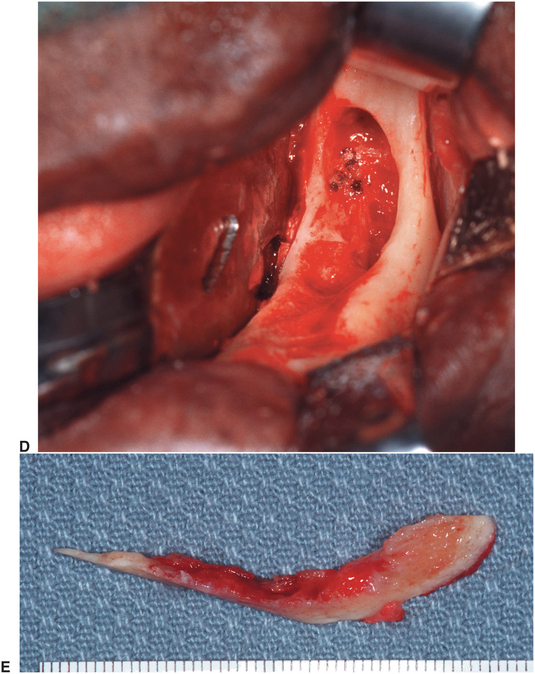
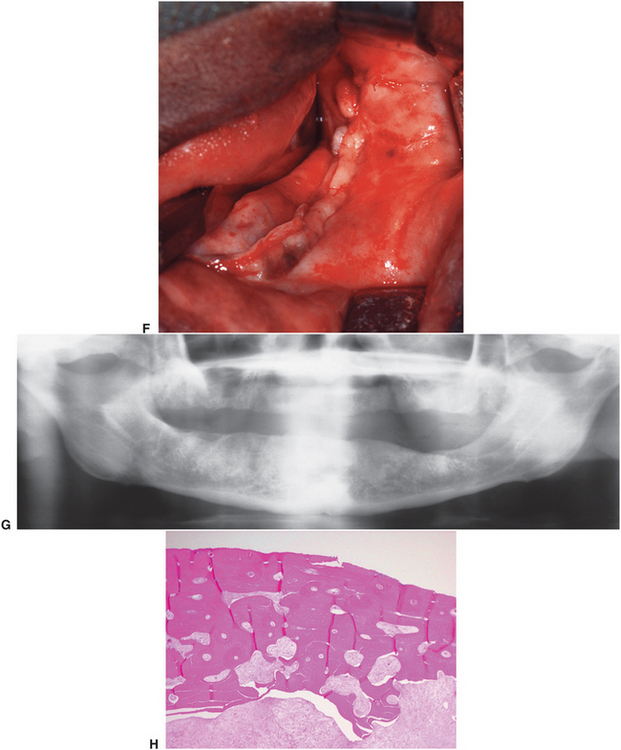
FIGURE 18-6 Osteoradionecrosis of the left mandible. This patient had a full course of tumoricidal radiotherapy for squamous cell carcinoma. The dentition was removed at the time of the cancer resection. This patient was prepared for treatment of the osteoradionecrosis with preoperative and postoperative hyperbaric oxygen treatments. A, Exposed devitalized bone along alveolar ridge of left mandible. B, Panoramic radiograph showing diffuse irregularity without good cortication of alveolar crest. C, Surgical exposure of the area shows devital bone margins and a central crater devoid of bone. D, The bone of the alveolar crest is removed, and the remainder is smoothed with a bur until bleeding bone is encountered. The central crater is similarly burred out. E, Resected specimen of alveolar crest. F, Closure of soft tissues. G, Panoramic radiograph 8 months after surgery showing slight remodeling and healing of the bone. H, Histology of the resected specimen showed osteoradionecrosis and fibrosis in marrow area of haversian systems.
Reconstructive efforts with bone grafts used for continuity defects can also be undertaken successfully in many patients who have undergone irradiation. Free microvascular grafting techniques are becoming more popular for restoring continuity defects in patients who have received radiotherapy. These bone grafts have their own blood supply from a reconnection of blood vessels and are therefore less dependent on the local tissues for incorporation and healing.
DENTAL MANAGEMENT OF PATIENTS RECEIVING SYSTEMIC CHEMOTHERAPY FOR MALIGNANT DISEASE
Destruction of malignant cells by tumoricidal chemotherapeutic drugs has proved an effective treatment for a variety of malignancies. Like radiotherapy, the antitumor effect of cancer chemotherapeutic agents is based on their ability to destroy or retard the division of rapidly proliferating cells, such as tumor cells, nonspecifically. Unfortunately, normal host cells that have a high mitotic index are also adversely affected. Normal cells most affected are the epithelium of the gastrointestinal tract (including oral cavity) and the cells of the bone marrow. The commonest oral side effects are altered taste sensation, xerostomia, and mucositis.46
Effects on Oral Mucosa
Many chemotherapeutic agents reduce the normal turnover rate of oral epithelium, which results in atrophic thinning of the oral mucosa manifested clinically as painful, erythematous, and ulcerative mucosal surfaces in the mouth. The effects are most noted on the unattached mucosa and are rarely seen on gingival surfaces. These changes are seen within 1 week of the onset of the administration of the antitumor agents. The effects are usually self-limiting, and spontaneous healing occurs in 2 to 3 weeks after cessation of the agent.
Effects on Hematopoietic System
Myelosuppression—as manifested by leukopenia, neutropenia, thrombocytopenia, and anemia—is a common sequela of several forms of cancer chemotherapy. Within 2 weeks of the beginning of chemotherapy administration, the white blood cell count falls to an extremely low level. The effect of myelosuppression in the oral cavity is marginal gingivitis. Mild infections may develop, and bleeding from the gingiva is common. If the neutropenia is severe and prolonged, severe infections may develop. The microorganisms involved in these infections may be overgrowths of the usual oral flora, especially fungi; however, other microorganisms may be causative. Thrombocytopenia can be significant, and spontaneous bleeding may occur. This is especially common in the oral cavity after oral hygiene measures. Recovery from myelosuppression is usually complete 3 weeks after cessation of chemotherapy.
The type of neoplasm for which the patient is being treated is important to determine. The type of neoplasm dictates the type of chemotherapeutic agents to be used. Many hematologic neoplasms (e.g., leukemia) are treated with chemotherapeutic agents that result in profound alterations in the function and number of bone marrow elements. Comparatively, chemotherapeutic management of some nonmarrow solid tumors may not be associated with as severe a marrow aplasia as is found in patients with hematologic neoplasms.
Effects on Oral Microbiology
Chemotherapeutic agents, because of their immunosuppressive side effect, cause profound changes in the oral flora. For example, overgrowth of indigenous microbes, superinfection with gram-negative bacilli, and opportunistic infections are common sequelae and lead to patient discomfort and morbidity. Systemic infections are responsible for about 70% of the deaths in patients receiving myelosuppressive cancer chemotherapy.47,48 Oral microorganisms have been shown to be a common source of bacteremia in these patients.47 Thus most patients who are receiving chemotherapy are treated concomitantly with systemic antimicrobial agents. However, in spite of these regimens, patients frequently have overgrowth of some organisms, most commonly the Candida species.49–51
General Dental Management
In general, the principles of dental management for the patient who has had or will have radiotherapy apply equally well to the patient who has had or will have chemotherapy.52,53 However, because of the intermittent nature of the chemotherapy delivered in many instances, the minimal effects on the vasculature, and the almost normal state of the individual between chemotherapeutic administrations, dental management can be much easier. The effects of the chemotherapy are almost always temporary, and with the passage of time, systemic health improves to optimal levels, which allows almost routine dental management.
Primary concerns for the dentist should be the severity and duration of bone marrow suppression. The dentist must be aware of the dates of chemotherapy and the hematologic status of the patient before beginning dental care. If the patient is being treated for a hematologic neoplasm (e.g., leukemia), both the disease and the chemotherapy lead to decreases in the functional blood elements. Therefore, these patients may be at great risk for infection and hemorrhage at any time in the course of their disease. Consultation with the patient’s physician in these instances is mandatory. In most cases of nonhematopoietic neoplasm, the patient is at risk for infection and hemorrhage only during the course of the chemotherapy, after which recovery of the blood elements occurs.
The decision of when to extract teeth before treatment is based on the condition of the residual dentition, the patient’s past dental hygiene practices, the immediacy of the need for chemotherapy, and the overall prognosis of the malignant disease.
Prechemotherapy dental measures that should routinely be performed are a thorough prophylaxis, fluoride treatment, and any necessary scaling. Unrestorable teeth should be removed before chemotherapy begins.
Patients who have begun chemotherapy must maintain scrupulous oral hygiene. This is difficult in the face of mucositis and ulceration, which frequently occur. No dental procedures should be performed on any patient receiving chemotherapy whose white blood cell and platelet status is unknown. In general, patients who have a white blood cell count greater than or equal to 2000/mm3, with at least 20% polymorphonuclear leukocytes and a platelet count greater than or equal to 50,000/mm3, can be treated in routine fashion. Antibiotics should be administered prophylactically if the patient has had chemotherapy within 3 weeks of dental treatment. If the white blood cell count and platelet levels fall below those specified, minimal oral care should be practiced because infection, severe bleeding, or both can occur. The patient may even need to avoid flossing and to use an extremely soft toothbrush during these periods. Any removable dental appliance should be left out at these times to prevent ulceration of the fragile mucosa.
Treatment of Oral Candidiasis
Initial treatment of candidiasis is with topical application of an antifungal medication.49 The advantage of using topical medication is that systemic side effects are minimized. Similarly, in patients with persistent infection, advantage can be gained by continuing topical agents in addition to systemic medications. The use of this combination may allow a reduced dose and duration of systemic administration of the antifungal medication and also may reduce the potential side effects.
Topical agents are available as oral rinses, oral tablets, and creams. In general, oral rinses provide a short contact time for the drug and are therefore of less efficacy. The tablets are one of the most accepted forms of topically treating candidiasis because they can be dissolved slowly in the mouth and provide increased exposure time of the drug with the oral flora. The cream forms of topical antifungals are helpful for Candida of the oral commissures or for application to the oral surfaces of prosthetic devices to prolong medication exposure.
The two most commonly administered topical medications for oropharyngeal Candida infections are clotrimazole and nystatin. Clotrimazole and nystatin are available in several forms and should be applied 4 times daily. Therapy should continue 2 weeks after cessation of clinical signs and symptoms. Clotrimazole troches are available and are dissolved in the mouth 4 or 5 times a day.
For more stubborn cases, ketoconazole or fluconazole (i.e., systemic antifungal medications) can be prescribed. However, the dentist must be careful with systemic administration of these antifungal medications because of their toxic side effects. These vary widely with the type of medication and can be serious.
Another widely prescribed medication for oral candidiasis is chlorhexidine mouth rinse. Chlorhexidine (Peridex) has been shown to have potent antibacterial and antifungal properties in vitro. The in vivo effects of chlorhexidine are less well documented, especially for use against Candida spp. in immunosuppressed individuals.13,54 However, chlorhexidine is used in most of such patients on the basis that it probably does no harm and may prove beneficial in many instances.
DENTAL MANAGEMENT OF PATIENTS WITH BISPHOSPHONATE-ASSOCIATED OSTEONECROSIS OF THE JAWS (BOJ)
Recently, a new oral complication of cancer treatment has been identified that looks similar to osteoradionecrosis, with exposure of devital areas of bone of the jaws. However, the complication is seen in patients who have not had any radiation treatment, and the methods used to treat osteoradionecrosis do not seem to be effective for the treatment of these lesions. This new oral lesion is called bisphosphonate-associated osteonecrosis of the jaws (BOJ)55 because what patients with these lesions have in common is that they are taking a bisphosphonate medication, usually as an adjunct to chemotherapy for malignant disease.
BOJ is a condition of chronically exposed necrotic bone; it is usually painful and often primarily or secondarily infected. Bone exposure might occur spontaneously or more commonly following an invasive dental procedure.56 Patients complain of halitosis and have difficulty eating and speaking.
Clinically, the lesions appear as oral mucosal ulcerations that expose the underlying bone and frequently are extremely painful. The lesions are persistent and do not respond to conventional treatment modalities such as débridement, antibiotic therapy, or HBO therapy.
Bisphosphonates
Bisphosphonates are a class of agents used to treat osteoporosis and malignant bone metastases. Bisphosphonates inhibit bone resorption and thus bone renewal by suppressing the recruitment and activity of osteoclasts, thus shortening their life span. Millions of postmenopausal women are taking bisphosphonates to stabilize bone loss caused by osteoporosis, decreasing their risk of pathologic fracture.57 Besides osteoporosis, bisphosphonates are used to manage Paget’s disease of bone and hypercalcemia of malignancy. Bisphosphonates are given to patients with cancer to help control bone loss resulting from metastatic skeletal lesions.58,59 The mechanism of action of bisphosphonates is by binding to bone mineral, where they are concentrated and accumulate over time. Bisphosphonates are potent inhibitors of osteoclastic activity,6 and this is why they are usually prescribed. Depending on the duration of the treatment and the specific bisphosphonate prescribed, the drug may remain in the body for years.8 Physiologic bone deposition and remodeling are severely compromised in patients receiving bisphosphonate therapy.60,61 Bisphosphonates also have antiangiogenic properties and may be directly tumoricidal, making them an important agent in cancer therapy.62,63
Many bisphosphonate medications are available, some given intravenously (pamidronate, zoledronic acid, clodronate) and some orally (alendronate; etidronate, risedronate, tiludronate, ibandronate; Table 18-1). The decision on which to be prescribed varies with the type of medical condition being treated and the potency of the drug required. For example, orally administered bisphosphonates often are used in patients with osteoporosis, whereas the injectable bisphosphonates are used in patients with cancer who have primary lesions of bone or skeletal metastasis.
Mechanism of BOJ
The exact mechanism that leads to the induction of BOJ is unknown. Bisphosphonates bind to bone and incorporate in the osseous matrix. During bone remodeling, the drug is taken up by osteoclasts and internalized in the cell cytoplasm, where it inhibits osteoclastic function and induces apoptotic cell death.64 Bisphosphonates also inhibit osteoblast-mediated osteoclastic resorption and have antiangiogenic properties.58,65,66 As a result, bone turnover becomes profoundly suppressed, and over time the bone shows little physiologic remodeling.61,67 The bone becomes brittle and unable to repair physiologic microfractures that occur in the human skeleton with daily activity.68,69 The need for repair and remodeling is increased greatly when there is infection in the maxilla or mandible and/or when an extraction is performed. Therefore, BOJ results from a complex interplay of bone metabolism, local trauma, increased demand for bone repair, infection, and hypovascularity.
Patients receiving bisphosphonates intravenously clearly are more susceptible to BOJ than are those receiving the drug orally. Thus, it is not common to see BOJ in patients taking bisphosphonates orally for prevention or treatment of osteoporosis; however, beginning in 2006, cases began to appear in the literature and now number around 200. Other metabolic factors may play a role in the development of BOJ, such as diabetes mellitus, as might the concomitant use of steroids, anticancer chemotherapeutic agents, and smoking.
Clinical Signs and Symptoms of BOJ
Apparently, BOJ exclusively affects the jaws.70 The most common clinical presentation associated with BOJ is an ulcer with exposed bone in a patient who has had a dental extraction (Fig. 18-7).55,56,71–74 An ulcer from a ill-fitting prosthetic device has also been implicated in the initiation of this pathologic process. However, spontaneous bone exposures that cannot be associated with any injury or infection occur in many cases.74 Similar to osteoradionecrosis, in the early stages of oral BOJ, no radiographic manifestations can be seen. Patients may be asymptomatic but may have severe pain because of the necrotic bone becoming infected secondarily after it is exposed to the oral environment. The osteonecrosis often is progressive and may lead to extensive areas of bony exposure and dehiscence (Fig. 18-8).
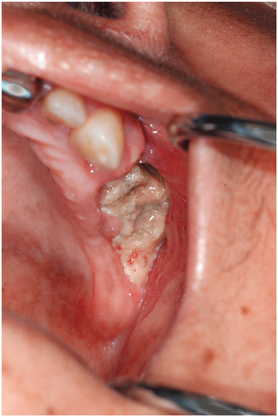
FIGURE 18-7 Bisphosphonate-related osteonecrosis of the maxilla. This area of exposed bone occurred 2 weeks after extractions. Sharp areas were débrided, but the wound had not healed after several months.
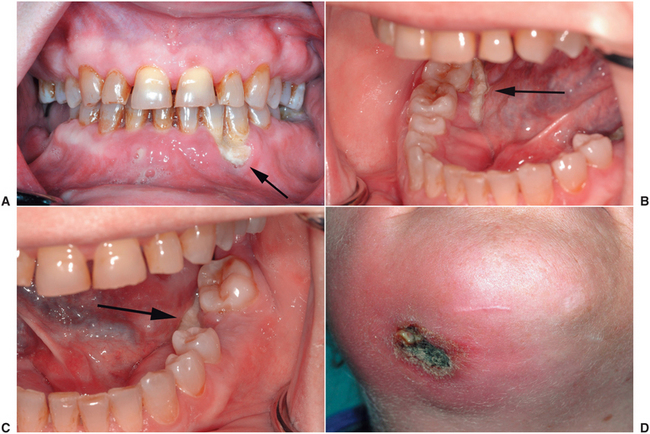
FIGURE 18-8 A progressive case of bisphosphonate-related osteonecrosis of the mandible. At initial presentation, areas of bone exposure occurred along the anterior teeth (A) and along the mylohyoid ridges bilaterally (B and C). Minor débridements were performed, but an infection of the right mandible developed, with spontaneous breakdown of the skin in the submental region (D).
In cancer patients taking intravenous forms of bisphosphonates, the median time from starting therapy to developing necrosis of bone in the jaws was 25 months.75 In addition, the elderly (over 65 years) also may have increased risk.76,77 The most common dental comorbidity in these patients reportedly is clinically and radiographically apparent periodontitis.74 Other local factors associated with BOJ are infected teeth, dental abscesses, previous endodontic treatments, and tori.
In patients in whom BOJ develops spontaneously, the most common initial complaint is the sudden presence of intraoral discomfort and the presence of roughness of the exposed bone that may progress to traumatize the oral soft tissues surrounding the area of necrotic bone.
Often a purulent discharge and local swelling occur in the adjacent soft tissues, with trismus and regional lymphadenopathy. One must differentiate BOJ from simple cases of transient mucosal ulcerations (in patients who have not been taking bisphosphonates) associated with ill-fitting prosthetic appliances, traumatic dental extractions, or spontaneously occurring denudation of bone in areas where the overlying mucosa is thin and prone to abrasion (e.g., mylohyoid ridge and tori). These areas heal spontaneously once the irritation has been removed. Lesions of BOJ will not.
Dental Care for Patients Who Are About to Start Taking Bisphosphonates
Because BOJ is a newly documented oral complication, consistently effective therapeutic measures have not yet been identified. Although several reports of this drug-associated complication have been published, there is no concensus on treatment strategies that yield predictable resolution and healing of BOJ. This presents a dilemma for the patient and the clinicians. The inability to manage lesions of BOJ worsens the patient’s medical status as the patient becomes more and more nutritionally compromised. Prevention of this condition is therefore critical for these patients so that they can receive the anticancer therapies they require for their neoplastic disease.
Similar to the management of patients who will receive radiation treatment, the dentist should see all patients before intravenous bisphosphonate therapy begins. The main emphasis at this time should be to minimize the risk of occurrence of BOJ. Most reports of BOJ occur after the patient has been taking bisphosphonates for 6 months or more,55,74 so it may be possible to provide dental care early in the treatment without unduly risking the development of BOJ from dental treatment. Although a small percentage of patients receiving bisphosphonates have BON spontaneously, the majority of affected patients experience this complication following routine dentoalveolar surgery (i.e., extraction, dental implant placement, or apical surgery). Therefore, teeth with a poor prognosis should be removed before bisphosphonate administration or as early as possible after institution of treatment. If possible, institution of bisphosphonate therapy should be delayed for approximately 4 to 6 weeks after invasive procedures such as dental extractions to give the bone a chance to recover.74
Dental prophylaxis, caries control, and conservative restorative dentistry are critical to maintaining functionally sound teeth. This level of care must be continued indefinitely. Patients with full or partial dentures should be examined for areas of mucosal trauma, especially along the lingual flange region. It is critical that patients be educated as to the importance of dental hygiene and regular dental evaluations and specifically instructed to report any pain, swelling, or exposed bone that would predict or characterize BOJ.
Dental Care for Patients Who Are Taking Bisphosphonates
The treatment of patients receiving oral or intravenous bisphosphonate therapy is principally preventive. Dentists should contact the patient’s physician to find out why the patient is taking the bisphosphonate, the type the patient is taking, and expected duration of treatment. It is recommended that dentists follow existing guidelines for a dental consultation for the prevention of oral complications of cancer therapy (chemotherapy, radiation therapy). Elimination of all potential sites of infection must be the primary objective of this consultation. Restorative dentistry should be performed to eliminate caries and defective restorations. Crowns and more extensive fixed prosthodontic work may not be appropriate for some patients. Prosthodontic appliances should be evaluated for fit, stability, and occlusion. Necessary adjustments should be made. Extraction of teeth should be avoided when possible. The goal of therapy should be to attain a state of good oral and dental health to prevent the need for invasive dental procedures in the future. Prophylaxis should be performed and oral hygiene instructions given. The patient also should be given information about BOJ and be made aware of the early signs of development of this condition. Once the active dental treatment is over, frequent periodic follow-up visits should be scheduled to reinforce the importance of oral hygiene maintenance and to conduct a new oral examination.
Role of Orally Administered Alendronate
It is unclear whether patients taking alendronate and having BOJ had other systemic or local comorbid factors.55,56,71,74 Because of the vast numbers of patients taking alendronate (Fosamax) for osteoporosis (approximately 22 million),78 a frequently asked question is whether such individuals can safely have invasive procedures such as dental extractions and dental implants placed to restore missing teeth. The risk of developing BOJ after dental extractions, implant placement, and periodontal and other surgical procedures for patients taking oral bisphosphonates such as alendronate is unknown. The duration of the physiologic effect of these drugs is variable. Evidence shows that severe suppression of bone remodeling may occur during long-term alendronate therapy61 and that bone resorption and formation markers may remain suppressed for the time during which the patient is taking the medication.60,67 At this time, it appears that the incidence of BOJ manifesting in patients taking alendronate for osteoporosis is low.79 However, the longer a patient takes this medication, the higher the risk for BOJ.
Dental Care for Patients with BOJ
For patients with established lesions of BOJ, the goal is to get the patient comfortable because it is likely the patient will have to live with the exposed bone. Treatment should be directed at eliminating or controlling pain, and preventing progression of the exposed bone. If the exposed bone has sharp edges that are irritating the adjacent soft tissues, eliminating sharp edges of bone may be performed using a rotating diamond bur. This is particularly important when the lingual aspect of the posterior mandibular arch is involved. However, superficial débridements should only be performed as a last resort. Attempts to cover the exposed bone with flaps may cause more bone exposure and worsening of symptoms, with risk of pathologic fracture. Several treatment modalities for BOJ are reported in the literature and include minor débridement under local anesthesia, major surgical sequestrectomies, marginal and segmental mandibular resections, partial and complete maxillectomies, and HBO therapy. Unfortunately, none of these therapeutic modalities have proved routinely successful. Despite the “appearance” of vascularized bone at the surgical margins, healing may not occur in the patients56,77 because the entire bone is affected, making it impossible to débride to “normal” bone. Many cases have a very poor outcome in spite of therapy, progressing to extensive dehiscence and exposure of bone.56,74,77
Patients should be closely followed up to reevaluate the areas and to ensure that they have not become suppurative. If the area around the exposed bone exhibits painful erythema and suppuration and/or sinus tracts, the patient should be treated with antibiotics until the areas resolve. Use of chlorhexidine mouth rinse 3 or 4 times a day also is recommended to reduce bacterial load and colonization.
The dentist can discuss the care of the patient with the patient’s oncologist. Because of the extremely long half-life of bisphosphonates (years), it is not reasonable to discontinue the medication in an attempt to facilitate healing of the BOJ. Further, patients taking bisphosphonates for metastatic cancer need their medication. However, if there is no cancer-related indication for continued bisphosphonate therapy or the original indication has resolved, it might be reasonable to discontinue the medication, although it will be present in the patient’s bone for a long time.
Routine restorative care may be provided to patients with BOJ. Local anesthetic can be used as necessary. Scaling and prophylaxis should be done as atraumatically as possible, with gentle soft tissue management. If the tooth is nonrestorable because of caries, root canal treatment and amputation of the crown may be a better option than removing the tooth unless it is very loose. One should try to avoid dental extractions if possible and, if necessary, should perform them as atraumatically as possible. Patients should be followed closely for the first several weeks afterward, then monthly until the sockets are completely closed and healed. If there is an indication for antibiotic use, penicillin V, amoxicillin, or clindamycin may help to reduce the incidence of local infection.
Any existing prosthetic appliances should be reevaluated to ensure that they fit well. Relining a denture with a soft liner to promote a better fit and to minimize soft tissue trauma and pressure points is recommended.
Odontogenic infections should be treated aggressively with systemic antibiotics. Although penicillin is the first-choice antibiotic in dentistry, amoxicillin and/or clindamycin provide better bone penetration and a wider spectrum of coverage.
REFERENCES
1. Okuno, SH, Foote, RL, Loprinzi, CL, et al. A randomized trial of a nonabsorbable antibiotic lozenge given to alleviate radiation-induced mucositis. Cancer. 1997;79:2193–2199.
2. Sciubba, JJ, Goldenberg, D. Oral complications of radiotherapy. Oncology. 2006;7:175–183.
3. Sweeney, MP, Bagg, J, Baxter, WP, et al. Clinical trial of a mucin-containing oral spray for treatment of xerostomia in hospice patients. Palliat Med. 1997;11:225–232.
4. Davies, AN. A comparison of artificial saliva and chewing gum in the management of xerostomia in patients with advanced cancer. Palliat Med. 2000;14:197–203.
5. Risheim, H, Amegerg, P. Salivary stimulation by chewing gum and lozenges in rheumatic patients with xerostomia. Scand J Dent Res. 1993;101:40–43.
6. Grisius, M. Salivary gland dysfunction: a review of systemic therapies. Oral Surg Oral Med Oral Pathol. 2001;92:156.
7. Greenspan, D, Daniels, TE. Effectiveness of pilocarpine in postradiation xerostomia. Cancer. 1987;59:1123–1125.
8. Johnson, JT, Ferretti, GA, Nethery, WJ, et al. Oral pilocarpine for post-irradiation xerostomia in patients with head and neck cancer. N Engl J Med. 1993;329:390–395.
9. LeVeque, FG, Montgomery, M, Potter, D, et al. A multicenter, randomized, double-blind, placebo-controlled, dose-titration study of oral pilocarpine for treatment of radiation-induced xerostomia in head and neck cancer patients. J Clin Oncol. 1993;11:1124–1131.
10. Khan, Z, Jacobsen, CS. Oral pilocarpine HCl for post-irradiation xerostomia in head and neck cancer patients. In: Proceedings of the First International Congress on Maxillofacial Prosthetics. New York: Memorial Sloan-Kettering Cancer Center; 1995.
11. Atkinson, JC, Baum, BJ. Salivary enhancers. J Dent Educ. 2001;65:1096–1101.
12. Leek, H, Albertsson, M. Pilocarpine treatment of xerostomia in head and neck patients. Micron. 2002;33:153–155.
13. Spijkervet, FK. Irradiation mucositis. Copenhagen: Munksgaard, 1991.
14. Spijkervet, FK, Van Saene, HK, Van Saene, JJ, et al. Effect of selective elimination of the oral flora on mucositis in irradiated head and neck cancer patients. J Surg Oncol. 1991;46:167.
15. Matheis, MJ, Esposito, SJ, Sherman, T. Evaluation of oral mucositis in patients receiving radiation therapy for head and neck cancer: a pilot study of 0.12% chlorhexidine gluconate oral rinse. In: Proceedings of the First International Congress on Maxillofacial Prosthetics. New York: Memorial Sloan-Kettering Cancer Center; 1995.
16. Ferretti, GA, Raybould, TP, Brown, AT, et al. Chlorhexidine prophylaxis for chemotherapy- and radiation-induced stomatitis: a randomized double-blind trial. Oral Surg Oral Med Oral Pathol. 1990;70:331.
17. Beumer, J, Brady, F. Dental management of the irradiated patient. Int J Oral Surg. 1978;7:208.
18. Beumer, J, Curtis, T, Harrison, RE. Radiation therapy of the oral cavity. I. Sequelae and management. Head Neck Surg. 1979;1:301.
19. Beumer, J, Curtis, T, Harrison, RE. Radiation therapy of the oral cavity. II. Sequelae and management. Head Neck Surg. 1979;1:392.
20. Beumer, J, Curtis, TA, Morrish, RB. Radiation complications in edentulous patients. J Prosthet Dent. 1976;36:193.
21. Dreizen, S, Brown, LR, Daly, TE, et al. Prevention of xerostomia-related dental caries in irradiated cancer patients. J Dent Res. 1977;56:99.
22. Bedwinek, JM, Shukovsky, LJ, Fletcher, GH, et al. Osteonecrosis in patients treated with definitive radiotherapy for squamous cell carcinomas of the oral cavity and naso- and oropharynx. Radiology. 1976;119:665.
23. Starcke, EN, Shannon, IL. How critical is the interval between extractions and irradiation in patients with head and neck malignancy? Oral Surg Oral Med Oral Pathol. 1977;43:333.
24. Marx, RE. A new concept in the treatment of osteoradionecrosis. J Oral Maxillofac Surg. 1983;41:351.
25. Marx, RE. Osteoradionecrosis: a new concept in its pathophysiology. J Oral Maxillofac Surg. 1983;41:283.
26. Marx, RE, Johnson, RP, Kline, SN. Prevention of osteoradionecrosis: a randomized prospective clinical trial of hyperbaric oxygen versus penicillin. J Am Dent Assoc. 1985;111:49.
27. Hobo, S, Ichida, E, Garcia, LT. Osseointegration and occlusal rehabilitation. Tokyo: Quintessence, 1989.
28. Hum, S, Larsen, P. The effect of radiation at the titanium-bone interface. In: Laney W, Tolman D, eds. Tissue integration in oral, orthopedic and maxillofacial reconstruction. Chicago: Quintessence, 1990.
29. Granström, G, Tjellstrom, A, Branemark, PI, et al. Bone-anchored reconstruction of the irradiated head and neck cancer patient. Otolaryngol Head Neck Surg. 1993;108:334.
30. Visch, LL, Levendag, PC, Denissen, HW. Five-year results of 227 HA-coated implants in irradiated tissues. In: Proceedings of the First International Congress on Maxillofacial Prosthetics. New York: Memorial Sloan-Kettering Cancer Center; 1995.
31. Esser, E, Wagner, W. Dental implants following radical oral cancer surgery and adjuvant radiotherapy. Int J Oral Maxillofac Implants. 1997;12:552–557.
32. Franzen, L, Rosenquist, JB, Rosenquist, KI, et al. Oral implant rehabilitation of patients with oral malignancies treated with radiotherapy and surgery without adjunctive hyperbaric oxygen. Int J Oral Maxillofac Implants. 1995;10:183–187.
33. Watzinger, F, Ewers, R, Henninger, A, et al. Endosteal implants in the irradiated lower jaw. J Craniomaxillofac Surg. 1996;24:237–244.
34. Keller, E, Tolman, DE, Zuck, SL, et al. Mandibular endosseous implants and autogenous bone grafting in irradiated tissue: a ten-year retrospective study. Int J Oral Maxillofac Implants. 1997;12:800–813.
35. Nimi, A, Ueda, M, Keller, EE, et al. Experience with osseointegrated implants placed in irradiated tissues in Japan and the United States. Int J Oral Maxillofac Implants. 1998;13:407–411.
36. Granstrom, G. Osseointegration in irradiated cancer patients: an analysis with respect to implant failures. J Oral Maxillofac Surg. 2005;63:579–585.
37. Moy, PK, Medina, D, Shetty, V, et al. Dental implant failure rates and associated risk factors. Int J Oral Maxillofac Implants. 2005;20:569–577.
38. Nimi, A, Fujimoto, T, Nosaka, Y, et al. A Japanese multicenter study of osseointegrated implants placed in irradiated tissues: a preliminary report. Int J Oral Maxillofac Implants. 1997;12:259.
39. Weischer, T, Mohr, C. Ten-year experience in oral implant rehabilitation of cancer patients: treatment concept and proposed criteria for success. Int J Oral Maxillofac Implants. 1999;14:521.
40. Granström, G, Jacobsson, M, Tjellström, A. Titanium implants in the irradiated tissue: benefits from hyperbaric oxygen. Int J Oral Maxillofac Implants. 1992;7:15.
41. Albrektsson, T. A multicenter report on osseointegrated oral implants. J Prosthet Dent. 1988;60:75.
42. Taylor, TD, Worthington, P. Osseointegrated implant rehabilitation of the previously irradiated mandible: results of a limited trial at 3 to 7 years. J Prosthet Dent. 1993;69:60.
43. Murray, CG, Herson, J, Daly, TE, Zimmerman, S. Radiation necrosis of the mandible: a 10-year study. I. Factors influencing the onset of necrosis. Int J Radiat Oncol Biol Phys. 1980;6:543.
44. Murray, CG, Herson, J, Daly, TE, Zimmerman, S. Radiation necrosis of the mandible: a 10-year study. II. Dental factors: onset, duration, and management of necrosis. Int J Radiat Oncol Biol Phys. 1980;6:549.
45. Beumer, J, 3rd., Harrison, R, Sanders, B, et al. Postradiation dental extractions: a review of the literature and a report of 72 episodes. Head Neck Surg. 1983;6:581.
46. Wilson, J, Rees, JS. The dental treatment needs and oral side effects of patients undergoing outpatient cancer chemotherapy. Eur J Prosthodont Restor Dent. 2005;13:129–134.
47. Greenberg, MS, Cohen, SG, McKitrick, JC, et al. The oral flora as a source of septicemia in patients with acute leukemia. Oral Surg Oral Med Oral Pathol. 1982;53:32.
48. McElroy, TH. Infection in the patient receiving chemotherapy: oral considerations. J Am Dent Assoc. 1984;109:454.
49. Epstein, JB. Antifungal therapy in oropharyngeal mycotic infections. Oral Surg Oral Med Oral Pathol. 1990;69:32.
50. Heimdahl, A, Nord, CE. Oral yeast infections in immunocompromised and seriously diseased patients. Acta Odontol Scand. 1990;48:77.
51. Odds, FC, Kibbler, CC, Walker, E, et al. Carriage of Candida species and C. albicans biotypes in patients undergoing chemotherapy or bone marrow transplantation for haematological disease. J Clin Pathol. 1989;42:1259.
52. DePaola, LG, Peterson, DE, Overholser, CD, Jr., et al. Dental care for patients receiving chemotherapy. J Am Dent Assoc. 1986;112:198.
53. Wright, WE, Haller, JM, Harlow, SA, et al. An oral disease prevention program for patients receiving radiation and chemotherapy. J Am Dent Assoc. 1985;110:43.
54. Thurmond, JM, Brown, AT, Sims, RE, et al. Oral Candida albicans in bone marrow transplant patients given chlorhexidine rinses: occurrence and susceptibilities to the agent. Oral Surg Oral Med Oral Pathol. 1991;72:291.
55. Migliorati, CA, Casiglia, J, Epstein, J, et al. Managing the care of patients with bisphosphonate-associated osteonecrosis: an American Academy of Oral Medicine position paper. J Am Dent Assoc. 2005;136:1658.
56. Ruggiero, SL, Mehrotra, B, Rosenberg, TJ, et al. Osteonecrosis of the jaws associated with the use of bisphosphonates: a review of 63 cases. J Oral Maxillofac Surg. 2004;62:527–534.
57. Watts, NB. Treatment of osteoporosis with bisphosphonates. Endocrinol Metab Clin North Am. 1998;27:419–439.
58. Rogers, MJ, Watts, DJ, Russell, RG. Overview of bisphosphonates. Cancer. 1997;80(suppl 8):1652–1660.
59. Licata, AA. Discovery, clinical development, and therapeutic uses of bisphosphonates. Ann Pharmacother. 2005;39:668–677.
60. Ensrud, KE, Barrett-Connor, EL, Schwartz, A, et al. Randomized trial of effect of alendronate continuation versus discontinuation in women with low BMD: results from the Fracture Intervention Trial long-term extension. J Bone Miner Res. 2004;19:1259–1269.
61. Odvina, CV, Zerwekh, JE, Rao, DS, et al. Severely suppressed bone turnover: a potential complication of alendronate therapy. J Clin Endocrinol Metab. 2005;90:1294–1301.
62. Wood, J, Bonjean, K, Ruetz, S, et al. Novel antiangiogenic effects of the bisphosphonate compound zoledronic acid. J Phamacol Exp Ther. 2002;302(3):1055–1061.
63. Fournier, P, Boissier, S, Filleur, S, et al. Bisphosphonates inhibit angiogenesis in vitro and testosterone-stimulated vascular regrowth in the ventral prostate in castrated rats. Cancer Res. 2002;62:6538–6544.
64. Russell, RG, Rogers, MJ, Frith, JC, et al. The pharmacology of bisphosphonates and new insights into their mechanisms of action. J Bone Miner Res. 1999;14(suppl 2):53–65.
65. Fleisch, H. Development of bisphosphonates. Breast Cancer Res. 2002;4(1):30–34.
66. Sietsema, WK, Ebetino, FH, Salvagno, AM, et al. Antiresorptive dose-dependent relationship across three generations of bisphosphonates. Drugs Exp Clin Res. 1989;15:389–396.
67. Ott, SM. Long-term safety of bisphosphonates. J Clin Endocrinol Metab. 2005;90:1897–1899.
68. Whyte, MP, Wenkert, D, Clements, KL, et al. Bisphosphonate-induced osteopetrosis. N Engl J Med. 2003;349:457–463.
69. Marini, JC. Do bisphosphonates make children’s bones better or brittle? N Engl J Med. 2003;349:423–426.
70. Ruggiero, SL, Fantasia, J, Carlson, E. Bisphosphonate-related osteonecrosis of the jaw: background and guidelines for diagnosis, staging and management. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;102:433–441.
71. Marx, RE. Pamidronate (Aredia) and zoledronate (Zometa) induced avascular necrosis of the jaws: a growing epidemic. J Oral Maxillofac Surg. 2003;61:1115–1157.
72. Melo, MD, Obeid, G. Osteonecrosis of the jaws in patients with a history of receiving bisphosphonate therapy: strategies for prevention and early recognition. J Am Dent Assoc. 2005;136:1675–1681.
73. Migliorati, CA, Schubert, MM, Peterson, DE, et al. Bisphosphonate-associated osteonecrosis of mandibular and maxillary bone: an emerging oral complication of supportive cancer therapy. Cancer. 2005;104:83–93.
74. Marx, RE, Sawatari, Y, Fortin, M, et al. Bisphosphonate-induced exposed bone (osteonecrosis/osteopetrosis) of the jaws: risk factors, recognition, prevention and treatment. J Oral Maxillofac Surg. 2005;63:1567–1575.
75. Bagan, JV, Murillo, J, Jimenez, Y, et al. Avascular jaw osteonecrosis in association with cancer chemotherapy: series of 10 cases. J Oral Pathol Med. 2005;34:120–123.
76. Markiewicz, MR, Margarone, JE, Campbell, JH, et al. Bisphosphonate-associated osteonecrosis of the jaws: a review of current knowledge. J Am Dent Assoc. 2005;136:1669–1674.
77. Bagan, JV, Jimenez, Y, Murillo, J, et al. Jaw osteonecrosis associated with bisphosphonates: multiple exposed areas and its relationship to teeth extractions: study of 20 cases. Oral Oncol. 2006;42:327–329.
78. Sachs, HC. One year post exclusivity adverse event review: alendronate. Center for Drug Evaluation and Research, Food and Drug Administration. http://www.fda.gov/ohrms/dockets/ac/04/slides/2004-4067s1_07_Sachs%202%20Final.pdf. [Accessed August 25, 2006.].
79. Jeffcoat, MK. Safety of oral bisphosphonates: controlled studies on alveolar bone. Int J Oral Maxillofac Implants. 2006;21:349–353.