Tissue Engineering
“Tissue engineering” is a term coined at a meeting sponsored by the National Institutes of Health in 1987. This discipline is a rapidly developing multidisciplinary branch of science that combines many of the basic principles of biology, medicine, and engineering. Its primary goal is the restoration, maintenance, or enhancement of tissue and organ function.
In addition to having therapeutic applications, in which a particular organ is custom grown to replace a failing or missing body part, tissue engineering has as a goal diagnostic applications in which tissues are made in vitro and used for in vitro biocompatibility testing of compounds for such uses as drug metabolism and uptake, toxicity, or pathogenicity. Tissue engineering research, therefore, translates fundamental knowledge in physics, chemistry, and biology into materials, devices, and strategies. It also integrates biomaterials, cell biology, and stem cell research, engineering characteristics of threedimensional structures and mass transport issues, biomechanical characteristics of native and replacement tissues, and biomolecules and growth factors and includes the bioinformatics to support gene/protein expression and analysis.
Tissue engineering as a discipline grew out of the pressing need for replacement tissues and organs. During 2004, in the United States alone, over 26,984 solid organs (heart, lung, intestine, kidney, pancreas, and liver) were transplanted. Nearly three quarters of these organs came from deceased donors. Meanwhile, during this same period, 47,066 new registrations were added to the waiting list and about 6000 patients in anticipation of a transplant died waiting (Box 23-1). Clearly, the number of organs available for transplanting is greatly exceeded by the number of waiting patients. Much of the enthusiasm over tissue engineering derives from the promise to make transplants easier, and more commonplace. In dentistry, periodontal and oral and maxillofacial surgery often utilizes bone tissue transplant materials to address defects.
HISTORY
The earliest documented reports of successful human transplants were with skin. These records were described by the Indian physician, Sushruta, in the Sanskrit medical texts, the Sushruta Samhita. These works, dating from approximately 500 BC, describe the use of skin flaps harvested from a patient to replace the patient’s own nose, amputation of which was a common punishment for theft and adultery.
From there, little advancement was accomplished in transplantation until the late 1500s when Gaspare Tagliacozzi, considered by many as the father of plastic surgery, used skin flaps from the upper arm to perform reconstructive surgery. During the sixteenth century there was a great need for reconstructive surgery procedures because of lesions caused by the widespread effects of syphilis. Tagliacozzi tried grafting skin from “donors” as well as from the patients themselves. These donors often experienced serious complications from the transplants. Although these early attempts to transplant tissue from one region to another on a patient were frequently successful, there was virtually no success with person-to-person transplantation. The distinction drawn from these experiences helped to establish a classification scheme for the origin of transplanted tissues. Today, four primary classes of tissue/organ transplants are recognized: autograft, allograft, xenograft, and alloplast (Box 23-2).
AUTOGRAFT
An autograft is a tissue or organ that is moved from one location to another within a single individual (Fig. 23-1). It is quite common practice to transplant tissues such as hair, blood, and even limited amounts of skin and bone. These tissues regenerate to some extent and can fill the void left by their removal. This method of transplantation avoids complication from immunologic effects and is considered the “gold standard” for success.
ALLOGRAFT
Allografts are tissues or organs that are transplanted from one individual to another within the same species (Fig. 23-2). Routinely, tissues and organs are removed from deceased individuals (as well as living donors) and pressed into continued service within a different individual. Blood, bone, skin, corneas, ligaments, and tendons are collected in banks and frozen, for use in future surgical procedures.
XENOGRAFT
The Center for Biologics Evaluation and Research (CBER), the Food and Drug Administration (FDA) branch that oversees and regulates human tissue transplants, defines xenotransplants as “transplantation, implantation, or infusion into a human recipient of either (a) live cells, tissues, or organs from a nonhuman animal source or (b) human body fluids, cells, tissues or organs that have had ex vivo contact with live nonhuman animal cells, tissues, or organs.” This therapeutic regimen has been used experimentally to treat neurodegenerative disorders, liver failure, and diabetes, when compatible human materials are not widely available.
Xenografts are now common in dentistry where BioOss (a product derived from cow bone) and BioCoral (a corraline product) are used to augment human bone tissue (Fig. 23-3).
ALLOPLASTS
Alloplasts are the newest type of grafting procedure materials. These grafts are composed of all synthetic materials, making them quite different from the other three types of grafts, because no living component is being placed. Procedures in dentistry involving alloplast materials are becoming increasingly common. For example, the procedure of placing dental implants composed of metal and ceramic materials is considered mainstream restorative therapy in many countries. These materials integrate within the bone tissues and provide long-term service to the patient.
Bone grafting alloplasts are also used in abundance today (Fig. 23-4). Ceramic and bioactive glass materials are often used to augment autograft bone in the reconstruction of craniofacial structures. They provide a nearly unlimited quantity of material that has no adverse immunological reaction and does not pose the risk of disease transmission from one individual to another.
STRATEGIES FOR TISSUE ENGINEERING
Tissue engineering began with the concept of using biomaterials and cells to assist the body to heal itself. As the discipline has matured, it now seeks to develop logical strategies for optimizing new tissue formation through the judicious selection of conditions that will enhance the performance of tissue progenitors in a graft site, ultimately encouraging the production of a desired tissue or organ. Rather than relying on a single approach to accomplish this goal, there are several different strategies, which have evolved to develop new organs and tissues (Box 23-3).
INJECTION OF CELLS
This strategy developed directly from its traditional tissue transplantation heritage. In essence, this approach seeks to inject disaggregated cells at an undifferentiated stage of development into the recipient. This is an area of current intensity in the research to combat systemic diseases such as Alzheimer’s and Parkinson’s disease, as well as juvenile-onset diabetes and multiple sclerosis. It also holds potential for treating damaged nerve and muscle sites. Commonly referred to as stem cells, the injected cells are more appropriately termed undifferentiated or progenitor cells (see later discussion under “Stem Cells”). These cells are capable of forming new tissue with one or more phenotypes. As a therapeutic regimen, the cells are injected into the vicinity in which they are intended, and they migrate to the area of injury and begin to replicate and replace the lost tissue, or produce a desired compound such as insulin (Fig. 23-5). This strategy has already shown initial success at regenerating small areas of cartilage in temporomandibular joints.
GUIDED TISSUE REGENERATION
Guided tissue regeneration is a surgical procedure that attempts to regenerate lost structures by enhancing the opportunity for one cell type to populate a region and provides contact guidance to the developing cells. These cell types can then populate a region without competition, because other cell types are excluded from this area. This concept has had experimental successes in providing a biodegradable polymer conduit through which nerve cell regeneration and reconnection can occur. It is currently a commonplace procedure in periodontal practice to regenerate lost periodontal structures such as the bone, periodontal ligament, and connective tissue attachment that support the teeth. The procedure involves placement of a membrane under the mucosa and over the remaining bone (Fig. 23-6). The barrier helps to exclude the faster-growing epithelium and gingival connective tissues during the postsurgical healing phase, thereby allowing the slower-growing periodontal ligament and bone cells to migrate into the protected areas.
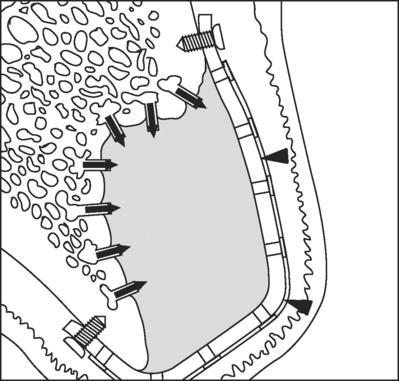
FIGURE 23-6 Guided bone regeneration, or GBR, is being used to isolate the site being regenerated from the overlying tissues by means of a titanium-reinforced membrane barrier (arrowheads). The newly forming bone may be augmented by the addition of particulate grafting material. The barrier is securely attached to the margins of the defect to prevent displacement during healing. Notice that decortication of the underlying bone bed allows invasion of the site by osteogenic precursor cells from the blood stream and surrounding bone.
CELL INDUCTION
There has been explosive growth in understanding the role of cytokines, developmental proteins, and growth factors in molecular biology during the last decade. Many of these growth factors are available as recombinant human lyophilized proteins. Tremendous advances have been accomplished by administering various mixtures of these growth factors directly to tissues in order to elicit a particular response from cells (i.e., to force them down a particular differentiation lineage). Often these growth and differentiation factors can be simply injected into the site. This technique, as a tissue-engineering regimen, targets local connective tissue progenitors already resident in the region where new tissues are desired and induces those cells to generate the desired tissue. Some of the injected proteins may serve as mitogens and recruit cells to migrate into the area, where they are acted upon by other growth factors to differentiate. Alternatively, they can be delivered to the site using a delivery substrate that releases them over time.
There is interest in using gene therapy in conjunction with this methodology. In this scenario, a gene sequence encoding for production of a particular growth factor or therapeutic compound is inserted wholly into the recipient cell’s genome. Insertion is accomplished using a carrier, called a vector, to deliver the therapeutic gene to the patient’s target cells. The most common vector is a genetically altered virus that has been modified to carry human DNA. Because viruses have evolved a way of encapsulating and delivering their genes to human cells, they can be manipulated to insert these therapeutic genes into the recipient cells. So, vectors are injected into a site along with an initial bolus of the therapeutic compound, and they upload their genes into resident cells. After incorporating this DNA into their genome, the newly transfected cells begin to replicate the desired growth factor endogenously. This method allows for continuous protein production at the site long after the initially injected growth factors have diffused from their target tissues or been degraded enzymatically.
CELLS WITHIN SCAFFOLD MATRICES
Another strategy involves the development of three-dimensional porous scaffolds. These may be used in conjunction with cells to provide many of the advantages of previously mentioned strategies. Usually these pre-formed scaffolds are composed of bioresorbable materials and they promote new tissue formation by providing a surface and void volume that encourages attachment, migration, proliferation, and desired differentiation of connective tissue progenitors throughout the region where new tissue is required. Typically, the scaffold is seeded with progenitor cells and they are allowed to attach and proliferate in vitro. Often, the cell constructs are grown in a nutrient media supplemented with growth factors necessary for cell and tissue development. At this stage, a mechanical load (either static or dynamic) may be applied to the construct in vitro, the effect of which is to align the cells in response to that challenge. The aligned cells will tend to produce a highly organized extracellular matrix, which will in turn result in improved tissue structure and function. After a suitable period of time in vitro, the entire construct is then implanted in vivo, where the tissue must continue to develop while forming a connection with the existing vascular system. As the tissues develop, the scaffold gradually degrades until it is completely replaced by the new tissues. During this degradation of the scaffold, the developing tissues begin to experience higher fractions of any applied loads on the tissue, and must respond to maintain their mechanical integrity.
Thus, the scaffold can serve a dual function, as both a rigid substrate for cell growth as well as a delivery vehicle for the release of therapeutic regulatory compounds in vivo. Local delivery of bioactive molecules functionalized to the scaffold surface or encapsulated within the matrix can change the function of connective tissue progenitor cells (activation, proliferation, migration, differentiation, or survival) in a manner that results in new or enhanced local tissue formation (Fig. 23-7).
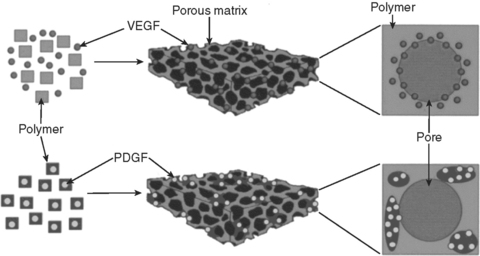
FIGURE 23-7 Scaffold systems may serve as delivery substrates for various therapeutic compounds, in addition to providing the cells with a suitable surface on which to grow. Multiple factors may even be delivered with different release profiles by varying the method in which they are incorporated into the substrate. In this example, VEGF is incorporated largely near the surface of the scaffold, and is subject to rapid release in vivo. In contrast, the pre-encapsulated PDGF is more uniformly incorporated throughout the scaffold and is subject to release regulated by the degradation of the matrix polymer. (From Richardson T, Peters M, Ennett A et al: Nature Biotech 19:1029, 2001.)
Critical variables in scaffold design and function include the bulk material or materials from which the scaffold is made, its three-dimensional architecture, its surface chemistry, the mechanical properties, and the physical, chemical, and biological environment in the area surrounding the scaffold during its functional lifetime (often determined by its degradation characteristics).
Design of the scaffold must also take into consideration knowledge that all cells require access to metabolic molecules (oxygen, glucose, and amino acids) and removal of cellular waste products (carbon dioxide, nitrogen compounds, and salts). Mass transport issues to cells within the interior of the matrix must be considered so that a balance between consumption and local delivery of these molecules is reached if cells are to survive. Eventually a rich blood supply will resolve this concern, but such a circulatory system will be absent during the initial period of implantation.
STEM CELLS
Patients who receive allogenous tissue and organ transplants must remain on immunosuppressive drugs for the balance of their lifetime to prevent rejection of the grafted tissue. Therefore, the ideal source for the cells used in the tissue engineering strategies previously mentioned is the patient. Using autograft tissue avoids the potential for adverse immunological reaction.
The type of cells used in tissue engineering therapies is a topic of current controversy. The generic name for these cells is stem cells. These cells are not terminally differentiated and are able to migrate within the body and to self-replicate. Additionally, they are able to produce progeny cells with multiple phenotypes. There are different types of stem cells within the body: pleuripotent cells can become any of the over 200 types of cells in the body, while multipotent cells are limited to forming specific tissues only. Fetal stem cells are pleuripotent, while adult stem cells are usually considered to be multipotent progenitor cells. For example, adult mesenchymal (or stromal) stem cells have been shown to be capable of transdifferentiation into neural tissue, cartilage, bone, and fat. These cells are found in small but significant numbers in bone marrow and also circulating in the blood stream.
In 2003, a new type of stem cell has been reported to be isolated from the pulp of normally lost deciduous teeth. These new cells have been termed SHED (stem cells from human exfoliated deciduous teeth). They appear to have greater proliferative capabilities than adult stem cells and also maintain the plasticity to produce the same range of progeny as mesenchymal stem cells (Fig. 23-8).
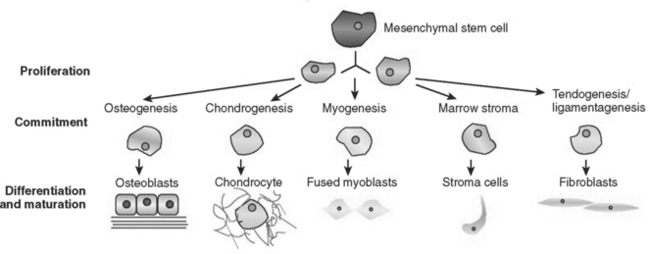
FIGURE 23-8 Differentiation pathways of mesenchymal cells. A circulating mesenchymal cell may become one of any of the cell types shown here. The pathway down which it travels is influenced by both local and systemic factors.
The ultimate cause of stem cell differentiation remains unclear. Left in a cell culture plate alone, several tissue types will result from a single group of mesenchymal stem cells. However, with suitably timed administration of the appropriate growth factors, a single cell type emerges and begins to coordinate into a tissue. It has also been observed that the fate of a cell is also controlled by changes in the migration, proliferation, differentiation, or survival of their progeny. Research is under way to investigate whether tactile stimulus or other factors upregulate or downregulate genes to initiate these changes. Also, transplanted stem cells may fuse with existing cells in a body and assume that tissue chrematistics.
BIOMATERIALS AND SCAFFOLDS
To date, research into scaffolds and carrier systems has focused on the use of three types of biomaterials: natural (or biologic) materials, ceramic or glass materials, and polymeric materials. No one material is optimal for all tissues and biomechanical circumstances. Each type has advantages and disadvantages that depend on the particular location and tissues that are being regenerated. However, several fundamental principles unite all the carriers. They must have no toxicity or immunogenicity, controlled biodegradability, amenability to surgical sterilization, and some mechanical performance in load-bearing situations, and they must be sufficiently porous to permit migration and growth of cells into their interior, along with the development of a new fractal circulatory system to promote the exchange of metabolic constituents.
BIOLOGICAL MATERIALS
Examples of natural materials that have been used as substrates include collagen, lyophilized bone (both allogenous and xenogenous), and coral. Collagen has been extensively tested as a scaffold for bone regeneration. In fact, the insoluble collagenous matrix, the inactive residue obtained after extraction of the bone matrix with various chemical agents, was one of the first materials used for bone tissue engineering. Along with freeze-dried bone, it was found to induce local endochondral bone formation in conjunction with growth factors in vivo. Coral, a calcium carbonate-based material, has a morphological structure strikingly similar to alveolar bone. It has historic use in orbital reconstruction. When treated with phosphoric acids, the resulting calcium phosphate material is very strong and biocompatible. However, considering the high potential for viral, prion, and disease transmission when implanting any natural materials in vivo, a nonbiological substrate is preferred by many patients.
CERAMIC AND GLASS MATERIALS
During the past three decades, research has firmly established that certain types of glasses, glassceramics, and pure ceramics can form an intimate bond with living bone tissue. Hydroxylapatite (HA), the major inorganic (ceramic) constituent of bone, was one of the first alloplastic materials proposed as a bone augmentation scaffold. Ceramic and glass-ceramic materials can provide some of the mechanical performance needed in these sites and are generally biocompatible. However, their long degradation time in vivo and lack of native porosity have limited their use as scaffolds. Recent investigations into nanoporous and nanoparticulate sol-gel synthesis methods for glasses and ceramics may overcome some of these limitations in the near future.
POLYMERIC MATERIALS
By far the most common materials from which to build tissue-engineering scaffolds are polymers. These substances include polylactic acid and polyglycolic acid (and co-polymers of these two) as well as polycaprolactone. These materials are metabolized in vivo, and their acidic degradation products are easily removed from the body. They can easily be cast into a mesh or other desired shape or can simply be extruded as fibers, which are used to loosely pack an anatomically designed mold. The same material from which the scaffold is designed can be used to encapsulate growth factors to provide a timed release of the protein as the capsule degrades. However, problems associated with polymers include inflammation around the implantation site due to the acidic and toxic degradation products, difficulty in precisely controlling the time of survival in the body, and stiffness of the material during degradation
CELL CULTURE METHODS
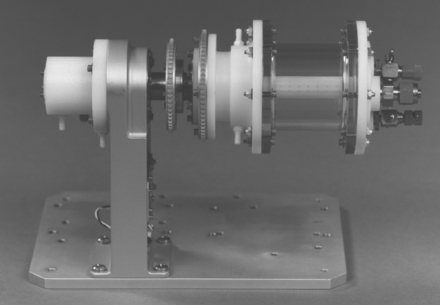
Traditional cell culture methods have been developed to grow a monolayer (or sheet) of cells on a polystyrene growth plate that has been treated to optimize cell attachment and proliferation. Culturing cells on three-dimensional scaffolds for subsequent implantation presents several challenges to these methods. In vitro tissue-engineering constructs thicker than 1 mm often result in a shell of viable cells and new extracellular matrix surrounding a necrotic core. In order to provide for mass transport throughout cell-seeded constructs, some type of perfusion bioreactor system (Fig. 23-9) must be employed to more closely mimic the in vivo environment. Alternatively, the tissues may be developed in vivo, in a location that is well vascularized. This allows a circulatory system to develop along with the cells.
Culture methods traditionally have included incubation in an oxygen-rich environment. Unless a reduced oxygen environment is used in the bioreactor, cell shock will occur when the culture is implanted in vivo, because the oxygen tension is dramatically reduced, which typically results in cell death.
In addition to adequate scaffold perfusion and appropriate oxygen levels, the optimal culture conditions for tissue engineering scaffolds also include high seeding efficiency to minimize growth time and a homogeneous cell distribution in the scaffolds to ensure a uniform, organized tissue. This tissue organization is further enhanced if some mechanical load is introduced to the developing cells as they are beginning to embed themselves within their extracellular matrix. Natural tissues are constantly undergoing physiological loading during development and use, and providing some mechanical challenge to the cells in vitro begins to prepare them for implantation. A mechanical challenge results in an alignment of the cells and a stronger, more organized matrix, which in turn results in improved tissue structure and function.
TISSUE-ENGINEERED DENTAL TISSUES
Although the creation of an entire new dentition from dental tissue engineered methods has not been accomplished in the laboratory, there is a great amount of work being done to regenerate tooth and supporting structures. The FDA has already approved two living skin tissue engineered products and these are commercially available. It is probable that tissue engineered bone and cartilage will soon follow.
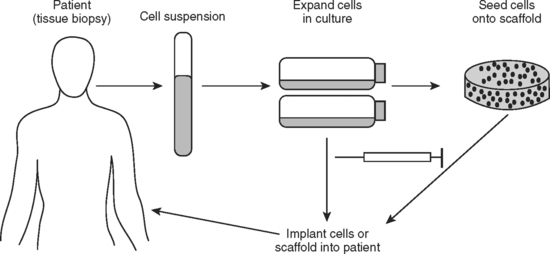
Periodontal ligament (PDL) engineering has been approached from two different strategies. The first entails harvesting existing PDL cells from the recipient/patient. This patient’s cells are grown and expanded in vitro (Fig. 23-10). They are cultured as a monolayer without any substrate. Once the layer is confluent and forms tight intercellular junctions, the sheet of cells is released from the culture plate and placed in vivo on the tooth surface to repair the periodontal defect.
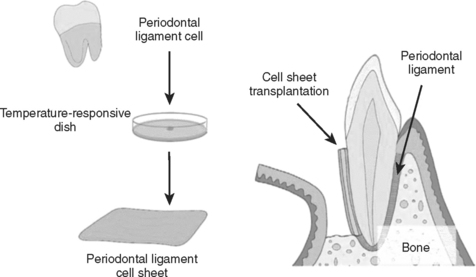
FIGURE 23-10 One method of periodontal ligament engineering under development is depicted here. Periodontal cells are extracted from a patient’s tooth and grown on temperature responsive culture plates in vitro. Upon lowering the temperature on these cultures, the confluent layer of cells spontaneously lifts off of the plate as a sheet of cells with intact cell junctions. These sheets are then implanted with various treatments in order to attempt regeneration of the periodontal tissues. (From Yamato M, Okano T: Mater Today 7:42, 2004.)
The second approach is a cell and scaffold method in which cells are again harvested from the PDL of the intended recipient. However, in this approach, they are seeded onto a three-dimensional polymer matrix. This is grown in vitro and eventually implanted back into a periodontal defect site in the original tissue donor (Fig. 23-11).
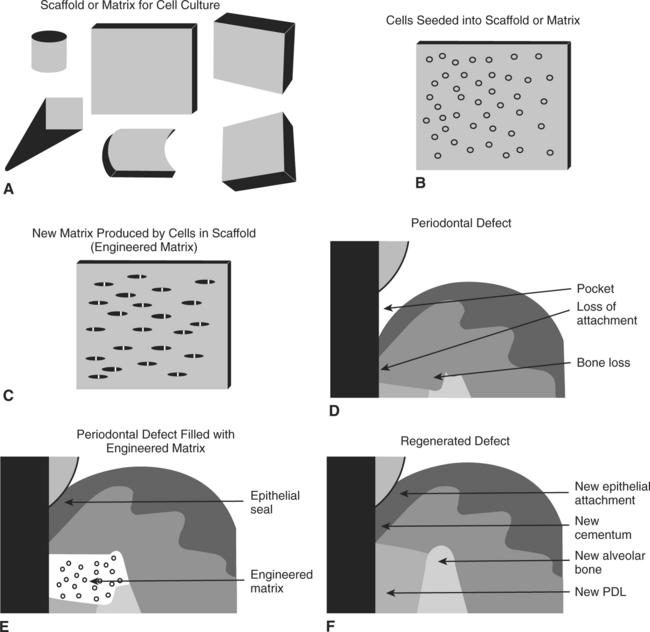
FIGURE 23-11 A more classical approach to periodontal tissue regeneration being developed. A, The support scaffold is developed and shaped to the desired geometry. B, In this method, cells are again harvested from the patient’s periodontal ligament and seeded into a scaffold, where they are expanded in vitro. C, The cells are allowed to grow for a time to allow synthesis of an appropriate matrix for implantation. D, A periodontal defect is shown. E, The tissue-engineered matrix is implanted into the defect site, leading to a regenerative response. F, In this method the fully regenerated periodontal defect is expected to be indistinguishable from other sites in the patient’s mouth. (From Bartold PM, McCulloch CA, Narayanan AS et al: Periodontology 24:253, 2000.)
Another oral tissue with a great deal of current research is a tissue-engineered salivary gland, which is also being attempted by the methodology of cells on a scaffold. The technique uses human parotid cells grown in vitro to develop an orally implantable, fluid-secreting tissue.
Finally, several groups announced in 2004 that they succeeded in growing primitive teeth in a tissue-engineered manner. They created a tooth-shaped porous scaffold of bioresorbable polymer and seeded it with individual cells taken from a tooth bud. This cell-seeded construct was implanted in vivo into the omentum of a rat to provide for fluid/nutrient transfer during growth and development. The cells used were a mixture of cell types from the bud initially. However, they migrated to the appropriate region during growth, and differentiated to form pulp tissue, dentin, and enamel in the correct anatomical relationships and ratios. Although these initial experiments have only produced teeth that were about 2 mm wide, they have shown that the concept will work to create entire teeth de novo.
Abukawa, H, Terai, H, Hannouche, D, et al. Formation of a mandibular condyle in vitro by tissue engineering. J Oral Maxillofac Surg. 2003;61:94.
Aframian, DJ, David, R, Ben-Bassat, H, et al. Characterization of murine autologous salivary gland graft cells: a model for use with an artificial salivary gland. Tissue Eng. 2004;10:914.
Agrawal, CM, Ray, RB. Biodegradable polymeric scaffolds for musculoskeletal tissue engineering. J Biomed Mater Res. 2001;55:141.
Almany, L, Seliktar, D. Biosynthetic hydrogel scaffolds made from fibrinogen and polyethylene glycol for 3D cell cultures. Biomaterials. 2005;26:2467.
Al-Salihi, KA, Samsudin, AR. Bone marrow mesenchymal stem cells differentiation and proliferation on the surface of coral implant. Med J Malaysia. 2004;59:45.
Alsberg, E, Hill, EE, Mooney, DJ. Craniofacial tissue engineering. Crit Rev Oral Biol Med. 2001;12:64.
Altman, GH, Diaz, F, Jakuba, C, et al. Silk-based biomaterials. Biomaterials. 2003;24:401.
Angelini, L, Eleuteri, E, Coppola, M. Surgery in Italy. Arch Surg. 2001;136:1318.
Anusaksathien, O, Giannobile, WV. Growth factor delivery to re-engineer periodontal tissues. Curr Pharm Biotechnol. 2002;3:129.
Auger, FA, Berthod, F, Moulin, V, et al. Tissue-engineered skin substitutes: from in vitro constructs to in vivo applications. Biotechnol Appl Biochem. 2004;39:263.
Badylak, SF. Xenogeneic extracellular matrix as a scaffold for tissue reconstruction. Transpl Immunol. 2004;12:367.
Bartold, PM, McCulloch, CA, Narayanan, AS, et al. Tissue engineering: a new paradigm for periodontal regeneration based on molecular and cell biology. Periodontology. 2000;24:253.
Baum, BJ, Mooney, DJ. The impact of tissue engineering on dentistry. J Am Dent Assoc. 2000;131:309.
Baum, BJ. Prospects for re-engineering salivary glands. Adv Dent Res. 2000;14:84.
Beele, H. Artificial skin: past, present and future. Int J Artif Organs. 2002;25:163.
Bhishagratna, KL. An English translation of the Sushruta Samhita. Varanasi, India: Chowkhamba Sanskrit Series Office, 1963;352–356.
Blum, JS, Barry, MA, Mikos, AG. Bone regeneration through transplantation of genetically modified cells. Clin Plast Surg. 2003;30:611.
Bohl, KS, Shon, J, Rutherford, B, et al. Role of synthetic extracellular matrix in development of engineered dental pulp. J Biomater Sci Polym Ed. 1998;9:749.
Bonassar, LJ, Vacanti, CA. Tissue engineering: the first decade and beyond. J Cell Biochem Suppl. 1998;30:297.
Buckley, MJ, Agarwal, S, Gassner, R. Tissue engineering and dentistry. Clin Plast Surg. 1999;26:657.
Chai, Y, Slavkin, HC. Prospects for tooth regeneration in the 21st century: a perspective. Microsc Res Tech. 2003;60:469.
Dard, M, Sewing, A, Meyer, J, et al. Tools for tissue engineering of mineralized oral structures. Clin Oral Investig. 2000;4:126.
Deporter, D. Surgical site development in the partially edentulous patient. In: Zarb G, Lekholm U, Albrektsson T, Tenenbaum H, eds. Aging, osteoporosis and dental implants. Carol Stream, IL: Quintessence Publishing Co, 2002.
Di Silvio, L, Gurav, N, Sambrook, R. The fundamentals of tissue engineering: new scaffolds. Med J Malaysia. 2004;59:89.
Duailibi, MT, Duailibi, SE, Young, CS, et al. Bioengineered teeth from cultured rat tooth bud cells. J Dent Res. 2004;83:523.
Earthman, JC, Sheets, CG, Paquette, JM, et al. Tissue engineering in dentistry. Clin Plast Surg. 2003;30:621.
Giannobile WV: What does the future hold for periodontal tissue engineering? 22:6, 2002.
Goldberg, M, Smith, AJ. Cells and extracellular matrices of dentin and pulp: A biological basis for repair and tissue engineering. Crit Rev Oral Biol Med. 2004;15:13.
Gosain, AK, Persing, JA. Biomaterials in the face: benefits and risks. J Craniofac Surg. 1999;10:404.
Gunatillake, PA, Adhikari, R. Biodegradable synthetic polymers for tissue engineering. Eur Cell Mater. 2003;20:1.
Hadlock, TA, Vacanti, JP, Cheney, ML. Tissue engineering in facial plastic and reconstructive surgery. Facial Plast Surg. 1998;14:197.
Hollister, SJ, Maddox, RD, Taboas, JM. Optimal design and fabrication of scaffolds to mimic tissue properties and satisfy biological constraints. Biomaterials. 2002;23:4095.
Hubbell, JA. Biomaterials in tissue engineering. Biotechnology. 1995;13:565.
Jin, Q, Anusaksathien, O, Webb, SA, et al. Engineering of tooth-supporting structures by delivery of PDGF gene therapy vectors. Mol Ther. 2004;9:519.
Jin, QM, Zhao, M, Webb, SA, et al. Cementum engineering with three-dimensional polymer scaffolds. J Biomed Mater Res A. 2003;67:54.
Kaigler, D, Mooney, D. Tissue engineering’s impact on dentistry. J Dent Educ. 2001;65:456.
Krebsbach, PH, Robey, PG. Dental and skeletal stem cells: potential cellular therapeutics for craniofacial regeneration. J Dent Educ. 2002;66:766.
Kuboki, Y, Sasaki, M, Saito, A, et al. Regeneration of periodontal ligament and cementum by BMP-applied tissue engineering. Eur J Oral Sci. 1998;106:197.
Lalan, S, Pomerantseva, I, Vacanti, JP. Tissue engineering and its potential impact on surgery. World J Surg. 2001;25:1458.
Langer, R. Tissue engineering. Mol Ther. 2001;1:12.
Lavik, E, Langer, R. Tissue engineering: current state and perspectives. Appl Microbiol Biotechnol. 2004;65:1.
Lee, KY, Mooney, DJ. Hydrogels for tissue engineering. Chem Rev. 2001;101:1869.
LeGeros, RZ. Properties of osteoconductive biomaterials: calcium phosphates. Clin Orthop. 2002;395:81.
Lenza, RF, Jones, JR, Vasconcelos, WL, et al. In vitro release kinetics of proteins from bioactive foams. J Biomed Mater Res A. 2003;1:121.
Letic-Gavrilovic, A, Todorovic, L, Abe, K. Oral tissue engineering of complex tooth structures on biodegradable DLPLG/beta-TCP scaffolds. Adv Exp Med Biol. 2004;553:267.
Miura, M, Gronthos, S, Zhao, M, et al. SHED: stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci USA. 2003;100:5807.
Murphy, WL, Mooney, DJ. Controlled delivery of inductive proteins, plasmid DNA and cells from tissue engineering matrices. J Periodontal Res. 1999;34:413.
Murray, PE, Garcia-Godoy, F. Stem cell responses in tooth regeneration. Stem Cells Dev. 2004;13:255.
Nakahara, T, Nakamura, T, Kobayashi, E, et al. In situ tissue engineering of periodontal tissues by seeding with periodontal ligament-derived cells. Tissue Eng. 2004;10:537.
Nakashima, M, Reddi, AH. The application of bone morphogenetic proteins to dental tissue engineering. Nat Biotechnol. 2003;21:1025.
Ohazama, A, Modino, SA, Miletich, I, et al. Stem-cell-based tissue engineering of murine teeth. J Dent Res. 2004;83:518.
Ohgushi, H, Miyake, J, Tateishi, T. Mesenchymal stem cells and bioceramics: strategies to regenerate the skeleton. Novartis Found Symp. 2003;249:118.
Pradeep, AR, Karthikeyan, BV. Tissue engineering: prospect for regenerating periodontal tissues. Indian J Dent Res. 2003;14:224.
Ratner, D. Skin grafting from here to there. Dermatol Clin. 1998;16:75.
Ratner, BD. Replacing and renewing: synthetic materials, biomimetics, and tissue engineering in implant dentistry. J Dent Educ. 2001;65:1340.
Richardson, T, Peters, M, Ennett, A, et al. Polymeric system for dual growth factor delivery. Nature Biotech. 2001;19:1029.
Ripamonti, U, Reddi, AH. Tissue engineering, morphogenesis, and regeneration of the periodontal tissues by bone morphogenetic proteins. Crit Rev Oral Biol Med. 1997;8:154.
Salgado, AJ, Coutinho, OP, Reis, RL. Bone tissue engineering: state of the art and future trends. Macromol Biosci. 2004;9:743.
Saltzman, WM, Olbricht, WL. Building drug delivery into tissue engineering. Nat Rev Drug Discov. 2002;1:177.
Schmelzeisen, R, Schimming, R, Sittinger, M. Soft tissue and hard tissue engineering in oral and maxillofacial surgery. Ann R Australas Coll Dent Surg. 2002;16:50.
Shin, H, Jo, S, Mikos, AG. Biomimetic materials for tissue engineering. Biomaterials. 2003;24:4353.
Smith, AJ. Tooth tissue engineering and regeneration—a translational vision. J Dent Res. 2004;83:517.
Stock, UA, Vacanti, JP. Tissue engineering: current state and prospects. Annu Rev Med. 2001;52:443.
Thesleff, I, Tummers, M. Stem cells and tissue engineering: prospects for regenerating tissues in dental practice. Med Princ Pract. 2003;12:43.
Ueda, M, Tohnai, I, Nakai, H. Tissue engineering research in oral implant surgery. Artif Organs. 2001;25:164.
Vacanti, JP, Langer, R. Tissue engineering: the design and fabrication of living replacement devices for surgical reconstruction and transplantation. Lancet. 1999;354:SI32.
Vacanti, CA, Vacanti, JP. The science of tissue engineering. Orthop Clin North Am. 2000;31:351.
Vunjak-Novakovic, G. The fundamentals of tissue engineering: scaffolds and bioreactors. Novartis Found Symp. 2003;249:34.
Whitaker, MJ, Quirk, RA, Howdle, SM, et al. Growth factor release from tissue engineering scaffolds. J Pharm Pharmacol. 2001;53:1427.
Yamato, M, Okano, T. Cell sheet engineering. Mater Today. 2004;7:42.
Yannas, IV. Synthesis of tissues and organs. Chembiochem. 2004;5:26.
Young, CS, Terada, S, Vacanti, JP, et al. Tissue engineering of complex tooth structures on biodegradable polymer scaffolds. J Dent Res. 2002;81:695.
Zhao, M, Jin, Q, Berry, JE, et al. Cementoblast delivery for periodontal tissue engineering. J Periodontol. 2004;75:154.
Organ Procurement and Transplantation Network: http://www.OPTN.org
Center for Biologics Evaluation and Research (CBER): http://www.fda.gov/cber/tiss.htm