Urinary tract infections
Introduction
Urinary tract infections are a common problem in surgery. They may be responsible for urinary tract symptoms presenting to a clinician for diagnosis or for abdominal pain that is not obviously urological. More often, urinary tract infections are a secondary problem. They can occur after operation, particularly if a urinary catheter has been employed, or may complicate surgical disorders of the urinary tract such as tumours or stones. Most infections are caused by common bacteria of faecal origin.
Urinary tract infections may also be caused by unusual organisms, in particular Mycobacterium tuberculosis. On a worldwide basis, other organisms are more important causes of infection, notably the trematode Schistosoma. One variety causes severe bladder disease in some developing countries.
Urethral infections are usually transmitted by sexual intercourse. Gonococci and Chlamydia are most commonly involved. A late result of gonorrhoea in males may be a fibrous urethral stricture. Urethral strictures are covered in this chapter, although most are traumatic and not infective in origin.
Bacterial infections of the lower urinary tract
Pathophysiology of lower urinary tract infections
Common urinary tract infections caused by faecal organisms involve the bladder, the upper tract (kidney, pelvicalyceal system and ureter) or both. The bladder is infected most often, with females being particularly susceptible. Probably half of all females are affected at some time. Infection rate rises with pregnancy and with increasing age. In females, the infecting organisms probably enter via the urethra, which is only 3 cm long. Organisms easily spread from perineal skin, particularly during sexual intercourse.
Normally, the bladder is flushed clean by the frequent passage of newly produced urine, preventing multiplication of bacteria. Stasis—such as incomplete bladder emptying, dehydration or immobility—interferes with this mechanism and predisposes to infection. Urethral instrumentation greatly predisposes to infection in either gender.
Clinical features of lower urinary tract infections
Typical symptoms of bladder infection are dysuria, frequency, urgency and a sensation of incomplete bladder emptying. The term ‘cystitis’ is often used by patients to mean symptoms in this list; however, infection is not always involved and the term is best avoided. Even when infection is present, symptoms may be trivial or absent, making diagnosis difficult. Abdominal pain may be the only symptom so most patients with abdominal pain should have urine tested as a matter of course.
There are often no localising symptoms in the elderly or the very young, and the patient may be non-specifically unwell. In any ill patient in these age groups, urine must be sent for examination before antibiotics are given. Recurrent fever in a child can result from urinary infection. A sudden onset of enuresis or urinary incontinence in children or the elderly should also suggest bladder infection. Presentations of bladder infection are summarised in Box 38.1.
Bacteriological diagnosis of lower urinary tract infections
Urinary tract infection is confirmed by examining a ‘midstream’ specimen of urine (MSU). If the specimen cannot be examined quickly, it should be refrigerated or it rapidly loses its diagnostic value. The specimen is examined microscopically for white blood cells (‘pus cells’) and bacteria, and cultured to identify the organism and determine antibiotic sensitivity. Bacterial contamination is common; a ‘significant’ infection is therefore defined as one with abundant pus cells (more than 100 000 (105) organisms per ml). Enteric organisms are almost always responsible, the usual culprits being Escherichia coli, Proteus spp., Enterococcus faecalis and Pseudomonas (the last in debilitated or catheterised patients). Staphylococcus saprophyticus is an important cause of uncomplicated bladder infection in young sexually active females.
Significant pus cells without bacterial growth most often result from patients taking antibiotics. If not, a stone, tumour, prostatitis or tuberculosis must be suspected and investigated. Infection often causes frank or occult haematuria but only warrants investigation if it persists after treating the infection. It must be noted that up to 10% of patients with infection have an underlying bladder cancer.
Some females experience symptoms typical of urinary infection but no evidence of bacterial infection of urine is found despite multiple MSUs. Non-specific urethral inflammation from the trauma of intercourse may be responsible (the ‘urethral syndrome’).
Management of bladder infections
Antibiotic therapy is the treatment for bladder infection, chosen on a ‘best-guess’ basis if treatment is urgent and changed if necessary. Treatment should be commenced only after an MSU specimen; this should be repeated if antibiotic treatment is ineffective or in complicated cases.
Patients who have had urinary tract infections should be encouraged to increase fluid intake. This is often effective with early or mild symptoms and probably allows mild infections to resolve without drugs.
In pregnancy, ureters and renal pelvis dilate under the effect of progestogens and become more susceptible to infection. Where bladder infection is suspected, significant bacterial growth should be treated with appropriate antibiotics, whether or not the patient is symptomatic. This is because of the risk of infection ascending to upper tracts and subsequent miscarriage. The antibiotic must be safe for use in pregnancy and non-teratogenic, e.g. a cephalosporin. Trimethoprim is also safe but may deplete folate. Standard texts such as the British National Formulary should be consulted on prescribing in pregnancy.
Recurrent bladder infections
Patients likely to suffer recurrent infections tend to fall into three groups:
• The elderly, debilitated and infirm
• Patients with urinary tract abnormalities predisposing to infection
The elderly, debilitated and infirm
These patients often have multiple simple predisposing factors such as constipation, incontinence, an indwelling catheter, poor fluid intake and diminished resistance. Correction of these problems and good nursing care (e.g. regular changes of indwelling catheter) may break the pattern. Antibiotic-resistant bacteria are often a problem because of previous courses of antibiotics.
Young and middle-aged women
Many can be helped by simple hygiene measures. These include ‘wiping from front to back’ after micturition or defaecation, frequent and complete emptying of the bladder, increasing urine flow by raising fluid intake, and emptying the bladder soon after intercourse.
Patients with urinary tract abnormalities predisposing to infection
Abnormalities should be suspected in children or young men with a single episode of urinary infection if they fail to respond to simple measures, and anyone with recurrent urinary infections. These patients may have a structural abnormality or pathological condition which encourages bacterial proliferation and require investigation. Bladder stone or prostatic hypertrophy is commonly responsible and predisposing conditions can often be corrected surgically. In the rest no predisposing factor can be found. Some patients with recurrent infections may need long-term, low-dose antibiotics to prevent recurrence.
Upper urinary tract infections
Pathophysiology of upper urinary tract infections
Infections of the pelvicalyceal system and renal parenchyma (acute pyelonephritis) arise by upward extension of a lower tract infection or via the bloodstream (haematogenous). Ascending infections are most common when there is an abnormality causing ureteric reflux or stasis such as ureteric obstruction, abnormal peristalsis (as in megaureter) and congenital incompetence of the cysto-ureteric antireflux mechanism. During pregnancy, ureters dilate under hormonal influences and this may increase the incidence of upper urinary tract infections.
With upper tract stasis, infection is often haematogenous. Common causes are stones in the renal pelvis or pelviureteric junction (PUJ) obstruction. In these, lower tract infection is a secondary phenomenon. Factors initiating renal infections are often unknown but pre-existing renal damage is a strong predisposing factor.
Pathological examination of an acutely infected kidney shows extensive neutrophilic infiltration of renal parenchyma, often with small abscesses. Usually only one kidney is involved and the causative organisms originate in the GI tract as in other urinary tract infections.
Clinical features of upper urinary tract infections
The classic clinical features of acute pyelonephritis are unilateral loin pain and tenderness (see Box 38.2). The patient is generally unwell with systemic features of infection, i.e. pyrexia and tachycardia. The urine is usually cloudy and there may be typical symptoms of bladder infection. Often the symptoms and signs are less specific, with unilateral abdominal pain or discomfort that may be mistaken for early acute appendicitis unless the urine is examined. Pyelonephritis may present without localising signs, especially in infants and the elderly, who may be more unwell and even develop signs of systemic sepsis.
Management of upper urinary tract infections
Diagnosis is based on clinical symptoms, signs and urine examination. Blood is also taken for culture when there are systemic signs of infection. Treatment is with antibiotics, initially on a ‘best-guess’ basis, based on local microbiological advice. Dosage and route of administration depend on the severity of the illness; severe cases are treated with intravenous antibiotics.
Once the acute illness has been treated, further investigation may be indicated to search for predisposing factors. This is usually ultrasonography, CT, sometimes intravenous urography, and flexible cystoscopy. In children, investigation should include a contrast micturating cystogram or equivalent radionuclide scintigram to identify ureteric reflux (see Ch. 51).
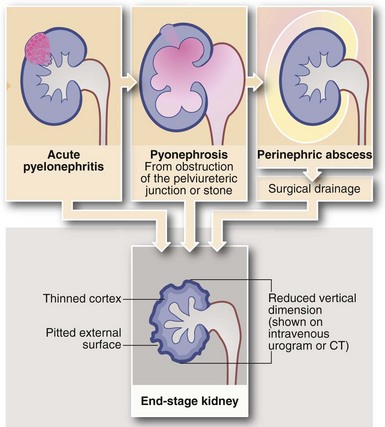
Complications of acute pyelonephritis (see Figs 38.1 and 38.2)
Severe infections may be complicated by pelviureteric outlet obstruction, resulting in accumulation of pus in the renal pelvis. If untreated, this destroys the renal parenchyma. Treatment involves surgical or percutaneous drainage followed by correction of the obstruction.
Perinephric abscess
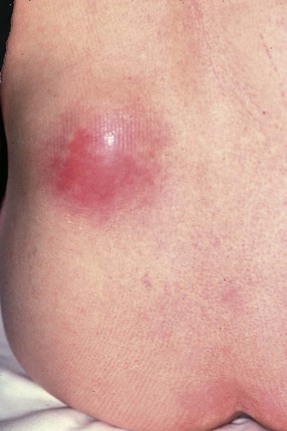
In severe infections, sometimes in the presence of a large ‘staghorn’ calculus, the accumulating pus may discharge through the renal capsule into surrounding fat, resulting in a perinephric abscess (Fig. 38.2). This presents as a slowly expanding loin mass, often with only low-grade local and systemic symptoms. The diagnosis should be considered in elderly patients with sepsis from an unknown source. Urine investigation will reveal pyuria, whilst ultrasound and radiology will show a non-functioning renal mass containing fluid-filled areas. A large renal calculus may also be seen. A perinephric abscess sometimes develops as a result of haematogenous infection of a traumatic perinephric haematoma. The treatment of perinephric abscess is drainage. If a perinephric inflammatory mass partially resolves, it can result in xanthomatous pyelonephritis, a solid mass often suspected of malignancy and hence removed surgically.
Genitourinary tuberculosis
Pathophysiology of genitourinary tuberculosis
About 4% of patients with tuberculosis have genitourinary involvement. Mycobacteria reach the kidney or epididymis via the bloodstream, causing typical centrally caseating granulomatous lesions which may later calcify. From the kidney, direct spread can occur to ureter (causing a fibrous stricture) or to bladder. Tuberculosis of the bladder usually begins around a ureteric opening and spreads to cause patchy ulceration of the bladder wall and later fibrotic contraction. Young adults are most commonly affected and there is a seriously increased incidence among patients with acquired immune deficiency syndrome (AIDS). These and patients ‘living rough’ are poorly compliant with treatment and provide a reservoir of infection for others.
Clinical features and investigation of genitourinary tuberculosis
Urinary tract tuberculosis is often asymptomatic and is diagnosed during investigation of ‘sterile pyuria’, although painless urinary frequency, nocturia and haematuria are sometimes present. Systemic features may also be present, including weight loss, night sweats and respiratory symptoms if the lungs are affected.
When tuberculosis is suspected, at least three early morning urine (EMU) specimens must be sent to the laboratory to be stained and cultured for tubercle bacilli (also known as acid-fast bacilli, AFB). The entire volume of the first urine passed in the morning is collected and centrifuged to concentrate the small number of organisms. Culture usually takes 6–8 weeks but unfortunately a negative result does not exclude tuberculosis.
If there is a red patch around a ureteric orifice at cystoscopy, this can be biopsied and examined histologically for caseating granulomas and stained for tuberculosis organisms, thus accelerating the diagnostic process. Blood is tested for anaemia, lymphocytosis and elevation of the erythrocyte sedimentation rate (ESR), and for biochemical indicators of renal function. A chest X-ray is taken to search for pulmonary disease. Renal calcification may be seen on plain abdominal X-ray, while urography may show renal abnormalities or ureteric strictures.
Management of genitourinary tuberculosis
Drug therapy is the mainstay of treatment, as for pulmonary tuberculosis, with agents chosen according to the local prevalence of particular strains and the results of culture and sensitivities. Surgery may be required later to treat ureteric strictures or a contracted bladder, or to excise damaged kidney tissue (partial or total nephrectomy). For ureteric tuberculosis, corticosteroids are usually given along with antituberculous therapy to reduce the risk of stricture formation. Plasma urea and creatinine should be monitored during the usual 6 months of therapy and signs of upper tract dilatation sought with periodic ultrasound examinations.
Schistosomiasis
Schistosomiasis (bilharzia) is the most important parasitic disease of the urinary tract. It causes chronic inflammatory lesions in the bladder that lead to severe fibrotic damage. Schistosomiasis also predisposes to stone formation and squamous carcinoma of the bladder.
Three Schistosoma species, S. haematobium, S. mansoni and S. japonicum, have a wide tropical distribution and are important human pathogens. The most destructive bladder disease is caused by S. haematobium, which is endemic to tropical and North Africa (particularly the Nile valley), and is also in some Middle Eastern and southern European countries. Effective treatment with praziquantel has dramatically reduced the reservoir of infection and the incidence of cases in Egypt, but treatment is relatively expensive and has so far made little impact in Sudan. Increasing worldwide travel makes it likely that the disease will be seen more frequently in travellers.
The schistosome has a sophisticated life cycle that depends on poor sanitation. Humans (the main definitive host) are infected by working or bathing in contaminated water. The free-swimming adult forms (cercaria) penetrate the skin and pass through the venous circulation and lungs to the systemic arterial circulation, which disseminates them throughout the body. In the portal veins, male and female worms mate. The females, crammed with fertilised ova, find their way via mesenteric veins to the venous plexuses of the pelvic viscera, notably the bladder, where ova are released. Aided by lytic enzymes, the ova then pass through the bladder wall into the urine and thence to the external environment. They then complete their life cycle via an intermediate host, a freshwater snail.
Clinical presentations of schistosomiasis
Initial skin penetration may cause mild local inflammation. Soon afterwards, the phase of haematogenous spread may cause general malaise, low-grade pyrexia and eosinophilia. About 2 months later, ova invading the bladder mucosa cause local inflammation, and manifest as frequency, and haematuria at the end of micturition. The early symptoms may be trivial and may pass unnoticed.
The main bladder damage caused by schistosomiasis is due to an intense chronic inflammatory reaction to dead ova which have become sequestered in the urothelium. Granulomatous ‘pseudotubercles’ develop around each ovum and later become fibrotic and calcified. Heavy or recurrent infestations result in a variety of destructive lesions including ulcers, papillomata, cysts, giant granulomata and severe bladder contracture. All predispose to secondary bacterial infection and bladder stones. Squamous metaplasia is common and strongly predisposes to carcinoma: two-thirds of these are squamous and one-third transitional cell. The clinical features of urinary schistosomiasis are summarised in Box 38.3.
Management of schistosomiasis
Bladder or ureteric calcification is almost diagnostic of schistosomiasis. Diagnosis is confirmed by microscopy of urine for ova or more reliably by cystoscopic biopsy of bladder lesions. Serological tests may also be of value in travellers from the developed world without previous exposure.
Treatment is with the drug praziquantel given in two doses of 20 mg/kg body weight on 1 day, 6 hours apart. Metrifonate is an alternative treatment. Surgery is occasionally needed later to correct or palliate residual lower urinary tract deformities.
About 5% of the world's population is affected by schistosomiasis and prevention must be the cornerstone of disease control. Effective treatment of affected individuals substantially reduces the pool of infection and the number of new cases, but better sanitation and clean water supplies are essential. Ironically, the rapid expansion of water conservation and irrigation schemes has spread the disease to previously unaffected populations.
Urethral infections and strictures
The only clinically important urethral infections are sexually transmitted diseases. The most common are gonorrhoea and chlamydial infection. The acute condition usually presents with urethral discharge and dysuria. The surgical importance of urethritis, particularly gonococcal, is that it may lead, months or years later, to fibrous stricturing of the urethra. Fortunately, these strictures are becoming less common as effective antibiotic therapy is more available. However, in recent years multi-antibiotic-resistant strains have begun to emerge, particularly in the Far East.
Serological tests for gonorrhoea and Chlamydia are relatively unreliable and have largely been abandoned. The standard tests for diagnosis now involve molecular amplification (e.g. PCR) which can be done on urethral swabs and first-catch urine samples.
Urethral stricture
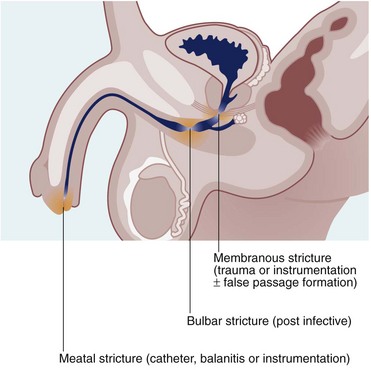
Urethral strictures are now most commonly caused by inflammation resulting from iatrogenic trauma. Transurethral resection for prostatic surgery is the most common cause. Urinary catheterisation in patients with poor tissue perfusion is another common cause, particularly during cardiopulmonary bypass for cardiac surgery. Iatrogenic strictures involve the distal urethra or the meatus. Strictures may also be a complication of traumatic instrumentation, where the membranous urethra is most vulnerable (see Fig. 38.3). A few strictures result from urethral tearing or rupture following displaced pelvic fractures. These usually require open surgical reconstruction.
The characteristic symptom of urethral stricture is a progressive decrease in urinary stream. If there is also chronic urinary retention, there may be frequency and urgency, symptomatic of bladder outlet obstruction. Diagnosis is made by direct inspection using a cystourethroscope or by urethrography.
Strictures may be short, elongated or multiple. An effective treatment involves stretching the scar tissue by repeated self-dilatation. An alternative with tight strictures is to cut the stricture longitudinally with a urethrotome under direct urethroscopic vision, and follow this by repeated dilatation. Recurrence after treatment is almost to be expected, so skilled urological follow-up is needed. More complicated strictures may require open surgical treatment: for example, excision of a short stricture and end-to-end anastomosis of the urethra. For some, inlays of oral mucosa or post-auricular skin may prove successful.
Urethral strictures cause lifelong disability, and most can be avoided by extreme care of the urethra during catheterisation and urethral instrumentation. As a general rule, the urethra should not be catheterised or interfered with unless absolutely essential. Sick patients should be catheterised only with a silicone-coated urethral catheter or else a suprapubic catheter employed. Any catheter or other urological instrument should be placed gently and with minimal force or else under direct vision.