Malignant Diseases of the Jaws
Definition
Malignant tumors represent an uncontrolled growth of tissue. Unlike benign neoplasms, they are more locally invasive, have a greater degree of cellular anaplasia, and have the ability to metastasize regionally to lymph nodes or distantly to other sites. Malignant tumors that arise de novo are termed primary tumors, and those that originate from distant primary tumors are termed secondary or metastatic malignancy. Cancers may be caused by viruses, significant radiation exposure, genetic defects, or exposure to carcinogenic chemicals. For instance, using tobacco is strongly associated with oral carcinoma.
The most convenient method of classification of cancers is on the basis of histopathologic characteristics. In the following text the malignancies that commonly affect the jaws have been divided into four categories: carcinomas (lesions of epithelial origin), metastatic lesions from distant sites, sarcomas (lesions of mesenchymal origin), and malignancies of the hematopoietic system. Of these four categories, carcinomas are by far the most commonly encountered in dental practice. Unusual malignant tumors have been omitted to concentrate on those lesions that a general practitioner may encounter.
Clinical Features
The following are clinical signs and symptoms that suggest that a lesion may be malignant: displaced teeth, loosened teeth over a short time, foul smell, ulceration, presence of an indurated or rolled border, exposure of underlying bone, sensory or motor neural deficits, lymphadenopathy, weight loss, dysgeusia, dysphagia, dysphonia, hemorrhage, lack of normal healing after oral surgery, and pain or rapid swelling with no demonstrable dental cause. Most oral cancers occur in men aged 50 years and older; however, malignant tumors may occur at any age in either sex.
Dentists must watch vigilantly for the possibility of malignancy in their patients. Because the prevalence of oral malignancy is low, many general practitioners practice years without encountering a patient with a malignant tumor. This rarity may make a dentist less likely to recognize a malignant condition when it is present. The risks of lack of attention to this possibility are delayed diagnosis, delayed treatment, increased need for aggressive treatment with added morbidity, and, in the worst case, premature death.
Radiographic Examination
Radiology has a number of important roles in the management of the patient with cancer. First, diagnostic images may aid in the establishment of an initial diagnosis of a tumor. Diagnostic imaging also aids in the appropriate staging of disease from early small cancers to large cancers that have spread. Appropriate radiologic investigations assist the surgeon or radiation oncologist to determine the anatomic spread of the tumor so that it can be excised or irradiated adequately. Radiologic investigation has the potential to determine the presence of osseous involvement from soft tissue tumors and allow the practitioner to assess the involvement of lymph nodes and treatment outcome. Finally, a thorough radiographic dental examination plays a part in the management of the cancer survivor, who often is rendered xerostomic, neutropenic, and susceptible to dental caries, periodontal disease, and systemic infection.
There are various diagnostic imaging modalities available to aid in the diagnosis. Intraoral radiographs still provide the best image resolution and are able to reveal subtle malignant changes such as irregular widening of the periodontal membrane space. Panoramic radiographs provide an overall assessment of the maxillofacial osseous structures and can reveal relevant changes such as destruction of the boundaries of the maxillary sinus. Either cone beam computed tomography (CT) or medical CT images can provide a superior three-dimensional analysis of the osseous structures and better determine the position and extent of the tumor. Positron emission tomographic (PET) scans, a technique capable of detecting abnormal cellular metabolic activity associated with malignant tumors, have been fused with CT images to provide an accurate location of the tumor in preparation for radiotherapy. Finally, magnetic resonance imaging (MRI) has provided three-dimensional soft tissue images of tumors and information regarding perineural spread and involvement of lymph nodes.
RADIOGRAPHIC FEATURES
The following features may suggest the presence of a malignant tumor. The absence of visible radiologic signs as described does not preclude malignancy. It only implies that no visible radiographic signs exist.
Location
Primary and metastatic malignant tumors may occur anywhere in the oral and maxillofacial region. Primary carcinomas are more commonly seen in the tongue, floor of mouth, tonsillar area, lip, soft palate, or gingiva and may invade the jaws from any of these sites. Sarcomas are more common in the mandible and in posterior regions of both jaws. Metastatic tumors are most common in the posterior mandible and maxilla. Some metastatic lesions grow at the apices of teeth or in the follicles of developing teeth (Fig. 23-1, D).
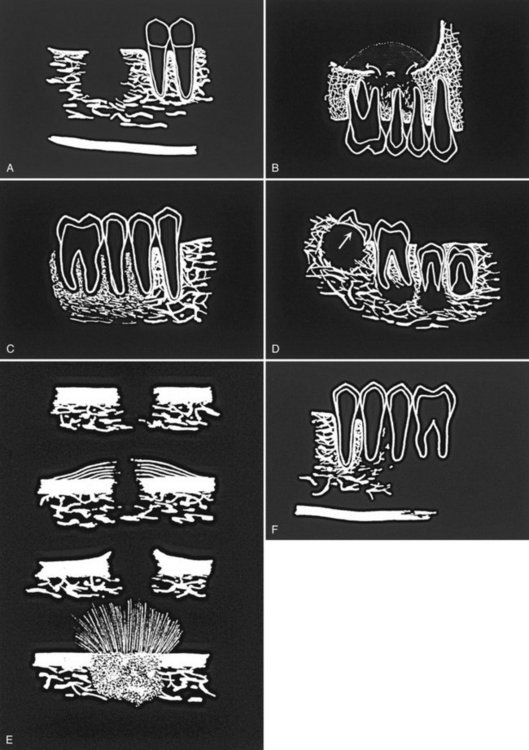
FIG. 23-1 Diagrammatic representation of radiologic features of oral malignancy. A, Ill-defined invasive borders followed by bone destruction. B, Destruction of the cortical boundary (floor of maxillary antrum) with an adjacent soft tissue mass (arrows). C, Tumor invasion along the periodontal membrane space causing irregular thickening of this space. D, Multifocal lesions located at root apices and in the papilla of a developing tooth destroying the crypt cortex and displacing the developing tooth in an occlusal direction (arrow). E, Four types of effects on cortical bone and periosteal reaction, from top to the bottom: cortical bone destruction without periosteal reaction, laminated periosteal reaction with destruction of the cortical bone and the new periosteal bone, destruction of cortical bone with periosteal reaction at the periphery forming Codman’s triangles, and a spiculated or sunray type of periosteal reaction. F, Bone destruction around existing teeth, producing an appearance of teeth floating in space.
Periphery and Shape
The typical appearance of the periphery (border) of a malignant lesion is an ill-defined border with lack of cortication and absence of encapsulation (a soft tissue or radiolucent periphery). This infiltrative border has uneven extensions of bone destruction. Fingerlike extension of the tumor occurs in many directions; this extension is followed by osseous destruction producing a region of radiolucency (Fig. 23-1, A). Evidence of destruction of a cortical boundary with adjacent soft tissue mass is highly suggestive of malignancy (Fig. 23-1, B). Such a mass may exhibit a smooth or ulcerated peripheral border if cast against a radiolucent background such as the air within the maxillary sinus. The shape of a malignant tumor of the jaw is commonly irregular.
Internal Structure
Because most malignancies do not produce bone nor do they stimulate the formation of reactive bone, the internal aspect is typically radiolucent in most instances. Occasionally residual islands of bone are present, resulting in a pattern of patchy destruction with some scattered residual internal osseous structure. Some tumors, such as metastatic prostate or breast lesions, can induce bone formation, resulting in an abnormal-appearing internal sclerotic osseous architecture, whereas others, such as osteogenic sarcomas, can produce abnormal bone, giving the involved bone a sclerotic (radiopaque) appearance.
Effects on Surrounding Structures
Malignancy is destructive, often rapidly so. The effect on surrounding structures mirrors this behavior. Slower-growing benign tumors or cysts may resorb tooth roots or displace teeth in a bodily fashion without causing loose teeth. In contrast, rapidly growing malignant lesions generally destroy supporting alveolar bone so that teeth may appear to be floating in space (Fig. 23-1, F).
Occasionally root resorption is present, more commonly in sarcomas. Internal trabecular bone is destroyed, as are cortical boundaries such as the sinus floor (Fig. 23-1, B), the inferior border of the mandible, the follicular cortices of developing teeth, and the cortex of the inferior alveolar neurovascular canal. Because malignant tumors tend to grow rapidly, they invade by means of the easiest routes, such as through the maxillary antrum or through the periodontal ligament space around teeth, resulting in irregular widening with destruction of the lamina dura (Fig. 23-1, C); they also may spread through the inferior alveolar neurovascular canal, causing similar widening. Where the tumor has destroyed the outer cortex of bone, usually no periosteal reaction occurs; however, some tumors stimulate unusual periosteal new bone formation (Fig. 23-1, E). Lesions such as osteosarcoma and metastatic prostate lesions and other tumors can stimulate the formation of thin straight spicules of bone, giving a hair-on-end or sunburst appearance. If there is a secondary inflammatory lesion coexisting with the malignancy, a periosteal reaction normally associated with an inflammatory lesion (e.g., “onion skin” like) may be seen.
Carcinomas
Definition
Squamous cell carcinoma, the most common oral malignancy, may be defined as a malignant tumor originating from surface epithelium. It is characterized initially by invasion of malignant epithelial cells into the underlying connective tissue with subsequent spread into deeper soft tissues and occasionally into adjacent bone, local-regional lymph nodes, and ultimately to distant sites such as the lung, liver, and skeleton.
Clinical Features
Squamous cell carcinoma appears initially as white or red (sometimes mixed) irregular patchy lesions of the affected epithelium. With time these lesions exhibit central ulceration; a rolled or indurated border, which represents invasion of malignant cells; and palpable infiltration into adjacent muscle or bone. Pain may be variable, and regional lymphadenopathy with hard lymph nodes that may or may not be tethered to underlying structures may be present. Other clinical features include a soft tissue mass, paresthesia, anesthesia, dysesthesia, pain, foul smell, trismus, grossly loosened teeth, or hemorrhage. Large lesions can obstruct the airway, the opening of the eustachian tube (leading to diminished hearing), or the nasopharynx. Patients often report a significant weight loss and feel unwell. Males are more commonly affected than females. The condition is often fatal, if untreated. Most squamous cell carcinomas occur in persons older than 50 years.
Radiographic Features
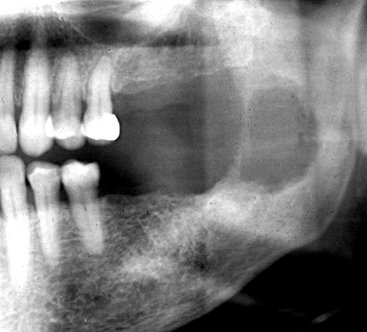
Location.: Squamous cell carcinoma commonly involves the lateral border of the tongue. Therefore a common site to observe bone invasion is the posterior lingual aspect of the mandible. Lesions of the lip and floor of the mouth may similarly invade the anterior mandible. Lesions involving attached gingiva and underlying alveolar bone may mimic inflammatory disease such as periodontal disease. This malignancy is also seen on the tonsils, soft palate, and buccal vestibule. It is uncommon on the hard palate.
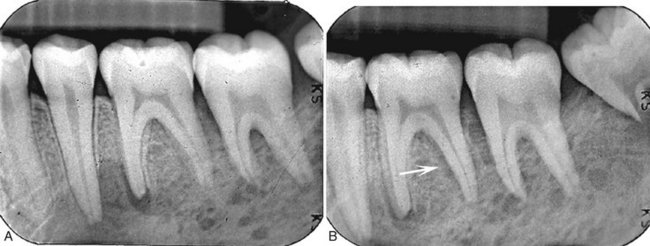
Periphery and Shape.: Squamous cell carcinoma may erode into underlying bone from any direction, producing a radiolucency that is polymorphous and irregular in outline. Invasion occurs in half of cases and is characterized most commonly by an ill-defined, noncorticated border (Fig. 23-2). Rarely, the border may appear smooth without a cortex, indicating underlying erosion rather than invasion. If bone involvement is extensive, the periphery appears to have fingerlike extensions preceding a zone of impressive osseous destruction. If pathologic fracture occurs, the borders show sharpened thinned bone ends with displacement of segments and an adjacent soft tissue mass. Sclerosis in underlying osseous structures (likely from secondary inflammatory disease) may be seen in association with erosions from surface carcinomas.
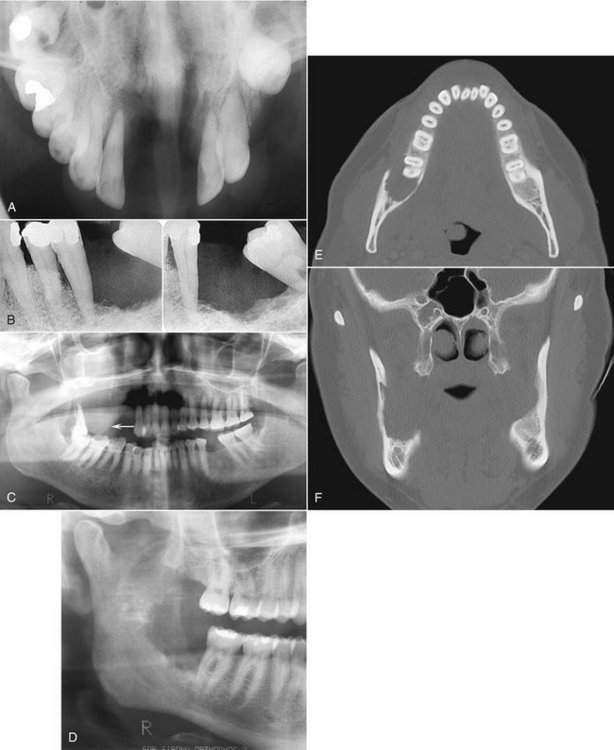
FIG. 23-2 A through F, Squamous cell carcinoma (arrows) resulting in irregular destruction of bone. In the occlusal film image (A) the anterior floor of the nasal fossa has been destroyed (note lack of anterior nasal spine). B, The supporting alveolar bone has been destroyed from around the teeth. C, There is destruction of the right alveolar process and floor of maxillary sinus and the soft tissue mass (arrow). D, Destruction of bone in the mandibular retromolar area by a squamous cell carcinoma. E and F, Axial and coronal CT images of the tumor displayed in D. Note destruction of lateral cortical plate in the axial image and medial cortical plate in the coronal image and lack of bone reaction at the margins of the tumor.
Internal Structure.: The internal structure of squamous cell carcinoma in jaw lesions is totally radiolucent; the original osseous structure can be completely lost. Occasionally small islands of residual normal trabecular bone are visible within this central radiolucency.
Effects on Surrounding Structures.: Evidence of invasion of bone around teeth may first appear as widening of the periodontal ligament space with loss of adjacent lamina dura. Teeth may appear to float in a mass of radiolucent soft tissue bereft of any bony support. In extensive tumors this soft tissue mass may grow with the teeth in it as “passengers,” so teeth are grossly displaced from their former position. Tumors may grow along the inferior neurovascular canal and through the mental foramen, resulting in an increase in the width and loss of the cortical boundary. Destruction of adjacent normal cortical boundaries such as the floor of the nose, maxillary sinus, or buccal or lingual mandibular plate may occur. The posterior aspect of the maxilla may also be effaced. The inferior border of the mandible may be thinned or destroyed. If the tumor is extensive, pathologic fracture may occur.
Differential Diagnosis
Squamous cell carcinoma is discernible from other malignancies by its clinical and histologic features. Occasionally it is difficult to differentiate inflammatory lesions such as osteomyelitis from squamous cell carcinoma, especially when oral bacteria secondarily infect the tumor. Both osteomyelitis and squamous cell carcinoma are destructive, leaving islands of osseous structure that may appear to be consistent with sequestra. Evidence of profound bone destruction or invasive characteristics helps to identify the presence of a malignancy when a mixture of inflammatory changes and carcinoma exists. Osteomyelitis usually produces some periosteal reaction, whereas squamous cell carcinoma does not. In cases of osteoradionecrosis, where the patient has had prior malignancy, periosteal new bone is absent. If osseous destruction is present, the differentiation of this condition from squamous cell carcinoma requires advanced imaging and biopsy. The bone loss from squamous cell carcinoma originating in the soft tissues of the alveolar process may appear very similar to that from periodontal disease (Fig. 23-3, A). Enlargement of a recent extraction socket instead of evidence of healing new bone formation can indicate the presence of an alveolar squamous cell carcinoma (Fig. 23-3, B).
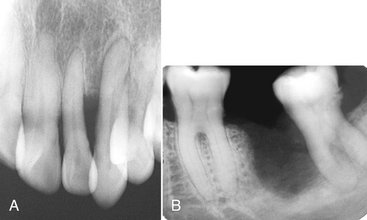
FIG. 23-3 A, This periapical film reveals bone destruction similar to periodontal disease around the lateral incisor from a squamous cell carcinoma originating in the soft tissues of the alveolar process. Note the lack of a sclerotic bone reaction at the periphery. B, The tooth socket from an extracted second molar has enlarged instead of healing due to the presence of a squamous cell carcinoma.
Management
Oral squamous cell carcinoma is usually managed with a combination of surgery and radiation therapy. The choice of which modality to use depends on the protocol of the treating center and the location and severity of the tumor. Generally, if an adequate margin of normal tissue can be obtained, surgery is the usual treatment, followed by radiation treatment. Alternately, radiation may be used as the primary treatment followed by surgical salvage. Currently, the trend is to add concomitant chemotherapy as an adjunct to either radiation or surgical treatment, which requires the dental practitioner to be aware of changes in the patient’s circulating blood count.
Synonyms
Primary intraosseous carcinoma, intra-alveolar carcinoma, primary intra-alveolar epidermoid carcinoma, primary epithelial tumor of the jaw, central squamous cell carcinoma, primary odontogenic carcinoma, intramandibular carcinoma, and central mandibular carcinoma
Definition
Primary intraosseous carcinoma is a squamous cell carcinoma that arises within the jaw and has no original connection with the surface epithelium of the oral mucosa. Primary intraosseous carcinomas are presumed to arise from intraosseous remnants of odontogenic epithelium. Carcinoma from surface epithelium, odontogenic cysts, or distant sites (metastases) must be excluded.
Clinical Features
These neoplasms are rare and may remain silent until they have reached a fairly large size. Pain, pathologic fracture, and sensory nerve abnormalities such as lip paresthesia and lymphadenopathy may occur with this tumor. It is more common in men and in patients in the fourth to eighth decade of life. The surface epithelium is invariably normal in appearance.
Radiographic Features
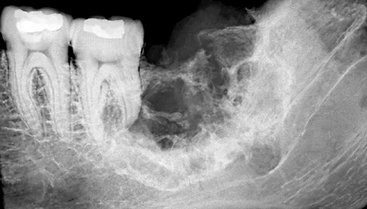
Location.: The mandible is far more commonly involved than the maxilla, with most cases being present in the molar region (Fig. 23-4) and less frequently in the anterior aspect of the jaws. Because the lesion is by definition associated with remnants of the dental lamina, it originates only in tooth-bearing parts of the jaw.
Periphery and Shape.: The periphery of the majority of lesions is ill defined, although some have been described as well defined. They are most often rounded or irregular in shape and have a border that demonstrates osseous destruction and varying degrees of extension at the periphery. The degree of raggedness of the border may reflect the aggressiveness of the lesion. If the lesion is of sufficient size, pathologic fracture occurs, with its associated step defects, thinned cortical borders, and subsequent soft tissue mass.
Internal Structure.: The internal structure is wholly radiolucent with no evidence of bone production and very little residual bone left within the center of the lesion. If the lesion is small, overlying buccal or lingual plates may cast a shadow that may mimic the appearance of internal trabecular bone.
Effects on Surrounding Structures.: These lesions are capable of causing destruction of the antral or nasal floors, loss of the cortical outline of the mandibular neurovascular canal, and effacement of the lamina dura. Root resorption is unusual. Teeth that lose both lamina dura and supporting bone appear to be floating in space.
Differential Diagnosis
If the lesions are not aggressive and have a smooth border and radiolucent area, they may be mistaken for periapical cysts or granulomas. Alternately, if lesions are not centered about the apex of a tooth, occasionally it is difficult to differentiate this condition from odontogenic cysts or tumors. If the border is obviously infiltrative with extensive bone destruction, a metastatic lesion must be excluded, as well as multiple myeloma, fibrosarcoma, and carcinoma arising in a dental cyst. Examination of the oral cavity and especially the surface epithelium assists in differentiating this condition from surface squamous cell carcinoma.
Management
Generally these tumors are excised with their surrounding osseous structure in an en bloc resection. Radiation and chemotherapy may be used as adjunctive therapies.
Definition
Squamous cell carcinoma arising in a preexisting dental cyst is uncommon and excludes invasion from surface epithelial carcinomas, metastatic tumors, and primary intraosseous carcinoma. They may arise from inflammatory periapical, residual, dentigerous, and odontogenic keratocysts. Histologically the lining squamous epithelium of the cyst gives rise to the malignant neoplasm.
Clinical Features
The most common presenting sign or symptom associated with this condition is pain. The pain may be characterized as dull and of several months’ duration. Swelling is occasionally reported. Pathologic fracture may occur, as may fistula formation and regional lymphadenopathy. If the upper jaw is involved, sinus pain or swelling may be present.
Radiographic Features
Location.: This tumor may occur anywhere an odontogenic cyst is found, namely, the tooth-bearing portions of the jaws. Most cases occur in the mandible (Fig. 23-5), with a few cases reported in the anterior maxilla.
Periphery and Shape.: The radiologic picture of squamous cell carcinoma originating in a cyst mirrors the histologic findings. Because the lesion arises from a cyst, the shape is often round or ovoid. If it is a small lesion in a cyst wall, the periphery may be mostly well defined and even corticated. In this case the radiographic differentiation from a normal cyst is impossible. As the malignant tissue progressively replaces cyst lining, the smooth border is lost or becomes ill defined. The advanced lesion has an ill-defined, infiltrative periphery that lacks any cortication. Its shape becomes less “hydraulic” looking and more diffuse.
Internal Structure.: This lesion lacks any ability to produce bone. It is wholly radiolucent, perhaps more so than invasive surface carcinoma, owing to prior osteolysis from the cyst.
Effects on Surrounding Structures.: Carcinoma arising in dental cysts is capable of thinning and destroying the lamina dura of adjacent teeth or adjacent cortical boundaries, such as the inferior border of the jaw or the floor of the nose. It can produce complete destruction of the alveolar process.
Differential Diagnosis
If a dental cyst is infected, it may lose its normal cortical boundary and appear ragged and identical to a malignant lesion arising in a preexisting cyst. However, inflamed cysts usually show a reactive peripheral sclerosis because of inflammatory products present in the cyst lumen. This is not normally present in a cyst, which has undergone malignant transformation. Nevertheless, the two may be difficult to differentiate radiologically, and therefore cysts should always be submitted for histologic examination. Multiple myeloma may appear as a solitary lesion and may be difficult to distinguish, especially if it has a cystic well-defined shape. Metastatic disease may be similar, although it is commonly multifocal.
Management
The treatment of squamous cell carcinoma originating in a cyst is identical to that described with primary intraosseous carcinoma.
Definition
Central mucoepidermoid carcinoma is an epithelial tumor arising in bone, likely originating from pluripotential odontogenic epithelium or from a cyst lining. It is histologically indistinguishable from its soft tissue counterpart. The criteria for diagnosis of a central mucoepidermoid tumor are the presence of intact cortical plates, radiographic evidence of bone destruction, and typical histologic findings consistent with mucoepidermoid tumor. Additionally, the practitioner must exclude the possibility of an invasive overlying mucoepidermoid tumor or odontogenic tumor.
Clinical Features
Unlike other malignant tumors of the jaws, the central mucoepidermoid tumor is more likely to mimic a benign tumor or cyst. The most common complaint is of a painless swelling. The swelling may have been present for months or even years and has been reported to cause facial asymmetry. Occasionally it may feel as if teeth have been moved or a denture may no longer fit. Tenderness rather than severe pain may also be present. Paresthesia of the inferior alveolar nerve and spreading of the lesion to regional lymph nodes has been reported. Central mucoepidermoid tumor, unlike other oral malignancies, is more common in females than males.
Radiographic Features
Location.: The lesion is twice as common in the mandible as the in maxilla, usually in the premolar and molar region with a few cases reported in the anterior mandible. The lesion most commonly occurs above the mandibular canal, similar to odontogenic tumors.
Periphery and Shape.: Mucoepidermoid tumor presents as a unilocular or multilocular expansile mass (Fig. 23-6). The border is most often well defined and well corticated and often crenated or undulating in nature, which is similar to benign odontogenic tumors. The peripheral cortication may be impressively thick, which belies its malignant nature. Rarely, the periphery is not corticated and has a more malignant appearance.
Internal Structure.: The internal structure has features like those of a benign odontogenic tumor such as an ameloblastoma. Lesions are often described as being multilocular or having either a soap bubble or honeycomb internal structure, implying the presence of compartments separated by thin or thick cortical septa. This bone is not produced by the tumor but is merely remodeled residual bone taking the form of septa.
Effects on Surrounding Structures.: Mucoepidermoid tumor is capable of causing expansion of adjacent normal bony walls. The buccal and lingual cortical plates, inferior border of the mandible, and alveolar crest are usually intact; however, they may be thinned and grossly displaced. The mandibular canal may be depressed or pushed laterally or medially. These characteristics are more similar to benign tumors than to malignant tumors. Teeth remain largely unaffected by this disease, although adjacent lamina dura may be lost.
Differential Diagnosis
The differential diagnosis of this lesion reflects its lack of features commonly associated with oral malignancy. The chief differential diagnosis is ameloblastoma and glandular odontogenic cyst, with which it shares similarities in its peripheral and internal features. It may not be possible to differentiate the two. Odontogenic myxoma and central giant cell granuloma also may be confused with mucoepidermoid tumor, as may other odontogenic cysts or tumors.
Management
Mucoepidermoid carcinoma is treated surgically with en bloc resection encompassing a margin of adjacent normal bone. Neck dissection and postoperative radiation therapy may be required to control spread to lymph nodes.
Definition
Malignant ameloblastoma is defined as an ameloblastoma with typical benign histologic features that is deemed malignant because of its biologic behavior, namely, metastasis. The histologic features may not correlate with the clinical behavior. On the other hand, ameloblastic carcinoma is an ameloblastoma exhibiting the histologic criteria of a malignant neoplasm such as increased and abnormal mitosis and large hyperchromatic, pleomorphic nuclei.
Clinical Features
Clinically these lesions may behave as benign ameloblastomas, exhibiting a hard expansile mass of the jaw with displaced and perhaps loosened teeth and normal overlying mucosa. Tenderness of the overlying soft tissue has been reported. Metastatic spread may be to the cervical lymph nodes, lung or other viscera, and the skeleton, especially the spine. Local extension may occur into adjacent bones, connective tissue, or salivary glands. These tumors occur most commonly between the first and sixth decades of life and are more common in males than in females.
Radiographic Features
Location.: These lesions are more common in the mandible than in the maxilla, with most occurring in the premolar and molar region, where ameloblastoma is typically found.
Periphery and Shape.: Similar to ameloblastoma, a well-defined border occurs with cortication, presence of crenations, or scalloping of the border. Malignant ameloblastoma may show some of the signs more commonly seen in malignant neoplasms, namely, loss of and subsequent breaching of the cortical boundary invading into the surrounding soft tissue.
Internal Structure.: The lesions are either unilocular or, more commonly, multilocular, giving the appearance of a honeycomb or soap-bubble pattern as seen in benign ameloblastomas. Most of the septa are robust and thick.
Effects on Surrounding Structures.: Teeth may be moved bodily by the tumor and may exhibit root resorption similar to a benign tumor. Bony borders may be effaced or breached, and, as in benign ameloblastoma, the lesions may erode the lamina dura and displace normal anatomic boundaries such as the floor of the nose and maxillary sinus. The mandibular neurovascular canal may be displaced or eroded.
Differential Diagnosis
The differential diagnosis of this lesion should include benign ameloblastoma, odontogenic keratocyst, odontogenic myxoma, and central mucoepidermoid tumor, from which it may not be distinguishable radiologically. If the lesion is locally invasive and this is apparent radiologically, a diagnosis of carcinoma arising in a dental cyst should be entertained. If the patient is young and the location of the lesion is anterior to the second permanent molar, central giant cell granuloma may mimic some of its radiologic features. Often the final diagnosis is the result of histologic evaluation or the detection of metastatic lesions.
Management
These lesions are most often treated with en bloc surgical resection. However, many may not be discovered to be malignant until the time of the first surgery or even later. Because the histologic appearance of these lesions may mimic benign ameloblastoma, the initial treatment often is inadequate. In addition, the metastatic lesions may not appear for many months or years after treatment of the primary tumor, adding another reason for treatment failure.
Metastatic Tumors
Definition
Metastatic tumors represent the establishment of new foci of malignant disease from a distant malignant tumor, usually by way of the blood vessels. An interesting feature of these lesions is that metastatic lesions in the jaws usually arise from sites that are anatomically inferior to the clavicle. Metastatic lesions of the jaws usually occur when the distant primary lesion is already known, although on occasion the presence of a metastatic tumor may reveal the presence of a silent primary lesion. Jaw involvement accounts for fewer than 1% of metastatic malignancies found elsewhere, with most affecting the spine, pelvis, skull, ribs, and humerus. Most frequently the tumor is a type of carcinoma; the most common primary sites are the breast, kidney, lung, colon and rectum, prostate, thyroid, stomach, melanoma, testes, bladder, ovary, and cervix. In children the tumors include neuroblastoma, retinoblastoma, and Wilms’ tumor. Metastatic carcinoma must be differentiated from the more common locally invading squamous carcinoma.
Clinical Features
Metastatic disease is more common in patients in their fifth to seventh decades of life. Patients may complain of dental pain, numbness or paresthesia of the third branch of the trigeminal nerve, pathologic fracture of the jaw, or hemorrhage from the tumor site.
Radiographic Features
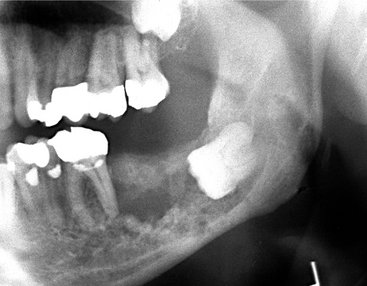
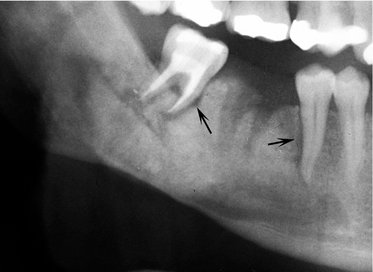
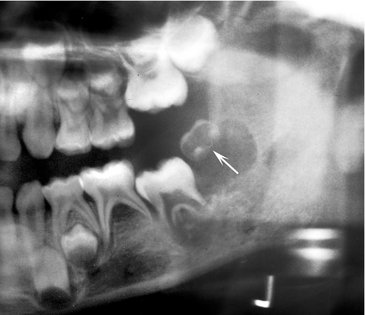
Location.: The posterior areas of the jaws are more commonly affected (Fig. 23-7), with the mandible favored over the maxilla. The maxillary sinus may be the next most common site, followed by the anterior hard palate and mandibular condyle. Frequently metastatic lesions of the mandible are bilateral (Fig. 23-7, B and C). Also, lesions may be located in the periodontal ligament space (sometimes at the root apex), mimicking periapical and periodontal inflammatory disease, or in the papilla of a developing tooth.
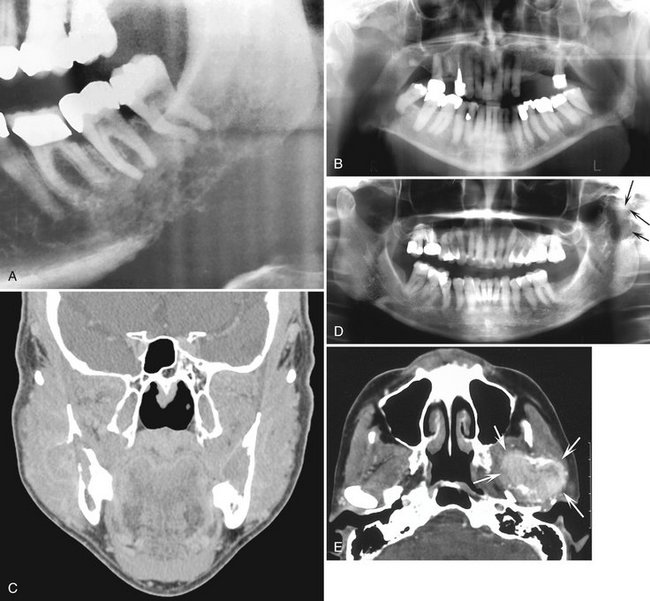
FIG. 23-7 Metastatic carcinomas. A, Metastatic breast carcinoma surrounding the apical half of the second and third molar roots and extending inferiorly. It has destroyed the inferior border of the mandible. B, Bilateral metastatic lesions from the lung destroying the mandibular rami. C, Coronal CT image with soft tissue algorithm of the same case. D, Destruction of the left mandibular condyle (arrows) from a thyroid metastatic lesion. E, Axial CT image with soft tissue algorithm of the same case showing invasion into surrounding soft tissue (arrows).
Periphery and Shape.: Metastatic lesions may be moderately well demarcated but have no cortication or encapsulation at their tumor margins; they may also have ill-defined invasive margins (see Fig. 23-7, A). The lesions are not usually round but polymorphous in shape. Both prostate and breast lesions may stimulate bone formation of the adjacent bone, which will be sclerotic. The tumor may begin as a few zones of osseous destruction separated by normal bone. After a time these small areas coalesce into a larger, ill-defined mass and the jaw may become enlarged.
Internal Structure.: Lesions are generally radiolucent, in which case the internal structure is a combination of residual normal trabecular bone in association with areas of bone lysis. If sclerotic metastases are present (i.e., prostate and breast), the normally ragged radiolucent area may appear as an area of patchy sclerosis, the result of new bone formation (Fig. 23-8). The origin of this new bone is not the tumor but stimulation of surrounding normal bone. If the tumor is seeded in multiple regions of the jaw, the result is a multifocal appearance (multiple small radiolucent lesions) with normal bone between the foci. Significant dissemination of metastatic tumor may give the jaws a general radiolucent appearance or even that of osteopenia.
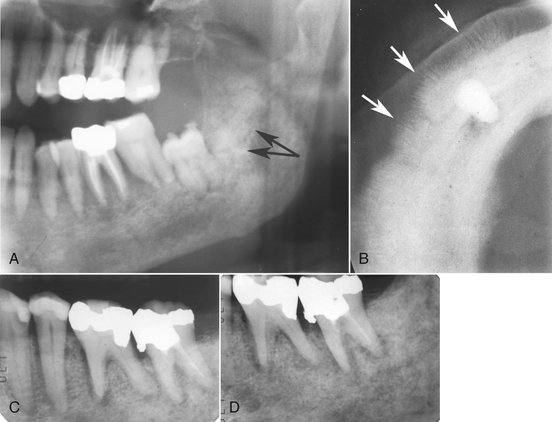
FIG. 23-8 A, Partial panoramic image of prostate metastatic lesions involving the body and ramus of the body; note the sclerotic bone reaction (arrows). B, Occlusal image of prostate lesions causing sclerosis and spiculated periosteal reaction (arrows). C and D, Two periapical images of a metastatic lesion of breast carcinoma; note the irregular widening of the periodontal membrane spaces and patchy sclerotic bone reaction, especially around the roots of the molars.
Effects on Surrounding Structures.: Metastatic carcinomas may stimulate a periosteal reaction that usually takes the form of a spiculated pattern (prostate and neuroblastoma) (Fig. 23-8). Typical of malignancy, the lesion effaces the lamina dura and can cause an irregular increase in the width of the periodontal ligament space. If the tumor has seeded in the papilla of a developing tooth, the cortices of the crypt may be totally or partially destroyed. Teeth may seem to be floating in a soft tissue mass and may be in an altered position because of loss of bony support. Extraction sockets may fail to heal and may increase in size. Resorption of teeth is rare (sometimes associated with multiple myeloma and chondrosarcoma); this is more common in benign lesions. The cortical bone of adjacent structures such as the neurovascular canal, sinus, and nasal fossa is destroyed. On occasion the tumor breaches the outer cortical plate of the jaws and extends into surrounding soft tissues or presents as an intraoral mass (see Fig. 23-7, E).
Differential Diagnosis
In most cases a known primary malignancy is present, and the diagnosis of metastasis is straightforward. Multiple myeloma may be confused with metastatic tumors; however, the border of multiple myeloma is usually better circumscribed than in metastatic disease. When a lesion starts within the periodontal ligament space of a tooth, the appearance may be identical to that of a periapical inflammatory lesion. A point of differentiation is that the periodontal ligament space widening from inflammation is at its greatest width and centered about the apex of the root. In contrast, the malignant tumor usually causes irregular widening, which may extend up the side of the root. Odontogenic cysts, if secondarily infected, may have an ill-defined border giving a similar appearance to a metastatic lesion. Invasion of the jaws by primary tumors of the overlying epithelium such as squamous cell carcinoma may be indistinguishable from metastatic disease but can be differentiated by clinical examination.
Management
The presence of a metastatic tumor in the jaw indicates a poor prognosis. If metastatic disease is present, the patient will usually die within 1 to 2 years. If the radiographic appearance is suspicious, an opinion from a dental radiologist should be sought and tissue submitted for histologic analysis. Nuclear medicine may be used to detect other metastatic lesions. Isolated malignant deposits, if symptomatic, may be treated with localized high-dose radiation. In the rare occasion that the jaw is the first diagnosed site of malignant spread, it is imperative that the patient be referred quickly to an oncologist so that anticancer treatment can be delivered promptly. This treatment may take the form of chemotherapy, radiation therapy, surgery, immunotherapy, or hormone treatment.
Sarcomas
Definition
Osteosarcoma is a malignant neoplasm of bone in which osteoid is produced directly by malignant stroma as opposed to adjacent reactive bone formation. The three major histologic types are chondroblastic, osteoblastic, and fibroblastic osteosarcoma. The cause of osteosarcoma is unknown, but genetic mutation and viral causes have been suggested. It is also known to occur in association with Paget’s disease and fibrous dysplasia after therapeutic irradiation.
Clinical Features
Osteosarcoma of the jaws is quite rare and accounts for approximately 7% of all osteosarcomas. Despite its rarity, the dentist may be the first health professional who observes tumors involving the jaws. The lesion occurs in all racial groups worldwide and in males twice as frequently as females. Jaw lesions typically occur with a peak in the fourth decade, about 10 years later on average than long bone lesions occur. The most commonly reported symptom or sign is swelling, which may be present as long as 6 months before diagnosis; the swelling is usually rapid. Other indicators are pain, tenderness, erythema of overlying mucosa, ulceration, loose teeth, epistaxis, hemorrhage, nasal obstruction, exophthalmos, trismus, and blindness. Hypoesthesia has also been reported in cases involving neurovascular canals.
Radiographic Features
Location.: The mandible is more commonly affected than the maxilla is. Although the lesion can occur in any part of either jaw, the posterior mandible, including the tooth-bearing region, angle, and vertical ramus, is most commonly affected. The posterior areas are also more commonly affected in the maxilla, with the most frequent sites being the alveolar ridge, antrum, and palate. The lesion may cross the midline.
Periphery and Shape.: Osteosarcoma has an ill-defined border in most instances. When viewed against normal bone, the lesion is usually radiolucent with no peripheral sclerosis or encapsulation. If the lesion involves the periosteum directly or by extension, the typical sunray spicules or “hair-on-end” trabeculae may be seen (Fig. 23-9). This occurs when the periosteum is displaced, partially destroyed, and disorganized. If the periosteum is elevated and maintains its osteogenic potential but is breached in the center, a Codman’s triangle at the edges is formed (see Fig. 23-1, E). Even more rarely, laminar periosteal new bone may be present. In many cases, extension is even more prominent, and a soft tissue mass is visible radiographically.
Internal Structure.: Osteosarcoma may be entirely radiolucent, mixed radiolucent-radiopaque, or quite radiopaque. The internal osseous structure may take the appearance of granular- or sclerotic-appearing bone, cotton balls, wisps, or honeycombed internal structures in areas with adjacent destruction of the preexisting osseous architecture. Whatever the resultant internal structure, the normal trabecular structure of the jaws is lost.
Effects on Surrounding Structures.: Widening of the periodontal membrane is associated with osteosarcoma but is also seen in other malignancies (Fig. 23-10). The antral or nasal wall cortices may be lost in maxillary lesions. Mandibular lesions may destroy the cortex of the neurovascular canal and adjacent lamina dura. Alternatively, the neurovascular canal may be symmetrically widened and enlarged. Effects on the periosteum are discussed under the discussion on periphery.
Differential Diagnosis
If internal structure is minimal or absent, fibrosarcoma or metastatic carcinoma may appear similar to osteosarcoma. If osseous structure is visible, the practitioner should also consider chondrosarcoma. If spiculated periosteal new bone is present, prostate and breast metastases should be considered. Comprehensive physical examination and laboratory tests assist in determining whether the lesion is primary or metastatic. Benign tumors such as ossifying fibroma and benign conditions such as fibrous dysplasia may mimic osteosarcoma radiographically. The former conditions, however, are usually better demarcated and have a more uniform internal structure. The histopathologic features of osteosarcoma may be interpreted as a benign fibro-osseous lesion, and in these cases, the correct diagnosis may rely on the radiographic characteristics alone. Ewing’s sarcoma, solitary plasmacytoma, and even osteomyelitis share some of the radiographic characteristics of osteosarcoma. Osteosarcoma is generally not associated with signs of infection.
Management
The management of osteosarcoma is resection with a large border of adjacent normal bone. This may be possible in orthopedic cases but may be complicated by the presence of important adjacent anatomic structures in the head and neck. Generally, radiation therapy and chemotherapy are used only for controlling metastatic spread or for palliation.
Definition
Chondrosarcoma is a malignant tumor of cartilaginous origin. The four histologic subtypes, which develop most commonly in the craniofacial region, are the clear cell, dedifferentiated, myxoid, and mesenchymal forms. They may occur centrally within bone, on the periphery of bone, or less commonly in soft tissue. They can arise directly from cartilage or may occur within benign cartilaginous tumors. In the case of the latter, they are termed secondary chondrosarcomas.
Clinical Features
Generally these tumors occur at any age, although they are more common in adults (mean age 47 years). They affect males and females equally. A patient with chondrosarcoma may have a firm or hard mass of relatively long duration. Enlargement of these lesions may cause pain, headache, and deformity. Less frequent signs and symptoms include hemorrhage from tumor or from the necks of the teeth, sensory nerve deficits, proptosis, and visual disturbances. Invariably the tumors are covered with normal overlying skin or mucosa unless secondarily ulcerated. If chondrosarcoma occurs in or near the temporomandibular joint region, trismus or abnormal joint function may result.
Radiographic Features
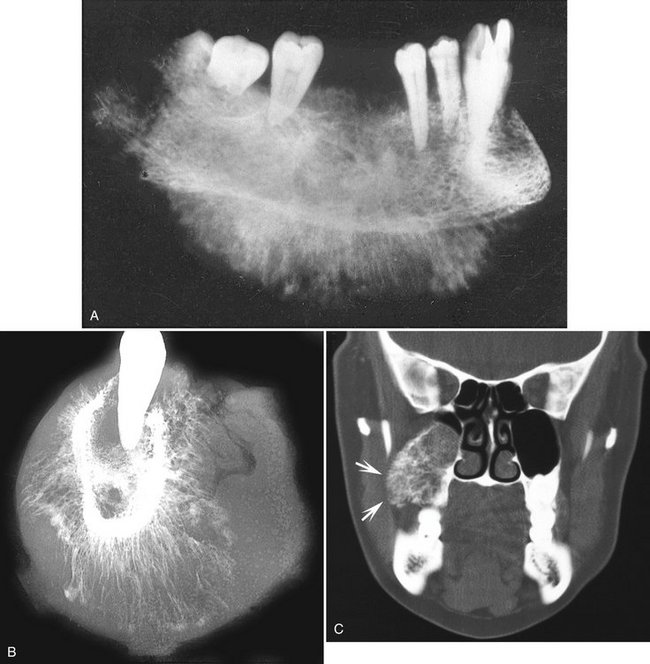
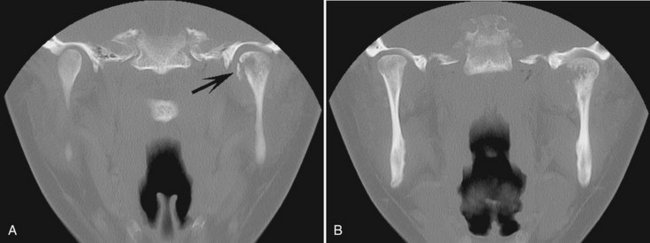
Location.: Chondrosarcomas are unusual in the facial bones, accounting for about 10% of all cases. They occur in the mandible and maxilla with equal frequency. Maxillary lesions typically occur in the anterior region in areas where cartilaginous tissues may be present in the maxilla. Mandibular lesions occur in the coronoid process, condylar head and neck (Fig. 23-11, B and C), and occasionally the symphyseal region.
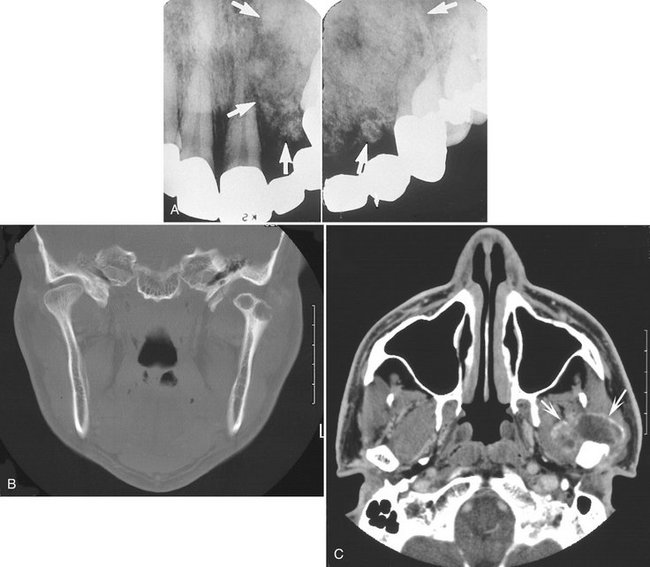
FIG. 23-11 A, Chondrosarcoma of the anterior maxilla, with irregular calcification in the internal structure of the tumor (arrows). B, Coronal CT image with bone algorithm of a chondrosarcoma involving the mandibular condyle (note the two areas of bone destruction). C, Axial CT scan with soft tissue algorithm demonstrating the soft tissue extent of the lesion (arrows) and sparse calcifications. (A courtesy L. Hollender, DDS, Seattle, Wash.)
Periphery and Shape.: Chondrosarcomas are slow-growing tumors, and their radiologic signs may be misleading and benign in nature. The lesions are generally round, ovoid, or lobulated. Generally their borders are well defined and at times corticated, whereas at other times melding with adjacent normal bone occurs. Occasionally peripheral periosteal new bone may be present perpendicular to the original cortex, giving the so-called sunray or hair-on-end appearance. Uncommonly these lesions are ill defined and invasive. Aggressive lesions such as these have infiltrative, ill-defined, and noncorticated borders.
Internal Structure.: Chondrosarcomas usually exhibit some form of calcification within their center, giving them a mixed radiolucent-radiopaque appearance. At times this mixture takes the form of moth-eaten bone alternating with islands of residual bone unaffected by tumor. Lesions are rarely completely radiolucent. The central radiopaque structure has been described as “flocculent,” implying snowlike features. This diffuse calcification may be superimposed on a bony background that resembles granular or ground-glass–appearing abnormal bone (Fig. 23-11, A). Careful examination of these areas of flocculence may reveal a central radiolucent nidus, which is probably cartilage surrounded by calcification. The result is rounded or speckled areas of calcification.
Effects on Surrounding Structures.: Chondrosarcoma, because it is relatively slow growing, often expands normal cortical boundaries rather than rapidly destroying them. In mandibular cases the inferior border or alveolar process may be grossly expanded while still maintaining its cortical covering. Maxillary lesions may push the walls of the maxillary sinus or nasal fossa and impinge on the infratemporal fossa. Lesions of the condyle cause its expansion and perhaps remodeling of the corresponding articular fossa and eminence. If lesions occur in the articular disk region, a widened joint space may be present with corresponding remodeling of the condylar neck. Erosion of the articular fossa may also occur. If lesions occur near teeth, root resorption and tooth displacement may occur, as may widening of the periodontal membrane space.
Differential Diagnosis
Osteosarcoma is often indistinguishable radiographically from chondrosarcoma. Although the typical calcifications of chondrosarcoma may be absent from osteosarcoma, the two share many other radiologic features. Fibrous dysplasia may also be difficult to differentiate from chondrosarcoma because similarities in the internal pattern. (The radiopaque portion of fibrous dysplasia is abnormal bone, not calcification. The calcifications in chondrosarcoma represent calcified cartilage.) Generally, the periphery of fibrous dysplasia is better defined and its margin with adjacent teeth differs from that of chondrosarcoma. For instance, fibrous dysplasia alters the bone pattern up to and including the lamina dura, leaving a normal or thin periodontal ligament space. The greatest danger results from the misleading benign characteristics of chondrosarcoma, which may delay correct diagnosis.
Definition
Ewing’s sarcoma is of indeterminate histogenesis. It is a tumor of long bones that is relatively rare in the jaws. Lesions arise in the medullary portion of the bone and spread to the endosteal and later to the periosteal surfaces.
Clinical Features
Ewing’s sarcoma is most common in the second decade of life; most patients are between the ages of 5 and 30 years. Males are twice as likely to manifest the disease as females. In addition, multicentric lesions have been reported. Other reported findings at the time of presentation include, in descending frequency, swelling, pain, loose teeth, paresthesia, exophthalmos, ptosis, epistaxis, ulceration, shifted teeth, trismus, and sinusitis. Cervical lymphadenopathy has also been reported.
Radiographic Features
Location.: Mandibular cases outnumber maxillary cases by about two to one, with the highest frequency found in posterior areas in both jaws. Generally the lesions develop within the marrow space first and then extend to involve overlying cortical plates. This neoplasm rarely occurs in the jaws.
Periphery and Shape.: Ewing’s sarcoma is a radiolucency that is poorly demarcated and never corticated. Its advancing edge destroys bone in an uneven fashion, resulting in a ragged border. The lesions are usually solitary and may cause pathologic fracture with adjacent radiographically visible soft tissue masses (Fig. 23-12). They may be round or ovoid but generally have no typical shape.
Internal Structure.: Ewing’s sarcoma is a destructive process with little induction of bone formation. Because it commences on the internal aspect of the bone and involves the endosteal and periosteal surfaces later in its course, it is usually entirely radiolucent.
Effects on Surrounding Structures.: Ewing’s sarcoma may stimulate the periosteum to produce new bone. This is usually the result of gross disturbances to the overlying periosteum and takes the form of Codman’s triangle or sunray or hair-on-end spiculation. Laminar periosteal new bone formation has been reported to occur but is not a common feature of active Ewing’s sarcoma lesions. Adjacent normal structures such as the mandibular neurovascular canal, inferior border of the mandible, and alveolar cortical plates may be effaced. If the lesion abuts teeth or tooth follicles, the cortices of these structures are destroyed. This tumor does not characteristically cause root resorption, although it does destroy the supporting bone of adjacent teeth.
Differential Diagnosis
Inflammatory or infectious lesions such as osteomyelitis of the jaw may share some of the radiographic features of Ewing’s sarcoma. Although both are radiolucent, osteomyelitis is likely to have demonstrable sequestra present within the confines of the lesion, whereas Ewing’s sarcoma does not. Inflammatory lesions contain some sign of reactive bone formation, resulting in some sclerosis internally or at the periphery, and differ in the associated periosteal bone formation.
Eosinophilic granuloma of the jaw is also a destructive process that occurs in the same part of the bone. It is associated with laminar periosteal bone reaction, whereas, in the jaws, Ewing’s sarcoma is not. The other central primary malignancies of bone such as osteosarcoma, chondrosarcoma, and fibrosarcoma may be difficult to differentiate from this condition.
Management
Too few cases of maxillofacial Ewing’s sarcoma are available at any single treatment center for any specific treatment policy to have been adopted. Surgery, radiation therapy, and chemotherapy may be used alone or in combination.
Definition
Fibrosarcoma is a neoplasm composed of malignant fibroblasts that produce collagen and elastin. The etiology is unknown, although it may arise secondarily in tissues that have received therapeutic levels of radiation.
Clinical Features
These lesions occur equally in males and females with a mean age in the fourth decade. A slowly to rapidly enlarging mass is the usual presenting symptom. The mass may be within bone, in which case it usually is accompanied by pain. Peripheral lesions or those exiting from bone may invade local soft tissues, causing a bulky, clinically obvious lesion. If central or peripheral lesions reach a large size, pathologic fracture may occur. If fibrosarcomas involve the course of peripheral nerves, sensory-neural abnormalities may occur. Overlying mucosa, although initially normal, may become erythematous or ulcerated. Involvement of the temporomandibular joint or paramandibular musculature is often accompanied by trismus.
Radiographic Features
Location.: Most cases of fibrosarcoma of the jaws occur in the mandible, with the greatest number of these occurring in the premolar/molar region.
Periphery and Shape.: Fibrosarcomas have ill-defined borders that are best described as ragged (Fig. 23-13). They are poorly demarcated, noncorticated, and lack any semblance of a capsule. These tumors are generally shaped in a fashion that suggests that they have grown along a bone, so they tend to be elongated through the marrow space. The radiographic border may underestimate the extent of the tumor because these lesions typically are infiltrative. If soft tissue lesions occur adjacent to bone, they may cause a saucerlike depression in the underlying bone or invade it as would a squamous cell carcinoma. Finally, sclerosis may occur in the adjacent normal bone whether the fibrosarcoma is peripheral to bone or central.
Internal Structure.: Fibrosarcomas have little internal structure. In most cases the lesions are entirely radiolucent. If the lesions have been present for some time and are not overly aggressive, either residual jawbone or reactive osseous bone formation occurs.
Effects on Surrounding Structures.: The most common effect on adjacent structures is destruction. In the mandible, the alveolar process, inferior border of the jaw, and cortices of the neurovascular canal are lost. In the maxilla, the inferior floor of the maxillary sinus, posterior wall of the maxilla, and nasal floor can be destroyed. In either jaw, lamina dura and follicular cortices are obliterated. Destruction of the outer cortical plate is usually accompanied by a protruding soft tissue mass. Root resorption is uncommon. Teeth are more likely to be grossly displaced and lose their support bone so that they appear to be floating in space. In addition, widening of the periodontal membrane space occurs with this tumor, as in other malignancies. Periosteal reaction is uncommon; however, if the lesion disrupts the periosteum, a Codman’s triangle or sunray spiculation may be evident.
Differential Diagnosis
This solitary, ragged radiolucency with little internal structure is difficult to differentiate from other central malignancies. If the lesion does not cause enlargement of the jaw, the practitioner must rule out metastatic carcinoma, multiple myeloma, and primary or secondary intraosseous carcinoma. Another possibility is a grossly infected dental cyst, although these usually show some degree of induced peripheral sclerosis in adjacent bone. If a fibrosarcoma exhibits enlargement of the affected jaw with an associated soft tissue mass, other sarcomas such as chondrosarcoma and osteosarcoma (both usually have internal structure) should be ruled out. Ewing’s sarcoma and radiolucent osteosarcomas may not be distinguishable from this tumor. Finally, peripheral invasive squamous cell carcinoma shares some of these radiologic features, but its ulcerative surface features differentiate it from fibrosarcoma, which usually lacks these.
Malignancies of the Hematopoietic System
Definition
Multiple myeloma is a malignant neoplasm of plasma cells. It is the most common malignancy of bone in adults. Single lesions are called plasmacytoma, and multiple lesions are termed multiple myeloma.
Clinical Features
Multiple myeloma is a fatal systemic malignancy. A patient with multiple myeloma is usually between the ages of 35 and 70 years (mean age 60 years). The patient may complain of fatigue, weight loss, fever, bone pain, and anemia, although the typical presenting feature is low back pain. Secondary signs include amyloidosis and hypercalcemia; in half of all patients, characteristic Bence Jones protein is present in the urine, which causes the urine to be foamy. The disease is more common in men. When this clonal cellular proliferation occurs, these cells occupy first cancellous and later cortical bone, replacing the normally radiopaque bone with areas of radiolucency.
Orally, patients may complain of dental pain, swelling, hemorrhage, paresthesia, and dysesthesia, or they may have no complaints. The number of patients with demonstrable radiologic findings in the jaws at the time of diagnosis is relatively small.
Radiographic Features
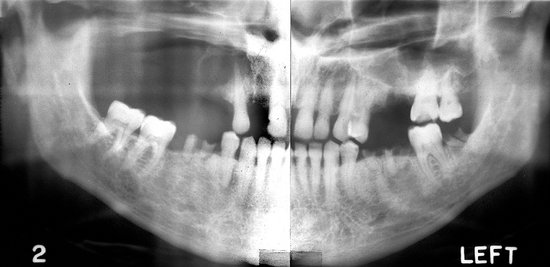
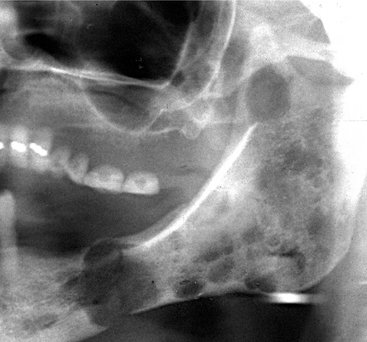
Location.: Multiple myeloma (Fig. 23-14) is seen more frequently in the mandible than the maxilla but is uncommon in either. The incidence of jaw involvement has been reported to vary from 2% to 78%. In the mandible the posterior body and ramus is favored. Maxillary lesions usually appear in posterior sites.
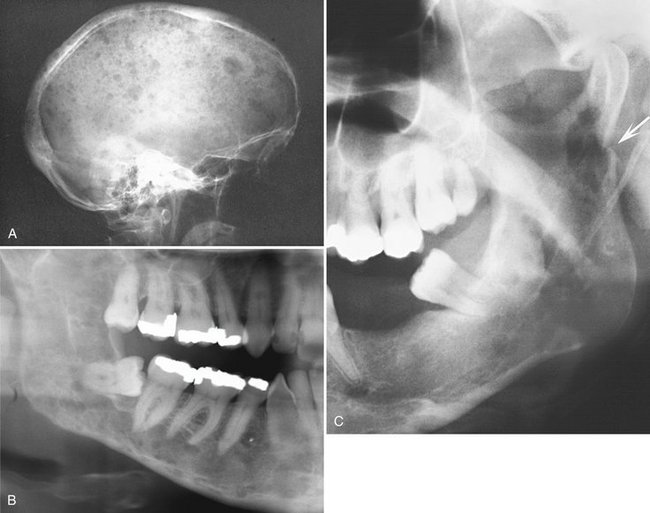
FIG. 23-14 Multiple myeloma, seen as multiple circular radiolucent lesions in the skull (A). In a different case (B) multiple small lesions of multiple myeloma are present through the body and ramus of the mandible in this cropped panoramic image. C, Cropped panoramic image shows a solitary lesion in the condylar neck region and a pathologic fracture (arrow).
Periphery and Shape.: The periphery of multiple myeloma lesions is well defined but not corticated; it lacks any sign of bone reaction (Fig. 23-15). The lesions have been described as appearing “punched out.” However, many appear ragged and even infiltrative. Some lesions have an oval or cystic shape. Untreated or aggressive areas of destruction may become confluent, giving the appearance of multilocularity. If the lesion is located in the periapical periodontal ligament space, it may have a border similar to that seen in inflammatory or infectious periapical disease. Soft tissue lesions have been reported in the jaws and nasopharynx. When visible on radiographs, they appear as smooth-bordered soft tissue masses, possibly with underlying bone destruction.
Internal Structure.: No internal structure is radiographically visible. Occasionally islands of residual bone, yet unaffected by tumor, give the appearance of the presence of new trabecular bone within the mass. Very rarely the lesions appear radiopaque internally.
Effects on Surrounding Structures.: If a good deal of bone mineral is lost, teeth may appear to be “too opaque” and may stand out conspicuously from their osteopenic background. Lamina dura and follicles of impacted teeth may lose their typical corticated surrounding bone in a manner analogous to that seen in hyperparathyroidism. The same may be said of the mandibular neurovascular canal, which, although usually visible, loses its cortical boundary in whole or in part. These changes are profound when there is associated renal disease. Mandibular lesions may cause thinning of the lower border of the mandible or endosteal scalloping. Any cortical boundary may be effaced if lesions involve them. Periosteal reaction is uncommon, but if it is present takes the form of a single radiopaque line or more rarely a sunray appearance.
Differential Diagnosis
The most likely disease to be mistaken for multiple myeloma is the radiolucent form of metastatic carcinoma. Knowledge of a prior malignancy in a patient may help differentiate multiple myeloma from metastatic carcinoma. Osteomyelitis, if severe, may yield a radiologic picture similar to that of multiple myeloma; however, a visible cause for it usually exists. In addition, inflammatory lesions and infections in general cause sclerosis in adjacent bone, which multiple myeloma does not. Simple bone cysts may be bilateral in the mandible and therefore may be mistaken for multiple myeloma. They are usually corticated in part and characteristically interdigitate between the roots of the teeth in a much younger population. Generalized radiolucency of the jaws may be caused by hyperparathyroidism but is differentiated on the basis of abnormal blood chemistry. However, brown tumors of hyperparathyroidism, if present with generalized radiolucency of the jaws and similar symptoms, can readily be confused with multiple myeloma radiographically. Other metabolic diseases such as Gaucher’s disease or oxalosis may cause many of the changes similar to multiple myeloma that are observed on dental radiographs.
Management
The management of multiple myeloma is usually chemotherapeutic with or without autologous or allogeneic bone marrow transplantation. Radiation therapy may be used for treatment of symptomatic osseous lesions when palliation is required.
Definition
Non-Hodgkin’s lymphoma is a malignant tumor of cells normally resident in the lymphatic system. In general, lymphomas occur within lymph nodes; however, extranodal sites such as bone, skin, gastrointestinal mucosa, tonsils, and Waldeyer’s ring can be involved. The term non-Hodgkin’s lymphoma describes a family of heterogeneous tumors of varying type and severity. The classification of these diseases is difficult, and numerous means exist of subdividing these tumors. Currently the working formulation for clinical usage classifies tumors on the basis of their histologic appearance into low grade, intermediate grade, or high grade tumors, with the last being the most aggressive.
Clinical Features
Non-Hodgkin’s lymphoma occurs in all age groups but is rare in patients in the first decade. The maxillary sinus, palate, tonsillar area, and bone may be sites of primary or secondary lymphoma spread. Lesions occurring outside lymph nodes in the head and neck are present in as much as one out of five cases. Patients may feel unwell, experiencing night sweats, pruritus, and weight loss. Palpable painless swelling, lymphadenopathy, and sensorineural deficits may accompany isolated lesions of the jaws. Lesions present for some time may cause pain and ulceration. Teeth resident in a lymphoma may become mobile as the supporting bone is lost.
Radiographic Features
Location.: Most non-Hodgkin’s lymphomas of the head and neck occur in the lymph nodes. Those that are extranodal are likely to affect the maxillary sinus, posterior mandible, and maxillary regions.
Periphery and Shape.: Most non-Hodgkin’s lymphomas initially take the shape and form of the host bone. If untreated, however, they are capable of causing destruction of the overlying cortex (Fig. 23-16). They may appear rounded or multiloculated and lack a defining outer cortex. Generally the borders are ill defined and invasive. Occasionally, lymphoma appears as multiple areas of destruction, which likely appear as fingerlike extensions of malignant tumor cells in a buccal or lingual direction. Visible lesions occurring in the maxillary sinus or nasopharynx have a smooth periphery.
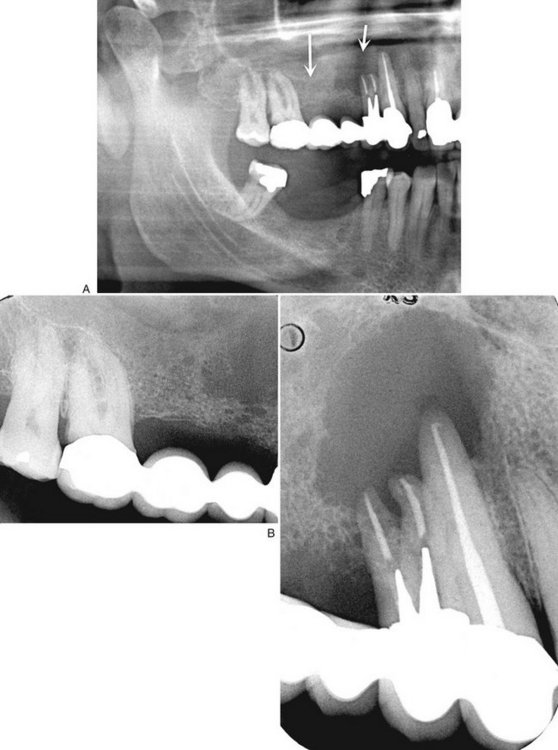
FIG. 23-16 A, A panoramic image revealing a malignant lymphoma invading the right maxilla. Note the ill-defined bone destruction and loss of the anterior aspect of the floor of the maxillary antrum (arrows). The intraoral radiographs (B) also show ill-defined bone destruction and the lack of any bone reaction or formation.
Internal Structure.: The internal structure of lymphoma is almost always entirely radiolucent. It is rare to see reactive bone formation. Occasionally patchy radiopacity may be present, but this is rare.
Effects on Surrounding Structures.: In maxillary sinus lesions the antral walls may be effaced and a soft tissue mass may be visible radiographically, either internally within the sinus or external to the maxillary sinus. Lesions involving the mandible destroy the cortex of the neurovascular canal. This tumor has a propensity to grow in the periodontal ligament space of mature teeth (Fig. 23-17). The cortex of the crypts of developing teeth may be lost when the lymphoma is located in the developing papilla, and the involved teeth may be displaced in an occlusal direction and exfoliated. Periosteal reaction is not common but may take the form of laminated or spiculated bone formation. With the advent of soft tissue imaging with MRI, it has become apparent that this tumor has a habit of growing along soft tissue spaces (fat layers) and along the surface of bone.
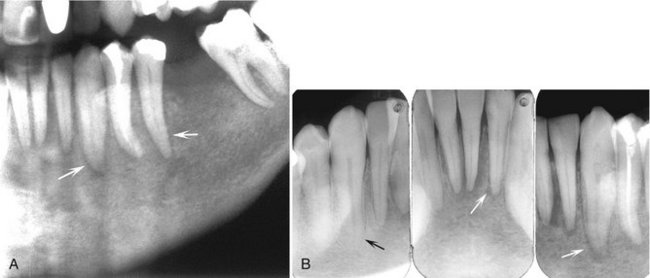
FIG. 23-17 A cropped panoramic image (A) revealing an ill-defined lymphoma invading the left body of the mandible; note the irregular widening of the periodontal ligament spaces (arrows). Intraoral films of the same case (B) and the widened periodontal ligament spaces (white arrows) compared with the normal periodontal ligament space of the right mandibular cuspid (black arrow).
Differential Diagnosis
Multiple myeloma and metastatic carcinoma are easily confused with non-Hodgkin’s lymphoma of the jaws. However, Ewing’s sarcoma and Langerhans’ histiocytosis, although also capable of producing the same effects, occur in a slightly younger age group. Osteolytic osteosarcoma and any of the central squamous cell carcinomas may not be distinguishable radiographically from non-Hodgkin’s lymphoma. Squamous cell carcinoma arising in the maxillary sinus may be difficult to differentiate from lymphoma of the maxillary sinus. Other lesions that can displace developing teeth in an occlusal direction include leukemia and Langerhans’ histiocytosis. Differentiation from apical rarefying osteitis may be difficult; however, careful inspection of the radiographic film may reveal the presence of an infiltrative border and adjacent bone destruction.
Management
The management of extranodal or isolated nodal disease is radiation therapy with or without concomitant chemotherapy. Treatment depends on histologic variants and the location and extent of disease.
Definition
Burkitt’s lymphoma is a high-grade B-cell lymphoma that differs from other B-cell lymphomas with respect to its histologic appearance and clinical behavior. It was first described by Denis Burkitt in East Africa as an African jaw lymphoma.
Two separate forms of the disease have been described: the endemic African Burkitt’s lymphoma and the American form. The latter is not characterized by jaw involvement (although it occurs), but by involvement of abdominal viscera. African Burkitt’s lymphoma affects young children, whereas American Burkitt’s lymphoma affects adolescents and young adults. Cases of endemic and nonendemic Burkitt’s tumor have been described throughout the world.
Clinical Features
The disease affects more males than females. Clinically the hallmark of this tumor is rapidity of growth with a tumor doubling time of less than 24 hours. It may involve children as young as 2 years and adults in the seventh decade, although it is primarily a disease of youth. Jaw tumors are rapidly growing and cause facial deformity very early in their course. They are capable of blocking nasal passages, displacing orbital contents, causing gross facial swelling, and eroding through skin. These rapidly growing tumors are more characteristic of African Burkitt’s lymphoma than the American form and cause pain and paresthesia. Teeth may become loosened rapidly and alveolar bone grossly distended. Paresthesia of the inferior alveolar nerve or other sensory facial nerves is common.
Radiographic Features
Location.: Extranodal disease is the norm in Burkitt’s tumor. African cases may involve one jaw or both the maxilla and mandible and affect the posterior parts of the jaws. By contrast, American cases may not involve the facial bones but are more likely to affect the abdominal viscera and testes.
Periphery and Shape.: The lesions may begin as multiple ill-defined noncorticated radiolucencies that later coalesce into larger ill-defined radiolucencies with an expansile periphery. They are of no specific shape, although they expand rapidly and have been likened to a balloon. This expansion breaches its outer cortical limits, causing gross balloonlike expansion with thinning of adjacent structures and production of a soft tissue tumor mass adjacent to the osseous lesion. Lesions that abut the orbital contents or the maxillary sinus may show a smooth surface soft tissue mass radiologically.
Internal Structure.: Burkitt’s lymphoma does not produce bone and rarely induces production of reactive bone within its center. For this reason, the lesions are radiolucent in almost all cases. It is particularly radiolucent in the jaw of a child.
Effects on Surrounding Structures.: Erupted teeth in the area of Burkitt’s tumor are grossly displaced, as are developing tooth crypts. Tumor cells within the crypt may displace the developing tooth bud to one side of its crypt. A tumor that is located apical to a developing tooth may cause it to be displaced such that it appears to erupt with little if any root formation. After tumor involvement of the developing dental structures occurs, root development ceases. Lamina dura of teeth in the area is destroyed, and cortical boundaries such as the maxillary sinus, nasal floor, orbital walls, and inferior border of the mandible are thinned and later destroyed. The cortex of the inferior alveolar canal is lost, although it is difficult to see in the radiographs of a normal pediatric patient in any case. If periosteum is involved, the border may show sunray spiculation, although this is rare. Cases that involve the orbit displace the orbital contents, seen both clinically and radiologically.
Differential Diagnosis
Metastatic neuroblastoma may give similar changes clinically and radiologically, as may Ewing’s tumor. Osteolytic osteosarcoma can grow rapidly and may be indistinguishable from Burkitt’s tumor on clinical and radiologic grounds. Cherubism has more internal structure, does not breach bony borders, is bilateral, and grows much more slowly. Finally, non-Hodgkin’s lymphoma must be considered, although it occurs in a much older age group in most cases.
Management
The management of Burkitt’s tumor is chemotherapeutic. Chemotherapy regimens vary from geographic locales, but the tumor is exquisitely sensitive to combinations of chemotherapeutic agents.
Synonyms
Acute myelogenous leukemia, acute lymphoblastic leukemia, chronic myelogenous leukemia, and chronic lymphocytic leukemia are types of leukemia.
Definition
Leukemia is a malignant tumor of hematopoietic stem cells. These malignant cells displace normal bone marrow constituents and spill out into the peripheral blood. They are subdivided into acute leukemias and chronic leukemias and further subdivided by the cell of origin. The acute leukemias occur with a bimodal age distribution, with very young patients and very old patients being the most commonly affected. Most cases of leukemia are associated with nonrandom chromosomal abnormalities.
Clinical Features
The patient with chronic leukemia may have no presenting signs or complaints. Acute leukemia patients generally feel unwell with weakness and bone pain. They may exhibit pallor, spontaneous hemorrhage, hepatomegaly, splenomegaly, lymphadenopathy, and fever. Oral symptoms are generally absent but, if present, include loose teeth, petechiae, ulceration, and boggy enlarged gingiva.
Radiographic Features
Radiologic signs associated with chronic leukemia are comparatively rare.
Location.: Leukemia affects the entire body because it is a malignancy of bone marrow, which discharges malignant cells into circulating blood. Its manifestations in the jaws may be seen more often in areas of developing teeth. Frequently, leukemia may be localized around the periapical region of a tooth, giving the appearance of a rarefying osteitis.
Periphery and Shape.: Leukemia must be considered a systemic malignancy, and as such its oral radiologic features may be present bilaterally as ill-defined patchy radiolucent areas. With time and lack of treatment, these patchy areas may coalesce to form larger areas of ill-defined radiolucent regions of bone (Fig. 23-18). The teeth may appear to stand out conspicuously from their surrounding, osteopenic bone.
Internal Structure.: The internal structure of leukemia is characterized by patchy areas of radiolucency and generalized radiolucency of the bone. Rarely, granular bone may be seen within these lesions. Occasionally, foci of leukemic cells may be present as a mass that may behave like a localized malignant tumor. These lesions, called chloromas, are rare in the jaws.
Effects on Surrounding Structures.: Leukemia does not cause expansion of bone, although occasionally a single layer of periosteal new bone may be seen in association with this disease, which is uncommon in chronic leukemia. Developing teeth in their crypts and teeth undergoing eruption may be displaced in an occlusal direction (Fig. 23-19) or into the oral cavity before root development. Less commonly, developing teeth may be displaced from their normal positions. The result of this is premature loss of teeth. The lamina dura and cortical outlines of follicles may be effaced. If lesions affect the periodontal structures, the crestal bone may be lost.
Differential Diagnosis
Generally, by the time oral radiologic signs of leukemia are present, a medical diagnosis has been reached. However, the development of radiologic changes may be the first indication of the relapse of treatment. Occasionally, lymphoma or neuroblastoma may mimic some of the features of destruction seen in leukemia. Metabolic disorders may be considered in those cases in which generalized rarefaction of bone is seen. These conditions are all excluded on the basis of blood testing. With apical lesions, careful examination of the involved tooth clinically and radiologically typically shows no apparent cause for rarefying osteitis.
Dental Radiology for the Cancer Survivor
The cancer survivor requires dental treatment just as any other patient. For the cancer survivor, dental radiologic examination may be more important than for a healthy patient receiving a routine examination. Some patients who have received a full course of radiation therapy are concerned about the additional exposure from a dental radiographic examination. However, this is not a valid concern because the small dose associated with dental radiographic examinations is negligible compared with the radiation dose received from cancer therapy.
The patient treated for head and neck malignancy with radiation therapy, even with today’s advanced radiotherapeutic methods, is prone to development of postradiation dental caries and osteoradionecrosis. Careful clinical examination and a thorough dental radiologic examination may be required periodically to ensure that the remaining dentition and periodontal apparatus is in good shape. Radiation caries occur in many patients and appears clinically different from typical dental caries. If untreated, these carious teeth become nonvital and may cause infection in the underlying jaw. If they require extraction, healing can be expected to be slow and occasionally osteoradionecrosis may result.
The role of radiology in these patients, however, is not restricted to examination of the teeth and supporting structures. Equally important is the monitoring of the outcome of treatment, specifically the examination of dental radiographs for evidence of tumor recurrence, development of metastases, and osteoradionecrosis.
Carter, RL. Patterns and mechanisms of spread of squamous carcinomas of the oral cavity. Clin Otolaryngol Allied Sci. 1990;15:185–191.
Casiglia, J, Woo, SB. A comprehensive review of oral cancer. Gen Dent. 2001;49:72–82.
Marchetta, FC, Sako, K, Murphy, JB. The periosteum of the mandible and intraoral carcinoma. Am J Surg. 1971;122:711–713.
McGregor, AD, MacDonald, DG. Routes of entry of squamous cell carcinoma to the mandible. Head Neck Surg. 1988;10:294–301.
Noyek, AM, Wortzman, G, Holgate, RC, et al. The radiologic diagnosis of malignant tumors of the paranasal sinuses and related structures. J Otolaryngol. 1977;6:399–406.
O’Brien, CJ, Carter, RL, Soo, KC, et al. Invasions of the mandible by squamous carcinomas of the oral cavity and oropharynx. Head Neck Surg. 1986;8:247–256.
Rao, LP, Das, SR, Mathews, A, et al. Mandibular invasion in oral squamous cell carcinoma: investigation by clinical examination and orthopantomogram. Int J Oral Maxillofac Surg. 2004;33:454–457.
Stambuk, HE, Karimi, S, Lee, N, et al. Oral cavity and oropharynx tumors. Radiol Clin North Am. 2007;45:1–20.
SQUAMOUS CELL CARCINOMA ORIGINATING IN BONE
Ariji, E, Ozeki, S, Yonetsu, K, et al. Central squamous cell carcinoma of the mandible: computed tomographic findings. Oral Surg Oral Med Oral Pathol. 1994;77:541–548.
Elzay, RP. Primary intraosseous carcinoma of the jaws. Review and update of odontogenic carcinomas. Oral Surg Oral Med Oral Pathol. 1982;54:299–303.
Lin, YJ, Chen, CH, Wang, WC, et al. Primary intraosseous carcinoma of the mandible. Dentomaxillofacial Radiol. 2005;34:112–116.
Suei, Y, Tanimoto, K, Taguchi, A, et al. Primary intra-osseous carcinoma: review of the literature and diagnostic criteria. J OralMaxillofac Surg. 1994;52:580.
SQUAMOUS CELL CARCINOMA ORGINATING IN A CYST
Cavalcanti, MG, Veltrini, VC, Ruprecht, A, et al. Squamous-cell carcinoma arising from an odontogenic cyst—the importance of computed tomography in the diagnosis of malignancy. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;100:365–368.
Dabbs, DJ, Schweitzer, RJ, Schweitzer, LE, et al. Squamous cell carcinoma arising in recurrent odontogenic keratocyst. Head Neck. 1994;16:375–378.
Eversole, LR, Sabes, WR, Rovin, S. Aggressive growth and neoplastic potential of odontogenic cysts: with special reference to central epidermoid and mucoepidermoid carcinomas. Cancer. 1975;35:270–282.
Kaffe, I, Ardekian, L, Peled, M, et al. Radiological features of primary intra-osseous carcinoma of the jaws: analysis of the literature and report of a new case. Dentomaxillofac Radiol. 1998;27:209–214.
van der Wal, KG, de Visscher, JG, Eggink, HF. Squamous cell carcinoma arising in a residual cyst. A case report. Int J Oral Maxillofac Surg. 1993;23:350–352.
Bilge, OM, Dayi, E. Central mucoepidermoid carcinoma of the jaws: review of the literature and case report. Ann Dent. 1992;51:36–39.
Browand, BC, Waldron, CA. Central mucoepidermoid tumors of the jaws: report of nine cases and review of the literature. Oral Surg Oral Med Oral Pathol. 1975;40:631–643.
Freije, JE, Campbell, BH, Yousif, NJ, et al. Central mucoepidermoid carcinoma of the mandible. Otolaryngol Head Neck Surg. 1995;112:453–456.
Inagaki, M, Yuasa, K, Nakayama, E, et al. Mucoepidermoid carcinoma in the mandible: findings of panoramic radiography and computed tomography. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;85:613–618.
Lopez, JI, Elizalde, JM, Landa, S. Central mucoepidermoid carcinoma: report of a case and review of the literature. Pathol Res Pract. 1993;189:365. 368
Sidoni, A, D’Errico, P, Simoncelli, C, et al. Central mucoepidermoid carcinoma of the mandible. J Oral Maxillofac Surg. 1996;54:1242–1245.
Strick, MJ, Kelly, C, Soames, JV, et al. Malignant tumours of the minor salivary glands—a 20 year review. Br J Plas Surg. 2004;57:624–631.
MALIGNANT AMELOBLASTOMA AND AMELOBLASTIC CARCINOMA
Ameerally, P, McGurk, M, Shaheen, O. Atypical ameloblastoma: report of 3 cases and review of the literature. Br J Oral Maxillofac Surg. 1996;34:235–239.
Buff, SJ, Chen, JT, Ravin, CC, et al. Pulmonary metastasis from ameloblastoma of the mandible: report of a case and review of the literature. J Oral Surg. 1980;38:374–376.
Slootweg, PJ, Müller, H. Malignant ameloblastoma or amelo-blastic carcinoma. Oral Surg Oral Med Oral Pathol. 1984;57:168–176.
Suomalainen, A, Hietanen, J, Robinson, S, et al. Ameloblastic carcinoma of the mandible resembling odontogenic cyst in a panoramic radiograph. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:638–642.
Ciola, B. Oral radiographic manifestations of a metastatic prostatic carcinoma. Oral Surg Oral Med Oral Pathol. 1981;52:105–108.
Mast, HL, Nissenblatt, MJ. Metastatic colon carcinoma to the jaw: a case report and review of the literature. J Surg Oncol. 1987;34:202–207.
Mucitelli, DR, Zuna, RE, Archard, HO. Hepatocellular carcinoma presenting as an oral cavity lesion. Oral Surg Oral Med Oral Pathol. 1988;66:701–705.
Nevins, A, Ruden, S, Pruden, P, et al. Metastatic carcinoma of the mandible mimicking periapical lesion of endodontic origin. Endod Dent Traumatol. 1988;4:238–239.
Redman, RS, Behrens, AS, Calhoun, NR. Carcinoma of the lung presenting as a mandibular metastasis. J Oral Maxillofac Surg. 1982;40:745–750.
Vigneul, JC, Nouel, O, Klap, P, et al. Metastatic hepatocellular carcinoma of the mandible. J Oral Maxillofac Surg. 1982;40:745–749.
Bainchi, SD, Boccardi, A. Radiological aspects of osteosarcoma of the jaws. Dentomaxillofac Radiol. 1999;28:42–47.
Batsakis, JG. Osteogenic and chondrogenic sarcomas of the jaws. Ann Otol Rhinol Laryngol. 1987;96:474–475.
Chindia, ML. Osteosarcoma of the jaw bones. Oral Oncol. 2001;37:545–547.
Clark, JL, Unni, KK, Dahlin, DC, et al. Osteosarcoma of the jaw. Cancer. 1983;51:2311–2316.
Gardner, DG, Mills, DM. The widened periodontal ligament of osteosarcoma of the jaws. Oral Surg Oral Med Oral Pathol. 1976;41:652–656.
Givol, N, Buchner, A, Taicher, S, et al. Radiological features of osteogenic sarcoma of the jaws: a comparative study of different radiographic modalities. Dentomaxillofac Radiol. 1998;27:313–320.
Seeger, LL, Gold, RH, Chandnani, VP. Diagnostic imaging of osteosarcoma. Clin Orthop Rel Res. 1991;270:254–263.
Vener, J, Rice, DH, Newman, AN. Osteosarcoma and chondrosarcoma of the head and neck. Laryngoscope. 1984;94:240–242.
Garrington, GE, Collett, WK. Chondrosarcoma, I: a selected literature review. J Oral Pathol. 1988;17:1–11.
Garrington, GE, Collett, WK. Chondrosarcoma, II: Chondrosarcoma of the jaws: analysis of 37 cases. J Oral Pathol. 1988;17:12–20.
Gorsky, M, Epstein, JB. Craniofacial osseous and chondromatous sarcomas in British Columbia—a review of 34 cases. Oral Oncol. 2000;36:27–31.
Hayt, MW, Becker, L, Katz, DS. Chondrosarcoma of the maxilla: panoramic radiographic and computed tomographic with multiplanar reconstruction findings. Dentomaxillofac Radiol. 1998;27:113–116.
Hertzanu, Y, Mendelsohn, DB, Davidge-Pitts, K, et al. Chondrosarcoma of the head and neck-the value of computed tomography. J Surg Oncol. 1985;28:97–102.
Dahlin, DC, Coventry, MB, Scanlon, PW. Ewing’s sarcoma. A critical analysis of 165 cases. J Bone Joint Surg Am. 1961;2:185–192.
Greer, RO, Mierau, GW, Favara, BE. Tumors of the head and neck in children. New York: Praeger; 1983.
Wood, RE, Nortje, CJ, Hesseling, P, et al. Ewing’s tumor of the jaw. Oral Surg Oral Med Oral Pathol. 1990;69:120–127.
Dahlin, DC. Bone tumors. Springfield, IL: Charles C Thomas; 1981.
Huvos, AG. Bone tumors. Philadelphia: WB Saunders; 1979.
O’Day, RA, Soule, EH, Goresg, RJ. Soft tissue sarcomas of the oral cavity. Mayo Clin Proc. 1964;39:169–181.
Slootweg, PJ, Müller, H. Fibrosarcoma of the jaws. A study of 7 cases. J Maxillofac Surg. 1984;12:157–162.
Taconis, WK, van Rijssel, TG. Fibrosarcoma of the jaws. Skeletal Radiol. 1986;15:10–13.
van Blarcom, CW, Masson, JMK, Dahlin, DC. Fibrosarcoma of the mandible. A clinicopathologic study. Oral Surg Oral Med Oral Pathol. 1971;32:428–439.
Bras, J, Batsakis, JG, Luna, MA. Rhabdomyosarcoma of the oral soft tissues. Oral Surg Oral Med Oral Pathol. 1987;64:585–596.
Grieman, RB, Katsikeris, NK, Symington, JM. Rhabdomyosarcoma of the maxillary sinus: review of the literature and report of a case. J Oral Maxillofac Surg. 1988;46:1090.
Masson, JK, Soule, EH. Embryonal rhabdomyosarcoma of the head and neck: report of 88 cases. Am J Surg. 1965;110:585.
Sekhar, MS, Desai, S, Kumar, GS:. Alveolar rhabdomyosarcoma involving the jaws: a case report. J Oral Maxillofac Surg. 2000;58:1062–1065.
Stobbe, GD, Dargeon, HW. Embryonal rhabdomyosarcoma of the head and neck in children and adolescents. Cancer. 1950;3:826–836.
Furutani, M, Ohnishi, M, Tanaka, Y. Mandibular involvement in patients with multiple myeloma. J Oral Maxillofac Surg. 1994;52:23–25.
Huvos, AG. Bone tumors. Philadelphia: WB Saunders; 1979.
Kaffe, I, Ramon, Y, Hertz, M. Radiographic manifestations of multiple myeloma in the mandible. Dentomaxillofac Radiol. 1986;15:31–35.
Witt, C, Borges, AC, Klein, K, et al. Radiographic manifestations of multiple myeloma in the mandible: a retrospective study of 77 patients. J Oral Maxillofac Surg. 1997;55:450–453.
Dahlin, DC. Bone tumors, ed 3. Springfield, IL: Charles C Thomas; 1981.
Daramola, JO, Ajagbe, HA. Presentation and behaviour of primary malignant lymphoma of the oral cavity in adult Africans. J Oral Med. 1983;38:177–179.
Maxymiw, WG, Goldstein, M, Wood, RE. Extranodal non-Hodgkin’s lymphoma of the maxillofacial region: analysis of 88 consecutive cases. SADJ. 2001;56:524–527.
Pazoki, A, Jansisyanont, P, Ord, RA:. Primary non-Hodgkin’s lymphoma of the jaws: report of 4 cases and review of the literature. J Oral Maxillofac Surg. 2003;61:112–117.
Yamada, T, Kitagawa, Y, Ogasawara, T, et al. Enlargement of the mandibular canal without hypesthesia caused by extranodal non-Hodgkin’s lymphoma: a case report. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;89:388–392.
Adatia, AK. Significance of jaw lesions in Burkitt’s lymphoma. Br Dent J. 1978;145:263–266.
Adatia, AK. Radiology of Burkitt’s tumour in the jaws. East Afr Med J. 1966;43:290–297.
Burkitt, D. A sarcoma involving the jaws in African children. Br J Surg. 1958;46:218–223.
Jan, A, Vora, K, Sandor, GKB. Sporadic Burkitt’s lymphoma of the jaws: the essentials of prompt life-saving referral and management. J Can Dent Assoc. 2005;71:165–168.
Levine, PH, Kamaraju, LS, Connelly, RR, et al. The American Burkitt’s lymphoma registry: eight years’ experience. Cancer. 1982;49:1016.
Sariban, E, Donahue, A, Magrath, IT. Jaw involvement in American Burkitt’s Lymphoma. Cancer. 1984;53:1777–1782.
Curtis, AB. Childhood leukemias: initial oral manifestations. J Am Dent Assoc. 1971;83:159–164.
Ficarra, G, Silverman, S, Jr., Quivey, JM, et al. Granulocytic sarcoma (chloroma) of the oral cavity: a case with a leukemic presentation. Oral Surg. 1987;53:709–714.
Greer, RO, Mierau, GW, Favara, BE. Tumors of the head and neck in children. New York: Praeger Scientific; 1983.
Nortjé, CJ. General practitioner’s radiology case 16. Acute leukemia. SADJ. 2001;58:393.