Chapter 4 Practical antimicrobial therapeutics
This chapter is not intended as a treatise on pharmacology, pharmacodynamics and antibacterial activity of antimicrobial agents. Other textbooks are available that deal with those subjects. However, antimicrobials are the most commonly employed group of drugs in large animal practice and their use is recommended on many occasions in the following chapters. To avoid repetition, the principles of usage and considerations for dose schedules and for the selection of antibacterial agents for certain circumstances are given here and in the formulary.
Some of the information or opinions presented are based on clinical use rather than experimental evidence. However, this is often unavoidable, because unfortunately many antimicrobial agents have in the past been released for use in large animals with minimal pharmacological or clinical evaluation in the species concerned. As a result it has been assumed, often erroneously, that information obtained from studies in laboratory animals, dogs and humans can be directly applied to the ruminant, horse and pig.
Principles of antimicrobial therapy
The success of antimicrobial therapy depends upon maintaining, at the site of infection, a drug concentration that will result, directly or indirectly, in the death or control of the infectious organism with minimal deleterious effect to the host. In order to achieve this aim the antimicrobial agent must have activity against the organism at its site of infection and it must be administered in such a way as to maintain an effective inhibitory or lethal concentration. These principles apply to therapy in all species and dictate the choice of antimicrobial agent to be used. However, in farm animal veterinary practice there are also other important considerations
• Cost is critical. This consideration includes not only the primary cost of the drug but also related factors such as the ease and frequency of administration and the duration of treatment
• Tissue residue problems and withdrawal periods must also be taken into consideration and are a primary determinant of treatment strategy
• Animal welfare becomes a consideration when a decision is made not to treat an animal because of concerns about cost or the occurrence of residues that would preclude marketing of the animal or its products in the future
• Antimicrobial resistance and the risk of contributing to the emergence and problem of antimicrobial resistance is a concern that has increasing attention, not so much with the therapeutic use of antimicrobials but certainly with the prolonged administration of antimicrobials in animal feeds for disease prevention.
In the theoretically ideal situation, the following steps would be taken before selecting an antimicrobial agent for therapy.
• First, the site of infection would be located and the identity of the infecting organism established by culture
• Second, the minimal inhibitory concentration (MIC) of each antimicrobial agent for the infecting organism would be identified
• Third, an initial selection would be made based on the sensitivity of the organism and the knowledge of the capacity of the individual antimicrobial agents to penetrate to the site of infection and to achieve and exceed these concentrations at nontoxic dose rates
• Fourth, the dose rates, route of administration and frequency of administration required to achieve these concentrations for each of the selected antibiotics, in the particular animal species being treated, would then be considered
• Finally, selection of a particular drug would be based on a consideration of the potential toxicity to the host, on the likely relative efficiency of each drug, on the cost and ease of administration and, in food animals, on costs associated with the relative withholding periods.
It is obvious that for many clinical situations all these steps cannot be followed before therapy is instituted. It may take several days to establish the identity of the infectious agent unless it can be ascertained by clinical diagnosis. The identification of the organism helps in determining its potential sensitivity but even without identification the establishment of exact MICs by tube dilution for each antimicrobial agent also takes several days, and the results would frequently be historical by the time that they were received. Also, a knowledge, for each antimicrobial agent, of the varying tissue and organ levels achieved following varying doses given by different routes of administration is not easily remembered and therefore not easily available in large-animal field situations. Nor, unfortunately, is complete information of this type available for each antimicrobial agent in all large-animal species.
Because of this uncertainty, some expedients are adopted in clinical antimicrobial therapy. One of them is the concept of the recommended dose and another is the use of disk sensitivity testing, both of which are discussed later in this chapter. Regardless of these expedients, it should be recognized that rational antimicrobial therapy is based upon the principles outlined above. These important principles in antimicrobial therapy are discussed in greater detail individually.
IDENTIFICATION OF THE INFECTION BY CLINICAL EXAMINATION
In infectious disease, clinical examination aims to identify the nature and site of the infection and its cause. The importance of making an accurate clinical diagnosis cannot be overemphasized as the first prerequisite for successful antimicrobial therapy. The establishment of a diagnosis in many instances immediately identifies the pathogen and previous clinical experience may suggest the specific antibiotic to be used and allow a confident prediction of success of the therapy. Equally, it may indicate the likelihood of unsuccessful or prolonged therapy. For example, the diagnosis of erysipelas or Glasser’s disease in pigs or strangles in horses immediately identifies the etiological cause of the infection and the type of antimicrobial agent that will be required. It also gives some indication of the likely ease or difficulty of successful therapy and of the duration of therapy that might be required.
The establishment of an accurate diagnosis is also important in animals where chemotherapeutic control of further disease may be required. Thus, in pigs, an accurate differentiation between the diarrhea of swine dysentery and that associated with coliform gastroenteritis is essential for effective prophylactic medication.
It is often not possible to establish an exact diagnosis at the first examination and yet in almost every instance it is essential that treatment be instituted at that time, not only for the wellbeing of the patient but also for the maintenance of good client relationships. The lack of a definitive etiological diagnosis should never preclude the initiation of therapy during the period when further tests are being carried out. Rational therapy in these circumstances depends very much on clinical acumen. A detailed examination leading to a determination of the site and nature of the infection can frequently allow an educated guess at the likely pathogen and allow rational therapy during the period that specific diagnosis is being determined by culture.
This approach is frequently used initially in field situations in large animal medicine but it requires good clinical knowledge. Clinicians should be familiar not only with the individual diseases of large animals but also with their differential diagnosis and with the relative prevalence of each condition in their area. They should also be familiar with the types of organism that may produce infections in various body areas with similar clinical manifestations and the relative prevalence of each of these. Thus peracute mastitis in recently calved cows is most commonly associated with infection by staphylococci but can also be associated with coliform organisms or, more rarely, Actinomyces (Corynebacterium) pyogenes or Pasteurella multocida. Treatment must be initiated immediately if the gland or even the cow is to be saved. There are subtle clinical and epidemiological differences that may allow some clinical differentiation between these agents but frequently treatment must begin with no sure knowledge of which agent is involved. There are two approaches in this type of situation:
• Therapy may be directed at the most prevalent or likely agent and, in situations where one particular infectious agent is the most prevalent cause of the condition, this is a rational approach
• In other situations, where a disease could be associated with any one of several different organisms, each with a different sensitivity, and where clinical experience suggests that no one organism is the predominant infectious agent, it is more common to initiate therapy with a broad-spectrum antimicrobial agent or a combination that will have activity against all the possibilities. If indicated, the antibacterial agent being used for therapy may have to be changed to a more specific one once the actual pathogen and its sensitivity have been determined.
There are also clinical situations where therapy must begin when there is little knowledge of the site of infection and consequently no knowledge of the identity of the infecting agent. This occurs where infection, such as abscessation, occurs in deep-seated and clinically inaccessible organs such as the liver or spleen. Also in these situations it may not be possible to determine the nature and cause of the disease by laboratory examination although biochemical examinations and ultrasound may give some indication of the site. In these cases therapy is generally started with a broad-spectrum antimicrobial agent or a combination of lesser ones, and the accuracy of the selection is determined by subsequent clinical response.
TAKING SAMPLES FOR DIAGNOSIS
In teaching hospitals, there is ready access to bacteriology laboratories, which frequently contain automated and rapid systems for sensitivity testing. However, in practice the taking of samples for this purpose is generally restricted and limited by such factors as the availability of a diagnostic laboratory, and by cost. Furthermore, in many cases the results of culture and sensitivity are historical by the time they are received. Nevertheless, information of this type is of value for future similar cases and it provides prevalence data and data of antimicrobial sensitivity that can be used for background clinical knowledge and justification for extralabel drug use in food-producing animals.
The recognition of when samples should be taken for microbiological examination and sensitivity testing comes with clinical experience. In general, the approach is different when dealing with individual sick animals from when dealing with groups of animals and a contagious disease. In individual animals, cost and the time for processing usually limit the taking of samples to valuable stud animals and to horses. They should be taken from individual sick animals with life-threatening conditions so that, if a response is not obtained to initial therapy, the subsequent choice of antimicrobial agent can be based on laboratory data. They should also be taken from animals with disease syndromes that may be caused by one of several agents or by an organism that may show variable resistance patterns. Examples would be infective arthritis in foals or Gram-negative sepsis.1 The increasing emergence of variable resistance patterns in veterinary pathogens places an increasing importance on sampling and sensitivity testing and many practices have now established their own laboratories for this purpose.
Samples are also frequently taken from chronic, poorly responsive conditions to determine the best course of treatment. In groups of animals where there is contagious disease, the taking of samples to establish or confirm the etiological diagnosis and to determine the best drug for chemotherapy is most important. Where there are a large number of animals at risk it is important to confirm the initial choice of therapy as soon as possible so that remedial steps can be taken if this was incorrect. It is also important in these situations to have a confirmed accurate etiological diagnosis so that control measures can be instigated to prevent future problems. Thus an outbreak of diarrhea in postweaned pigs may be due to coliform gastroenteritis, salmonellosis or swine dysentery. Clinical and pathological examination may eliminate swine dysentery but not allow complete differentiation between salmonellosis and coliform gastroenteritis. An aminoglycoside could be used for the initial therapy of the outbreak but, at the same time, samples are taken for culture and sensitivity to determine the exact antimicrobial sensitivity of the infectious agent in case there is resistance to this antibiotic. Also, by this procedure the exact etiological diagnosis will be determined, which will then determine recommendations for future control of the disease.
Consideration should be given to the nature of the sample for examination. In outbreaks of diarrhea there is little point in taking fecal samples from chronically scouring and runted animals. Samples should be taken from animals at the onset of diarrhea. The site of sampling can also have an influence that may affect the relevance of the results. In animals with pneumonia, the nasal flora may not reflect that in the lung and cultures are best taken as transtracheal aspirates of the lower respiratory system.2,3
Similarly, fecal Escherichia coli strains are not always representative of small-intestinal strains in scouring calves.4
ANTIMICROBIAL SENSITIVITY TESTS
RATIONALE
Antimicrobial sensitivity testing is not required with all infections because many organisms are invariably sensitive to one or more antimicrobial agents and in most cases these can be used for therapy. The clinician should be familiar not only with the spectrum of each antimicrobial drug but also with the spectrum of sensitivity for the common organisms involved in diseases of large animals. Sensitivity testing is generally reserved for members of those groups of organisms that show considerable variation in sensitivity to individual antimicrobial agents.
There can be considerable area-to-area variation and spatial and temporal clustering in the sensitivity patterns of individual organisms.5-7 It is wise to establish the broad patterns of general sensitivity or resistance for these groups in any practice area and to monitor any change periodically so that therapy can be guided by this information. This also can provide information justifying the extralabel use of antimicrobials in food-producing animals.
The purpose of sensitivity testing is to attempt to determine if the organism under consideration is likely to be susceptible to the action of an antimicrobial agent at the drug levels that can be achieved using the usual therapeutic dose rates. In clinical terms, organisms are considered to be either sensitive or resistant to the action of an antimicrobial. However, with many organism–antimicrobial associations, resistance or susceptibility is not an all-or-none phenomenon but is dependent upon drug concentration. Organisms that may be resistant to low levels of an antimicrobial agent are frequently susceptible to its action at higher concentrations. Thus an organism that is susceptible to the action of benzylpenicillin at a concentration of 0.1 μg/mL would be considered sensitive because equivalent levels of benzylpenicillin can be easily achieved in the blood and tissues. One that was susceptible only at a concentration above 5 μg/mL might be considered resistant, even though it is possible to achieve and maintain this concentration of benzylpenicillin in the tissues with high and frequent dosing.
SENSITIVITY TEST METHODS
Tube sensitivity tests
Sensitivity tests may be quantitative or qualitative. Tube sensitivity tests, using serial dilutions of the antimicrobial drug against a standard dose of the test organism, provide quantitative information in terms of an exact MIC of the drug being tested. The MIC is the lowest antibiotic concentration that prevents the growth of bacteria within a defined period of time and under the conditions of the test. Tube sensitivity testing is the gold standard. With most antibiotics, a mean plasma level 2–5 times the MIC needs to be sustained through the dosing interval for effective therapy. These tests are laborious and time-consuming and are seldom used in practice situations for these reasons.
Disk sensitivity tests
Disk sensitivity tests provide more limited qualitative information. They are generally a valuable adjunct in the choice of an antimicrobial agent for therapy, particularly for systemic diseases. However, the limitations of the usual method of testing, and the limitations of interpretation should be recognized by the clinician.
The Kirby–Bauer technique is the most commonly used method of disk diffusion sensitivity testing. With this technique, disks are impregnated with a standard amount of antibiotic that diffuses into the media to produce a zone of inhibition of growth. With a standard concentration of antibiotic in the disk and standard antibiotic sensitivity test media and test conditions, the concentration of the diffused antibiotic at any given distance from the disk is relatively predictable and constant. There is a linear relationship between the diameter of the zone of inhibition and the log2 of the MIC. For each antibiotic MIC, breakpoints have been established and corresponding zone size breakpoints established above or below which an organism is classified as resistant, susceptible or of intermediate sensitivity.
Although the Kirby–Bauer disk sensitivity testing system has a quantitative genesis the results are qualitative – especially as used in most practice laboratories. MIC breakpoints are specific values used to assign bacteria to one of three classifications – susceptible, intermediate and resistant.
The MIC breakpoints and thus the published reference zone sizes for resistance and susceptibility are often based on the pharmacokinetic properties of each antimicrobial in humans. These frequently have limited relationship to their pharmacokinetic properties in animals, particularly ruminants.8
Also, a single antimicrobial considered to be representative of its class is used to test sensitivity to that class of antimicrobials, but commonly this representative is not the antibiotic agent present in commercially available antibiotic treatments for livestock.8 Further, the use of specific zone diameters to establish resistance and susceptibility assumes a standard test with standard media and under standard conditions. These conditions are frequently not met in veterinary practice laboratories.
Despite these limitations, disk sensitivity tests can be used as a guide to the selection of antimicrobials for therapy in large-animal veterinary practice. They are of particular value in selecting a choice of antibiotic with organisms that exhibit variable patterns of resistance and where this pattern for any one antibiotic is essentially bimodal in distribution.9 They may have limited value in the testing of organisms where the sensitivities are clustered around the MIC breakpoint. However, there is a lack of validation for susceptibility testing being predictive for treatment outcome in almost all large-animal diseases as the breakpoints have not been validated.8,10
There should not be over-reliance on the results of testing for sulfonamide sensitivity, as these are frequently misleading and a good clinical response can be achieved with therapy even though the sensitivity test suggests resistance.
Frequently, with disk sensitivity tests, the organism proves sensitive to a number of different antimicrobial agents. The selection of one of these for therapy is based on such factors as ease of administration and cost. The relative efficacy of any one of the agents cannot be determined by comparison of the size of the zones of inhibition.
Microtiter techniques
The development of semiautomated microtiter methodology for direct MIC determinations allows many reference diagnostic laboratories and teaching hospitals to determine MIC concentrations directly in bacterial sensitivity testing. The results are more directly applicable to rational therapy and, in particular, have more relevance than disk diffusion tests for determining the sensitivity of organisms that cluster around the MIC breakpoint for a given antibiotic.9,11
OTHER CONSIDERATIONS
The antimicrobial sensitivity of an organism can vary considerably depending upon the species of animal from which it is isolated. E. coli isolates from pigs generally show a greater degree of antibiotic resistance than those isolated from adult cattle. Similarly, Campylobacter spp. isolates from pigs show substantially different antibiotic sensitivity patterns from those isolated from sheep. Isolates from the same species may also vary significantly in sensitivity, so that E. coli isolated from mastitis in cattle generally have a broader sensitivity pattern than those isolated from enteric disease in calves. In addition, there are area differences and changes with time. Low levels of antibiotic fed for growth-promoting purposes may influence sensitivity patterns, and in herds where growth promoters are being used it is generally wise not to use the same drug or members of the same group for therapeutic purposes without prior testing.
Woolcock JB, Mutimer MD. Antibiotic sensitivity testing: caeci caecos ducentes? Vet Res. 1983;113:125-128.
Prescott JG, Baggot JD. Antimicrobial susceptibility testing and antimicrobial drug dosage. J Am Vet Med Assoc. 1985;187:363-368.
Baggot JD, Prescott JF. Antimicrobial selection and dosage in the treatment of Equine bacterial infections. Equine Vet J. 1987;19:92-96.
Wilcke JR. Therapeutic decisions. Choosing appropriate antimicrobial therapy. Probl Vet Med. 1990;2:279-289.
Dow SW, Papich MG. Keeping current on developments in antimicrobial therapy. Vet Med. 1991;86:600-609.
Constable PD, Morrin DE. Treatment of clinical mastitis: Using antimicrobial susceptibility testing profiles for treatment decisions. Vet Clin North Am Food Anim Pract. 2003;19:139-155.
1 Brewer BD, Koterba AM. Compend Contin Educ Equine Pract. 1990;12:1773.
2 Spurlock SL. Equine Pract. 1989;11:6.
3 Thomas A, et al. Vet Res Commun. 2002;26:333.
4 Constable PD. J Vet Intern Med. 2004;18:8.
5 Gunn GL, Low JC. Vet Rec. 2003;152:537.
6 Singer RS, et al. J Am Vet Med Assoc. 1998;212:1001.
7 Dunlop RH, et al. Prev Vet Med. 1998;34:265. 283
8 Constable PD. Proceedings of the 37th Annual Convention of the American Association of Bovine Practitioners. 2004;37:11.
9 Libal MC, et al. Proc Am Assoc Vet Lab Diagn. 1986;29:9.
10 Constable PD, Morrin DE. J Am Vet Med Assoc. 2002;221:103.
ANTIBIOTIC RESISTANCE
Antimicrobial resistance is a natural biological phenomenon and the introduction of antibiotics into clinical use has almost invariably been followed by the emergence of resistance to these drugs in bacterial populations.1 When a microbial population is exposed to an antibiotic, the more susceptible organisms will succumb, and antimicrobial use in human medicine and in agriculture naturally must result in the selection of antimicrobial-resistant phenotypes. This occurs in nonpathogens as well as pathogens.2 Resistance is generally slow to reverse or is irreversible.
There are a number of mechanisms whereby resistance is engendered. Resistance that results from spontaneous mutation of chromosomal genes encoding a target site is probably of limited importance in clinical settings. It occurs more frequently with certain antibacterials, i.e. rifampin, and may be combated by the inclusion of a second antibacterial in the treatment regimen. Plasmid- and transposon-determined drug resistance is of much more importance in clinical situations and has led to widespread multiresistance patterns in certain bacterial populations.
Plasmids are extrachromosomal genetic elements that replicate independently of the chromosome. They can be transferred within, and in some cases between, bacterial species and may also act as vectors for transposons. They may encode for single or multiple patterns of antibiotic resistance and, increasingly, multiple patterns of resistance are emerging. With veterinary pathogens, plasmid-determined resistance is particularly important in the Enterobacteriaceae, Staphylococcus aureus and to some extent in Pasteurella spp.3-5
Virtually all antibiotics given in therapeutic doses cause marked changes in the microflora of sites in the host normally colonized by bacteria. There is suppression of the sensitive flora with subsequent selection and colonization by resistant bacteria. In pigs, there is some evidence that therapeutic use of antibiotics in individual animals does not greatly influence herd flora resistance patterns but in-feed medication of postweaned pigs selects for antibiotic resistance that maintains in finisher pigs.6 The feeding of antibiotics for growth promotion and the feeding of antibiotic-treated milk to calves will select for resistance among organisms within the alimentary tract. These resistant organisms can persist in the animal and in the environment and subsequently form part of the normal colonizing flora of other animals, so that it is not unusual to isolate organisms, E. coli for example, that are resistant to one or more antibiotics even though the animal from which they were isolated had never received antibiotic medication.7,8
There is a higher prevalence of antibiotic-resistant E. coli in the normal intestinal flora of young animals than adults.9 The prevalence is higher in young animals reared intensively, such as veal calves and pigs, and in environments where antibiotic usage has exerted selection pressure. The prevalence falls with increasing age and the intestinal flora of adults generally shows a broader sensitivity pattern. Although many of these resistant organisms are not pathogens, they contribute a pool of R plasmids that can be transmitted to pathogens, and therapy decisions should take into account what antibiotics are in routine use on the farm as growth-promoting additives. Tetracyclines and neomycin are commonly incorporated in calf milk replacers with the label claim that they are growth promoters and aid in the control of calf diarrhea. However, there are no published studies that support health benefits.10 There are studies that show improved growth of calves on medicated milk replacers compared with control calves but this difference is lost after weaning and not of any production benefit.
Feeding antimicrobials to livestock and poultry to reduce disease and promote weight gain has been standard practice in developed countries for several decades but is engendering increasing concern and the occurrence of antimicrobial resistance is beginning to be considered to be a societal issue. The concern is that antimicrobial use in food-producing animals may affect human health by the presence of drug residues in foods, and by promoting the presence of antibiotic-resistant strains in animals that can subsequently infect humans through food or from effluent contamination of the environment.11-13 The consequences of this also include an increased risk for resistant pathogens to be transferred to humans by direct contact with animals. Although many of the growth-promoting antibiotics used in animals are not the same as those used for human therapy, antimicrobial exposure can initiate bacterial resistance to compounds of dissimilar structures.14
There is a particular risk to farmers, farm workers and veterinarians from exposure to contamination in the farm environment.13,15-17 and a risk from transfer of resistant bacteria through farm food and via environmental contamination from farm effluents.18
Public and medical concern about the ways in which antimicrobials are used in agriculture has particularly been aroused by the development of vancomycin-resistant enterococci in humans associated with the use of the related drug avoparcin as a growth-promoter in animal feeds.19 In response to concerns about the emergence of antimicrobial resistance, Sweden banned all growth-promoting antibiotics in 1986. This was followed by a ban on avoparcin and virginiamycin in Denmark in 1995 and 1998. Finally, the European Union (EU) banned the use of avoparcin in 1997 and bacitracin, spiramycin, tylosin and virginiamycin for growth promotion in 1999. The effects of these bans on the antibiotic resistance of flora in animals and humans will take some time to determine. There has been an apparent reduction in vancomycin resistance in fecal enterococci isolated from humans and animals.20,21 There has also been an apparent increase in morbidity and mortality among pigs, associated with enteric infections, diarrhea and chronic infections due to Lawsonia intracellularis. This increase in animal disease since the ban has resulted in a substantial increase in the use of therapeutic antibiotics for food animals in Europe, primarily tetracyclines, trimethoprim/sulfonamides and macrolides.21
With respect to the emergence of antibiotic resistance in zoonotic organisms, a particular concern has been plasmid-determined multiple antibiotic-resistant strains of salmonella that have emerged and caused rapidly spreading epidemics of disease in young calves in England and Europe.22 These multiple resistance patterns have been associated with particular phage types and biotypes of Salmonella typhimurium and Salmonella dublin.
Preventing the spread of multiresistant organisms is not easily achieved and there are examples of spread involving virtually every major pathogenic bacterial group.23 An example is the emergence and spread of S. typhimurium DT 104, in which multiple antibiotic resistance is chromosomally determined. A pathogen of a variety of different animal species, including humans, this organism spread globally in the 1990s. Because of the advanced salmonella surveillance system in the UK, this organism was first recognized as causing outbreaks of disease in cattle and humans in the UK and its emergence was initially attributed to the use of antimicrobials in cattle. There is however no evidence in support of this24 and its spread was due to its colonizing ability, not to selection by the feeding of antimicrobials. The history of the emergence and spread of this organism, which was unrelated to the use of antimicrobials in livestock and related more to the colonizing ability of DT104, should act as a brake on proposals to use changing patterns of antimicrobial resistance as a measure of the risk of the use of antimicrobials in livestock.
Plasmid-determined multiple patterns of resistance are likely to increase in organisms in environments where selection pressure is high as a result of frequent antibiotic usage. The use of antibiotics in agriculture is an obvious target to reduce this selection, and is frequently blamed for the problem of developing antibiotic resistance in human pathogens. Nosocomial infection with antibiotic-resistant animal pathogens is an emerging problem in veterinary hospitals and procedures for limiting their spread are available.25
Whereas the major concern has been directed at antibiotic use for growth promotion there are also moves, in some countries, to restrict the use of certain antimicrobials, e.g. fluoroquinolones, from therapeutic use in farm animals. However, a European survey of antimicrobial susceptibility among zoonotic and commensal bacteria from food-producing animals found that, although there was variation among European countries in the resistance of enteric organisms, this largely involved the older antimicrobials, and that resistance to the newer compounds used to treat humans was low.26 Equally, a study of mastitis pathogens over a 7-year period in the USA showed no trend towards increased resistance and reported a reduction of resistance to beta-lactam antimicrobials for several Gram-positive mastitis pathogens.27
Saunders JR. Genetics and evaluation of antibiotic resistance. Br Med Bull. 1984;40:54-60.
Hinton M. The ecology of Escherichia coli in animals including man with particular reference to drug resistance. Vet Rec. 1986;119:420-426.
Pohl P, Lintermann P. R plasmid reservoirs and circulation. In: Rico RG, editor. Drug residues in animals. Veterinary Science and Comparative Medicine A Series. New York: Academic Press, 1986.
Corpet DE. Microbiological hazards for humans of antimicrobial growth promoter use in animal production. Rev Med Vet. 1996;147:851-862.
National Research Council. The use of drugs in food animals: benefits and risks. Washington, DC: National Academy Press, 1998;211.
Tallefson L, Angulo FJ, Fedorka-Cray PJ. National surveillance for antibiotic resistance in zoonotic enteric pathogens. Vet Clin North Am Food Anim Pract. 1998;14:141-150.
Bailar JC, Travers K. Review of assessments of the human health risk associated with the use of antimicrobial agents in agriculture. Clin Infect Dis. 2002;34(Suppl 3):S135-S143.
Wise R, Soulsby EJL. Antibiotic resistance — an evolving problem. Vet Rec. 2002;151:371-372.
Besser TE, Hancock D, Davis M. The veterinarians role in controlling the emergence and dissemination of drug-resistant bacteria. J Vet Med Educ. 2003;30:136-139.
Thiele-Bruhn S. Pharmaceutical antibiotic compounds in soils — a review. J Plant Nutr Soil Sci. 2003;166:145-167.
Rooklidge SJ. Environmental antimicrobial contamination from terraccumulation and diffuse pollution pathways. Sci Total Environ. 2004;325:1-13.
1 Kayser FH. Vet Microbiol. 1993;35:257.
2 Schroeder CM, et al. Emerg Infect Dis. 2002;8:1409.
3 Hinckley LS, et al. J Am Vet Med Assoc. 1985;187:709.
4 Boyce JR, Morter RL. Am J Vet Res. 1986;47:1204.
5 Libal M, et al. J Am Vet Med Assoc. 1982;180:908.
6 Dunlop RH, et al. Prev Vet Med. 1998;34:265. 283
7 Gellin G, et al. Appl Environ Microbiol. 1989;55:2287.
8 Hinton M, et al. J Appl Bacteriol. 1985;58:131.
9 Khachatryan AR, et al. Appl Environ Microbiol. 2004;70:752.
10 Constable PD. In: Proceedings of the Annual Conference of the American Association of Bovine Practitioners. 2003;37:137.
11 Corper DE. Vet Microbiol. 1993;35:199.
12 Gorbach SL. Vet Hum Toxicol. 1993;35(Suppl 1):13.
13 Rooklidge SJ. Sci Total Environ. 2004;325:1.
14 Courvalin P. Emerg Infect Dis. 2001;7:489.
15 Hamscher G. Environ Health Perspect. 2003;111:1590.
16 Haller M, et al. J Chromatogr A. 2002;952:111.
17 Meyer M, et al. Sci Total Environ. 2000;248:181.
18 Thiele-Bruhn S. J Plant Nutr Soil Sci. 2003;166:145.
19 Lu K, et al. Emerg Infect Dis. 2004;10:679.
20 Aarestrup FM, et al. Antimicrob Agents Chemother. 2001;45:2054.
21 Casewell M, et al. J Antimicrob Chemother. 2003;52:159.
22 Threlfall EJ. Soc Appl Bacteriol Symp Ser. 1992;21:S96.
23 Tenover FC. Clin Infect Dis. 2001;33(Suppl 3):S108.
24 Hancock D, et al. Brown C, Bolin CA, editors. Emerging diseases of animals. Washington, DC: ASM Press. 2000:217-243.
25 Brewer BD, Koterba AM. Compend Contin Educ Equine Pract. 1990;12:1773.
Practical usage of antimicrobial drugs
ANTIBIOTIC DOSAGE: THE RECOMMENDED DOSE
Theoretically, there is no set dose for any antimicrobial agent. The concentration of an antimicrobial drug required for effective activity against different microorganisms varies and these requirements could be met by varying the dose rate of the drug. However, this is an impractical situation and in practice one works from the recommended dose. The recommended dose is one that will give blood and tissue levels that will be effective against very susceptible organisms, with minimal side effects to the host. In this respect the recommended dose should be considered as a minimum dose.
If one is dealing with organisms that require higher concentrations of the drug for therapeutic effectiveness, the recommended dose can be exceeded. With low-toxicity antibacterials this dose may be exceeded severalfold and with drugs such as benzyl penicillin this is a frequent therapeutic ploy. However, with antibacterials that have toxic potential the recommended dose should only be exceeded with caution and frequently it is wise to search for a different antimicrobial agent to which the organism is more sensitive.
Similarly, the recommended dose may be exceeded in an attempt to increase the concentration gradient in sensitive infections where necrotic tissue produces long diffusion paths. The recommended dose may also be exceeded for management reasons, as in the case of the treatment of sheep with footrot or mycotic dermatitis, where only a single treatment is administered for practical purposes.
The label dose is the dose stated on the label of the drug and is the legal dose that can be used for that product. The label states the required withdrawal periods for avoidance of tissue or milk residues. The recommended doses given in the sections on individual diseases are based on our expectations of therapeutic efficiency, and may exceed the label dose recommendations for certain drugs. The problem of persisting tissue residues should be recognized when label recommendations are exceeded and withdrawal periods should be adjusted accordingly.
Label dose levels and dose intervals for many of the antimicrobial agents used in large animals are frequently too low and too long. In many cases, there are no obvious pharmacological reasons for these dosing regimes. Unfortunately, pharmacokinetic studies of the earlier antimicrobial agents released for use in large animal species were limited at the time of their release and it would appear that in many instances the label dose established at that time was inadequate. Some estimate of the dose required for an antimicrobial drug can be obtained by a comparison of the MICs required for activity against various organisms with the blood and tissue levels of the drug obtained at various dose levels. Usually, levels 3–5 times the MIC are considered necessary for effective therapy, and it is generally considered desirable to maintain these levels over the treatment period, especially with bacteriostatic antimicrobials, although this is probably not essential.
The ultimate proof for dose levels and dose intervals of an antimicrobial is by clinical trials of its efficacy in the treatment of infectious disease. It is apparent that antimicrobial drugs are effective in many diseases in large animals at the dose rates and intervals currently in use. Nevertheless, as the results of pharmacokinetic studies in farm animals become available it is quite probable that they will suggest changes in the dose levels and intervals for several of the antimicrobial drugs in use, which may result in more efficacious therapy and lead to label doses that have a broader spectrum of activity against disease.
ROUTES OF ADMINISTRATION
INTRAVENOUS INJECTION
Intravenously administered antibiotics attain high and immediate blood and tissue levels. This route should be used in the treatment of septicemia and other life-threatening diseases. The concentrations obtained are much higher than those obtained with equivalent doses of the same drug given intramuscularly or orally, and consequently greater diffusion concentrations are achieved at sites of infection. For this reason this route of administration may also be used in an attempt to increase the drug concentration in areas where the antibiotic normally achieves only low concentrations, and where areas of necrosis increase the length of the diffusion pathway. Intravenous administration may also be indicated in chronic infections such as corynebacterial pneumonia in foals, where high diffusion concentrations are required in order to penetrate the abscess areas and the capsular material of the organism.
An initial intravenous loading dose may combat the development of stepwise resistant mutants. Because of the initial higher blood and tissue levels, the intravenous route may also be used for the treatment of infections that are only moderately sensitive to the antibacterial drug being used. This is because effective concentrations may be achieved by repeated intravenous dosing which would not be achieved by equivalent doses given intramuscularly or orally.
For practical reasons the intravenous route of administration is used for low-concentration, high-volume antimicrobial agents such as sulfamethazine and oxytetracycline. It is also preferred to the intramuscular route in racehorses where there is a need to avoid muscular soreness. The need to avoid muscle damage in beef cattle close to marketing may also dictate intravenous administration.
Administration by this route is not without its dangers. Acute toxic reactions either to the drug or to its vehicle are more common when intravenous administration is used. Drugs specifically formulated for intravenous use should be used, or the manufacturer’s recommendations on the advisability of the use of this route for any preparation should be followed. Severely toxemic terminal cases may die immediately following injection, and in the owner’s mind death may be attributed to the therapy.
Injections should be given slowly and not as a bolus. Therapy by repeated intravenous administration is generally restricted to hospital situations and can be expensive because of the added cost of the intravenous preparations. In field situations an initial intravenous loading dose followed by sustaining intramuscularly administered doses is frequently indicated in the treatment of infectious diseases and is sound therapeutic policy.
The jugular vein is used in all species except the pig, where the inaccessibility of superficial veins other than the ear veins makes the jugular route of administration generally impractical. Perivascular reactions and intravascular thrombosis are a hazard with this route, especially following the administration of irritant drugs such as sulfonamides and tetracyclines.
INTRAMUSCULAR INJECTION
Intramuscular injection is the most commonly used method for antimicrobial administration in large animals. Where possible this route should be avoided in meat-producing animals, especially with irritant preparations. Lesions can be detected at slaughter 12 months after the intramuscular injection of long-acting tetracyclines.1 If the drug must be given intramuscularly in a meat-producing animal it should be given in the muscles of the neck, as scar tissue and blemish are more likely to be detected at this site in the cutting process after slaughter and they can be trimmed. With certain antibiotics, drug residues may persist at these sites for long periods, and the label recommendation for withdrawal or withholding time should be followed.2
Irritant drugs should be used with care in horses, or avoided, as this species more commonly develops severe reactions at the site of injection. The development of such reactions is usually an indication to change to alternative therapy. Oil-based vehicles frequently produce severe reactions at the site of injection in horses and should not be used.
There is evidence, for some antibiotics at least, that the site of intramuscular administration can influence the rate of absorption, the bioavailability and the subsequent pharmacokinetics of the administered antibiotic. In both cattle and horses, injection in the neck gives more favorable pharmacokinetic parameters than does injection into the gluteal or shoulder muscles.3-5 Injection into the dewlap gives the poorest bioavailability. These differences presumably result from differences in the spread of the injected drug within and between the muscles and differences in blood supply. With intermuscular spread there is a greater absorption area and less compromise of capillary and lymphatic structures.5 Injection into the side of the neck of horses is considered to be malpractice in some countries. When irritant preparations must be given to horses it is wise to inject them into the muscle of the chest between the forelegs, as reactions in this area have less tendency to spread and are more accessible to drainage and treatment.
At all sites, care should be taken to ensure that the injection is not inadvertently given intravascularly, by applying negative pressure to the syringe prior to injection. In adult animals no more than 10 mL should be given at any injection site. Large injection volumes can result in the formation of encapsulated antibiotic-filled cysts in muscle.2 Label directions of the maximum amount to be given at any one site should not be exceeded.
With most antimicrobial drugs, excepting the repository forms and drugs of an irritant nature, peak blood concentrations are obtained within 30–120 minutes of injection. However, the bioavailability of drugs given by intramuscular injection is markedly influenced by their formulation and irritant nature. This is especially marked with oxytetracycline preparations.
INTRAPERITONEAL INJECTION
Intraperitoneal injection is occasionally used for antimicrobial administration, especially in cattle close to market size, and where intravenous administration for various reasons may be impractical. It is also occasionally used in pigs with diarrhea, where the antibacterial drug is combined with fluids for rehydration. In cattle the injection is given in the right flank midway between the last rib and the tuber coxae and at least 10 cm ventral to the lateral processes of the lumbar vertebrae so as to avoid retroperitoneal and perirenal deposition of the drug. An aseptic injection technique should be used. Animals with peritonitis are also occasionally additionally treated by this route of injection. In horses with peritonitis the peritoneal cavity can be drained through a cannula inserted in the ventral midline as used for abdominal paracentesis, and the antimicrobial agent is injected via this route. Intraperitoneal injection may also be used for the parenteral administration of the tetracycline group in acutely toxemic animals or in animals with severe respiratory distress where intravenous injection may result in collapse and even death.
SUBCUTANEOUS INJECTION
Subcutaneous injection has not been commonly used in large-animal practice but concerns regarding lesions in meat following intramuscular injections is leading to a greater use of this route. Providing the drug is not deposited in a fat depot, this route provides a reasonable alternative to intramuscular injection.6 With irritant preparations there is a danger of excessive reaction and the occurrence of sterile abscesses. Very small animals (piglets) are often treated by this route.
ORAL ADMINISTRATION
Oral administration of antimicrobial agents is generally restricted to preruminant animals, young foals and pigs. The blood and tissue levels achieved following oral administration are considerably less than those achieved by an equivalent dose of the same antimicrobial agent given parenterally, and for this reason the oral dose rate is generally 2–5 times greater than the parenteral dose. Oral drugs are less reliable because absorption characteristics may vary with the volume of ingesta, the presence or absence of gastric and intestinal stasis or hypermotility and the nature of the ingesta, which variably bind the orally administered drug. For example, oxytetracycline and trimethoprim have a much lower bioavailability to calves when administered in milk, rather than in water, because of the high degree of binding to milk.7 There is some evidence that the oral administration of antibiotics to calves in glucose– glycine–electrolyte solutions is associated with more favorable absorption characteristics. The aminoglycoside and polymyxin groups of antimicrobial agents are not absorbed from the alimentary tract and benzylpenicillin is largely destroyed within the stomach.
The oral route is the easiest method for administration, and where the cost of revisits is a significant consideration this route is often chosen for continuing medication, as it is within the capability of any owner. In general, however, systemic infections are better treated by parenteral injection and certainly treatment should be initiated by this route. The oral route is the one of choice for the treatment of enteric infections. Experimental studies have shown that the oral administration of antibiotics to healthy neonatal calves may induce villous atrophy within the intestine and a malabsorption diarrhea.8 This occurred particularly with neomycin and to a lesser extent with tetracycline and ampicillin. Although this does not negate the use of antibiotics for specific therapy of enteritis in young calves (when this is indicated), it does suggest that prophylactic use of oral antibiotics has a risk in young calves.
Prolonged oral medication at therapeutic levels may result in superinfection in all animal species. Commonly a yeast, staphylococcus or Pseudomonas aeruginosa is involved. It occurs most commonly in calves given courses of differing antimicrobial agents. It is more common following medication involving tetracyclines and usually a treatment period of at least 2 weeks is required for its development.
Antimicrobial drugs are seldom given orally to ruminant animals. Exceptions are the use of sulfonamides, especially as sustaining medication following initial parenteral treatment, and low-level antibiotic therapy to feedlot animals to reduce the incidence of liver abscess and respiratory disease. Blood levels following oral administration in ruminants are variable and frequently not achieved until 12–18 hours after dosing. Also, many antibacterials are destroyed or inactivated within the rumen. Orally administered antimicrobials cause a significant disruption of the ruminal flora and by itself this may result in a syndrome of ruminal stasis, anorexia and depression. If antibacterial agents are given orally to ruminants, the course should be followed by re-establishment of the ruminal flora by cud transfer.
Contamination of feedstuffs
Antibiotic contamination of rations is a potential problem in feed mills that process medicated and nonmedicated feeds consecutively. The inadvertent feeding of antibiotics to cattle and horses can result in clinical disease and the cause may not be immediately apparent to the investigating clinician. This can occur when cattle and horses are fed medicated pig feed, but may also occur when regular rations become contaminated with antibiotics. Residual carryover of medicated material into other feedstuffs can occur with feed-mixers of various types and also via residues in conveyors, hoppers and trucks. The risk for feedstuffs being contaminated can be quite high and the most common contaminating drugs are chlortetracycline, sulfonamides, penicillin and ionophores.9
Within 24 hours of being fed medicated feed, dairy cattle show anorexia, rumen stasis and subsequently pass custard-consistency feces containing undigested fiber. There is a precipitous fall in milk production. Dullness, muscle fasciculation, ketosis, hypocalcemia and recumbency have also been observed. Affected cattle usually recover when placed on non medicated feed, but milk production may be adversely affected for the remainder of the lactation. Feeds contaminated with dimetridazole, lincomycin and tylosin have been incriminated,10,11 although there is debate as to the role of tylosin in this syndrome.12 The carryover of medicated material into other feeds can also create violative tissue residues at slaughter.9 Sulfonamide contamination of swine rations is a particular problem.13
The use of orally administered antimicrobial agents in horses over 3 months of age should be approached with great care. Their use can be followed by diarrhea, which is often intractable and results in chronic debilitation or death. Clindamycin and lincomycin carry a high risk and are probably totally contraindicated but macrolides, tetracyclines, tylosin and metronidazole are also associated with risk in stressed horses.
Water medication of pigs
The oral route is the most common and convenient one for group medication of pigs. The antibacterial agent may be incorporated in the water or in the feed. For the treatment of disease in pigs, water medication is preferred as sick pigs may drink, whereas they frequently will not eat. Also, water medication can usually be started immediately, whereas the mixing of an antibacterial agent with the diet for piggeries purchasing prepared diets may take 1–2 days. Antibiotic bioavailability is also less in pelleted feeds.14
In outbreaks of contagious disease in pigs, the sick pigs within the group are usually initially treated individually by parenteral injection followed by mass medication of the water supply. Large swine units usually have facilities for in-line medication; small swine units may not. With pigs using troughs, water medication is no problem. However, with automatic watering systems, medication must be through the header tank, if this can be isolated, or more commonly the water is turned off and medicated water is provided for the pigs via portable 200 L drums with a drinking bowl or nipple drinker inserted in the side.
In determining the concentration of antibiotic required in the water, the total daily dose of the drug is computed by multiplying the total weight of the group of pigs in kilograms by the daily dose of the drug in milligrams per kilogram. This dose must then be added to the amount of water that will be consumed in one day. It is obvious that this amount will vary according to climatic conditions and to the nature of the disease in the pigs. For example, diarrheic pigs may drink more than normal quantities. In practice, a rule of thumb of 10% body weight water consumption of pigs between weaning and market age has been found to be satisfactory, with estimates of 15% for situations in which high water consumption can be expected. The total daily dose is thus added to the number of liters of water equivalent to 10–15% of the estimated total body weight of the group. In pregnant sows, water consumption is usually 5–8 L/d, but lactating sows may drink 15–20 L/d. When there is doubt as to the exact water consumption the medication can be added to the lower estimate and, when consumed, fresh water provided for the remainder of the day. Water medication is generally continued for a period of at least 5 days. Antibiotics may deteriorate rapidly in water and a fresh mix should be prepared each day. Most drugs for water medication have label directions.
Water medication in cattle
There are some major limitations in the mass medication of water supplies of cattle. The daily amount of water consumed is usually directly proportional to the amount of dry matter intake. Anorexia or inappetence will result in a marked decrease in water intake to mere maintenance requirements. Depending on the drug used, the palatability of the medicated water may affect intake. With large drinking water tanks that are replenished on a continuous basis, or even two or three times daily, it is difficult to determine how much drug should be added on a daily basis in order to maintain a reasonably steady concentration. On a theoretical basis, automatic in-line water medicators should provide a uniform concentration of drug in the water supply. However, some medicators are extremely unreliable and regular surveillance and servicing may be necessary. In countries where below-freezing temperatures occur during the winter months, the medication of water supplies may be difficult and impractical under certain management conditions.
Dietary medication
This is generally used for long-term disease control. In many countries, the amount of an antimicrobial that can be added to a feed is restricted to the approved label level and the veterinarian has no legal right to alter this concentration. The drug is usually added at the feed mill.
OTHER ROUTES
Other routes of administration may be used to increase the level of antibacterial drug in areas where diffusion following parenteral administration of the drug may be limited and when high local levels are required. These include intra-articular, intrapleural and subconjunctival injection. Non-irritant preparations should be used with strict aseptic technique. In most cases these treatments should be supported by parenteral treatment. The indications are described in the special medicine section.
Intramammary infusion of drugs is dealt with under mastitis.
Intratracheal administration of antibiotics has its advocates for the treatment of pneumonia in cattle. In theory, this could result in higher levels of antibiotics at the site of infection, although with many pneumonias diffusion through the affected lung must be minimal. The antibiotics are administered in sterile physiological saline equivalent to 2.0 mL/kg body weight. An extensive study has shown variation in absorption and persistence between antibiotics administered by this route, when compared to parenteral administration, but has concluded that there is no potentially useful advantage to its use.15
The local administration of antibiotics may not always be the preferred route despite historical precedence. For example, in the treatment of the genital tract, it has been shown that parenteral administration of antibiotics achieves tissue concentrations of drug in all areas of the genital tract, whereas intrauterine infusion results in comparable concentrations only in the endometrium and uterine secretions. Local and/or parenteral administration may be indicated in different cases of genital tract infection.16
1 George MH, et al. J Anim Sci. 1995;73:3510.
2 Mawhinney H, et al. Aust Vet J. 1996;74:140.
3 Hoffman B, et al. Dtsch Tierarztl Wochenschr. 1986;93:310.
4 Nouws JM, Vree TB. Vet Q. 1983;5:165.
5 Firth EC, et al. Am J Vet Res. 1986;47:2380.
6 Gilman JM, et al. J Vet Pharmacol Ther. 1987;10:101.
7 Groothuis DG, van Miert ASJPAM. Vet Q. 1987;9:91.
8 Mero KN, et al. Vet Clin North Am Food Anim Pract. 1985;1:581.
9 Lynas L, et al. Food Addit Contamin. 1998;15:162.
10 Crossman PJ, Payser MR. Vet Rec. 1981;108:285.
11 Anon. Vet Res. 1984;114:132.
12 MacKinnon JD, et al. Vet Rec. 1984;115:278.
13 Rosenburg MC. J Am Vet Med Assoc. 1985;187:704.
14 Mevius DJ, et al. Vet Q. 1986;8:274.
DRUG DISTRIBUTION
ABSORPTION
Antibiotics of the aminoglycoside group and polymyxins are not absorbed from the alimentary tract and if circulating levels of these antibiotics are required they must be given by parenteral injection. Where both intestinal and systemic levels are required, as may be the case in neonatal colibacillosis, these drugs should be given both orally and parenterally. Benzylpenicillin and methicillin are destroyed by acid pH and significant blood levels are not achieved following oral administration but blood levels are achieved with ampicillin and amoxicillin. Certain sulfonamides (phthalylsulfathiazole, phthalylsulfacetamide, sulfaguanidine and succinyl sulfathiazole) are not absorbed from the alimentary tract. The remaining antibiotics and sulfonamides are absorbed following oral administration in preruminant calves and lambs and in pigs and horses. However, in general, blood and tissue levels obtained are considerably lower than those achieved with equivalent doses given parenterally. Whey feeding (calcium) will inhibit the absorption of tetracylines in pigs.
DISTRIBUTION
Factors governing the distribution of antimicrobial agents in the body fluids are complex, and distribution should be considered as involving a multicompartmental system with all body compartments being in contact directly or indirectly with the blood. The occurrence of exchange, and its rate, between the blood and the various tissue compartments is governed by the factors that influence the diffusion of solutes, such as the concentration of the drug and the volume of blood flow through the tissues and the volume of the tissue. It is also considerably influenced by the extent of protein binding of the drug in blood and in the tissues, the ionization constant of the drug, pH differences in the compartments, and the lipid solubility of the drug. Drug distribution is also influenced by age and the disease state of the animal.
In most diseases infection occurs in the extravascular tissue compartments and it is the concentration of the unbound drug at these sites that determines the efficacy of therapy. The majority of antibiotics diffuse relatively freely in extracellular fluids but sulfonamides, the chloramphenicol group, tetracyclines, fluoroquinolones and macrolides have a distribution that more closely approximates total body water, and they can enter cells.
There are several so-called barriers to antimicrobial diffusion and these include the brain and cerebrospinal fluid, serous cavities, joints and synovial fluid, the eye and the placenta and fetus. In general sulfonamides, the tetracyclines and chloramphenicol have some ability to penetrate these barriers in the normal state, whereas penicillin may not. Erythromycin has the ability to penetrate intracellularly and across most barriers but will not produce effective levels in the brain or cerebrospinal fluid. Members of the aminoglycoside group of antibiotics generally achieve effective levels in synovial fluid and the pleural and peritoneal fluid but not in the brain or eye. The importance of these barriers, especially those of serous cavities and synovia, in the presence of inflammation is open to doubt and effective therapy can often be achieved by the use of antibiotics that do not in normal situations reach these areas unless they are inflamed. An exception to this rule is infections involving the eyes where, in order to achieve effective levels, high circulating levels of the antimicrobial agent are required and intravenous injection to achieve this is usually necessary. Lipophilic drugs diffuse into tears and parenterally administered erythromycin, oxytetracycline and gentamicin, for example, may achieve bacteriostatic concentrations in tears. In many areas, especially joints and the peritoneal, pleural and pericardial cavities, high levels of the required antimicrobial agent can be achieved by local administration.
Almost all antimicrobial agents are excreted via the kidney, and the urine usually contains high levels of them. This feature is not of great significance in large animals, where urinary tract infections are comparatively rare, but violative residue levels can persist in the kidney for long periods with drugs such as the aminoglycosides. Penicillins and tetracyclines have a significant enterohepatic cycle, and erythromycin also may obtain significant levels in bile.
Baggot JD. Principles of drug distribution in domestic animals. Philadelphia: WB Saunders, 1977.
Baggot JD. Factors involved in the choice of routes of administration of antimicrobial drugs. J Am Vet Med Assoc. 1984;185:1076-1082.
Koritz GD. Relevance of peak and trough concentrations to antimicrobial therapy. J Am Vet Med Assoc. 1984;185:1072-1075.
Short CR, Clark CR. Calculation of dosage regimens of antimicrobial drugs for the neonatal patient. J Am Vet Med Assoc. 1984;185:1088-1093.
Riviere JD. Veterinary clinical pharmacokinetics. Compend Contin Educ Pract Vet. 1988;10:24-30. 314–328
Koritz GD. Practical aspects of pharmacokinetics for the large animal practitioner. Compend Contin Educ Pract Vet. 1989;11:202-204.
Prescott JF. Antimicrobial chemotherapy. In: Biberstein EL, Yuan CZ, editors. Review of veterinary microbiology. Boston, MA: Blackwell Scientific, 1990.
Riviere JD. Pharmacologic principles of residue avoidance for veterinary practitioners. J Am Vet Med Assoc. 1991;198:809-816.
DURATION OF TREATMENT
For certain infectious diseases there is an established regimen of therapy that is known from clinical experience to be therapeutically effective. Where such regimens are known they are stated in the treatment section for the individual diseases in the Special Medicine section. As a rule of thumb in undifferentiated diseases, therapy should be continued for at least a 3–5-day period, or longer if there is evidence of chronic infectious disease with localization. An alternative rule of thumb is that treatment should be continued for at least 1 day beyond the return of body temperature to normal, especially if bacteriostatic antibiotics are being used. Chronic pyogenic processes may require treatment for a 2–4-week period or even longer.
DRUG COMBINATIONS
Combinations of antimicrobial drugs are frequently used in veterinary practice. Combinations of antimicrobial agents are used either to achieve a synergistic effect in the case of a single infection, or to achieve a broad spectrum of activity in the case of infections involving more than one agent. Combinations may also be of value in combating the emergence of resistant mutants during therapy.
The combination of two drugs may result in indifference, where the effect is either that of the single most effective drug or is equal to the sum of the effects of the two individual drugs, or it may result in synergism or antagonism. There are, however, no hard and fast rules for combinations that will result in any of these effects. Knowledge of these effects results largely from laboratory animal studies and from some human therapeutic trials. From these trials it is evident that the occurrence of synergism is very much dependent on the type of infectious organism, and to some extent the site of infection, and, whereas two drugs may show a synergistic effect with one type of infection, the effect may be indifferent or even occasionally antagonistic with other infective agents. Antagonism is equally not easily predictable but the drugs that most commonly result in antagonistic effect when combined with others are the tetracycline group, chloramphenicol and the macrolide groups.
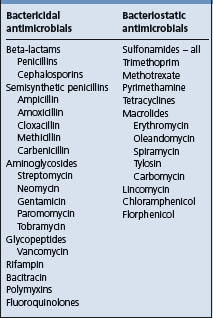
A traditional approach has been that combinations of bactericidal drugs will generally result in an indifferent effect or in synergism; combinations of bacteriostatic drugs generally give an indifferent effect, whereas combinations of a bactericidal with a bacteriostatic drug may result in antagonism (Table 4.1). This approach is, however, too general for validity as interactions are specific to individual infections and are dose-dependent.
In farm animals, synergistic activity between penicillin and streptomycin has been demonstrated in the therapy of mycotic dermatitis and footrot in sheep.
The synergism between aminoglycoside and beta-lactam antimicrobials is widely used in the approach to the therapy of sepsis in neonates. Carbenicillin and gentamicin in combination can be of value in therapy against P. aeruginosa, Klebsiella and Proteus spp., and tylosin and oxytetracycline can be of value in treating infection with Mannheimia and Pasteurella spp. Trimethoprim and sulfonamide combinations are of special value in treating several infectious diseases in large animals. Rifampin and erythromycin show in-vitro synergism against Rhodococcus equi, as does a combination of gentamicin and penicillin. Tiamutilin and tetracycline show in-vitro synergism against several swine respiratory pathogens and herd studies show a measured response in the control of respiratory disease greater than that achieved by chlortetracycline alone.
Drug combinations are also used for broad-spectrum therapy. An accurate diagnosis with consequent recognition of the likely infectious organism allows specific antibacterial therapy and obviates the need for broad-spectrum antibacterial therapy. However, there are clinical situations where broad-spectrum therapy, including the possibility of combined drug therapy, is indicated. These include such problems as the acute septicemia, where a number of different organisms, with differing antibacterial sensitivities, can produce identical clinical disease, and those infections associated with organisms that have a varying sensitivity depending upon the isolate. The requirement for immediate treatment without knowledge of the bacterial sensitivity dictates the use of antimicrobial drugs designed to obtain a broad spectrum of activity.
The availability of broad-spectrum drugs such as ampicillin or amoxicillin and trimethoprim-potentiated sulfonamides has lessened the need to use drug combinations but the latter may still be necessary in certain situations and are fully indicated. Although antagonism has not been demonstrated in clinical veterinary situations it is wise to avoid bacteriostatic and bactericidal drug combinations.
Fixed-dose combinations are available commercially for some antibiotics but they are not recommended for use and are gradually being withdrawn from the market or being declared not legal for use in food-producing animals. Fixed-dose combinations suffer from the deficiency that the dose level of any one of the drugs in the combination is dictated by the level of the other. Also, the excretion rates of the two drugs may be markedly different. The most common of these, fixed-dose penicillin/streptomycin combinations, suffer from this deficiency.
Where combinations of antibacterial drugs are used they should be given individually and at their respective recommended doses and repeats. Some antibiotics are physically incompatible when mixed together. The incompatibility may rest with the drugs or their vehicles and may be visible, as with crystalline benzylpenicillin and neomycin, or it may be inapparent, as with gentamicin and carbenicillin. The two drugs should be given separately at separate sites. Incompatibilities can also occur with antibiotics and intravenous fluid solutions – especially those containing protein hydrolysates.
Antibiotics may influence the activity of other drugs. In particular, chloramphenicol and tetracyclines inhibit liver microsomal metabolism and may significantly increase the half-life of drugs metabolized by this mechanism, such as digitalis or barbiturates, with resultant potential toxicity.
Reilly PEB, Issacs JP. Adverse drug interactions of importance in veterinary practice. Vet Rec. 1983;112:29-33.
Whittem T, Hanlon D. Dihydrostreptomycin or streptomycin in combination with penicillin G in dairy cattle therapeutics: a review and re-analysis of published data. NZ Vet J. 1997;45:178-184. 223–229
McKenzie HC, Furr MO. Aminoglycoside antibiotics in neonatal foals. Compend Contin Educ Pract Vet. 2003;25:457-469.
ADDITIONAL FACTORS DETERMINING SELECTION OF AGENTS
In addition to the considerations of bacterial sensitivity to the antimicrobial agent, there are other important factors that dictate the selection of the antimicrobial agent to be used in a particular case. In most clinical situations several agents would be effective and a choice needs to be made amongst them.
COST
This is a major factor and includes not only the primary cost of the drug but also the ancillary costs that may be associated with its administration. This is a most important factor in agricultural animals but of less importance with pleasure horses. The importance of the primary cost of the drug is obvious. For example, in most countries a 5-day course of treatment with procaine benzylpenicillin will cost considerably less than one with, for example, oxytetracycline. If there is no specific indication for the use of the more expensive drug then the less expensive one should be used. The ancillary costs associated with repeat visits to administer the drug may also be important. The practice of dispensing drugs for continuing intramuscular therapy varies between countries and veterinary practices and has an influence on this consideration.
EASE OF ADMINISTRATION
This is a further factor that influences the nature of the drug and treatment used. In general, one avoids starting a course of therapy with an antibacterial such as tetracycline, which may require daily intravenous administration, in favor of one that can be administered more simply – unless there are good therapeutic reasons for choosing the former. In situations where facilities are poor, where mustering or yarding is difficult, or where mass medication is required, long-acting repository preparations may be indicated. Irritant preparations are avoided where possible.
TOXICITY
This is always a consideration when dealing with infections that may require high dose rates of antimicrobial drugs, or in chronic infections that require a prolonged course of therapy. Where a choice is available, antimicrobial agents with a low incidence of toxic side effects at high doses are chosen. As in all clinical situations involving large animals it is essential to make an assessment of the case and to attempt a prognosis. The possible cost and duration of treatment should be estimated and the owner advised of this. When examined in this light the decision may be against treatment and for salvage slaughter.
BACTERICIDAL OR BACTERIOSTATIC ANTIMICROBIALS
Antibiotics are either primarily bactericidal or primarily bacteriostatic in their activity (Table 4.1). Some of the bactericidal group are bacteriostatic at low concentration. Both classes rely on intact and effective body defense mechanisms for full effect. Although in terms of clinical response little if any difference can be detected between the two groups in most diseases, in certain situations it is probably advisable to choose a bactericidal antibiotic for therapy. This is especially true when dealing with acute septicemic infection where there is frequently a significant leukopenia, and quick maximal bactericidal effect is required. There is also the need to prevent subsequent localization.
Bactericidal antimicrobials are also indicated for antibacterial treatment of secondary infection in granulocytopenic syndromes such as bracken fern poisoning or chronic furazolidone poisoning in calves. Bactericidal antibiotics are also preferable in the treatment of heavily capsulated organisms, such as Klebsiella spp. and R. equi, which show antiphagocytic activity. Infections in which significant intracellular parasitism occurs are a problem. The majority of antimicrobials that diffuse relatively freely into cells are bacteriostatic in activity and, although the disease may be controlled by their use, infection may still persist in a latent carrier state.
DRUG DETERIORATION
Many antibacterials lose their activity rapidly when kept under adverse conditions. Quality control in terms of purity, efficacy and freedom from toxicity costs money but for these reasons it is preferable to purchase from known reputable companies and follow their recommendations with respect to storage and expiration periods. The use of cheap antibacterial preparations, often purchased in bulk and simply packaged, and distributed with little consideration for factors influencing drug stability, often results in poor therapeutic results. Crystalline or dry preparations that require reconstitution to a solution before parenteral administration are frequently presented this way because their activity degenerates rapidly once they are in solution. Therefore, once they have been prepared they should be used immediately, or the manufacturer’s recommendations should be followed regarding storage. Attention should be paid to the length of activity expected following reconstitution.1 Temperature and exposure to sunlight can be important factors in antibiotic stability and become especially important in farm ambulatory practice: car cold boxes should be used to store antibiotic preparations and other sensitive drugs.
UNFAVORABLE RESPONSE TO THERAPY
In clinical cases that do not respond to antimicrobial therapy the initial consideration should be that the wrong antimicrobial agent has been chosen for therapy. This is especially true of infectious conditions of undetermined etiology where the drug has been chosen on the basis of an educated guess. In these circumstances adequate time should be given for an evaluation of the efficacy of the treatment before a change is made. In general a 3-day period of treatment is allowed for this evaluation provided there is no marked deterioration in the clinical state or further elevation of temperature during this period. If there is no response to initial therapy then, in the case of conditions of undetermined etiology, it is generally best to change to an entirely different class of antimicrobial agent. However, the possibility of viral or noninfectious etiology should always be considered in these cases and the case and diagnosis should be reviewed before any change is made.
In any situation where there is a poor response to therapy the usual causes of this failure should be considered in any further adjustments to therapy or future therapy of similar cases. The first and most obvious of these is that the organism is either insensitive to the drug or that it is not susceptible to the level of the drug that is being used for therapy. There are two possible approaches. The first is to increase the dose rate and dose frequency and/or to change the route of administration so that higher and possibly effective levels will be achieved, bearing in mind the possible toxic consequences. The second, and safer, approach is to change the antimicrobial agent being used. This problem can be avoided if the organism and its potential sensitivity can be identified, either by clinical examination or by appropriate sampling with culture and sensitivity testing. The development of resistance during antimicrobial treatment of an individual animal is not a recognized problem in large-animal medicine.
Another common cause of poor response is that the infection is situated in an area to which the drug is poorly accessible. If this is associated with an area behind a barrier to the entry of the antibiotic, such as the joints or the eye, it may be necessary to resort to higher dose rates and frequency, or intravenous administration of the drug, or to ancillary local treatment into this area. Alternatively, another drug with superior penetrability may be used.
Organisms must be actively metabolizing in order for antimicrobial agents to exert their effect. This feature can result in poor response to therapy or relapse following discontinuation of therapy in chronic infections such as endocarditis or where there is excessive necrotic or fibrotic tissue associated with the infection. In these instances, dormant organisms and the long diffusion tracks make effective cure difficult and high antimicrobial levels sustained over longer periods are required. In purulent conditions surgical drainage, where possible, is an essential adjunct to antimicrobial therapy.
The importance of ancillary and supportive therapy to counteract the effects of shock, toxemia and dehydration that may be associated with infection cannot be overemphasized and frequently such therapy may markedly influence the outcome of a case. It is obvious, for example, that 3 mL of antibiotic will do little to counter the effects of a 4 L fluid deficit in a scouring calf.
DRUG WITHDRAWAL REQUIREMENTS AND RESIDUE AVOIDANCE
In most countries there are requirements for the withdrawal of antimicrobial agents from the feed for specified periods prior to slaughter, and animals or their milk cannot be marketed for certain periods following antimicrobial therapy.
Antibiotic contamination of food products can be a public health risk, although proven risk for toxicity or allergy from antibiotics in humans is minuscule. An example would be allergic reactions to antibiotic residues – particularly penicillin. There are also commercial considerations where residues of antibiotics in milk can cause considerable problems in the manufacture of milk products. Effects on starter cultures for cheese and yoghurt can be particularly deleterious and can result in downgrading or total loss of large quantities of manufacturing milk.
The purpose of withdrawal requirements is to ensure that meat and milk for human consumption is wholesome and does not contain violative residues of drugs. The public’s concern for the wholesomeness of the food that it consumes will determine the food that it buys. Cooperative quality assurance programs involving both the producer and the veterinarian are a major answer to this concern.
A withdrawal period is the time during which the animal must be held free of the drug before it can be marketed. In the case of milk, the term withholding period is commonly used and defines the period during which milk cannot be sent for human consumption following the treatment of the animal with a drug. A tolerance for the pharmacologically active ingredient in tissues is set by regulatory authorities for each drug. The tolerance level is the level below which tissue concentrations must fall before they are considered safe for human consumption, and there is a large margin of safety.2
The required withdrawal and withholding periods will vary between antimicrobial agents and also with the same antimicrobial agent depending upon the amount of drug given; factors such as age and the disease state of the animal are also important. Unfortunately, the required withdrawal and withholding periods to ensure freedom of food products from violative drug residues are not known for the variety of dose concentrations and dose intervals of the various antimicrobials that could be used in clinical practice – nor are they likely to be known in the near future. In many countries this has led to regulations that limit the quantity of antibiotics in drug products. Label instructions explaining product usage and drug withdrawal times are required. These label instructions include what is generally called the label dose.
The label dose and extralabel use
The label dose (and dose interval) is a dose of an antimicrobial for which the specific withdrawal and withholding periods have been established, and these are stated in conjunction with the label dose. The label dose is the officially approved or legal dose rate for that drug.
When an antimicrobial is used, it is incumbent upon the practitioner to notify the owner that the animal cannot be marketed (or milk sent for human consumption) before the accompanying withdrawal (or withholding) period has expired. The practitioner may be legally liable if a violation occurs and this notification has not been given.
In the USA the label dose of a drug also includes use only in the species of animal for which the drug is labeled, the class of animal (lactating versus nonlactating dairy cow), the disease conditions indicated by the label, the route of injection, the amount of drug to be injected at one site and the number of repeat treatments that can be given. These label directions, and the need to follow them, are directed primarily at lay users of these drugs and lay users may not use the drug in a nonlabel fashion. The label directions should also be followed by the veterinarian whenever possible.
Requirements for extralabel use of drugs in the USA
• Extralabel use of drugs (ELDU) is permitted only by or under the supervision of a veterinarian
• ELDU is allowed only for US Food and Drug Administration (FDA)-approved animal and human drugs
• A valid veterinarian–client–patient relationship is a prerequisite for all ELDU
• ELDU must be for therapeutic purposes only (animal’s health is suffering or threatened), not drugs for production use
• Rules apply to dosage form drugs and drugs administered in water – ELDU in feed is prohibited
• ELDU is not permitted if it results in a violative food residue, or any residue that may present a risk to public health
Extralabel use
There are times where extralabel use of drugs is necessary and veterinarians can do this where they have established a proper veterinarian–client–patient relationship.3 It is the intention that the label dose should be one that is therapeutically effective for that drug. However, this is not always the case, and the label dose should not be confused with the term Ôrecommended dose’ as used elsewhere in this book. There are also circumstances where, although the label dose may be therapeutically efficient in many cases, it is not for the particular case in hand. In fact, optimal therapeutic dose regimes often require extralabel use of the drug.4,5 In these situations, antimicrobial drugs may need to be used at dose concentrations and dose intervals different from the label dose. Extralabel use of the drug may be therapeutically necessary for the successful treatment of the problem, but it is not officially approved and the establishment of the required withdrawal period is entirely incumbent upon the veterinarian. The withdrawal period in these circumstances cannot always be extrapolated from that for the label dose.2
Definition of valid veterinarian–client– patient relationship (American Veterinary Medical Association)
An appropriate veterinarian–client–patient relationship will exist when:
1. The veterinarian has assumed the responsibility for making medical judgments regarding the health of the animal(s) and the need for medical treatment, and the client (owner or other caretaker) has agreed to follow the instructions of the veterinarian
2. There is sufficient knowledge of the animal(s) by the veterinarian to initiate at least a general or preliminary diagnosis of the medical condition of the animal(s). This means that the veterinarian has recently seen and is personally acquainted with the keeping and care of the animal(s) by virtue of an examination of the animal(s) and/or by medically appropriate and timely visits to the premises where the animal(s) are kept, and
3. The practicing veterinarian is readily available for followup in case of adverse reactions or failure of the regimen of therapy
WITHDRAWAL PERIODS
Label dose withdrawal periods are determined from pharmacokinetic studies of excretion following administration of the label dose. However, the rate of drug elimination from the body can be influenced by drug dose and dose frequency. For example, the metabolism and excretion half-life of sulfonamides in cattle is dose-dependent. With repeated dosing of antibiotics such as tetracycline and the aminoglycosides, there is deposition of the antibiotic in certain tissues and following cessation of drug administration there is a slow release from these tissues and a long washout period.6,7 During this washout period there are decreasing concentrations of the drug in tissues and in milk, which, although not of therapeutic importance, are sufficiently high to be violative. This presents a dilemma to the veterinarian trying to establish withdrawal periods.
The occurrence of significant washout periods following prolonged therapy with antibiotics has only recently been recognized and there are few data on their duration at different dose concentrations and dose frequencies. A further problem is that most pharmokinetic parameters have been determined in healthy animals and altered physiology in diseased animals can markedly alter elimination half-lives; there is also considerable animal-to-animal variation.2 Rather than try to guess the possible withdrawal period for an extralabel use, computer-based data information banks with easy access are established to provide this information.8 One of these is the Food Animal Residue Avoidance Databank (FARAD), which provides recommendations for withdrawal intervals for extralabel drug use based on analysis of published pharmacokinetic data, foreign and domestic label drug withdrawal intervals and established maximum residue limits. Another, the Veterinary Antimicrobial Support System (VADS), aims to provide information on optimal therapeutic regimens against pathogens in cattle and swine using approved drugs and treatment regimens but also providing information on extralabel regimens that might be required in the face of a refractory pathogen.
RESIDUE TESTING
Currently, the only way to attempt to ensure nonviolation with extralabel use of antimicrobials is to test for residues.9,10 There are a very large number of testing systems becoming available, which vary in their method of detection of the presence of antibiotics.11-14 Tests such as the Swab Test on Premises (STOP), Calf Antibiotic and Sulfa Test (CAST), Live Animal Swab Test (LAST), Fast Antimicrobial Screen Test (FAST) (which has a higher sensitivity and shorter analytical time and has largely replaced the use of STOP and CAST), the Delvotest-P, the Charm Inhibition Assay and the Charm Farm and Disk Assays are based on the inhibition of growth of Bacillus stearothermophilus var. calidolactes or Bacillus stearothermophilus. While relatively cheap and easy to perform, they have a risk for false-positive results due to inhibition of growth by inhibitory substances other than antibiotics in milk, particularly substances in milk from inflamed mammary glands.12,13,15-17 They are sensitive for detecting penicillin and its derivative compounds but less sensitive to other classes of antibiotic. Other commercially available tests use a variety of different immunological detection methods and test for a single antibiotic or class of antibiotics.
TESTING FOR COMPLIANCE
Most countries have a monitoring program to detect the occurrence of residues in meat. In the USA, sampling is such as to provide a 95% probability of finding a violative residue when 1% of the population is violative. The occurrence of violative residues in red meat is very low as the prevalence of infectious disease is low in the period before slaughter. Feedlot cattle can have a high prevalence of disease in the early feeding period but there is a substantial subsequent period on-feed before the animals are slaughtered, which exceeds the withholding period of most drugs used for treatment of disease occurring during the early feeding period. Violative drug residues occur predominantly in cull dairy cows and in bob veal calves.
The concentrations for the various antibiotics that are violative are not stated in this chapter for two reasons. First, they vary from country to country. Secondly, the violative concentrations tend to be set by the sensitivity of the detection assay used by the regulatory authority and, as assay technology improves, legally acceptable minimal concentrations will be lowered. Local regulatory publi-cations should be consulted for current requirements.
Assay techniques can be remarkably sensitive. An example is the occurrence of violative residues of chloramphenicol in the milk, blood and urine of cows that had teat or skin lesions sprayed with a 5% chloramphenicol solution18 – an illegal drug for use in animals for food in most countries.
CAUSES OF RESIDUE VIOLATIONS IN MILK
In a retrospective study of reasons for the presence of violative antibiotic residues in milk19 failure to withhold milk for the full withdrawal period and accidental inclusion of treated milk in the shipment were the most common. Accidental inclusion of treated milk can occur when there is inadequate identification of treated cows. The veterinarian should work with the producer to establish a system that easily identifies cows whose milk is subject to a withholding period. Colored leg markers are one system and are immediately visible to the milker.
Contamination of recorder jars and milking equipment with the high concentration of antibiotic secreted in milk in the first milking after treatment is a further reason for residue violations. Treated cows should be milked last in large dairies, or milked with separate equipment, and are preferably kept separate as a hospital string.
Common causes of antibiotic residues in milk
• Extended usage or excessive dosage
• Failure to observe withdrawal times
• Failure to identify treated animals
• Contaminated milking equipment
• Products not used according to label directions
• Lack of advice on withdrawal period
• Withholding milk from treated quarters only
• Early calving or short dry periods
Other reasons for residue violations include short dry periods, where dry cow therapy has been used but the cow has calved earlier than expected. The infusion of dry cow treatments into the udder of heifers prior to calving for the prevention of summer mastitis has also been followed by the presence of violative residues for as long as 26 days.9 A less common cause is the accidental milking of dry cows, where the latter are not kept as a separate group, and the withholding of milk from only treated quarters.19 The use of dry cow infusion preparations for treatments during lactation can occur by mistake if drugs intended for the treatment of lactating cows are not kept in a separate storage area from other drugs.
The risk for residues is higher for farms that have higher frequency of antibiotic usage and for those that use part time labor.10 The use of records to document treatments and the day of exit from the withholding period is an important preventive measure. Sulfonamides, tetracyclines, penicillins, aminoglycosides, cephalosporin and chloramphenicol have been found in milk in the USA.20
CAUSES OF RESIDUE VIOLATIONS IN BEEF CATTLE
Violative drug residues occur predominantly in cull dairy cows and in bob veal calves.21 In one study22 the primary reasons for violations in this group were:
• Failure to observe the withdrawal periods (61%)
• Use of an unapproved drug (10%)
• The feeding to calves of milk or colostrum from a treated cow (9%). A greater risk for residues occurs in herds that feed larger volumes of colostrum, possibly reflecting contamination from dry cow therapy; waste milk, discarded from treated cows and fed to calves, is also a risk23,24 especially if extralabel doses of antimicrobials are used for udder infusions25
The major drugs involved with residues in meat are neomycin, streptomycin, penicillin, oxytetracycline, gentamicin and sulfamethazine, with intramuscular injection being the route of administration in 60% of the residue cases, oral administration in 28% and intramammary infusion in 9%.21,22 The use of orally administered antimicrobial boluses in calves that were subsequently slaughtered as bob veal calves is also a problem.
CAUSES OF RESIDUE VIOLATIONS IN SWINE
Similar causes are recorded for the occurrence of violative residues in pigs but an additional problem in pigs is tissue residues resulting from antibiotic inclusions in feeds for growth promotion and disease control purposes. Sulfonamides are a particular problem. Nonobservance of the required withdrawal period can result in the rejection of market batches of animals with a substantial financial loss to producers. If feed inclusions have been for the purposes of medication, the prescribing veterinarian may be liable if adequate information on withdrawal periods has not been given.
There is also a problem with sulfonamide residues resulting from carryover of sulfonamides from medicated to nonmedicated feeds at the feed mill or on the farm.26 Mistakes in feed delivery, feed mixing sequences, ingredient contamination and contamination within the bulk feed distribution system, and delivery augers can cause residual contamination.26,27 Carryover concentrations of sulfamethazine (sulfadimidine) of greater than 2 g per tonne in the finisher ration can result in violative residues in the liver at slaughter.28 The use of granular forms of sulfamethazine markedly reduces the potential for carryover.29
A further source of contamination in the piggery is environmental contamination. Manure and pooled urine from swine fed 100 g per tonne of sulfamethazine contains sufficient drug to contaminate swine to violative levels when contact with the material is maintained and this can continue for 6–7 weeks when pens are not cleaned after a drug is withdrawn from the feed.27 Dried urine has the potential for airborne contamination of pigs. Sulfamethazine is stable in manure and flush water for long periods and coprophagy by pigs can lead to significant intake of the drug. In order to avoid the risk of this occurring, it is recommended that, 3 days after the medicated feed has been withdrawn, the pens should be thoroughly cleaned or the pigs moved to new housing. Water medication can also lead to buildup of residues in the water delivery system, so the watering systems should be flushed. Pigs destined for slaughter can be tested on the farm prior to shipping using commercially available testing systems, which can also be used for detection of the occurrence of sulfonamides in feed and water.27
TYPE OF THERAPY
In the USA veterinarians are responsible for a very minor proportion of detected residue violations.30 Possible causes of violations resulting from veterinary therapy include the selection of an inadequate withdrawal period following extralabel use of an antimicrobial and treatment modalities that may not be considered a risk. The local infusion of antibiotic solutions into the uterus of cows may result in circulating concentrations of antibiotic and residues in body tissues and in milk. This results from the absorption of the antibiotic through the endometrium and from the peritoneal cavity following passage through the fallopian tubes.31 Similarly, following infusion of antibiotic solutions into one quarter of the udder, low concentrations of the antibiotic can occur in milk secreted from the remaining quarters. Gentamicin is generally considered not to be absorbed from the mammary gland but more than 87% of an intramammary dose of gentamicin is absorbed from the inflamed udder.32
APPROVED DRUGS
Whenever possible, approved antimicrobials should be used for therapy at label dose and a known withdrawal time in order to comply with regulatory requirements and to minimize the possibility of antibiotic residues in meat and milk. It may be necessary to use nonapproved antimicrobial drugs in certain circumstances and in minor species. The use of an approved antibiotic in a minor species for which it is not approved constitutes an extralabel use of the drug. The legality of the use of unapproved drugs, or of approved drugs in minor species for which they are not approved, is questionable. If such use is contemplated it is probably wise to have culture and sensitivity data indicating that the use of the unapproved drug is therapeutically necessary. Certain nonapproved antibiotics are totally banned for use in food-producing animals in some countries (e.g. in the USA: chloramphenicol, the nitroimidazoles, sulfamethazine in dairy cattle over 20 months of age, furazolidone and the use of fluoroquinolones in an extralabel fashion) and local regulations should be followed. The use of sulfamethazine in food-producing animals may be banned in some countries. The American Association of Bovine Practitioners has passed a voluntary moratorium on the use of aminoglycosides in cattle.
Bevill RF. Factors influencing the occurrence of drug residues in animal tissues after the use of antimicrobial agents. J Am Vet Med Assoc. 1984;185:1124.
Bishop JR, White CH. Antibiotic residue detection in milk — a review. J Food Pract. 1984;47:647.
Bishop JR, et al. Retention data for antibiotics commonly used for bovine infections. J Dairy Sci. 1984;67:437.
Sundloff SF. Drug and chemical residues in livestock. Vet Clin North Am Food Anim Pract. 1989;5:424-430.
Riviere JD. Pharmacologic principles of residue avoidance for veterinary practitioners. J Am Vet Med Assoc. 1991;198:809-816.
Payne MA. The rational use of antibiotics in dairies. Vet Med. 1993;88:161-169.
Nicholls TJ, et al. Food safety and residues in Australian agricultural produce. Aust Vet J. 1994;71:393-396.
Hung E, Tollefson L. Microbial food borne pathogens. Vet Clin North Am Food Anim Pract. 1998;14:1-176.
Sundberg P. Food safety: Antimicrobial residues. Compend Contin Educ Pract Vet. 2000;22(9):S118.
Food Animal Residue Avoidance Databank Homepage. Available on line at: http://www.farad.org/. Accessed May 19 2005.
1 Waltner-Toews D, McEwen SA. Prev Vet Med. 1994;20:159.
2 Riviere JD. J Am Vet Med Assoc. 1991;198:809.
3 Sterner KE. J Am Vet Med Assoc. 1991;198:825.
4 Constable PD. Proceedings of the 37th Annual Convention of the American Association of Bovine Practitioners. 2004;37:11.
5 Constable PD, Morrin DE. J Am Vet Med Assoc. 2002;221:103.
6 Nouws JFM, et al. Am J Vet Res. 1986;47:642.
7 Haddad NA, et al. Am J Vet Res. 1987;48:21.
8 Haskell SRR, et al. J Am Vet Med Assoc. 2003;223:1277.
9 Hogg RA, et al. Vet Rec. 1992;130:4.
10 McEwen SA, et al. Can Vet J. 1992;33:527.
11 Korsrud GO, et al. J AOAC Int. 1998;81:21.
12 Katz SE, Siewierski M. J AOAC Int. 1995;78:1408.
13 Cullor JC. Vet Med. 1992;87:1235.
14 Dey BP, et al. J Environ Health B. 2003;38:391.
15 Tyler JW, et al. J Am Vet Med Assoc. 1992;201:1378.
16 Slenning BD, Gardner IA. J Am Vet Med Assoc. 1997;211:419.
17 Andrew SM. Torrence ME, Isaacson RE, editors. Microbial safety in animal agriculture: current topics. Ames, IA: Iowa State Press. 2003:397.
18 Pengov A, et al. Anal Chem Acta. 2005;529:347.
19 Booth JM, Harding F. Vet Rec. 1986;119:565.
20 Kaneene JB, Miller R. J Am Vet Med Assoc. 1992;201:68.
21 Gibbons SN, et al. J Am Vet Med Assoc. 1996;209:589.
22 Guest GB, Paige JC. J Am Vet Med Assoc. 1991;198:805.
23 Selim SA, Cullor JS. J Am Vet Med Assoc. 1997;21:1029.
24 Carpenter LV, et al. Prev Vet Med. 1995;23:143.
25 Musser JM, et al. J Am Vet Med Assoc. 2001;219:346.
26 McCaughey WJ, et al. Ir Vet J. 1990;43:127.
27 McKean JD. Agri-Pract. 1988;9:15.
28 Ashworth RB, et al. Am J Vet Res. 1986;47:2596.
29 Rosenburg MC. J Am Vet Med Assoc. 1985;187:704.
30 Gibbons SN, et al. J Am Vet Med Assoc. 1996;209:589.