Diseases of the peritoneum
PERITONITIS
Inflammation of the peritoneum is accompanied by abdominal pain, fever, toxemia and a reduction in the amount of feces. Symptoms vary in degree with the severity and extent of the peritonitis.
ETIOLOGY
Peritonitis may occur as a primary disease or secondarily as part of an etiologically specific disease. As a primary disease it results most commonly from injury of the serosal surfaces of the alimentary tract within the abdomen, allowing gastrointestinal contents to enter the peritoneal cavity. Less commonly there is perforation of the abdominal wall from the exterior from traumatic injury, perforation of the reproductive tract, or the introduction of pathogens or irritating substances as result of injections into the peritoneal cavity or exploratory laparotomy. Some of the more common individual causes are as follows.
Cattle
• Traumatic reticuloperitonitis
• Secondary to ruminal trocarization
• Perforation or leakage of abomasal ulcer
• Concurrent abomasal displacement and perforating ulcer1
• Necrosis and rupture of abomasal wall after abomasal volvulus
• Rumenitis of cattle subsequent to acute carbohydrate indigestion
• Complication of caesarean section
• Rupture of vagina in young heifers during violent coitus with a young, active bull
• Deposition of semen into the peritoneal cavity by any means
• Injection of sterile hypertonic solutions, e.g. calcium preparations for milk fever. The chemical peritonitis that results may lead to formation of constrictive adhesions between loops of the coiled colon
• Transection of small intestine that becomes pinched between the uterus and the pelvic cavity at parturition
• Intraperitoneal injection of nonsterile solutions
• Spontaneous uterine rupture during parturition, or during manual correction of dystocia
• Spontaneous rupture of rectum at calving2
Horses
Peritonitis in horses is usually secondary to infectious, chemical, or parasitic peritoneal injuries, and can be a major complication after abdominal surgery.3
• Rupture of dorsal sac of cecum or colon4 at foaling, usually related to a large meal given just beforehand
• Cecal rupture in foals subjected to anesthesia and gastric endoscopy5
• Administration of NSAIDs causing cecal stasis and dilatation and eventually perforation6
• Rectal rupture or tear during rectal examination, predisposed to by inflammation of mucosa and overenthusiasm by the operator; this subject is presented separately under the heading of rectal tear
• Extension from a retroperitoneal infection, e.g. Streptococcus equi after an attack of strangles, Rhodococcus equi in foals under 1 year of age, both probably assisted by migration of Strongylus vulgaris larvae
• Gastric erosion or rupture related to ulceration associated with larvae of Gasterophilus or Habronema spp.
• Colonic perforation associated with aberrant migration of Gasterophilus intestinalis7
• Leakage from a cecal perforation apparently associated with a heavy infestation of Anoplocephala perfoliata tapeworms
• Actinobacillus equuli infection by unknown means.8,9 Septicemia and peritonitis due to A. equuli infection in an adult horse has been described.10
All species
• Traumatic perforation from the exterior of the abdominal wall by horn gore, stake wound
• Faulty asepsis at laparotomy, peritoneal injection, trocarization for tympany of rumen or cecum
• Leakage through wall of infarcted gut segment
• Spread from subperitoneal sites in spleen, liver, umbilical vessels.
PATHOGENESIS
At least six factors account for the clinical findings and the various consequences of peritonitis. They are toxemia or septicemia, shock and hemorrhage, abdominal pain, paralytic ileus, accumulation of fluid exudate and the development of adhesions.
Toxemia and septicemia
Toxins produced by bacteria and by the breakdown of tissue are absorbed readily through the peritoneum. The resulting toxemia is the most important factor in the production of clinical illness and its severity is usually governed by the size of the area of peritoneum involved. In acute diffuse peritonitis, the toxemia is profound; in local inflammation, it is negligible. The type of infection present is obviously important because of variations between bacteria in their virulence and toxin production.
With rupture of the alimentary tract wall and the spillage of a large quantity of gut contents into the peritoneal cavity, some acute peritonitis does develop, but death is usually too sudden, within 2–3 hours in horses, for more than an early lesion to develop. These animals die of endotoxic shock due to absorption of toxins from the gut contents. In acute diffuse peritonitis due solely to bacterial contamination from the gut, the reaction depends on the bacteria that gain entry and the capacity of the omentum to deal with the peritonitis, and the amount of body movement that the animal has to perform. Cows that suffer penetration of the reticular wall at calving have lowered immunological competence, a greater than normal negative pressure in the peritoneal cavity, are invaded by F. necrophorum, Corynebacterium spp. and E. coli, and are required to walk to the milking parlor, to the feed supply and so on. They are likely to develop a massive diffuse purulent peritonitis and a profound toxemia and die within 24 hours. By contrast, horses that develop acute peritonitis due to streptococci or A. equuli show little toxemia and manifest only abdominal pain due to the inflammatory reaction of the peritoneum.
Shock and hemorrhage
The shock caused by sudden deposition of gut contents, or infected uterine contents, into the peritoneal cavity, plus the hemorrhage resulting from the rupture, may be significant contributors to the common fatal outcome when an infected viscus ruptures. Following rupture of the uterus in cows, the shock and hemorrhage may be minor and peritonitis may not develop if the uterine contents are not contaminated. Failure of the uterus to heal or be repaired may be followed by peritonitis several days later.
Abdominal pain
Abdominal pain is a variable sign in peritonitis. In acute, diffuse peritonitis, the toxemia may be sufficiently severe to depress the response of the animal to pain stimuli, but in less severe cases the animal usually adopts an arched-back posture and shows evidence of pain on palpation of the abdominal wall. Inflammation of the serous surfaces of the peritoneum causes pain, which may be severe enough to result in rigidity of the abdominal wall and the assumption of an abnormal humped-up posture.
Paralytic ileus
Paralytic ileus occurs as a result of reflex inhibition of alimentary tract tone and movement in acute peritonitis. It is also an important sequel to intestinal obstruction and to traumatic abdominal surgery, in which much handling of viscera is unavoidable. Rarely, it arises because of ganglionitis and a loss of neural control of peristalsis, similar to the idiopathic intestinal pseudo-obstruction of humans.11 The net effect is functional obstruction of the intestine, which, if persistent, will increase the likelihood of death. The end result is a complete absence of defecation, often with no feces present in the rectum.
Accumulation of fluid exudate
Accumulation of large quantities of inflammatory exudate in the peritoneal cavity may cause visible abdominal distension and, if severe enough, interfere with respiration by obstruction of diaphragmatic movement. It is a comparatively rare occurrence but needs to be considered in the differential diagnosis of abdominal distension.
Adhesions
Trauma to the peritoneum results in a serosanguineous exudate, which contains two closely bound proteins: fibrinogen and plasminogen. Fibrinogen is converted by thrombin to fibrin, forming an early fibrinous adhesion. Plasminogen may be converted by plasminogen activators to plasmin, a specific fibrinolytic enzyme favoring lysis of the early adhesion. Peritoneal mesothelial cells are a source of plasminogen activators and each species of domestic animal has its own baseline peritoneal plasminogen activity. Cattle have a high capacity to respond to trauma with fibrin deposition.6 Intra-abdominal fibrin deposition and adhesion formation is the most important factor in localizing peritonitis after peritoneal trauma from penetrating foreign bodies or abomasal ulcers. However, these adhesions can cause mechanical or functional intestinal obstruction.
In chronic peritonitis, the formation of adhesions is more important than either of the two preceding pathogenetic mechanisms. Adhesions are an essential part of the healing process and are important to localize the inflammation to a particular segment of the peritoneum. If this healing process is developing satisfactorily and the signs of peritonitis are diminishing, it is a common experience to find that vigorous exercise causes breakdown of the adhesions, spread of the peritonitis and return of the clinical signs. Thus, a cow treated conservatively for traumatic reticuloperitonitis by immobilization may show an excellent recovery by the third day but, if allowed to go out to pasture at this time, may suffer an acute relapse. The secondary adverse effects of adhesions may cause partial or complete obstruction of the intestine or stomach, or by fixation to the body wall interfere with normal gut motility. Adhesions are important in the pathogenesis of vagus indigestion in cattle.
CLINICAL FINDINGS
Peritonitis is common in cattle, less common in horses and rarely, if ever, identified clinically in sheep, pigs or goats. There are general signs applicable to all species and most forms of the disease in a general way. In addition, there are special findings peculiar to individual species and to various forms of the disease.
Acute and subacute peritonitis
Inappetence and anorexia
Inappetence occurs in less severe and chronic cases, and complete anorexia in acute diffuse peritonitis.
Toxemia and fever
Toxemia, usually with a fever, is often present but the severity varies depending on the area of peritoneum involved, the identity of the pathogens and the amount of tissue injury. For example, in cattle with acute local peritonitis the temperature will be elevated (39.5°C; 103°F) for the first 24–36 hours, but then return to normal even though the animal may still be partly or completely anorexic. A high fever (up to 41.5°C; 106°F) suggests an acute diffuse peritonitis, but in the terminal stages the temperature usually falls to subnormal. It is most noteworthy that a normal temperature does not preclude the presence of peritonitis. In horses with peritonitis, the temperature will usually exceed 38.5°C but the fever may be intermittent.12 There is usually a moderate increase in heart and respiratory rates, the latter contributed to by the relative fixation of the abdominal wall because of pain. In some cases there is spontaneous grunting at the end of each expiratory movement.
Feces
The amount and composition of feces is usually abnormal. The transit time of ingesta through the alimentary tract is increased and the dry matter content of the feces increases. The amount of feces is reduced, although in the early stages there may be transient period of increased frequency of passage of small volumes of soft feces, which may give the false impression of increased fecal output. In some horses with peritonitis, periods of diarrhea may occur but the feces are usually reduced in amount.12 Feces may be completely absent for periods of up to 3 days, even in animals that recover, and the rectum may be so dry and tacky, because of the presence of small amounts of tenacious mucus, that it is difficult to do a rectal examination. This may suggest a complete intestinal obstruction.
In pastured cattle with peritonitis the feces are characteristically scant, dark and like small fecal balls accompanied by thick, jelly-like mucus. The feces may alternatively have a thick, sludge-like consistency, be tenacious and difficult to remove from a rubber glove, and have a foul smell.
Alimentary tract stasis
As well as absence of feces, there are other indicators of intestinal stasis. In cows with acute peritonitis ruminal contractions are reduced or absent; in chronic peritonitis the contractions may be present but are weaker than normal. In the horse, intestinal stasis is evidenced by an absence or reduction of typical intestinal peristaltic sounds on auscultation, although the tinkling sounds of paralytic ileus may be audible. It is very important to differentiate the two.
Abdominal pain evidenced by posture and movement
In cattle with acute peritonitis there is a disinclination to move, disinclination to lie down, lying down with great care and grunting with pain. The posture includes a characteristically arched back, the gait is shuffling and cautious, with the back held rigid and arched. Grunting at each step and when feces or urine are passed is common, and when urine is eventually passed it is usually in a very large volume. Sudden movements are avoided and there is an absence of kicking or bellowing or licking the coat.
In horses these overt signs of peritonitis that characterize the condition in cattle are uncommon, which makes the diagnosis difficult. In the horse peritonitis is often manifested as an episode of abdominal pain including flank watching, kicking at the belly and going down and rolling, which suggests colic caused by intestinal obstruction.8,11
In a series of 51 cases of peritonitis associated with A. equuli in horses, most had tachycardia, increased respiratory rates, fever and reduced intestinal borborygmi.9 Affected horses were depressed, lethargic and inapparent. Mild to moderate abdominal pain was manifested as reluctance to move, pawing on the ground, lying down or splinting of the abdominal musculature. The onset of clinical signs was acute (<24 h) in 30 horses, 1–4 days in 8 horses, or longer and associated with weight loss in 3 horses. In 10 horses, there was no record of the duration of clinical signs.
Abdominal pain as evidenced by deep palpation
In cattle, deep firm palpation of the abdominal wall elicits an easily recognized pain response. It may be possible to elicit pain over the entire abdominal wall if the peritonitis is widespread. If it is localized the response may be detectable over only a very small area. Increased tenseness of the abdominal wall is not usually detectable in the cow, although it is responsible for the characteristic arched-back posture and apparent gauntness of the abdomen, because the wall is already tightly stretched anyway.
Several methods are used to elicit a grunt in cattle with abdominal pain. In average-sized cows with acute local peritonitis (most commonly traumatic reticuloperitonitis), while listening over the trachea with a stethoscope, a controlled upward push with the closed fist of the ventral body wall caudal to the xiphoid sternum is most successful. In large bulls, especially if the peritonitis is subsiding, it may be difficult to elicit a grunt with this method. In these cases, the best technique is to use a heavy pole held horizontally under the area immediately caudal to the xiphoid sternum to provide a sharp lift given by assistants holding the pole on either side. Pinching of the withers while auscultating over the trachea is also used and with some clinical experience is highly reliable.
In horses with acute or subacute peritonitis, it is usually easy to elicit a pain response manifested by the animal lifting its leg and turning its head with anger when its lower flank is firmly lifted, but not punched. The abdominal wall also feels tense if it is lifted firmly with the heel of the hand. In all cases of peritonitis in all species a pain response is always much more evident in the early stages of the disease and severe chronic peritonitis can be present without pain being detected on palpation.
Rectal examination
The general absence of feces is characteristic. In cattle, it may be possible to palpate slightly distended, saggy, thick-walled loops of intestine in some cases. Also, it may be possible to feel fibrinous adhesions separating as the intestines are manipulated. Adhesions are not often palpable and their absence should not be interpreted as precluding the presence of peritonitis. Only adhesions in the caudal part of the abdomen may be palpable. Tough, fibrous adhesions may be present in long-standing cases. In horses, there are no specific rectal findings, other than a reduced fecal output, to indicate the presence of peritonitis. Distension of segments of both the small and large intestines may provide indirect evidence of paralytic ileus. However, there is a lack of clarity as to what can be felt in chronic cases because of the presence of fibrin deposits and thickening of the peritoneum. There may also be more than usual pain when an inflamed area is palpated or a mesenteric band or adhesion is manipulated.
In rupture of the rectum associated with a difficult dystocia, the rupture is usually easily palpable rectally in the ventral aspect of the rectum deep in the abdomen.2 Distended loops of intestine may become entrapped in the rectal tear.
Peracute diffuse peritonitis
In those cases in which profound toxemia occurs, especially in cows immediately after calving or when rupture of the alimentary tract occurs, the syndrome is quite different. There is severe weakness, depression and circulatory failure. The animal is recumbent and often unable to rise, depressed almost to the point of coma, has a subnormal temperature of 37–37.5°C (99–100°F), a high heart rate (110–120/min) and a weak pulse. No abdominal pain is evidenced spontaneously or on palpation of the abdominal wall. In mares that rupture the dorsal sac of the cecum during foaling, the owner observes that the mare has been straining and getting results when suddenly she stops making violent muscular contractions, and progress towards expelling the foal ceases.13 Moderate abdominal pain followed by shock are characteristic developments. Death follows 4–15 hours after the rupture.
The outcome in cases of acute, diffuse peritonitis varies with the severity. Peracute cases accompanied by severe toxemia usually die within 24–48 hours. The more common, less severe cases may be fatal in 4–7 days, but adequate treatment may result in recovery in about the same length of time.
In a series of 31 cases of generalized peritonitis in cattle most cases occurred peripartum.14 The most consistent clinical findings were depression, anorexia, decreased fecal output and varying degrees of dehydration. The duration of illness ranged from 1–90 days with a median of 4 days. In 19 animals, the duration of clinical disease was less than 1 week and in 12 cases the duration of illness was more than 1 week. All animals died or were euthanized.
Chronic peritonitis
Cattle
The development of adhesions, which interfere with normal alimentary tract movements, and gradual spread of infection as adhesions break down combine to produce a chronic syndrome of indigestion and toxemia that is punctuated by short, recurrent attacks of more severe illness. The adhesions may be detectable on rectal examination but they are usually situated in the anterior abdomen and are impalpable. If partial intestinal obstruction occurs, the bouts of pain are usually accompanied by a marked increase in alimentary tract sounds and palpable distension of intestinal loops with gas and fluid. The course in chronic peritonitis may be several weeks and the prognosis is not favorable because of the presence of physical lesions caused by scar tissue and adhesions. In some cases there is marked abdominal distension with many liters of turbid-infected fluid present. This may be restricted in its location to the omental bursa.15 Detection of fluid in the peritoneal cavity of a cow is not easy because of the fluid nature of the ruminal contents. Results obtained by testing for a fluid wave should be interpreted cautiously. Collection of fluid by paracentesis abdominis is the critical test.
Horses
Horses with chronic peritonitis usually have a history of ill-thrift for a period of several weeks. Weight loss is severe and there are usually intermittent episodes of abdominal pain suggesting intestinal colic. Gut sounds are greatly diminished or absent, and subcutaneous edema of the ventral abdominal wall occurs in some cases. There may also be a contiguous pleurisy. Identification of the cause of the colic depends on the examination of a sample of peritoneal fluid.
Diagnostic medical imaging
In cattle with traumatic reticuloperitonitis, inflammatory fibrinous changes, and abscesses can be imaged16 (see also Ch. 6).
In cattle, standing reticular radiography is a useful aid for the diagnosis and management of traumatic reticuloperitonitis.5 It can accurately detect the presence of a foreign body and in most instances if that foreign body is perforating the reticular wall.
CLINICAL PATHOLOGY
Hematology
The total and differential leukocyte count is a useful aid in the diagnosis of peritonitis and in assessing its severity. In acute diffuse peritonitis with toxemia there is usually a leukopenia, neutropenia and a marked increase in immature neutrophils (a degenerative left shift). There is ‘toxic’ granulation of neutrophils. In less severe forms of acute peritonitis of a few days’ duration there may be a leukocytosis due to a neutrophilia with the appearance of immature neutrophils. In acute local peritonitis, commonly seen in acute traumatic reticuloperitonitis in cattle, there is commonly a normal total leukocyte count, or a slight increase, with regenerative left shift. In chronic peritonitis, depending on the extent of the lesion (diffuse or local), the total and differential leukocyte count may be normal, or there may be a leukocytosis with a marked neutrophilia and occasionally an increase in the total numbers of lymphocytes and monocytes. The plasma fibrinogen levels in cattle, in general, tend to increase as the severity of acute peritonitis increases and may be a useful adjunct to the cell counts for assessing severity.5
In horses with peritonitis associated with A. equuli, there was hemoconcentration, hypoproteinemia and a neutrophilia count with a left shift.
Abdominocentesis and peritoneal fluid
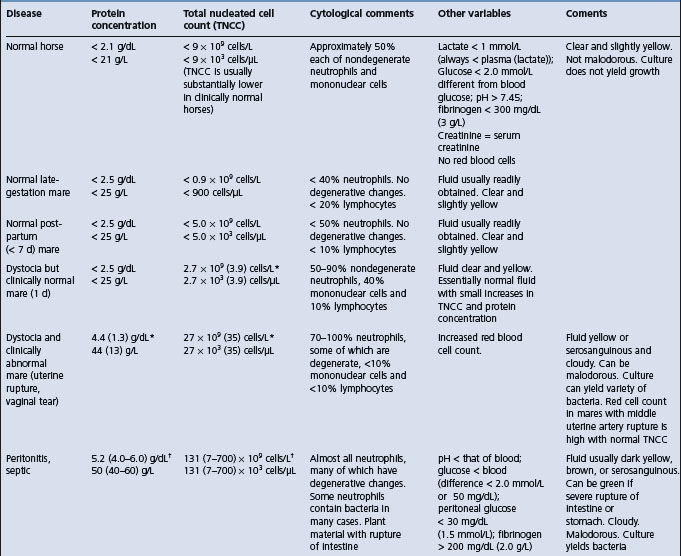
Examination of peritoneal fluid obtained by paracentesis is a valuable aid in the diagnosis of peritonitis and in assessing its severity. It may also provide an indication of the kind of antibacterial treatment required. The values in healthy horses, and horses with various intestinal or peritoneal diseases are provided in Table 5.2. The maximum peritoneal fluid nucleated cell counts in healthy foals is much lower than reported maximum values for adult horses17 and similarly for calves. Particular attention should be paid to:
• The ease of collection of the sample as a guide to the amount of fluid present
• Whether it is bloodstained, indicating damage to a wall of the viscus
• The presence of feed or fecal material, indicating intestinal ischemic necrosis or rupture
• Whether it clots and has a high protein content, indicating inflammation rather than simple transudation
• The number and kinds of leukocytes present, as an indication of the presence of inflammation, and also its duration
When these results are available they should be interpreted in conjunction with the history, clinical signs and other results, including hematology, serum chemistry and possibly radiology. In particular, it must be noted that failure to obtain a sample does not preclude a possible diagnosis of peritonitis.
Interpretation of peritoneal fluid is also influenced by simple manipulation of the abdominal viscera and the response is greater than that following opening and closing of the abdomen without manipulation of the viscera. Surgical manipulation results in a significant and rapid postoperative peritoneal inflammatory reaction.11
In peritonitis in horses associated with A. equuli, the peritoneal fluid was turbid and had an abnormal color in 98% of cases. The protein content was elevated above normal in 50 samples (range 25–84 g/L, mean 44 g/L, normal < 20 g/L). Total nucleated cell count was elevated in all samples (range 46–810 × 109 cells/L, mean 230 × 109 cells/L, normal < 10 × 109 cells/L). A nucleated cell count above 100 × 109 cells/L, was present in 88% of animals.9 Pleomorphic Gram-negative rods were seen on cytology in 53% of samples, and a positive culture of A equuli was obtained in 72% of samples.
Experimentally, resection and anastomosis of the small colon in healthy horses causes a different inflammatory response than does manipulation. Absolute values in the peritoneal fluid for cell count, total protein and differential count are inadequate to differentiate between a normal surgical reaction and a postoperative infection. Cytological examination of peritoneal fluid is necessary to demonstrate degenerative cell changes and the presence of bacteria and ingesta. The peripheral leukon and fibrinogen concentration should always be compared with the peritoneal fluid for evidence of postsurgical infection. The nucleated cell and red blood counts of peritoneal fluid are commonly elevated for several days in horses following open castration.15 These elevated counts may be mistaken for peritonitis.
Septic peritonitis in the horse
Diagnosis of septic peritonitis is routinely made on the basis of physical examination and hematologic findings, and peritoneal fluid analysis.18 After abdominal surgery, differentiation between septic peritonitis and other postoperative complications can be difficult using physical and hematological findings alone. As a result of the exploratory process itself, diagnosis of septic peritonitis is often complicated in horses after surgery because the total nucleated cell count and protein concentration in the peritoneal fluid are often high. Consequently, identification of bacteria on cytological evaluation or isolation of bacteria from peritoneal fluid is a more definitive indicator of septic peritonitis, but sometimes there are false-negative results. Although bacterial cultures are considered the standard criterion for the diagnosis of sepsis, positive results may not always be obtained and results may be delayed by a minimum of 24 hours for aerobic organisms and up to 10–14 days for anaerobic organisms. Thus ancillary tests such as pH, glucose concentrations and lactate dehydrogenase (LDH) activity in equine pleural and synovial fluid have been used to detect sepsis with the potential advantages of speed, ease of measurement and lower cost relative to bacterial cultures.18
Horses with septic peritonitis have significantly lower peritoneal fluid pH and glucose concentrations than horses with nonseptic peritonitis and healthy horses.18 Compared with other tests, serum-to-peritoneal fluid glucose concentration differences of more than 50 mg/dL had the highest diagnostic use for detection of septic peritonitis. Peritoneal fluid pH below 7.3, glucose concentration below 30 mg/dL and fibrinogen concentration above 200 mg/dL were also highly indicative of septic peritonitis.
NECROPSY FINDINGS
In acute diffuse peritonitis, the entire peritoneum is involved but the most severe lesions are usually in the ventral abdomen. Gross hemorrhage into the subserosa, exudation and fibrin deposits in the peritoneal cavity and fresh adhesions that are easily broken down are present. In less acute cases, the exudate is purulent and may be less fluid, often forming a thick, cheesy covering over most of the viscera. In cattle, F. necrophorum and Actinomyces (Corynebacterium) pyogenes are often present in large numbers and produce a typical, nauseating odor. Acute local peritonitis and chronic peritonitis are not usually fatal and the lesions are discovered only if the animal dies of intercurrent disease such as traumatic pericarditis or intestinal obstruction.
DIAGNOSIS
The diagnosis of peritonitis can be difficult because the predominant clinical findings are often common to other diseases. The clinical features that are the most reliable as indicators of peritonitis are:
• Abnormal feces – in amount and composition
• Alimentary tract stasis based on auscultation and evaluation of the passage of feces
• Abdominal pain evinced as a groan with each respiration or on light or deep percussion of the abdomen
• Abnormality of intestines on rectal palpation
• Fibrinous or fibrous adhesions on rectal palpation
• Abnormal peritoneal fluid with an increased leukocyte count collected by paracentesis
• A normal or low blood leukocyte count with a degenerative left shift
• The peritonitis may be chemical, so that, although microbiological examination usually yields positive results, these are not essential to a diagnosis of peritonitis.
PROGNOSIS
Case fatality rate in horses
Peritonitis in the horse is a potentially life-threatening disease that must be treated promptly and aggressively.20 Therapy must be aimed at reducing systemic shock and hypovolemia, correction of the primary cause, antibiotic therapy, and abdominal drainage and lavage. The reported case fatality rates for peritonitis in horses range from 30–67%. In a series of 67 cases of peritonitis in horses, of those which developed peritonitis after abdominal surgery the case fatality was 56%.3 Peritonitis not associated with intestinal rupture or abdominal surgery had a lower case fatality rate of 43%. Horses that died had higher heart rates, red blood cell count, serum creatinine concentration, PCV and anion gap; lower venous blood pH; and a greater number of bacterial species cultured from the peritoneal fluid compared with survivors. Those that died were more likely to have clinical evidence of abdominal pain, shock and bacteria in the peritoneal fluid.
The diseases which could be considered in the differential diagnosis of peritonitis are as follows.
Cattle
• Acute local peritonitis– Traumatic reticuloperitonitis, acute intestinal obstruction, splenic or hepatic abscess, simple indigestion, abomasal displacement (right and left), postpartum metritis, ketosis
• Acute diffuse peritonitis – Parturient paresis, coliform mastitis (peracute form), acute carbohydrate indigestion, perforation of or rupture at abomasal ulcer, acute intestinal obstruction, uterine rupture, postpartum metritis
• Chronic peritonitis – Vagus indigestion, lipomatosis or extensive fat necrosis of the mesentery and omentum, persistent minor leakage from an intestinal lesion, large accumulations of fluid as in ascites, rupture of bladder, chronic pneumonia and chronic toxemias due to a great variety of causes
• Ascites associated most commonly with primary or secondary cardiac disease, cor pulmonale with chronic pneumonia, endocarditis, thrombosis of the caudal vena cava, and diffuse abdominal epithelioid mesothelioma19
Horses
• Acute and subacute peritonitis– Acute intestinal obstruction and thromboembolic colic
• Chronic peritonitis – Repeated overeating causing colic, internal abdominal abscess (retroperitoneal or mesenteric abscess) may be classified as chronic peritonitis but is dealt with separately under the heading of retroperitoneal abscess. Horses with both intra-abdominal neoplasms and abscesses will have clinical findings including anorexia, weight loss, fever, colic and depression.13 Both groups may also have peritoneal fluid that can be classified as an exudate
TREATMENT
The specific cause must be treated in each case and the treatments used are described under the specific diseases listed above. An exploratory laparotomy may be indicated to determine the cause of the peritonitis and to effect repair. The literature on the treatment of peritonitis in horses has been reviewed.20
Antimicrobials
Broad-spectrum antimicrobials given intravenously or intramuscularly are indicated for the infection and toxemia. However, there are no published reports of clinical trials to evaluate the effectiveness of various antimicrobials for the treatment of peritonitis in cattle or horses. Thus the recommendations are empirical. In general, peritonitis in cattle is commonly treated with any of the broad-spectrum antimicrobials, with the choice dependent on ease of administration and drug withdrawal times necessary in lactating dairy cattle. Treatment for traumatic reticuloperitonitis has commonly been restricted to the use of antimicrobials; supportive therapy has not been indicated with the exception of diffuse peritonitis.
Peritonitis in horses associated with abdominal surgery or rupture of the gastrointestinal tract is likely be accompanied by a mixed flora of bacteria, and broad-spectrum antimicrobials are necessary. They must be given at doses high enough to achieve high blood and tissue levels and maintained daily until recovery has occurred. In a series of cases of peritonitis in horses, the most commonly used antimicrobials were gentamicin at 2.2–3.3 mg/kg BW intravenously every 8–12 hours; penicillin at 22000 IU/kg BW intravenously or intramuscularly every 6–12 hours. Metronidazole given orally at 15–25 mg/kg BW has also been used in horses with peritonitis.3
Horses with peritonitis associated with A. equuli respond quickly to treatment with penicillin at 20 mg/kg BW intramuscularly twice daily for 5 days to 2 weeks.9 Most isolates of the organism are sensitive to penicillin but some are resistant and gentamicin sulfate at 6.6 mg/kg BW intravenously once daily for 5 days to 2 weeks in combination with the penicillin has also been used successfully.9 In a series of 51 cases in horses, the recovery rate following treatment with penicillin and gentamicin and supportive therapy was 100%.9 Most horses responded favorably within 48 hours following commencement of treatment.
Administration of antimicrobials into the peritoneal cavity has been attempted on the basis that higher levels of the drug may be achieved at the site of the inflammation. However, there is no scientific evidence that it is superior to daily parenteral administration and there is some danger of causing adhesions and subsequent intestinal obstruction.
Fluid and electrolytes
Intensive intravenous fluid and electrolyte therapy is a vital part of treatment of peritonitis when accompanied by severe toxemia and shock, especially during the first 24–72 hours following abdominal surgery in the horse. It is continued until recovery is apparent and the animal is drinking water voluntarily; water can then be supplemented with electrolytes. (See Ch. 2 for details of fluid and electrolyte therapy for the treatment of dehydration and toxemia.)
Nonsteroidal anti-inflammatory drugs
Flunixin meglumine is recommended at 0.25–1.1 mg/kg BW intravenously every 8–12 hours when the peritonitis is accompanied by shock. However, no information is available on efficacy.
Lavage
Peritoneal lavage with large volumes of fluid containing antimicrobials is rational and has been attempted when large quantities of exudate are present. However, it is not easy to maintain the patency of drains, especially in cattle. Also, peritoneum is highly susceptible to inflammation and chemical peritonitis is common following the introduction of certain materials into the peritoneal cavity. Peritoneal lavage of ponies with saline and antimicrobials induces a mild, transient inflammatory response with minimal change visible at necropsy.21 Solutions containing povidone-iodine induced chemical peritonitis, which was severe when 10% povidone-iodine solution was used. A 3% solution also causes peritonitis and the use of these solutions is not recommended. Extreme caution is required when foreign materials are introduced into the cavity in order to avoid exacerbating the existing inflammation. The peritoneum is also a very vascular organ and toxic material is rapidly absorbed from it.
An active intra-abdominal drain has been used successfully to treat abdominal contamination in horses.22 Closed-suction abdominal drains were placed, mostly under general anesthesia. Abdominal lavage was done every 4–12 hours and about 83% of the peritoneal lavage solution was retrieved.
1 Cable CS, et al. J Am Vet Med Assoc. 1998;212:1442.
2 Tyler JW, et al. Vet Rec. 1998;143:280.
3 Hawkins JF, et al. J Am Vet Med Assoc. 1993;203:284.
4 Platt H. J Comp Pathol. 1983;93:343.
5 Edwards JF, Ruoff WW. J Am Vet Med Assoc. 1991;198:1421.
6 Ross MW, et al. J Am Vet Med Assoc. 1985;187:249.
7 Lapointe JM, et al. Vet Pathol. 2003;40:338.
8 Fubini SL, et al. J Am Vet Med Assoc. 1990;197:1060.
9 Matthews S, et al. Aust Vet J. 2001;79:536.
10 Patterson-Kane JC, et al. Vet Pathol. 2001;38:230.
11 Hanson RR, et al. Am J Vet Res. 1992;53:216.
12 Mair TS, et al. Vet Rec. 1990;126:567.
13 Zicker SC, et al. J Am Vet Med Assoc. 1990;196:1130.
14 Ebeid M, Rings DM. Bovine Pract. 1999;33:144.
15 Schumacher J, et al. J Vet Intern Med. 1988;2:22.
16 Braun U. Vet J. 2003;166:112.
17 Grinden CB, et al. Equine Vet J. 1990;22:359.
18 Van Hoogmoed L, et al. J Am Vet Med Assoc. 1999;214:1032.
19 Milne MH, et al. Vet Rec. 2001;148:341.
20 Davis JL. Vet Clin North Am Equine Pract. 2003;19:765.