Chapter 12 Diseases of the nervous system
INTRODUCTION 575
PRINCIPLES OF NERVOUS DYSFUNCTION 576
CLINICAL MANIFESTATIONS OF DISEASE OF THE NERVOUS SYSTEM 577
SPECIAL EXAMINATION OF THE NERVOUS SYSTEM 583
PRINCIPLES OF TREATMENT OF DISEASES OF THE NERVOUS SYSTEM 594
PATHOPHYSIOLOGICAL MECHANISMS OF NERVOUS SYSTEM DISEASE 596
DIFFUSE DISEASES OF THE BRAIN* 596
FOCAL DISEASES OF THE BRAIN 606
DISEASES OF THE MENINGES 609
TOXIC AND METABOLIC ENCEPHALOMYELOPATHIES 611
PSYCHOSES OR NEUROSES 612
EPILEPSY 613
DISEASES OF THE SPINAL CORD 613
DISEASES OF THE PERIPHERAL NERVOUS SYSTEM 618
CONGENITAL DEFECTS OF THE CENTRAL NERVOUS SYSTEM 619
This chapter presents the principles of clinical neurology and their application to large animal practice. In general, this activity has not kept pace with the study of neurology in humans and small animals, although remarkable progress has been made in equine neurology over the last 20 years. To a large extent this shortfall is due to the failure of large animal clinicians to relate observed clinical signs to a neuroanatomical location of the lesion. In many cases this failure has been because of adverse environmental circumstances, or the large size or nature of the animal, all of which adversely impact the quality of the neurological examination. It may be very difficult to do an adequate neurological examination on an ataxic belligerent beef cow that is still able to walk and attack the examiner. An aggressive, paretic bull in broad sunlight can be a daunting subject if one wants to examine the pupillary light reflex; ophthalmoscopic examination of the fundus of the eye in a convulsing steer in a feedlot pen can be an exasperating task. Thus, at one end of the spectrum is the clinical examination of pigs affected with nervous system disease, which is limited to an elementary clinical examination and necropsy examination.1 At the other end, neurological examination of the horse with nervous system disease is very advanced. The global occurrence of bovine spongiform encephalopathy has highlighted the importance of accurate clinical diagnosis in adult cattle with neurological abnormalities.
Discrete lesions of the central nervous system resulting in well-defined neurological signs are not common in agricultural animals. Many of the diseases are characterized by diffuse lesions associated with viruses, bacteria, toxins, nutritional disorders and embryological defects, and the clinical findings of each disease are similar. Rather than attempting to localize lesions in the nervous system, large-animal practitioners more commonly devote much of their time to attempting to identify whether an animal has meningoencephalitis, as in Histophilus somni meningoencephalitis; whether it has diffuse brain edema or increased intracranial pressure, as in polioencephalomalacia; or whether the dysfunction is at the neuromuscular level, as in hypomagnesemic tetany.
Radiographic examination, including myelography, is not used routinely or available as a diagnostic aid in large-animal practice. The collection of cerebrospinal fluid (CSF) from the different species and ages of large animal without causing damage to the animal or contaminating the sample with blood is a technique that few large-animal veterinarians have mastered. However, the collection of CSF from the lumbosacral cistern is not difficult if the animals are adequately restrained, and the information obtained from analysis of CSF can be very useful in the differential diagnosis of diseases of the brain and spinal cord.2 Referral veterinary centers are now providing detailed neurological examinations of horses with nervous system disease and the clinical and pathological experience has expanded the knowledge base of large-animal clinical neurology.3
In spite of the difficulties, the large animal practitioner has an obligation to make the best diagnosis possible using the diagnostic aids available. The principles of large-animal neurology are presented in this chapter and the major objective is to recognize the common diseases of the nervous system by correlating the clinical findings with the location and nature of the lesion. Accurate neuroanatomical localization of the lesion(s) remains the fundamental requirement for creating a differential diagnosis list and diagnostic and treatment plan.
A disease such as rabies has major public health implications and it is important for the veterinarian to be able to recognize the disease as early as possible and to minimize human contact. It is also important to be able to recognize treatable diseases of the nervous system such as polioencephalomalacia, listeriosis and nervous ketosis, and to differentiate them from untreatable and globally important diseases such as bovine spongiform encephalopathy.
The nontreatable diseases must also be recognized as such, and slaughter for salvage or euthanasia recommended if necessary. There must be a major emphasis on prognosis because it is inhumane and uneconomic to hospitalize or continue to treat an adult cow or horse with incurable neurological disease for an indefinite period. If they are recumbent the animals commonly develop secondary complications such as decubitus ulcers and other self-inflicted injuries because of repeated attempts to rise. Very few diseases of the nervous system of farm animals are treatable successfully over an extended period of time. This has become particularly important in recent years with the introduction of legislation prohibiting the slaughter of animals that have been treated with antibiotics until after a certain withdrawal period, which may vary from 5–30 days. This creates even greater pressure on the clinician to make a rapid, inexpensive and accurate diagnosis and prognosis.
Because of limitations in the neurological examination of large animals, there must be much more emphasis on the history and epidemiological findings. Many of the diseases have epidemiological characteristics that give the clinician a clue to the possible causes, thus helping to narrow the number of possibilities. For example, viral encephalomyelitis of horses occurs with a peak incidence during the insect season, lead poisoning is most common in calves after they have been turned out on to pasture, and polioencephalomalacia occurs in grain-fed feedlot cattle.
The functions of the nervous system are directed at the maintenance of the body’s spatial relation with its environment. These functions are performed by the several divisions of the nervous system including:
• The sensorimotor system, responsible for the maintenance of normal posture and gait
• The autonomic nervous system, controlling the activity of smooth muscle and endocrine glands, and thereby the internal environment of the body
• The largely sensory system of special senses
• The psychic system, which controls the animal’s mental state.
The nervous system is essentially a reactive one geared to the reception of internal and external stimuli and their translation into activity and consciousness; it is dependent upon the integrity of both the afferent and efferent pathways. This integrative function makes it often difficult to determine in a sick animal whether abnormalities are present in the nervous system, the musculoskeletal system or acid–base and electrolyte status. Accordingly, the first step when examining an animal with apparent abnormalities in the nervous system is to determine whether other relevant systems are functioning normally. In this way a decision to implicate the nervous system is often made on the exclusion of other systems.
The nervous system itself is not independent of other organs and its functional capacity is regulated to a large extent by the function of other systems, particularly the cardiovascular system. Hypoxia due to cardiovascular disease commonly leads to altered cerebral function because of the dependence of the brain on an adequate oxygen supply.
It is important to distinguish between primary and secondary diseases of the nervous system since both the prognosis and the treatment will differ with the cause.
In primary disease of the nervous system the lesion is usually an anatomical one with serious, long-range consequences.
In secondary disease the lesion, at least in its early stages, is more likely to be functional and therefore more responsive to treatment, provided the defect in the primary organ can be corrected.
The clinical findings that should arouse suspicion of neurological disturbance include abnormalities in the three main functions of the system.
Posture and gait
An animal’s ability to maintain a normal posture and to proceed with a normal gait depend largely upon the tone of skeletal muscle but also upon the efficiency of the postural reflexes. Abnormalities of posture and gait are among the best indications of nervous system disease because these functions are governed largely by the coordination of nervous activity. Besides contributing to posture and gait, skeletal muscle tone is characteristic in its own right. However, its assessment in animals is subject to great inaccuracy because of our inability to request complete voluntary relaxation by the patient. In humans it is a very valuable index of nervous system efficiency, but in animals it has serious limitations. The most difficult step when there is a defect of gait or posture is to decide whether it originates in the skeleton, the muscles or the nerve supply.
Principles of nervous dysfunction
Nervous tissue is limited in the ways in which it can respond to noxious influences. Because of its essentially coordinating function, the transmission of impulses along nerve fibers can be enhanced or depressed in varying degrees, the extreme degree being complete failure of transmission. Because of the structure of the system, in which nerve impulses are passed from neuron to neuron by relays at the nerve cells, there may also be excessive or decreased intrinsic activity of individual cells giving rise to an increase or decrease in nerve impulses discharged by the cells. The end result is the same whether the disturbance be one of conduction or discharge and these are the only two ways in which disease of the nervous system is manifested. Nervous dysfunction can thus be broadly divided into two forms, depressed activity and exaggerated activity. These can be further subdivided into four common modes of nervous dysfunction; excitation (irritation) signs, release of inhibition signs, paresis or paralysis due to tissue damage, and nervous shock.
MODES OF NERVOUS DYSFUNCTION
Excitation (irritation) signs
Increased activity of the reactor organ occurs when there is an increase in the number of nerve impulses received either because of excitation of neurons or because of facilitation of passage of stimuli.
The excitability of nerve cells can be increased by many factors, including stimulant drugs, inflammation and mild degrees of those influences that in a more severe form may cause depression of excitability. Thus early or mild hypoxia may result in increased excitability while sustained or severe hypoxia will cause depression of function or even death of the nerve cell.
Irritation phenomena may result from many causes, including inflammation of nervous tissue associated with bacteria or viruses, certain nerve poisons, hypoxia and edema. In those diseases that cause an increase in intracranial pressure, irritation phenomena result from interference with circulation and the development of local anemic hypoxia. The major manifestations of irritation of nervous tissue are tetany, local muscle tremor, and whole body convulsions in the motor system and hyperesthesia and paresthesia in the sensory system. For the most part the signs produced fluctuate in intensity and may occur periodically as nervous energy is discharged and reaccumulated in the nerve cells.
The area of increased excitability may be local or sufficiently generalized to affect the entire body. Thus a local lesion in the brain may cause signs of excitatory nervous dysfunction in one limb and a more extensive lesion may cause a complete convulsion.
Release of inhibition signs
Exaggeration of normal nervous system activity occurs when lower nervous centers are released from the inhibitory effects of higher centers. The classic example of a release mechanism is experimental decerebrate rigidity caused by transection of the brain stem between the colliculi of the midbrain. This results in an uninhibited extensor tonus of all the antigravity muscles. The head and neck are extended markedly in a posture of opisthotonos, and all four limbs in the quadruped are extended rigidly. The tonic mechanism or myotactic reflex involving the lower motor neuron has been released from the effects of the descending inhibitory upper motor neuron pathways.
Cerebellar ataxia is another example of inhibitory release. In the absence of cerebellar control combined limb movements are exaggerated in all modes of action including rate, range, force, and direction. In general, release phenomena are present constantly while the causative lesion operates, whereas excitatory phenomena fluctuate with the building up and exhaustion of energy in the nerve cells.
Paresis or paralysis due to tissue damage
Depression of activity can result from depression of metabolic activity of nerve cells, the terminal stage being complete paralysis when nervous tissue is destroyed. Such depression of activity may result from failure of supply of oxygen and other essential nutrients, either directly from their general absence or indirectly because of failure of the local circulation. Infection of the nerve cell itself may cause initial excitation, then depression of function and finally complete paralysis when the nerve cell dies.
Signs of paralysis are constant and are manifested by muscular paresis or paralysis when the motor system is affected and by hypoesthesia or anesthesia when the sensory system is involved. Deprivation of metabolites and impairment of function by actual invasion of nerve cells or by toxic depression of their activity produce temporary, partial depression of function that is completely lost when the neurons are destroyed.
Nervous shock
An acute lesion of the nervous system causes damage to nerve cells in the immediate vicinity of the lesion but there may be, in addition, a temporary cessation of function in parts of the nervous system not directly affected. The loss of function in these areas is temporary and usually persists for only a few hours. Stunning is the obvious example. Recovery from the flaccid unconsciousness of nervous shock may reveal the presence of permanent residual signs caused by the destruction of nervous tissue.
Determining the type of lesion is difficult because of the limited range of modes of reaction to injury in the nervous system. Irritation signs may be caused by bacterial or virus infection, by pressure, by vascular disturbance or general hypoxia, by poisons and by hypoglycemia. It is often impossible to determine whether the disturbance is structural or functional. Degenerative lesions produce mainly signs of paresis or paralysis but unless there are signs of local nervous tissue injury, such as facial nerve paralysis, paraplegia or local tremor, the disturbance may only be definable as a general disturbance of a part of the nervous system. Encephalopathy is an all-embracing diagnosis, but it is often impossible to go beyond it unless other clinical data, including signalment of the animal, epidemiology and systemic signs, are assessed or special tests, including radiographic examination and examination of the CSF, are undertaken.
Some information can be derived from a study of the sign-time relationship in the development of nervous disease. A lesion that develops suddenly tends to produce maximum disturbance of function, sometimes accompanied by nervous shock. Slowly developing lesions permit a form of compensation in that undamaged pathways and centers may assume some of the functions of the damaged areas. Even in rapidly developing lesions partial recovery may occur in time but the emphasis is on maximum depression of function at the beginning of the disease. Thus a slowly developing tumor of the spinal cord will have a different pattern of clinical development from that resulting from an acute traumatic lesion. Another aspect of the rapidity of onset of the lesion is that irritation phenomena are more likely to occur when the onset is rapid and less common when the onset is slow.
Clinical manifestations of disease of the nervous system
The major clinical signs of nervous system dysfunction include:
ALTERED MENTATION
Excitation states
Excitation states include mania, frenzy, and aggressive behavior, which are manifestations of general excitation of the cerebral cortex. The areas of the cortex that govern behavior, intellect and personality traits in humans are the frontal lobes and temporal cortex. The clinical importance of these areas, which are poorly developed in animals, is not great. The frontal lobes, temporal cortex and limbic system are highly susceptible to influences such as hypoxia and increased intracranial pressure.
Mania
In mania the animal acts in a bizarre way and appears to be unaware of its surroundings. Maniacal actions include licking, chewing of foreign material, sometimes themselves, abnormal voice, constant bellowing, apparent blindness, walking into strange surroundings, drunken gait and aggressiveness in normally docile animals. A state of delirium cannot be diagnosed in animals, but mental disorientation is an obvious component of mania as we see it.
Diseases characterized by mania include:
• Encephalitis, e.g. the furious form of rabies, Aujeszky’s disease in cattle (pseudorabies, mad itch)
• Degenerative diseases of the brain, e.g. mannosidosis, early polioencephalomalacia, poisoning by Astragalus sp.
• Toxic and metabolic diseases of brain, e.g. nervous ketosis, pregnancy toxemia, acute lead poisoning, poisoning with carbon tetrachloride, and severe hepatic insufficiency, especially in horses.
Frenzy
Frenzy is characterized by violent activity and with little regard for surroundings. The animal’s movements are uncontrolled and dangerous to other animals in the group and to human attendants, and are often accompanied by aggressive physical attacks.
Examples of frenzy in diseases of the nervous system include:
• Encephalomyelitides, e.g. Aujeszky’s disease
• Toxic and metabolic brain disease, e.g. hypomagnesemic tetany of cattle and sheep, poisoning with ammoniated roughage in cattle.
Examples of frenzy in diseases of other body systems are:
• Acute pain of colic in horses
• Extreme cutaneous irritation, e.g. photosensitization in cattle. Apparently reasonless panic, especially in individual horses or groups of cattle, is difficult to differentiate from real mania. A horse taking fright at a botfly or a swarm of bees, a herd of cattle stampeding at night are examples.
Aggressive behavior
Aggression and a willingness to attack other animals, humans and inert objects is characteristic of: the early stages of rabies and Aujeszky’s disease in cattle; in sows during postparturient hysteria; in the later stages of chronic hypoxia in any species; and in some mares and cows with granulosa-cell tumors of the ovary. The latter are accompanied by signs of masculinization and erratic or continuous estrus.4 It is often difficult to differentiate between an animal with a genuine change in personality and one that is in pain or is physically handicapped, e.g. pigs and cattle with atlantoaxial arthroses.
Depressive states
Depressive mental states include somnolence, lassitude, narcolepsy/catalepsy, syncope and coma. They are all manifestations of depression of cerebral cortical function in various degrees and occur as a result of those influences that depress nervous system function generally, as well as those that specifically affect behavior, probably via the limbic system. It is not possible to classify accurately the types of depressive abnormality and relate them to specific causes, but the common occurrences in farm animals are listed below.
Depression leading to coma
In all species this may result from:
• Encephalomyelitis and encephalomalacia
• Toxic and metabolic diseases of the brain such as uremia, hypoglycemia, hepatic insufficiency, toxemia, septicemia and most toxins that damage tissues generally
• Hypoxia of the brain, as in peripheral circulatory failure of milk fever
• Specific poisons that cause somnolence, including bromides, Amitraz in horses, methyl alcohol, Filix mas (male fern), kikuyu grass.
Narcolepsy (catalepsy)
Affected animals experience episodes of uncontrollable sleep and literally ‘fall’ asleep. The disease is recorded in Shetland ponies and is thought to be inherited in them, in other horses, and in cattle.5
Compulsive walking or head-pressing
Head-pressing is a syndrome characterized by the animal pushing its head against fixed objects, into a corner of a pen, leaning into a stanchion or between fence posts. Head-pressing should be differentiated from compulsive walking, where affected animals put their heads down and walk slowly while appearing blind. If they walk into an object they lean forward and indulge in head-pressing; if confined to a stall they will often walk around the pen continuously or head-press into a corner. The syndrome represents a change in behavior pattern due to an unsatisfied compulsive drive characteristic of a disorder of the limbic system. Causes include:
INVOLUNTARY MOVEMENTS
Involuntary movements are due to involuntary muscle contractions, which include gradations from fasciculations, shivering and tremor, to tetany, seizures or convulsions. Opisthotonos or ‘backward tone’ is a sustained spasm of the neck and limb muscles resulting in dorsal and caudal extension of the head and neck with rigid extension of the limbs.
Tremor
This is a continuous, repetitive twitching of skeletal muscles, which is usually visible and palpable. The muscle units involved may be small and cause only local skin movement, in which case the tremor is described as fasciculations; or the muscle units may be extensive, the movement much coarser and sufficient to move the extremities, eyes or parts of the trunk. The tremor may become intensified when the animal undertakes some positive action, this usually being indicative of cerebellar involvement and is the counterpart of intention tremor in humans. True tremor is often sufficiently severe to cause incoordination and severe disability in gait. Examples of causes of tremor include:
• Diffuse diseases of the cerebrum, cerebellum, spinal cord
• Degenerative nervous system disease, e.g. hypomyelinogenesis of the newborn as in congenital tremor of pigs and calves, poisoning by Swainsona sp.
• Toxic nervous system disease caused by a large number of poisons, especially poisonous plants and fungi, Clostridium botulinum toxin in shaker foal syndrome; metabolic disease such as hyperkalemic periodic paralysis in the horse; early stages of hypocalcemia in the cow (fasciculations of the eyelids and ears).
Tics
Tics are spasmodic twitching movements made at much longer intervals than in tremor, the intervals being usually at least several seconds in duration and often much longer. The movements are sufficiently widespread to be easily visible and are caused by muscles that are ordinarily under voluntary control. They are rare in large animals but may occur after traumatic injury to a spinal nerve.
Tetany
Tetanus is a sustained contraction of muscles without tremor. The most common cause is Clostridium tetani intoxication following localized infection with the organism. The degree of muscular contraction can be exaggerated by stimulation of the affected animal and the limbs are rigid and cannot be passively flexed easily – ‘lead pipe’ rigidity.
Myoclonus is a brief, intermittent tetanic contraction of the skeletal muscles that results in the entire body being rigid for several seconds, followed by relaxation. Inherited congenital myoclonus (hereditary neuraxial edema) of Polled, Horned and crossbred Hereford calves is a typical example. Affected calves are bright and alert and can suck normally but if they undertake a voluntary movement or are handled, their entire body becomes rigid for 10–15 seconds.
Convulsions
Convulsions, seizures, fits, or ictus are violent muscular contractions affecting part or all of the body and occurring for relatively short periods as a rule, although in the late stages of encephalitis they may recur with such rapidity as to give the impression of being continuous.
Convulsions are the result of abnormal electrical discharges in forebrain neurons that reach the somatic and visceral motor areas and initiate spontaneous, paroxysmal, involuntary movements. These cerebral dysrhythmias tend to begin and end abruptly and they have a finite duration. A typical convulsion may have a prodromal phase or aura that lasts for minutes to hours, during which the animal is oblivious to its environment and seems restless. The beginning of the convulsion may be manifested as a localized partial convulsion of one part of the body that soon spreads to involve the whole body, when the animal usually falls to the ground thrashing rhythmically. Following the convulsion there may be depression and temporary blindness, which may last for several minutes up to a few hours.
The convulsion may be clonic, the typical ‘paddling’ involuntary movement in which repeated muscle spasms alternate with periods of relaxation. Tetanic or tonic convulsions are less common and are manifested by prolonged muscular spasm without intervening periods of relaxation. True tetanic convulsions occur only rarely, chiefly in strychnine poisoning and in tetanus, and in most cases they are a brief introduction to a clonic convulsion.
Convulsions can originate from disturbances anywhere in the prosencephalon, including cerebrum, thalamus or even the hypothalamus alone. However, the initiating cause may be in the nervous system outside the cranium or in some other system altogether, so that convulsions are therefore often subdivided into intracranial and extracranial types. Causes are many and include the following.
Intracranial convulsions are caused by:
• Encephalomyelitis, meningitis
• Brain ischemia, including increased intracranial pressure
• Local lesions caused by trauma (concussion, contusion), abscess, tumor, parasitic injury, hemorrhage
Extracranial convulsions are caused by brain hypoxia, as in acute circulatory or cardiac failure, and toxic and metabolic diseases of the nervous system, including:
• Hypoglycemia (as in newborn piglets and in hyperinsulinism due to islet cell adenoma of the pancreas as described in a pony)
• Hypomagnesemia (as in lactation tetany in cows and mares)
• Inorganic poisons, poisonous plants and fungi. There are too many to give a complete list but well-known examples are the chlorinated hydrocarbons, pluronics used in bloat control in cattle, Clostridium spp. intoxications, e.g. Clostridium perfringens type D and Clostridium sordellii, and subacute fluoroacetate poisoning
• Congenital and inherited defects without lesions, e.g. familial convulsions and ataxia in Angus cattle.
ABNORMAL POSTURE AND GAIT
Posture
Posture is evaluated with the animal at rest. Abnormal postures may be adopted intermittently by animals in pain but in diseases of the nervous system the abnormality is usually continuous and repeatable. Deviation of the head and neck from the axial plane or rotation of the head and neck from the horizontal plane (head tilt) and drooping of the lips, eyelids, cheeks and ears, and opisthotonos and orthotonos are examples, although the latter two are often intermittent in that they occur as part of a convulsive seizure. Head-pressing and assumption of a dog-sitting posture are further examples. Abnormalities of posture and gait are the result of lesions of the brainstem, cerebellum, all levels of the spinal cord, spinal nerve roots, peripheral nerves, neuromuscular junctions and muscles. The clinical emphasis is on vestibular disease, cerebellar disease and spinal cord disease. It is important to recognize that cerebral lesions do not cause abnormalities in posture and gait.
Vestibular disease
The vestibular system is a special proprioceptive system that assists the animal to maintain orientation in its environment with respect to gravity. The system helps to maintain the position of the eyes, trunk and limbs in relationship to movements and positioning of the head.
From the vestibular nuclei, the vestibulospinal tracts descend ipsilaterally through the length of the spinal cord. These neurons are facilitatory to ipsilateral motor neurons going to extensor muscles of the limbs, are inhibitory to ipsilateral motor flexor muscles and are inhibitory to contralateral extensor muscles. The principal effect of unilateral stimulation of this system on the limbs is a relative ipsilateral extensor tonus and contralateral flexor tonus, which promotes ipsilateral support of the trunk against gravity. Conversely, a unilateral vestibular lesion usually results in ipsilateral flexor and contralateral extensor tonus, forcing the animal toward the side of the lesion.
The nuclei of cranial nerves III, IV, and VI, which control eye movement, are connected with the vestibular system by way of a brainstem tract – the medial longitudinal fasciculus. Through this tract, coordinated eye movements occur with changes in positioning of the head. Through these various pathways, the vestibular system coordinates movements of the eye, trunk, and limbs with head movements and maintains equilibrium of the entire body during motion and rest.
Signs of vestibular disease vary depending on whether there is unilateral or bilateral involvement and whether the disease involves peripheral or central components of the system.
The vestibular influence on balance can be affected:
Unilateral excitation or loss of function can be caused by lesions at any of these points.
General signs of vestibular system dysfunction are staggering, leaning, rolling, circling, drifting sideways when walking and a head tilt, and various changes in eye position such as strabismus and nystagmus. The walking in a circle toward the affected side is accompanied by increased tone in the contralateral limbs, which is most easily observed in the contralateral forelimb. Rotation or tilt of the head occurs and severely affected animals fall to the affected side.
When the lesion affects the inner ear, as it may do in otitis media, the affected side is turned down, the animal falls to that side and there may be facial paralysis on the same side if the lesion is extensive and affects the seventh cranial nerve. In the recumbent position, the affected side is held to the ground, and if these animals are rolled over to the opposite side they quickly roll back to the affected side. When the vestibular nuclei are affected, which may occur in listeriosis, the animal falls to the affected side.
Nystagmus and forced circling are common when there is irritation of the vestibular nucleus or the medial longitudinal fasciculus.
Causes of vestibular disease include:
• Otitis media–interna with involvement of the inner ear
• Focal lesion at the vestibular nucleus, e.g. listeriosis
• Traumatic injury to the vestibular apparatus in the horse caused by fracture of the basisphenoid, basioccipital and temporal bones in a traumatic injury. The clinical signs include lack of control of balance, rotation of the head, circling to the affected side, nystagmus and facial paralysis.
In paradoxical vestibular syndrome there is also head tilting, but circling in a direction away from the side of the lesion.6 Deviation of the head and neck must be distinguished from a head tilt. Asymmetric lesions of the forebrain such as a brain abscess, some cases of polioencephalomalacia, verminous larval migration or head trauma may cause an animal to hold its head and neck turned to one side, but there is no head tilt and the circle is large in diameter. In fact, the presence of a head tilt (deviation of eyes away from a horizontal plane) accompanied by a tight circle provide clinically useful methods of differentiating a cerebral lesion from a vestibular lesion.
Gait
Gait is assessed when the animal is moving. Neurological gait abnormalities have two components, weakness and ataxia. Weakness (paresis) is evident when an animal drags its limbs, has worn hooves or has a low arc to the swing phase of the stride. When an animal bears weight on a weak limb, the limb often trembles and the animal may even collapse on that limb because of lack of support. While circling, walking on a slope, and walking with the head elevated, an animal frequently will stumble on a weak limb and knuckle over at the fetlock. During manipulation of the limb, the clinician will usually make the subjective observation that the muscle tone is reduced.
Ataxia
Ataxia is an unconscious, general proprioceptive deficit causing incoordination when the animal moves. Ataxia is manifest as a swaying from side to side of the pelvis, trunk and sometimes the whole body (truncal sway). Ataxia may also appear as a weaving of the affected limb during the swing phase of the stride. This often results in abducted or adducted foot placement, crossing of the limbs or stepping on the opposite foot.
Hypermetria is an increased range of movement and is seen as an overreaching of the limbs with excessive joint movement. Hypermetria without paresis is characteristic of spinocerebellar and cerebellar disease.
Hypometria is a decreased range of movement that is characterized by a stiff or spastic movement of the limbs with little flexion of the joints, particularly the carpal and tarsal joints.
Dysmetria is a term that includes both hypermetria and hypometria, with goose-stepping being the most common sign of dysmetria. Dysmetria usually is caused by a lesion in the cerebellum or cerebellar pathway.
In equine degenerative myeloencephalopathy, there is dysmetria of the hindlimbs and tetraparesis due to neuraxonal dystrophy originating in the accessory cuneate nuclei.7 Severely affected horses lift their feet excessively high and stamp them to the ground.
Cerebellar disease
When cerebellar function is abnormal there is ataxia, which is an incoordination when the animal moves. In general terms there are defects in the rate, range and direction of movement. In typical cerebellar diseases, ataxia of the limbs is common and no weakness is evident. In true cerebellar ataxia (e.g. cerebellar hypoplasia) the affected animal stands with the legs wide apart, sways and has a tendency to fall. Ataxia of the head and neck are characterized by wide, swinging, head excursions, jerky head bobbing and an intention tremor (nodding) of the head.
The head tremor may be the most obvious sign in mild cases of cerebellar hypoplasia in young foals. The limbs do not move in unison, the movements are grossly exaggerated, muscular strength is usually preserved and there is a lack of proper placement of the feet (hypermetria and hypometria), so that falling is common. The fault in placement is the result of poor motor coordination and not related in any way to muscle weakness or proprioceptive deficit. Attempts to proceed to a particular point are usually unsuccessful and the animal cannot accurately reach its feed or drinking bowl. Examples of cerebellar disease include:
• Inherited defects of cerebellar structure or abiotrophy8 in most breeds of cattle and in Arabian horses
• Congenital cerebellar defects resulting from maternal viral infections such as bovine virus diarrhea (BVD) infection in cattle
• Dysplastic disease of the cerebellum of the horse
• Traumatic injury, e.g. by parasite larvae such as Hypoderma bovis, which have caused unilateral cerebellar ataxia in adult cattle
• Tremorogenic mycotoxicoses and ryegrasses
• Encephalomyelitis in which other localizing signs also occur.
Spinal cord disease
Ataxia due to cerebellar dysfunction can be difficult to differentiate from the proprioceptive defects and partial motor paralysis (weakness) that occur in animals with spinal cord lesions and it is most important that this differentiation be made. Spinal cord disease, causing varying degrees of weakness, and ataxia are common in large animals. The weakness is caused by damage to the upper or lower motor neurons and the proprioceptive deficit by damage to the ascending sensory neurons. With a mild or even moderate cervical spinal cord lesion in an adult cow or horse, signs of ataxia and weakness may be evident in the pelvic limbs only and it can be difficult to determine whether the thoracic limbs are involved.
Close examination of the gait, posture and postural reactions in the limbs, together with a search for localizing abnormalities, will often be productive in localizing the lesion. Signs of weakness or ataxia may be elicited by gently pushing the hindquarters to one side or pulling the tail to one side as the animal is walked (the sway response). The normal animal resists these movements or steps briskly to the side as it is pushed or pulled. The weak animal can be easily pulled to one side and may stumble or fall. The weak animal may also tend to buckle or collapse when strong pressure is applied with the hand over the withers and loin regions. The ataxic animal may sway to one side, be slow to protract a limb, cross its hindlegs or step on its opposite limb.
It is often difficult to distinguish paresis from ataxia but in most instances it is unimportant because of the close anatomical relationship of the ascending general proprioceptive and descending upper motor neuron tracts in the white matter of the spinal cord. These same abnormal sway responses can be elicited in the standing animal.
The ataxic animal may abduct the outside pelvic limb too far as it is pushed to one side or moved in a small circle. This may appear as a hypermetric movement similar to a stringhalt action and is assumed to be a sign of a general proprioceptive tract lesion. The pushed or circled animal may keep a clinically affected pelvic limb planted in one position on the ground and pivot around it without moving it. The same failure to protract the limb may be seen on backing. It may even force the animal into a ‘dog-sitting’ posture.
Examples of ataxia due to spinal cord disease include:
• Limited trauma to the spinal cord
• The early stages of a developing compression lesion in the vertebral canal
• Degenerative and inflammatory diseases of the nervous system, especially those causing enzootic incoordination in horses and staggers in sheep (both of them dealt with under their respective headings)
• Functional diseases in toxic and metabolic diseases of the nervous system in which lesions have not yet been identified and caused mainly by poisons, especially plant materials. Typical examples are poisoning by the fungi Claviceps paspali, Diplodia spp., Acremonium lolii, the grass Phalaris aquatica, the ferns Zamia and Xanthorrhea spp. and herbaceous plants such as Kallstroemia, Vicia, Baccharis, Solanum, Aesculus and Ficus spp.
• Nutritional deficiency especially of thiamin, occurring naturally in horses poisoned by bracken and horsetail, and experimentally in pigs
• Developmental defects including congenital abnormalities and abiotrophic abnormalities that develop some time after birth. Examples are Brown Swiss weavers and Pietrain pig creepers.
In many of these diseases incoordination and paresis are a stage in the development of tetraplegia or paraplegia.
PARESIS AND PARALYSIS
• The pyramidal tracts, which originate in the motor cortex
• The extrapyramidal system, which originates in the corpus striatum, red nucleus, vestibular nucleus and roof of the midbrain
• The peripheral nerves, which originate in the ventral horn cells.
The pyramidal tracts are of minor importance in hoofed animals (ungulates), reaching only to the fourth cervical segment. Accordingly, lesions of the motor cortex in farm animals do not produce any deficit of gait. Neither is there any paresis, although in an acute lesion weakness may be evident for the first day or two. If the lesion is unilateral the paresis will be on the contralateral side. This is in marked contradistinction to the severe abnormalities of posture and gait that occur with lesions of the pons, medulla, and spinal cord.
The main motor nuclei in these animals are subcortical and comprise the extrapyramidal system, and most combined movements are controlled by nerve stimuli originating in the tectal nuclei, reticular nuclei, vestibular nuclei and possibly red nuclei. The pyramidal and extrapyramidal tracts comprise the upper motor neurons, which reach to the ventral horn cells of the spinal cord, which cells together with their peripheral axons form the lower motor neurons. Paralysis is a physiological end result in all cases of motor nerve injury, which if severe enough is expressed clinically. The type of paralysis is often indicative of the site of the lesion.
A lesion of the upper motor neuron causes:
The spasticity of an upper motor neuron lesion usually occurs with the affected limb in extension. These are all release phenomena resulting from liberation of spinal reflex arcs from higher control.
A lesion of the lower motor neuron causes:
As injuries to specific peripheral nerves are treated surgically, these are dealt with in surgical textbooks and are not repeated here.
A special form of paralysis is the Schiff– Sherrington syndrome, which is common in dogs but recorded rarely in large animals. It is caused by acute, severe, compressive injury of the thoracolumbar spinal cord and manifested by extensor rigidity or hypertonia of the forelimbs and hypotonic paralysis of the hindlimbs. Neurons located in the lumbar spinal cord are responsible for the tonic inhibition of extensor muscle alpha motor neurons in the cervical intumescence. The cell bodies of these neurons are located in the ventral gray column from L1–L7, with a maximum population from L2–L4. Their axons ascend to the cervical intumescence. Acute severe lesions cranial to these neurons and caudal to the cervical intumescence will suddenly deprive the cervical intumescence neurons of this source of tonic inhibition, resulting in a release of these latter neurons. This results in extensor hypertonia observed in the thoracic limbs which can function normally in the gait and postural reactions, except for the hypertonia.
The degree of paresis or paralysis needs to be defined. Paralysis is identified as an inability to make purposeful movements. Thus convulsive, uncontrolled movements as they occur in polioencephalomalacia may still fit a description of paralysis. Paresis, or weakness short of paralysis, can be classified into four categories:
• Animals that cannot rise, nor support themselves if helped up, but can make purposeful movements in attempting to rise
• Animals that cannot rise but can support themselves if helped up
• Animals that can rise but are paretic and can move the limbs well and stumble only slightly on walking
• Animals that move with difficulty and have severe incoordination and stumbling.
Probably the most difficult decision in farm animal neurology is whether a patient’s inability to move is because of a nervous or muscular deficit. For example, the horse recumbent because of exertional rhabdomyolysis often resembles a horse with an injured spinal cord. Examples of paresis and paralysis include:
• Focal inflammatory, neoplastic, traumatic lesions in the motor pathway. These lesions usually produce an asymmetric nervous deficit
• Toxic and metabolic diseases of the nervous system in their most severe form, e.g. flaccid paralysis associated with tickbite (Ixodes holocyclus, Ornithodoros sp.), poisoning, botulism, snakebite. Comparable tetanic paralyses include tetanus, lactation tetany of mares, hypomagnesemic tetany of cows and calves. In contrast to inflammatory, neoplastic and traumatic lesions in the motor pathway, toxic and metabolic lesions usually produce a symmetric nervous deficit.
ALTERED SENSATION
Lesions of the sensory system are rarely diagnosed in animals, except for those affecting sight and the vestibular apparatus, because of the impossibility of measuring subjective responses.
Thus, although animals must experience paresthesia, as in Aujeszky’s disease (pseudorabies) in cattle and sheep, the animal’s response of licking or scratching does not make it possible to decide whether the diagnosis should be paresthesia or pruritus. Lesions of the peripheral sensory neurons cause hypersensitivity or decreased sensitivity of the area supplied by the nerve. Lesions of the spinal cord may affect only motor or only sensory fiber tracts or both, or may be unilateral.
Although it is often difficult to decide whether failure to respond to a normally painful stimulus is due to failure to perceive or inability to respond, certain tests may give valuable information. The test commonly used is pricking the skin with a needle, or pinching the skin with a pair of forceps, and observing the reaction. In exceptional circumstances light stroking may elicit an exaggerated response. The ‘nibbling’ reaction stimulated by stroking the lumbar back of sheep affected with scrapie is a striking example of hypersensitivity.
In every test of sensitivity it must be remembered that there is considerable variation between animals and in an individual animal from time to time, and much discretion must be exercised when assessing the response. In any animal there are also cutaneous areas that are more sensitive than others. The face and the cranial cervical region are highly sensitive, the caudal cervical and shoulder regions less so, with sensitivity increasing over the caudal thorax and lumbar region to a high degree on the perineum. The proximal parts of the limbs are much less sensitive than the distal parts and sensitivity is highest over the digits, particularly on the medial aspect.
Absence of a response to the application of a painful stimulus to the limbs (absence of the withdrawal reflex) indicates interruption of the reflex arc; absence of the reflex with persistence of central perception, as demonstrated by groaning or body movement such as looking at the site of stimulus application, indicates interruption of motor pathways and that central perception of pain persists. In the horse the response can be much more subtle than in other species, with movements of the ears and eyelids being the best indicators of pain perception. Increased sensitivity is described as hyperesthesia, decreased as hypoesthesia, and complete absence of sensitivity is described as anesthesia. Special cutaneous reflexes include the anal reflex, in which spasmodic contraction of the anus occurs when it is touched, and the corneal reflex, in which there is closure of the eyelids on touching the cornea. The (cutaneous trunci) panniculus reflex is valuable in that the sensory pathways, detected by the prick of a pin, enter the cord at spinal cord segments T1–L3, but the motor pathways leave the cord only at spinal cord segments C8, T1, and T2. The quick twitch of the superficial cutaneous muscle along the whole back, which is the positive response (panniculus reflex), is quite unmistakable. Examination of the eye reflexes and hearing are discussed under examination of the cranial nerves (see below).
BLINDNESS
Blindness is manifested as a clinical abnormality by the animal walking into objects that it should avoid.
The menace or blink reflex is used to test the visual pathway. A threatening gesture of the hand (or even better by the index finger in a pointing manner) toward the eye elicits immediate closure of the eyelids. The hand must come close enough to the eye without touching the tactile hairs of the eyelids or creating a wind which can be felt by the animal. Some stoic, depressed or even excited animals may not respond to a menace reflex with closure of the eyelids; others may keep the eyelids partially or almost closed. It may be necessary to alert the patient to the risk of injury by touching the eyelids first. The menace reflex is a learned reflex that is absent in neonates.
The most definitive test is to make the animal walk an obstacle course and place objects in front of it so that it must step over the objects easily. A similar procedure is the only way to test for night blindness (nyctalopia). The area should be dimly lit but the observer should be able to see the obstructions clearly. A decision that the animal is blind creates a need for examination of the visual pathways.
Central or peripheral blindness
Blindness may be central or peripheral. Animals with forebrain lesions are centrally blind, with depressed menace response in one or both eyes while the pupillary light reflexes are commonly intact. In peripheral blindness, such as hypovitaminosis-A, the menace reflex is absent, and the pupillary light reflexes are also absent.
Blindness can be caused by lesions along the visual pathway, from the eye to the cerebral cortex:
• Diseases of the orbit including keratoconjunctivitis, hypopyon, cataract, panophthalmia, mixed ocular defects inherited in white Shorthorn and Jersey cattle, night blindness in Appaloosa horses, sporadic cases of blindness due to idiopathic retinal degenerative disease in cattle
• Diseases of the retina including retinal dysplasia of goats, lenticular cataracts caused by poisoning with hygromycin in pigs,9 congenital ocular malformations in calves after intrauterine infection with BVD virus (usually accompanied by cerebellar defects)
• Diseases of the optic nerve and chiasma, e.g. abscess of pituitary rete mirabile, constriction of optic nerve by diet deficient in vitamin A. Tumor of pituitary gland, injury to the optic nerve, especially in horses after rearing and falling backwards. There is a sudden onset of unilateral or bilateral blindness with no ophthalmological change until 3–4 weeks after the injury, when the optic disc becomes paler and less vascular10
• Metabolic or ischemic lesions of the cerebral cortex as in polioencephalomalacia, cerebral edema, hydrocephalus
• Localized infectious or parasitic lesions caused by abscesses, migrating larvae
• Functional blindness in which there is complete, often temporary, blindness in the absence of any physical lesions. Causes are acetonemia, pregnancy toxemia and acute carbohydrate indigestion (hyper d-lactatemia) of ruminants
• Specific poisonings causing blindness include Filix mas (male fern), Cheilanthes spp. (rockfern) and rape. Stypandra spp. cause a specific degeneration of the optic nerves. Lead poisoning in cattle.
ABNORMALITIES OF THE AUTONOMIC NERVOUS SYSTEM
Lesions affecting the cranial parasympathetic outflow do so by involvement of the oculomotor, facial, vagus, and glossopharyngeal nerves or their nuclei and the effects produced are discussed under examination of the individual nerves.
In general, the lesions cause abnormality of pupillary constriction, salivation and involuntary muscular activity in the upper part of the alimentary and respiratory tracts. Lesions of the spinal sympathetic system interfere with normal function of the heart and alimentary tract. For the most part, affections of the autonomic nervous system are of minor importance in farm animals. Central lesions of the hypothalamus can cause abnormalities of heat exchange, manifested as neurogenic hyperthermia or hypothermia and obesity, but they are also of minor importance.
Some manifestations of autonomic disease are important. Autonomic imbalance is usually described as the physiological basis for spasmodic colic of horses; grass sickness of horses is characterized by degenerative lesions in the sympathetic ganglia; involvement of the vagus nerve in traumatic reticuloperitonitis of cattle can lead to impaired forestomach and abomasal motility and the development of vagus indigestion.
Defects of sphincter control and motility of the bladder and rectum may also be of importance in the diagnosis of defects of sacral parasympathetic outflow and the spinal sympathetic system. The sacral segments of the spinal cord are the critical ones, and loss of their function will cause incontinence of urine and loss of rectal tone. The parasympathetic nerve supply to the bladder stimulates the detrusor muscle and relaxes the sphincter; the sympathetic nerve supply has the reverse function. A spinal cord lesion may cause loss of the parasympathetic control and result in urinary retention. Incontinence, if it occurs, does so from overflow. When the sympathetic control is removed incontinence occurs but the bladder should empty. Similar disturbances of defecation occur. Both micturition and defecation are controlled by medullary and spinal centers but some measure of control is regained even when the extrinsic nerve supply to the bladder and rectum is completely removed.
Special examination of the nervous system
Veterinarians commonly include several components of a neurological examination in a complete clinical examination. Most often a diagnosis and differential diagnosis can be made from consideration of the history and the clinical findings. However, if the diagnosis is uncertain it may be necessary to conduct a complete neurological examination, which may uncover additional clinical findings necessary to make a diagnosis and give a prognosis.
The accuracy of clinical diagnosis of neurological diseases in the horse is high.11 In a study of 210 horses in which a definitive pathological diagnosis was confirmed, the overall accuracy of clinical diagnosis for all diseases was 0.95; the accuracy ranged from 0.79–1.00, the sensitivity varied from 0.73–0.95 and the specificity varied from 0.88–1.00 for individual disease categories. Some neurological diseases are therefore underdiagnosed while others are overdiagnosed. The use of careful and thorough clinical examinations and diagnostic techniques, combined with confirmed pathological diagnoses, will result in more accurate diagnosis and therapy. Retrospective studies of series of ataxic horses, for example, will add to the body of knowledge and improve diagnosis.12
THE NEUROLOGICAL EXAMINATION
The primary aim of the neurological examination is to confirm whether or not a neurological abnormality exists and to determine the neuroanatomical location of the lesion.13 A clinicoanatomical diagnosis is necessary before one can develop a list of differential diagnoses and decided whether or not treatment is possible. The format for a precise practical examination procedure that is logical in sequence, easy to remember with practice, and emphasizes the need for an anatomical diagnosis is outlined below. The rationale for the sequence is that the examination starts from a distance to assess posture and mentation, and then proceeds to a closer examination that may require placing the animal in stocks or a chute. The examination sequence is therefore suitable for minimally handled beef cattle, dairy cattle, horses, sheep, goats, and New World camelids. The results of the neurological examination should be documented and not left to memory. There are many standard examination forms available that outline each step in the examination and provide for documentation of the results.
SIGNALMENT AND EPIDEMIOLOGY
The age, breed, sex, use, and value of the animal are all important considerations in the diagnosis and prognosis of neurological disease. Some diseases occur more frequently under certain conditions: for example, lead poisoning in nursing beef calves turned out to pasture in the spring of the year. Histophilus somni meningoencephalitis occurs most commonly in feedlot cattle from 6–10 months of age and hypovitaminosis-A occurs most commonly in beef calves 6–8 months of age after grazing dry summer pastures. In the horse there are several clearly defined diseases that affect the spinal cord including cervical stenotic myelopathy, degenerative myeloencephalopathy, protozoal myelitis, equine rhinopneumonitis myelopathy, rabies polioencephalomyelitis and equine motor neuron disease.14 Some of these diseases have distinguishing epidemiological characteristics that are useful in diagnosis and differential diagnosis.9 The neurological examination of the newborn foal is fraught with hazards because of the different responses elicited from those in adults. The differences relate mostly to the temporary dysmetria of gait and exaggerated responses of reflexes.
HISTORY
Special attention should be given to the recording of an accurate history. The questioning of the owner should focus on the primary complaint and when it occurred and how it has changed over time (the time–sign relationship). The duration of signs, the mode of onset, particularly whether acute with later subsidence, or chronic with gradual onset, the progression of involvement and the description of signs that occur only intermittently should be ascertained. When the disease is a herd problem the morbidity and mortality rates and the method of spread may indicate an intoxication when all affected animals show signs within a very short period. Diseases associated with infectious agents may have an acute or chronic onset. Neoplastic diseases of the nervous system may begin abruptly but are often slowly progressive. For some diseases, such as epilepsy, consideration of the history may be the only method of making a diagnosis.15 Traumatic injuries have a sudden onset and then often stabilize or improve.
When obtaining a history of convulsive episodes an estimate should be made of their duration and frequency. The pattern is also of importance, and may be diagnostic, e.g. in salt poisoning in swine. The occurrence of pallor or cyanosis during the convulsion is of particular importance in the differentiation of cardiac syncope and a convulsion originating in the nervous system.
HEAD
Behavior
The owner should be questioned about the animal’s abnormal behavior, which can include bellowing, yawning, licking, mania, convulsions, aggressiveness, head-pressing, wandering, compulsive walking and head-shaking.16 Head-shaking may be photic in origin and can be tested by the application of blindfolds, covering the eyes with a face mask and observing the horse in total darkness outdoors.17 In one horse, head-shaking ceased with blindfolding or night darkness outdoors, and became less with the use of gray lenses. Outdoor behavior suggested efforts to avoid light.
Mental status
Assessment of mental status is based on the animal’s level of awareness or consciousness. Coma is a state of complete unresponsiveness to noxious stimuli. Other abnormal mental states include stupor, somnolence, deliriousness, lethargy and depression. Large animals that are recumbent because of spinal cord disease are commonly bright and alert unless affected with complications, which may cause fever and anorexia. Mature beef cattle that are recumbent with a spinal cord lesion and not used to being handled may be quite aggressive and apprehensive.
Head position and coordination
Lesions of the vestibular system often result in a head tilt. Lesions of the cerebrum often result in deviation of the head and neck. In cerebellar disease, there may be jerky movements of the head, which are exaggerated by increasing voluntary effort. These fine jerky movements of the head are called intention tremors. Animals with severe neck pain will hold their neck in a fixed position and be reluctant to move the head and neck. Head-shaking in horses has been associated with ear mite infestation, otitis externa, cranial nerve dysfunction, cervical injury, ocular disease, guttural pouch mycosis, dental periapical osteitis and vasomotor rhinitis.16 However, idiopathic head-shaking in the horse is often associated with evidence of nasal irritation, sneezing and snorting, nasal discharge, coughing and excessive lacrimation.
Cranial nerves
Abnormalities of cranial nerve (CN) function assist in localizing a lesion near or within the brainstem. Some of the information on cranial nerve dysfunction is presented in tabular form (Tables 12.1-12.6) in addition to the more detailed examination described here.
Table 12.1 Correlation between clinical findings and location of lesions in the nervous system of farm animals: abnormalities of mental state (behavior)
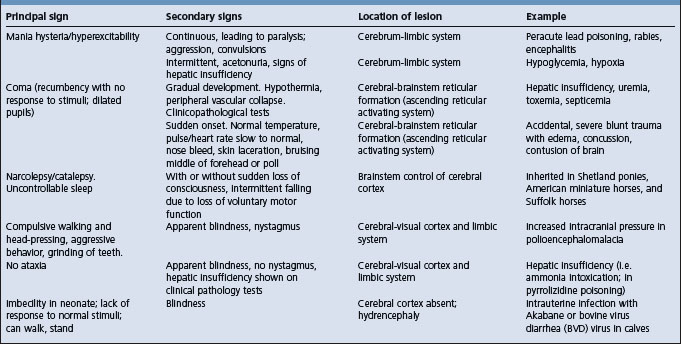
Table 12.2 Correlation between clinical findings and location of lesion in the nervous system of farm animals: involuntary movements
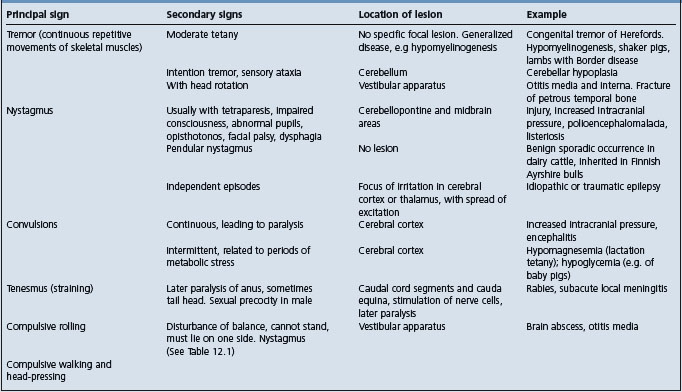
Table 12.3 Correlation between clinical findings and location of lesion in the nervous system of farm animals: abnormalities of posture
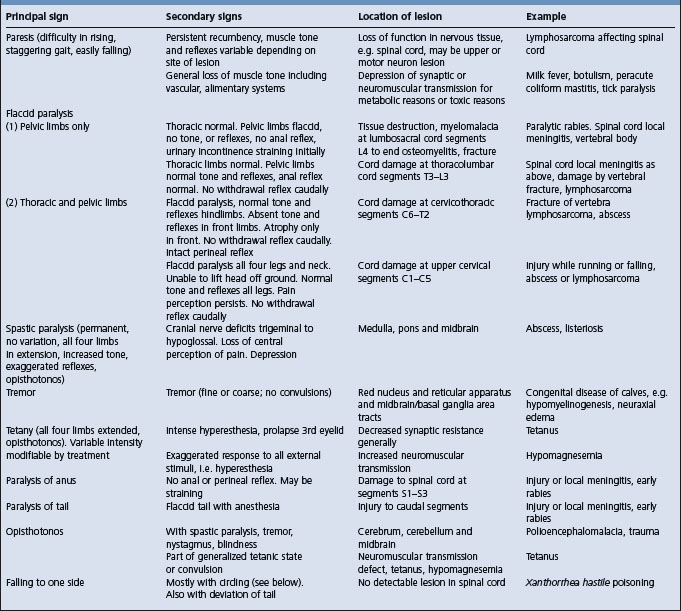
Table 12.4 Correlation between clinical findings and location of lesion in the nervous system of farm animals: abnormalities of gait
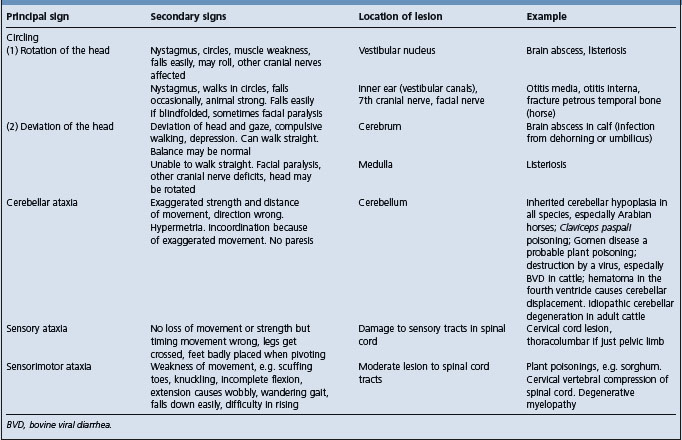
Table 12.5 Correlation between clinical findings and location of lesion in the nervous system of farm animals: abnormalities of the visual system
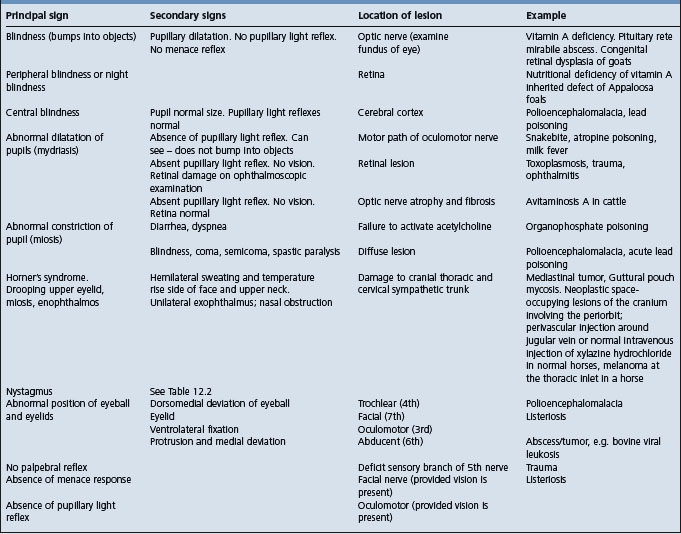
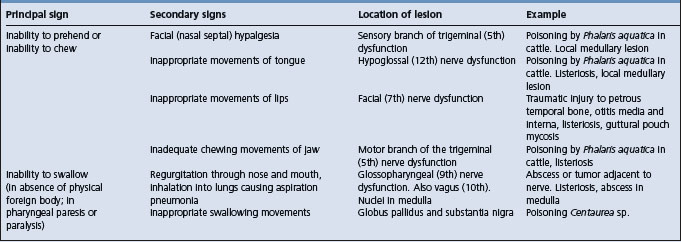
Table 12.6 Correlation between clinical findings and location of lesion in the nervous system of farm animals: disturbances of prehension, chewing, or swallowing
Olfactory nerve (CN I)
Tests of smell are unsatisfactory in large animals because of their response to food by sight and sound.
Optic nerve (CN II)
The only tests of visual acuity applicable in animals are testing the eye preservation (menace) reflex: provoking closure of the eyelids and withdrawal of the head by stabbing the finger at the eye; and by making the animal run a contrived obstacle course. Both tests are often difficult to interpret and must be carried out in such a way that other senses are not used to determine the presence of the obstacles or threatened injury. In more intelligent species, a good test is to drop some light object such as a handkerchief or feather in front of the animal. It should gaze at the object while it is falling and continue to watch it on the ground. The same method can be applied to young ruminants, which demonstrate normal vision by following the examiner’s moving hand at an age so early that they have not yet developed a menace reflex. Ophthalmoscopic examination is an integral part of an examination of the optic nerve.
Oculomotor nerve (CN III)
This nerve supplies the pupilloconstrictor muscles of the iris and all the extrinsic muscles of the eyeball except the dorsal oblique, the lateral rectus and the retractor muscles. Loss of function of the nerve results in pupillary dilatation and defective pupillary constriction when the light intensity is increased, abnormal position (ventrolateral deviation) or defective movement of the eyeballs and palpebral ptosis.
The pupillary light reflex is best tested by shining a bright point source of light into the eye, which causes constriction of the iris of that eye (direct pupillary reflex). Constriction of the opposite eye (consensual pupillary light reflex) will also occur. The consensual light reflex may be used to localize lesions of the optic pathways.
Examination of the menace reflex (eye preservation reflex to a menace) and the results of the pupillary light reflex can be used to distinguish between blindness due to a lesion in the cerebral cortex (central blindness) and that due to lesions in the optic nerve or other peripheral parts of the optic pathways (peripheral blindness).
As examples, in polioencephalomalacia (central blindness) the menace reflex is absent but the pupillary light reflex is present. In the ocular form of hypovitaminosis A (peripheral blindness) in cattle the menace reflex is also absent, the pupils are widely dilated and the pupillary light reflex is absent. In polioencephalomalacia, the optic nerve, oculomotor nucleus and oculomotor nerve are usually intact but the visual cortex is not; in hypovitaminosis A the optic nerve is usually degenerate, which interferes with both the menace and pupillary light reflexes.
Testing of ocular movements can be carried out by moving the hand about in front of the face. In paralysis of the oculomotor nerve there may also be deviation from the normal ocular axes and rotation of the eyeball. There will be an absence of the normal horizontal nystagmus reaction with a medial jerk of the eyeball in response to quick passive movement of the head. Failure to jerk laterally indicates a defect of the abducens nerve.
Trochlear nerve (CN IV)
This nerve supplies only the dorsal oblique muscle of the eye so that external movements and position of the eyeball are abnormal (dorsolateral fixation) when the nerve is injured. This is common in polioencephalomalacia in cattle, resulting in a dorsomedial fixation of the eyeball. In other words, the medial angle of the pupil is displaced dorsally when the head is held in normal extension.
Trigeminal nerve (CN V)
The sensory part of the trigeminal nerve supplies sensory fibers to the face and can be examined by testing the palpebral reflex and the sensitivity of the face. The motor part of the nerve supplies the muscles of mastication and observation of the act of chewing may reveal abnormal jaw movements and asymmetry of muscle contractions. There may also be atrophy of the muscles, which is best observed when the lesion is unilateral.
Abducent nerve (CN VI)
Because the abducent nerve supplies motor fibers to the retractor and lateral rectus muscles of the eyeball, injury to the nerve may result in protrusion and medial deviation of the globe. This is not readily observable clinically. An inherited exophthalmos and strabismus occurs in Jersey cattle.
Facial nerve (CN VII)
The facial nerve supplies motor fibers for movement of the ears, eyelids, lips, and nostrils, in addition to the motor pathways of the menace, palpebral, and corneal reflexes. The symmetry and posture of the ears, eyelids, and lips are the best criteria for assessing the function of the nerve. Ability to move the muscles in question can be determined by creating a noise or stabbing a finger at the eye. Absence of the eye preservation reflex may be due to facial nerve paralysis or blindness. Facial paralysis is evidenced by ipsilateral drooping of the ear, ptosis of the upper eyelid, drooping of the lips and pulling of the filtrum to the unaffected side. There may also be drooling of saliva from the commissures of the lips and in some cases a small amount of feed may remain in the cheeks of the affected side.
The common causes of damage to the nerve are fracture of the petrous temporal bone, guttural pouch mycosis and damage to the peripheral nerve at the mandible. A common accompaniment is injury to the vestibular nerve or center. A diagnosis of central, as compared to peripheral nerve involvement, can be made by identifying involvement of adjacent structures in the medulla oblongata. Signs such as depression, weakness and a head tilt would result, and are frequently present in ruminants and New World camelids with listeriosis.
Vestibulocochlear nerve (CN VIII)
The cochlear part of the vestibulocochlear nerve is not easily tested by simple clinical examination, but failure to respond to sudden sharp sounds, created out of sight and without creating air currents, suggests deafness. The cochlear portion can be tested electronically (the brainstem auditory evoked response, or BAER, test) to diagnose a lesion of the auditory nerve, eliminating the possibility of a central brain lesion. Abnormalities of balance and carriage of the head (rotation around the long axis and not deviation laterally) accompany lesions of the vestibular part of the vestibulocochlear nerve, and nystagmus is usually present.
In severe cases, rotation of the head is extreme, the animal is unable to stand and lies in lateral recumbency; moving to achieve this posture is compulsive and forceful. There is no loss of strength. In some species there is a relatively common occurrence of paralysis of the facial and the vestibular nerves as a result of otitis interna and otitis media. This does occur in the horse but less commonly than traumatic injury to the skull as a result of falling.
Pendular nystagmus should not be mistaken as a sign of serious neurological disease. Pendular nystagmus is characterized by oscillations of the eyeball that are always the same speed and amplitude and appear in response to a visual stimulus, e.g. a flashing light. Pendular nystagmus is observed most frequently in Holstein–Friesian cattle (prevalence of 0.51% in 2932 Holstein–Friesian and Jersey cows),18 is not accompanied by other signs and there is no detectable histological lesion. A familial relationship was observed in Ayrshire bulls in Finland.19
Glossopharyngeal nerve (CN IX) and vagus nerve (CN X)
The glossopharyngeal nerve is sensory from the pharynx and larynx, and the vagus nerve is motor to these structures. Dysfunction of these nerves is usually accompanied by paralysis of these organs with signs of dysphagia or inability to swallow, regurgitation through the nostrils, abnormality of the voice and interference with respiration.
Because of the additional role of the vagus nerve in supplying nerve fibers to the upper alimentary tract, loss of vagal nerve function will lead to paralysis of the pharynx and esophagus. Parasympathetic nerve fibers to the stomach are also carried in the vagus and damage to them could cause hypomotility of that organ. The principal clinical finding in vagus nerve injury is laryngeal and pharyngeal paralysis.
Spinal accessory nerve (CN XI)
Damage to this nerve is extremely rare and the effects are not documented. Based on its anatomical distribution loss of function of this nerve could be expected to lead to paralysis of the trapezius, brachiocephalic and sternocephalic muscles and lack of resistance to lifting the head.
Hypoglossal nerve (CN XII)
As the motor supply to the tongue, the function of this nerve can be best examined by observing the motor activity of the tongue. There may be protrusion, deviation or fibrillation of the organ, all resulting in difficulty in prehending food and drinking water. The most obvious abnormality is the ease with which the tongue can be pulled out. The animal also has difficulty in getting it back into its normal position in the mouth, although diffuse cerebral disease can also produce this clinical sign. In lesions of some duration there may be obvious unilateral atrophy.
POSTURE AND GAIT
The examiner evaluates posture and gait to give a general assessment of brainstem, spinal cord and peripheral nerve and muscle function. Evaluation of posture and gait consists of determining which limbs are abnormal and looking for evidence of lameness suggesting a musculoskeletal gait abnormality. Weakness and ataxia are the essential components of gait abnormality. Each limb is examined for evidence of these abnormalities. This is done while the animal is standing still, walking, trotting, turning tightly (pivoting) and backing up. To detect subtle asymmetry in the length of the stride, the observer should walk parallel to or behind the animal, step for step. If possible, the gait should also be evaluated while the animal is walking up and down a slope, walking with the head and neck held extended, while blindfolded and while running free in an enclosure.
The best observations are made when the animal is running free, preferably at a fast gait, to avoid abnormalities resulting from being led. Also, slight abnormalities such as a high-stepping gait, slight incoordination of movement, errors of placement of feet, stumbling and failure to flex joints properly are all better observed in a free animal.
Weakness or paresis is evident when an animal drags its limbs, has worn hooves or has a low arc to the swing phase of the stride. When an animal bears weight on a weak limb, the limb often trembles and the animal may even collapse on that limb because of lack of support. While circling, walking on a slope and walking with the head held elevated, an animal frequently will stumble on a weak limb and knuckle over on the fetlock.
The presence of weakness in the limbs of horses or cattle can be determined by pulling the tail while the animal is walking forward. A weak animal is easily pulled to the side and put off stride. While the animal is circling, the examiner can pull on the lead rope and tail simultaneously to assess strength. Ease in pulling the animal to the side occurs because of weakness due to lesions of descending upper motor neuron pathway, the ventral horn gray matter level with the limb or peripheral nerves or muscle. With lower motor neuron lesions, the weakness is often so marked that it is easy to pull an animal to the side while it is standing or walking. In contrast, a weak animal with a lesion of the upper motor neuron pathways will often fix the limb in extension, reflexly, when pulled to one side. It resists the pull and appears strong.
Severe weakness in all four limbs, but with no ataxia and spasticity, suggests neuromuscular disease. Obvious weakness in only one limb is suggestive of a peripheral nerve or muscle lesion in that limb.
Ataxia is an unconscious, general proprioceptive deficit causing poor coordination when moving the limbs and the body. It results in swaying from side to side of the pelvis, trunk and sometimes the entire body. It may also appear as a weaving of the affected limb during the swing phase. This often results in abducted or abducted foot placement, crossing of the limbs or stepping on the opposite foot, especially when the animal is circling or turning tightly. Circumduction of the outside limbs when turning and circling is also considered a proprioceptive deficiency. Walking an animal on a slope, with the head held elevated, often exaggerates ataxia, particularly in the pelvic limbs. When a weak and ataxic animal is turned sharply in circles, it leaves the affected limb in one place while pivoting around it. An ataxic gait may be most pronounced when an animal is moving freely, at a trot or canter, especially when attempting to stop. This is when the limbs may be wildly abducted or adducted. Proprioceptive deficits are caused by lesions affecting the general proprioceptive sensory pathways, which relay information on limb and body position to the cerebellum (unconscious proprioception) and to the thalamus and cerebral cortex (conscious proprioception).
Knuckling the flexed foot while the animal stands on the dorsum to determine how long the animal leaves the foot in this state before returning it to a normal position is a test for conscious proprioception in dogs and cats. The test has not been useful in horses and adult cattle but is useful in sheep, goats, New World camelids, and calves. Depressed animals will often allow the foot to rest on the dorsum for prolonged periods. Crossing the limbs and observing how long the animal maintains a crosslegged stance has been used to test conscious proprioception.
Hypermetria is used to describe a lack of direction and increased range of movement, and is seen as an overreaching of the limbs with excessive joint movement. Hypermetria without paresis is characteristic of spinocerebellar and cerebellar disease.
Hypometria is seen as stiff or spastic movement of the limbs with little flexion of the joints, particularly the carpal and tarsal joints. This generally is indicative of increased extensor tone and of a lesion affecting the descending motor or ascending spinocerebellar pathways to that limb. A hypometric gait, particularly in the thoracic limbs, is best seen when the animal is backed up or when it is maneuvered on a slope with the head held elevated. The thoracic limbs may move almost without flexing.
Dysmetria is a term that incorporates both hypermetria and hypometria. Animals with severe cerebellar lesions may have a high-stepping gait but have limited movement of the distal limb joints, especially in thoracic limbs.
The degree of weakness, ataxia, hypometria and hypermetria should be graded for each limb. The types of gait abnormalities and the degree of weakness reflect various nervous and musculoskeletal lesions. Generally, with focal, particularly compressive, lesions in the cervical spinal cord or brainstem, neurological signs are one grade more severe in the pelvic limbs than in the thoracic limbs. Thus, with a mild, focal, cervical spinal cord lesion there may be more abnormality in the pelvic limbs with no signs in the thoracic limbs. The anatomical diagnosis in such cases may be a thoracolumbar, cervical, or diffuse spinal cord lesion.
A moderate or severe abnormality in the pelvic limbs, and none in the thoracic limbs, is consistent with a thoracolumbar spinal cord lesion. With a mild and a severe change in the thoracic and the pelvic limb gaits respectively, one must consider a severe thoracolumbar lesion plus a mild cervical lesion, or a diffuse spinal cord disease.
Lesions involving the brachial intumescence (spinal cord segments C6–T2) with involvement of the gray matter supplying the thoracic limbs, and diffuse spinal cord lesions may both result in severe gait abnormality in the thoracic limbs and the pelvic limbs.
A severely abnormal gait in the thoracic limbs, with normal pelvic limbs, indicates lower motor neuron involvement of the thoracic limbs; a lesion is most likely to be present in the ventral gray columns at spinal cord segments C6–T2 or thoracic limb peripheral nerves of muscle.
Gait abnormalities can occur in all four limbs, with lesions affecting the white matter in the caudal brainstem, when head signs, such as cranial nerve deficits, are used to define the site of the lesion. Lesions affecting the cerebrum cause no change in gait or posture.
NECK AND FORELIMBS
If a gait abnormality was evident in the thoracic limbs and there was no evidence of brain involvement, then examination of the neck and forelimbs can confirm involvement of the spinal cord, peripheral nerves (spinal cord segments C1–T2) or thoracic limb muscles. The neck and forelimbs are examined for evidence of gross skeletal defects, asymmetry of the neck and muscle atrophy. The neck should be manipulated from side to side and up and down to detect any evidence of resistance or pain. Localized unilateral sweating of the neck and cranial shoulder is evidence of Horner’s syndrome,20 in which there are varying degrees of ptosis, prolapse of the third eyelid, miosis, enophthalmos and increased temperature of the face, neck and shoulder. The syndrome is associated with lesions affecting the descending sympathetic fibers in the white matter of the spinal cord or gray matter in the cranial thoracic segments, thoracocervical sympathetic trunk, cervical vagosympathetic trunk or cranial cervical ganglion and its pre- and postganglionic fibers.
Sensory perception from the neck and forelimbs is assessed using a painful stimulus such as a blunt needle or forceps. The local responses as well as the cerebral responses are noted when the skin over the shoulders and down the limbs is pricked.
Gait deficits are evaluated by making the horse or halter-broken ruminant perform a series of movements. Such exercises should include walking and trotting in a straight line, in large circles, in tight circles, backing on a level ground and on a slight slope, walking and trotting over curbs or low obstacles, walking in straight lines and circles, and walking on a slope with the head held elevated. The sway reaction for the thoracic limb is assessed by pushing against the shoulders and forcing the animal first to resist and then to take a step laterally. This can be done while the animal is standing still and walking forward. Pulling the tail and lead rope laterally at the same time will assess the strength on each side of the body. Making the animal turn in a tight circle by pulling the lead rope and tail at the same time will indicate strength; an adult horse should be able to pull the examiner around and should not pivot on a limb or be pulled to the side. Pressing down with the fingers on the withers of a normal animal causes some arching, followed by resistance to the downward pressure. An animal with weakness in the thoracic limbs may not be able to resist this pressure by fixing its vertebral column but will arch its back more than normal and often buckle in the thoracic limbs.
In smaller farm animal species, other postural reactions can be performed. These include wheelbarrowing and the hopping response test. The spinal reflexes are assumed to be intact in animals that are ambulating normally.
If a large mature horse, cow or pig has a gait abnormality, it is very rare to cast the animal to assess the spinal reflexes. However, spinal reflexes are usually examined in calves, sheep, and goats.
A recumbent animal that can use its thoracic limbs to sit up in the dog-sitting position may have a lesion caudal to spinal cord segment T2. If a recumbent animal cannot attain a dog-sitting position, the lesion may be in the cervical spinal cord. In lambs aged between 4 and 10 weeks with thoracic vertebral body abscesses extending into the epidural space causing spinal cord compression, the thoracic limbs are normal and the lambs frequently adopt a ‘dog-sitting’ position and move themselves around using the thoracic limbs only.21 Lambs with a cervical spinal cord lesion are unable to maintain sternal recumbency and have paresis of all four limbs.
However, mature cattle with the downer cow syndrome secondary to hypocalcemia may be unable to use both the thoracic and pelvic limbs. If only the head, but not the neck, can be raised off the ground, there may be a severe cranial cervical lesion. With a severe caudal cervical lesion, the head and neck can usually be raised off the ground but thoracic limb function is decreased and the animal is unable to maintain sternal recumbency.
Assessment of limb function is done by manipulating each limb separately, in its free state, for muscle tone and sensory and motor activity. A limb that has been lain upon for some time cannot be properly evaluated because there will be poor tone from the compression. A flaccid limb, with no motor activity, indicates a lower motor lesion to that limb. A severe upper motor neuron lesion to the thoracic limbs causes decreased, or absent, voluntary effort, but there is commonly normal or increased muscle tone in the limbs. This is due to release of the lower motor neuron, which reflexly maintains normal muscle tone from the calming influence of the descending upper motor neuron pathways.
The tone of skeletal muscle may be examined by passively flexing and extending the limbs and moving the neck from side to side and up and down. Increased muscle tone, spasticity or tetany may be so great that the limb cannot be flexed without considerable effort. If the spastic-extended limb does begin to flex but the resistance remains, this is known as ‘lead-pipe’ rigidity, which is seen in tetanus. If after beginning to flex an extended spastic limb, the resistance suddenly disappears (‘clasp-knife release’) this suggests an upper motor neuron lesion, as occurs in spastic paresis in cattle.
Flaccidity, or decreased muscle tone, indicates the presence of a lower motor neuron lesion with interruption of the spinal reflex arc.
Localized atrophy of muscles may be myogenic or neurogenic and the difference can be determined only by electromyography, a technique not well suited to large-animal practice. If the atrophic muscle corresponds to the distribution of a peripheral nerve it is usually assumed that the atrophy is neurogenic. In addition, neurogenic atrophy is usually rapid (will be clinically obvious in a few days) and much more marked than either disuse or myogenic atrophy.
Spinal reflexes of the thoracic limbs
These include the flexor reflex, the biceps reflex and the triceps reflex. The flexor reflex is tested by stimulation of the skin of the distal limb and observing for flexion of the fetlock, knee, elbow, and shoulder. The reflex arc involves sensory fibers in the median and ulnar nerves, spinal cord segments C6–T2 and motor fibers in the axillary, musculocutaneous, median and ulnar nerves. Lesions cranial to spinal cord segment C6 may release this reflex from the calming effect of the upper motor neuron pathways and cause an exaggerated reflex with rapid flexion of the limb, and the limb may remain flexed for some time. A spinal reflex may be intact without cerebral perception. Cerebral responses to the flexor reflex include changes in the facial expression, head movement toward the examiner and vocalization. Conscious perception of the stimulus will be intact only as long as the afferent fibers in the median and ulnar nerves, the dorsal gray columns at spinal cord segments C6–T2 and the ascending sensory pathways in the cervical spinal cord and brainstem are intact.
The laryngeal adductory reflex is of special interest in the examination of ataxic horses. In normal horses a slap on the saddle region just caudal to the withers causes a flickering adductory movement of the contralateral arytenoid cartilage that is visible by an endoscope. Reflex muscle contraction can be palpated on the dorsolateral surfaces of the larynx. The reflex is absent when there is damage to afferent tracts up the spinal cord, when there is damage to the recurrent laryngeal nerves, and in tense or frightened horses. Elicitation of the reflex is called the slap test.
TRUNK AND HINDLIMBS
If examination of the posture, gait, head, neck or thoracic limbs reveals evidence of a lesion, then an attempt should be made to explain any further signs found during examination of the trunk and hindlimbs that could have been caused by the lesion. If there are only signs in the trunk and hindlimbs, then the lesion(s) must be either between spinal cord segments T2 and S2 or in the trunk and pelvic limb nerves or muscles. It must be remembered that a subtle neurological gait in the pelvic limbs may be anywhere between the midsacral spinal cord and the rostral brainstem.
The trunk and hindlimbs are observed and palpated for malformations and asymmetry. Diffuse or localized sweating, the result of epinephrine release and sympathetic denervation, is often present in horses affected with a severe spinal cord injury.
Gentle pricking of the skin over the trunk and over the lateral aspects of the body wall on both sides, including on either side of the thoracolumbar vertebral column, will test-stimulate the cutaneous trunci reflex. The sensory stimulus travels to the spinal cord in thoracolumbar spinal nerves at the level of the site of stimulation. These impulses are transmitted up the spinal cord to spinal cord segments C8–T1, where the lateral thoracic nerve is stimulated, causing contraction of the cutaneous trunci muscle, which is seen as a flicking of the skin over the trunk. Lesions anywhere along this pathway will result in suppression or absence of this reflex caudal to the site of the lesion. Degrees of hypalgesia and analgesia have been detected caudal to the sites of thoracolumbar spinal cord lesions, especially if they are severe. In mature cattle with fractured thoracolumbar vertebrae associated with traumatic injury or vertebral body abscesses in calves, the site of the lesion may be able to be localized with this reflex. Sensory perception of pinpricking the trunk and hindlimbs may also be absent caudal to the lesion.
The sway reaction for the pelvic limbs involves pushing against the pelvis and pulling on the tail with the animal standing still and walking forward. An animal which is weak in the pelvic limbs will be easily pulled and pushed laterally, especially while walking. Proprioceptive deficits can be observed as overabduction and crossing of the limbs when a step is taken to the side.
Pinching and pressing down on the thoracolumbar or sacral paravertebral muscles with the fingers causes a normal animal to extend slightly, then fix, the thoracolumbar vertebral column. It also resists the ventral motion and usually does not flex the thoracic or pelvic limbs. A weak animal usually is not able to resist the pressure by fixing the vertebral column and thus it overextends the back and begins to buckle in the pelvic limbs.
In the recumbent animal, examination of the pelvic limbs includes the pelvic limb spinal reflexes, the degree of voluntary effort and the muscle tone present. Observing the animal attempting to rise on its own or following some coaxing will help to assess the pelvic limbs. The flexor spinal reflex is performed by pricking the skin and observing the flexion of the limb; central perception of the painful stimulus is also noted. The afferent and efferent pathways for this reflex are in the sciatic nerve and involve spinal cord segments L5–S3.
The patellar reflex is evaluated by placing the animal in lateral recumbency and supporting the limb in a partly flexed position. The intermediate patellar ligament (horses) or patellar ligament (ruminants, pigs, New World camelids) is then tapped with a heavy metal plexor. This results in extension of the stifle joint. The sensory and motor fibers for this reflex are in the femoral nerve, and the spinal cord segments are L4 and L5. The patellar reflex is hyperactive in newborn farm animals. The gastrocnemius reflex and the cranial tibial reflex are not evaluated because they cannot be reliably induced.
The spinal cord of the calf has more control of basic physical functions than in humans, dogs and horses. For example, calves are able to retain control of the pelvic limb in spite of experimentally induced lesions that cause hemiplegia in dogs and humans. Also transection of the spinothalamic tract in the calf cord does not produce an area of hypalgesia or analgesia on the contralateral side as such a lesion would do in a human.22
Skin sensation of the pelvic limbs should be assessed independently from reflex activity. The femoral nerve is sensory to the skin of the medial thigh region, the peroneal nerve to the dorsal tarsus and metatarsus, and the tibial nerve to the plantar surface of the metatarsus.
TAIL AND ANUS
Tail tone is evaluated by lifting the tail and noting the resistance to movement. A flaccid tail, with no voluntary movement, is indicative of a lesion of the sacrococcygeal spinal cord segments, nerves, or muscles. Decreased tone in tail can be detected with severe spinal cord lesions cranial to the coccygeal segment.
The perineal reflex is elicited by lightly pricking the skin of the perineum and observing reflex contraction of the anal sphincter and clamping down of the tail. The sensory fibers are contained within the perineal branches of the pudendal nerve (spinal cord segments S1–S3). Contraction of the anal sphincter is mediated by the caudal rectal branch of the pudendal nerve, and tail flexion is mediated by the sacral and coccygeal segments and nerves (spinal cord segments S1–Co). An animal with a flaccid tail and anus, due to lower motor neuron lesion, will not have an anal or tail reflex. However, it may still have normal sensation from the anus and tail provided that the sensory nerves and spinal cord and brainstem white matter nociceptive pathways are intact.
Observation of defecation and urination movements and postures contributes to knowledge of the state of the cauda equina. Thus neuritis of the cauda equina is characterized by flaccid paralysis and analgesia of the tail, anus and perineum, rectum and bladder. There is no paresis or paralysis of the hindlimbs unless lumbosacral segments of the cord are damaged.
PALPATION OF THE BONY ENCASEMENT OF THE CENTRAL NERVOUS SYSTEM
Palpable or visible abnormalities of the cranium or spinal column are not commonly encountered in diseases of the nervous system but this examination should not be neglected. There may be displacement, abnormal configuration or pain on deep palpation. These abnormalities are much more readily palpable in the vertebral column and if vertebrae are fractured. Abnormal rigidity or flexibility of the vertebral column, such as occurs in atlanto-occipital malformations in Arabian horses and cattle, may also be detectable by manipulation.
COLLECTION AND EXAMINATION OF CEREBROSPINAL FLUID
The collection and laboratory analysis of CSF from farm animals with clinical evidence of nervous system disease can provide useful diagnostic and prognostic information.2
CSF is formed mostly from the choroid plexuses of the lateral ventricles by the ultrafiltration of plasma and the active transport of selected substances across the blood–brain barrier. The CSF in the ventricular system flows caudally and diffuses out of the lateral aperture in the fourth ventricle to circulate around the brain and spinal cord. The presence of CSF in the subarachnoid space separates the brain and spinal cord from the bony cranium and vertebral column, which reduces trauma to the underlying delicate nervous tissue. CSF also has excretory functions with the removal of products of cerebral metabolism.
Collection of cerebrospinal fluid
CSF can be collected from lumbosacral cistern with sedation (horses) or restraint (ruminants) and the atlanto-occipital cistern (cisterna magna) using injectable general anesthesia. For collection it is necessary to puncture the subarachnoid space in either the lumbosacral space or cisterna magna. Although there is no substantial difference between the composition of lumbosacral or cisternal CSF samples unless there is a compressive lesion of the spinal cord, the general policy is to sample as close to the lesion as possible. CSF should be collected into a sterile tube and there is no need to add an anticoagulant, even in samples visibly contaminated with blood. Cytology should be performed as soon as possible after collection (ideally within 15 min) because the cells rapidly degenerate.
Collection from the lumbosacral cistern
The lumbosacral site is preferred because general anesthesia is not required. CSF can be collected from the lumbosacral cistern with relative ease provided that adequate restraint can be achieved and the anatomical landmarks can be identified. CSF can be collected from the standing or recumbent animal. If recumbent, the animal should be placed in sternal recumbency with hips flexed and the pelvic limbs extended alongside the abdomen. This widens the lumbosacral space to permit correct placement of the spinal needle.
The site for collection is the midpoint of the lumbosacral space, which can be identified as the midline depression between the last palpable dorsal lumbar spine (L6 in cattle, goats and horses; L6 or L7 in sheep and pigs; L7 in New World camelids) and the first palpable sacral dorsal spine (usually S2). In well conditioned animals, these landmarks cannot always be identified; in which case the site is identified as the midpoint of a line connecting the caudal aspect of the tuber coxae. The site is clipped, surgically prepared and 1–2 mL of local anesthetic is administered subcutaneously. Sterile surgical gloves should be worn. Hypodermic spinal needles with stilettes are recommended because ordinary needles commonly plug with tissue. The length and gauge of needle depends on the size of the animal, but 15 cm (6 in) 18-gauge needles are needed for adult horses and cattle. The following guide is recommended (Table 12.7).
Table 12.7 Needle length gauge for lumbosacral cerebrospinal fluid collection
| Species and body weight | Length (cm) and gauge of needle |
|---|---|
| Lambs <30 kg | 2.5 and 20 |
| Ewes 40–80 kg | 4.0 and 20 |
| Rams > 80 kg | 5.0 and 20 |
| Calves < 100 kg | 4.0 and 20 |
| Calves 100–200 kg | 5.0 and 18 |
| Cattle > 200 kg | 10.0–15.0 and 18 |
Provided the animal is well restrained and care is exercised in introducing the needle, little difficulty should be encountered. For collection from the lumbosacral space the needle is slowly advanced perpendicular or up to 15° caudal to perpendicular to the plane of the vertebral column. The needle must be introduced in a perfectly vertical position relative to the plane of the animal’s vertebral column because of the danger of entering one of the lateral blood vessels in the vertebral canal. Changes in tissue resistance can be felt as the needle point passes sequentially through the subcutaneous tissue, interarcuate ligament, then the sudden ‘pop’ due to the loss of resistance as the needle point penetrates the ligamentum flavum into the epidural space. Once the needle point has penetrated the dorsal subarachnoid space, CSF will well up in the needle hub within 2–3 seconds. Failure to appreciate the changes in resistance as the needle moves down may result in puncture of the conus medullaris, which may elicit an immediate pain response and some discomfort. Movement of the pelvic limbs may dislodge the needle point, with the risk of causing local trauma and hemorrhage in the leptomeninges, which results in blood in the sample. Repeated CSF taps of the lumbosacral space may make it more difficult to obtain an adequate sample volume because of fibrosis of epidural tissue.23
Careful aspiration with a syringe attached to the needle held between the thumb and index finger is usually required to obtain a sample of 2–3 mL, which is sufficient for laboratory analysis. This can be facilitated by firmly resting the forearms and wrists on the animal’s back. Failure to obtain fluid is usually due to incorrect direction of the needle, in which the case the bony landmarks of the lumbosacral space (depression) must be rechecked and, with the needle correctly realigned, the procedure repeated. In animals with a vertebral body abscess and neurological disease confined to the hind limbs, CSF may be difficult to obtain from the lumbosacral space because flow is occluded. In these circumstances, if a sample is obtained, the CSF protein may be increased as a result of stagnation of CSF distal to the lesion with exudation or transudation of protein from the lesion (Froin’s syndrome).24
Collection from the atlanto-occipital cistern (cisterna magna)
This site is preferred for intracranial lesions because the fluid is produced in the subarachnoid space and flows caudally down the spinal cord.25 However, this site is rarely used because of the inherent risk of needle penetration of the brainstem. Xylazine at 0.20 mg/kg body weight (BW) intramuscularly is effective in providing adequate sedation and analgesia for this procedure in cattle. A general anesthetic (such as combined intravenous administration of xylazine and ketamine) is recommended for horses.
The site is prepared as with the lumbosacral cistern. Ventriflexion of the head and neck of cattle enlarges the space of the cisterna magna and allows easy entry using a stiletted spinal needle inserted at a point created by the transection of the transverse line of the cranial rim of the wing of the atlas and the dorsal midline. The needle is advanced carefully and steadily and the tip is directed rostrally towards the symphysis of the lower jaw. The needle point goes through the skin, ligamentum nuchae and leptomeninges. In most mature cattle of body weight over 500 kg, a 20-gauge, 10 cm (4 in) spinal needle will enter the cisterna magna at 5–7 cm after going through the ligamentum nuchae, which provides some increased resistance. When the needle point punctures the leptomeninges, the animal may move its head slightly. At that point the needle is advanced only 1–2 mm and the stilette is then removed. If the end of the needle is in the cisterna magna, CSF will flow out of the needle freely and the manometer can be attached and the pressure measured.
Cerebrospinal fluid pressure
The CSF pressure can be determined by the use of a manometer attached to the spinal needle. Normal CSF pressures of the cisterna magna in cattle and xylazine/ketamine-anesthetized horses range from 5–15 cm (uncertain reference point) and 28 ± 4 cm (referenced to the right atrium), respectively, using 0.9% NaCl solution in a manometer.26 When the fluid system is properly connected, occlusion of both jugular veins causes a marked rise in CSF pressure; this is called Queckenstedt’s test. The Queckenstedt test involves bilateral jugular vein compression; this results in a sudden increase in intracranial subarachnoid pressure that is transmitted to the cranial subarachnoid space. The resultant CSF pressure wave is transmitted to the lumbar area (when obtaining CSF from the lumbosacral space) in the absence of an obstruction in the spinal subarachnoid space, thereby resulting in an increased flow of CSF.
Variations in CSF pressure are not of much use in clinical diagnosis except in hypovitaminosis A, and measurement of CSF pressure is only indicated in animals with signs of cerebral disease (abnormal mentation). Care is needed in interpreting results because the pressure is greatly affected by voluntary movement such as tenesmus. CSF pressure is increased in a number of diseases, including polioencephalomalacia, bacterial meningitis, and hypovitaminosis A. Xylazine given intravenously causes a decrease in intracranial pressure in healthy conscious horses.27 Epidural pressure of cattle changes with change in position from standing to lateral recumbency to dorsal recumbency, and epidural pressure is positive in laterally recumbent animals.23 Although the effect of epidural pressure on CSF pressure has not been evaluated in large animals, it is likely that CSF pressure is also affected by position.
Analysis of cerebrospinal fluid
Analysis of CSF has greater diagnostic value than hematology in animals with nervous system disease. CSF can be examined for the presence of protein, cells and bacteria.2 The white blood cell count in normal animals is usually less than 5 cells/μL but a report exists of higher counts (12–200 cells/μL) in sheep.28 An increase in CSF leukocyte count above 5 cells/μL is termed a pleocytosis and is categorized as mild (6–49 cells/μL), moderate (50–200 cells/μL) or marked (>200 cells/μL). The differential white cell count comprises mostly lymphocytes and monocytes; there are no erythrocytes in normal animals. Samples that show visible turbidity usually contain large numbers of cells (>500 cells/μL) and much protein. In cattle, protein concentrations range from 23–6 mg/dL, sodium concentrations from 132–144 mmol/L, potassium 2.7–3.2 mmol/L, magnesium 1.8–2.1 mEq/L and glucose concentrations 37–51 mg/dL29 In the horse, the reference values for CSF are similar.30 Neonatal foals under 3 weeks of age have higher CSF protein concentrations than do adult horses. Glucose concentrations peak in the first 48 hours after birth, then decrease to adult values by the second week of life. Concentrations of sodium and potassium are not affected by age and are similar to values reported for adult horses and ponies.
With bacterial infections of the nervous system the CSF concentration of protein will be increased and the white blood cell count increased up to 2000 cells/μL with more than 70% neutrophils.28 A neutrophilic pleocytosis is considered 95–100% indicative of an inflammatory process within the central nervous system.28 Theoretically, the CSF glucose concentration will be decreased and CSF lactate concentration will be increased in animals with bacterial meningitis because of bacterial metabolism, but these are unreliable signs and usually do not provide additional information to that provided by determination of CSF leukocyte and protein concentrations. Bacteria may also be cultured from the CSF. Because meningoencephalitis may occur concurrently or following acute diarrhea in calves under a few weeks of age, the evaluation of CSF should be considered in calves that remain depressed and inactive following rehydration and treatment.31
The creatine kinase and lactate dehydrogenase activities in CSF have been examined as an aid in the differentiation of some neurological diseases. However, creatine kinase activity is considered to be unreliable; contamination of the sample with epidural fat and dura may increase CSF creatine kinase activity in the horse.32 Insufficient information is available to evaluate the clinical utility of CSF lactate dehydrogenase activity in large animals.
The CSF glucose concentration is usually 60–80% of serum glucose concentration; this steady state value reflects facilitated transport across the blood–brain barrier, absence of binding proteins for glucose in CSF and nervous tissue metabolism of glucose.29 However, sudden changes in plasma glucose concentrations are not immediately reflected in CSF glucose concentrations, because CSF turns over at around 1% per minute. Typically, a lag time of up to 3 hours is needed for CSF glucose concentration to be in equilibrium with plasma glucose concentrations. Hyperglycemia as a result of the stress of handling and restraint may therefore not be reflected by an increased CSF glucose concentration.
Blood contamination of CSF can make interpretation difficult. A formula has been developed that ‘corrects’ the CSF values for the degree of blood contamination, based on the red blood cell count in CSF (RBCCSF) and blood (RBCblood), whereby the corrected value for substance X in CSF (Xcorrected, where X is a concentration or activity) is derived from the measured value of X in CSF (XCSF) and blood (Xblood) and applying the following formula:
Calculation of a ‘corrected’ value rarely provides additional insight into the CSF analysis and is not commonly practiced in large animals.
Protein fractionation of CSF is not routinely performed because it requires sensitive electrophoresis methodology or species-specific radial immunodiffusion assays. However, calculation of the albumin quotient and IgG index may be informative in specific neurologic diseases.34,35 Theoretically, these calculations can differentiate four blood–brain permeability patterns; normal blood–brain barrier permeability (normal albumin quotient and IgG index); intrathecal IgG production with normal blood–brain barrier permeability (normal albumin quotient and increased IgG index); increased blood–brain barrier permeability without intrathecal IgG production (increased albumin quotient and normal IgG index) and increased blood–brain barrier permeability with intrathecal production of IgG (increased albumin quotient and increased IgG index). The albumin quotient is calculated from the albumin concentration in CSF (ALBCSF) and serum (ALBserum), whereby:
The normal value for albumin quotient in the adult horse is less than 2.234,36 but the mean is 0.4 to 0.5 in cattle and adult llamas.29,35 Because CSF protein is most commonly derived by disturbance of the blood–brain barrier and inflammation (resulting in an increased CSF albumin concentration), an increased CSF protein concentration is usually accompanied by an increased albumin quotient.
In animals suspected to have increased immunoglobulin production in the central nervous system (a rare occurrence, and almost always accompanied by disturbance of the blood–brain barrier), the IgG index can be calculated from the IgG concentration in CSF (IgGCSF) and serum (IgGserum), and the albumin concentration in CSF (ALBCSF) and serum (ALBserum), whereby:
An IgG index of more than 0.3 is suspected to indicate intrathecal IgG production in the adult horse.34
This formula corrects the CSF IgG concentration for an increased permeability of the blood–brain barrier, and therefore theoretically provides a more sensitive method for detecting local production of IgG within the central nervous system. Calculating the albumin quotient and IgG index is expensive and rarely provides additional information to that provided by CSF protein concentration alone, and for this reason is not commonly performed in large animals.
In summary, collection and analysis of CSF from the lumbosacral region provides a practical, safe and informative diagnostic tool in conscious large animals with neurological disease. Analysis of CSF in animals with central nervous system disease has greater diagnostic value than analysis of the leukon or serum biochemical analysis. Routine assessment of CSF should include total protein concentration, erythrocyte count, leukocyte count and leukocyte differential count. Other analytical procedures on CSF can be performed in specific diseases related to the nervous system.
EXAMINATION OF THE NERVOUS SYSTEM WITH SERUM BIOCHEMICAL ANALYSIS
Arterial plasma ammonia concentration In animals suspected of having hepatic encephalopathy, measurement of the arterial plasma ammonia concentration provides a clinically useful diagnostic test and a means of monitoring the response to treatment. In monogastrics, ammonia is produced by bacterial degradation of amines, amino acids, and purines in the gastrointestinal tract, by the action of bacterial and intestinal urease on urea in the gastrointestinal tract and by the catabolism of glutamine by enterocytes.37 In ruminants, ammonia is derived predominantly from bacterial metabolism in the rumen and catabolism of amino acids in tissue. Absorbed ammonia is normally converted to urea by the liver and to glutamine by the liver, skeletal muscle, and brain. In the presence of hepatic dysfunction, ammonia is inadequately metabolized, resulting in high plasma ammonia concentrations. Ammonia is a direct neurotoxin that alters inhibitory and excitatory neurotransmission in the brain.
Hyperammonemia can be used as a specific indicator of hepatic dysfunction. Normal values for arterial plasma ammonia concentration are less than 29 μmol/L in adult cattle37 but may reach higher values in the immediate periparturient period. Arterial values are higher than venous values, and are preferred for analysis.
Blood gas analysis and serum electrolyte determination should be routinely undertaken in animals with clinical signs of encephalopathy, in order to rule out metabolic causes of cerebral dysfunction.
EXAMINATION OF THE NERVOUS SYSTEM WITH IMAGING TECHNIQUES
Radiography
Examination of the bony skeleton of the head and vertebral column to detect abnormalities which are affecting the nervous system of large animals is being used more commonly in referral centers. Conventional diagnostic radiography remains the best method for the initial evaluation of trauma to the brain and spinal cord. The injection of contrast media into the CSF system (myelography) is used for the detection of spinal cord compression but is rarely indicated in large animals because spinal cord depression surgery is rarely performed. In cases of peripheral nerve injury the radiograph of the appropriate limb may reveal the presence of a fracture or space-occupying lesion that has caused dysfunction of the peripheral nerve.
Computed tomography
Computed tomography (CT) of the skull has several advantages over radiography because structures are viewed in cross section without superimposition.38 The development of computer software and technology allows a large amount of information to be obtained from a CT examination. Numerous diseases of the head of the horse, including those of the brain, can be diagnosed using this technique, but the limiting factors are the weight of the patient, accessibility for large animals and the need for general anesthesia. In general, CT provides an excellent image of skeletal defects. CT has been used for the antemortem diagnosis of many conditions in foals and horses, and otitis interna in calves.39
Magnetic resonance imaging
Magnetic resonance imaging (MRI) scanning uses nuclear magnetic resonance to create cross sectional images based on the magnetic properties of tissues. In general, MRI provides an excellent image of soft tissue defects, but the limiting factors are the weight of the patient, accessibility for large animals and the need for general anesthesia. MRI has been used for the antemortem diagnosis of many neurological conditions in foals and horses40 and cerebellar hypoplasia in a calf.41
RHINOLARYNGOSCOPY (ENDOSCOPY) AND OPHTHALMOSCOPY
Endoscopy (rhinolaryngoscopy) is now a routine technique for the examination of horses with suspected laryngeal hemiplegia.3 Ophthalmoscopy for the examination of the structures of the eye is important in the diagnosis of diseases affecting the optic nerve such as in vitamin A deficiency, and the optic disc edema (papilledema) associated with diffuse cerebral edema.
ELECTROENCEPHALOGRAPHY
Electroencephalography (EEG) has not been utilized to any significant degree in large animals. The EEG requires sophisticated equipment, a quiet dim environment free from electrical interference and a quiet patient that has minimal muscular activity. Because of the difficulty in obtaining quality recordings in a conscious large animal, it is preferred that the animal is anesthetized for the recording, which may confound interpretation of the EEG pattern depending on the anesthetic protocol. Electroencephalography has therefore been primarily used as an antemortem or research tool in large animals, and its use will probably remain as a complementary test to other neurological examinations and diagnostic tests at referral institutions.42
Recommendations have been made in order to standardize EEG techniques for animals; these typically involve meticulous preparation of the recording sites on the scalp, and placement of electrodes over the left and right frontal areas, the left and right occipital areas, the vertex area and a reference electrode is placed behind the tip of the nose.20 However, the addition of other recording sites increases the ability to localize a focal lesion.42 Neurological disease is associated with changes in either EEG frequency or amplitude, or both, with frequency changes being a more reliable indicator of disease. In general, focal EEG abnormalities indicate a focal lesion in the cortex, whereas diffuse EEG abnormalities indicate diffuse cortical or subcortical lesions or focal subcortical lesions.
Electroencephalography has been used to study epilepsy in goats and cattle, congenital hydranencephaly and hydrocephalus in cattle, scrapie in sheep, thiamine-responsive polioencephalomalacia in cattle43 and bovine spongiform encephalopathy in cattle. When performed under controlled conditions, EEG has been shown to be a useful diagnostic tool for the early diagnosis of equine intracranial diseases,42 with adequate sensitivity and specificity.
ELECTROMYOGRAPHY
Electromyographic needle examination (EMG) is a technique that records the electrical activity generated by single muscle fibers and the summated electrical activity of muscle fibers in individual motor units.44 The technique involves inserting a recording needle into the muscle of interest and recording the resultant EMG. Typically, animals are unsedated and restrained in stocks or a chute. Abnormal EMG signals include short-duration and low-amplitude motor unit action potentials, which indicate diseased muscle fibers of early or incomplete reinnervation after denervation. Other abnormalities include the presence of fibrillation potentials, positive sharp waves and complex repetitive discharges that occur when the skeletal cell membrane becomes unstable because of denervation or myopathy.44
Electromyography provides a more practical diagnostic test than EEG, and is especially useful for evaluating peripheral nerve injury and diagnosing hyperkalemic periodic paresis in horses. Electromyography can discriminate between neurogenic or myogenic disorders, and nerve conduction studies can differentiate axonal loss from demyelination. In addition, repetitive stimulation can provide information regarding neuromuscular transmission.
Electromyography has been coupled with transcranial magnetic stimulation to induce magnetic motor evoked potentials in the horse.45 This provides a useful noninvasive evaluation of cervical spinal cord dysfunction in horses that evaluates the integrity of the descending motor tracts.
BRAINSTEM AUDITORY EVOKED POTENTIALS
The brainstem auditory evoked potential (BAEP) is a recording of the electrical activity of the brainstem following an acoustic stimulation.46 The use of the BAEP is well documented in human medicine as a diagnostic aid and has been used in dogs to evaluate deafness. The BAEP is obtained by recording neuroelectrical activity from generators in the auditory pathway immediately following an acoustic click stimulus, and BAEP waveforms for horses and ponies have been recorded.47 Such recordings can be useful in evaluating horses suspected to have deafness, vestibular disease or brainstem disease, and to monitor the response to treatment.48
INTRACRANIAL PRESSURE AND CEREBRAL PERFUSION PRESSURE
Intracranial pressure has been measured in neonatal foals,49 although the clinical utility of such measurements has not been demonstrated. Increases in intracranial pressure can cause decreases in cerebral perfusion pressure and irreversible injury to the central nervous system.
Principles of treatment of diseases of the nervous system
Treatment of disease of the nervous system presents some particular problems because of the failure of nervous tissue in the brain and spinal cord to regenerate and because of the impermeability of the blood–brain barrier to many antimicrobial agents, antiprotozoal agents, and anthelmintics.
When peripheral nerves are severed, regeneration occurs if the damage is not extensive but no specific treatment, other than surgical intervention, can be provided to facilitate repair. When neurons are destroyed in the brain and spinal cord no regeneration occurs and the provision of nervous system stimulants can have no effect on the loss of function that occurs. The emphasis in the treatment of diseases of the nervous system must be on prevention of further damage. On occasion this can be done by providing specific or ancillary treatments.
ELIMINATION AND CONTROL OF INFECTION
Most of the viral infections of the nervous system are not susceptible to chemotherapeutics. Some of the larger organisms such as Chlamydia spp. are susceptible to broad-spectrum antimicrobial agents such as the tetracyclines and chloramphenicol.
Bacterial infections of the central nervous system are usually manifestations of a general systemic infection as either bacteremia or septicemia. Treatment of such infections is limited by the existence of the blood–brain and blood–CSF barriers, which prevent penetration of some substances into nervous tissue and into the CSF. Very little useful data exist on the penetration of parenterally administered antibiotics into the central nervous system of either normal farm animals or those in which there is inflammation of the nervous system.
In humans it is considered that most antimicrobials do not enter the subarachnoid space in therapeutic concentrations unless inflammation is present, and the degree of penetration varies among drugs. Chloramphenicol is an exception; levels of one-third to one-half of the blood level are commonly achieved in normal individuals. The relative diffusion of Gram-negative antimicrobial agents from blood into CSF in humans is shown in Table 12.8.
Table 12.8 Relative diffusion of Gram-negative antimicrobials
| Excellent with or without inflammation | Good only with inflammation |
|---|---|
| Minimal or not good with inflammation | No passage with inflammation |
The most promising antimicrobial agents for the treatment of bacterial meningitis in farm animals are the third-generation cephalosporins, trimethoprim–sulfonamide combinations and gentamicin.50
In most instances of bacterial encephalitis or meningitis in farm animals it is likely that the blood–brain barrier is not intact and that parenterally administered drugs will diffuse into the nervous tissue and CSF. Certainly, the dramatic beneficial response achieved by the early parenteral treatment of Histophilus somni meningoencephalitis in cattle using intravenous oxytetracycline, intramuscular penicillin or a broad-spectrum antibiotic suggests that the blood–brain barrier may not be a major limiting factor when inflammation is present. Another example of an antibiotic that does not normally pass the blood–brain barrier well but is able to do so when the barrier is damaged is penicillin in the treatment of listeriosis. When cases of bacterial meningoencephalitis fail to respond to antimicrobial agents, to which the organisms are susceptible, other reasons should also be considered. Often the lesion is irreversibly advanced or there is a chronic suppurative process which is unlikely to respond.
Intrathecal injections of antimicrobial agents have been suggested as viable alternatives when parenteral therapy appears to be unsuccessful. However, there is no evidence that such treatment is superior to appropriate parenteral therapy. In addition, intrathecal injections can cause rapid death and therefore are not recommended.
DECOMPRESSION
Increased intracranial pressure probably occurs in most cases of inflammation of the brain but it is only likely to be severe enough to cause physical damage in acute cerebral edema, space-occupying lesions such as abscesses, and hypovitaminosis A. In these circumstances some treatment should be given to withdraw fluid from the brain tissue and decrease the intracranial pressure.
One treatment that may be attempted is the combination of mannitol and corticosteroids used in man and in small animals. Mannitol given as a 20% solution intravenously over a 30–60-minute period is a successful intracranial decompressant with an effect lasting about 4 hours; the effect can be prolonged by the intravenous administration of dexamethasone 3 hours after the mannitol. The treatment has been used in calves with polioencephalomalacia, combined with thiamin, with excellent results to relieve the effects of acute cerebral edema. The dose rates have been those recommended for dogs and are very expensive: mannitol 2 g/kg BW, dexamethasone 1 mg/kg BW, both intravenously. There are dangers with mannitol: it should not be repeated often; it must not be given to an animal in shock; it should be given intravenously slowly. Dexamethasone on its own is safe and has a good effect but does not decompress sufficiently. Hypertonic glucose given intravenously is dangerous because an initial temporary decompression is followed after a 4–6-hour interval by a return to pretreatment CSF pressure when the glucose is metabolized.
TREATMENT OF BRAIN INJURY AFTER HEAD TRAUMA
The principles of treatment of animals exhibiting neurological abnormalities after a traumatic event are derived from the results of large, controlled, multicenter clinical trials in human beings. Similar studies have not been performed in large animals. The general principles are: 1) stabilize the patient by ensuring a patent airway, obtaining vascular access and attending to wounds, 2) specific treatment for hyperthermia as brain defects may result in an inability to regulate core temperature, 3) prevent or treat systemic arterial hypotension, 4) optimize oxygen delivery, 5) ensure adequate ventilation by placing in sternal recumbency whenever possible, 6) decrease pain, 7) monitor plasma glucose concentration and maintain euglycemia, and 8) prevent or treat cerebral edema by having the head elevated or by the intravenous administration of a hyperosmolar agent (hypertonic saline, 7.2% NaCl, 2 mL/kg BW every 4 hours for 5 infusions; 20% mannitol as a series of bolus infusions of 0.25 to 1 g/kg BW every 4–6 hours, the latter is an expensive treatment). Intravenous catheterization should be confined to one jugular vein and the neck should not be bandaged in an attempt to minimize promotion of cerebral edema by jugular venous hypertension. Seizures should be treated when they occur by administering diazepam, midazolam, phenobarbital, or pentobarbital.
Many anecdotal treatments have been used in large animals, but evidence attesting to their efficacy is clearly lacking. Amongst the more popular empiric antioxidant treatments are dimethyl sulfoxide (1 g/kg BW as a 10% solution in 0.9% NaCl) administered intravenously or by nasogastric tube every 12 h, vitamin E (α-tocopherol, 50 IU/kg BW administered orally every day), vitamin C (ascorbic acid, 20 mg/kg BW administered orally every day), and allopurinol (5 mg/kg BW administered orally every 12 h). Corticosteroids have also been advocated; promoted treatments include an anti-inflammatory dose of dexamethasone (0.05 mg/kg BW every day) or a high dose of methylprednisolone sodium succinate (30 mg/kg BW initial bolus, followed by continuous infusion of 5.4 mg/kg BW per hour for 24–48 h); the latter treatment is prohibitively expensive in large animals and must be given within 8 hours of the traumatic event to be effective. Intravenous magnesium sulfate (50 mg/kg BW) in the first 5–10 L of intravenous fluids has also been advocated on the basis that it inhibits several aspects of the secondary injury cascade.
CENTRAL NERVOUS SYSTEM STIMULANTS
These substances are used to excess in many instances. They exert only a transitory improvement in nervous function and are indicated only in nervous shock and after anesthesia or other short-term reversible anoxias such as cyanide or nitrate poisoning. It is unlikely that terminal respiratory failure caused by anoxia over a long period, and in which anoxia is likely to continue, will respond permanently to their use.
CENTRAL NERVOUS SYSTEM DEPRESSANTS
Animals with convulsions should be sedated to avoid inflicting traumatic injuries on themselves. Most of the general anesthetic agents in common use will satisfactorily control convulsions, and allow some time to examine the animal properly, assess the diagnosis and institute specific therapy if possible.
Mayhew IG. Large animal neurology. In A handbook for veterinary clinicians. Philadelphia, PA: Lea & Febiger; 1989.
Summers BA, Cummings JF, de Lahunta A. Veterinary neuropathology. St Louis, MO: Mosby, 1995.
Tucker RL, Farell E. Computed tomography and magnetic resonance imaging of the Equine head. Vet Clin North Am Equine Pract. 2001;17:131-144.
Saegerman C, Claes L, Dewaele A, et al. Differential diagnosis of neurologically expressed disorders in Western European cattle. Rev Sci Tech Off Int Epiz. 2003;22:83-102.
Wijnberg ID, van der Kolk JH, Franssen H, Breukink HJ. Needle electromyography in the horse compared with its principles in man: a review. Equine Vet J. 2003;35:9-17.
Constable PD. Clinical examination of the ruminant nervous system. Vet Clin North Am Food Anim Pract. 2004;20:215-230.
MacKay RJ. Brain injury after head trauma: pathophysiology, diagnosis, and treatment. Vet Clin North Am Equine Pract. 2004;20:199-216.
Scott PR. Diagnostic techniques and clinicopathologic findings in ruminant neurologic disease. Vet Clin North Am Food Anim Pract. 2004;20:215-230.
1 Done S. In Pract. 1995;17:318.
2 Scott PR. Br Vet J. 1995;151:603.
3 Tyler CM, et al. Aust Vet J. 1993;70:445.
4 Perino LJ, et al. Equine Pract. 1985;7:14.
5 Strain GM, et al. J Am Vet Med Assoc. 1984;185:538.
6 Roeder BL, et al. Compend Contin Educ Pract Vet. 1990;12:1175.
7 Beech J. Am J Vet Res. 1987;48:109.
8 De Lahunta A. Can J Vet Res. 1990;54:65.
9 Fayer R, et al. J Vet Intern Med. 1990;4:54.
10 Martin L, et al. Equine Vet J. 1986;18:133.
11 Mayhew IG. J Vet Intern Med. 1991;5:332.
12 Nappert G, et al. Can Vet J. 1989;30:802.
13 Mayhew IG. Large animal neurology: A handbook for veterinary clinicians. Philadelphia, PA: Lea & Febiger, 1989.
14 Cummings JF, et al. Cornell Vet. 1990;80:357.
15 Strain GM, et al. J Am Vet Med Assoc. 1987;191:833.
16 Lane JG, Mair TS. Equine Vet J. 1987;19:331.
17 Madigan JE, et al. Equine Vet J. 1995;27:306.
18 McConnon JM, et al. J Am Vet Med Assoc. 1983;182:812.
19 Nurmio P, et al. Nord Vet Med. 1982;34:130.
20 Green SL, et al. Can Vet J. 1992;33:330.
21 Scott PR, et al. N Z Vet J. 1991;39:105.
22 Cash WC, et al. J Vet Med A. 1986;33:491.
23 Lee I, et al. Vet J. 2005;169:257.
24 Scott PR, Will RG. Aust Vet J. 1991;147:582.
25 St Jean G, et al. Can J Vet Res. 1997;61:108.
26 Foreman JH, et al. J Vet Intern Med. 2004;18:223.
27 Moore RM, Trims CM. Am J Vet Res. 1992;53:1558.
28 Scott P. Br Vet J. 1992;148:15.
29 Welles EG, et al. Am J Vet Res. 1992;53:2050.
30 Furr MO, Bender H. Am J Vet Res. 1994;55:781.
31 Scott PR, Penny CD. Vet Rec. 1993;133:119.
32 Jackson C, et al. J Vet Intern Med. 1996;10:246.
33 Wilson JW, Stevens JB. J Am Vet Med Assoc. 1977;171:256.
34 Andrews FM, et al. Prog Vet Neurol. 1990;1:98.
35 Welles EG, et al. Am J Vet Res. 1994;55:1075.
36 Foreman JH, et al. J Vet Intern Med. 2004;18:223.
37 Mudron P, et al. Vet Med Czech. 2004;49:187.
38 Tietje S, et al. Equine Vet J. 1996;28:98.
39 Van Biervliet J, et al. J Vet Intern Med. 2004;18:907.
40 Spoormakers TJP, et al. Equine Vet J. 2003;35:146.
41 Gordon PJ, Dennis R. Vet Rec. 1995;137:671.
42 Lacombe VA, et al. J Vet Intern Med. 2001;15:385.
43 Suzuki M, et al. Jpn J Vet Sci. 1990;52:1077.
44 Wijnberg ID, et al. J Vet Intern Med. 2003;17:185.
45 Nollet H, et al. Equine Vet J. 2004;36:51.
46 Mayhew IG, Washbourne JR. Aust Vet J. 1992;148:315.
47 Mayhew IG, Washbourne JR. Vet J. 1997;153:107.
48 Mayhew IG, Washbourne JR. Aust Vet J. 1990;146:509.
49 Kortz GD, et al. Am J Vet Res. 1995;56:1351.
50 Jamison JM, Prescott JF. Compend Contin Educ Pract Vet. 1988;10:225.
Pathophysiological mechanisms of nervous system disease
Etiology of nervous system disease
There are many different causes of nervous system disease in large domestic animals.
• Infectious causes include bacteria, viruses, fungi, and helminth, arthropod and protozoan parasites
• Exogenous substances such as lead, salt, selenium, organophosphate insecticides, feed additives such as urea, poisonous plants and many other chemicals are common causes
• Endogenous substances such as products of disease in other body systems or of abnormal metabolism such as bacterial toxins, ammonia and carbon dioxide can cause abnormalities of the nervous system
• Metabolic and nutritional causes include ischemia secondary to cardiopulmonary disease, hypoglycemia, hypomagnesemia, copper deficiency in pregnant animals, and hyper d-lactatemia in calves with neonatal diarrhea and adult ruminants with grain overload
• Acidemia associated with diarrhea can cause mental depression and ataxia
• Traumatic and physical injuries to the brain or spinal cord are common
• Neoplasms of the nervous system in large animals are uncommon, with the exception of spinal lymphosarcoma in adult cattle due to enzootic bovine leukosis
• Idiopathic diseases account for several diseases of the spinal cord of horses
• Malformation occurs primarily in the developing fetus and results in congenital nervous system disease, which is usually present at birth. Many different teratogens can cause congenital defects. In some cases of inherited disease, the clinical signs are not manifest until some time after birth.
Responses of central nervous system to injury
The central nervous system may respond to injury by morphological changes that include cerebral edema and brain swelling, inflammation, and demyelination. Malformations occur when the central nervous system is affected during fetal life.
The remainder of this chapter will present the general clinical aspects of the diseases of the nervous system according to anatomical sites. The salient features of the etiology, pathogenesis, clinical findings, diagnosis, and treatment of these clinicoanatomical diseases are described. The objective is to generalize about the clinical findings that are common or typical of diseases affecting each of the major anatomical sites of the brain and spinal cord. Cerebral hypoxia and ischemia, hydrocephalus and cerebral edema are common to many diseases of the nervous system, and are described here.
Diffuse diseases of the brain*
CEREBRAL HYPOXIA
Cerebral hypoxia occurs when the supply of oxygen to the brain is reduced for any reason. An acute or chronic syndrome develops depending on the acuteness of the deprivation. Initially there are irritation signs followed terminally by signs of loss of function.
ETIOLOGY
All forms of hypoxia, including anemic, anoxic, histotoxic, and stagnant forms cause some degree of cerebral hypoxia but signs referable to cerebral dysfunction occur only when the hypoxia is severe. The hypoxia of the brain may be secondary to a general systemic hypoxia or be caused by lesions restricted to the cranial cavity.
Cerebral hypoxia secondary to general hypoxia
• Poisoning by hydrocyanic acid or nitrite
• Acute heart failure in severe copper deficiency in cattle
• Terminally in pneumonia, congestive heart failure
• During or at birth in foals, neonatal maladjustment syndrome in foals, or intrapartum hypoxia in calves and lambs due to prolonged parturition.
PATHOGENESIS
The central nervous system is extremely sensitive to hypoxia, and degeneration occurs if the deprivation is extreme and prolonged for more than a few minutes. The effects of the hypoxia vary with the speed of onset and with the severity. When the onset is sudden there is usually a transitory period during which excitation phenomena occur and this is followed by a period of loss of function. If recovery occurs, a second period of excitation usually develops as function returns. In more chronic cases the excitation phase is not observed, the signs being mainly those of loss of function. These signs include dullness and lethargy when deprivation is moderate, and unconsciousness when it is severe. All forms of nervous activity are depressed but the higher centers are more susceptible than medullary centers and the pattern of development of signs may suggest this.
CLINICAL FINDINGS
Acute and chronic syndromes occur depending on the severity of the hypoxia. Acute cerebral hypoxia is manifested by a sudden onset of signs referable to paralysis of all brain functions, including tetraparesis and unconsciousness. Muscle tremor, beginning about the head and spreading to the trunk and limbs, followed by recumbency, clonic convulsions and death or recovery after further clonic convulsions is the most common pattern, although affected animals may fall to the ground without premonitory signs. In chronic hypoxia there is lethargy, dullness, ataxia, weakness, blindness and in some cases muscle tremor or convulsions. In both acute and chronic hypoxia the signs of the primary disease will also be evident. Cerebral hypoxia of fetal calves is thought to be a cause of weakness and failure to suck after birth, leading to the eventual death of the calf from starvation. Such hypoxia can occur during the birth process, especially if it is difficult or delayed, or during late pregnancy.
CLINICAL PATHOLOGY AND NECROPSY FINDINGS
There is no distinctive clinical pathology or characteristic necropsy lesion other than those of the primary disease.
Clinically there is little to differentiate cerebral hypoxia from hypoglycemia or polioencephalomalacia in which similar signs occur. Irritation and paralytic signs follow one another in many poisonings including lead and arsenic and in most diffuse diseases of the brain including encephalitis and encephalomalacia. The differential diagnosis of cerebral hypoxia depends upon the detection of the cause of the hypoxia.
INCREASED INTRACRANIAL PRESSURE, CEREBRAL EDEMA, AND BRAIN SWELLING
Diffuse cerebral edema and brain swelling usually occur acutely and cause a general increase in intracranial pressure. Cerebral edema is rarely a primary disease, but commonly an accompaniment of other diseases. Cerebral edema is commonly a transient phenomenon and may be fatal but complete recovery or recovery with residual nervous signs also occurs. It is manifested clinically by blindness, opisthotonos, muscle tremor, paralysis, and clonic convulsions.
ETIOLOGY
Diffuse cerebral edema and brain swelling may be vasogenic, when there is increased permeability of capillary endothelium, cytotoxic when all the elements of brain tissue, glia, neurons and endothelial cells undergo swelling. Causes include the following.
Vasogenic edema
• Brain abscess, neoplasm, hemorrhage, lead encephalopathy, purulent meningitis
• Minor edema after most traumatic injuries, in many encephalitides and many poisonings, including propylene glycol in the horse; probably contributes to the pathogenesis
• Accidental intracarotid injection of promazine in horses
• Leukoencephalomalacia in horses due to fumonisin consumption1,2
• Septicemia in neonatal foals.3
PATHOGENESIS
Cerebral edema and brain swelling
This disease is potentially life-threatening because of the limited ability for accommodation of increased volume within the confines of the dura and the cranium. The central nervous system parenchyma does not possess a lymphatic system, and the interstitial space between cells, especially in the gray matter, is much narrower than in other tissues. When central nervous system edema develops, of necessity it largely accumulates within cells, although interstitial fluid will form if cells lyse or if the edema is severe.
Cerebral edema commonly occurs to some degree in all pathological states, whether degenerative or inflammatory, traumatic or neoplastic. Edema around chronic, focal lesions such as abscesses, parasitic cysts and primary or metastatic tumors in white matter often produces marked swelling. Cerebral hemispheric swelling compresses the underlying brainstem, flattening the rostral colliculi and distorting the aqueduct. As the swollen brain expands and fills the confines of the calvaria, some regions are prone to herniation. If this occurs, the accompanying blood vessels are likely to become occluded, which may result in hemorrhage or infarction. Commonly with brain swelling, the caudal lobe of the cerebellar vermis protrudes as a flattened lip over the medulla oblongata toward the foramen magnum.
In vasogenic edema the primary insult is to the wall of cerebral capillaries, allowing the escape of plasma fluid and proteins under the hydrostatic pressure of the circulation. The inciting vascular injury may be brain or spinal cord trauma, vasculitis, a neoplasm or a cerebrovascular accident. Vasogenic edema affects predominantly the white matter, where fluid accumulates within the cytoplasm of astrocytes and spreads in the interstitial spaces. Vasogenic edema moves over very long distances and from one hemisphere to the other via the corpus callosum. A chronic epidural abscess involving the frontal lobe can produce sufficient brain swelling from vasogenic edema to induce herniation of the occipital cortex beneath the tentorium cerebelli.
Cytotoxic edema results from an injury to a glial cell that disturbs osmoregulation of that cell by depletion of energy stores and failure of energy-dependent ionic pumps. This leads to cell swelling with fluid, and differs from edema in other tissues in which fluid accumulation is interstitial. Cytotoxic edema reflects a specific cellular insult and may result from ischemia or hypoxia, nutritional deficiency, an intoxication or an inherited metabolic abnormality. Brain swelling from cytotoxic edema is less dramatic than that seen in vasogenic edema. It may affect just the gray matter, just the white matter, or both.
The extracellular fluid volume in vasogenic edema is increased by the edema fluid, which is a plasma filtrate containing plasma protein. In cytotoxic edema it is the cellular elements themselves that increase in size. In hypoxia this is because of failure of the adenosine triphosphate (ATP)-dependent sodium pump within the cells. As a result sodium accumulates within the cells and water follows to maintain osmotic equilibrium. In polioencephalomalacia and salt poisoning the edema of the brain is primary. In salt poisoning in pigs there is an increase in concentration of cations in brain tissue with a sudden passage of water into the brain to maintain osmotic equilibrium. The cause of the edema in polioencephalomalacia of ruminants, associated with a thiamin inadequacy, is unknown. When promazine is injected accidentally into the carotid artery of the horse it produces a vasogenic edema and infarction generally, but especially in the thalamus and corpora quadrigemina on the injected side. The vasogenic edema surrounding an abscess is localized and is not evident in the white matter.
Cerebral edema and cerebellar herniation has been described in four neonatal foals admitted to an intensive care unit for treatment.3 All foals had septicemia. It was suggested that hypoglycemia, hypoxia, or the alterations in cerebral blood flow associated with septicemia might have initiated injury to cell membranes, resulting in vascular damage and subsequent edema. It is hypothesized that cerebellar herniation occurs in neonatal foals with sepsis because of the inelastic nature of the dural folds and the anatomical rigidity of the neonatal equine skull. This is in contrast to the human infant, in whom cerebral edema occurs in bacterial meningitis but cerebral or cerebellar herniation is not normally a feature. The relatively small brain of the newborn foal is only 1% of total body mass compared to the human infant which is 12% and in which the brain is enclosed within a large but relatively thin calvarium with sutures that, in the preterm infant at least, can be separated by excess internal pressure.4
An increase in intracranial pressure occurs suddenly and, as in hydrocephalus, there is a resulting ischemic anoxia of the brain due to compression of blood vessels and impairment of blood supply. This may not be the only factor that interferes with cerebral activity in polioencephalomalacia and salt poisoning. The clinical syndrome produced by the rapid rise in intracranial pressure is manifested by involuntary movements such as tremor and convulsions followed by signs of weakness. If the compression of the brain is severe enough and of sufficient duration, ischemic necrosis of the superficial layers of the cortical gray matter may occur, resulting in permanent nervous defects in those animals that recover. Opisthotonos and nystagmus are commonly observed and are probably due to the partial herniation of the cerebellum into the foramen magnum.
CLINICAL FINDINGS
Although the rise of intracranial pressure in diffuse edema of the brain is usually more acute than in hydrocephalus, the development of clinical signs takes place over a period of 12–24 hours and nervous shock does not occur. There is central blindness, and periodic attacks of abnormality occur in which opisthotonos, nystagmus, muscle tremor, and convulsions are prominent.
In the intervening periods the animal is dull, depressed, and blind, and optic disc edema may be present. The involuntary signs of tremor, convulsions, and opisthotonos are usually not extreme but this varies with the rapidity of onset of the edema. Because of the involvement of the brainstem, in severe cases muscle weakness appears, the animal becomes ataxic, goes down and is unable to rise, and the early signs persist. Clonic convulsions occur terminally and animals that survive may have residual defects of mentality and vision.
CLINICAL PATHOLOGY
Clinicopathological observations will depend on the specific disease causing the edema.
NECROPSY FINDINGS
Microscopically the gyri are flattened and the cerebellum is partially herniated into the foramen magnum with consequent distortion of its caudal aspect. The brain has a soft, swollen appearance and tends to sag over the edges of the cranium when the top has been removed. Caudal portions of the occipital lobes herniate ventral to the tentorium cerebelli.
Diffuse brain edema causes a syndrome not unlike that of encephalitis although there are fewer irritation phenomena. Differentiation from encephalomalacia and vitamin A deficiency may be difficult if the history does not give a clue to the cause of the disease. Metabolic diseases, particularly pregnancy toxemia, hypomagnesemic tetany of calves and lactation tetany, resemble it closely, as do some cases of acute ruminal impaction. In the history of each of these diseases there are distinguishing features that aid in making a tentative diagnosis. Some of the poisonings, particularly lead, organic mercurials and arsenicals and enterotoxemia associated with Clostridium perfringens type D produce similar nervous signs and gut edema of swine may be mistaken for diffuse cerebral edema.
TREATMENT
Decompression of the brain is desirable in acute edema. The treatment will depend in part on the cause; the edema associated with polioencephalomalacia will respond to early treatment with thiamin. In general terms, edema of the brain responds to parenteral treatment with hypertonic solutions and corticosteroids. Hypertonic solutions are most applicable to cytotoxic edema and corticosteroids to vasogenic edema. This is in addition to treatment for the primary cause of the disease. Mannitol at 2 g/kg BW and dexamethasone at 1 mg/kg BW, both intravenously, are recommended. The mannitol is given intravenously as a 20% solution followed 3 hours later by the dexamethasone, also intravenously. Diuretics usually produce tissue dehydration too slowly to be of much value in acute cases, but they may be of value as an adjunct to hypertonic solutions or in early or chronic cases. The removal of CSF from the cisterna magna in an attempt to provide relief may cause complications. In some cases the removal of 25–75 mL of CSF provides some temporary relief but the condition becomes worse later because portions of the swollen brain herniate into the foramen magnum. There is no published information available on how much fluid can be safely removed and recommendations cannot therefore be made.
HYDROCEPHALUS
Obstructive hydrocephalus may be congenital or acquired and is manifested in both cases by a syndrome referable to a general increase in intracranial pressure. Irritation signs of mania, head-pressing, muscle tremor and convulsions occur when the onset is rapid, and signs of paralysis including dullness, blindness and muscular weakness are present when the increased pressure develops slowly.
ETIOLOGY
Obstructive hydrocephalus may be congenital or acquired but in both instances it is due to defective drainage or absorption of CSF. In the congenital disease there is an embryological defect in the drainage canals and foramina between the individual ventricles or between the ventricles and the subarachnoid space, or in the absorptive mechanism, the arachnoid villi.
Congenital hydrocephalus
• Alone, with lateral narrowing of the mesencephalon
• Inherited defects of Hereford, Holstein, Ayrshire and Jersey cattle
• Inherited combined defects with chondrodysplasia, or in white Shorthorn cattle combined with hydrocephalus, microphthalmia and retinal dysplasia
• Virus infections of the fetus suggest themselves as possible causes of embryological defects in the drainage system, but there are no verified examples of this. The cavitation of brain tissue and subsequent accumulation of fluid, hydranencephaly, which occurs after infection with bluetongue virus in lambs and Akabane virus in calves, is compensatory, not obstructive
• Vitamin A deficiency may contribute
• Other occurrences, sometimes at high levels of prevalence, but without known cause.
Acquired hydrocephalus
• Hypovitaminosis A in young growing calves causing impaired absorption of fluid by the arachnoid villi
• Cholesteatoma in choroid plexuses of the lateral ventricles in the horse. These may produce an acute, transient hydrocephalus on a number of occasions before the tumor reaches sufficient size to cause permanent obstruction
• Other tumor or chronic inflammatory lesion obstructing drainage from the lateral ventricles.
PATHOGENESIS
Increased intracranial pressure in the fetus and before the syndesmoses of the skull have fused causes hydrocephalus with enlargement of the cranium. After fusion of the suture lines the skull acts as a rigid container and an increase in the volume of its contents increases intracranial pressure. Although the increase in volume of the contents may be caused by the development of a local lesion such as an abscess, tumor, hematoma or cestode cyst, which interferes with drainage of the CSF, the more common lesion is a congenital defect of CSF drainage.
Clinical and pathological hydrocephalus has been produced experimentally in animals by creating granulomatous meningitis. The clinical signs included depression, stiffness of gait, recumbency and opisthotonus with paddling convulsions. The general effects in all cases are the same, the only difference being that local lesions may produce localizing signs as well as signs of increased intracranial pressure. These latter signs are caused by compression atrophy of nervous tissue and ischemic anoxia due to compression of blood vessels and impairment of blood supply to the brain.
In congenital hydrocephalus the signs observed are usually those of paralysis of function, while acquired hydrocephalus, being more acute, is usually manifested first by irritation phenomena followed by signs of paralysis. Edema of the optic papilla is a sign of increased intracranial pressure and may be detected ophthalmoscopically. Bradycardia occurs inconstantly and cannot be considered to be diagnostic.
CLINICAL FINDINGS
In acquired hydrocephalus there is, in most cases, a gradual onset of general paresis. Initially there is depression, disinclination to move, central blindness, an expressionless stare and a lack of precision in acquired movements. A stage of somnolence follows and is most marked in horses. The animal stands with half-closed eyes, lowered head and a vacant expression and often leans against or supports itself upon some solid object. Chewing is slow, intermittent and incomplete and animals are often observed standing with food hanging from their mouths. The reaction to cutaneous stimulation is reduced, and abnormal postures are frequently adopted. Frequent stumbling, faulty placement of the feet and incoordination are evidenced when the animal moves, and circling may occur in some cases. Bradycardia and cardiac arrhythmia have been observed.
Although the emphasis is on depression and paresis, signs of brain irritation may occur, particularly in the early stages. These signs often occur in isolated episodes during which a wild expression, charging, head-pressing, circling, tremor and convulsions appear. These episodes may be separated by quite long intervals, sometimes of several weeks’ duration. In vitamin A deficiency in calves blindness and papilledema are the early signs and an acute convulsive stage occurs terminally.
Congenitally affected animals are usually alive at birth but are unable to stand and most die within 48 hours. The cranium is sometimes domed, the eyes protrude and nystagmus is often evident. Meningocele is an infrequent accompaniment.
CLINICAL PATHOLOGY
Examination of the composition and pressure of the CSF will be of value. The fluid is usually normal biochemically and cytologically but the pressure is increased. A marked increase in serum muscle enzyme activity has been observed in calves with congenital hydrocephalus, due probably to an accompanying muscular dystrophy. Convulsions, if they occur, may contribute to this increase.
NECROPSY FINDINGS
The cranium may be enlarged and soft in congenital hydrocephalus. The ventricles are distended with CSF under pressure and the overlying cerebral tissue is thinned if the pressure has been present for some time.
Congenital hydrocephalus resembles vitamin A deficiency in newborn pigs, toxoplasmosis and hydranencephaly if there is no distortion of the cranium.
Acquired hydrocephalus needs to be differentiated from other diffuse diseases of the brain, including encephalitis and encephalomalacia, and from hepatic dystrophies, which resemble it very closely. In these latter diseases there may be other signs of diagnostic value, including fever in encephalitis and jaundice in hepatic dystrophy. In most cases it is necessary to depend largely on the history and recognition of individual disease entities.
ENCEPHALITIS
Encephalitis is, by definition, inflammation of the brain but in general usage it includes those diseases in which inflammatory lesions occur in the brain, whether there is inflammation of the nervous tissue or primarily of the vessel walls. Clinically, encephalitis is characterized initially by signs of involuntary movements, followed by signs caused by loss of nervous function. The meninges and spinal cord may be involved in an encephalitis, causing varying degrees of meningoencephalomyelitis.
ETIOLOGY
Many encephalitides of large animals are associated with viruses but other infectious agents are also common. Some causes are as follows.
All species
• Viral infections – rabies, pseudorabies, Japanese B encephalitis, West Nile virus encephalomyelitis
• Bacterial infections of neonatal farm animals
• Toxoplasmosis, which is not a common cause in any species
• Verminous encephalomyelitis – migration of larvae of parasitic species that normally have a somatic migration route, e.g. Micronema deletrix, Setaria spp. Paraelaphostrongylus tenuis
Cattle
• Bovine spongiform encephalopathy
• Viral infections – bovine malignant catarrh, sporadic bovine encephalomyelitis and bovine herpes virus
• Bacterial infections including Listeria monocytogenes, Histophilus somni (formerly Haemophilus somnus),1 heartwater, clostridial infections following dehorning of calves2
• Migration of Hypoderma bovis occasionally to brain and spinal cord
Horses
• Viral infections – infectious equine encephalomyelitis, Borna disease, equine herpes virus, equine infectious anemia, eastern, western and West Nile equine encephalomyelitides, rarely louping ill virus
• Protozoal encephalomyelitis6
• Verminous encephalomyelitis due to Strongylus vulgaris in horses and Draschia megastoma. Angiostrongylus cantonensis, which normally migrates through the central nervous system of the rat, has been found as a cause of verminous encephalomyelitis in foals7
PATHOGENESIS
Compared to other extraneural tissues, the inflammatory response mounted by the nervous system is unique. The central nervous system is in a sequestered and immunologically dormant state within the body. The capillary endothelial blood– brain barrier restricts free access by blood constituents. The central nervous system lacks specialized dendritic antigen-presenting cells, and the intrinsic expression by central nervous system cells of major histocompatibility complex molecules, especially class II, is low. There is no lymphatic system within nervous tissue, but cells and antigens within the central nervous system drain into the circulation and to the cervical lymph nodes.
The central nervous system has unique populations of cells consisting of parenchymal cells, which are neurons, and neuroglia. The neuroglia are supporting cells and are subdivided into macroglia and microglia: the macroglia are astrocytes and oligodendrocytes; the third glial cell type is a microglial cell. The brain and spinal cord are enclosed by meninges (dura, arachnoid and pia), which provide protection, a compartment for CSF circulation (the subarachnoid space), support for blood vessels and a sheath for the cranial and spinal nerves. Within the brain and spinal cord are the ventricular system and central canal, which are lined by ependymal cells and the choroid plexuses, which produce the CSF. Circulation of the CSF moves from the lateral, third and fourth ventricles into the central canal or through lateral apertures at the cerebellomedullary angle into the subarachnoid space of the brain. CSF in the subarachnoid space drains via specialized arachnoid granulations into intracranial venous sinuses, with some draining into venous plexuses associated with cranial and spinal nerves. CSF may also cross the ventricular surface into the adjacent parenchyma.
The histological characteristics of central nervous system inflammation are:
A perivascular compartment, actual or potential, exists around all central nervous system arteries, arterioles, venules and veins. A characteristic feature of central nervous system inflammation is perivascular cuffing, the accumulation of leukocytes of one or multiple types in the perivascular space. All perivascular cuffing results in vasculitis of some degree. In bacterial diseases, polymorphonuclear cells predominate with a minor component of mononuclear cells. In general, viral diseases are characterized by lymphocyte-rich cells with some plasma cells and monocytes; some arbovirus infections cause a polymorphonuclear cell response. In immune-mediated diseases, there are mixtures of polymorphonuclear and mononuclear cells. In thrombogenic diseases such as thrombotic meningoencephalitis, vascular occlusion precludes the development of cuffing around injured vessels.
Gliosis is the increased prominence of glial cells, resulting from cytoplasmic swelling and the acquisition of more cell processes, from cell proliferation, or both. Either of the macroglia (oligodendrocytes or astrocytes) or microglia may participate in gliosis.
Neuronal satellitosis occurs when oligodendrocytes react and proliferate in response to degenerating neurons which may be infected by a virus.
Neuronophagia is the progressive degeneration of the neuron characterized by its piecemeal division and phagocytosis, eventually leaving a dense nodule of glial cells and fragments of the former neuron. Details of the form, functions and roles of astrocytes in neurological disease have been reviewed.8Primary demyelination is characteristic of only a small number of inflammatory neurological diseases and is associated with only a few viruses. The inflammatory neuraxial diseases of large animals include visna in sheep and caprine arthritis–encephalitis. The demyelinating process may be initiated directly by the infectious agent alone or by an immunological response initiated by the agent.
With the exception of the viruses of bovine malignant catarrh and equine herpesvirus 1, which exert their effects principally on the vasculature, those viruses that cause encephalitis do so by invasion of cellular elements, usually the neurons, and cause initial stimulation and then death of the cells. Those bacteria that cause diffuse encephalitis also exert their effects primarily on vascular endothelium. L. monocytogenes does so by the formation of microabscesses. In some diseases, such as meningoencephalitis in cattle associated with H. somni, the lesions may be present in the brain and throughout the spinal cord.9
Entrance of the viruses into the nervous tissue occurs in several ways. Normally the blood–brain barrier is an effective filtering agent but when there is damage to the endothelium infection readily occurs. The synergistic relationship between the rickettsias of tick-borne fever and the virus of louping ill probably has this basis. Entry may also occur by progression of the agent up a peripheral nerve trunk, as occurs with the viruses of rabies and pseudorabies and with L. monocytogenes. Entry via the olfactory nerves is also possible.
The clinical signs of encephalitis are usually referable to a general stimulatory or lethal effect on neurons in the brain. This may be in part due to the general effect of inflammatory edema and in part to the direct effects of the agent on nerve cells. In any particular case one or other of these factors may predominate but the tissue damage and therefore the signs are generalized. Clinical signs are often diverse and can be acute or chronic, localized or diffuse, and progressive or reversible. Because of diffuse inflammation in encephalitis, the clinical signs are commonly multifocal and asymmetric. This is not the case in listeriosis, in which damage is usually localized in the pons–medulla. Localizing signs may appear in the early stages of generalized encephalitis and remain as residual defects during the stage of convalescence. In calves with thromboembolic meningoencephalitis due to H. somni, prolonged recumbency may be associated with widespread lesions of the spinal cord. Visna is a demyelinating encephalitis, and caprine leukoencephalomyelitis is both demyelinating and inflammatory and also invades other tissues including joints and lung.
In verminous encephalomyelitis, destruction of nervous tissue may occur in many parts of the brain and in general the severity of the signs depends upon the size and mobility of the parasites and the route of entry. One exception to this generalization is the experimental ‘visceral larva migrans’ produced by Toxocara canis in pigs when the nervous signs occur at a time when lesions in most other organs are healing. The signs are apparently provoked by a reaction of the host to static larvae rather than trauma due to migration. Nematodes not resident in nervous tissues may cause nervous signs due possibly to allergy or to the formation of toxins.
CLINICAL FINDINGS
Because the encephalitides are associated with infectious agents they are often accompanied by fever, anorexia, depression and increased heart rate. This is not the case in the very chronic diseases such as scrapie and bovine spongiform encephalopathy. In those diseases associated with agents that are not truly neurotropic, there are characteristic signs, which are not described here.
The clinical findings that can occur in encephalitis are combinations of:
Bacterial meningoencephalitis in lambs 2–4 weeks of age is characterized by lack of suck reflex, weakness, altered gait and depression extending to stupor, but hyperesthesia to auditory and tactile stimuli.5 Opisthotonus is common during the terminal stages.
There may be an initial period of excitement or mania. The animal is easily startled and responds excessively to normal stimuli. It may exhibit viciousness and uncontrolled activity including blind charging, bellowing, kicking and pawing. Self-mutilation may occur in diseases such as pseudorabies. Mental depression, including head-pressing, may occur between episodes.
Involuntary movements are variable in their occurrence or may not appear at all. When they do occur they include convulsions, usually clonic, and may be accompanied by nystagmus, champing of the jaws, excessive frothy salivation and muscle tremor, especially of the face and limbs. In cattle with malignant catarrhal fever, there is severe depression for a few days followed by the onset of tremors associated with the terminal encephalitis. Unusual irritation phenomena are the paresthesia and hyperesthesia of pseudorabies and scrapie.
Signs caused by loss of nervous function follow and may be the only signs in some instances. Excessive drooling and pharyngeal paralysis are common in rabies. In horses with equine encephalomyelitis, feed may be left hanging from the mouth, although swallowing may not be impaired. The loss of function varies in degree from paresis with knuckling at the lower limb joints, to spasticity of the limbs with resultant ataxia, to weakness and recumbency. Recumbency and inability to rise may be the first clinical finding encountered as in many cases of meningoencephalitis associated with H. somni. Hypermetria, a staggering gait and apprehensiveness progressing to belligerency may occur in a disease such as bovine spongiform encephalopathy.
Clinical signs referable to certain anatomical sites and pathways of the brain and spinal cord are manifested by deviation of the head, walking in circles, abnormalities of posture, ataxia and incoordination but these are more commonly residual signs after recovery from the acute stages. Progressive ascending spinal cord paralysis, in which the loss of sensation and weakness occur initially in the hindlimbs followed by weakness in the forelimbs, occurs commonly in rabies. Residual lesions affecting the cranial nerves do not commonly occur in the encephalitides, except in listeriosis and protozoal encephalitis of horses, both infections predominating in the caudal brainstem.
An acute hemorrhagic necrotizing encephalitis following dehorning calves has been described.2 Affected calves were found dead or moribund within a few days following dehorning using a gouge. A secondary clostridial infection was suspected.
In the horse with cerebral nematodiasis due to S. vulgaris the clinical signs are referable to migration of the parasite in the thalamus, brainstem and cerebellum. There is incoordination, leaning and head-pressing, dysmetria, intermittent clonic convulsions, unilateral or bilateral blindness and paralysis of some cranial nerves. The onset may be gradual or sudden. The clinical diagnosis is extremely difficult because examination of CSF and hematology are of limited value. A pathological diagnosis is necessary. In foals with neural angiostrongylosis, tetraparesis was the end result of progressive and multifocal neurological disease.7
CLINICAL PATHOLOGY
Clinical pathology may be of considerable assistance in the diagnosis of encephalitis but the techniques used are for the most part specific to the individual diseases.
Hemogram
In the horse, complete and differential blood counts and serum chemistry profiles are recommended for most neurological cases.
Serology
Acute and convalescent sera can be submitted when a specific infectious disease is suspected for which a serologic diagnosis is possible.
Cerebrospinal fluid
Laboratory examination of CSF for cellular content and pathogens may also be indicated. In bacterial meningoencephalitis of young lambs, analysis of CSF obtained from the lumbosacral space reveals a highly significant increase in protein concentration with neutrophilic pleocytosis.5
NECROPSY FINDINGS
In some of the commonly occurring encephalitides there are no gross lesions of the brain apart from those that occur in other body systems and that are typical of the specific disease. In other cases, on transverse section of the brain, extensive areas of hemorrhagic necrosis may be visible, as in meningoencephalitis in cattle due to H. somnus. Histological lesions vary with the type and mode of action of the causative agent. Material for laboratory diagnosis should include the fixed brain and portions of fresh brain material for culture and for transmission experiments.
The diagnosis of encephalitis cannot depend entirely on the recognition of the typical syndrome because similar syndromes may be caused by many other brain diseases. Acute cerebral edema and focal space-occupying lesions of the cranial cavity, and a number of poisonings, including salt, lead, arsenic, mercury, rotenone and chlorinated hydrocarbons all cause similar syndromes, as do hypovitaminosis A, hypoglycemia, encephalomalacia and meningitis.
Fever is common in encephalitis but is not usually present in rabies, scrapie, or bovine spongiform encephalopathy; but it may occur in the noninflammatory diseases if convulsions are severe.
Generally, the clinical diagnosis rests upon the recognition of the specific encephalitides and the elimination of the other possible causes on the basis of the history and clinical pathology, especially in poisonings, and on clinical findings characteristic of the particular disease. In many cases a definite diagnosis can only be made on necropsy. For differentiation of the specific encephalitides reference should be made to the diseases listed under Etiology, above.
Infestation with nematode larvae causes a great variety of signs depending on the number of invading larvae and the amount and location of the damage.
TREATMENT
Specific treatments are dealt with under each disease. Antimicrobials are indicated for bacterial meningoencephalomyelitis. Generally the aim should be to provide supportive treatment by intravenous fluid and electrolyte therapy or stomach tube feeding during the acute phase. Sedation during the excitement stage may prevent the animal from injuring itself, and nervous system stimulants during the period of depression may maintain life through the critical phase. Although there is an increase in intracranial pressure, the removal of CSF is contraindicated because of the deleterious effects of the procedure on other parts of the brain.
1 Ahrens AO, et al. Int J Syst Evol Microbiol. 2003;53:1449.
2 Nation PN, Calder WA. Aust Vet J. 1985;26:378.
3 Duffel SJ. Vet Rec. 1984;115:547.
4 Cassidy JP, et al. Vet Rec. 1997;140:193.
5 Scott PR, et al. Vet Rec. 1994;135:154.
6 Boy MG, et al. J Am Vet Med Assoc. 1990;196:632.
7 Geiser DR, et al. Compend Contin Educ Pract Vet. 1988;10:740.