Mastitis–metritis–agalactia syndrome in sows
The mastitis–metritis–agalactia (MMA) syndrome (also called toxemic agalactia, farrowing fever, lactation failure, periparturient hypogalactia syndrome (PHS), or postpartum dysgalactia syndrome (PPDS)) occurs in sows between 12 and 48 hours (sometimes 72 h) after farrowing and is characterized clinically by anorexia, lethargy, restlessness, lack of interest in the piglets, fever, swelling of the mammary glands and agalactia. Most affected animals respond to therapy within 12–24 hours. Pathologically, there are varying degrees of mastitis. In some sows the level of oxytocin may be half the level in unaffected sows. The disease is of major economic importance when outbreaks occur because the inadequate milk production leads to high piglet mortality from starvation and secondary infectious diseases. In cases of subclinical MMA there is often a failure to achieve weaning weights (< 4 kg at 24 d). The term mastitis– metritis–agalactia was originally developed to describe sows with agalactia that had swollen udders, assumed to be due to mastitis, and the appearance of a vulval discharge, assumed to be due to metritis. Necropsy of spontaneously occurring cases has frequently confirmed the presence of mastitis but the incidence of metritis has been insignificant.
The prevalence of the condition appears to have reduced recently with the increased attention to hygiene in the farrowing house and the use of more porous and less traumatic floorings. When it does occur it can be quite common, with up to 11–58% of the sows being affected.1 A recent case definition2 suggests that the pathognomonic signs are poor piglet growth and sow rectal temperatures greater than 39.5°C.
ETIOLOGY
The etiology is unclear. Several different cause-and-effect relationships have been proposed, based on clinical and epidemiological observations, but only infectious mastitis has been substantiated. The list of proposed causes includes infectious mastitis, metritis, overfeeding during pregnancy, nutritional deficiencies, constipation and endocrine dysfunction. The composite view of this is that there are potential sources of bacterial infection with subsequent endotoxin absorption that lead to the subsequent systemic signs. There is a very considerable farm effect in the appearance of the condition as sow care and management are so important.
A major problem in the determination of the etiology is the difficulty of being precise in the description of the clinical findings of the abnormal mammary glands of affected sows. The common clinical findings are:
There is considerable overlap in the clinical findings from one affected sow to another but the lesion present in the mammary glands may vary from uncomplicated physiological congestion and edema to severe necrotizing mastitis.
Ringarp3 published the classic work on this disease based on 1180 cases of postparturient illness in sows in which agalactia was present. At least five causes of agalactia or hypogalactia were recognized which are as follows (the incidence of each group as a percentage of the total cases is given in brackets):
• Eclampsia (0.6%), usually of older sows, responding to calcium and magnesium therapy
• Failure of milk ejection reflex (3.3%), affecting primarily first-litter gilts and usually treated satisfactorily with oxytocin
• Mammary hypoplasia (1.5%) in gilts, resulting in deficient milk secretion
• Primary agalactia (6%), in which reduced milk supply is the only abnormality
• Toxic agalactia (88.6%), the most important numerically and economically. It is characterized by anorexia, depression, fever, swelling of the mammary glands and a course of 2–4 days. Mastitis was commonly present but there was no evidence of metritis.
Infectious mastitis is suggested as a major cause in many clinicopathological investigations and there is a greater incidence of intramammary infection in affected sows compared to normal sows. Peracute mastitis in sows is readily recognized as a clinical entity but less severe infections may result in small foci of inflammation within the gland that cannot be detected on clinical examination.
Escherichia coli and Klebsiella pneumoniae have been recovered from the mammary glands of naturally affected cases and both bacterial species are associated with histopathological changes of mastitis. Experimental intramammary inoculation of sows with field isolates of E. coli and K. pneumoniae has resulted in cases of lactation failure and mastitis that closely resemble naturally occurring cases. Unfortunately, they cannot always be demonstrated in the plasma of affected sows and neither can endotoxin. Streptococcus spp. and Staphylococcus spp. have also been isolated, but these are frequently isolated from healthy glands unassociated with pathological changes. It is unlikely that Mycoplasma spp. are important.
Coliforms are the most significant pathogens isolated from sows with mastitis. Pathological examination of affected sows that were euthanized within 3 days after parturition revealed the presence of varying degrees of mastitis, and E. coli and Klebsiella spp. were the most common organisms recovered. A recent study has shown that E. coli strains from mastitis in sows are highly variable in serotype, biochemical profile, virulence factors and random amplified polymorphic DNA (RAPD) type. No relationship between serotypes, virulence factors and RAPD types was found.4 Toxic agalactia can be produced experimentally by the introduction of E. coli endotoxin into the mammary gland of sows at parturition. The clinical, hematological, and serum biochemical changes are similar to those that occur in naturally occurring cases of toxic agalactia. E. coli endotoxin acting at the level of the hypothalamus can suppress prolactin release, which results in a pronounced decline in milk production. Experimental Klebsiella mastitis in sows is an excellent model for the study of toxic agalactia due to infectious mastitis.
Agalactia may also be the result of a deficiency of prolactin. Prolactin levels may be dramatically reduced by even the smallest amounts of endotoxin. Any factor that interferes with the release of prostaglandin from the uterus may affect the increase in prolactin that must occur to stimulate lactogenesis immediately prior to parturition.5
In summary, field observations have suggested many different causes and predisposing factors, including infectious mastitis, nutritional disturbances, metabolic disorders and the stress of farrowing in total confinement in a crate. Based on the examination of spontaneously occurring cases, infectious mastitis appears to be a major cause. Both prolactin and oxytocin release can be stopped by stressors and toxins from bacteria such as E. coli.
EPIDEMIOLOGY
Occurrence
The disease occurs most commonly in sows at farrowing or within the first 48 hours after parturition. It is most common in sows that farrow in crates indoors and only occasionally occurs in sows farrowing outdoors, which may be a reflection of the greater number of pigs raised in confinement. A peak incidence during the summer months has also been observed. The disease will often occur in one batch and then disappear again for months, which suggests that some unidentified factor may be affecting a whole group.
Morbidity and case fatality
Morbidity and mortality data are not readily available nor precise because of the difficulty of making a reliable clinical diagnosis. Epidemiological observations indicate that the risk of sows developing toxic mastitis increases with increasing age up to the third or fourth litter. The population incidence of toxic agalactia ranges from 4–10% of all farrowings while the herd incidence may vary from 0–100%. Some surveys report an overall incidence of 6.9% of all farrowings with a range from 1.1% to 37.2%. The incidence rates in Swedish herds ranges from 5.5% in small herds to 10.3% in large herds.6 In Denmark, an incidence rate of 9.5% from a total of 80 000 farrowings, independent of herd size, has been reported.6 In a survey of 70 herds in Norway over a period of 2 years, the incidence rate was about 17.5%7 and there was no consistent relationship between disease incidence and herd size.8,9 Sporadic outbreaks of the disease may occur in which almost all sows farrowing over a period of several weeks or a few months may be affected and then suddenly no further cases develop for no apparent reason.
The fatality rate is usually less than 2%, but piglet losses due to starvation and crushing may be as high as 80%. It is not a major cause of sow mortality.10 The disease does not usually recur in the same animal, which may suggest that immunity develops and possibly that affected sows should not necessarily be culled because of the disease.
Risk factors
The risk factors that have been proposed based on field observations include overfeeding during pregnancy, a drastic change of feed at farrowing, insufficient time for the sow to adjust to the farrowing crate after being transferred from the gestation unit and constipation of the sow at farrowing.
Animal risk factors
The initiating factors have not been identified. The incidence of the disease may also be higher in sows with larger litters than sows in the same herd that remain healthy, and in those with a higher number of stillbirths and pigs found dead after birth.6
Each section of the mammary gland of the sow is divided into a separate anterior and posterior section, each with its own teat cistern and teat canal. In a sow with 14 teats there are 28 potential portals of entry for environmental infectious agents and perhaps it is little wonder that mastitis should occur commonly immediately after parturition when the teat canals have become patent. Bacteria in the gut and in endometritis have been proposed as a source of endotoxin, particularly as beta-hemolytic streptococci and coliforms have been associated with the condition.
Some clinicopathological examinations of affected sows have revealed the presence of a slightly enlarged, flaccid uterus from which coliform and streptococcal organisms can be recovered. However, pathological evidence of metritis in affected sows is uncommon; the organisms that can be recovered are commonly present in the reproductive tract of normal sows after parturition and their recovery from vaginal mucus is difficult to interpret.
Constipation of sows at farrowing time has been suggested as a cause but has not been substantiated. However, clinical and pathological examinations of both spontaneously occurring cases of agalactia and experimental agalactia induced by the introduction of E. coli endotoxin into the mammary gland have been unable to support the observation of constipation. Both sick and normal sows defecate less frequently from 1 day before farrowing until 2 days later. There is no difference in the weight of feces in the terminal colon and rectum between sick and normal sows. Low exercise has also been suggested as a cause and this also contributes to constipation. The role of water intake or lack of it and stress or disturbance during parturition has also not been investigated.
The nursing behavior of the sow and the sucking behavior of the piglets may provide an explanation for the pathogenesis and clinical findings of some cases of agalactia in sows. Successful ejection of milk by the sow is dependent on proper stimulation of the sow’s udder by the piglets followed by a complex response by the sow. A period of time ranging from 15–45 minutes must elapse from the last successful milk ejection to the next. Failure of milk ejection may occur in up to 27% of sows that attempt to suckle their piglets within 40 minutes after the previous milk ejection. The failure of milk ejection in sows within the first few crucial adjustment days after farrowing might possibly contribute to the cause of mastitis and engorgement of the mammary glands. The possible causes of failure of milk ejection, even when a suitable interval has elapsed since the previous milk ejection, include:
Management and dietary factors
The disease occurs under management, environmental and sanitation conditions ranging from very poor to excellent; however, the possible relationship between the level of bacterial contamination in the farrowing barn and on the skin of the sow and the incidence of the disease has apparently not been examined. Dirty conditions greatly increase the bacterial contamination of the udder.
Digestive disturbances and certain feeding practices have been associated with the disease. Sows that have been on high-level feeding during pregnancy appear to be susceptible to the disease, especially if they are subjected to a change of feed immediately prior to parturition. Also, any management practice that results in a marked change in feed intake at or near farrowing may appear to precipitate the disease. A sudden change of feed severe enough to result in gastrointestinal stasis has been used to reproduce the condition experimentally.
The effects of different feed allowances during late pregnancy may affect the incidence rate of the disease. Feeding sows during the last 15 days of gestation a diet at a level of 3.4 kg daily compared to 1.0 kg daily resulted in an incidence rate of 26.6% and 14.0%, respectively.6 The explanation for the effects of feeding is unknown. It has been proposed that intense feeding may promote toxin production in the alimentary tract but how this is related to mastitis is unknown. Another hypothesis suggests that increased feeding in late gestation may intensify the initiation of lactation and result in udder engorgement and increased susceptibility to intramammary infection.6 A further suggestion is that moldy food may play a part, but this has never been proved.
The clinical status of the mammary glands, the bacteriological findings and the total cell count and its percentage of polymorphonuclear leukocytes and pH in colostrum and milk secretion during the first 3 weeks of lactation of sows on high or low feeding regimes during late pregnancy have been examined.11 E. coli infection was present in 80% of the sows affected with toxic agalactia and 30% of the healthy sows. The E. coli were eliminated at between 3 and 8 days of lactation and were not isolated from sows examined at the time of weaning.12 The different feeding regimes did not influence the total cell count, the polymorphonuclear cells or the pH in milk from bacteriologically negative glands or glands with E. coli mastitis. The two feeding regimes had no influence on total cell count, the percentage of polymorphonuclear cells or the pH of colostrum and milk of healthy sows.13
PATHOGENESIS
The pathogenesis of infectious mastitis due to E. coli or Klebsiella spp. is probably similar to that of bovine mastitis in which the infection gains entry through the teat canal and invades the mammary tissue causing mastitis. Endotoxemia occurs accounting for the fever initially, and the depression, anorexia and agalactia, even in glands that are unaffected.14 The lipopolysaccharide endotoxins acting at the level of the hypothalamus and hypophysis suppress the release of prolactin which results in a marked decline in milk production.15,16 The endotoxin may also have a direct inhibitory effect on the mammary gland. There is a higher prevalence of bacterial endotoxin in the blood of affected sows compared to control animals. The endotoxin can be detected in the blood of about 33% of sows affected with coliform mastitis.14 However, the oral administration of endotoxin daily to prepubertal gilts did not result in any clinical abnormalities.17 Experimentally, mastitis can be produced in sows by contamination of the skin of the teats with K. pneumoniae either shortly before or after parturition. The clinical signs are similar to those described for MMA; mastitis is present in more than 50% of the mammary gland subsections and a marked leukopenia and degenerative left shift occurs. A total of 120 organisms is sufficient to produce the mastitis when the organisms are inoculated into the teats. In recent experimental infections with E. coli it was shown that the time of infection of the mammary gland relative to parturition and the number of circulating neutrophils at the time of infection influenced the development of clinical coliform mastitis in the sow.18 Similarly parturition allows the penetration of vaginal organisms further up the reproductive tract and the absorption of endotoxin reduces F2α in the uterus and this stimulates prolactin, which may contribute to the hypogalactia and agalactia.
If noninfectious acute painful swelling of the mammary glands accompanied by agalactia occurs in the sow as a result of the possible noninfectious factors that were described earlier, the pathogenesis is unclear. It is difficult to synthesize a pathophysiological mechanism that would explain how stress, overfeeding, changes in diet or constipation could result in acute swelling of the mammary gland in sows.
CLINICAL FINDINGS
Sometimes there may be a delay in parturition of more than 5 hours. The sow is usually normal, with a normal milk flow, for the first 12–18 hours after farrowing. Normally, the sow will suckle her piglets for about 20 seconds once an hour. One of the first indications of the disease is the failure of the sow to suckle her piglets. She is uninterested in the piglets, generally lies in sternal recumbency and is unresponsive to their squealing and sucking demands. Litters of affected sows are more noisy and are generally scattered around the pen searching for an alternative food supply. Such piglets may drink surface water or urine in the pen and infectious diarrhea may occur. If sucking is permitted, it does not progress from the vigorous nosing phase to the quiet letdown stage, and it is accompanied by much teat-to-teat movement by the piglets. Many piglets may die from starvation and hypoglycemia. A failure to grow at more than 105 g/d is a sure sign of piglet problems. Some sows are initially restless and stand up and lie down frequently, which contributes to a high mortality from crushing and trampling.19,20
Affected sows do not eat, drink very little and are generally lethargic. The body temperature is usually elevated and ranges from 39.5–41°C (103.1–105°F), especially if there is mastitis. Mild elevations in body temperatures of sows in the first 2 days after parturition are difficult to interpret because a slight elevation occurs in normal healthy sows.21 This is known as uncomplicated farrowing fever. However, temperatures above 40°C (104°F) are usually associated with acute mastitis that requires treatment. One detailed investigation of the disease in Sweden concluded that 78% of sows with a temperature exceeding 39.5°C had clinical evidence of mastitis. It is suggested that a temperature of 39.4°C at 12–18 hours after farrowing is an appropriate threshold at which to give preventive treatment for the disease. The heart and respiratory rates are usually increased.
Initial temperatures greater than 40.5°C (104.9°F) are usually followed by severe illness and toxemia. Normally, the sows get better within 3 days, but not always if the temperature is very high.
The characteristic findings are present in the mammary glands and consist of varying degrees of swelling and inflammation. In most cases, several sections are affected, which results in the appearance of diffuse involvement of the entire udder. Individual sections are enlarged, warm and painful, and may feel ‘meaty’ and lack the resilience of normal mammary tissue. There may be extensive subcutaneous edema around and between each section, which results in a ridge of edema on the lateral aspects of the udder extending for its entire length. The skin overlying the sections is usually reddened and is easily blanched by finger pressure. The teats are usually empty and may be slightly edematous. A few drops of milk may be expressed out of some teats after gentle massage of the section or the administration of oxytocin but rarely can a normal stream of milk be obtained. In severe cases of mastitis the milk contains flakes and pus or is watery.
The feces are usually scant and drier than normal but whether or not constipation is present in most cases is uncertain. The inappetence and anorexia and failure to drink normally could account for the reduced volume of feces. Constipation with impaction of the rectum with large quantities of feces is uncommon in sows and when it does occur as the only abnormality it has little effect on appetite and milk production.
A vaginal discharge is normal following parturition, and normal sows frequently expel up to 50 mL of a viscid, nonodorous, clear mucus that contains variable amounts of white material within the first 3 days following farrowing. Tenacious strands of this discharge may also be observed within the vagina. The presence of this discharge has been misleading and has been interpreted as evidence of the presence of metritis. Necropsy examination after euthanasia of affected sows has failed to reveal evidence of significant metritis. The clinical diagnosis of metritis in sows is difficult but generally large quantities of dark-brown, foul-smelling fluid are expelled several times daily, accompanied by severe toxemia. This is uncommon in sows. Diagnosis is usually on clinical signs.
CLINICAL PATHOLOGY
Examination of milk
The number of somatic cells in the milk from sows with mastitis will range from 2–20 × 109/mL compared to the normal of less than 2 × 109/mL. Significant numbers of bacteria are present in the milk of more than 80% of sows with toxic agalactia. Milk obtained for laboratory examination and culture should be taken after thorough cleaning and disinfection of the teats to minimize contamination by skin flora. However, because mastitis may be present in only one or a few of the mammary gland subsections in the sow and because it is often impossible to clinically identify affected subsections and distinguish them from unaffected adjacent glands, which may be swollen and agalactic because of continuous swelling, a valid assessment of intramammary infection is not possible unless milk samples are obtained from each subsection. Subclinical mastitis may not be easy to detect with cells not reaching 2 × 109/mL but 75% may be polymorphs. Normally, milk is around 1 × 109/mL.
Hematology and serum biochemistry
Some hematological and biochemical changes are present in affected sows but may not be marked enough to be a routine reliable diagnostic aid. In severe cases of infectious mastitis, a marked leukopenia with a degenerative left shift is common. In moderate cases there is a leukocytosis and a regenerative left shift. The serum biochemical changes that occur in naturally occurring cases and in the experimental disease are recorded. The plasma cortisol levels are commonly elevated, which may be due to a combination of the stress of parturition and infectious mastitis. The plasma protein-to-fibrinogen ratio is lower than normal and the plasma fibrinogen levels are commonly increased in severe cases that occur 8–16 hours after parturition.
NECROPSY FINDINGS
Neither lesions in the udder nor the reproductive tract are consistent. If they are found, the most important lesions are in the mammary gland. There may be extensive edema and some slight hemorrhage of the subcutaneous tissue. Grossly, on cross-section of the mammary tissue there is focal to diffuse reddening and often only one subsection of a mammary gland may be affected. Histologically, the mastitis may be focal or diffuse in distribution and the intensity of the lesion varies from a mild catarrhal inflammation to a severe purulent and necrotizing mastitis usually involving more than 50% of all the mammary glands. There are no significant lesions of the uterus when compared with the state of the uterus in normal healthy sows immediately after parturition. The adrenal gland is enlarged and heavier than normal, presumably due to adrenocortical hyperactivity. In a series of spontaneous cases, E. coli and Klebsiella spp. were most commonly isolated from the mammary tissues. The abscesses of the mammary glands of sows examined at slaughter are not sequelae to coliform mastitis but rather probably due to injuries and secondary infection.
Samples for confirmation of diagnosis
The characteristic clinical findings in toxic mastitis and agalactia are a sudden onset of anorexia and lack of interest in the piglets, acute swelling of the mammary gland, hypogalactia or agalactia, a moderate fever and a course of about 2 days. The mammary secretion from mastitic glands may be watery or thickened and contain pus, and the cell count will be increased up to 20 × 109/mL.
The acute swelling and agalactia of infectious mastitis must be differentiated from other non-infectious causes of acute swelling or ‘caking’ of the mammary glands, which also results in agalactia as follows:
• Agalactia due to a failure in milk letdown is most common in first-litter gilts and is characterized by a fullness of the mammary glands but an inability of the gilt to suckle her piglets in spite of her grunting at them. The gilt is usually bright and alert and systemically normal. The response to oxytocin is dramatic and repeat treatment is rarely necessary
• Farrowing fever is characterized clinically by loss of appetite, inactivity and a body temperature of 39.3–39.9°C (102.7–103.8°F) with minimal detectable changes of the mammary gland
• Parturient psychosis of sows is characterized by aggressive and nervous behavior of the sow after the piglets are born. The sow does not call the piglets and does not allow them to suck. When the piglets approach the sow’s head, she will back away, snap and make noisy staccato nasal expirations. Some sows will bite and kill their piglets. The mammary gland is usually full of milk but the sow will not let it down. Ataractic drugs and/or short-term general anesthesia are indicated and the response is usually excellent. Some sows need repeated tranquilization or sedation for the first few days until the maternal–neonatal bond is established
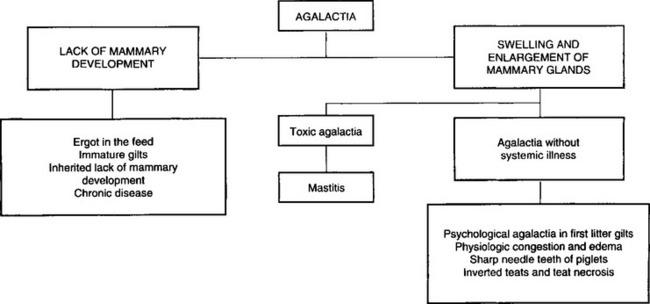
• Other causes of agalactia accompanied by enlargement of the mammary gland include inherited inverted teats and blind teats due to necrosis of the teats occurring when the gilt was a piglet. These are readily obvious on clinical examination. The sharp needle teeth of piglets may cause the sow to refuse to suckle her piglets. The sow attempts to suckle but leaps up suddenly, grunting and snapping at the piglets. The piglets squeal and fight to retain a teat, thus causing more damage to the teats, which is obvious on clinical examination. Other causes of agalactia accompanied by systemic illness include retained piglets and infectious disease such as outbreaks of transmissible gastroenteritis and erysipelas. The common causes of agalactia in pigs where there is lack of mammary development include ergotism, immature gilts and inherited lack of mammary development. These are set out in Figure 15.1.
TREATMENT
Most affected sows will recover within 24–48 hours if treated with a combination of antimicrobials, oxytocin and anti-inflammatory agents. The treatment should begin when the temperature reaches 39.4°C.22
Antimicrobials are indicated in most cases because infectious mastitis and metritis are the two most common causes of the disease. The choice is generally determined by previous experience in the herd or region but broad-spectrum antimicrobials are indicated because E. coli and Klebsiella spp. are the most common pathogens involved. They should be given daily for at least 3 days.23 Usually ampicillin, tetracyclines, trimethoprim– sulphonamide, or enrofloxacin is used.
As soon as possible after the disease is recognized every effort must be made to restore normal mammary function through the use of oxytocin and warm water massaging of the affected mammary glands.
Oxytocin 30–40 U intramuscularly or 20–30 U intravenously, is given, frequently, to promote the letdown of milk. If there is a beneficial response the piglets should be placed on the sow if she is willing to allow them to suck. This will assist in promoting milk flow. Massage of the mammary glands with towels soaked in warm water and hand milking for 10–15 minutes every few hours may assist in reducing the swelling and inflammation and promote the flow of milk. It will also relieve the pain and encourage the sow to suckle her piglets. Intramuscular injections of oxytocin may be repeated every hour, along with massaging of the glands with warm water. Failure of milk letdown or a low response following the use of oxytocin may be due to a reduced sensitivity of the sow to oxytocin during the first week of lactation. In the normal, healthy sow the peak response to oxytocin occurs in the second week of lactation and gradually decreases to a low response at the eighth week.
The use of a long-acting carba oxytocin analog is being explored as a possible substitute for oxytocin. Oxytocin has an effect for about 14 minutes, while the analog has an effect for about 6 hours. Preliminary results of its use in agalactic sows indicate superior results compared to oxytocin.
Anti-inflammatory agents are in common use for their anti-inflammatory effect but are rarely used on their own; flunixin meglumine has been shown to be beneficial and ketoprofen to alleviate pyrexia and endotoxemia. Recently, meloxicam and oxytocin were shown to reduce mortality compared to flunixin only.24 Plasma cortisol levels are increased in the experimental disease and for this reason may be contraindicated. However, field reports suggest that their use along with antimicrobials and oxytocin provides a better response than when they are not used. Corticosteroids used alone do not appear to prevent the disease or enhance recovery. To be effective they must be used in combination with antimicrobials and oxytocin. Dexamethasone at the rate of 20 mg intramuscularly daily for 3 days for sows weighing 150–200 kg has been recommended.
Supplementation of piglets
The hypoglycemic piglets must be given a supply of milk and/or balanced electrolytes and dextrose until the milk flow of the sow is resumed, which may take 2–4 days, and most important of all must be kept warm until body reserves are re-established. Piglets should receive 300–500 mL of milk per day divided into hourly doses of 40–50 mL given through a 12–14 French plastic tube passed orally into the stomach. A solution of balanced electrolytes containing 5% glucose can also be given for 1–2 days if a supply of cows’ milk is not available. Condensed canned milk diluted with water 1:1 is a satisfactory and readily available supply of milk. In severe cases where the return to milk production and flow are unlikely, the piglets should be fostered on to other sows. If these are unavailable, the use of milk substitute fortified with porcine gammaglobulin is recommended to prevent the common enteric diseases. This is discussed under colibacillosis. Many more piglets are treated for diarrhea when the sows are treated for MMA, perhaps up to 19% compared to up to 9% normally.1
CONTROL
It has been difficult to develop a rational approach to control because the disease has been considered to be a complex syndrome caused by several different factors. However, the control of infectious mastitis would seem to be of major importance. The routine use of antibiotics and oxytocin without indication does not appear to be helpful.24 Farrowing crates should be vacated, cleaned, disinfected and left vacant for a few days before pregnant sows are transferred from the dry sow barn and placed in the crates. Pregnant sows should be washed with soap and water before being placed in the crate. Farrowing crates must be kept clean and hosed down if necessary, particularly a few days before and after farrowing to minimize the level of intramammary infection. In problem herds, it may be necessary to wash and disinfect the skin over the mammary glands immediately after farrowing. All in/all out in the farrowing area with proper cleaning and disinfection facilitated by batch farrowing will reduce the disease. An opportunity for exercise will help, as under outdoor conditions (sows in paddocks) the condition is rare.
To minimize the stress to the sow of adjusting to the farrowing crate and the farrowing facilities, the sow should be placed in the crate at least 1 week before the expected date of farrowing.
The nature and composition of the diet fed to the sow while in the farrowing crate should not be changed. In order to minimize the risk of toxic agalactia, it is recommended that the daily feed allowance be related to body condition score. It may be necessary to reduce the feed to 1 kg/day (from 100 d gestation) before farrowing.25 The daily intake (compared to the intake during the dry period) may be increased on the day after the sow has farrowed and in increments thereafter as the stage of lactation proceeds. The inclusion of bran at the rate of one-third to one-half of the total diet for 2 days before and after farrowing has been recommended to prevent constipation. In some herds the use of lucerne meal or other vegetable protein at the rate of 15% of the diet may help control the disease. However, under intensified conditions it may be impractical to prepare and provide these special diets on a regular basis. While field observations suggest that a bulky diet at the time of farrowing will minimize the incidence of toxic agalactia, there is little scientific evidence to support the practice.
Antimicrobial agents used prophylactically have apparently been success-ful in controlling some outbreaks. A trimethoprim–sulfadimidine and sulfathiazole combination at 15 mg/kg in feed from day 112 of gestation to day 1 after farrowing may reduce the prevalence. Using oxytocin early may also help. In a recent study where E. coli, streptococci and staphylococci were the most cultured pathogens, marbofloxacin(10% solution) was found to be superior to amoxicillin. All E. coli, were susceptible to the former but 32% were resistant to the latter antibiotic.
The use of prostaglandins for the induction of parturition in sows has not been associated with a marked consistent change in the incidence of the disease. Some field trials have shown a reduction, while others have had no effect.
Smith BP. Pathogenesis and therapeutic management of lactation failure in periparturient sows. Compend Contin Educ Pract Vet. 1985;9:S523-S532.
Liptrap RM. Lactational failure. Vet Annu. 1987;47:134-138.
Martineau GP, et al. Mastitis, metritis and agalactia. Vet Clin North Am Food Anim Pract. 1992;8:661-684.
1 Thorup F. Proceedings of the 16th IPVS Congress, Melbourne 2000:97.
2 Klopfenstein C, et al. Proceedings of the 15th IPVS Congress, Birmingham. 1998;2:230.
3 Ringarp N. Acta Agric Scand Suppl. 1960:7.
4 Ramsoota P, et al. Acta Vet Scand. 2000;41:249.
5 Peter AT, et al. Res Vet Sci. 1985;39:222.
6 Persson A, et al. Acta Vet Scand. 1989;30:9.
7 Lingaas F, Ronningen K. Acta Vet Scand. 1991;32:89.
8 Lingaas F. Acta Vet Scand. 1991;32:97.
9 Lingaas F. Acta Vet Scand. 1991;32:107. 115
10 Chagnon M, et al. Can J Vet Res. 1991;55:180.
11 Persson A, et al. Acta Vet Scand. 1996;37:293.
12 Persson A. J Vet Med A. 1997;44:143.
13 Persson A, et al. Acta Vet Scand. 1996;37:279.
14 Pejsak Z, Tarasiuk K. Theriogenology. 1989;32:335.
15 Smith BB, Wagner WC. Am J Vet Res. 1985;46:175.
16 Smith BB. Compend Contin Educ Pract Vet. 1985;9:S523.
17 Madec F, et al. Rec Med Vet Ec Alfort. 1992;168:341.
18 Magnusson U, et al. J Vet Med B. 2001;48:501.
19 Backstrom L, et al. J Am Vet Med Assoc. 1984;185:70.
20 Delgado J, Jones JET. Aust Vet J. 1981;137:639-643.
21 King GJ, et al. Can Vet J. 1972;13:72.
22 Martineau GP, et al. Vet Clin North Am Food Anim Pract. 1992;8:661.
23 Rose M, et al. Proceedings of the 14th IPVS Congress, Bologna 1996:317.
24 Hirsch AC, et al. J Vet Pharmacol Ther. 2003;26:355.